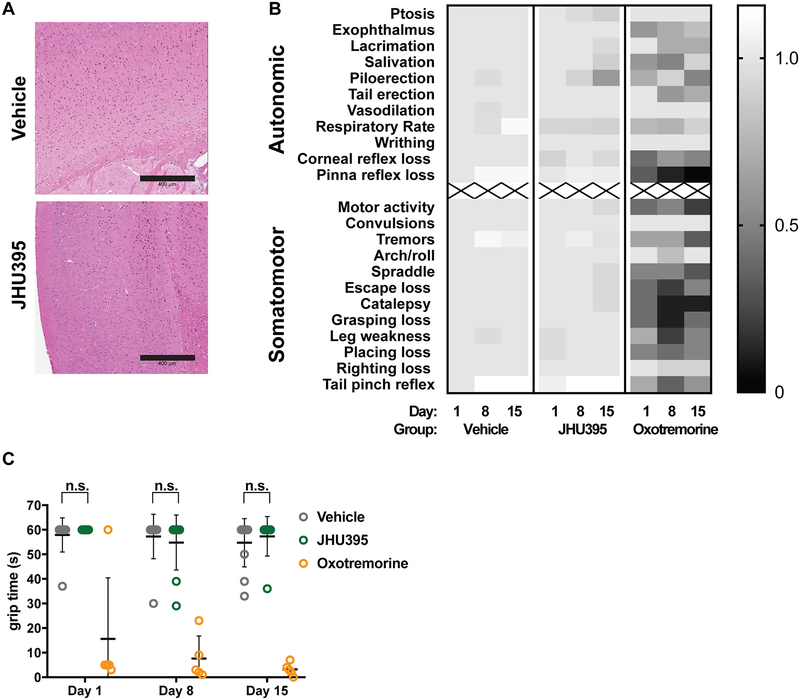
Figure 5:
Orally administered JHU395 has no overt neurotoxicity. A) Brain histology was evaluated in vehicle (PBS + 1% Tween-80 + 2.5% ethanol p.o. daily × 14 days; n=3) and JHU395 (1.2 mg/kg/day p.o. × 5 days, then 0.5 mg/kg p.o. × 9 days; n=3) treated mice. No cortical vacuolization was observed. Scalebar = 400 μm. B) Heatmap showing scoring on modified Irwin behavioral test of vehicle (n=11), JHU395 (n=9), or oxotremorine (n=5; assay positive control) B6 flank MPNST mice over a 14 day dosing period. Mean scores are normalized to day 0 vehicle group and shown per behavior per day. C) Time in seconds that vehicle, JHU395, and oxotremorine treated mice were able to hang from inverted wire in seconds on days 0, 8, and 15 as part of modified Irwin assay. Data shown is mean +/− S.D. p > 0.05 for vehicle and JHU395 grip times by multiple t-tests.