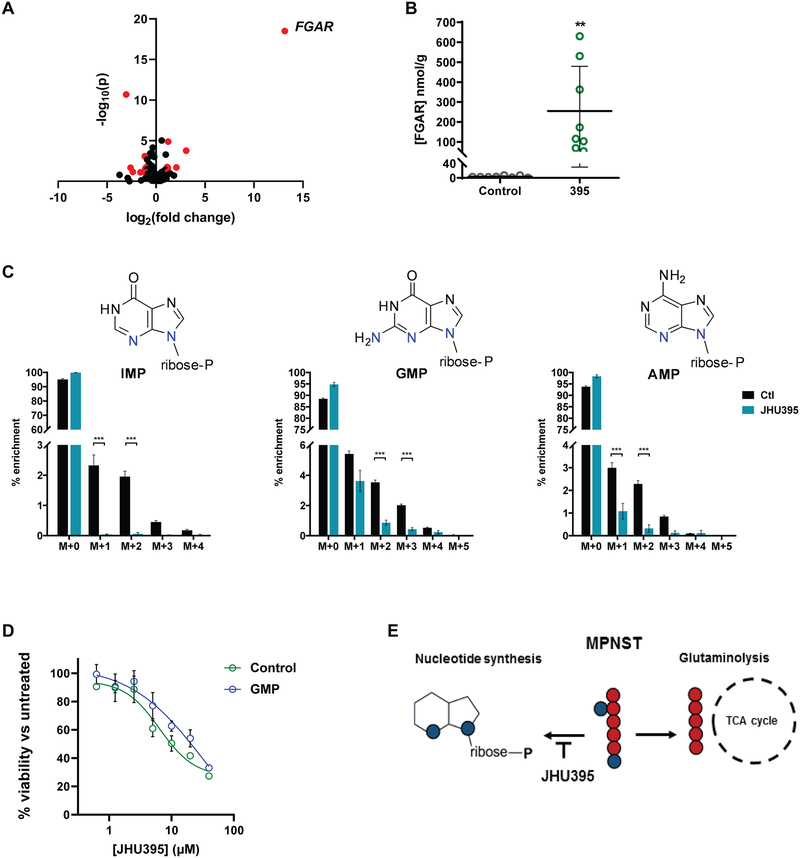
Figure 6:
Metabolomics analyses demonstrate JHU395 inhibition of tumor glutamine utilization in purine synthesis. A) Volcano plot of metabolites identified from murine flank MPNST (n = 8 tumors/group) harvested two hours after oral administration of JHU395 (1.2 mg/kg/dose) or vehicle. Metabolites selected by volcano plot with fold change threshold (x) 2 and t-tests threshold (y) 0.1 are shown in red circles. Both fold changes and p values are log transformed. B) Quantitative LC-MS analysis of FGAR from tumors (n = 8 per group) of vehicle or JHU395 treated murine flank MPNST. Data shown is mean +/− S.D.; p < 0.01 by t-test. C) 15N2-glutamine labeled JHU395-treated tumors show significantly decreased m+1, m+2 isotopologue enrichment (inosine monophosphate, adenosine monophosphate) and significantly decreased m+2, m+3 isotopologue enrichment (guanosine monophosphate) compared to vehicle-treated tumors. Data shown is for 6–7 animals per treatment representative of two independent experiments. Graphed data is mean percent enrichment +/− S.D. Statistical testing was done by multiple t-tests where *** p ≤ 0.001, ** 0.001 < p ≤ 0.01, * 0.01 < p ≤ 0.05. D) Percent viable human MPNST cells (sNF96.2) based on alamar blue fluorescence normalized to untreated controls following treatment with JHU395 in standard media (control) or media containing 100μM guanosine monophosphate (GMP). Viability was measured at 72 hours JHU395 treatment. E) Schematic of JHU395 inhibition of glutamine utilization for nucleotide synthesis versus glutaminolysis in human MPNST.