Abstract
Objective
To clarify whether cardiac cachexia (CC) alters the prognostic impact of other general risk factors in patients with heart failure (HF).
Methods
This was an observational study. CC was defined as the combination of a body mass index of < 20 kg/m2 and at least one of the following biochemical abnormalities: C-reactive protein > 5 mg/L; hemoglobin < 12 g/dL; and/or albumin < 3.2 g/dL. We divided 1608 hospitalized HF patients into a CC group (n = 176, 10.9%) and a non-CC group (n = 1432, 89.1%). The primary endpoints were cardiac event and all-cause death.
Results
The presence of CC showed significant interactions with other risk factors including cancer, estimated glomerular filtration rate (eGFR), and sodium in predicting these endpoints. Multiple Cox proportional analysis revealed that use of â blockers [hazard ratio (HR) = 1.900, 95% confidence interval (CI): 1.045–3.455, P = 0.035) and eGFR (HR = 0.989, 95% CI: 0.980–0.998, P = 0.018) were independent predictors of cardiac event in the CC group, while age (HR = 1.020, 95% CI: 1.002–1.039, P = 0.029) and hemoglobin (HR = 0.844, 95% CI: 0.734–0.970, P = 0.017) were independent predictors of all-cause death. The survival classification and regression tree analysis showed the optimal cut-off points for cardiac event (eGFR: 59.9 mL/min per 1.73 m2) and all-cause death (age, 83 years old; hemoglobin, 10.1 g/dL) in the CC group.
Conclusions
In predicting prognosis, CC showed interactions with several risk factors. Renal function, age, and hemoglobin were pivotal markers in HF patients with CC.
Keywords: Body mass index, Cachexia, Heart failure, Mortality, Prognosis
1. Introduction
Cachexia is a complex metabolic syndrome of which chronic illnesses such as chronic obstructive pulmonary disease (COPD), chronic heart failure (HF), cancer, and chronic kidney disease (CKD) are the common leading causes, in order.[1],[2] Cachexia associated with chronic HF is known as cardiac cachexia (CC), with a prevalence ranging from 5% to 15% in patients with chronic HF.[2],[3] CC is related to hemodynamic alterations such as congestion[4],[5] and consequent proinflammation, malabsorption, anorexia, and neurohormonal activation.[5]–[8] The presence of CC is a predictor of adverse prognosis, including all-cause death.[3],[4],[9],[10]
On the other hand, patients with HF have various prognostic risk factors in addition to CC, including impaired renal function, aging, and anemia.[3],[10]–[12] However, since CC has a multifactorial underlying pathophysiology in nature,[1],[5],[13] we hypothesized that the presence of CC alters the prognostic impact of these general risk factors in patients with HF. Thus, in the current study, we aimed to elucidate: (1) the interactions between the respective impacts of CC and coexisting prognostic risk factors; and (2) the independent prognostic risk factors and their impact in patients with CC.
2. Methods
2.1. Study design and patient population
This was a prospective observational cohort study of 2213 patients who were hospitalized at Fukushima Medical University Hospital for decompensated HF between January 2010 and December 2017. Diagnosis of HF was made by each attending cardiologist on the basis of the current guidelines.[3],[10] The exclusion criteria (n = 605) were as follows: (1) patients who were receiving maintenance dialysis; and (2) patients whose medical records were incomplete regarding body mass index (BMI), C-reactive protein (CRP), hemoglobin, and/or albumin. Finally, 1608 patients were included in this study. CC was defined on the basis of the previous studies as the combination of BMI < 20 kg/m2 and at least one of the following biochemical abnormalities: CRP > 5 mg/L, hemoglobin < 12 g/dL, and/or albumin < 3.2 g/dL.[1],[4],[14] We divided these patients based on the presence (the CC group n = 176, 10.9%) or absence (the non-CC group n = 1432, 89.1%) of CC. We compared the patients' characteristics and clarified post-discharge prognosis for cardiac event and all-cause death. A cardiac event was defined as rehospitalization due to worsening HF or cardiac death.[15] Cardiac death was defined as death due to worsening HF, acute coronary syndrome, or ventricular fibrillation documented by electrocardiogram or implantable devices.[15]
All subjects gave written informed consent to participate in the study. The study protocol was approved by the ethical committee of Fukushima Medical University. The investigation conforms with the principles outlined in the Declaration of Helsinki. Reporting of the study conforms with STROBE along with references to STROBE and the broader EQUATOR guidelines.
2.2. Data collection and classification
The patients' characteristics included demographic data and medications at the time of discharge. Blood samples and echocardiographic data were obtained within one week prior to discharge. Estimated glomerular filtration rate (eGFR) was calculated using the modified Modification of Diet in Renal Disease equation: eGFR (mL/min per 1.73 m2) = 194 × serum creatinine (−1.094) × age (−0.287) × 0.739 (if female).[16] As post-discharge follow-ups, status and dates of endpoints were obtained from the patients' medical records. If these data were unavailable, status was ascertained by a telephone call to the patient's referring hospital physician.[15]
Comorbidities were defined in accordance with the preceding studies.[15],[17],[18] Peripheral artery disease was diagnosed according to the guidelines using computed tomography, angiography, and/or ankle-brachial index.[17] Cancer was identified from the patient's medical records.[15] COPD was diagnosed based on the patient's medical records, the usage of drugs to treat COPD, or the results of spirometry (forced expiratory volume in 1 second/forced vital capacity < 0.70).[19],[20]
2.3. Statistical analysis
Normality was confirmed using the Shapiro-Wilk test in each group. Normally distributed variables were presented as mean ± SD, non-normally distributed variables were presented as median (interquartile range), and categorical variables were expressed as counts and percentages. Normally distributed variables were compared using the Student's t-test, non-normally distributed variables were compared using the Mann-Whitney U test, and the chi-square test was used for comparisons of categorical variables. Kaplan-Meier analysis was used to assess the two primary endpoints (cardiac event and all-cause death), and a log-rank test was used for initial comparisons. To fit the multifactorial pathophysiology of CC, clinically important prognostic risk factors were evaluated by the univariable Cox proportional hazard analysis separately based on the presence or absence of CC. Then, each prognostic risk factor, CC, and interaction between each prognostic risk factor and CC, were entered into a multivariable Cox proportional hazard model to obtain interaction P values. Moreover, we performed univariable and multivariable Cox proportional hazard analyses in the CC group. Risk factors which had P values of < 0.05 in univariable model were entered into multivariable model. The survival classification and regression tree (CART) analysis were then performed in the CC group to determine the optimal cut-off points in predicting the endpoints if factors had P values of < 0.05 in the multivariable model. These cut-off points were verified by the Kaplan-Meier analysis. P values of < 0.05 were considered statistically significant for all analyses. The survival CART analysis were performed with EZR ver. 1.40 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R ver. 3.5.2 (The R Foundation for Statistical Computing, Vienna, Austria). All other analyses were performed using SPSS ver. 26 (IBM, Armonk, NY, USA).
3. Results
3.1. Baseline patient characteristics
In the current study, 176 of 1608 patients (10.9%) belonged to the CC group. The comparisons of patients' characteristics are summarized in Table 1. The CC group patients were older, had a lower prevalence of male sex, lower BMI, and lower systolic blood pressure, compared with the non-CC group patients. With respect to past medical history, peripheral artery disease and cancer were more common in the CC group, although the prevalence of COPD did not differ between the two groups. Prescription rate of loop diuretics was higher, as were B-type natriuretic peptide levels, while eGFR and sodium levels were lower in the CC group. Echocardiography revealed no significant differences between the two groups, except for higher tricuspid regurgitation pressure gradient in the CC group.
Table 1. Patient characteristics.
| Non-CC (n = 1432) | CC (n = 176) | P value | |
| Demographic data | |||
| Age, yrs | 68.0 (58.0–76.0) | 76.0 (67.0–81.0) | < 0.001 |
| Male sex | 892 (62.3%) | 86 (48.9%) | 0.001 |
| Body mass index, kg/m2 | 23.4 (21.5–26.0) | 18.2 (17.2–19.1) | < 0.001 |
| Systolic blood pressure, mmHg | 123.0 (108.0–140.0) | 117.5 (101.5–137.0) | 0.021 |
| Past medical history | |||
| Hypertension | 994 (69.4%) | 107 (60.8%) | 0.020 |
| Diabetes mellitus | 567 (39.6%) | 70 (39.8%) | 0.964 |
| Atrial fibrillation | 576 (40.2%) | 69 (39.2%) | 0.795 |
| Coronary artery disease | 444 (31.0%) | 45 (25.6%) | 0.139 |
| Peripheral artery disease | 152 (17.2%) | 25 (28.4%) | 0.009 |
| Cerebrovascular disease | 257 (17.9%) | 39 (22.2%) | 0.174 |
| Cancer | 257 (18.7%) | 43 (25.9%) | 0.026 |
| COPD | 357 (29.0%) | 46 (32.4%) | 0.397 |
| Medications at discharge | |||
| β blockers | 1086 (75.8%) | 125 (71.0%) | 0.162 |
| ACEIs/ARBs | 1035 (72.3%) | 116 (65.9%) | 0.077 |
| Loop diuretics | 949 (66.3%) | 138 (78.4%) | 0.001 |
| Laboratory data | |||
| C-reactive protein, mg/L | 1.5 (0.6–6.0) | 6.7 (1.0–19.4) | < 0.001 |
| Hemoglobin, g/dL | 13.2 (11.6–14.6) | 11.0 (9.9–11.9) | < 0.001 |
| Albumin, g/dL | 3.9 (3.5–4.3) | 3.4 (2.9–3.8) | < 0.001 |
| BNP, pg/mL | 189.3 (67.0–495.1) | 468.7 (202.7–827.9) | < 0.001 |
| eGFR, mL/min per 1.73 m2 | 59.8 (46.3–73.2) | 56.3 (35.8–74.0) | 0.036 |
| Sodium, mEq/L | 140.0 (138.0–142.0) | 138.0 (135.0–140.0) | < 0.001 |
| Echocardiographic data | |||
| LVEF, % | 53.6 (39.0–63.9) | 56.2 (40.7–63.0) | 0.513 |
| TR-PG, mmHg | 24.6 (19.0–35.0) | 33.0 (21.6–40.3) | < 0.001 |
| RV-FAC, % | 41.7 (31.9–48.5) | 41.7 (34.1–47.9) | 0.968 |
Data are presented as n (%) or median (interquartile range). ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; BNP: B-type natriuretic peptide; CC: cardiac cachexia; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; LVEF: left ventricular ejection fraction; RV-FAC: right ventricular fractional area change; TR-PG: tricuspid regurgitation pressure gradient.
3.2. Post-discharge prognosis
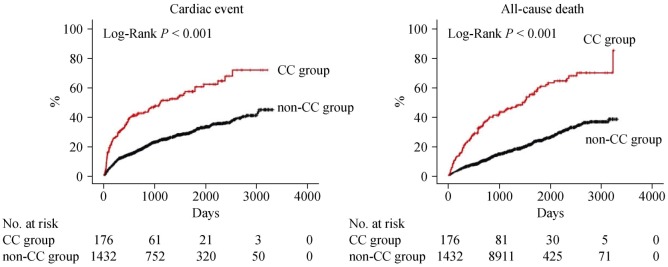
During the post-discharge follow-up period of median 1,295 days, there were 483 cardiac events and 419 all-cause deaths. The Kaplan-Meier analysis revealed that cardiac event rate and all-cause mortality were higher in the CC group than in the non-CC group (Figure 1, log-rank P < 0.001, respectively). In the univariable Cox proportional hazard analysis, CC was associated with both cardiac event [hazard ratio (HR) = 2.609, 95% confidence interval (CI): 2.078–3.277, P < 0.001] and all-cause death (HR = 3.246, 95% CI: 2.587–4.071, P < 0.001). Additionally, with respect to other risk factors, CC showed significant interactions with sex, cancer, loop diuretics, eGFR, and sodium in predicting cardiac event (Table 2). On the other hand, there were significant interactions between CC and age, hypertension, cancer, albumin, B-type natriuretic peptide, eGFR, and sodium in predicting all-cause death (Table 3).
Figure 1. Kaplan-Meier analysis.
Comparisons of rates of cardiac event and all-cause death between the CC and non-CC groups. CC: cardiac cachexia.
Table 2. Interactions between presence of CC and other risk factors in predicting cardiac event (event n = 483/1608).
| Subgroup | HR (95% CI) | P value | Interaction P value | |
| Age | Non-CC | 1.030 (1.021–1.038) | < 0.001 | 0.250 |
| CC | 1.017 (1.002–1.033) | 0.030 | ||
| Male Sex | Non-CC | 0.962 (0.785–1.179) | 0.709 | 0.020 |
| CC | 1.588 (1.051–2.400) | 0.028 | ||
| Body mass index | Non-CC | 0.981 (0.956–1.007) | 0.142 | 0.547 |
| CC | 1.023 (0.873–1.199) | 0.776 | ||
| Systolic blood pressure | Non-CC | 0.995 (0.992–0.999) | 0.020 | 0.202 |
| CC | 1.000 (0.994–1.007) | 0.948 | ||
| Hypertension | Non-CC | 1.417 (1.117–1.798) | 0.004 | 0.194 |
| CC | 1.050 (0.686–1.607) | 0.822 | ||
| Diabetes mellitus | Non-CC | 1.717 (1.408–2.094) | < 0.001 | 0.592 |
| CC | 1.472 (0.976–2.220) | 0.065 | ||
| Atrial fibrillation | Non-CC | 1.614 (1.323–1.968) | < 0.001 | 0.452 |
| CC | 1.351 (0.895–2.040) | 0.152 | ||
| Coronary artery disease | Non-CC | 1.116 (0.904–1.379) | 0.306 | 0.107 |
| CC | 1.618 (1.034–2.533) | 0.035 | ||
| Peripheral artery disease | Non-CC | 1.593 (1.187–2.137) | 0.002 | 0.256 |
| CC | 1.046 (0.525–2.085) | 0.898 | ||
| Cerebrovascular disease | Non-CC | 1.223 (0.955–1.565) | 0.111 | 0.321 |
| CC | 0.922 (0.561–1.514) | 0.749 | ||
| Cancer | Non-CC | 1.375 (1.074–1.761) | 0.011 | 0.016 |
| CC | 0.649 (0.376–1.119) | 0.120 | ||
| COPD | Non-CC | 1.514 (1.207–1.900) | < 0.001 | 0.795 |
| CC | 1.434 (0.888–2.317) | 0.141 | ||
| β-blockers | Non-CC | 1.642 (1.258–2.144) | < 0.001 | 0.888 |
| CC | 1.731 (1.043–2.875) | 0.034 | ||
| ACEIs/ARBs | Non-CC | 1.314 (1.030–1.677) | 0.028 | 0.606 |
| CC | 1.163 (0.745–1.816) | 0.507 | ||
| Loop diuretics | Non-CC | 4.148 (3.066–5.610) | < 0.001 | 0.035 |
| CC | 2.047 (1.137–3.685) | 0.017 | ||
| C-reactive protein | Non-CC | 0.999 (0.996–1.003) | 0.694 | 0.590 |
| CC | 0.997 (0.991–1.003) | 0.386 | ||
| Hemoglobin | Non-CC | 0.854 (0.817–0.893) | < 0.001 | 0.846 |
| CC | 0.870 (0.772–0.981) | 0.023 | ||
| Albumin | Non-CC | 0.612 (0.525–0.713) | < 0.001 | 0.114 |
| CC | 0.834 (0.598–1.164) | 0.286 | ||
| Log-BNP | Non-CC | 2.725 (2.249–3.302) | < 0.001 | 0.282 |
| CC | 1.891 (1.139–3.138) | 0.014 | ||
| eGFR | Non-CC | 0.974 (0.970–0.979) | < 0.001 | 0.028 |
| CC | 0.986 (0.978–0.994) | 0.001 | ||
| Sodium | Non-CC | 0.925 (0.901–0.949) | < 0.001 | <0.001 |
| CC | 1.017 (0.973–1.064) | 0.454 | ||
| LVEF | Non-CC | 0.985 (0.978–0.991) | < 0.001 | 0.939 |
| CC | 0.985 (0.970–1.000) | 0.050 | ||
| TR-PG | Non-CC | 1.001 (1.000–1.002) | 0.006 | 0.474 |
| CC | 0.996 (0.983–1.010) | 0.584 | ||
| RV-FAC | Non-CC | 0.992 (0.981–1.003) | 0.134 | 0.314 |
| CC | 1.004 (0.980–1.028) | 0.761 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CC: cardiac cachexia; COPD: chronic obstructive pulmonary disease; CI: confidence interval; eGFR: estimated glomerular filtration rate; HR: hazard ratio; Log-BNP: log-transformed B-type natriuretic peptide; LVEF: left ventricular ejection fraction; TR-PG: tricuspid regurgitation pressure gradient; RV-FAC: right ventricular fractional area change.
Table 3. Interactions between presence of CC and other risk factors in predicting all-cause death (event n = 419/1,608).
| Subgroup | HR (95% CI) | P value | Interaction P value | |
| Age | Non-CC | 1.056 (1.045–1.067) | < 0.001 | 0.003 |
| CC | 1.022 (1.006–1.039) | 0.009 | ||
| Male Sex | Non-CC | 1.110 (0.884–1.393) | 0.370 | 0.108 |
| CC | 1.552 (1.042–2.313) | 0.031 | ||
| Body mass index | Non-CC | 0.949 (0.920–0.978) | 0.001 | 0.557 |
| CC | 0.909 (0.785–1.053) | 0.203 | ||
| Systolic blood pressure | Non-CC | 0.997 (0.993–1.001) | 0.186 | 0.150 |
| CC | 1.003 (0.997–1.009) | 0.385 | ||
| Hypertension | Non-CC | 1.345 (1.028–1.760) | 0.031 | 0.009 |
| CC | 0.734 (0.490–1.100) | 0.134 | ||
| Diabetes mellitus | Non-CC | 1.409 (1.132–1.754) | 0.002 | 0.066 |
| CC | 0.925 (0.614–1.394) | 0.710 | ||
| Atrial fibrillation | Non-CC | 1.451 (1.165–1.806) | 0.001 | 0.077 |
| CC | 0.954 (0.637–1.431) | 0.821 | ||
| Coronary artery disease | Non-CC | 1.232 (0.980–1.548) | 0.074 | 0.468 |
| CC | 1.452 (0.940–2.245) | 0.093 | ||
| Peripheral artery disease | Non-CC | 1.592 (1.142–2.218) | 0.006 | 0.428 |
| CC | 1.188 (0.616–2.294) | 0.607 | ||
| Cerebrovascular disease | Non-CC | 1.460 (1.130–1.886) | 0.004 | 0.187 |
| CC | 1.025 (0.637–1.649) | 0.919 | ||
| Cancer | Non-CC | 2.594 (2.047–3.287) | < 0.001 | 0.012 |
| CC | 1.239 (0.772–1.990) | 0.375 | ||
| COPD | Non-CC | 1.229 (0.946–1.596) | 0.123 | 0.757 |
| CC | 1.130 (0.698–1.830) | 0.618 | ||
| β blockers | Non-CC | 1.002 (0.772–1.301) | 0.988 | 0.872 |
| CC | 0.952 (0.610–1.486) | 0.830 | ||
| ACEIs/ARBs | Non-CC | 0.800 (0.627–1.022) | 0.074 | 0.667 |
| CC | 0.719 (0.476–1.086) | 0.117 | ||
| Loop diuretics | Non-CC | 2.103 (1.594–2.775) | < 0.001 | 0.093 |
| CC | 1.275 (0.764–2.127) | 0.353 | ||
| C-reactive protein | Non-CC | 1.003 (1.000–1.005) | 0.035 | 0.625 |
| CC | 1.001 (0.996–1.006) | 0.610 | ||
| Hemoglobin | Non-CC | 0.768 (0.732–0.806) | < 0.001 | 0.238 |
| CC | 0.839 (0.743–0.947) | 0.005 | ||
| Albumin | Non-CC | 0.469 (0.398–0.553) | < 0.001 | 0.022 |
| CC | 0.718 (0.525–0.982) | 0.038 | ||
| Log-BNP | Non-CC | 3.184 (2.546–3.983) | < 0.001 | 0.015 |
| CC | 1.733 (1.067–2.814) | 0.026 | ||
| eGFR | Non-CC | 0.973 (0.968–0.979) | < 0.001 | <0.001 |
| CC | 0.991 (0.984–0.999) | 0.029 | ||
| Sodium | Non-CC | 0.913 (0.889–0.938) | < 0.001 | <0.001 |
| CC | 0.996 (0.956–1.037) | 0.833 | ||
| LVEF | Non-CC | 0.985 (0.977–0.992) | < 0.001 | 0.987 |
| CC | 0.986 (0.971–1.000) | 0.055 | ||
| TR-PG | Non-CC | 1.001 (1.001–1.002) | 0.001 | 0.445 |
| CC | 0.996 (0.983–1.010) | 0.613 | ||
| RV-FAC | Non-CC | 0.997 (0.984–1.009) | 0.606 | 0.590 |
| CC | 1.004 (0.981–1.027) | 0.738 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CC: cardiac cachexia; COPD: chronic obstructive pulmonary disease; CI: confidence interval; eGFR: estimated glomerular filtration rate; HR: hazard ratio; Log-BNP: log-transformed B-type natriuretic peptide; LVEF: left ventricular ejection fraction; TR-PG: tricuspid regurgitation pressure gradient; RV-FAC: right ventricular fractional area change.
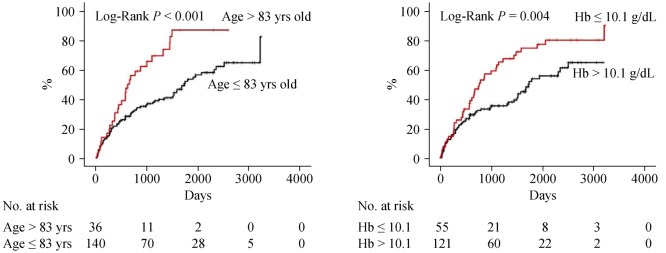
Next, we focused on the CC group (n = 176) and performed a multivariable Cox proportional hazard analysis (Table 4). Factors which had P values of < 0.05 in the univariable Cox analysis of a subgroup of CC in Tables 2 and 3 were analyzed. With regard to predicting cardiac event, use of â blockers (HR = 1.900, 95% CI: 1.045–3.455, P = 0.035) and eGFR (HR = 0.989, 95% CI: 0.980–0.998, P = 0.018) were independent predictors. On the other hand, age (HR = 1.020, 95% CI: 1.002–1.039, P = 0.029) and hemoglobin (HR = 0.844, 95% CI: 0.734–0.970, P = 0.017) were independent predictors of all-cause death. The survival CART analysis revealed the optimal cut-off points in predicting both cardiac event (eGFR, 59.9 mL/min per 1.73 m2) and all-cause death (age, 83 years old; hemoglobin, 10.1 g/dL) in the CC group. Finally, these cut-off points were verified by Kaplan-Meier analysis. CC patients with eGFR of ≤ 59.9 mL/min per 1.73 m2 experienced more cardiac event (Figure 2, log-rank P = 0.001). Similarly, CC patients with age > 83 years old, and those with hemoglobin of ≤ 10.1 g/dL had a higher rate of all-cause death (Figure 3, log-rank P < 0.001 and P = 0.004, respectively).
Table 4. Cox proportional hazard analysis in the CC group (n = 176).
| Univariable |
Multivariable |
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Cardiac event (event n = 92/176) | ||||||
| Age | 1.017 (1.002–1.033) | 0.030 | 1.013 (0.995–1.031) | 0.171 | ||
| Male sex | 1.588 (1.051–2.400) | 0.028 | 1.265 (0.801–1.999) | 0.314 | ||
| CAD | 1.618 (1.034–2.533) | 0.035 | 1.332 (0.823–2.157) | 0.244 | ||
| β-blockers | 1.731 (1.043–2.875) | 0.034 | 1.900 (1.045–3.455) | 0.035 | ||
| Loop diuretics | 2.047 (1.137–3.685) | 0.017 | 1.769 (0.909–3.443) | 0.093 | ||
| Hemoglobin | 0.870 (0.772–0.981) | 0.023 | 0.897 (0.789–1.018) | 0.093 | ||
| Log-BNP | 1.891 (1.139–3.138) | 0.014 | 1.411 (0.825–2.413) | 0.209 | ||
| eGFR | 0.986 (0.978–0.994) | 0.001 | 0.989 (0.980–0.998) | 0.018 | ||
| All-cause death (event n = 98/176) | ||||||
| Age | 1.022 (1.006–1.039) | 0.009 | 1.020 (1.002–1.039) | 0.029 | ||
| Male sex | 1.552 (1.042–2.313) | 0.031 | 1.313 (0.849–2.032) | 0.221 | ||
| Hemoglobin | 0.839 (0.743–0.947) | 0.005 | 0.844 (0.734–0.970) | 0.017 | ||
| Albumin | 0.718 (0.525–0.982) | 0.038 | 0.860 (0.576–1.284) | 0.461 | ||
| Log-BNP | 1.733 (1.067–2.814) | 0.026 | 1.611 (0.955–2.716) | 0.074 | ||
| eGFR | 0.991 (0.984–0.999) | 0.029 | 0.995 (0.987–1.004) | 0.264 | ||
CAD: coronary artery disease; CC: cardiac cachexia; CI: confidence interval; eGFR: estimated glomerular filtration rate; HR: hazard ratio; Log-BNP: log-transformed B-type natriuretic peptide.
Figure 2. Kaplan-Meier analysis for cardiac event in the CC group.
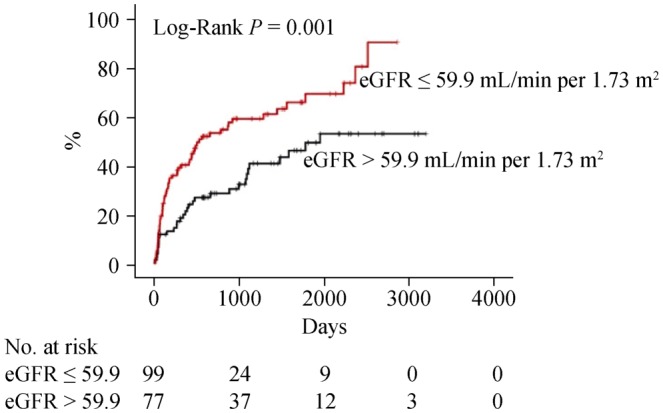
CC: cardiac cachexia; eGFR: estimated glomerular filtration rate.
Figure 3. Kaplan-Meier analysis for all-cause death in the CC group.
CC: cardiac cachexia.
4. Discussion
To the best of our knowledge, the present study is the first to focus on the interactions between CC and other important risk factors, as well as the first to determine independent prognostic factors in HF patients with CC. The main findings of this study were that (1) the presence of CC showed significant interactions with several important risk factors in predicting post-discharge prognosis, and (2) eGFR, age, and hemoglobin were independent predictors of post-discharge prognosis in patients with CC accompanied by useful cut-off points determined by the survival CART analysis.
The term “cachexia” comes from the Greek words kakós (bad) and hexis (condition or appearance), and is described as wasting.[2],[9] The CC group in this study demonstrated several unfavorable features as suggested by the name cachexia. Lower BMI in patients with HF suggests systemic inflammation, catabolism and higher right heart pressure, and is associated with higher cardiac and all-cause mortality.[18] Peripheral artery disease also predicts higher mortality and deteriorates exercise capacity because of its arterial obstruction, endothelial dysfunction, mitochondrial dysfunction, and inflammatory activation.[17],[21] Regarding other cachexia-associated comorbidities, the prevalence of COPD was similar between the CC and non-CC groups. However, COPD in patients with HF can be underrecognized, because both conditions exhibit similar symptoms (e.g., dyspnea and fatigue).[19],[20] COPD can lead to cachexia and is a predictor of adverse prognosis in patients with HF.[20],[22],[23] In the current study, the prevalence of cancer was higher and eGFR was lower in the CC group. These results suggest that cancer cachexia, CKD cachexia, and CC can coexist, or that one type of cachexia can lead to other types of cachexia. Patients with cancer cachexia experience cardiac atrophy and HF through underlying heart disease, cancer itself, or cardiotoxic effects of cancer treatment.[24]–[26] Kottgen, et al.[27] collected the data of a community-based prospective cohort and found that patients with eGFR of < 60 mL/min per 1.73 m2 had a 1.94-fold risk of incident HF compared to those with normal eGFR. Like this condition in which CKD contributes to HF, CKD cachexia causes HF.[14] Hasin, et al.[28] reported in their case-control study that patients with HF had a 1.68-fold risk of developing new cancer. In addition, the authors of the present study recently reported that HF patients with preexisting cancer demonstrated higher prevalence of CKD, COPD, and anemia compared to those without preexisting cancer.[15] These cachexia-associated vicious cross-talk have been explained by shared pathophysiology: metabolic disturbance, oxidative stress, chronic inflammation, and neurohormonal activation.[2],[14],[15],[25],[29] Thus, effective cachexia treatment requires the collaboration of various physicians, including cardiologists, oncologists, nephrologists, and pulmonologists.
With respect to prognosis prediction, we found several significant interactions between CC and other important factors. If the interactions were significant, the HRs of the CC group were all attenuated compared to those in the non-CC group except for male sex in predicting cardiac event. Cancer and sodium showed significant interactions with CC in predicting both cardiac event and all-cause death, suggesting that they were no longer associated with these endpoints in the CC group. Since cachexia is a condition that occurs following cancer and activation of renin-angiotensin-aldosterone system,[2],[5] the prognostic impacts of these factors would decrease when cachexia develops. However, these explanations remain a matter of speculation. Considering the results shown in Tables 2 and 3, physicians should keep in mind that the prognostic impact of general risk factors can be altered on the basis of the presence or absence of CC in patients with HF.
In our patients with CC, the cut-off value of eGFR in predicting cardiac event was similar to the cut-off value of CKD of 60 mL/min per 1.73 m2, which seemed to be reasonable and acceptable.[30] The cut-off value of hemoglobin for predicting all-cause death was slightly lower than the cut-off value for CC diagnosis. Although the causes of decreased hemoglobin (e.g., deficiency of iron, vitamin B12 or folic acid, renal anemia, or occult bleeding) were unclear, anemic patients with CC were presumed to be associated with advanced myocardial remodeling, inflammation, and volume overload.[31] The results from the multivariable Cox proportional hazard analysis of the current study suggest that eGFR and hemoglobin are pivotal biomarkers in patients with CC.
The limitations of this study are worth noting to avoid overstating the results. For the first, the diagnostic criteria of CC included BMI at discharge, not weight loss within a certain period. Secondly, since this was a single-center study with a relatively small number of patients, our results should be considered as preliminary. Further studies including large population and consideration of pre- and post-discharge weight change are required.
Acknowledgments
The authors thank Ms. Kumiko Watanabe, Ms. Hitomi Kobayashi, and Ms. Tomiko Miura for their technical assistance. This study was supported in part by a Grant-in-Aid for Scientific Research (No. 16K09447) from the Japan Society for the Promotion of Science. AY and TM belong to a Department supported by Fukuda-denshi Co, Ltd. This company is not associated with contents of this study.
References
- 1.Evans WJ, Morley JE, Argiles J, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle. 2010;1:1–5. doi: 10.1007/s13539-010-0002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 4.Melenovsky V, Kotrc M, Borlaug BA, et al. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J Am Coll Cardiol. 2013;62:1660–1670. doi: 10.1016/j.jacc.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 5.Valentova M, von Haehling S, Bauditz J, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–1691. doi: 10.1093/eurheartj/ehw008. [DOI] [PubMed] [Google Scholar]
- 6.Anker SD, Ponikowski PP, Clark AL, et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683–693. doi: 10.1053/euhj.1998.1446. [DOI] [PubMed] [Google Scholar]
- 7.Pittman JG, Cohen P. The Pathogenesis of Cardiac Cachexia. N Engl J Med. 1964;271:403–409. doi: 10.1056/NEJM196408202710807. [DOI] [PubMed] [Google Scholar]
- 8.Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. doi: 10.1378/chest.115.3.836. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 10.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 11.Yao YN, Zhang RC, An T, Zhang Q, Zhao XK, Zhang J. Different prognostic association of systolic blood pressure at different time points with postdischarge events in patients hospitalized for decompensated heart failure. J Geriatr Cardiol. 2019;16:676–688. doi: 10.11909/j.issn.1671-5411.2019.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mene-Afejuku TO, Moisa EA, Akinlonu A, et al. The relevance of serum albumin among elderly patients with acute decompensated heart failure. J Geriatr Cardiol. 2019;16:522–528. doi: 10.11909/j.issn.1671-5411.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lena A, Coats AJS, Anker MS. Metabolic disorders in heart failure and cancer. ESC Heart Fail. 2018;5:1092–1098. doi: 10.1002/ehf2.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cicoira M, Anker SD, Ronco C. Cardio-renal cachexia syndromes (CRCS): pathophysiological foundations of a vicious pathological circle. J Cachexia Sarcopenia Muscle. 2011;2:135–142. doi: 10.1007/s13539-011-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshihisa A, Ichijo Y, Watanabe K, et al. Prior history and incidence of cancer impacts on cardiac prognosis in hospitalized patients with heart failure. Circ J. 2019;83:1709–1717. doi: 10.1253/circj.CJ-19-0279. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S, Imai E, Horio M, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura Y, Kunii H, Yoshihisa A, et al. Impact of peripheral artery disease on prognosis in hospitalized heart failure patients. Circ J. 2015;79:785–793. doi: 10.1253/circj.CJ-14-1280. [DOI] [PubMed] [Google Scholar]
- 18.Takiguchi M, Yoshihisa A, Miura S, et al. Impact of body mass index on mortality in heart failure patients. Eur J Clin Invest. 2014;44:1197–1205. doi: 10.1111/eci.12354. [DOI] [PubMed] [Google Scholar]
- 19.Roversi S, Fabbri LM, Sin DD, et al. Chronic obstructive pulmonary disease and cardiac diseases. An urgent need for integrated care. Am J Respir Crit Care Med. 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Yoshihisa A, Oikawa M, et al. Prognostic impact of chronic obstructive pulmonary disease on adverse prognosis in hospitalized heart failure patients with preserved ejection fraction—A report from the JASPER registry. J Cardiol. 2019;73:459–465. doi: 10.1016/j.jjcc.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation. 2011;123:87–97. doi: 10.1161/CIRCULATIONAHA.109.881888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am Heart J. 2013;165:193–199. doi: 10.1016/j.ahj.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64:256–264. doi: 10.1016/j.jjcc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Murphy KT. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am J Physiol Heart Circ Physiol. 2016;310:H466–477. doi: 10.1152/ajpheart.00720.2015. [DOI] [PubMed] [Google Scholar]
- 25.Kazemi-Bajestani SM, Becher H, Fassbender K, Chu Q, Baracos VE. Concurrent evolution of cancer cachexia and heart failure: bilateral effects exist. J Cachexia Sarcopenia Muscle. 2014;5:95–104. doi: 10.1007/s13539-014-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkhudaryan A, Scherbakov N, Springer J, Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail. 2017;4:458–467. doi: 10.1002/ehf2.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottgen A, Russell SD, Loehr LR, et al. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J Am Soc Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 28.Hasin T, Gerber Y, McNallan SM, et al. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belloum Y, Rannou-Bekono F, Favier FB. Cancer-induced cardiac cachexia: pathogenesis and impact of physical activity (Review) Oncol Rep. 2017;37:2543–2552. doi: 10.3892/or.2017.5542. [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 31.O'Meara E, Rouleau JL, White M, et al. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail. 2014;7:773–781. doi: 10.1161/CIRCHEARTFAILURE.114.001100. [DOI] [PubMed] [Google Scholar]