Abstract
Legionella pneumophila is the causative agent of the lung malady Legionnaires' disease, it modulates host function to create a niche termed the Legionella-containing vacuole (LCV) that permits intracellular L. pneumophila replication. One important aspect of such modulation is the co-option of the host ubiquitin network with a panel of effector proteins. Here, using recombinantly expressed and purified proteins, analytic ultracentrifugation, structural analysis, and computational modeling, along with deubiquitinase (DUB), and bacterial infection assays, we found that the bacterial defective in organelle trafficking/intracellular multiplication effector Ceg23 is a member of the ovarian tumor (OTU) DUB family. We found that Ceg23 displays high specificity toward Lys-63–linked polyubiquitin chains and is localized on the LCV, where it removes ubiquitin moieties from proteins ubiquitinated by the Lys-63–chain type. Analysis of the crystal structure of a Ceg23 variant lacking two putative transmembrane domains at 2.80 Å resolution revealed that despite very limited homology to established members of the OTU family at the primary sequence level, Ceg23 harbors a catalytic motif resembling those associated with typical OTU-type DUBs. ceg23 deletion increased the association of Lys-63–linked polyubiquitin with the bacterial phagosome, indicating that Ceg23 regulates Lys-63–linked ubiquitin signaling on the LCV. In summary, our findings indicate that Ceg23 contributes to the regulation of the association of Lys-63 type polyubiquitin with the Legionella phagosome. Future identification of host substrates targeted by Ceg23 could clarify the roles of these polyubiquitin chains in the intracellular life cycle of L. pneumophila and Ceg23's role in bacterial virulence.
Keywords: bacteria, bacterial pathogenesis, structural biology, deubiquitylation (deubiquitination), polyubiquitin chain, effector protein, Legionella pneumophila, ovarian-tumor family deubiquitinases, type IV secretion system
Introduction
Ubiquitination is a widely used post-translational modification of proteins that is highly conserved in eukaryotes (1). Through sequential action of the E1-activating enzyme, E2-conjugation enzyme, and the E3-ubiquitin ligase, the ubiquitin molecule is covalently linked to a target protein mostly via an isopeptide bond between the glycine residue in its C terminus and the ϵ-amino group of lysine residues (1). It is believed that the great majority of proteins are ubiquitinated at least one time prior to being degraded, this post-translational modification therefore regulates virtually all cellular functions, particularly cellular homeostasis, cell cycle, immune responses, vesicle trafficking, and DNA damage responses (2). Such diversity in function in part is accomplished by the formation of topologically distinct architecture formed by different types of ubiquitin modifications, which dictate the fate of modified proteins. A protein can be either modified by a single ubiquitin moiety (monoubiquitination) or by polymeric ubiquitin chains that are assembled by multiple ubiquitin molecules (polyubiquitination). Seven lysine residues (Lys-6, Lys-11, Lys-27, Lys-29, Lys-33, Lys-48, and Lys-63), as well as the primary methionine (Met-1), in the ubiquitin can be used for the formation of polyubiquitin chains, resulting in the generation of eight homotypic and multiple heterotypic ubiquitin chains (3–5). In addition, components of the ubiquitination machinery including E2 and E3 enzymes and ubiquitin itself can be modified by either ubiquitination or other forms of post-translational modifications, which further increases the scope and intricacy of cellular events regulated by ubiquitination (6).
Ubiquitin moieties can be removed from substrate proteins by the action of specific deubiquitinating enzymes (DUBs)5 that cleave the isopeptide bond (7). Therefore, DUBs together with the ubiquitination machinery dictate ubiquitin-dependent processes. DUBs are currently grouped into seven structurally distinct families: the ubiquitin–C-terminal hydrolases, ubiquitin-specific proteases, Machado–Joseph domain DUBs, ovarian-tumor (OTU) domain DUBs, the motif interacting with ubiquitin–containing novel DUB family, ZUFSP/ZUP1, and the Jab1/Mov34/Mpr1 Pad1 N-terminal+ (JAMM) domain proteases (8). All DUBs families, except for the JAMM domain protease family, are cysteine proteases that utilize either a catalytic triad (Cys, His, Asn, or Asp) or dyad (Cys and His) for catalysis (8, 9).
Because of its extensive involvement in the regulation of cellular processes in eukaryotes, the ubiquitin system is a common target for pathogenic or symbiotic microorganisms that employ diverse mechanisms to co-opt the ubiquitin network to establish intimate relationships with their hosts (10). Among these, bacterial pathogens have evolved multiple strategies to manipulate the host ubiquitination machinery during infection, including functional mimicry of E3 ubiquitin ligases and DUBs (11). For instance, SopA of Salmonella typhimurium mimics mammalian HECT E3 ubiquitin ligases to modulate immune responses by targeting two host TRIM family E3 ligases (12, 13). Members of the NleG family effectors from pathogenic Escherichia coli are U-box domain–containing E3 ubiquitin ligases (14). In addition, pathogenic bacteria have evolved novel types of E3 ligase without significant structural similarity to members of the HECT or RING protein family. One such example is the IpaH family of effectors from Shigella species (15–17), SspH1 and SspH2 from Salmonella (18, 19). Similarly, DUBs have been found in the arsenal of virulence factors of many bacterial pathogens (20).
Legionella pneumophila is the causative agent of Legionnaires' disease, a potentially fatal form of pneumonia (21). In the environment, the bacteria survive and grow in diverse species of amoebae, which is believed to provide the primary evolutionary pressure for its acquisition and maintenance of traits required for its adaption to an intracellular life cycle in phagocytes (22, 23). After phagocytosis, the bacterium resides in a membrane-bound compartment called the Legionella-containing vacuole (LCV) that embarks a unique trafficking route featured by sequential interactions with components of the secretory pathway, the endoplasmic reticulum (ER), mitochondria, and ribosomes, leading to the biogenesis of a compartment resembling the ER that supports intracellular bacterial growth (24). Biogenesis of the LCV requires the Dot/Icm type IV secretion system that translocates hundreds of effectors into host cells where they modulate diverse host cellular processes to facilitate the development of the LCV (25).
The observation that the AAA+ ATPase Cdc48/p97 involved in segregating polyubiquitinated proteins from complexes or membranes is crucial for intracellular bacterial replication highlights the importance of the ubiquitin network in L. pneumophila virulence (26). Moreover, consistent with the fact that the Dot/Icm transporter is required for the enrichment of ubiquitin species on the LCV (26), a cohort of Dot/Icm effectors have been characterized as E3 ubiquitin ligases, either through the adoption of classical E3 domains such as U-box and F-box or by novel catalytic mechanisms (27). In particular, members of the SidE family (SidEs) catalyze ubiquitination of target proteins without the need of E1 and E2 enzymes (28–30). SidEs also harbor a DUB domain in their N termini that cleaves the isopeptide bond installed by ubiquitination by a Cys–His–Asp catalytic triad (31). The DUB activity of SidEs negatively regulates the association of polyubiquitin species on the LCV (31). LotA, another Dot/Icm effector, is a DUB of the OTU family that uniquely harbors two catalytic pockets (32). In addition, RavD is a DUB that specifically attacks linear ubiquitin chains to down-regulate host immune responses (33). Here, we present evidence to demonstrate that the Dot/Icm effector Ceg23 (Lpg1621) (34) is an OTU domain-containing DUB that regulates the association of Lys-63–linked polyubiquitin with the LCV by specifically targeting Lys-63–linked polyubiquitin chains.
Results
The Dot/Icm substrate Ceg23 is a deubiquitinase of the OTU subfamily
L. pneumophila extensively manipulates the host ubiquitin network with scores of proteins that catalyze ubiquitination (27). In addition, at least three categories of deubiquitinases have been characterized, including RavD, which is specific for linear ubiquitin chains (33); LotA (32); and those associated with members of the SidE effector family (31). Considering the possibility that additional DUBs are employed by the bacterium to explore host function, we analyzed Dot/Icm substrates (35) for the presence of motifs potentially involved in deubiquitination. By pairwise comparison of profile hidden Markov models (HHpred) (36), we found that the N-terminal portion of Ceg23, a protein translocated by the Dot/Icm transporter, is distantly similar to members of the ovarian tumor (OTU) protein subfamily. Ceg23 is 439 amino acids in length with two predicted transmembrane domains located at its C terminus (Fig. 1A).
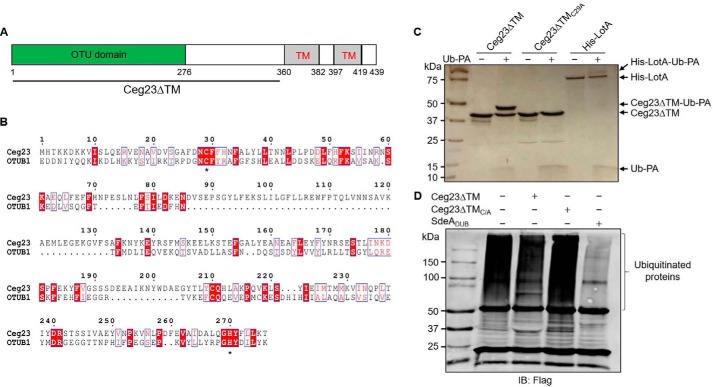
Figure 1.
Ceg23 shows DUB activity in vitro. A, a diagram of the predicted domain structure of Ceg23. B, sequence alignment of Ceg23 with OTUB1 (NCBI reference sequence NP_060140.2), a member of the eukaryotic OTU protein DUB superfamily. Alignment was generated by Clustal Omega and ESPript 3.0. Residues with the red background indicate identical sites, and residues highlighted by blue boxes represent conserved sites. Asterisks indicate the cysteine and histidine residues potentially critical for catalysis. C, Ceg23ΔTM but not Ceg23ΔTMC29A reacted with the suicide probe Ub-PA. LotA, a Legionella OTU-like DUB, was used as a positive control. D, Ceg23ΔTM removes ubiquitin chains from modified proteins. Polyubiquitinated proteins immunoprecipitated using agarose beads coated with the anti-FLAG antibody from HEK293T cells transfected to express 4× FLAG–Ub were incubated with Ceg23ΔTM or Ceg23ΔTMC29A. Ubiquitin signals were detected by the anti-FLAG antibody. The established DUB SdeADUB was included as a positive control. The results in C and D show one representative from three independent experiments.
Proteins of the OTU family are cysteine proteases with DUB activity, which have emerged as important regulators of many essential signaling pathways (37). Sequence alignment between Ceg23 and OTUB1, which is the founding member of the OTU family, revealed that the putative catalytic cysteine (Cys-29) and histidine (His-270) residues are surrounded by two short conserved elements (Fig. 1B), suggesting that Ceg23 may have DUB activity.
To further examine the potential cysteine-based DUB activity of Ceg23, we circumvented the problem of expression and insolubility of full-length protein by constructing Ceg23ΔTM (Ceg231–352), a mutant lacking the two predicted transmembrane domains, which allowed the production of recombinant protein from E. coli (Fig. S1). Incubation of Ceg23ΔTM with the suicide probe ubiquitin propargylamide (Ub-PA), a specific inhibitor of most OTU DUBs (38), led to the formation of a conjugate species that is ∼8 kDa larger than Ceg23ΔTM (Fig. 1C). When the putative catalytic Cys-29 was mutated to alanine (Ceg23ΔTMC29A), its ability to form the conjugate with Ub-PA was abolished (Fig. 1C). We also examined the ability of Ceg23ΔTM to remove ubiquitin from modified proteins obtained from mammalian cells. We transfected HEK293T cells with a construct expressing 4× FLAG–Ub and prepared proteins modified by this tagged ubiquitin using beads coated with the FLAG-specific antibody. When equal amounts of beads carrying ubiquitinated proteins were incubated with Ceg23ΔTM or Ceg23ΔTMC29A, Ceg23ΔTM but not its cysteine-substituted mutant reduced the polyubiquitin signals (Fig. 1D). Thus, Ceg23 is a DUB, and Cys-29 acts as its catalytic cysteine residue.
Ceg23ΔTM specifically cleaves Lys-63–linked ubiquitin chains
Polyubiquitin chains can be generated by isopeptide bonds via the primary methionine (which forms linear ubiquitin) and one of its seven lysine residues, and DUBs often exhibit specificity toward chains linked by certain lysine sites. To determine the linkage specificity of Ceg23ΔTM, we used a gel-based cleavage assay to examine the sensitivity of diubiquitins formed by one of the eight possible linkage types to Ceg23ΔTM. Instant cleavage was observed when Ceg23ΔTM was incubated with Lys-63–linked diubiquitin, and 10 min of incubation led to almost complete cleavage (Fig. 2A). However, cleavage of diubiquitins linked by the primary methionine or each of the other six lysines did not occur even when the reactions were allowed to proceed for 2 h (Fig. 2B). Ceg23ΔTM also cleaved Lys-63–linked polyubiquitin chains produced by biochemical reactions, as well as more biologically relevant ubiquitinated substrates purified from mammalian cells expressing 4× FLAG–Ub63K, an ubiquitin derivative that harbors only Lys-63 (Fig. 2, C and D). Mutation in the catalytic cysteine abolished its ability to cleave both Lys-63–linked diubiquitin and polyubiquitin chains (Fig. 2, B–D). These results establish a Lys-63–specific DUB activity of Ceg23, which suggests its role in the regulation of host signaling pathways controlled by Lys-63 ubiquitination.
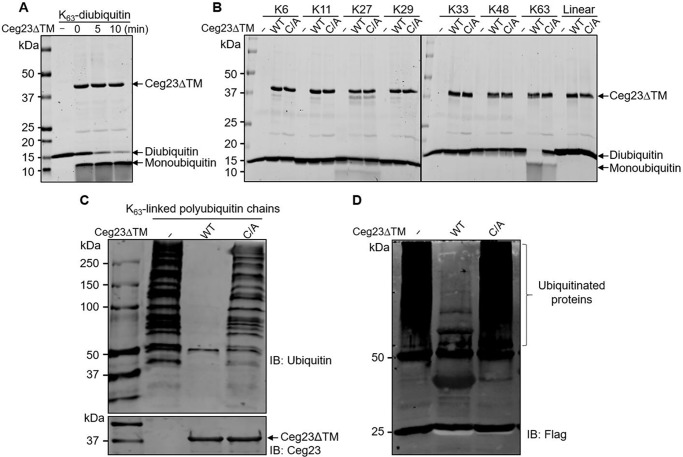
Figure 2.
Ceg23 specifically cleaves Lys-63–linked ubiquitin chains. A, time-dependent cleavage of Lys-63–linked diubiquitin by Ceg23ΔTM. 1 μm of Ceg23ΔTM was incubated with 1 μm of diubiquitin at 37 °C for the indicated time. B, Ceg23ΔTM induced cleavage of diubiquitins linked by the primary methionine and each of the seven lysine residues. 1 μm of Ceg23ΔTM was incubated with 1 μm of diubiquitin at 37 °C for 2 h, and DUB activity was indicated by the release of free ubiquitin. C and D, disassembly of Lys-63–linked polyubiquitin chains by Ceg23ΔTM. Polyubiquitin chains assembled by Lys-63 were either synthesized by an in vitro reaction containing ubiquitin, E1, UBE2V2, and UBE2N (C) or isolated from cells transfected with pCMV–4× FLAG–Ub63K (a ubiquitin mutant that harbors only Lys-63) (D). After incubation with Ceg23ΔTM, polyubiquitin was probed by antibodies specific for ubiquitin or for the FLAG tag. The results in A–D show one representative from three independent experiments. IB, immunoblot.
Ceg23ΔTM harbors a classical OTU fold
To determine the molecular basis of the DUB activity of Ceg23, we solved the structure of Ceg23ΔTM at a 2.80 Å resolution using the selenium single-wavelength anomalous dispersion method. Data collection and structure refinement statistics are summarized in Table 1. The structure belongs to the space group P 21 21 21 and contains two Ceg23ΔTM molecules in one asymmetric unit (Fig. S2A). When applied to a Superdex 200 increase column, Ceg23ΔTM was eluted at 14.50 ml with an estimated molecular mass higher than 75 kDa (Fig. S2B). However, results from the PISA server (39) and analytical ultracentrifugation (Fig. S2C) indicate that Ceg23ΔTM mainly exists as a monomer in solution. Although Ceg23 has only very limited sequence identity with other members of the OTU family, the structure of Ceg23ΔTM reveals that it contains a classical OTU fold (Fig. 3A). A unique insertion region that comprises residues Gly-126 to Ala-203 extending from the central catalytic domain is present in Ceg23ΔTM but not in members of the OTU family whose structures are available (Fig. 3A). In addition, a hydrophobic helical arm and a potential S1 binding site that are structurally conserved in all OTU domains are also found in Ceg23ΔTM (Fig. 3A). A search of the DALI server (40) hit the structures of ten OTU family members including OTUD3, OTUD5, OTU1, and an OTU DUB in the Crimean-Congo hemorrhagic fever virus (Table 2). The structure with the highest similarity to Ceg23ΔTM is OTUD1 from humans (PDB code 4BOP, RMS deviation 2.6 Å over 122 Cα) (37) (Fig. 3B). Superposition of Ceg23ΔTM and human OTUD1 revealed the common papain-like OTU core fold in which the catalytic triad Cys-29, His-270, and Asp-21 of Ceg23ΔTM fit well with the corresponding Cys-320, His-431, and Asp-433 of OTUD1 (Fig. 3, C and D). Furthermore, cleavage of Lys-63–linked diubiquitin requires Cys-29 or His-270, but not Asp-21 (Fig. 3E), which is similar to Ala-20, a DUB whose Asp-70 involved in the formation of the catalytic pocket is dispensable for its activity (41). Interestingly, the S1 site potentially involved in ubiquitin binding is within the insertion region (Fig. 3A), suggestive of a role in substrate recognition.
Table 1.
Data collection and refinement statistics
| Ceg23ΔTM | |
|---|---|
| Data collection | |
| Wavelength (Å) | 0.9798 |
| Resolution range (Å)a | 64.02–2.80 (2.90–2.80) |
| Space group | P 21 21 21 |
| Cell dimensions | |
| a, b, c (Å) | 67.35, 71.61, 142.87 |
| α, β, γ (°) | 90.00, 90.00, 90.00 |
| Total reflections | 223,467 |
| Unique reflections | 17,429 (1654) |
| Multiplicity | 12.8 (13.3) |
| Completeness (%) | 98.76 (96.73) |
| Wilson B-factor | 88.43 |
| Mean I/σ(I) | 14.2 (2.5) |
| Rmerge | 0.122 (0.878) |
| Rmeas | 0.128 (0.912) |
| Rpim | 0.068 (0.246) |
| CC½ | 0.995 (0.901) |
| Anomalous CC | 0.557 (0.005) |
| Refinement | |
| Resolution (Å) | 64.02–2.80 |
| Reflections used in refinement | 17,418 (1655) |
| Rwork | 0.2748 (0.3748) |
| Rfree | 0.2986 (0.3625) |
| Number of nonhydrogen atoms | 4689 |
| Macromolecule atoms | 4689 |
| Solvent | 4 |
| RMS | |
| Bonds (Å) | 0.012 |
| Angles (°) | 0.68 |
| Ramachandran (%) | |
| Favored | 95.76 |
| Allowed | 4.24 |
| Rotamer outliers (%) | 0.00 |
a The values in parentheses are for highest-resolution shell.
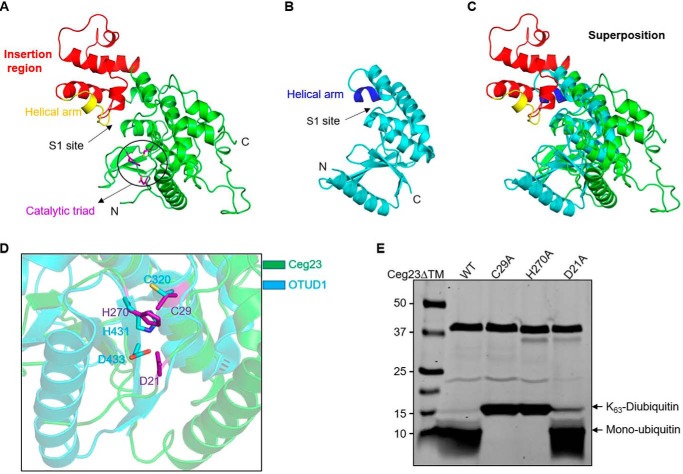
Figure 3.
Structural features of Ceg23ΔTM. A, structure of Ceg23ΔTM in ribbon representations. The insertion region is colored in red, and the helical arm for ubiquitin binding is highlighted in yellow. Residues participating in the formation of the catalytic site shown with purple sticks are highlighted by a circle. B, crystal structure of the human OTUD1 (PDB code 4BOP), one of the OTU family DUBs. The ubiquitin-contacting helical arm is colored in blue. C, superposition of Ceg23ΔTM (colored as in A) and OTUD1 (colored as in B). D, zoom-in view of the aligned active site regions of Ceg23ΔTM and OTUD1. E, cleavage of Lys-63–linked diubiquitin by C29A, H270A, and D21A mutants of Ceg23ΔTM. 1 μm of Ceg23ΔTM was incubated with 1 μm of Lys-63–linked diubiquitin at 37 °C for 5 min. Note that mutation in Cys-29 or H270A almost completely abolished its activity, whereas mutation in Asp-21 only slightly affects its ability to cleave Lys-63–linked diubiquitin. E is one representative from three independent experiments.
Table 2.
DALI search results of the Ceg23 structure
| PDB entry | Z score | RMS deviation | Residue number | Identity | Description |
|---|---|---|---|---|---|
| Å | % | ||||
| 4BOP | 7.5 | 2.6 | 151 | 14 | OTU domain of OTUD1 |
| 4BOU | 10.0 | 2.9 | 141 | 14 | OTU domain of OTUD3 |
| 3PHU | 9.2 | 2.6 | 186 | 12 | OTU family DUB encoded by CCHFV |
| 6DRM | 9.1 | 3.6 | 270 | 11 | OTU domain of Fam105A |
| 3TMP | 8.9 | 2.8 | 169 | 17 | Human deubiquitinase DUBA (OTUD5) |
| 3C0R | 8.4 | 2.8 | 175 | 20 | OTU1 from yeast |
| 4DDG | 7.8 | 3.5 | 399 | 14 | Human OTUB1/UbcH5b∼Ub/Ub |
| 4KSL | 7.6 | 3.7 | 270 | 17 | FAM105B in complex with linear diubiquitin |
| 3DKB | 7.1 | 5.6 | 352 | 15 | OTU domain of A20 |
| 3ZRH | 6.2 | 6.1 | 428 | 13 | OTU domain-containing DUB TRABID |
Ubiquitin recognition by Ceg23
To better understand the mechanism of substrate recognition by Ceg23, we attempted to solve the structure of the Ceg23ΔTM complex with the inhibitor Ub-PA but were unable to obtain crystals that diffract at sufficient resolutions. We thus performed molecular docking analyses of Ceg23ΔTM and ubiquitin, and these exercises revealed that the C-terminal tail of ubiquitin appears to reach Cys-29 of Ceg23ΔTM, a residue that is potentially involved in the formation of a covalent bond with the Ub-PA (Fig. 4A). Notably, the hydrophobic patch formed by residues Phe-143, Met-144, Leu-149, and Phe-154 in Ceg23ΔTM may interact with Ile-44, His-68, Leu-8, and Val-70 in ubiquitin (Fig. 4B). To test their potential roles in ubiquitin recognition, we mutated Phe-143, Met-144, Leu-149, and Phe-154 into hydrophilic arginine. Mutation of a single residue detectably attenuated the cleavage of both Lys-63–linked diubiquitin and polyubiquitin chains, and simultaneous mutations in Met-144 and Leu-149 almost completely abolished the DUB activity of Ceg23ΔTM (Fig. 4, C and D). These results suggest that the hydrophobic Met-144 and Leu-149 in the helical arm are important for ubiquitin recognition.
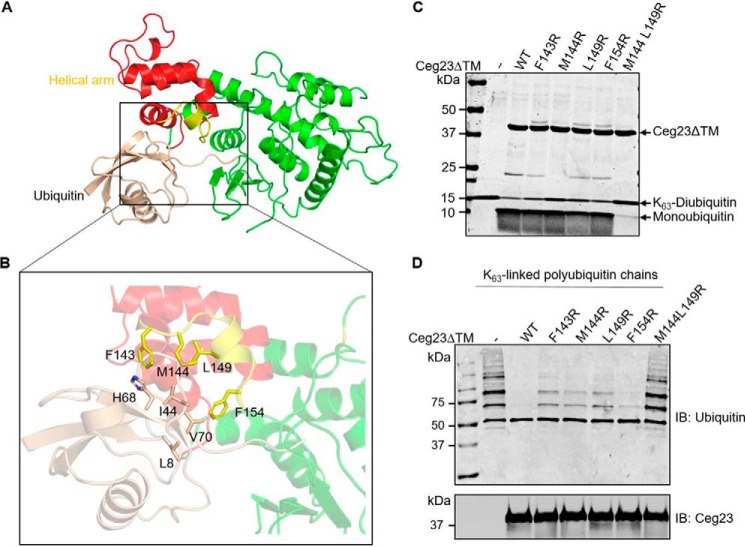
Figure 4.
Docking of ubiquitin into structure of Ceg23ΔTM. A, the predicted ubiquitin recognition mode of Ceg23ΔTM. B, ubiquitin inserted into a hydrophobic cavity of Ceg23ΔTM formed by residues Phe-143, Met-144, Leu-149, and Phe-154. C and D, cleavage of Lys-63–linked diubiquitin (C) or polyubiquitin chains (D) by F143R, M144R, L149R, F154R, and M144R/L149R mutants of Ceg23ΔTM. The reactions were performed at 37 °C for 5 min. Note that single mutation of Phe-143, Met-144, Leu-149, or Phe-154 only slightly reduced its activity, whereas simultaneous mutations in Met-144 and Leu-149 resulted in an almost complete loss of its DUB activity. C and D are one representative from three independent experiments.
Ceg23 is associated with the LCV
To investigate whether Ceg23 targets to specific organelles in host cells, we first transiently expressed a GFP–Ceg23 fusion by transfection in HeLa cells and immunostained the cells with antibodies specific for the ER resident protein calnexin. Immunofluorescence microscopy showed complete co-localization of GFP–Ceg23 and calnexin, suggesting that Ceg23 localizes on the ER (Fig. 5A). Interestingly, deletion of the two putative transmembrane domains at the C-terminal end of Ceg23 abolished the ability of the protein to localize to the ER (Fig. 5A).
Figure 5.
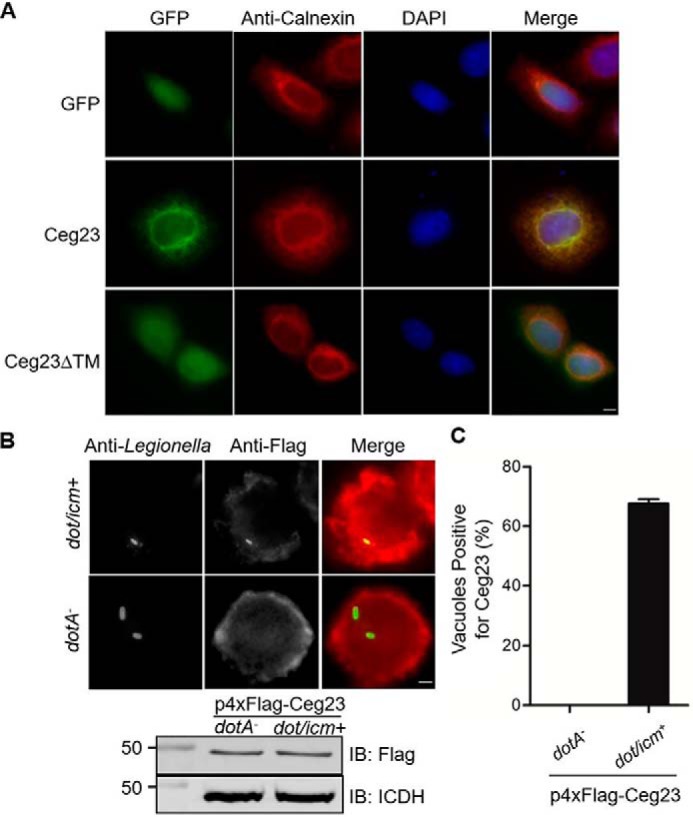
Cellular localization of Ceg23. A, Ceg23 localized to the ER in a manner that requires the two predicted transmembrane domains. HeLa cells were transfected with plasmids that direct the expression of GFP–Ceg23 or GFP–Ceg23ΔTM. The ER was labeled by immunostaining with antibodies specific for the ER-resident protein calnexin. Bar, 5 μm. B, the association of Ceg23 with the LCV. U937 cells infected with the indicated L. pneumophila strains expressing 4× FLAG–Ceg23 were sequentially immunostained with antibodies against L. pneumophila and FLAG. Images were obtained by a fluorescence microscope. The lower panel indicated that 4× FLAG–Ceg23 was similarly expressed in dotA− and dot/icm+ strains. C, quantification of LCVs positively stained for 4× FLAG–Ceg23 shown in B. The results in B and C are from one representative experiment done in triplicate from three independent experiments; at least 100 vacuoles were counted in each sample. Bar, 5 μm. The error bars represent S.E. (n = 3). DAPI, 4′,6′-diamino-2-phenylindole; IB, immunoblot.
To further analyze the intracellular localization of Ceg23 under conditions closer to the natural context, we made a plasmid that directs the expression of 4× FLAG–Ceg23 and introduced the construct into both WT and the dotA− mutant defective in the Dot/Icm transporter. We then infected U937 cells with these strains and determined the translocation and localization of the fusion by immunostaining with a FLAG-specific antibody. Signals of 4× FLAG–Ceg23 were detected on ∼60% of the vacuoles containing bacteria with a functional Dot/Icm transporter 2 h after infection. In contrast, no FLAG-specific signal was observed in vacuoles harboring the dotA− mutant that similarly expressed 4× FLAG–Ceg23 (Fig. 5, B and C). These results indicate that Ceg23 localizes on the LCV after being delivered into host cells by L. pneumophila.
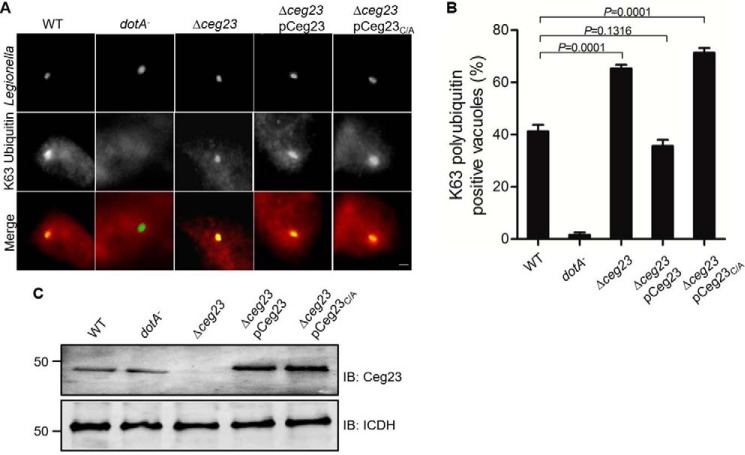
Ceg23 affects the association of Lys-63–linked polyubiquitin on the LCV
To investigate the role of Ceg23 in L. pneumophila infection, we constructed a ceg23 deletion strain and monitored its replication in host cells. Similar to the majority of Dot/Icm substrates, deletion of ceg23 did not cause detectable growth defects in mouse bone marrow-derived macrophages (BMDMs) (Fig. S3), suggesting that Ceg23 is dispensable for intracellular replication of L. pneumophila in this host.
The LCV is known to be enriched with ubiquitinated proteins (26). In light of the DUB activity of Ceg23, as well as its localization on the LCV, we hypothesized that this protein might modulate the association of ubiquitin species on the bacterial vacuole. To test this, we infected BMDMs with relevant L. pneumophila strains and determined the association of Lys-63–linked polyubiquitinated proteins with the LCV by immunostaining using an antibody that specifically recognizes Lys-63–linked polyubiquitin. Approximately 41% of the vacuoles harboring WT bacteria stained positively with this Lys-63–specific polyubiquitin antibody. In cells infected with the Δceg23 mutant, the ratio of Lys-63–type positive vacuoles significantly increased to 63% (Fig. 6). Furthermore, expression of Ceg23 in strain Δceg23 from a plasmid reduced the ubiquitin positive rates to WT levels (Fig. 6). In contrast, the catalytically inactive mutant Ceg23C29A expressed at similar levels cannot reduce such association (Fig. 6). Thus, Ceg23 may function to regulate Lys-63–type ubiquitination of one or more proteins on the bacterial phagosome.
Figure 6.
Ceg23 down-regulates the association of Lys-63–linked polyubiquitins with the LCV. A, BMDMs were challenged with the indicated L. pneumophila strains for 2 h. The cells were immunostained with antibodies against L. pneumophila and Lys-63–linked polyubiquitin, respectively. Representative images were taken by a fluorescence microscope. Bar, 5 μm. B, quantification of phagosomes associated with Lys-63–linked polyubiquitin. The data in B show one representative experiment done in triplicate from three independent experiments. At least 100 vacuoles were counted in each sample. The error bars represent S.E. (n = 3). The p values were calculated by unpaired two-tailed Student's t tests, and p < 0.05 was considered as significant difference. C, expression of Ceg23 in the L. pneumophila strains used for infection. The metabolic enzyme isocitrate dehydrogenase (ICDH) was probed as a loading control. C shows one representative from three independent experiments. IB, immunoblot.
Discussion
Akin to most PTMs, ubiquitination is a reversible process mediated by multiple types of DUBs that catalyze the removal of ubiquitin moieties from ubiquitinated substrates (7). Hence, the protein levels or activities are dependent on the balance between ubiquitination and deubiquitination (8). To deal with the complexity of ubiquitin modification, DUBs need to display specificity to distinguish the different architecture of the ubiquitin chains (9). Most DUBs of the motif interacting with ubiquitin–containing novel DUB family are specific for Lys-48 linkage (42), whereas JAMM family DUBs often show Lys-63 linkage specificity (43); characterized ubiquitin-specific protease family DUBs have little or no linkage preference (44). By contrast, most human OTU family DUBs display intrinsic specificity against one or a subset of linkage types. Similar to human OTU family enzymes, bacteria-encoded OTU domain-containing proteins also show chain preferences. For example, the chlamydia T3SS effector ChlaOTU is capable of cleaving both Lys-48– and Lys-63–linked polyubiquitin chains (45); LotA from L. pneumophila, a distant homolog of OTU cysteine proteases attacks most ubiquitin linkage types with a preference for Lys-6 (32). Our study demonstrates that the OTU-like protein Ceg23 from L. pneumophila is a Lys-63–specific DUB. Whereas our crystal structure of Ceg23 revealed that it uses a catalytic motif resembling other members of the OTU family despite remote similarity in primary sequences, it appears to catalyze peptide bond cleavage with a structurally similar domain. However, the structural basis of the chain specificity remains to be determined, and crystal structures of Ceg23 in complex with Lys-63–linked diubiquitin substrate may provide detailed mechanistic insights.
Ubiquitin chains assembled by Lys-48 linkage are the most abundant linkage type in cells that mainly serve as signals for proteasomal degradation (46). The second most predominant ubiquitin chains linked by Lys-63 are nondegradative and often function as molecular glue that mediates fast and reversible assembly of critical signaling complexes (4). Lys-63–linked polyubiquitin plays key roles in diverse processes such as the activation of the transcription factor NF-κB, protein sorting, and DNA repair (47). The finding that Ceg23 is a Lys-63–specific DUB indicates that Ceg23 might terminate the signaling events mediated by this chain topology. Based on a NF-κB luciferase reporter assay, ectopic expression of Ceg23 did not suppress NF-κB activation stimulated by phorbol 12-myristate 13-acetate (PMA) or tumor necrosis factor α (Fig. S4), suggesting that Ceg23 may affect Lys-63 polyubiquitin-regulated signaling pathways other than NF-κB. The inability of Ceg23 to interfere with NF-κB activation may result from its exclusive association with the ER (Fig. 5A), an organelle not known to extensively be involved in NF-κB signaling.
The biogenesis and maintenance of the LCV requires the host ubiquitin system (26). Dot/Icm effectors involved in ubiquitination attribute to the dynamics of the association of ubiquitinated proteins on the LCV effectors exhibiting E3 ligase activity such as AnkB, SidC/SdcA, and RavN (48–50) or DUB activity, including those of RavD, LotA and members of the SidE family are important for the accumulation of ubiquitinated species on the LCV (31–33). Our results indicate that Ceg23 contributes to the regulation the association of Lys-63–type polyubiquitin with the phagosome. Future studies aiming at identifying host substrates targeted by this DUB will clarify roles of Lys-63 type polyubiquitin chains in the intracellular life cycle of L. pneumophila and how this DUB impacts such signaling to promote L. pneumophila virulence.
Materials and methods
Ethics statement
All animal studies were conducted according to the experimental practices and standards approved by the Institutional Animal Care and Use Committee of Jilin University (permit no. SY201902008).
Bacterial strains, plasmids, and growth media
The bacterial strains and plasmids used in this study are listed in Tables S1 and S2, respectively. E. coli strains were grown on LB agar plates or in LB broth. When necessary, antibiotics were supplemented at the following concentrations: ampicillin, 100 μg/ml; and kanamycin, 30 μg/ml. L. pneumophila strains used in this study were constructed from strain Lp02, a derivative of strain Philadelphia 1 (51). Bacteria were cultured on charcoal-yeast extract plates or in N-(2-Acetamido)-2-aminoethanesulfonic acid-buffered yeast extract broth as previously described (52). In-frame deletion mutant of ceg23 was constructed by allelic exchange as described earlier (53). For expression or complementation of Ceg23 in L. pneumophila, ceg23 was first cloned into pCMV4xFLAG (54) to generate pCMV–4× FLAG–Ceg23, from which 4× FLAG–Ceg23 was amplified and inserted into pZL507 (54) as a BamHI/SalI fragment to give pZL507–4× FLAG–Ceg23. pZL507 harbors the thyA gene (55), which allows the growth of the thymidine auxotrophic Lp02 and its derivatives in the absence of exogenous thymidine, thus a selection marker for the plasmid.
Protein purification
The E. coli strain BL21 (DE3) was used for expression of recombinant proteins. SdeADUB was expressed as a GST-fusion protein, whereas LotA, Ceg23ΔTM, and its mutants were purified as His6-tagged proteins. In each case, an overnight culture of the E. coli strain carrying the relevant plasmid was transferred to fresh LB medium (1:50) supplemented with appropriate antibiotics. Bacteria were grown at 37 °C in a shaker (200 rpm/min) to an A600 nm of 0.6–0.8. After adding isopropyl thio-d-galactopyranoside to a final concentration of 0.2 mm, bacteria were further cultured at 18 °C for 16–18 h. E. coli cells were harvested by centrifugation and lysed by a low temperature and ultra-high pressure continuous flow homogenizer (JN-mini, JNBIO, Guangzhou, China). After removing insoluble materials by centrifugation at 12,000 × g for 20 min, the supernatant was incubated with prewashed Ni2+–nitrilotriacetic acid beads (Qiagen) for 2 h at 4 °C. After washing with lysis buffer containing 50 mm imidazole, His6-tagged proteins were eluted with 300 mm imidazole, and proteins were dialyzed twice in a buffer containing 25 mm Tris-HCl (pH 7.5), 150 mm NaCl, 5% glycerol, and 1 mm DTT. Protein concentrations were measured by the Bradford assay. GST-tagged proteins were purified with GSH-Sepharose 4 Fast Flow beads (GE Healthcare). The selenomethionine derivant of Ceg23ΔTM was purified as the same procedure but with 2 mm DTT added to the gel filtration buffer.
Analytic ultracentrifugation
Sedimentation velocity experiments were used to assess the molecular sizes of the Ceg23ΔTM at 20 °C on a Beckman XL-A analytical ultracentrifuge equipped with absorbance optics and an An60 Ti rotor (Beckman Coulter, Inc., Fullerton, CA). Samples were diluted to an optical density at 280 nm (A280 nm) of 1 in a 1.2-cm path length. The rotor speed was set at 72,000 × g for all samples. The sedimentation coefficient was obtained using the c(s) method with the Sedfit Software.
Crystallization
The purified Selenomethionine-labeled Ceg23ΔTM protein was concentrated to ∼30 mg/ml and used for crystal screening using the hanging-drop vapor-diffusion method at 18 °C by mixing 0.5 μl of protein with 0.5 μl of reservoir. Crystals of Ceg23ΔTM appeared overnight in the condition containing 20% PEG 4000, 0.2 m sodium cacodylate (pH 6.0), and 0.2 m NaCl, which grew to full size in 4 days. After optimization, crystals of the selenomethionine-labeled protein formed in a solution containing 22% PEG 3350, 0.2 m sodium cacodylate (pH 6.2), and 0.2 m NaCl. The crystals were flash-frozen in liquid nitrogen and cryoprotected by 20% glycerol.
Data collection and structure determination
The diffraction data were collected at the Shanghai Synchrotron Research Facility on Beamline BL17-U1. After many crystal diffraction tests, a crystal of the selenomethionine-labeled protein was diffracted to a 2.80 Å resolution. A data set was then integrated and processed using the HKL-2000 program suite (56). Further data processing was performed using the PHENIX suite (57). The phase of Ceg23ΔTM was determined using the single-wavelength anomalous diffraction method with AutoSol. The structure was iteratively built using Coot (58) and refined using the PHENIX program. All of the structure figures were generated using PyMOL.
Computational modeling
To predict the complex structure of Ceg23ΔTM and ubiquitin (PDB code 2AYO), we used the rigid protein–protein docking software ZDOCK (version 3.0.3) to generate a large amount of binding conformations (59). Those binding conformations (2,000 conformations) were clustered and screened by searching for the ones with cysteine at the interfaces, because the ubiquitin is assumed to form disulfide bond with its target. Thus, it left six binding conformations near Cys-29 of Ceg23ΔTM. To remove the atomic clashes between Ceg23ΔTM and ubiquitin, side-chain repacking was carried out (59, 60), and 1-ns molecular dynamics simulation was performed (61). Finally, the binding conformation with the lowest energy was predicted to be the complex structure of Ceg23ΔTM and ubiquitin.
Cell cultures and transfection
HEK293T or HeLa cells were cultured in Dulbecco's modified minimum Eagle's medium containing 10% fetal bovine serum. Transfection was performed using Lipofectamine 3000 (Life Technology) to cells grew to ∼80% confluence following the instructions provided by the manufacturer. U937 cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum and were differentiated into macrophages with PMA as described (62). BMDMs were prepared from 6–10-week-old female A/J mice using L-cell supernatant-conditioned medium as previously described (52).
NF-κB luciferase reporter assay
HEK293T cells grown to 80% confluence in 24-well plate were transfected with NF-κB reporter plasmids, pEGFPC1 or pEGFPC1-Ceg23, and the Renilla luciferase plasmid pRL-SV40 (Promega) at a ratio of 1:2:2. The cells were either untreated or stimulated with (50 nm PMA) or tumor necrosis factor α (20 ng/ml) at 24 h post-transfection for 4 h. Then the NF-κB activation was determined by Dual-Luciferase reporter assay system according to the manufacturer's instructions (Promega, catalog no. E1910). Renilla luciferase activities were used as internal controls to normalize all the values, and the NF-κB activity is presented as fold induction.
In vitro DUB assays
For reactions of DUBs with Ub-PA (Boston Biochem), 1 μm Ub-PA was mixed with 1 μm Ceg23ΔTM, Ceg23ΔTMC29A, or His-LotA (32) in 20 μl of DUB buffer (50 mm Tris-Cl, pH 7.5, 50 mm NaCl, and 5 mm DTT) and incubated at 37 °C for 1 h. The reaction was stopped by adding 5 μl of 5× SDS sample buffer and boiling the samples for 5 min. Proteins resolved by SDS-PAGE were visualized by silver staining.
To assay cleavage of ubiquitin chains on polyubiquitinated proteins by Ceg23ΔTM, HEK293T cells were transfected with pCMV–4× FLAG–Ub or pCMV–4× FLAG–Ub63K. 24 h after transfection, the cells were collected and lysed by an NP-40 lysis buffer supplemented with a complete protease inhibitors (EMD Millipore). The cell lysates were cleared by centrifugation at 12,000 × g for 10 min. The supernatant was incubated with 40 μl of anti-FLAG M2–agarose (Sigma) for 14 h at 4 °C. After washing with the lysis buffer four times, the beads were resuspended in a cleavage buffer containing 50 mm Tris (pH 7.5), 100 mm NaCl, and 1 mm DTT. Aliquots of the samples were treated with 2 μm of Ceg23ΔTM or Ceg23ΔTMC29A for 1 h at 37 °C. The beads were washed again using the cleavage buffer and boiled in 1× SDS sample buffer. Polyubiquitinated proteins retained on the beads were detected by immunoblotting using the FLAG antibody.
For in vitro cleavage of diubiquitin (Boston Biochem) by Ceg23ΔTM, 1 μm of Ceg23ΔTM were mixed with 1 μm of diubiquitin in a buffer containing 50 mm Tris-HCl (pH 7.5), 100 mm NaCl, and 1 mm DTT, and the mixture was incubated at 37 °C for indicated time. 5× SDS sample buffer was added to stop the reaction, and boiled samples were resolved by SDS-PAGE and visualized by Coomassie Brilliant Blue staining.
For in vitro synthesis of Lys-63–linked polyubiquitin chains, 2 μm ubiquitin (Boston Biochem), 0.5 μm GST-E1 (Boston Biochem), 1 μm UBE2N/UBE2V2 (Boston Biochem), 2 mm ATP, and 5 mm Mg2+ were added into a 100-μl reaction containing 50 mm Tris-HCl (pH 7.5) and 1 mm DTT. The reaction was allowed to proceed at 37 °C for 4 h. Then 10 μl of prewashed GSH-Sepharose 4 Fast Flow beads were added to remove GST-E1. Aliquots of the samples were further incubated with 1 μm Ceg23ΔTM or its mutants at 37 °C for 5 min. 5× SDS sample buffer was added to stop the reaction, boiled samples were resolved by SDS-PAGE, and Lys-63–linked polyubiquitin chains were detected by immunoblotting using ubiquitin antibody.
Bacterial infection
For infection experiments, L. pneumophila strains were cultured to the postexponential phase (A600 = 3.3–3.8) in N-(2-Acetamido)-2-aminoethanesulfonic acid-buffered yeast extract broth, and the motility of the bacteria was checked under a microscope before infection. To determine the growth of L. pneumophila in BMDMs, 4 × 105 cells seeded on 24-well plates were infected with L. pneumophila at a multiplicity of infection of 0.05. Two h after infection, extracellular bacteria were removed by washing the samples three times with warm PBS. Infected macrophages were lysed, and the bacteria were enumerated at 2, 24, 48, and 72 h, respectively. Infected cells were lysed with 0.02% saponin for 30 min, and dilutions of the lysates were plated onto CYE plates and grown at 37 °C until the colonies were suitable for counting. The number of colony-forming units of each strain at indicated time points was determined.
For immunostaining, 2 × 105 BMDMs or U937 macrophages were seeded on glass coverslips in 24-well plates, and the cells were challenged with relevant L. pneumophila strains at an multiplicity of infection of 1 for 2 h. After washing with PBS, cells fixed with paraformaldehyde were subjected to immunostaining with antibodies specific for L. pneumophila, the FLAG tag, or Lys-63–specific ubiquitin.
Antibodies, immunoblotting, and immunostaining
For immunoblotting, proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Pall Life Sciences). After blocking with 5% nonfat milk, the membranes were incubated with the appropriate primary antibody for 2 h at room temperature, followed by incubation with appropriate fluorescence dye-conjugated secondary antibody (Li-Cor) for 1 h. Signals were detected by the Odyssey detection system (Li-Cor). Antibodies specific for Ceg23 was generated by immunization of rabbits with purified His6-Ceg23ΔTM using a standard procedure (AbMax Biotechnology Co., LTD, Beijing, China). The sources for other antibodies used and their dilutions were as follows: anti-GFP (Sigma, catalog no. G7781, 1:5000); anti-FLAG (Sigma, catalog no. F1804, 1:3000); anti-Ub (Santa Cruz, catalog no. sc-8017, 1:1000); anti-tubulin (DSHB, E7, 1:10,000); anti-ICDH (1:20,000) (54); and anti-Ceg23 (1:5000).
To visualize the cellular localization of Ceg23, HeLa cells seeded on glass coverslips were transfected to express GFP–Ceg23 for 14 h and fixed by 4% paraformaldehyde for 30 min at room temperature. Following permeabilization with 0.2% Triton-X100, the cells were incubated with the anti-calnexin (Abcam, catalog no. ab22595) antibodies at a dilution of 1:100. The nuclei were labeled by 4′,6′-diamino-2-phenylindole staining. For infection samples, paraformaldehyde-fixed cells were permeabilized with prechilled methanol for 10 s, and extracellular and intracellular bacteria were differentially stained with anti-L. pneumophila antibodies (31) used at a dilution of 1:10,000; Ceg23 and Lys-63–specific polyubiquitin were stained by antibodies specific for the FLAG tag (Sigma, catalog no. F1804, 1:200), Lys-63–specific ubiquitin chains (EMD Millipore, catalog no. 05–1308, 1: 50), respectively. After labeling with appropriate secondary antibodies, samples were inspected using an Olympus IX-81 fluorescence microscope.
Data analysis
Statistical analysis was calculated using the unpaired two-tailed Student's t tests, and the significant difference was set at p < 0.05.
Author contributions
K. M., X. Z., and B. Z. data curation; K. M., X. Z., B. Z., N. G., Y. C., and C. F. investigation; S. O., Z.-Q. L., and J. Q. conceptualization; S. O., Z.-Q. L., and J. Q. supervision; S. O. and J. Q. funding acquisition; S. O. and Z.-Q. L. writing-review and editing; J. Q. methodology; J. Q. writing-original draft.
Supplementary Material
Acknowledgments
We thank Chittaranjan Das, Kedar Puvar, and the members of our laboratories for helpful discussion. The diffraction data were collected at the Beamline BL-17U1 of the Shanghai Synchrotron Radiation Facility.
This work was supported by National Natural Science Foundation of China Grants 31770149 and 31970134 (to J. Q.) and 31770948 and 31570875 (to S. O.), funds from the Thousand Young Talents Program of the Chinese government (to J. Q.), a startup fund from Jilin University and the First Hospital of Jilin University, and by Special Funds of the Central Government Guiding Local Science and Technology Development Grant 2017L3009. Portions of the structural work were supported by Fujian Normal University Grant Z0210509. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Tables S1 and S2 and Figs. S1–S4.
The atomic coordinates and structure factors (code 6KS5) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- DUB
- deubiquitinase
- LCV
- Legionella-containing vacuole
- Dot/Icm
- defective in organelle trafficking/intracellular multiplication
- OTU
- ovarian-tumor
- RMS
- root mean squared
- PDB
- protein data bank
- JAMM
- Jab1/Mov34/Mpr1 Pad1 N-terminal+
- ER
- endoplasmic reticulum
- Ub-PA
- ubiquitin propargylamide
- BMDM
- bone marrow-derived macrophage
- PMA
- phorbol 12-myristate 13-acetate.
References
- 1. Hershko A., and Ciechanover A. (1998) The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 10.1146/annurev.biochem.67.1.425 [DOI] [PubMed] [Google Scholar]
- 2. Pisano A., Albano F., Vecchio E., Renna M., Scala G., Quinto I., and Fiume G. (2018) Revisiting bacterial ubiquitin ligase effectors: weapons for host exploitation. Int. J. Mol. Sci. 19, E3576 10.3390/ijms19113576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komander D., and Rape M. (2012) The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 10.1146/annurev-biochem-060310-170328 [DOI] [PubMed] [Google Scholar]
- 4. Yau R., and Rape M. (2016) The increasing complexity of the ubiquitin code. Nat. Cell Biol. 18, 579–586 10.1038/ncb3358 [DOI] [PubMed] [Google Scholar]
- 5. Walczak H., Iwai K., and Dikic I. (2012) Generation and physiological roles of linear ubiquitin chains. BMC Biol. 10, 23 10.1186/1741-7007-10-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Song L., and Luo Z. Q. (2019) Post-translational regulation of ubiquitin signaling. J. Cell Biol. 218, 1776–1786 10.1083/jcb.201902074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkinson K. D. (2009) DUBs at a glance. J. Cell Sci. 122, 2325–2329 10.1242/jcs.041046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clague M. J., Urbé S., and Komander D. (2019) Breaking the chains: deubiquitylating enzyme specificity begets function. Nat. Rev. Mol. Cell Biol. 20, 338–352 10.1038/s41580-019-0099-1 [DOI] [PubMed] [Google Scholar]
- 9. Mevissen T. E. T., and Komander D. (2017) Mechanisms of deubiquitinase specificity and regulation. Annu. Rev. Biochem. 86, 159–192 10.1146/annurev-biochem-061516-044916 [DOI] [PubMed] [Google Scholar]
- 10. Li J., Chai Q. Y., and Liu C. H. (2016) The ubiquitin system: a critical regulator of innate immunity and pathogen-host interactions. Cell Mol. Immunol 13, 560–576 10.1038/cmi.2016.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashida H., Kim M., and Sasakawa C. (2014) Exploitation of the host ubiquitin system by human bacterial pathogens. Nat. Rev. Microbiol. 12, 399–413 10.1038/nrmicro3259 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y., Higashide W. M., McCormick B. A., Chen J., and Zhou D. (2006) The inflammation-associated Salmonella SopA is a HECT-like E3 ubiquitin ligase. Mol. Microbiol. 62, 786–793 10.1111/j.1365-2958.2006.05407.x [DOI] [PubMed] [Google Scholar]
- 13. Kamanova J., Sun H., Lara-Tejero M., and Galán J. E. (2016) The Salmonella effector protein SopA modulates innate immune responses by targeting TRIM E3 ligase family members. PLoS Pathog. 12, e1005552 10.1371/journal.ppat.1005552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu B., Skarina T., Yee A., Jobin M. C., Dileo R., Semesi A., Fares C., Lemak A., Coombes B. K., Arrowsmith C. H., Singer A. U., and Savchenko A. (2010) NleG Type 3 effectors from enterohaemorrhagic Escherichia coli are U-Box E3 ubiquitin ligases. PLoS Pathog. 6, e1000960 10.1371/journal.ppat.1000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rohde J. R., Breitkreutz A., Chenal A., Sansonetti P. J., and Parsot C. (2007) Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1, 77–83 10.1016/j.chom.2007.02.002 [DOI] [PubMed] [Google Scholar]
- 16. Singer A. U., Rohde J. R., Lam R., Skarina T., Kagan O., Dileo R., Chirgadze N. Y., Cuff M. E., Joachimiak A., Tyers M., Sansonetti P. J., Parsot C., and Savchenko A. (2008) Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1293–1301 10.1038/nsmb.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Y., Li H., Hu L., Wang J., Zhou Y., Pang Z., Liu L., and Shao F. (2008) Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1302–1308 10.1038/nsmb.1517 [DOI] [PubMed] [Google Scholar]
- 18. Keszei A. F., Tang X., McCormick C., Zeqiraj E., Rohde J. R., Tyers M., and Sicheri F. (2014) Structure of an SspH1–PKN1 complex reveals the basis for host substrate recognition and mechanism of activation for a bacterial E3 ubiquitin ligase. Mol. Cell. Biol. 34, 362–373 10.1128/MCB.01360-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bhavsar A. P., Brown N. F., Stoepel J., Wiermer M., Martin D. D., Hsu K. J., Imami K., Ross C. J., Hayden M. R., Foster L. J., Li X., Hieter P., and Finlay B. B. (2013) The Salmonella type III effector SspH2 specifically exploits the NLR co-chaperone activity of SGT1 to subvert immunity. PLoS Pathog 9, e1003518 10.1371/journal.ppat.1003518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kubori T., Kitao T., and Nagai H. (2019) Emerging insights into bacterial deubiquitinases. Curr. Opin. Microbiol. 47, 14–19 10.1016/j.mib.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 21. Fraser D. W., Tsai T. R., Orenstein W., Parkin W. E., Beecham H. J., Sharrar R. G., Harris J., Mallison G. F., Martin S. M., McDade J. E., Shepard C. C., and Brachman P. S. (1977) Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297, 1189–1197 10.1056/NEJM197712012972201 [DOI] [PubMed] [Google Scholar]
- 22. Rowbotham T. J. (1980) Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol 33, 1179–1183 10.1136/jcp.33.12.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cirillo J. D., Cirillo S. L., Yan L., Bermudez L. E., Falkow S., and Tompkins L. S. (1999) Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67, 4427–4434 10.1128/IAI.67.9.4427-4434.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Isberg R. R., O'Connor T. J., and Heidtman M. (2009) The Legionella pneumophila replication vacuole: making a cosy niche inside host cells. Nat. Rev. Microbiol. 7, 13–24 10.1038/nrmicro1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiu J., and Luo Z. Q. (2017) Legionella and Coxiella effectors: strength in diversity and activity. Nat. Rev. Microbiol. 15, 591–605 10.1038/nrmicro.2017.67 [DOI] [PubMed] [Google Scholar]
- 26. Dorer M. S., Kirton D., Bader J. S., and Isberg R. R. (2006) RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2, e34 10.1371/journal.ppat.0020034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Qiu J., and Luo Z. Q. (2017) Hijacking of the host ubiquitin network by Legionella pneumophila. Front. Cell. Infect. Microbiol. 7, 487 10.3389/fcimb.2017.00487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qiu J., Sheedlo M. J., Yu K., Tan Y., Nakayasu E. S., Das C., Liu X., and Luo Z. Q. (2016) Ubiquitination independent of E1 and E2 enzymes by bacterial effectors. Nature 533, 120–124 10.1038/nature17657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhogaraju S., Kalayil S., Liu Y., Bonn F., Colby T., Matic I., and Dikic I. (2016) Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636–1649.e13 10.1016/j.cell.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 30. Kotewicz K. M., Ramabhadran V., Sjoblom N., Vogel J. P., Haenssler E., Zhang M., Behringer J., Scheck R. A., and Isberg R. R. (2017) A single Legionella effector catalyzes a multistep ubiquitination pathway to rearrange tubular endoplasmic reticulum for replication. Cell Host Microbe 21, 169–181 10.1016/j.chom.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sheedlo M. J., Qiu J., Tan Y., Paul L. N., Luo Z. Q., and Das C. (2015) Structural basis of substrate recognition by a bacterial deubiquitinase important for dynamics of phagosome ubiquitination. Proc. Natl. Acad. Sci. U.S.A. 112, 15090–15095 10.1073/pnas.1514568112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kubori T., Kitao T., Ando H., and Nagai H. (2018) LotA, a Legionella deubiquitinase, has dual catalytic activity and contributes to intracellular growth. Cell Microbiol. 20, e12840 10.1111/cmi.12840 [DOI] [PubMed] [Google Scholar]
- 33. Wan M., Wang X., Huang C., Xu D., Wang Z., Zhou Y., and Zhu Y. (2019) A bacterial effector deubiquitinase specifically hydrolyses linear ubiquitin chains to inhibit host inflammatory signalling. Nat. Microbiol. 4, 1282–1293 10.1038/s41564-019-0454-1 [DOI] [PubMed] [Google Scholar]
- 34. Zusman T., Aloni G., Halperin E., Kotzer H., Degtyar E., Feldman M., and Segal G. (2007) The response regulator PmrA is a major regulator of the icm/dot type IV secretion system in Legionella pneumophila and Coxiella burnetii. Mol. Microbiol. 63, 1508–1523 10.1111/j.1365-2958.2007.05604.x [DOI] [PubMed] [Google Scholar]
- 35. Zhu W., Banga S., Tan Y., Zheng C., Stephenson R., Gately J., and Luo Z. Q. (2011) Comprehensive identification of protein substrates of the Dot/Icm type IV transporter of Legionella pneumophila. PLoS ONE 6, e17638 10.1371/journal.pone.0017638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Söding J., Biegert A., and Lupas A. N. (2005) The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 33, W244–W248 10.1093/nar/gki408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mevissen T. E., Hospenthal M. K., Geurink P. P., Elliott P. R., Akutsu M., Arnaudo N., Ekkebus R., Kulathu Y., Wauer T., El Oualid F., Freund S. M., Ovaa H., and Komander D. (2013) OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell 154, 169–184 10.1016/j.cell.2013.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ekkebus R., van Kasteren S. I., Kulathu Y., Scholten A., Berlin I., Geurink P. P., de Jong A., Goerdayal S., Neefjes J., Heck A. J., Komander D., and Ovaa H. (2013) On terminal alkynes that can react with active-site cysteine nucleophiles in proteases. J. Am. Chem. Soc. 135, 2867–2870 10.1021/ja309802n [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Krissinel E., and Henrick K. (2007) Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- 40. Holm L., and Rosenström P. (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res. 38, W545–W549 10.1093/nar/gkq366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Komander D., and Barford D. (2008) Structure of the A20 OTU domain and mechanistic insights into deubiquitination. Biochem. J. 409, 77–85 10.1042/BJ20071399 [DOI] [PubMed] [Google Scholar]
- 42. Abdul Rehman S. A., Kristariyanto Y. A., Choi S. Y., Nkosi P. J., Weidlich S., Labib K., Hofmann K., and Kulathu Y. (2016) MINDY-1 is a member of an evolutionarily conserved and structurally distinct new family of deubiquitinating enzymes. Mol. Cell 63, 146–155 10.1016/j.molcel.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ritorto M. S., Ewan R., Perez-Oliva A. B., Knebel A., Buhrlage S. J., Wightman M., Kelly S. M., Wood N. T., Virdee S., Gray N. S., Morrice N. A., Alessi D. R., and Trost M. (2014) Screening of DUB activity and specificity by MALDI-TOF mass spectrometry. Nat. Commun. 5, 4763 10.1038/ncomms5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Faesen A. C., Luna-Vargas M. P., Geurink P. P., Clerici M., Merkx R., van Dijk W. J., Hameed D. S., El Oualid F., Ovaa H., and Sixma T. K. (2011) The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem. Biol. 18, 1550–1561 10.1016/j.chembiol.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 45. Furtado A. R., Essid M., Perrinet S., Balañá M. E., Yoder N., Dehoux P., and Subtil A. (2013) The chlamydial OTU domain-containing protein ChlaOTU is an early type III secretion effector targeting ubiquitin and NDP52. Cell Microbiol. 15, 2064–2079 10.1111/cmi.12171 [DOI] [PubMed] [Google Scholar]
- 46. Swatek K. N., and Komander D. (2016) Ubiquitin modifications. Cell Res. 26, 399–422 10.1038/cr.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lauwers E., Jacob C., and André B. (2009) K63-linked ubiquitin chains as a specific signal for protein sorting into the multivesicular body pathway. J. Cell Biol. 185, 493–502 10.1083/jcb.200810114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsu F., Luo X., Qiu J., Teng Y. B., Jin J., Smolka M. B., Luo Z. Q., and Mao Y. (2014) The Legionella effector SidC defines a unique family of ubiquitin ligases important for bacterial phagosomal remodeling. Proc. Natl. Acad. Sci. U.S.A. 111, 10538–10543 10.1073/pnas.1402605111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Price C. T., Al-Khodor S., Al-Quadan T., Santic M., Habyarimana F., Kalia A., and Kwaik Y. A. (2009) Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5, e1000704 10.1371/journal.ppat.1000704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lin Y. H., Lucas M., Evans T. R., Abascal-Palacios G., Doms A. G., Beauchene N. A., Rojas A. L., Hierro A., and Machner M. P. (2018) RavN is a member of a previously unrecognized group of Legionella pneumophila E3 ubiquitin ligases. PLoS Pathog. 14, e1006897 10.1371/journal.ppat.1006897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berger K. H., and Isberg R. R. (1993) Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol. Microbiol. 7, 7–19 10.1111/j.1365-2958.1993.tb01092.x [DOI] [PubMed] [Google Scholar]
- 52. Conover G. M., Derré I., Vogel J. P., and Isberg R. R. (2003) The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48, 305–321 10.1046/j.1365-2958.2003.03400.x [DOI] [PubMed] [Google Scholar]
- 53. Liu Y., and Luo Z. Q. (2007) The Legionella pneumophila effector SidJ is required for efficient recruitment of endoplasmic reticulum proteins to the bacterial phagosome. Infect. Immun. 75, 592–603 10.1128/IAI.01278-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xu L., Shen X., Bryan A., Banga S., Swanson M. S., and Luo Z. Q. (2010) Inhibition of host vacuolar H+-ATPase activity by a Legionella pneumophila effector. PLoS Pathog. 6, e1000822 10.1371/journal.ppat.1000822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mintz C. S., Chen J. X., and Shuman H. A. (1988) Isolation and characterization of auxotrophic mutants of Legionella pneumophila that fail to multiply in human monocytes. Infect. Immun. 56, 1449–1455 10.1128/IAI.56.6.1449-1455.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 57. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 59. Chen R., Li L., and Weng Z. (2003) ZDOCK: an initial-stage protein-docking algorithm. Proteins 52, 80–87 10.1002/prot.10389 [DOI] [PubMed] [Google Scholar]
- 60. Cao Y., Song L., Miao Z., Hu Y., Tian L., and Jiang T. (2011) Improved side-chain modeling by coupling clash-detection guided iterative search with rotamer relaxation. Bioinformatics 27, 785–790 10.1093/bioinformatics/btr009 [DOI] [PubMed] [Google Scholar]
- 61. Phillips J. C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., Chipot C., Skeel R. D., Kalé L., and Schulten K. (2005) Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 10.1002/jcc.20289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tilney L. G., Harb O. S., Connelly P. S., Robinson C. G., and Roy C. R. (2001) How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114, 4637–4650 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.