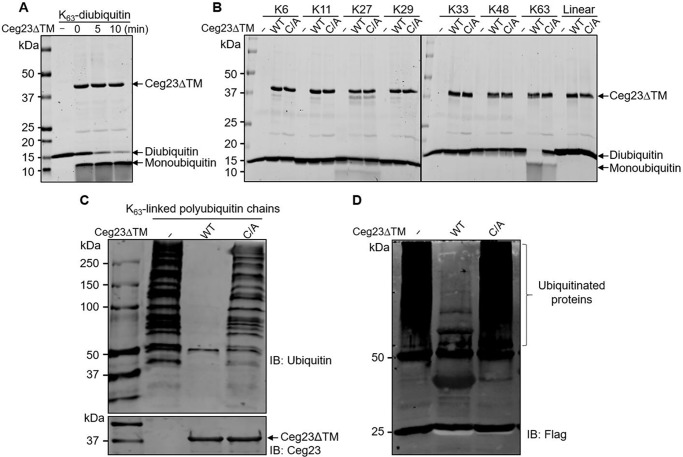
Figure 2.
Ceg23 specifically cleaves Lys-63–linked ubiquitin chains. A, time-dependent cleavage of Lys-63–linked diubiquitin by Ceg23ΔTM. 1 μm of Ceg23ΔTM was incubated with 1 μm of diubiquitin at 37 °C for the indicated time. B, Ceg23ΔTM induced cleavage of diubiquitins linked by the primary methionine and each of the seven lysine residues. 1 μm of Ceg23ΔTM was incubated with 1 μm of diubiquitin at 37 °C for 2 h, and DUB activity was indicated by the release of free ubiquitin. C and D, disassembly of Lys-63–linked polyubiquitin chains by Ceg23ΔTM. Polyubiquitin chains assembled by Lys-63 were either synthesized by an in vitro reaction containing ubiquitin, E1, UBE2V2, and UBE2N (C) or isolated from cells transfected with pCMV–4× FLAG–Ub63K (a ubiquitin mutant that harbors only Lys-63) (D). After incubation with Ceg23ΔTM, polyubiquitin was probed by antibodies specific for ubiquitin or for the FLAG tag. The results in A–D show one representative from three independent experiments. IB, immunoblot.