Figure 6.
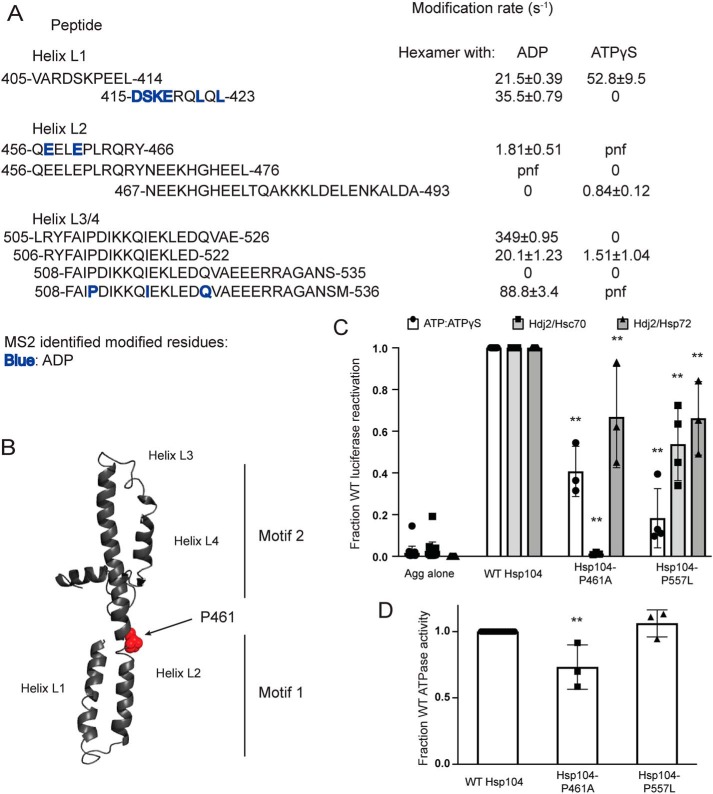
Hsp104 MD hinge region is crucial for Hsp70 collaboration and intrinsic Hsp104 disaggregase activity. A, peptide sequences and modification rates for regions of interest of Hsp104 MD. Residues shown in bold have been identified as modified by MS2 and are colored by the state in which they were found: blue, hexamer with ADP. B, isolated MD modeled off the tClpB crystal structure (PDB code 1qvr). Helices L1–L4 are labeled, and residue Pro-461 is shown as spheres. C, urea-denatured firefly luciferase aggregates were incubated with either WT or an Hsp104 variant, Hsp104-P461A or Hsp104-P557L for 90 min at 25 °C in the presence of either 5.1 mm ATP or ATP/ATPγS; 2.6 mm ATP and 2.5 mm ATPγS, or Hdj2/Hsc70; 5.1 mm ATP, 1 μm Hdj2, and 1 μm Hsc70, or Hdj2/Hsp72; 5.1 mm ATP, 1 μm Hdj2, and 1 μm Hsp72. Reactivation of luciferase was then determined by measuring luminescence and converted to fraction WT activity for each condition. Data are displayed as scatterplot with bar representing mean ± S.D. (n = 3). One-way ANOVA with Dunnett's test comparing the variants to Hsp104-WT were performed with ** denoting p < 0.01. D, ATPase activity for Hsp104-WT and variants. Data are displayed as scatterplot with bar representing mean ± S.D. (n = 3). One-way ANOVA with Dunnett's test comparing the variants to Hsp104-WT were performed with ** denoting p < 0.01.