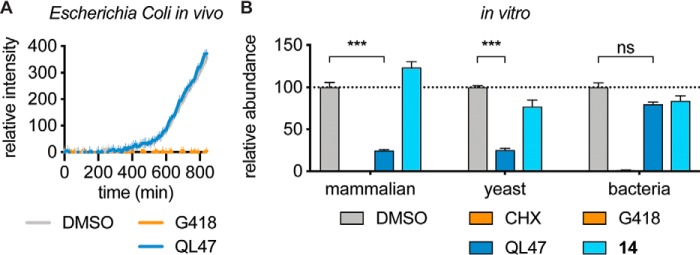
Figure 3.
QL47 inhibits eukaryotic but not prokaryotic protein synthesis. A, E. coli cells carrying the pUA66-rrnB plasmid that constitutively expresses GFP (24) were treated with DMSO, 250 μg/ml G418, or 50 μm QL47. The intracellular GFP fluorescence signal was then measured continuously for 14 h at 37 °C. The signal obtained from growth medium was subtracted, and data are presented as means ± S.D. of 12 experimental replicates. One representative experiment is shown from two independent experiments. B, analysis of in vitro translation assays performed in rabbit reticulocyte lysates, yeast cell lysates, or a reconstituted E. coli cell-free synthesis system (PURExpress®). Translation in rabbit reticulocyte lysates was performed in the presence of DMSO, 30 μg/ml CHX, 40 μm QL47, or 40 μm compound 14. An in vitro transcribed reporter DV subgenomic RNA was used as a template, and the luciferase signal was measured after 90-min incubation at 30 °C. Data are presented as means normalized to DMSO ± S.D. of four experimental replicates. Translation in yeast cell lysates was performed in the presence of DMSO, 40 μm QL47, or 40 μm compound 14. An in vitro transcribed vesicular stomatitis virus (VSV) RNA bearing a luciferase reporter gene (44) was used as a template, and the luciferase signal was measured after 2-h incubation at 25 °C. Data are presented as means normalized to DMSO ± S.D. of three experimental replicates. Translation in a reconstituted E. coli cell-free synthesis system (PURExpress®) was performed in the presence of DMSO, 250 μg/ml G418, 100 μm QL47, or 100 μm compound 14. A plasmid expressing GFP under control of a T7 promoter was used as a template. After 1-h incubation at 37 °C, the total protein content was analyzed by Western blotting. The reporter protein was detected using a GFP antibody, and its abundance was normalized to the loading control (histidine tag). Data are presented as means normalized to DMSO ± S.D. of two technical replicates. One representative experiment is shown from four (rabbit reticulocyte lysates) or two (yeast cell lysates and E. coli cell-free synthesis system) independent experiments. Asterisks indicate that the differences between experimental samples and the DMSO-treated control samples are statistically significant when compared using unpaired t test: ***, p < 0.001; nonsignificant (ns), p > 0.05.