Figure 4.
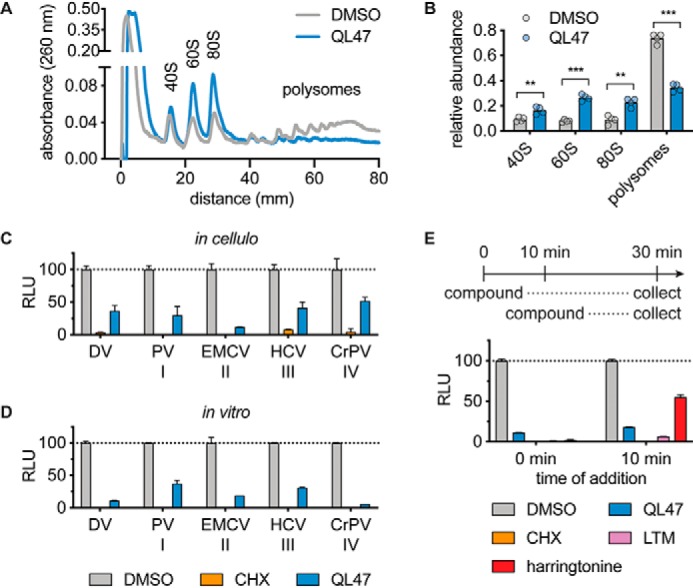
QL47 inhibits an early step in the translation process. A, polysome profiling. Huh7 cells were treated with DMSO or 2 μm QL47 for 3 h. Cell extracts were loaded on a sucrose density gradient and separated by ultracentrifugation. Sucrose gradients were eluted from the top using a fractionator, and RNA absorption at 260 nm was continuously recorded. Actively translated polysomal mRNAs were separated from mRNAs associated with the 80S monosome or single ribosomal units (40S and 60S). One representative experiment is shown from five independent experiments. B, quantification of polysomes and ribosomal units obtained in the polysome profiling experiments shown in A. The area under the curve for each peak was quantified, and data are presented as means normalized to the sum of all peaks area ± S.D. of four independent experiments. Asterisks indicate that the differences between experimental samples and the DMSO-treated control samples are statistically significant when compared using unpaired t test: ***, p < 0.001; **, p < 0.01. C, analysis of translation assays performed in live cells. Huh7 cells were transfected with various in vitro transcribed reporter RNAs. Reporter luciferase translation was driven by either the DV 5′ UTR, poliovirus type I IRES (41), EMCV type II IRES, HCV type III IRES (43), or CrPV type IV IRES. Cells were immediately treated with DMSO, 30 μg/ml CHX, or 2 μm QL47. The intracellular luciferase signal was measured 6 h post-treatment, and data are presented as means normalized to DMSO ± S.D. of two experimental replicates. One representative experiment is shown from four independent experiments. RLU, relative light units. D, analysis of in vitro translation assays performed in rabbit reticulocyte lysates. The in vitro transcribed reporter RNAs presented in B were used as templates. Translation was performed in the presence of DMSO, 30 μg/ml CHX, or 40 μm QL47. The reactions were incubated for 90 min at 30 °C, and the luciferase signal was measured. Data are presented as means normalized to DMSO ± S.D. of two technical replicates. One representative experiment is shown from two independent experiments. E, in vitro translations were performed in rabbit reticulocyte lysates using in vitro transcribed reporter DV subgenomic RNA as a template. Compounds were added either immediately after the translation reaction was assembled or after 10-min incubation at 30 °C. Samples were treated with DMSO, 40 μm QL47, 30 μg/ml CHX, 40 μm LTM, or 40 μm harringtonine. Data are presented as means normalized to DMSO values obtained for each treatment ± S.D. of two technical replicates. One representative experiment is shown from two independent experiments.
