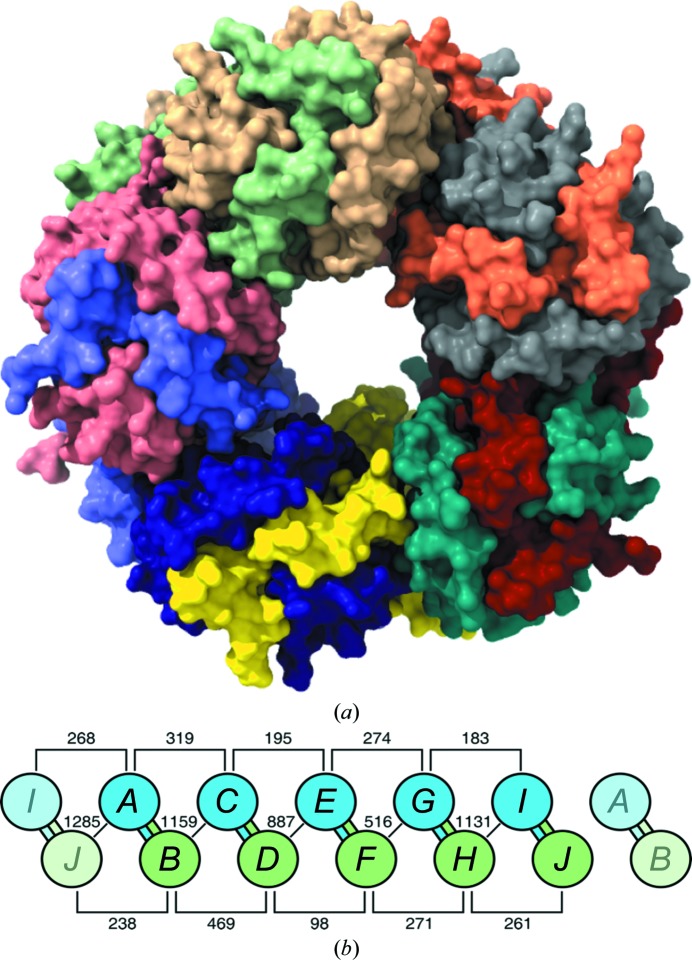
Figure 4.
Structure of the SpTrpA decamer. (a) Contents of the asymmetric unit viewed down the fivefold axis of the decamer. Chain colors are as in Fig. 2 ▸(a). (b) The domain-swapped dimers are formed by the following pairings: AB, CD, EF, GH and IJ. The decamer comprises two pentameric rings: –A–C–E–G–I– (blue) and –B–D–F–H–J– (green). To illustrate the circular connectivity, the unique subunit sequences (for example A–C–E–G–I–) are flanked by their circular neighbors (for example I and A) shown in dim colors. Chain surface interface (buried) areas are given in Å2, as calculated by the PISA server (Krissinel & Henrick, 2007 ▸). The mean interface area between chains forming the α2 domain-swapped dimers (AB, CD, EF, GH and IJ) is ∼5271 Å2. The variabilities of the buried surface areas in the same group (for example I/A, J/B, A/C etc.) reflect to a certain degree the variable incompleteness (missing fragments) of the models.