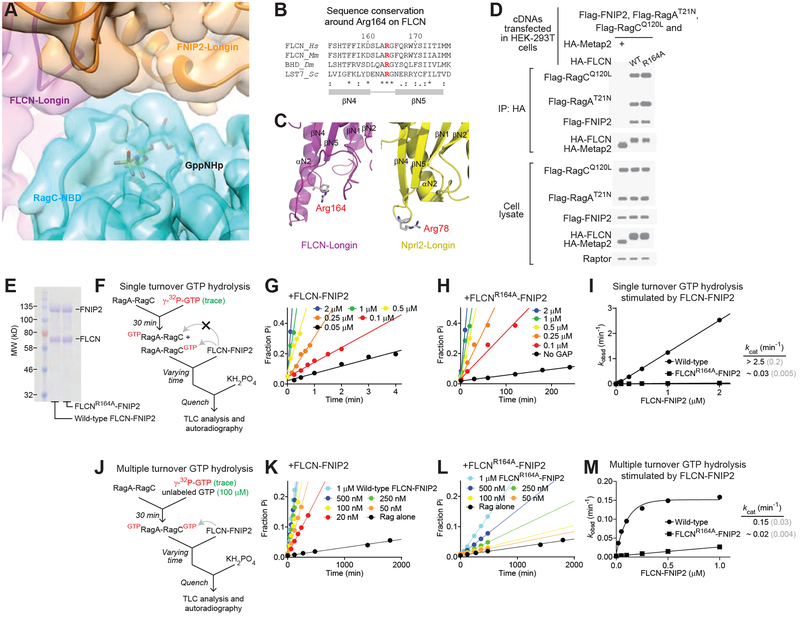
Figure 4. Arg164 of FLCN is necessary for the GAP activity.
A. Cryo-EM density map (colored surface) and atomic model (colored ribbon) around the nucleotide binding pocket of RagC. Clear boundaries between RagC and FLCN-FNIP2 are observed with no EM density extending into the nucleotide binding pocket of RagC.
B. Sequence conservation of Arg164 of FLCN. Arg164 is conserved to yeast (Lst7) and localizes between the two β-strands of the Longin domain of FLCN.
C. Structural comparison between Arg164 of FLCN and Arg78 of Nprl2. Arg78 of Nprl2 is the catalytic residue for GATOR1’s GAP function on RagA. These two arginines localize at similar positions on FLCN and Nprl2, respectively.
D. Co-immunoprecipitation experiment to probe the interaction between FLCN-FNIP2 and the Rag GTPases. FLCN-FNIP2 carrying the R164A mutation binds to the Rag GTPases to a similar extent as wild-type FLCN-FNIP2. This experiment was repeated twice, and a representative data set is shown here.
E. Coomassie blue stained gel of wild-type FLCN-FNIP2 and the mutant carrying the FLCN(R164A) mutation.
F. Single turnover GTP hydrolysis assay to determine the effect of FLCN-FNIP2. The Rag GTPase heterodimer was first loaded with radiolabeled GTP. FLCN-FNIP2 was then added to stimulate the hydrolysis. Time points were taken to track the reaction process and were fitted to extract the observed reaction constants.
G & H. Time courses of GTP hydrolysis by the Rag GTPases under single turnover conditions, stimulated by wild-type FLCN-FNIP2 (G) or the mutant carrying the FLCN(R164A) mutation (H).
I. Concentration dependence of stimulated GTP hydrolysis by FLCN-FNIP2 under single turnover conditions. A 100-fold decrease was observed with the FLCN(R164A)-FNIP2 mutant under single turnover conditions. Gray numbers in parenthesis denote the SDs of the reported values calculated from at least three independent experiments.
J. Multiple turnover GTP hydrolysis assay to determine the effect of FLCN-FNIP2. The Rag GTPase heterodimer was doubly-loaded with GTP. FLCN-FNIP2 was then added to stimulate hydrolysis. Time points were taken to track the reaction process and were fitted to extract the observed reaction constants.
K & L. Time courses of GTP hydrolysis by the Rag GTPases under multiple turnover conditions, stimulated by wild-type FLCN-FNIP2 (K) or the mutant carrying the FLCN(R164A) mutation (L).
M. Concentration dependence of stimulated GTP hydrolysis by FLCN-FNIP2 under multiple turnover conditions. A 7-fold decrease was observed with the FLCN(R164A)-FNIP2 mutant under these conditions. Gray numbers in parenthesis denote the SDs of the reported values calculated from at least three independent experiments.