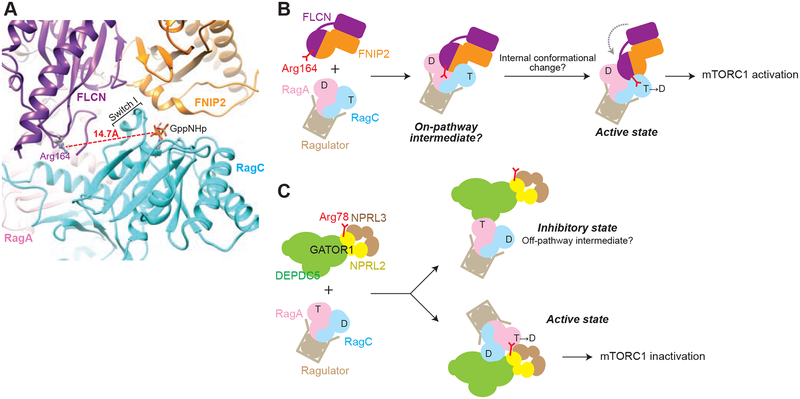
Figure 5. The resolved FLCN-FNIP2-Rag-Ragulator complex represents an on-pathway intermediate during GTP hydrolysis.
A. Relative positioning of the catalytic arginine and the nucleotide binding pocket of RagC. The distance between Arg164 and the phosphate of the GppNHp molecule bound to RagC is 14.7 Å. Nucleotide binding pocket of RagC is mis-oriented to allow for insertion of Arg164.
B. Model for stimulated GTP hydrolysis of the Rag GTPases by FLCN-FNIP2. We interpret our structure as an on-pathway intermediate. Subsequent local conformational changes are required to insert the catalytic residue into RagC-NBD.
C. Model for stimulated GTP hydrolysis of the Rag GTPases by GATOR1. We interpret our previous structure as an off-pathway intermediate during GTP hydrolysis. Global conformational change is required to access the catalytic arginine on Nprl2.