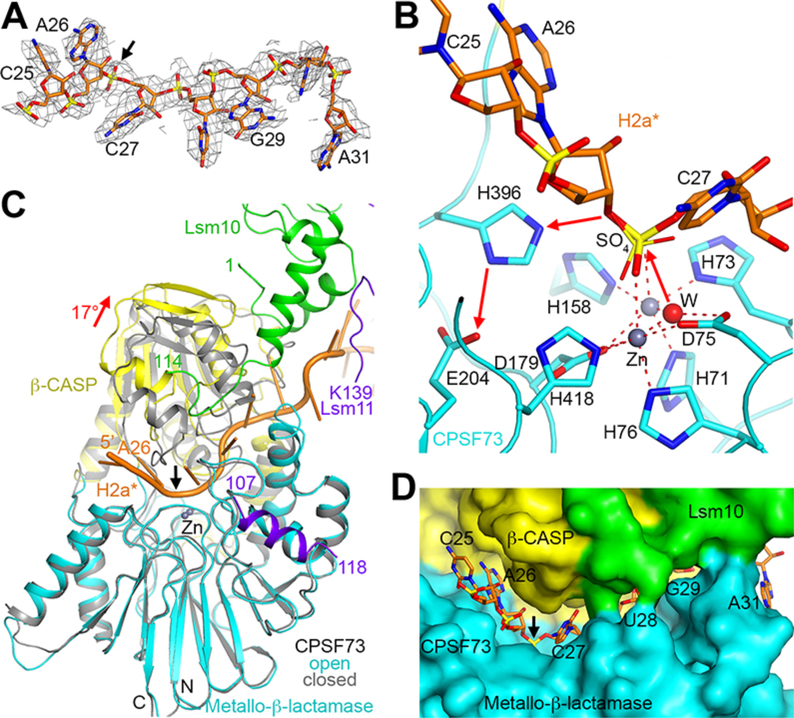
Figure 3. CPSF73 is in an active state, poised for the cleavage reaction.
(A) Cryo-EM density for H2a* nucleotides bound in the CPSF73 active site. The scissile phosphate is indicated with the black arrow. (B) The endonuclease mechanism of CPSF73. The positions of the zinc ions (gray spheres) and the bridging hydroxide (red sphere) are based on the crystal structure of CPSF73 alone (6) (PDB entry 2I7V). The position of the sulfate ion observed in the earlier structure is shown in thin sticks. (C) Overlay of the structure of CPSF73 in the active state observed here (in color) with the inactive, closed state reported earlier (gray) (6). The metallo-β-lactamase domain was used for the overlay. The rearrangement of the β-CASP domain is indicated with the red arrow, corresponding to a rotation of 17°. (D) Molecular surface of the active site region of CPSF73, colored by the domains. Lsm10 is located at the rim of the canyon, contacting nucleotides downstream of the cleavage site.