Abstract
The applicant DuPont de Nemours (Deutschland) GmbH submitted a request to the competent national authority in Austria to evaluate confirmatory data identified for cymoxanil in the framework of the maximum residue level (MRL) review under Article 12 of Regulation (EC) No 396/2005 and implemented in the EU MRL Regulation. To address the data gap on table and wine grapes, lettuce and spinach, the applicant submitted new residue trials for cymoxanil on these commodities. The data gap was considered satisfactorily addressed. Confirmatory data on analytical methods for enforcement in hops and herbal infusions (dried flower), and storage stability data in dry matrices were not submitted with the present application. Based on the available information, a revision of the existing MRLs for table and wine grapes, spinach and pulses should be considered by risk managers.
Keywords: cymoxanil, confirmatory data, pesticide, MRL review, risk assessment
Summary
In 2015, when the European Food Safety Authority (EFSA) reviewed the existing maximum residue levels (MRLs) for cymoxanil according to Article 12 of Regulation (EC) No 396/2005, EFSA identified some information as unavailable (data gaps) and derived tentative MRLs for those uses which were not fully supported by data but for which no risk to consumers was identified. The following data gaps were identified:
a confirmatory analytical method for enforcement in hops and herbal infusions (dried, flower);
a study investigating storage stability of cymoxanil in dry matrices;
additional residue trials supporting the authorisations on table and wine grapes (southern Europe (SEU) use), lettuce (SEU use), spinach (SEU use), sunflower seed (SEU use) and soyabean (SEU use).
Tentative MRL proposals have been implemented in the MRL legislation by Commission Regulation (EU) No 2016/1785, including footnotes related to data gaps number 1, 2 and 3, indicating the type of confirmatory data that should be provided by a party having an interest in maintaining the proposed tentative MRL by 8 October 2018. The footnote related to data gap number 3 was introduced only for table and wine grapes, lettuces and spinaches. For sunflower seeds and soyabeans, data gap number 3 was not translated into a footnote in the MRL regulation because risk managers decided to set the MRL at the level of limit of quantification (LOQ) for these commodities.
Thus, in the framework of the current assessment, EFSA focused on the confirmatory data gaps number 1, 2 and 3, the latter one for table, wine grapes, lettuces and spinaches only.
In accordance with the agreed procedure set out in the working document SANTE/10235/2016, DuPont de Nemours (Deutschland) GmbH submitted an application to the competent national authority in Austria (rapporteur Member State, RMS) to evaluate confirmatory data on table and wine grapes, lettuces and spinaches identified under data gap number 3 during the MRL review. The applicant provided residue trials supporting the SEU uses for these crops. The RMS assessed the new information in an evaluation report which was submitted to the European Commission and forwarded to the EFSA on 11 March 2019. No information on data gaps number 1 and 2 was submitted in the context of the current application.
The summary table below provides an overview of the assessment of confirmatory data and the recommended MRL modifications to Regulation (EU) No 396/2005.
| Codea | Commodity | Existing MRLb | Proposed MRL | Conclusion/recommendation |
|---|---|---|---|---|
| Enforcement residue definition: Cyproxanil | ||||
| 0151000 | Grapes | 0.3 ft 1 | 0.05 | The data gap identified by EFSA concerning three additional trials from SEU in grapes has been addressed. The SEU residue trials suggest an MRL of 0.02 mg/kg. Considering that MRL proposed in the NEU residue trials for grapes evaluated in the MRL review is higher (0.05 mg/kg), a revision of the existing MRL in table and wine grapes from 0.3 to 0.05 mg/kg would be appropriate; the footnote can be deleted |
| 0151010 | Table grapes | 0.3 ft 1 | 0.05 | |
| 0151020 | Wine grapes | 0.3 ft 1 | 0.05 | |
| 0251020 | Lettuces | 0.03 ft 1 | 0.03 | The data gap identified by EFSA concerning two additional trials from SEU in lettuces has been addressed. The MRL is confirmed and the footnote can be deleted |
| 0252010 | Spinaches | 1 ft 1 | 0.9 | The data gap identified by EFSA concerning four trials from SEU in spinaches has been addressed. The SEU residue trials suggest an MRL of 0.9 mg/kg. Therefore, a revision of the existing MRL in spinaches from 1 to 0.9 mg/kg would be appropriate; the footnote can be deleted |
| 0300000 | Pulses | 0.05* ft 2 | 0.01* |
Information on storage stability in dry matrices requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on pulses Considering that the validated analytical method assessed in the MRL review reports an LOQ of 0.01 mg/kg for dry matrices, a revision of the existing MRL from 0.05* to 0.01* mg/kg and deletion of the footnote would be appropriate |
| 0300010 | Beans | 0.05* ft 2 | 0.01* | |
| 0300020 | Lentils | 0.05* ft 2 | 0.01* | |
| 0300030 | Peas | 0.05* ft 2 | 0.01* | |
| 0300040 | Lupins/lupini beans | 0.05* ft 2 | 0.01* | |
| 0631000 | Herbal infusions (dried flowers) | 0.1* ft 3 | 0.1* | Information on storage stability and analytical methods for enforcement in herbal infusions from flowers requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on herbal infusions (dried flowers). A modification of the existing MRL is not required |
| 0631010 | Chamomile | 0.1* ft 3 | 0.1* | |
| 0631020 | Hibiscus/roselle | 0.1* ft 3 | 0.1* | |
| 0631030 | Rose | 0.1* ft 3 | 0.1* | |
| 0631040 | Jasmine | 0.1* ft 3 | 0.1* | |
| 0631050 | Lime/linden | ft 3 | 0.1* | |
| 0700000 | Hops (dried) | 0.1* ft 3 | 0.1* | Information on storage stability and analytical methods for enforcement in hops requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on hops. A modification of the existing MRL is not required |
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing EU MRL and corresponding footnote on confirmatory data.
The European Food Safety Authority identified some information on residue trials as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it. (Footnote related to data gap No 3).
The European Food Safety Authority identified some information on storage stability as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it. (Footnote related to data gap No 2).
ft 1 The European Food Safety Authority identified some information on storage stability and analytical methods as unavailable. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it. Footnote related to data gap No 1 and 2).
Assessment
The review of existing maximum residue levels (MRLs) for the active substance cymoxanil according to Article 12 of Regulation (EC) No 396/20051 (MRL review) has been performed in 2015 (EFSA, 2015). The European Food Safety Authority (EFSA) identified some information as unavailable (data gaps) and derived tentative MRLs for those uses not fully supported by data but for which no risk to consumers was identified. The list of Good Agricultural Practices (GAPs) assessed in the framework of the MRL review that were not fully supported by data and for which confirmatory data were requested are listed in Appendix A.
Following the review of existing MRLs, the legal limits have been modified by Commission Regulation (EU) No 2016/17852, including footnotes for tentative MRLs that specified the type of information that was identified as missing. Any party having an interest in maintaining the proposed tentative MRLs was requested to address the confirmatory data by 8 October 2018.
In accordance with the specific provisions set out in the working document of the European Commission SANTE/10235/2016 (European Commission, 2016), the applicant, DuPont de Nemours (Deutschland) GmbH, submitted an application to the competent national authority in Austria (designated rapporteur Member State, RMS) to evaluate some of the confirmatory data identified during the MRL review. To address the data gaps identified by EFSA, the applicant provided residue trials supporting the southern Europe (SEU) GAPs on table and wine grapes, lettuces and spinaches.
The RMS assessed the new information in an evaluation report, which was submitted to the European Commission and forwarded to EFSA on 11 March 2019 (Austria, 2019). EFSA assessed the application as requested by the European Commission in accordance with Article 9 of Regulation (EC) No 396/2005.
EFSA based its assessment on the evaluation report submitted by the RMS (Austria, 2019), the reasoned opinion on the MRL review according to Article 12 of Regulation (EC) No 396/2005 and an additional assessment on the modification of the existing MRL for cymoxanil in beans without pods performed after the MRL review (EFSA, 2015, 2017).
For this application, the data requirements established in Regulation (EU) No 544/20113 and the relevant guidance documents at the date of implementation of the confirmatory data requirements by Regulation (EU) No 2016/1785 are applicable. The assessment is performed in accordance with the legal provisions of the Uniform Principles for the Evaluation and the Authorisation of Plant Protection Products adopted by Commission Regulation (EU) No 546/20114.
An updated list of end points, including the end points of relevant studies assessed previously and the confirmatory data evaluated in this application, is presented in Appendix B.
The evaluation report submitted by the RMS (Austria, 2019) is considered a supporting document to this reasoned opinion and, thus, is made publicly available as a background document to this reasoned opinion.
1. Residues in plants
1.1. Nature of residues and methods of analysis in plants
1.1.1. Nature of residues in primary crops
Not relevant for the current assessment.
1.1.2. Nature of residues in rotational crops
Not relevant for the current assessment.
1.1.3. Nature of residues in processed commodities
Not relevant for the current assessment.
1.1.4. Methods of analysis in plants
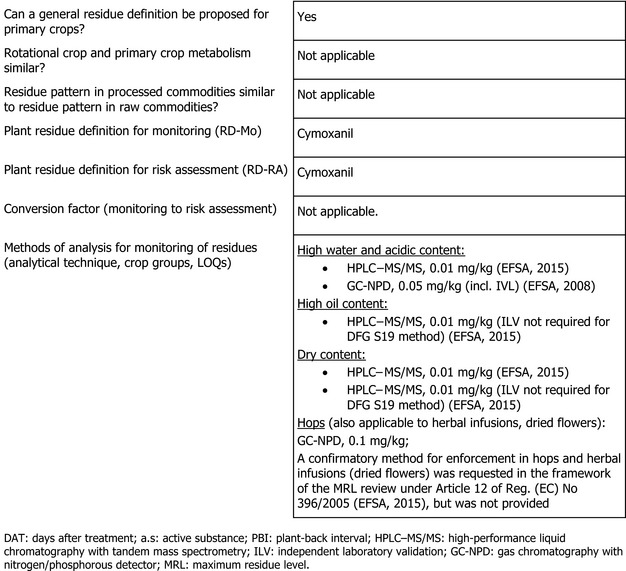
Data gap number 1 requested a confirmatory method for enforcement in hops and herbal infusions (dried, flower) (EFSA, 2015). No confirmatory method for the above‐mentioned commodities was submitted in the context of the current assessment.
1.1.5. Storage stability of residues in plants
Data gap number 2 requested a study investigating storage stability of cymoxanil in dry matrices. No new information was submitted on data gap number 2 in the context of the current assessment.
1.1.6. Proposed residue definitions
The previously derived residue definitions are still applicable.
1.2. Magnitude of residues in plants
1.2.1. Magnitude of residues in primary crops
In order to address data gap number 3, the applicant provided additional residue trials on grapes, lettuces and spinaches.
1.2.1.1. Table and wine grapes
In the MRL review for cymoxanil (EFSA, 2015), three additional residue trials compliant with the southern outdoor GAP on table and wine grapes were requested as confirmatory data.
Four additional residue trials on wine grapes in SEU were submitted to address the data gap identified for this commodity. Samples were stored in compliance with the demonstrated storage conditions and analysed with a sufficiently validated analytical method. Trials were found to be independent and compliant with the SEU critical GAP.
EFSA concluded that the data gap identified for table and wine grapes in the framework of the MRL review was addressed. The SEU residue trials suggest an MRL of 0.02 mg/kg.
Residue trials on table and wine grapes from northern Europe (NEU) have been submitted and assessed in the context of the MRL review (EFSA, 2015). Since the preharvest interval (PHI) for grapes in the SEU GAP (= 21 days) is different from the PHI for grapes in the NEU GAP (= 28 days), the SEU and NEU residue trials cannot be combined. Considering that MRL proposal from the NEU residue trials for grapes evaluated in the MRL review is higher (0.05 mg/kg) than the one from the SEU (0.02 mg/kg), a revision of the existing MRL in table and wine grapes from 0.3 to 0.05 mg/kg would be appropriate.
1.2.1.2. Lettuces
In the framework of the MRL review for cymoxanil (EFSA, 2015), two additional residue trials compliant with the SEU outdoor GAP on lettuce were requested as confirmatory data.
Three additional SEU residue trials on lettuce were submitted to address the data gap identified for this commodity. Samples were stored in compliance with the demonstrated storage conditions and analysed with a sufficiently validated analytical method. Trials were found to be independent and compliant with the critical GAP.
EFSA concluded that the data gap identified for lettuces in the framework of the MRL review was addressed. The estimated MRL for cymoxanil in lettuce was found to be 0.03 mg/kg. Therefore, the MRL set in lettuces is confirmed.
1.2.1.3. Spinaches
In the framework of the MRL review for cymoxanil (EFSA, 2015), four additional residue trials compliant with the SEU outdoor GAP on spinaches were requested as confirmatory data.
Five additional SEU residue trials on spinach were submitted to address the data gap identified for this commodity. Samples were stored in compliance with the demonstrated storage conditions and analysed with a sufficiently validated analytical method. Trials were found to be independent and compliant with the SEU critical GAP.
EFSA concluded that the data gap identified for spinaches in the framework of the MRL review was addressed. The SEU residue trials suggest an MRL of 0.9 mg/kg. Residue trials on spinach from NEU have been submitted and assessed in the context of the MRL review (EFSA, 2015). Since the PHI for spinach in the SEU GAP (= 7 days) is different from the PHI for spinaches in the Northern Europe GAP (= 14 days), the SEU and NEU residue trials cannot be combined. Considering that MRL proposal from the SEU residue trials for spinaches evaluated in the MRL review is higher (0.9 mg/kg) than the one from the NEU (0.07 mg/kg), a revision of the existing MRL in spinaches from 1 to 0.9 mg/kg would be appropriate.
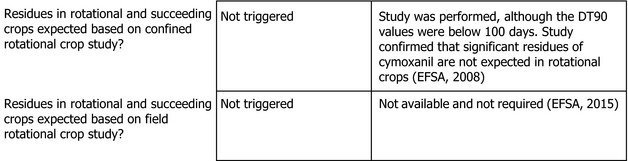
1.2.2. Magnitude of residues in rotational crops
Not relevant for the current assessment.
1.2.3. Magnitude of residues in processed commodities
Not relevant for the current assessment.
2. Residues in livestock
Sunflower seeds and soyabeans may be fed to livestock. In the context of the EFSA MRL review of cymoxanil in 2015, the dietary burden for livestock was calculated including potatoes and potato by‐products; soyabeans and sunflower seeds were not considered in the estimation of the animal intake of cymoxanil residues due to the lack of residue data for cymoxanil in these commodities (EFSA, 2015). EFSA highlighted that this might underestimate the animal intake of the active substance. However, there was sufficient evidence to conclude that MRLs for cymoxanil in animal commodities are not required (EFSA, 2015).
The previous assessment of residues in livestock (EFSA, 2015) is still valid.
3. Consumer risk assessment
EFSA updated the previous risk assessment, taking into account information on beans without pods, submitted in the framework of the modification of the existing MRL for cymoxanil in this commodity (EFSA, 2017) and the new data on grapes, spinaches and lettuces submitted under this application. The consumer risk assessment was performed with revision 2 of the EFSA PRIMo (EFSA, 2007). The toxicological reference values for cymoxanil have not changed since the MRL review (EFSA, 2015).
3.1. Short‐term (acute) dietary risk assessment
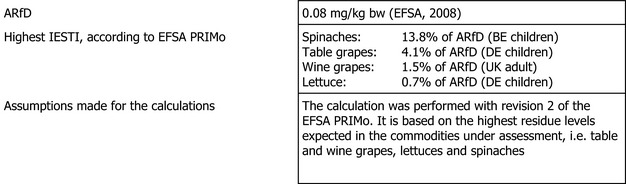
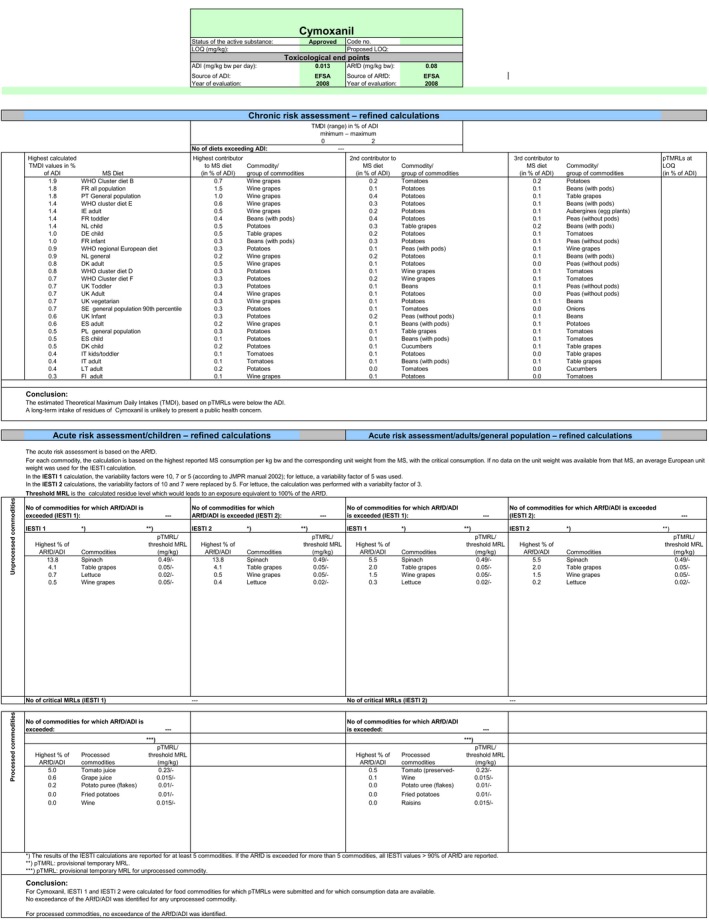
The short‐term risk assessment was performed only for the commodities under assessment and for which new residue trials were submitted: table and wine grapes, lettuces and spinaches. The estimation of the exposure is based on the highest residue (HR) derived from the supervised field trials on the above‐mentioned commodities (see Appendix D). The international estimated short‐term intake (IESTI) accounted for 13.8% of the acute reference dose (ARfD) in spinaches (BE children), 4.1% of the ARfD in table grapes (DE children), 1.5% of the ARfD in wine grapes (UK adult) and 0.7% of the ARfD in lettuce (DE children).
3.2. Long‐term (chronic) dietary risk assessment
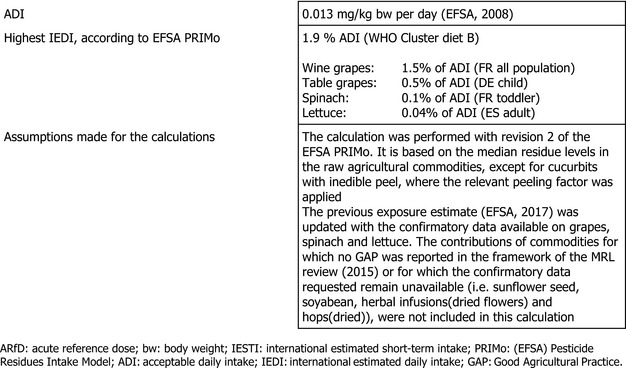
The long‐term exposure assessment was performed based on the existing uses of cymoxanil at EU level. EFSA updated the previous exposure assessment (EFSA, 2017), with the supervised trials median residue value (STMR), derived from the new trials available in grapes, lettuce and spinach (Austria, 2019). The complete list of input values is reported in Appendix D. The contributions of commodities for which no GAP was reported in the framework of the MRL review (EFSA, 2015) or for which the confirmatory data requested remain unavailable (i.e. sunflower seeds, soyabeans, herbal infusions (dried flowers) and hops (dried)) were not included in the calculation.
The estimated long‐term exposure to cymoxanil accounted for up to 1.9% of the acceptable daily intake (ADI) for WHO Cluster diet B. The contribution of residues expected in (i) table grapes is up to the 0.5% of the ADI for DE child, (ii) wine grapes up to the 1.5% of the ADI for FR all population, (iii) lettuces up to the 0.04% of the ADI for ES adult and (iv) spinaches up to the 0.1% of the ADI for FR toddler.
4. Conclusion and Recommendations
To address data gaps identified in the framework of the MRL review (EFSA, 2015), the following confirmatory data on data gap number 35 were submitted by the applicant:
-
–
Four residue trials on wine grapes in SEU to support the authorisations on table and wine grapes;
-
–
Three residue trials on lettuces in SEU and
-
–
Five additional residue trials on spinaches in SEU.
The above data gaps were sufficiently addressed. Based on the available information, a revision of the existing MRLs for table and wine grapes and spinaches would be appropriate. The MRL for lettuces is confirmed.
For sunflower seeds and soyabeans, the data gap number 3 was not translated into footnote in the MRL regulation because risk managers decided to set the MRL at the level of LOQ for these commodities. Thus, these crops are not subject to the current assessment.
No new information was submitted on data gap number 1 requesting a confirmatory analytical method for enforcement in hops and herbal infusions (dried, flower). Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on hops and herbal infusions (dried flowers). A modification of the existing MRLs is not required.
No new information was submitted on data gap number 2 requesting a study investigating storage stability of cymoxanil in dry matrices. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on pulses. Considering that the validated analytical method assessed in the MRL review reports an LOQ of 0.01 mg/kg for dry matrices, a revision of the existing MRL from 0.05* mg/kg to 0.01* mg/kg and deletion of the footnote would be appropriate. The overview of the assessment of confirmatory data and the recommended MRL modifications are summarised in Appendix B.4.
Abbreviations
- a.s.
active substance
- ADI
acceptable daily intake
- ARfD
acute reference dose
- BBCH
growth stages of mono‐ and dicotyledonous plants
- bw
body weight
- DAT
days after treatment
- DT90
period required for 90% dissipation (define method of estimation)
- Eq
residue expressed as a.s. equivalent
- GAP
Good Agricultural Practice
- GC‐NPD
gas chromatography with nitrogen/phosphorous detector
- HPLC‐MS/MS
high performance liquid chromatography with tandem mass spectrometry
- HR
highest residue
- IEDI
international estimated daily intake
- IESTI
international estimated short‐term intake
- ILV
independent laboratory validation
- LOQ
limit of quantification
- MRL
maximum residue level
- MS
Member States
- MS
mass spectrometry detector
- NEU
northern Europe
- PBI
plant back interval
- PF
processing factor
- PHI
preharvest interval
- PRIMo
(EFSA) Pesticide Residues Intake Model
- RA
risk assessment
- RD
residue definition
- RMS
rapporteur Member State
- SANCO
Directorate‐General for Health and Consumers
- SEU
southern Europe
- STMR
supervised trials median residue
- WG
water‐dispersible granule
- WHO
World Health Organization
- WP
wettable powder
Appendix A – Summary of GAPs assessed in the evaluation of confirmatory data
1.
| Code | Crop name | Region/country | Outdoor/indoora | Pests controlled | Active substance (a.s.) | Formulation typeb | a.s. conc. in formulation (g/kg) | Appl. method | Growth stagec | No of appl. | Interval (days) Minim. | Water amount (L/ha) | Max. appl. rate (g a.s./ha) | PHI (days)d | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 151010 | Table grapes | NEU/AT | Outdoor | Plasmopara viticola, Downy mildew | Cymoxanil | WG | 45 | Foliar treatment – broadcast spraying | BBCH 11–71 | 1–4 | 10 | Not specified | 90 | 28 | |
| 151010 | Table grapes | SEU/IT | Outdoor | Plasmopara viticola, Phomopsis viticola | Cymoxanil | WP | 200 | Foliar treatment – broadcast spraying | BBCH 15–85 | 1–4 | 7 | Not specified | 170 | 21 | Assessed for confirmatory data |
| 151020 | Wine grapes | NEU/DE | Outdoor | Plasmopara viticola | Cymoxanil | WG | 330 | Foliar treatment – broadcast spraying | BBCH 14–89 | 1–5 | 7 | Not specified | 150 | 28 | |
| 151020 | Wine grapes | SEU/IT | Outdoor | Plasmopara viticola, Phomopsis viticola | Cymoxanil | WP | 200 | Foliar treatment – broadcast spraying | BBCH 15–85 | 1–4 | 7 | Not specified | 170 | 21 | Assessed for confirmatory data |
| 251020 | Lettuces | SEU/IT | Outdoor | Bremia lactucae | Cymoxanil | WP | 200 | Foliar treatment – broadcast spraying | BBCH 19–49 | 1–4 | 5 | Not specified | 240 | 7 | Assessed for confirmatory data |
| 252010 | Spinaches | NEU/BE | Outdoor | Downy mildew | Cymoxanil | WG | 250 | Foliar treatment – broadcast spraying | N/A | 1–2 | 10 | Not specified | 150 | 14 | |
| 252010 | Spinaches | SEU/IT | Outdoor | Downy mildew | Cymoxanil | WP | 200 | Foliar treatment – broadcast spraying | BBCH 19–49 | 1–4 | 7 | Not specified | 180 | 7 | Assessed for confirmatory data |
NEU: northern European Union; SEU: southern European Union; MS: Member State.
Outdoor or field use (F), greenhouse application (G) or indoor application (I).
CropLife International Technical Monograph no 2, 6th Edition. Revised May 2008. Catalogue of pesticide formulation types and international coding system.
Growth stage range from first to last treatment (BBCH Monograph, Growth Stages of Plants, 1997, Blackwell, ISBN 3‐8263‐3152‐4), including, where relevant, information on season at time of application.
PHI: minimum preharvest interval.
Appendix B – List of end points
B.1. Residues in plants
B.1.1. Nature of residues and methods of analysis in plants
B.1.1.1. Metabolism studies, methods of analysis and residue definitions in plants
| Primary crops (available studies) | Crop groups | Crop(s) | Application(s) | Sampling (DAT) | Comment/Source |
|---|---|---|---|---|---|
| Fruit crops | Tomatoes | Foliar, 3 × 0.63 kg a.s./ha | 3 | EFSA (2015) | |
| Tomatoes | Foliar, 4 × 0.24 kg a.s./ha | 13 | EFSA (2015) | ||
| Tomatoes | Foliar, 7 × 0.14 kg a.s/ha | 7, 14, 21, 35 | EFSA (2015) | ||
| Grapes | Foliar, 8 × 0.21 kg a.s./ha | 0, 1, 4, 10, 18 | EFSA (2015) | ||
| Root crops | Potatoes | Foliar, 8 × 0.24 kg a.s./ha | 10 | EFSA (2008) | |
| Potatoes | Foliar, 3 × 0.40 kg a.s./ha | 3 | EFSA (2008) | ||
| Leafy crops | Lettuces | Foliar, 3 × 0.24 kg a.s./ha | 11 | EFSA (2008) | |
| Lettuces | Foliar, 4 × 0.84 kg a.s./ha | 3 | EFSA (2008) |
| Rotational crops (available studies) | Crop groups | Crop(s) | Application(s) | PBI (DAT) | Comment/source |
|---|---|---|---|---|---|
| Root/tuber crops | Sugar beet | Bare soil, 1.2 kg a.s./ha | 30, 120 | A ‘no residue’ situation in rotational crops was established (EFSA, 2008) | |
| Leafy crops | Lettuces | Bare soil, 1.2 kg a.s./ha | 30, 120 | A ‘no residue’ situation in rotational crops was established (EFSA, 2008) | |
| Cereal (small grain) | Wheat | Bare soil, 1.2 kg a.s./ha | 30, 120 | A ‘no residue’ situation in rotational crops was established (EFSA, 2008) | |
| Other | – | – | – | – |
| Processed commodities (hydrolysis study) | Conditions | Stable? | Comment/source |
|---|---|---|---|
| Pasteurisation (20 min, 90°C, pH 4) | Not investigated | In the context of the MRL review for cymoxanil, a study investigating the nature of residues in processed commodities through standard hydrolytic conditions was considered desirable but not essential (EFSA, 2015) | |
| Baking, brewing and boiling (60 min, 100°C, pH 5) | Not investigated | ||
| Sterilisation (20 min, 120°C, pH 6) | Not investigated | ||
| Other processing conditions | Not investigated |
B.1.1.2. Storage stability
| Plant products (available studies) | Category | Commodity | T (°C) | Stability period | Compound covered | Comment/source | |
|---|---|---|---|---|---|---|---|
| Value | Unit | ||||||
| High water content | Tomatoes | −18 | ≤ 18 | Months | cymoxanil | Study performed on several crops investigating storage periods of up to 873 days. However, an unexplained decline of residues was observed in tomatoes between 18 and 24 months. (EFSA, 2015) | |
| High acid content | Grapes | −18 | 24 | Months | cymoxanil | ||
| High oil content | Sunflower seed | −18 | 18 | Months | cymoxanil | ||
| Dry content | – | – | – | – | – | A confirmatory study investigating storage stability of cymoxanil in dry matrices was requested in the framework of the MRL review under Article 12 of Reg. (EC) No 396/2005 (EFSA, 2015), but was not provided | |
B.1.2. Magnitude of residues in plants
B.1.2.1. Summary of residues data from the supervised residue trials
| Commodity | Region/indoora | Residue levels observed in the supervised residue trials (mg/kg) | Comments/Source | Calculated MRL (mg/kg) | HRb (mg/kg) | STMRc (mg/kg) |
|---|---|---|---|---|---|---|
| Table and wine grapes | NEU | EFSA (2015): 2 × < 0.001; 6 × < 0.01; < 0.04; 14 × < 0.05 | Residue trials on wine grapes compliant with GAP. Extrapolation to table grapes for which the GAP is less critical is possible because residue levels are < LOQ (EFSA, 2015) | 0.05 * | 0.05 | 0.05 |
| SEU |
EFSA (2015): 4 × < 0.01; 0.015 Austria (2019): 4 × < 0.01 |
Residue trials on wine grapes compliant with GAP. Extrapolation to table grapes is possible (EFSA, 2015) | 0.02 | 0.015 | 0.01 | |
| Lettuces | SEU |
EFSA (2015): 4 × < 0.01; 0.01; 0.02 Austria, (2019): 3 × < 0.01 |
Residue trials on lettuces compliant with GAP | 0.03 | 0.02 | 0.01 |
| Spinaches | NEU | EFSA (2015): 4 × < 0.02; 0.04 | Residue trials on spinaches compliant with GAP | 0.07 | 0.04 | 0.02 |
| SEU |
EFSA (2015): Unscaled residues: 2 × < 0.01; 0.01; 0.02; 0.04; 0.05; 0.65 Scaled residues (sf 0.75): 2 × < 0.01; 0.0075; 0.15; 0.03; 0.0375; 0.488 Austria (2019): 2 × < 0.01; 0.021; 0.049; 0.47 |
Residue trials on spinaches compliant with GAP. Trials evaluated in the framework of the MRL review (EFSA, 2015) were overdosed (240 g a.s./ha instead of 180 g a.s./ha). A scaling factor (sf) of 0.75 was applied to derive the scaled residue values for these trials which were used in the present assessment | 0.9 | 0.49 | 0.02 |
* Indicates that the MRL is proposed at the limit of quantification (EFSA, 2015). Values in bold are the final MRL proposals.
NEU: Outdoor trials conducted in northern Europe, SEU: Outdoor trials conducted in southern Europe, Indoor: indoor EU trials or Country code: if non‐EU trials.
Highest residue. The highest residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue. The median residue for risk assessment refers to the whole commodity and not to the edible portion.
Supervised trials median residue according to the residue definition for monitoring.
B.1.2.2. Residues in rotational crops
B.1.1.3. Processing factors
No processing studies were submitted in the framework of the present MRL application.
B.2. Residues in livestock
No additional data were submitted on sunflower seeds and soyabeans which may be fed to livestock; grapes, lettuces and spinaches are not expected to be fed to livestock.
B.3. Consumer risk assessment
B.4. Recommended MRLs
| Codea | Commodity | Existing MRLb | Proposed MRL | Conclusion/recommendation |
|---|---|---|---|---|
| Enforcement residue definition: Cyproxanil | ||||
| 0151000 | Grapes | 0.3 ft 1 | 0.05 | The data gap identified by EFSA concerning three additional trials from SEU in grapes has been addressed. The SEU residue trials suggest an MRL of 0.02 mg/kg. Considering that MRL proposed in the NEU residue trials for grapes evaluated in the MRL review is higher (0.05 mg/kg), a revision of the existing MRL in table and wine grapes from 0.3 mg/kg to 0.05 mg/kg would be appropriate; the footnote can be deleted |
| 0151010 | Table grapes | 0.3 ft 1 | 0.05 | |
| 0151020 | Wine grapes | 0.3 ft 1 | 0.05 | |
| 0251020 | Lettuces | 0.03 ft 1 | 0.03 | The data gap identified by EFSA concerning two additional trials from SEU in lettuces has been addressed. The MRL is confirmed and the footnote can be deleted |
| 0252010 | Spinaches | 1 ft 1 | 0.9 | The data gap identified by EFSA concerning four trials from SEU in spinaches has been addressed. The SEU residue trials suggest an MRL of 0.9 mg/kg. Therefore, a revision of the existing MRL in spinaches from 1 to 0.9 mg/kg would be appropriate; the footnote can be deleted |
| 0300000 | Pulses | 0.05* ft 2 | 0.01* |
Information on storage stability in dry matrices requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on pulses Considering that the validated analytical method assessed in the MRL review reports an LOQ of 0.01 mg/kg for dry matrices, a revision of the existing MRL from 0.05* mg/kg to 0.01* mg/kg and deletion of the footnote would be appropriate |
| 0300010 | Beans | 0.05* ft 2 | 0.01* | |
| 0300020 | Lentils | 0.05* ft 2 | 0.01* | |
| 0300030 | Peas | 0.05* ft 2 | 0.01* | |
| 0300040 | Lupins/lupini beans | 0.05* ft 2 | 0.01* | |
| 0631000 | Herbal infusions (dried flowers) | 0.1* ft 3 | 0.1* | Information on storage stability and analytical methods for enforcement in herbal infusions from flowers requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on herbal infusions (dried flowers). A modification of the existing MRL is not required |
| 0631010 | Chamomile | 0.1* ft 3 | 0.1* | |
| 0631020 | Hibiscus/roselle | 0.1* ft 3 | 0.1* | |
| 0631030 | Rose | 0.1* ft 3 | 0.1* | |
| 0631040 | Jasmine | 0.1* ft 3 | 0.1* | |
| 0631050 | Lime/linden | 0.1* ft 3 | 0.1* | |
| 0700000 | Hops (dried) | 0.1* ft 3 | 0.1* | Information on storage stability and analytical methods for enforcement in hops requested as confirmatory data was not provided. Since the data gap was not addressed, risk management action would be appropriate, e.g. revocation of the existing uses on hops. A modification of the existing MRL is not required |
* Indicates that the MRL is set at the limit of analytical quantification (LOQ).
Commodity code number according to Annex I of Regulation (EC) No 396/2005.
Existing EU MRL and corresponding footnote on confirmatory data.
ft 1 The European Food Safety Authority identified some information on residue trials as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it (footnote related to data gap No 3).
ft 2 The European Food Safety Authority identified some information on storage stability as unavailable. When reviewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it (footnote related to data gap No 2).
ft 3 The European Food Safety Authority identified some information on storage stability and analytical methods as unavailable. When re‐viewing the MRL, the Commission will take into account the information referred to in the first sentence, if it is submitted by 8 October 2018, or, if that information is not submitted by that date, the lack of it (footnote related to data gaps No 1 and 2).
Appendix C – Pesticide Residue Intake Model (PRIMo)
1.
Appendix D – Input values for the exposure calculations
1.
| Commodity | Chronic risk assessment | Acute risk assessment | ||
|---|---|---|---|---|
| Input value (mg/kg) | Comment | Input value (mg/kg) | Comment | |
| Table grapes | 0.05 | STMR | 0.05 | HR |
| Wine grapes | 0.05 | STMR | 0.05 | HR |
| Lettuces | 0.01 | STMR | 0.02 | HR |
| Spinaches | 0.02 | STMR | 0.49 | HR |
| Potatoes | 0.01 | STMR (EFSA, 2015) | Acute risk assessment only for the crops under consideration | |
| Garlic | 0.01 | STMR (EFSA, 2015) | ||
| Onions | 0.01 | STMR (EFSA, 2015) | ||
| Tomatoes | 0.01 | STMR (EFSA, 2015) | ||
| Aubergines (egg plants) | 0.05 | STMR (EFSA, 2015) | ||
| Cucumbers | 0.01 | STMR (EFSA, 2015) | ||
| Gherkins | 0.01 | STMR (EFSA, 2015) | ||
| Courgettes | 0.01 | STMR (EFSA, 2015) | ||
| Melons | 0.002 | STMR x PF (EFSA, 2015) | ||
| Pumpkins | 0.002 | STMR x PF (EFSA, 2015) | ||
| Watermelons | 0.002 | STMR x PF (EFSA, 2015) | ||
| Broccoli | 0.01 | STMR (EFSA, 2015) | ||
| Cauliflower | 0.01 | STMR (EFSA, 2015) | ||
| Beans (fresh, with pods) | 0.05 | STMR (EFSA, 2015) | ||
| Beans (fresh, without pods) | 0.05 | STMR (EFSA, 2017) | ||
| Peas (fresh, with pods) | 0.05 | STMR (EFSA, 2015) | ||
| Peas (fresh, without pods) | 0.05 | STMR (EFSA, 2015) | ||
| Globe artichokes | 0.01 | STMR (EFSA, 2015) | ||
| Leek | 0.01 | STMR (EFSA, 2015) | ||
| Beans (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Lentils (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Peas (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Lupins (dry) | 0.02 | STMR (EFSA, 2015) | ||
| Sunflower seeds | – | Not considered in the risk assessment since the confirmatory data requested in the framework of the MRL review (EFSA, 2015) remain unavailable | ||
| Soyabeans | – | |||
| Herbal infusions (dried, flowers) | – | |||
| Hops (dried), including hop pellets and unconcentrated powder | – | |||
Appendix E – Used compound codes
1.
| Code/trivial name | Chemical name/SMILES notationa | Structural formulaa |
|---|---|---|
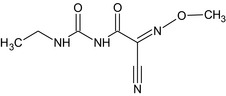
| cymoxanil |
1‐[(EZ)‐2‐cyano‐2‐methoxyiminoacetyl]‐3‐ethylurea N#C\C(=N\OC)C(=O)NC(=O)NCC PSOONIQXVGMYIU‐JIBDQCPFSA‐N |
|
(ACD/ChemSketch, Advanced Chemistry Development, Inc., ACD/Labs Release: 12.00 Product version: 12.00 (Build 29305, 25 Nov 2008).
Suggested citation: EFSA (European Food Safety Authority) , Anastassiadou M, Brancato A, Carrasco Cabrera L, Greco L, Jarrah S, Kazocina A, Leuschner R, Magrans JO, Miron I, Nave S, Pedersen R, Reich H, Rojas A, Sacchi A, Santos M, Stanek A, Theobald A, Vagenende B and Verani A, 2019. Evaluation of confirmatory data following the Article 12 MRL review for cymoxanil. EFSA Journal 2019;17(10):5823, 23 pp. 10.2903/j.efsa.2019.5823
Requestor: European Commission
Question number: EFSA‐Q‐2019‐00186
Approved: 16 September 2019
Notes
Regulation (EC) No 396/2005 of the Parliament and of the Council of 23 February 2005 on maximum residue levels of pesticides in or on food and feed of plant and animal origin and amending Council Directive 91/414/EEC. OJ L 70, 16.3.2005, p. 1–16.
Commission Regulation (EU) 2016/1785 of 7 October 2016 amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for cymoxanil, phosphane and phosphide salts and sodium 5‐nitroguaiacolate, sodium o‐nitrophenolate and sodium p‐nitrophenolate in or on certain products. OJ L 273, 8.10.2016, p. 10–30.
Commission Regulation (EU) No 544/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards the data requirements for active substances. OJ L 155, 11.6.2011, p. 1–66.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
Additional residue trials supporting the authorisations on table and wine grapes (SEU use), lettuce (SEU use), spinach (SEU use), sunflower seed (SEU use) and soyabean (SEU use).
References
- Austria , 2019. Evaluation report on the modification of MRLs for cymoxanil in grapes, lettuce and spinach. January 2019, 44 pp.
- EFSA (European Food Safety Authority), 2007. Reasoned opinion on the potential chronic and acute risk to consumers' health arising from proposed temporary EU MRLs. EFSA Journal 2007;5(3):32r, 1141 pp. 10.2903/j.efsa.2007.32r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Conclusion on the peer review of the pesticide risk assessment of the active substance cymoxanil. EFSA Journal 2008;6(10):167r, 116 pp. 10.2903/j.efsa.2008.167r [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2015. Review of the existing maximum residue levels for cymoxanil according to Article 12 of Regulation (EC) No 396/2005. EFSA Journal 2015;13(12):4355, 48 pp. 10.2903/j.efsa.2015.4355 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Brancato A, Brocca D, De Lentdecker C, Erdos Z, Ferreira L, Greco L, Jarrah S, Kardassi D, Leuschner R, Lythgo C, Medina P, Miron I, Molnar T, Nougadere A, Pedersen R, Reich H, Sacchi A, Santos M, Stanek A, Sturma J, Tarazona J, Theobald A, Vagenende B, Verani A and Villamar‐Bouza L, 2017. Reasoned opinion on the modification of the existing maximum residue level for cymoxanil in beans without pods. EFSA Journal 2017;15(12):5066, 19 pp. 10.2903/j.efsa.2017.5066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2016. Commission staff working document on the evaluation of data submitted to confirm MRLs following the review of existing MRLs Finalised in the Standing Committee on Plants, Animals, Food and Feed at its meeting on 17 June 2016. SANTE/E4/VW 10235/2016 ‐ Rev. 2, 3 pp., Brussels, 17 June 2016.


