Figure 1: Electrical detection of the twenty proteinogenic amino acids.
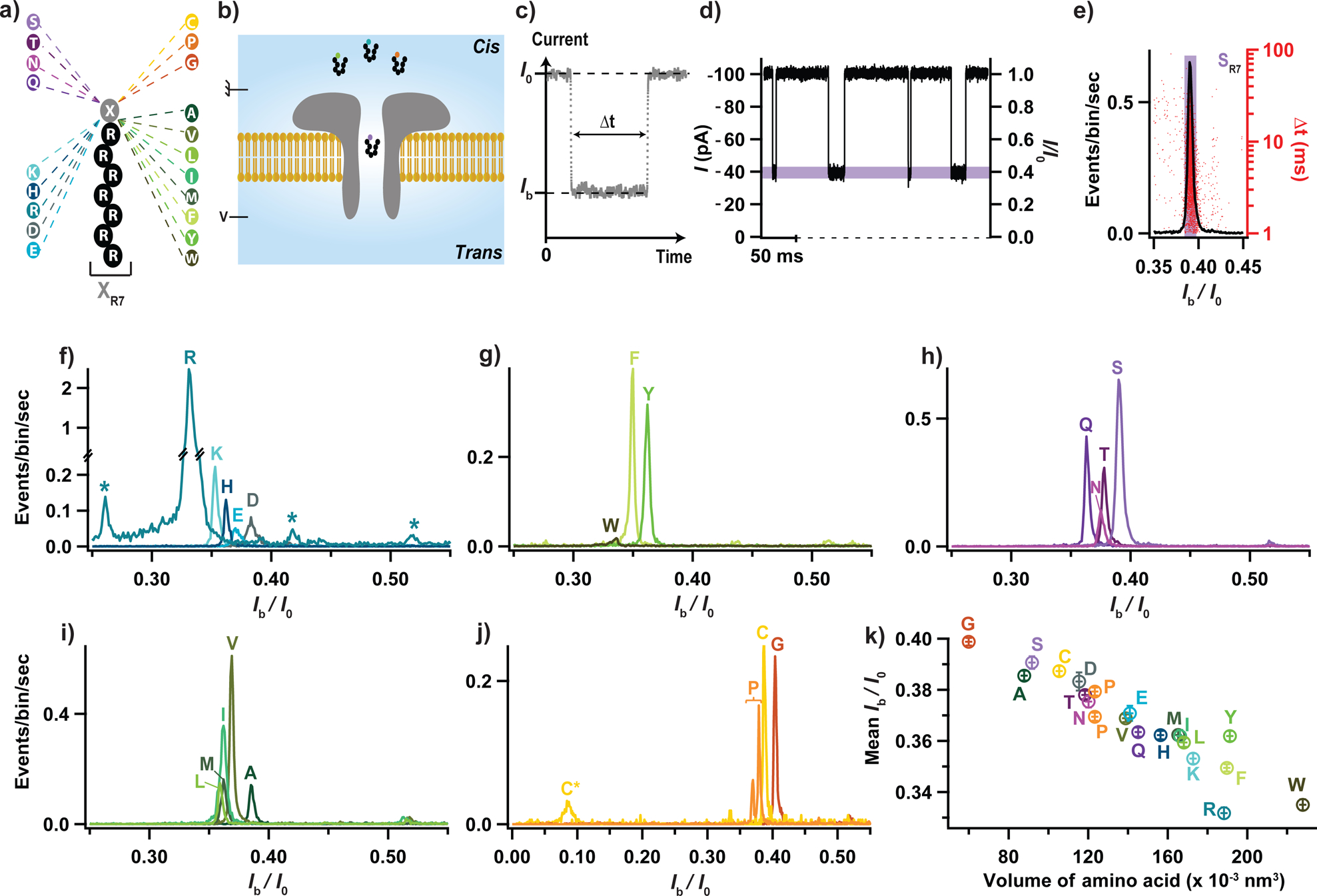
(a) Schematic illustration of the peptide constructs used to probe the current blockade of the twenty amino acids. A cationic carrier of seven arginine amino acids (R7) is chemically linked at the C-terminus to the eighth amino acid, X, to form twenty XR7 peptides. (b) Schematics of the experimental setup (not to scale). (c) Illustration of a typical current blockade. (d) Representative fragment of ionic current recording. (e) Typical histogram of the relative residual current, Ib/I0 (left axis), and blockade duration, Δt (right axis), produced by the transport of SR7 peptides through the aerolysin nanopore. (f-j) Superimposed histograms of Ib/I0 obtained from nanopore experiments performed for each of the twenty XR7 peptides, analyzed individually and grouped according to the properties of amino acid X: charged (f), hydrophobic aromatic (g), polar uncharged (h), hydrophobic non-aromatic (i), and when amino acid X is either cytosine, proline or glycine (j). In panel f, stars indicate blockade levels likely produced by R9, R7 and R6 polyarginine impurities (from left to right) present in the RR7 sample. In panel j, C* and C indicate populations recorded before and after dithiothreitol treatment, see Supplementary Fig. 1. (k) Mean relative residual current and its standard deviation produced by the XR7 probes versus volume of amino acid X. All data were acquired in 4 M KCl, 25 mM HEPES buffer, at 7.5 pH, 1 μM peptide concentration, 20.0 ± 0.5°C, and under a −50 mV bias applied to the trans compartment. For each histogram, at least 1000 events were analyzed.
