Abstract
The Panel on Plant Health performed a pest categorisation of non‐EU Acleris spp. Acleris is a well‐defined insect genus in the family Tortricidae (Insecta: Lepidoptera). Species can be identified using taxonomic keys based on adult morphology and genitalia. The genus includes 261 species attacking conifers and non‐conifer plants in many areas in the world, among which 40 species are present in the EU. The non‐EU species are collectively listed in Annex IAI of Council Directive 2000/29/EC as Acleris spp. (non‐European). Some species are important defoliators in North America, mainly on conifers but also on several broadleaf trees. Females lay eggs on the leaves or on the bark. The larvae bind together with silk the leaves upon which they feed. Pupation occurs in leaves attached with silk or in the soil. Some species are univoltine; others are bivoltine or multivoltine. Flight capacity is not documented, but outbreak expansion suggests that the adults can probably fly long distances. The main pathways for entry are host plants for planting with or without soil, cut branches, fruits of host plants (including cones), round wood with bark and bark. The presence of host plants and suitable EU climate would allow the establishment of the known non‐EU harmful species. In the literature, nine non‐EU Acleris species are reported as pests on various host plants, namely A. gloverana, A. variana, A. minuta, A. nishidai, A. issikii, A. semipurpurana, A. robinsoniana, A. senescens and A. nivisellana. These non‐EU Acleris spp. satisfy all the criteria to be considered as Union quarantine pests. Concerning the other 212 non‐EU Acleris species, there is scarce information on host plants, pests status and climatic suitability. Measures are in place to prevent the introduction of non‐EU Acleris spp. through the pathways described in the document. As non‐EU Acleris spp. are not present in the EU and plants for planting are not the major pathway for spread, non‐EU Acleris spp. do not meet the criteria to be considered as regulated non‐quarantine pests.
Keywords: Budworms, European Union, pest risk, plant health, plant pest, quarantine, Tortricidae
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. Background
Council Directive 2000/29/EC1 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community establishes the present European Union plant health regime. The Directive lays down the phytosanitary provisions and the control checks to be carried out at the place of origin on plants and plant products destined for the Union or to be moved within the Union. In the Directive's 2000/29/EC annexes, the list of harmful organisms (pests) whose introduction into or spread within the Union is prohibited, is detailed together with specific requirements for import or internal movement.
Following the evaluation of the plant health regime, the new basic plant health law, Regulation (EU) 2016/20312 on protective measures against pests of plants, was adopted on 26 October 2016 and will apply from 14 December 2019 onwards, repealing Directive 2000/29/EC. In line with the principles of the above mentioned legislation and the follow‐up work of the secondary legislation for the listing of EU regulated pests, EFSA is requested to provide pest categorisations of the harmful organisms included in the annexes of Directive 2000/29/EC, in the cases where recent pest risk assessment/pest categorisation is not available.
1.1.2. Terms of Reference
EFSA is requested, pursuant to Article 22(5.b) and Article 29(1) of Regulation (EC) No 178/2002,3 to provide scientific opinion in the field of plant health.
EFSA is requested to prepare and deliver a pest categorisation (step 1 analysis) for each of the regulated pests included in the appendices of the annex to this mandate. The methodology and template of pest categorisation have already been developed in past mandates for the organisms listed in Annex II Part A Section II of Directive 2000/29/EC. The same methodology and outcome is expected for this work as well.
The list of the harmful organisms included in the annex to this mandate comprises 133 harmful organisms or groups. A pest categorisation is expected for these 133 pests or groups and the delivery of the work would be stepwise at regular intervals through the year as detailed below. First priority covers the harmful organisms included in Appendix 1, comprising pests from Annex II Part A Section I and Annex II Part B of Directive 2000/29/EC. The delivery of all pest categorisations for the pests included in Appendix 1 is June 2018. The second priority is the pests included in Appendix 2, comprising the group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), the group of Tephritidae (non‐EU), the group of potato viruses and virus‐like organisms, the group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., and the group of Margarodes (non‐EU species). The delivery of all pest categorisations for the pests included in Appendix 2 is end 2019. The pests included in Appendix 3 cover pests of Annex I part A section I and all pest categorisations should be delivered by end 2020.
For the above‐mentioned groups, each covering a large number of pests, the pest categorisation will be performed for the group and not the individual harmful organisms listed under “such as” notation in the Annexes of the Directive 2000/29/EC. The criteria to be taken particularly under consideration for these cases, is the analysis of host pest combination, investigation of pathways, the damages occurring and the relevant impact.
Finally, as indicated in the text above, all references to ‘non‐European’ should be avoided and replaced by ‘non‐EU’ and refer to all territories with exception of the Union territories as defined in Article 1 point 3 of Regulation (EU) 2016/2031.
1.1.2.1. Terms of Reference: Appendix 1
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Aleurocanthus spp. | Numonia pyrivorella (Matsumura) |
| Anthonomus bisignifer (Schenkling) | Oligonychus perditus Pritchard and Baker |
| Anthonomus signatus (Say) | Pissodes spp. (non‐EU) |
| Aschistonyx eppoi Inouye | Scirtothrips aurantii Faure |
| Carposina niponensis Walsingham | Scirtothrips citri (Moultex) |
| Enarmonia packardi (Zeller) | Scolytidae spp. (non‐EU) |
| Enarmonia prunivora Walsh | Scrobipalpopsis solanivora Povolny |
| Grapholita inopinata Heinrich | Tachypterellus quadrigibbus Say |
| Hishomonus phycitis | Toxoptera citricida Kirk. |
| Leucaspis japonica Ckll. | Unaspis citri Comstock |
| Listronotus bonariensis (Kuschel) | |
| (b) Bacteria | |
| Citrus variegated chlorosis | Xanthomonas campestris pv. oryzae (Ishiyama) Dye and pv. oryzicola (Fang. et al.) Dye |
| Erwinia stewartii (Smith) Dye | |
| (c) Fungi | |
| Alternaria alternata (Fr.) Keissler (non‐EU pathogenic isolates) | Elsinoe spp. Bitanc. and Jenk. Mendes |
| Anisogramma anomala (Peck) E. Müller | Fusarium oxysporum f. sp. albedinis (Kilian and Maire) Gordon |
| Apiosporina morbosa (Schwein.) v. Arx | Guignardia piricola (Nosa) Yamamoto |
| Ceratocystis virescens (Davidson) Moreau | Puccinia pittieriana Hennings |
| Cercoseptoria pini‐densiflorae (Hori and Nambu) Deighton | Stegophora ulmea (Schweinitz: Fries) Sydow & Sydow |
| Cercospora angolensis Carv. and Mendes | Venturia nashicola Tanaka and Yamamoto |
| (d) Virus and virus‐like organisms | |
| Beet curly top virus (non‐EU isolates) | Citrus tristeza virus (non‐EU isolates) |
| Black raspberry latent virus | Leprosis |
| Blight and blight‐like | Little cherry pathogen (non‐ EU isolates) |
| Cadang‐Cadang viroid | Naturally spreading psorosis |
| Palm lethal yellowing mycoplasm | Tatter leaf virus |
| Satsuma dwarf virus | Witches’ broom (MLO) |
| Annex IIB | |
| (a) Insect mites and nematodes, at all stages of their development | |
| Anthonomus grandis (Boh.) | Ips cembrae Heer |
| Cephalcia lariciphila (Klug) | Ips duplicatus Sahlberg |
| Dendroctonus micans Kugelan | Ips sexdentatus Börner |
| Gilphinia hercyniae (Hartig) | Ips typographus Heer |
| Gonipterus scutellatus Gyll. | Sternochetus mangiferae Fabricius |
| Ips amitinus Eichhof | |
| (b) Bacteria | |
| Curtobacterium flaccumfaciens pv. flaccumfaciens (Hedges) Collins and Jones | |
| (c) Fungi | |
| Glomerella gossypii Edgerton | Hypoxylon mammatum (Wahl.) J. Miller |
| Gremmeniella abietina (Lag.) Morelet | |
1.1.2.2. Terms of Reference: Appendix 2
List of harmful organisms for which pest categorisation is requested per group. The list below follows the categorisation included in the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Cicadellidae (non‐EU) known to be vector of Pierce's disease (caused by Xylella fastidiosa), such as: | |
| 1) Carneocephala fulgida Nottingham | 3) Graphocephala atropunctata (Signoret) |
| 2) Draeculacephala minerva Ball | |
| Group of Tephritidae (non‐EU) such as: | |
| 1) Anastrepha fraterculus (Wiedemann) | 12) Pardalaspis cyanescens Bezzi |
| 2) Anastrepha ludens (Loew) | 13) Pardalaspis quinaria Bezzi |
| 3) Anastrepha obliqua Macquart | 14) Pterandrus rosa (Karsch) |
| 4) Anastrepha suspensa (Loew) | 15) Rhacochlaena japonica Ito |
| 5) Dacus ciliatus Loew | 16) Rhagoletis completa Cresson |
| 6) Dacus curcurbitae Coquillet | 17) Rhagoletis fausta (Osten‐Sacken) |
| 7) Dacus dorsalis Hendel | 18) Rhagoletis indifferens Curran |
| 8) Dacus tryoni (Froggatt) | 19) Rhagoletis mendax Curran |
| 9) Dacus tsuneonis Miyake | 20) Rhagoletis pomonella Walsh |
| 10) Dacus zonatus Saund. | 21) Rhagoletis suavis (Loew) |
| 11) Epochra canadensis (Loew) | |
| (c) Viruses and virus‐like organisms | |
| Group of potato viruses and virus‐like organisms such as: | |
| 1) Andean potato latent virus | 4) Potato black ringspot virus |
| 2) Andean potato mottle virus | 5) Potato virus T |
| 3) Arracacha virus B, oca strain | 6) non‐EU isolates of potato viruses A, M, S, V, X and Y (including Yo, Yn and Yc) and Potato leafroll virus |
| Group of viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L., such as: | |
| 1) Blueberry leaf mottle virus | 8) Peach yellows mycoplasm |
| 2) Cherry rasp leaf virus (American) | 9) Plum line pattern virus (American) |
| 3) Peach mosaic virus (American) | 10) Raspberry leaf curl virus (American) |
| 4) Peach phony rickettsia | 11) Strawberry witches’ broom mycoplasma |
| 5) Peach rosette mosaic virus | 12) Non‐EU viruses and virus‐like organisms of Cydonia Mill., Fragaria L., Malus Mill., Prunus L., Pyrus L., Ribes L., Rubus L. and Vitis L. |
| 6) Peach rosette mycoplasm | |
| 7) Peach X‐disease mycoplasm | |
| Annex IIAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Group of Margarodes (non‐EU species) such as: | |
| 1) Margarodes vitis (Phillipi) | 3) Margarodes prieskaensis Jakubski |
| 2) Margarodes vredendalensis de Klerk | |
1.1.2.3. Terms of Reference: Appendix 3
List of harmful organisms for which pest categorisation is requested. The list below follows the annexes of Directive 2000/29/EC.
| Annex IAI | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Acleris spp. (non‐EU) | Longidorus diadecturus Eveleigh and Allen |
| Amauromyza maculosa (Malloch) | Monochamus spp. (non‐EU) |
| Anomala orientalis Waterhouse | Myndus crudus Van Duzee |
| Arrhenodes minutus Drury | Nacobbus aberrans (Thorne) Thorne and Allen |
| Choristoneura spp. (non‐EU) | Naupactus leucoloma Boheman |
| Conotrachelus nenuphar (Herbst) | Premnotrypes spp. (non‐EU) |
| Dendrolimus sibiricus Tschetverikov | Pseudopityophthorus minutissimus (Zimmermann) |
| Diabrotica barberi Smith and Lawrence | Pseudopityophthorus pruinosus (Eichhoff) |
| Diabrotica undecimpunctata howardi Barber | Scaphoideus luteolus (Van Duzee) |
| Diabrotica undecimpunctata undecimpunctata Mannerheim | Spodoptera eridania (Cramer) |
| Diabrotica virgifera zeae Krysan & Smith | Spodoptera frugiperda (Smith) |
| Diaphorina citri Kuway | Spodoptera litura (Fabricus) |
| Heliothis zea (Boddie) | Thrips palmi Karny |
| Hirschmanniella spp., other than Hirschmanniella gracilis (de Man) Luc and Goodey | Xiphinema americanum Cobb sensu lato (non‐EU populations) |
| Liriomyza sativae Blanchard | Xiphinema californicum Lamberti and Bleve‐Zacheo |
| (b) Fungi | |
| Ceratocystis fagacearum (Bretz) Hunt | Guignardia laricina (Saw.) Yamamoto and Ito |
| Chrysomyxa arctostaphyli Dietel | Gymnosporangium spp. (non‐EU) |
| Cronartium spp. (non‐EU) | Inonotus weirii (Murril) Kotlaba and Pouzar |
| Endocronartium spp. (non‐EU) | Melampsora farlowii (Arthur) Davis |
| Mycosphaerella larici‐leptolepis Ito et al. | Septoria lycopersici Speg. var. malagutii Ciccarone and Boerema |
| Mycosphaerella populorum G. E. Thompson Thecaphora solani Barrus | |
| Phoma andina Turkensteen | Trechispora brinkmannii (Bresad.) Rogers |
| Phyllosticta solitaria Ell. and Ev. | |
| (c) Viruses and virus‐like organisms | |
| Tobacco ringspot virus | Pepper mild tigré virus |
| Tomato ringspot virus | Squash leaf curl virus |
| Bean golden mosaic virus | Euphorbia mosaic virus |
| Cowpea mild mottle virus | Florida tomato virus |
| Lettuce infectious yellows virus | |
| (d) Parasitic plants | |
| Arceuthobium spp. (non‐EU) | |
| Annex IAII | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Meloidogyne fallax Karssen | Rhizoecus hibisci Kawai and Takagi |
| Popillia japonica Newman | |
| (b) Bacteria | |
| Clavibacter michiganensis (Smith) Davis et al. ssp. sepedonicus (Spieckermann and Kotthoff) Davis et al. | Ralstonia solanacearum (Smith) Yabuuchi et al. |
| (c) Fungi | |
| Melampsora medusae Thümen | Synchytrium endobioticum (Schilbersky) Percival |
| Annex I B | |
| (a) Insects, mites and nematodes, at all stages of their development | |
| Leptinotarsa decemlineata Say | Liriomyza bryoniae (Kaltenbach) |
| (b) Viruses and virus‐like organisms | |
| Beet necrotic yellow vein virus | |
1.2. Interpretation of the Terms of Reference
Acleris spp. (non‐European Union (EU)) are listed in the Appendices to the Terms of Reference (ToR) to be subject to pest categorisation to determine whether they fulfil the criteria of quarantine pests or those of regulated non‐quarantine pests for the area of the EU excluding Ceuta, Melilla and the outermost regions of Member States (MSs) referred to in Article 355(1) of the Treaty on the Functioning of the European Union (TFEU), other than Madeira and the Azores.
2. Data and methodologies
2.1. Data
2.1.1. Literature search
A literature search on Acleris spp. was conducted at the beginning of the categorisation in the ISI Web of Science bibliographic database, using the scientific name of the genus as search term. Relevant papers were reviewed, and further references and information were obtained from experts, as well as from citations within the references and grey literature.
2.1.2. Database search
Pest information, on host(s) and distribution, was retrieved from the European and Mediterranean Plant Protection Organization (EPPO) Global Database (EPPO, online) and relevant publications.
The Europhyt database was consulted for pest‐specific notifications on interceptions and outbreaks. Europhyt is a web‐based network run by the Directorate General for Health and Food Safety (DG SANTÉ) of the European Commission and is a subproject of PHYSAN (Phyto‐Sanitary Controls) specifically concerned with plant health information. The Europhyt database manages notifications of interceptions of plants or plant products that do not comply with EU legislation, as well as notifications of plant pests detected in the territory of the MS and the phytosanitary measures taken to eradicate or avoid their spread.
2.2. Methodologies
The Panel performed the pest categorisation for Acleris spp., following guiding principles and steps presented in the EFSA guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018) and in the International Standard for Phytosanitary Measures No 11 (FAO, 2013) and No 21 (FAO, 2004).
This work was initiated following an evaluation of the EU plant health regime. Therefore, to facilitate the decision‐making process, in the conclusions of the pest categorisation, the Panel addresses explicitly each criterion for a Union quarantine pest and for a Union regulated non‐quarantine pest (RNQP) in accordance with Regulation (EU) 2016/2031 on protective measures against pests of plants, and includes additional information required in accordance with the specific ToR received by the European Commission. In addition, for each conclusion, the Panel provides a short description of its associated uncertainty.
Table 1 presents the Regulation (EU) 2016/2031 pest categorisation criteria on which the Panel bases its conclusions. All relevant criteria have to be met for the pest to potentially qualify either as a quarantine pest or as a RNQP. If one of the criteria is not met, the pest will not qualify. A pest that does not qualify as a quarantine pest may still qualify as a RNQP that needs to be addressed in the opinion. For the pests regulated in the protected zones only, the scope of the categorisation is the territory of the protected zone; thus, the criteria refer to the protected zone instead of the EU territory.
Table 1.
Pest categorisation criteria under evaluation, as defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Criterion in Regulation (EU) 2016/2031 regarding protected zone quarantine pest (articles 32–35) | Criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest |
|---|---|---|---|
| Identity of the pest (Section 3.1) | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? | Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible? |
| Absence/presence of the pest in the EU territory (Section 3.2) |
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU? Describe the pest distribution briefly! |
Is the pest present in the EU territory? If not, it cannot be a protected zone quarantine organism | Is the pest present in the EU territory? If not, it cannot be a RNQP. (A regulated non‐quarantine pest must be present in the risk assessment area) |
| Regulatory status (Section 3.3) | If the pest is present in the EU but not widely distributed in the risk assessment area, it should be under official control or expected to be under official control in the near future |
The protected zone system aligns with the pest‐free area system under the International Plant Protection Convention (IPPC) The pest satisfies the IPPC definition of a quarantine pest that is not present in the risk assessment area (i.e. protected zone) |
Is the pest regulated as a quarantine pest? If currently regulated as a quarantine pest, are there grounds to consider its status could be revoked? |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Is the pest able to enter into, become established in, and spread within, the EU territory? If yes, briefly list the pathways! |
Is the pest able to enter into, become established in, and spread within, the protected zone areas? Is entry by natural spread from EU areas where the pest is present possible? |
Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects? Clearly state if plants for planting is the main pathway! |
| Potential for consequences in the EU territory (Section 3.5) | Would the pests’ introduction have an economic or environmental impact on the EU territory? | Would the pests’ introduction have an economic or environmental impact on the protected zone areas? | Does the presence of the pest on plants for planting have an economic impact as regards the intended use of those plants for planting? |
| Available measures (Section 3.6) | Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated? |
Are there measures available to prevent the entry into, establishment within or spread of the pest within the protected zone areas such that the risk becomes mitigated? Is it possible to eradicate the pest in a restricted area within 24 months (or a period longer than 24 months where the biology of the organism so justifies) after the presence of the pest was confirmed in the protected zone? |
Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated? |
| Conclusion of pest categorisation (Section 4) | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential quarantine pest were met and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as potential protected zone quarantine pest were met, and (2) if not, which one(s) were not met | A statement as to whether (1) all criteria assessed by EFSA above for consideration as a potential RNQP were met, and (2) if not, which one(s) were not met |
It should be noted that the Panel's conclusions are formulated respecting its remit and particularly with regard to the principle of separation between risk assessment and risk management (EFSA founding regulation (EU) No 178/2002); therefore, instead of determining whether the pest is likely to have an unacceptable impact, the Panel will present a summary of the observed pest impacts. Economic impacts are expressed in terms of yield and quality losses and not in monetary terms, whereas addressing social impacts is outside the remit of the Panel.
The Panel will not indicate in its conclusions of the pest categorisation whether to continue the risk assessment process, but following the agreed two‐step approach, will continue only if requested by the risk managers. However, during the categorisation process, experts may identify key elements and knowledge gaps that could contribute significant uncertainty to a future assessment of risk. It would be useful to identify and highlight such gaps so that potential future requests can specifically target the major elements of uncertainty, perhaps suggesting specific scenarios to examine.
3. Pest categorisation
3.1. Identity and biology of the pest
3.1.1. Identity and taxonomy
3.1.1.1.
Is the identity of the pest established, or has it been shown to produce consistent symptoms and to be transmissible?
Yes, the identity of the non‐EU Acleris species is well established. The different species can be identified using taxonomic keys based on adult morphology and their genitalia.
Acleris Hübner is an insect genus in the family Tortricidae (Insecta: Lepidoptera, Subfamily: Tortricinae, Tribe: Tortricini). It includes 261 species distributed in all around the world (Razowski et al., 2010; Gilligan and Epstein, 2012; Gilligan et al., 2018). The different species can be identified using taxonomic keys based on adult morphology and the genitalia of both sexes.
Although the species Acleris gloverana is mentioned under this name in most of the literature (Brown, 2006), it is referred to as Acleris gloveranus in the Tortricid.net database (Brown et al., 2008). The bases for this discrepancy have not been found in the literature. For the purpose of this opinion, we use the name A. gloverana.
3.1.2. Biology of the pest
The genus Acleris includes 221 non‐EU species (Appendix A). They primarily feed on leaves. The biology of the species known as major pests, which has been used as a criterion to identify those species subjected to this categorisation, is summarised here. Unless specified otherwise, much of the information in this section has been drawn from Gilligan and Epstein (2012).
The non‐EU Acleris include two major defoliators of conifers that occur in North America, A. variana and A. gloverana also known as eastern and western blackheaded budworm, respectively (Nealis and Turnquist, 2010; Johns et al., 2016). Both species complete one generation per year. Adults appear in mid‐summer till early September and lay eggs in the underside of the needles at the upper part of the host plants. Eggs overwinter and hatch in next spring. Larvae feed initially in the buds and later on the needles which are folded or tied together with silk. Pupation occurs in a sheath made of needles attached with silk.
Acleris minuta attacks various deciduous hosts in the families Rosaceae, Myricaceae, Ericaceae and Salicaceae. It completes two to three generations per year depending on latitude. Adults of the first and second generation are orange or yellow and are present in June and August. Adults of the third generation are grey and are present in October. It overwinters as adult. Eggs are laid singly on bark in the spring or on leaves in the summer. Eggs hatch in 7–10 days and first instar larvae feed on the underside of leaves. Later instars web together leaves to create a shelter or fold single leaves where they feed and then pupate (Weatherby, 1982).
Acleris semipurpurana attacks mainly oak trees. It completes one generation per year and eggs are the overwintering stage. In Spring, young larvae feed in buds and later use silk to tie sections of leaves together and feed inside the folds. The fully grown larvae are whitish to light green. The pale head capsule has black bars on the sides. Usually in May, the mature larvae spin down to the ground and pupate in the soil litter. Pupation lasts from 1 to 2 weeks. Adults emerge and mate, and eggs are deposited individually on the bark of second‐year branches. Only one generation per year has been reported (USDA, 1979).
Other species such as Acleris robinsoniana, Acleris senescens and Acleris nivisellana attack several deciduous plants among which the Rosaceae. They complete one or two generations per year.
Acleris nishidai is known only from the mountains of central Costa Rica. It feeds on Rubus spp. (Rosaceae). Larvae may be relatively abundant on cultivated blackberry, and are responsible for heavy damage. A. nishidai is therefore considered as a pest in Costa Rica (Brown and Nishida, 2008).
Acleris issikii has two generations per year with adults on wing from June to July and again from September to October. Larvae feed on Salix integra, Populus nigra and Populus sieboldii.
Figure 1.

Acleris gloverana male. The wingspan is about 20 mm. (Todd M. Gilligan and Marc E. Epstein, TortAI: Tortricids of Agricultural Importance, USDA APHIS PPQ, Bugwood.org)
Figure 2.

Acleris gloverana late instar larva (size 10–15 mm) (Tom Gray, Canadian Forest Service, Bugwood.org)
Figure 3.

Damage on Abies balsamea by Acleris variana larvae. (Rick West, Canadian Forest Service, Bugwood.org)
3.1.3. Intraspecific diversity
No intraspecific diversity has been reported.
3.1.4. Detection and identification of the pest
3.1.4.1.
Are detection and identification methods available for the pest?
Yes, the pest can be detected and identified visually. For some species, pheromones have been identified and could be used for detection.
For all non‐EU Acleris species, detailed description of the adults and male and female genitalia is available for identification. For several species including those which have been reported as pests, online identification keys based on adult morphological characters are available. For several species, molecular tools have been developed for their identification (Obraztsov, 1963; Razowski et al., 2010; Gilligan and Epstein, 2012).
Detection of non‐EU Acleris species is based on the presence of larval feeding damage, which is typical of a leafroller insect. Feeding larvae or damaged leaves could be detected visually by the presence of silk used to tie or fold infested leaves. In addition, for several Acleris species (e.g. A. gloverana, A. minuta and A. variana), the pheromone has been identified and could be used for detection with pheromone traps (Gries et al., 1994; Gray et al., 1996; Nealis et al., 2010; Pherobase 2019).
3.2. Pest distribution
3.2.1. Pest distribution outside the EU
In Appendix A, information about the distribution of all non‐EU Acleris can be found based on Gilligan and Epstein (2012) and the EPPO Global Database (EPPO, online). For the non‐EU Acleris that are reported as pests, distribution data are summarized in Table 2. The distribution of two major pests, A. gloverana and A. variana, are presented in Figures 4 and 5.
Table 2.
Geographic distribution of nine non‐EU Acleris species reported as pests
| Species | Distribution | Reference |
|---|---|---|
| A. gloverana (A. gloveranus) | Alaska and northwestern Canada south to northern California and east to western Montana | Gilligan and Epstein (2012) |
| A. issikii | Japan, North Korea, South Korea, China, East Russia | Oku (1957), Byun and Yan (2004) |
| A. minuta | Widely distributed in eastern North America. No record was found for Sweden or any European countries; in particular, the pest is not included in the Fauna Europaea database (de Jong et al., 2014, accessed on 24 September 2019) | Gilligan and Epstein (2012), EPPO (2015) |
| A. nishidai | Costa Rica | Brown and Nishida (2008) |
| A. nivisellana | Northeastern United States across southern Canada to British Columbia and south to California. In the United States, it is found mainly north of the 40th parallel | Gilligan and Epstein (2012) |
| A. robinsoniana | Northeastern United States across southern Canada to British Columbia and south to California | Gilligan and Epstein (2012) |
| A. semipurpurana | USA, Ohio | Gilligan and Epstein (2012) |
| A. senescens | Pacific Coast of North America from British Columbia south to California | Gilligan and Epstein (2012) |
| A. variana | Eastern Canada and Northeastern United States | Gilligan and Epstein (2012) |
Figure 4.
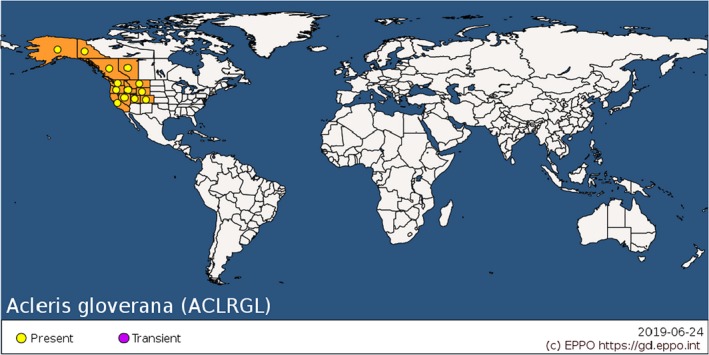
Global distribution map for Acleris gloverana (extracted from the EPPO Global Database accessed on 24 June 2019)
Figure 5.
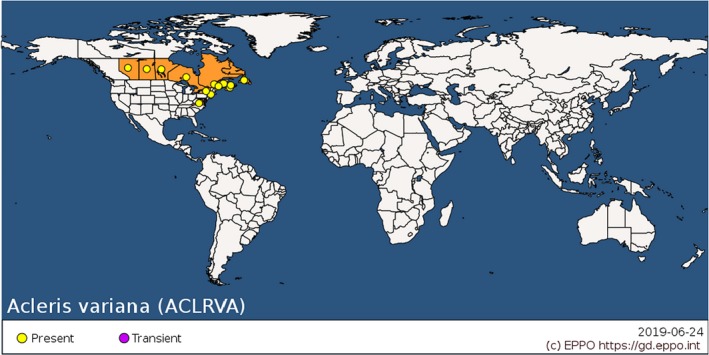
Global distribution map for Acleris variana (extracted from the EPPO Global Database accessed on 24 June 2019)
3.2.2. Pest distribution in the EU
3.2.2.1.
Is the pest present in the EU territory? If present, is the pest widely distributed within the EU?
No, non‐EU species of the genus Acleris are not present in the EU territory.
For a list of Acleris species present in the EU, please see Appendix B.
3.3. Regulatory status
3.3.1. Council Directive 2000/29/EC
Acleris species are listed in Council Directive 2000/29/EC. Details are presented in Table 3.
Table 3.
Acleris spp. in Council Directive 2000/29/EC
| Annex I, Part A | Harmful organisms whose introduction into, and spread within, all member states shall be banned |
|---|---|
| Section I | Harmful organisms not known to occur in any part of the community and relevant for the entire community |
| (a) | Insects, mites and nematodes, at all stages of their development |
| Species | |
| 1. | Acleris spp. (non‐European) |
3.3.2. Legislation addressing the hosts of Acleris spp
Acleris spp. (non‐European) are listed on Annex IAI, which indicates that they are regulated for all plant genera and commodities. Some host plants used by Acleris spp. are listed in the import prohibitions of Annex III or specific requirements in Annex IV of Council Directive 2000/29/EC (see Section 3.4.2).
3.4. Entry, establishment and spread in the EU
3.4.1. Host range
The nine species considered as potential pests attack a range of host plant families that include Betulaceae; Cupressaceae; Ericaceae; Fagaceae; Myricaceae; Pinaceae, Rosaceae and Salicaceae (Brown et al., 2008), and the following species or genera:
Abies spp.; Alnus spp.; Betula spp.; Calluna sp.; Crataegus spp.; Kalmia sp.; Larix spp.; Malus sp.; Myrica gale; Physocarpus malvaceus; Picea spp.; Pinus spp.; Populus spp.; Prunus spp.; Pseudotsuga menziesii; Pyrus sp.; Quercus spp.; Rosa spp.; Rubus spp.; Salix spp.; Sorbus spp.; Thuja plicata; Tsuga spp.; Vaccinium spp. (Brown et al., 2008).
A more complete list of host plants is provided in Appendix A.
3.4.2. Entry
3.4.2.1.
Is the pest able to enter into the EU territory? If yes, identify and list the pathways.
Yes, non‐EU Acleris are able to enter as eggs or pupae on plants for planting belonging to one of the families listed in section 3.4.1. and as eggs on the bark of round wood with bark of host plants. A. semipurpurana also pupates in the soil and therefore could travel with potted plants.
The main pathways of entry are:
Plants for planting (including seeds) of the host plants, with or without soil
Cut branches of host plants
Fruits (including cones of conifers) of host plants
Round wood with bark of host plants
Bark of host plants
For the pathways listed above, the following prohibitions (Annex III) or special requirements (Annex IV) are in place:
Plants for planting
Plants of Abies, Larix, Picea, Pinus, Pseudotsuga, Tsuga, and plants with leaves of Quercus, other than fruit and seeds – prohibited from non‐European countries (Annex IIIAI 1 and 2.)
Plants with leaves (other than fruit and seeds) of Populus – prohibited from North American countries (Annex IIIAI 3.)
Plants for planting of Crateagus, Malus, Prunus, Pyrus, Rosa other than dormant plants free from leaves, flowers and fruit – prohibited from non‐European countries (Annex IIIAI 9.)
Plants for planting of Malus, Prunus, Pyrus other than seeds – prohibited from non‐European countries, other than Mediterranean countries, Australia, New Zealand, Canada, the continental states of the USA (Annex IIIAI 18.)
Plants of conifers other than fruit and seeds – special requirements in relation to other pests (Annex IVAI 8.1., 8.2.)
Plants of Abies, Larix, Picea, Pinus, Pseudotsuga, Tsuga, Quercus – special requirements in relation to other pests (Annex IVAI 9., 10., 11.01., 11.1., 11.2.)
Plants of Betula, Populus – special requirements in relation to other pests (Annex IVAI 11.5., 13.1., 13.2.)
Plants of Crataegus, Malus, Prunus, Pyrus, Rubus – special requirements in relation to other pests (Annex IVAI 14.1., 17., 19.1., 19.2., 20., 22.1., 22.2., 23.1., 23.2., 24.)
Wood
Wood of conifers – special requirements in relation to other pests (Annex IVAI 1.1., 1.3., 1.5., 1.6.)
Wood of Quercus, Populus – special requirements (Annex IVAI 3., 6.)
Wood of Betula – special requirements in relation to other pests (Annex IVAI 4.1., 4.3.)
Wood of Crataegus, Malus, Prunus, Pyrus – special requirements in relation to other pests (Annex IVAI 7.4.)
Bark
Isolated bark of Quercus – prohibited from North American countries (Annex IIIAI 6.)
Isolated bark of Populus – prohibited from countries of the American continent (Annex IIIAI 8.)
Isolated bark of conifers – special requirements (Annex IVAI 7.3.)
For all other identified pathways (e.g. fruits and cut branches of host plants, dormant Populus plants from North America, dormant plants of Malus from Mediterranean countries, Australia, New Zealand, Canada, the continental states of the USA, etc.) no import requirements are currently specified.
Between 1994 and May 2019, there was one record of interception of Acleris sp. in the Europhyt database, on a consignment of Cassia fistula (Fabaceae) plants from Vietnam.
3.4.3. Establishment
3.4.3.1.
Is the pest able to become established in the EU territory?
Yes, many of the host plants of the non‐EU Acleris spp. are present in the EU, and climatic conditions are locally favourable for pest establishment.
3.4.3.2. EU distribution of main host plants
The two main pest species A. gloverana and A. variana feed on various coniferous hosts (Abies, Picea, Pinus, etc.). These are distributed throughout the EU territory (Figure 6). Apart from conifers several other plant species (see Section 3.4.1) that are present throughout EU are known hosts for non‐EU Acleris spp. Therefore, available hosts are present throughout the EU.
Figure 6.
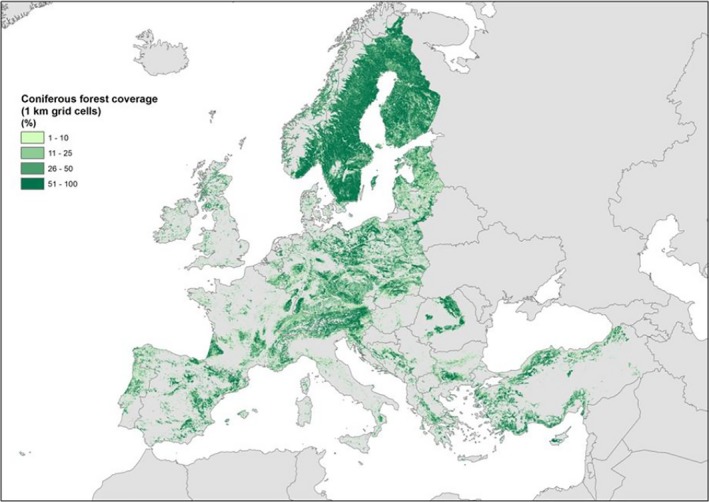
The cover percentage of coniferous forests in Europe with a range of values from 0 to 100 at 1 km resolution (source: Corine Land Cover year 2012 version 18.5 by European Environment Agency)
3.4.3.3. Climatic conditions affecting establishment
Non‐EU Acleris spp. have a broad distribution in North America and Asia. Climatic categories based on Köppen–Geiger climate classification can be found in these areas that are also present in EU. For instance, Köppen–Geiger climate types Bsk, Cfa, Csa and Dfb which occur in North America also occur in EU. Moreover, the two blackheaded budworm species, A. gloverana and A. variana, occur in North America with Cfb and Dfb climatic classes that are also found in EU with Cfb being more prevalent in EU than Dfb (MacLeod and Korycinska 2019) suggesting that most of EU is more climatic suitable for A. gloverana than for A. variana.
3.4.4. Spread
3.4.4.1.
Is the pest able to spread within the EU territory following establishment?
Yes, the pest can fly and can also be transported as eggs or pupae on plants for planting and other commodities (see section 3.4.2)
RNQPs: Is spread mainly via specific plants for planting, rather than via natural spread or via movement of plant products or other objects?
No, the spread is also due to flight and transportation on other commodities than plants for planting.
The literature does not provide any direct information on flight. However, outbreak expansion provides an indirect measure of flight. During the period 1957–1987, the maximal size of an A. gloverana outbreak was 27,530 ha, and the maximal size of an A. variana outbreak was 115,789 ha (Mattson et al., 1991), suggesting a flight capacity of tens of kilometres, and probably more when the insects are carried by the wind. For comparison, although Acleris spp. adults are two or three times smaller than Choristoneura spp. adults, C. fumiferana could fly a distance of 20 km with a maximum recorded distance of 450 km using prevailing winds (Anderson and Sturtevant, 2011).
In A. gloverana at least, the larvae do not disperse by ballooning (Shepherd and Gray, 2001).
3.5. Impacts
3.5.1.
Would the pests’ introduction have an economic or environmental impact on the EU territory?
Yes, there are some non‐EU Acleris species that are destructive defoliators and pests in their native area
RNQPs: Does the presence of the pest on plants for planting have an economic impact, as regards the intended use of those plants for planting?
Yes, the presence of the pest on plants for planting has an economic impact on its intended use
In the literature, nine non‐EU Acleris species are reported as pests on various host plants. The eastern and western blackheaded budworm species, A. gloverana and A. variana, are known destructive pests on coniferous plants in North America with extensive outbreaks occurring in areas of 90,000 ha (Gries et al., 1994; Nealis et al., 2004, Nealis and Turnquist, 2010). Mattson et al. (1991) report an outbreak of A. gloverana that expanded over 170,000 ha of Tsuga heterophylla in British Columbia between 1996 and 2001. Nearly 70% mortality was observed by Wood and Garbutt (1990) during a survey of young T. heterophylla defoliated by A. gloverana in the 1970s in British Columbia. A. minuta has been reported as significant pest on various host plants, including cultivated ones such as Malus sp., Prunus sp., Pyrus sp. (Gilligan and Epstein, 2012). Acleris nishidai is reported as an important pest on Rubus sp. in Costa Rica (Brown and Nishida, 2008) and A. issikii is reported as a pest on Salix miyabeana from Japan (Nakamura and Ohgushi, 2004). Acleris semipurpurana is reported as a pest on Quercus sp. causing significant tree mortality in the Appalachian region (USDA, 1979). A. robinsoniana, A. senescens and A. nivisellana are also reported as pests (Gilligan and Epstein, 2012).
3.6. Availability and limits of mitigation measures
3.6.1.
Are there measures available to prevent the entry into, establishment within or spread of the pest within the EU such that the risk becomes mitigated?
Yes, see sections 3.3 and 3.6.1
RNQPs: Are there measures available to prevent pest presence on plants for planting such that the risk becomes mitigated?
Yes, plants for planting from pest free areas and grown in isolation would mitigate the risk in case the pest entered the EU.
3.6.2. Identification of additional measures
Phytosanitary measures are currently applied to coniferous plants and to several other host plants (see Sections 3.3 and 3.4.2).
3.6.2.1. Additional control measures
Potential additional control measures are listed in Table 4.
Table 4.
Selected control measures (a full list is available in EFSA PLH Panel, 2018) for pest entry/establishment/spread/impact in relation to currently unregulated hosts and pathways. Control measures are measures that have a direct effect on pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Control measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| http://doi.org/10.5281/zenodo.1175887 | Description of possible exclusion conditions that could be implemented to isolate the crop from pests and if applicable relevant vectors. E.g. a dedicated structure such as glass or plastic greenhouses | Entry/spread |
| http://doi.org/10.5281/zenodo.1175910 |
Use of chemical compounds that may be applied to plants or to plant products after harvest, during process or packaging operations and storage The treatments addressed in this information sheet are: a) fumigation; b) spraying/dipping pesticides; c) surface disinfectants; d) process additives; e) protective compounds |
Entry/spread |
| http://doi.org/10.5281/zenodo.1181436 | Roguing is defined as the removal of infested plants and/or uninfested host plants in a delimited area, whereas pruning is defined as the removal of infested plant parts only, without affecting the viability of the plant | Establishment/spread |
| Chemical treatments on crops including reproductive material | Application of insecticides on nurseries for plants for planting may be considered to reduce the presence of the pest | Entry/spread |
| Post‐entry quarantine and other restrictions of movement in the importing country |
This information sheet covers post‐entry quarantine of relevant commodities; temporal, spatial and end‐use restrictions in the importing country for import of relevant commodities; prohibition of import of relevant commodities into the domestic country Relevant commodities are plants, plant parts and other materials that may carry pests, either as infection, infestation or contamination |
Entry/spread |
3.6.2.2. Additional supporting measures
Potential additional supporting measures are listed in Table 5.
Table 5.
Selected supporting measures (a full list is available in EFSA PLH Panel, 2018) in relation to currently unregulated hosts and pathways. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk reduction options that do not directly affect pest abundance
| Information sheet title (with hyperlink to information sheet if available) | Supporting measure summary | Risk component (entry/establishment/spread/impact) |
|---|---|---|
| http://doi.org/10.5281/zenodo.1181430 |
Inspection is defined as the official visual examination of plants, plant products or other regulated articles to determine if pests are present or to determine compliance with phytosanitary regulations (ISPM 5) The effectiveness of sampling and subsequent inspection to detect pests may be enhanced by including trapping and luring techniques |
Entry |
| http://doi.org/10.5281/zenodo.1181213 | Examination, other than visual, to determine if pests are present using official diagnostic protocols. Diagnostic protocols describe the minimum requirements for reliable diagnosis of regulated pests | Entry |
| http://doi.org/10.5281/zenodo.1180845 | Mandatory/voluntary certification/approval of premises is a process including a set of procedures and of actions implemented by producers, conditioners and traders contributing to ensure the phytosanitary compliance of consignments. It can be a part of a larger system maintained by a National Plant Protection Organization in order to guarantee the fulfilment of plant health requirements of plants and plant products intended for trade. Key property of certified or approved premises is the traceability of activities and tasks (and their components) inherent the pursued phytosanitary objective. Traceability aims to provide access to all trustful pieces of information that may help to prove the compliance of consignments with phytosanitary requirements of importing countries | Entry |
| http://doi.org/10.5281/zenodo.1180597 | ISPM 5 defines a buffer zone as ‘an area surrounding or adjacent to an area officially delimited for phytosanitary purposes in order to minimise the probability of spread of the target pest into or out of the delimited area, and subject to phytosanitary or other control measures, if appropriate’ (ISPM 5). The objectives for delimiting a buffer zone can be to prevent spread from the outbreak area and to maintain a pest‐free production place, site or area | Entry/spread |
| Sampling |
According to ISPM 31, it is usually not feasible to inspect entire consignments, so phytosanitary inspection is performed mainly on samples obtained from a consignment. It is noted that the sampling concepts presented in this standard may also apply to other phytosanitary procedures, notably selection of units for testing For inspection, testing and/or surveillance purposes the sample may be taken according to a statistically based or a non‐statistical sampling methodology |
Entry |
| Phytosanitary certificate and plant passport | An official paper document or its official electronic equivalent, consistent with the model certificates of the IPPC, attesting that a consignment meets phytosanitary import requirements (ISPM 5) a) export certificate (import) b) plant passport (EU internal trade) | Entry |
| Surveillance | Pheromone traps and visual inspection | Entry/establishment/spread |
3.6.2.3. Biological or technical factors limiting the effectiveness of measures to prevent the entry, establishment and spread of the pest
Eggs on the leaves or the bark, or pupae in the foliage or the soil, can pass inspection unnoticed.
The adults probably fly long distance.
3.7. Uncertainty
The host range of many non‐EU Acleris spp. is not known
The impact of many non‐EU Acleris spp. is not known
4. Conclusions
From the group of 221 non‐EU Acleris species, nine non‐EU species (A. gloverana, A. variana, A. minuta, A. nishidai, A. issikii, A. semipurpurana, A. robinsoniana, A. senescens and A. nivisellana) are consistently reported as pests on various host plants, and meet all criteria assessed by EFSA above for consideration as potential quarantine pests. All non‐EU Acleris spp. do not meet all criteria assessed by EFSA above for consideration as potential regulated non‐quarantine pests as they are not present in the EU.
Table 6 provides a summary of the conclusions of each part of this pest categorisation for the nine species listed above.
Table 6.
The Panel's conclusions on the pest categorisation criteria defined in Regulation (EU) 2016/2031 on protective measures against pests of plants (the number of the relevant sections of the pest categorisation is shown in brackets in the first column)
| Criterion of pest categorisation | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union quarantine pest | Panel's conclusions against criterion in Regulation (EU) 2016/2031 regarding Union regulated non‐quarantine pest | Key uncertainties |
|---|---|---|---|
| Identity of the pests (Section 3.1) | Acleris is a clearly defined insect genus (Lepidoptera: Tortricidae) and detailed morphological descriptions are available for species identification | Acleris is a clearly defined insect genus (Lepidoptera: Tortricidae) and detailed morphological descriptions are available for species identification | |
| Absence/presence of the pest in the EU territory (Section 3.2) | Non‐EU Acleris spp. are not known to be present in the EU | Non‐EU Acleris spp. are not known to be present in the EU | |
| Regulatory status (Section 3.3) | Non‐EU Acleris spp. are listed on Annex IAI of Council Directive 2000/29/EC as Acleris spp (Non‐ European) | Non‐EU Acleris spp. are listed on Annex IAI of Council Directive 2000/29/EC as Acleris spp (Non‐European). There are no grounds to consider its status could be revoked | |
| Pest potential for entry, establishment and spread in the EU territory (Section 3.4) | Non‐EU Acleris spp. are able to enter into the EU through plants for planting with soil attached, cut branches, fruits, round wood and bark. Establishment is possible as host plants are available and climatic conditions similar to their native range do occur in the EU. Active dispersal by flight is the main means for spread | Non‐EU Acleris spp. mainly spread by active dispersal through adult flight over long distances. Plants for planting are not considered as the main pathway of spread | Dispersal abilities for several species are not known |
| Potential for consequences in the EU territory (Section 3.5) | There are nine species of non‐EU Acleris (A. gloverana, A. variana, A. minuta, A. nishidai, A. issikii, A. semipurpurana, A. robinsoniana, A. senescens and A. nivisellana) that are known as pests in their native area. The introduction of these species could cause economic impact on several forest plants and other crops in the EU. For the remaining 212 species, the potential impact is unknown | The presence of non‐EU Acleris on plants for planting would have an economic impact on its intended use | The host plants and potential damaging ability of several species is not known |
| Available measures (Section 3.6) | There are measures available to prevent entry, establishment and spread of non‐EU Acleris spp. in the EU which are described in Council Directive 2000/29/EC and in Section 3.6 | There are measures available to prevent pest presence on plants for planting (e.g. plants for planting from pest‐free areas and grown in isolation) that could mitigate the risk in case the pest entered the EU | |
| Conclusion on pest categorisation (Section 4) | Nine non‐EU Acleris species, reported as pests on various host plants (A. gloverana, A. variana, A. minuta, A. nishidai, A. issikii, A. semipurpurana, A. robinsoniana, A. senescens and A. nivisellana) meet all criteria assessed by EFSA above for consideration as potential quarantine pests | Non‐EU Acleris spp. do not meet all criteria assessed by EFSA above for consideration as potential regulated non‐quarantine pests, as they are not present in the EU | |
| Aspects of assessment to focus on/scenarios to address in future if appropriate | Scarce information on host plants, pests status and climatic suitability of the other 212 non‐EU Acleris species | ||
Abbreviations
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- IPPC
International Plant Protection Convention
- ISPM
International Standards for Phytosanitary Measures
- MS
Member State
- PLH
EFSA Panel on Plant Health
- PZ
Protected Zone
- RNQP
regulated non‐quarantine pest
- TFEU
Treaty on the Functioning of the European Union
- ToR
Terms of Reference
Glossary
- Containment (of a pest)
Application of phytosanitary measures in and around an infested area to prevent spread of a pest (FAO, 1995, 2017)
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017)
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017)
- Eradication (of a pest)
Application of phytosanitary measures to eliminate a pest from an area (FAO, 2017)
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017)
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017)
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate Risk Reduction Options that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017)
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests, or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017)
- Protected zones (PZ)
A protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017)
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017)
- Risk reduction option (RRO)
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A RRO may become a phytosanitary measure, action or procedure according to the decision of the risk manager
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017)
Appendix A – List of Acleris spp. (221 species) reported outside the EU with information on their host plants
1.
| Species name (species known as pests are in bold) | Occurrencea (based on Brown et al., 2008) | Host species | Host family | Region/continent | |
|---|---|---|---|---|---|
| 1 | A. aenigmana | USA | Hypericum perforatum; Prunus emarginata | Clusiaceae; Rosaceae | North America |
| 2 | A. aestuosa | Japan | Fagus crenata | Fagaceae | Asia |
| 3 | A. affinatana | China | Quercus acutissima; Quercus cerris; Quercus dentata; Quercus glauca; Quercus serrata; Quercus variabilis; Zelkova schneideriana | Fagaceae; Ulmaceae | Asia |
| 4 | A. albicomana | USA | Gaylussacia sp.; Vaccinium sp.; Quercus sp.; Rosa californica; Rosa sp.; Rosa californica; Rosa gymnocarpa | Ericaceae; Fagaceae; Rosaceae | North America |
| 5 | A. albinvia | USA | |||
| 6 | A. albiscapulana | Russia | |||
| 7 | A. albopterana | China | |||
| 8 | A. alnivora. | Japan | Alnus hirsuta; Alnus japonica var. rufa | Betulaceae | Asia |
| 9 | A. amurensis | Russia | Populus tremula | Salicaceae | Asia |
| 10 | A. arcuata | Japan | |||
| 11 | A. argyrograpta | Vietnam | |||
| 12 | A. askoldana | Russia | Abelia spathulata; Deutzia scabra; Deutzia sp. | Caprifoliaceae; Hydrangeaceae (also Saxifragaceae) | Asia |
| 13 | A. atayalicana | Taiwan | |||
| 14 | A. atomophora | Nepal | |||
| 15 | A. auricaput | Taiwan | |||
| 16 | A. aurichalcana | Russia | Tilia japonica; Tilia tuan | Tiliaceae | Asia |
| 17 | A. avicularia | Guatemala | |||
| 18 | A. bacurana. | Libya | |||
| 19 | A. baleina | Ethiopia | |||
| 20 | A. bengalica | India, Bengal | |||
| 21 | A. bicolor | Japan | Viburnum burejaeticum; Populus sp. | Caprifoliaceae; Salicaceae | Asia |
| 22 | A. blanda | Japan | |||
| 23 | A. bowmanana | Canada | Myrica gale; Picea engelmannii; Picea glauca; Aronia melanocarpa; Aronia melanocarpa; Spiraea sp.; Rubus sp. | Myricaceae; Pinaceae; Rosaceae | North America |
| 24 | A. braunana | Canada | Alnus incana; Alnus rubra; Alnus sp.; Betula papyrifera; Betula sp. | Betulaceae | North America |
| 25 | A. britannia | Canada | Potentilla sp.; Rosa sp.; Rubus occidentalis; Rubus parviflorus; Rubus parviflorus; Rubus ursinus | Rosaceae | North America |
| 26 | A. bununa | Taiwan | |||
| 27 | A. busckana | Canada | Spiraea alba; Spiraea sp. | Rosaceae | North America |
| 28 | A. caerulescens | Japan | Pterocarya rhoifolia | Juglandaceae | Asia |
| 29 | A. caliginosana | North America | Alnus incana; Alnus incana subsp. tenuifolia; Alnus rubra; Betula papyrifera | Betulaceae | North America |
| 30 | A. cameroonana | Cameroon | |||
| 31 | A. capizziana | USA | |||
| 32 | A. caucasica | Georgia | |||
| 33 | A. celiana | USA | Betula nana; Betula papyrifera; Betula sp.; Prunus virginiana; Salix sp. | Betulaceae; Rosaceae; Salicaceae | North America |
| 34 | A. cervinana | USA | Alnus sp.; Betula alleghaniensis; Corylus sp. | Betulaceae | North America |
| 35 | A. chalybeana | USA | Acer rubrum; Acer saccharinum; Acer sp.; Acer spicatum; Betula papyrifera; Betula sp.; Corylus sp.; Fagus sp.; Quercus rubra | Aceraceae; Betulaceae; Fagaceae | North America |
| 36 | A. chionocentra | India | |||
| 37 | A. chloroma: | Uganda | |||
| 38 | A. clarkei | USA | |||
| 39 | A. comandrana | USA | Comandra sp.; Comandra sp. | Santalaceae | North America |
| 40 | A. compsoptila | India | |||
| 41 | A. conchyloides | China | Quercus mongolica | Fagaceae | Asia |
| 42 | A. coniferarum | Kazakhstan | Picea sp. | Pinaceae | Asia |
| 43 | A. cornana | Canada | Alnus incana; Cornus canadensis; Cornus racemosa; Cornus sericea; Cornus sericea; Cornus sp. | Betulaceae; Cornaceae | North America |
| 44 | A. crassa | Japan | |||
| 45 | A. crataegi | Russia | |||
| 46 | A. cribellata | Russia | |||
| 47 | A. curvalana | USA | Gaylussacia baccata; Gaylussacia sp.; Vaccinium angustifolium; Vaccinium pallidum; Vaccinium sp.; Quercus sp.; Rosa sp. | Ericaceae; Fagaceae; Rosaceae | North America |
| 48 | A. dealbata | Japan | Salix sp. | Salicaceae | Asia |
| 49 | A. decolorata | India | |||
| 50 | A. delicata | Japan | |||
| 51 | A. delicatana | Russia | Carpinus japonica; Corylus sieboldiana var. mandshurica; Quercus cerris; Quercus sp. | Betulaceae; Fagaceae | Asia |
| 52 | A. dentata | Japan | |||
| 53 | A. denticulosa | Nepal | |||
| 54 | A. diadecta | North Vietnam | |||
| 55 | A. diaphora | North Vietnam | |||
| 56 | A. dispar | China | |||
| 57 | A. dryochyta | China | |||
| 58 | A. duoloba | North Vietnam | |||
| 59 | A. duracina | China | |||
| 60 | A. elaearcha | India | |||
| 61 | A. elegans | Japan | |||
| 62 | A. emera | Bolivia | |||
| 63 | A. enitescens | India | Rubus microphyllus; Rubus sp. | Rosaceae | Asia |
| 64 | A. expressa | Japan | Fraxinus mandshurica | Oleaceae | Asia |
| 65 | A. exsucana | Russia | Viburnum opulus var. sargentii; Deutzia scabra | Caprifoliaceae; Hydrangeaceae | Asia |
| 66 | A. extensana | Sri Lanka | Malus sp.; Malus sylvestris; Pyrus sp.; Rosa sp. | Rosaceae | Asia |
| 67 | A. extranea | China | |||
| 68 | A. ferox | China | |||
| 69 | A. ferrumixtana | Scandinavia | |||
| 70 | A. filipjevi | Russia | |||
| 71 | A. fistularis | Nepal | |||
| 72 | A. flavivittana | USA | Malus pumila; Prunus pensylvanica | Rosaceae | North America |
| 73 | A. flavopterana | China | |||
| 74 | A. foliana | USA | Cercocarpus betuloides; Cercocarpus ledifolius; Cercocarpus montanus; Cercocarpus montanus; Cercocarpus sp. | Rosaceae | North America |
| 75 | A. forbesana | Canada | Cornus californica; Cornus sericea subsp. occidentalis; Cornus sericea subsp. Stolonifera; Cornus sp. | Cornaceae | North America |
| 76 | A. formosae | Taiwan | |||
| 77 | A. fragariana | USA | Aronia melanocarpa; Fragaria sp.; Fragaria virginiana; Myrica gale; Pouteria sp.; Prunus sp.; Rosa sp.; Rubus sp. | Myricaceae; Sapotaceae | North America |
| 78 | A. fuscopterana | China | |||
| 79 | A. fuscopunctata | China | |||
| 80 | A. fuscotogata | Japan | Quercus serrata; | Fagaceae | Asia |
| 81 | A. ganeshia | Nepal | |||
| 82 | A. gatesclarkei | Taiwan | |||
| 83 | A. gibbopterana | China | |||
| 84 | A. glaucomis | India | |||
| 85 | A. gloverana (A. gloveranus) | USA | Abies balsamea; Abies concolor; Abies magnifica; Abies sp.; Larix sp.; Picea sitchensis; Picea sp.; Pseudostuga sp.; Pseudotsuga menziesii; Tsuga heterophylla; Tsuga sp. | Pinaceae | North America |
| 86 | A. gobica | Mongolia | |||
| 87 | A. gothena | Nepal | |||
| 88 | A. griseopterana | China | |||
| 89 | A. hapalactis | India | |||
| 90 | A. harenna | Ethiopia | |||
| 91 | A. helvolaris | China | |||
| 92 | A. hispidana | Russia | Quercus mongolica | Fagaceae | Asia |
| 93 | A. hohuanshana | Taiwan | |||
| 94 | A. hokkaidana | Japan | |||
| 95 | A. hudsoniana | Canada | Alnus sp.; Betula papyrifera; Picea glauca; Populus balsamifera; Populus tremuloides; Salix sp. | Betulaceae; Pinaceae; Salicaceae | North America |
| 96 | A. idonea | Mongolia | |||
| 97 | A. imitatrix | China | |||
| 98 | A. inana | USA | Alnus sp.; Betula sp.; Corylus sp. | Betulaceae | North America |
| 99 | A. incognita | USA | |||
| 100 | A. indignana | Russia | |||
| 101 | A. issikii | Japan | Populus nigra; Populus sieboldii; Salix integra | Salicaceae | Asia |
| 102 | A. japonica | Japan | Zelkova serrata | Ulmaceae | Asia |
| 103 | A. kearfottana | Canada | Hamamelis sp.; Myrica gale; Myrica sp.; Comptonia peregrina | Hamamelidaceae; Myricaceae; Myrtaceae | North America |
| 104 | A. keiferi | USA | Fragaria sp.; Rosa californica; Rubus ursinus | Rosaceae | North America |
| 105 | A. kerincina | West Sumatra | |||
| 106 | A. kinangopana | Kenya | |||
| 107 | A. klotsi | USA | |||
| 108 | A. kodamai | Japan | Pinus koraiensis | Pinaceae | Asia |
| 109 | A. kuznetzovi | Russia | |||
| 110 | A. leechi | Japan | Quercus acutissima; Quercus variabilis | Fagaceae | Asia |
| 111 | A. leucophracta | China | |||
| 112 | A. longipalpana. | Russia | |||
| 113 | A. loxoscia | Sri Lanka | |||
| 114 | A. lucipara | India | |||
| 115 | A. lucipeta | India | |||
| 116 | A. luoyingensis | Taiwan | |||
| 117 | A. lutescentis | China | |||
| 118 | A. macdunnoughi | USA | Rubus sp.; Salix sp.; Spiraea alba; Vaccinium sp. | Ericaceae; Rosaceae; Salicaceae | North America |
| 119 | A. macropterana | China | |||
| 120 | A. maculidorsana | USA | Chamaedaphne calyculata; Hypericum perforatum; Hypericum perforatum; Hypericum sp.; Kalmia sp.; Vaccinium sp. | Clusiaceae; Ericaceae | North America |
| 121 | A. maculopterana | China | |||
| 122 | A. malagassana | Madagascar | |||
| 123 | A. matthewsi | Peru | |||
| 124 | A. maximana | Canada | Malus pumila; Malus sp.; Populus balsamifera; Populus fremontii; Populus sp.; Populus tremula L.; Populus tremuloides; Prunus emarginata; Salix sp. | Rosaceae; Salicaceae | North America |
| 125 | A. medea | Nepal | |||
| 126 | A. micropterana | China | |||
| 127 | A. minuta | USA | Calluna sp.; Kalmia angustifolia; Kalmia sp.; Malus pumila; Malus sp.; Myrica gale; Prunus sp.; Pyrus sp.; Salix sp.; Vaccinium macrocarpon; Vaccinium sp. | Ericaceae; Myricaceae; Rosaceae; Salicaceae | North America |
| 128 | A. monagma | Nepal | |||
| 129 | A. mundana | Russia | |||
| 130 | A. nakajimai | Taiwan | |||
| 131 | A. napaea | India, European Russia | |||
| 132 | A. nectaritis | India | |||
| 133 | A. negundana | USA | Acer sp.; Acer negundo | Aceraceae | North America |
| 134 | A. nigriradix | Russia | |||
| 135 | A. nigrolinea | Canada, Ontario | |||
| 136 | A. nigropterana | China | |||
| 137 | A. nishidai | Costa Rica | Rubus eriocarpus, R. vulcanicola, R. praecipuus; Rubus spp. | Rosaceae | |
| 138 | A. nivisellana | USA | Crataegus sp.; Malus pumila; Malus sp.; Physocarpus malvaceus; Prunus pensylvanica; Sorbus scopulina; Sorbus sp. | Rosaceae | North America |
| 139 | A. obligatoria | South Korea | |||
| 140 | A. ochropicta | China | |||
| 141 | A. ochropterana | China | |||
| 142 | A. okanagana | Canada | |||
| 143 | A. ophthalmicana | Japan | |||
| 144 | A. orphnocycla | China | Chamaedaphne calyculata (L.) Moench; Chamaedaphne calyculata (L.) Moench; Malus pumila Mill.; Malus sp.; Prunus pumila L.; Rosa sp. | Ericaceae; Rosaceae | Asia; North America |
| 145 | A. osthelderi | Syria | |||
| 146 | A. oxycoccana | USA | |||
| 147 | A. pallidorbis | Nepal | |||
| 148 | A. paracinderella | USA | Betula sp.; Prunus dumosa; Prunus fremontii; Prunus virginiana | Betulaceae; Rosaceae | North America |
| 149 | A. paradiseana | Japan | Malus pumila; Sorbus sambucifolia | Rosaceae | Asia |
| 150 | A. perfundana | Russia, Asia | Quercus mongolica; Quercus serrata; Zelkova schneideriana; Zelkova serrata | Fagaceae; Ulmaceae | Non‐EU Europe; Asia |
| 151 | A. phalera | Russia | |||
| 152 | A. phanerocrypta | Madagascar | |||
| 153 | A. phantastica | Japan | |||
| 154 | A. phyllosocia | Vietnam | |||
| 155 | A. placata | India | |||
| 156 | A. placidana | USA | |||
| 157 | A. placidus | Japan | |||
| 158 | A. platynotana | Japan | Ilex pedunculosa; Lyonia ovalifolia; Quercus glauca; Rhododendron kaempferi; Rhododendron molle | Aquifoliaceae; Ericaceae; Fagaceae | Asia |
| 159 | A. porphyrocentra | China | |||
| 160 | A. potosiana | Mexico | |||
| 161 | A. praeterita | South Korea | |||
| 162 | A. proximana | China | |||
| 163 | A. ptychogrammos | USA | Cornus sericea | Cornaceae | North America |
| 164 | A. pulchella | Japan | |||
| 165 | A. pulcherrima | Taiwan | |||
| 166 | A. quadridentana | China | |||
| 167 | A. rantaizana. | Taiwan | |||
| 168 | A. razowskii. | Japan | |||
| 169 | A. recula | China | |||
| 170 | A. retrusa | Mexico | Rubus sp. | Rosaceae | North America |
| 171 | A. robinsoniana | USA | Populus tremuloides; Rosa californica | Rosaceae; Salicaceae | North America |
| 172 | A. rosella | China | Rosa acicularis var. taquetii | Rosaceae | Asia |
| 173 | A. roxana | Japan | |||
| 174 | A. rubi | South Africa | Rubus rigidus | Rosaceae | Africa |
| 175 | A. rubrivorella | Kazakhstan | |||
| 176 | A. ruwenzorica | Uganda, Congo | |||
| 177 | A. sagarmathae | Nepal | |||
| 178 | A. sagmatias | Sri Lanka | |||
| 179 | A. salicicola | Russia | |||
| 180 | A. santacrucis | USA | |||
| 181 | A. schisma | Thailand | |||
| 182 | A. semiannula | USA | Acer rubrum; Acer saccharinum; Acer sp.; Quercus alba | Aceraceae; Fagaceae | North America |
| 183 | A. semipurpurana | USA | Quercus alba; Quercus coccinea; Quercus palustris; Quercus rubra; Quercus sp.; Quercus velutina; Rosa sp. | Fagaceae; Rosaceae | North America |
| 184 | A. semitexta | India | |||
| 185 | A. senescens | Canada | Alnus rubra; Betula sp.; Malus sp.; Populus tremuloides; Prunus virginiana; Salix hookeriana; Salix lasiolepis; Salix sp. | Betulaceae; Rosaceae; Salicaceae | North America |
| 186 | A. similis | Russia | |||
| 187 | A. simpliciana | USA | |||
| 188 | A. sinica | China | |||
| 189 | A. sinuopterana | China | |||
| 190 | A. sinuosaria | China | |||
| 191 | A. sordidata | Afghanistan | |||
| 192 | A. stachi | Central Asia | |||
| 193 | A. stadiana | Canada | Alnus sp.; Betula alleghaniensis; Betula papyrifera; Betula populifolia; Betula sp. | Betulaceae | North America |
| 194 | A. stibiana | Russia | |||
| 195 | A. strigifera | Russia | |||
| 196 | A. submaccana | Russia | Alnus japonica var. rufa; Alnus maximowiczii; Betula platyphylla; Betula sp.; Duchesnea indica; Populus sp.; Rhododendron sinsii; Ribes sp.; Salix koreensis; Vaccinium vitis‐idaea; Viburnum dilatatum | Betulaceae; Caprifoliaceae; Ericaceae; Grossulariaceae (or Saxifragaceae); Rosaceae; Salicaceae | Asia |
| 197 | A. subnivana | Canada | Vernonia sp.; Quercus rubra; Quercus sp. | Asteraceae; Fagaceae | North America |
| 198 | A. auriga | Western New Guinea | |||
| 199 | A. supernova | Ecuador | |||
| 200 | A. tabida | China | |||
| 201 | A. taiwana | Taiwan | |||
| 202 | A. takeuchii | Japan | |||
| 203 | A. thiana | China | |||
| 204 | A. thomasi | India | |||
| 205 | A. thylacitis | Kenya | |||
| 206 | A. tibetica | Tibet | |||
| 207 | A. tigricolor | Japan | Sorbus alnifolia | Rosaceae | Asia |
| 208 | A. tremewani | Myanmar | |||
| 209 | A. trujilloana | Venezuela | |||
| 210 | A. tsuifengana | Taiwan | |||
| 211 | A. tungurahuae | Ecuador | |||
| 212 | A. tunicatana | Japan | |||
| 213 | A. ulmicola | China | Hemiptelea davidii; Ulmus davidiana; Ulmus davidiana var. japonica; Ulmus pumilla; Ulmus sp. | Ulmaceae | Asia |
| 214 | A. uniformis | Russia | |||
| 215 | A. variana | USA | Abies alba; Abies amabilis; Abies balsamea; Abies grandis; Abies lasiocarpa; Abies sp.; Larix occidentalis; Larix sp.; Picea abies; Picea engelmanni; Picea engelmannii; Picea glauca; Picea mariana; Picea pungens; Picea rubens; Picea sitchensis; Picea sp.; Pinus contorta; Populus balsamifera; Pseudotsuga menziesii; Pseudotsuga menziesii; Pseudotsuga sp.; Thuja plicata; Tsuga canadensis; Tsuga heterophylla; Tsuga mertensiana | Cupressaceae; Pinaceae; Salicaceae | North America |
| 216 | A. venatana | Taiwan | |||
| 217 | A. yasudai | Japan | Enkianthus campanulatus; Enkianthus campanulatus var. sikokianus | Ericaceae | Asia |
| 218 | A. yasutoshii | Taiwan | |||
| 219 | A. youngana | Canada | |||
| 220 | A. zeta | China | |||
| 221 | A. zimmermani | Hawaiian Islands | Rubus sp.; Rubus vitifolius | Rosaceae | North America; Pacific Islands |
NB – No information was found on host plants for 151 species in the table above.
The actual distribution of the species may differ from what is reported here as this table is based only on Brown et al. (2008).
Appendix B – List of Acleris spp. reported from the EU
1.
This table is based on Brown et al. (2008) and the Fauna Europaea database.
| No. | Species | Distribution in the EU | Distribution in non‐EU Europe |
|---|---|---|---|
| 1. | Acleris abietana | UK, Austria, Belgium, Czech Republic, Italy, Hungary, Germany, France, Denmark, Lithuania, Poland, Romania Slovakia Slovenia The Netherlands | European Russia, Switzerland |
| 2. | Acleris arcticana | Finland, Sweden | Norway |
| 3. | Acleris aspersana | Bulgaria, Croatia, Estonia, Finland, Ireland, Latvia, Sweden, The Netherlands, UK, Austria, Belgium, Czech Republic, Italy, Hungary, Germany, France, Denmark | European Russia, Liechtenstein, Norway, Switzerland |
| 4. | Acleris bergmanniana | Austria, Belgium, UK, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany Hungary Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Albania, Bosnia and Herzegovina, European Russia, North Macedonia, Norway, Switzerland, Ukraine |
| 5. | Acleris boscanoides | Bulgaria, Croatia, Greece | European Turkey, North Macedonia, Ukraine |
| 6. | Acleris caledoniana | UK, Ireland, Poland | |
| 7. | Acleris comariana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Slovakia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 8. | Acleris cristana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland, Ukraine |
| 9. | Acleris effractana | UK, Denmark, Germany, Poland, Finland, Lithuania, Poland, Estonia, Sweden | Liechtenstein, Norway |
| 10. | Acleris emargana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Liechtenstein, Norway, Switzerland |
| 11. | Acleris ferrugana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland, Ukraine |
| 12. | Acleris fimbriana | Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Sweden | European Russia, Norway, Ukraine |
| 13. | Acleris forsskaleana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Albania, European Russia, Liechtenstein, North Macedonia, Norway, Switzerland, Ukraine |
| 14. | Acleris hastiana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Portugal, Slovakia, Slovenia, Spain, Netherlands, Sweden | Bosnia and Herzegovina, European Russia, North Macedonia, Norway, Switzerland, Ukraine |
| 15. | Acleris hippophaeana | Austria, Belgium, France, Germany, Italy, Romania, Slovakia | European Russia, Switzerland |
| 16. | Acleris holmiana | Austria, Belgium, Bulgaria Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Slovakia, Slovenia, Spain, Netherlands, Sweden | Albania, Bosnia and Herzegovina, European Russia, North Macedonia, Norway, Switzerland |
| 17. | Acleris hyemana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Italy, Latvia, Lithuania, Poland, Slovakia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 18. | Acleris implexana | Finland, Sweden | European Russia, Norway |
| 19. | Acleris kochiella | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, France, Germany, Hungary, Italy, Latvia, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Bosnia and Herzegovina, European Russia, Switzerland, Ukraine |
| 20. | Acleris lacordairana | Austria, Estonia, Germany, Hungary, Italy, Latvia, Poland | European Russia, Switzerland |
| 21. | Acleris laterana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden | European Russia, Norway, Switzerland, Ukraine |
| 22. | Acleris lipsiana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 23. | Acleris literana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Albania, European Russia, North Macedonia, Norway, Switzerland, Ukraine |
| 24. | Acleris logiana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 25. | Acleris lorquiniana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia, Netherlands, Sweden | European Russia, Liechtenstain, Switzerland |
| 26. | Acleris maccana | Austria, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Latvia, Lithuania, Poland, Slovakia, Slovenia, Sweden | European Russia, Norway, Switzerland |
| 27. | Acleris notana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland, Ukraine |
| 28. | Acleris obtusana | Estonia, Finland, Latvia, Sweden | Norway |
| 29. | Acleris permutana | Austria, Belgium, Croatia, UK, Czech Republic, Denmark, France, Germany, Hungary, Italy, Luxembourg, Portugal, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, North Macedonia |
| 30. | Acleris quercinana | Austria, Belgium, Bulgaria, Czech Republic, Denmark, France, Germany, Greece, Hungary, Italy, Poland, Portugal, Romania, Slovenia, Spain, Netherlands, Sweden | Albania, Bosnia and Herzegovina, North Macedonia, Switzerland, Ukraine |
| 31. | Acleris rhombana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Bosnia and Herzegovina, European Russia, European Turkey, North Macedonia, Norway, Switzerland, Ukraine |
| 32. | Acleris roscidana | Austria, Czech Republic, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Sweden | European Russia, Norway, Switzerland, Ukraine |
| 33. | Acleris rufana | Austria, Belgium, UK, Bulgaria, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 34. | Acleris scabrana | Austria, Belgium, Czech Republic, Finland, France, Germany, Hungary, Latvia, Lithuania, Poland, Romania, Slovakia, Netherlands | European Russia, Switzerland, Ukraine |
| 35. | Acleris schalleriana | Austria, Belgium, UK, Czech Republic, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Bosnia and Herzegovina, European Russia, North Macedonia, Norway, Switzerland, Ukraine |
| 36. | Acleris shepherdana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Lithuania, Luxembourg, Poland, Slovakia, Netherlands, Sweden | European Russia, Norway, Switzerland |
| 37. | Acleris sparsana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Norway, Poland, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | European Russia, Liechtenstein, North Macedonia, Norway, Switzerland |
| 38. | Acleris umbrana | Austria, Belgium, UK, Czech Republic, Denmark, Estonia, Finland, France, Germany, Hungary, Italy, Latvia, Lithuania, Poland, Romania, Slovakia, Sweden | European Russia, Norway, Switzerland |
| 39. | Acleris undulana | Cyprus, Spain | |
| 40. | Acleris variegana | Austria, Belgium, UK, Bulgaria, Czech Republic, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Netherlands, Sweden | Albania, Bosnia and Herzegovina, European Russia, North Macedonia, Norway, Switzerland, Ukraine |
Suggested citation: EFSA Panel on Plant Health (PLH) , Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Civera AV, Yuen J, Zappalà L, Grégoire J‐C, Kertész V and Milonas P, 2019. Scientific Opinion on the pest categorisation of non‐EU Acleris spp. EFSA Journal 2019;17(10):5856, 37 pp. 10.2903/j.efsa.2019.5856
Requestor: European Commission
Question Number: EFSA‐Q‐2018‐00794
Panel members: Claude Bragard, Katharina Dehnen‐Schmutz, Francesco Di Serio, Paolo Gonthier, Marie‐Agnès Jacques, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A. Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L. Reignault, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent, Jonathan Yuen and Lucia Zappalà.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figures 1–3: © bugwood.org, Figures 4 and 5: © European and Mediterranean Plant Protection Organization (EPPO), Figure 6: © European Environment Agency (EEA).
Adopted: 25 September 2019
Notes
Council Directive 2000/29/EC of 8 May 2000 on protective measures against the introduction into the Community of organisms harmful to plants or plant products and against their spread within the Community. OJ L 169/1, 10.7.2000, p. 1–112.
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants. OJ L 317, 23.11.2016, p. 4–104.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31/1, 1.2.2002, p. 1–24.
References
- Anderson DP and Sturtevant BR, 2011. Pattern analysis of eastern spruce budworm Choristoneura fumiferana dispersal. Ecography, 34, 488–497. [Google Scholar]
- Brown JW, 2006. Scientific names of pest species in Tortricidae (Lepidoptera) frequently cited erroneously in the entomological literature. American Entomologist, 52, 182–189. [Google Scholar]
- Brown JW and Nishida K, 2008. A new species of Acleris Hübner,[1825] from high elevations of Costa Rica (Lepidoptera: Tortricidae, Tortricini). SHILAP Revista de Lepidopterología, 36, 341–348. [Google Scholar]
- Brown JW, Robinson G and Powell JA, 2008. Food plant database of the leafrollers of the world (Lepidoptera: Tortricidae) (Version 1.0). Available online: http://www.tortricid.net/foodplants.asp.
- Byun BK and Yan SC, 2004. Check list of the tribe Tortricini (Lepidoptera: Tortricidae) in Northeast China, with two newly recorded species from China. Korean Journal of Applied Entomology, 43, 91–101. [Google Scholar]
- de Jong Y, Verbeek M, Michelsen V, Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel F, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P and Penev L, 2014. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal, 2, e4034 10.3897/BDJ.2.e4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Caffier D, Candresse T, Chatzivassiliou E, Dehnen‐Schmutz K, Gregoire J‐C, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Rossi V, Urek G, Van Bruggen A, Van Der Werf W, West J, Winter S, Hart A, Schans J, Schrader G, Suffert M, Kertesz V, Kozelska S, Mannino MR, Mosbach‐Schulz O, Pautasso M, Stancanelli G, Tramontini S, Vos S and Gilioli G, 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. Available online: 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization), online. EPPO Global Database. Available online: https://gd.eppo.int [Accessed: 9 January 2019]
- EPPO (European and Mediterranean Plant Protection Organization), 2015. Mini data sheet on Acleris minuta. Available online: https://gd.eppo.int/taxon/ACLRMI/documents
- FAO (Food and Agriculture Organization of the United Nations), 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations), 2004. ISPM (International Standards for Phytosanitary Measures) 21—Pest risk analysis of regulated non‐quarantine pests. FAO, Rome, 30 pp. Available online: https://www.ippc.int/sites/default/files/documents//1323945746_ISPM_21_2004_En_2011-11-29_Refor.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2013. ISPM (International Standards for Phytosanitary Measures) 11—Pest risk analysis for quarantine pests. FAO, Rome, 36 pp. Available online: https://www.ippc.int/sites/default/files/documents/20140512/ispm_11_2013_en_2014-04-30_201405121523-494.65%20KB.pdf
- FAO (Food and Agriculture Organization of the United Nations), 2017. ISPM (International standards for phytosanitary measures) No 5. Glossary of phytosanitary terms. Available online: https://www.ippc.int/en/publications/622/
- Gilligan TM and Epstein ME, 2012. TortAI, Tortricids of Agricultural Importance to the United States (Lepidoptera: Tortricidae). Identification Technology Program (ITP). Center for Plant Health Science and Technology, Fort Collins, Colorado, United States of America. Accessed online: http://idtools.org/id/leps/tortai/
- Gilligan TM, Baixeras J and Brown JW, 2018. T@RTS: Online World Catalogue of the Tortricidae (Ver. 4.0). Available online: http://www.tortricid.net/catalogue.asp.
- Gray TG, Shepherd RF, Gries G and Gries R, 1996. Sex pheromone component of the western blackheaded budworm, Acleris gloverana Walsingham (Lepidoptera: Tortricidae). The Canadian Entomologist, 128, 1135–1142. [Google Scholar]
- Gries G, Jianxiong LI, Gries R, Bowers WW, West RJ, Wimalaratne PD, Khaskin G, Skip King GG, Slessor KN. 1994. (E)‐11, 13‐tetradecadienal: major sex pheromone component of the eastern blackheaded budworm, Acleris variana (Fern.) (Lepidoptera: Tortricidae). Journal of Chemical Ecology, 20, 1–8. [DOI] [PubMed] [Google Scholar]
- Johns RC, Flaherty L, Carleton D, Edwards S, Morrison A and Owens E, 2016. Population studies of tree‐defoliating insects in Canada: a century in review. The Canadian Entomologist, 148(S1), S58–S81. [Google Scholar]
- MacLeod A and Korycinska A, 2019. Detailing Koppen‐Geiger climate zones at a country and regional level: a resource for pest risk analysis. EPPO Bulletin, 49, 73–82. [Google Scholar]
- Mattson WJ, Herms DA, Witter JA and Allen DC, 1991. Woody plant grazing systems: North American outbreak folivores and their host plants In: Baranchikov YN, Mattson WJ, Hain FP. and Payne TL. (eds.). Forest Insect Guilds: Patterns of Interaction with Host Trees; 1989 August 13‐17; Abakan, Siberia, USSR Gen. Tech. Rep. NE‐153. US Department of Agriculture, Forest Service, Northeastern Forest Experiment Station, Radnor, PA: pp. 53–84, 153. [Google Scholar]
- Nakamura M and Ohgushi T, 2004. Species composition and life histories of shelter‐building caterpillars on Salix miyabeana . Entomological Science, 7, 99–104. [Google Scholar]
- Nealis VG, Turnquist R and Garbutt R, 2004. Defoliation of juvenile western hemlock by western blackheaded budworm in Pacific coastal forests. Forest Ecology and Management, 198, 291–301. [Google Scholar]
- Nealis VG and Turnquist R, 2010. Impact and recovery of western hemlock following disturbances by forestry and insect defoliation. Forest Ecology and Management, 260, 699–706. [Google Scholar]
- Nealis VG, Silk P, Turnquist R and Wu J, 2010. Baited pheromone traps track changes in populations of western blackheaded budworm (Lepidoptera: Tortricidae). The Canadian Entomologist, 142, 458–465. [Google Scholar]
- Obraztsov NS, 1963. Some North American moths of the genus Acleris (Lepidoptera: Tortricidae). Proceedings of the United States National Museum.
- Oku T, 1957. Description of a new species of Acleris huebner with notes on synonymy. Insecta Matsumarana, 21. [Google Scholar]
- Pherobase , 2019. Available online: http://www.pherobase.com/database/genus/genus-Acleris.php. Accessed on 25 June 2019
- Razowski J, Tarcz S and Greczek‐Stachura M, 2010. Molecular approach to the systematics of European Tortricini (Lepidoptera: Tortricidae). Folia biologica, 58, 189–194. [DOI] [PubMed] [Google Scholar]
- Shepherd RF and Gray TG, 2001. Comparative rates of density change in declining populations of the blackheaded budworm Acleris gloverana (Lepidoptera: Tortricidae) among different sites on Vancouver Island. Environmental Entomology, 30, 883–891. [Google Scholar]
- USDA (U.S. Department of Agriculture, Forest Service), 1979. A guide to common insects and diseases of forest trees in the northeastern United States. Northeast. Area State and Private Forestry, Forest Insect and Disease Management., Broomall, PA. p. 123, illus. (USDA Forest Service, Northeast Area State and Private Forestry Publication. NA‐FR‐4).
- Weatherby JC, 1982. The life system of the yellow‐headed fireworm, Acleris minuta (Robinson) (Lepidoptera: Tortricidae). Retrospective Theses and Dissertations. 8396. Available online: https://lib.dr.iastate.edu/rtd/8396
- Wood C and Garbutt R, 1990. Defoliator damage assessment and detection and mapping of insect epidemics, Queen Charlotte Islands and mainland coast, 1989. Can. For. Serv. Ann. Rep. For. Insect Dis. Surv. 90–8.


