Figure 6. Effect of FS mutation on TRBP-PACT interaction.
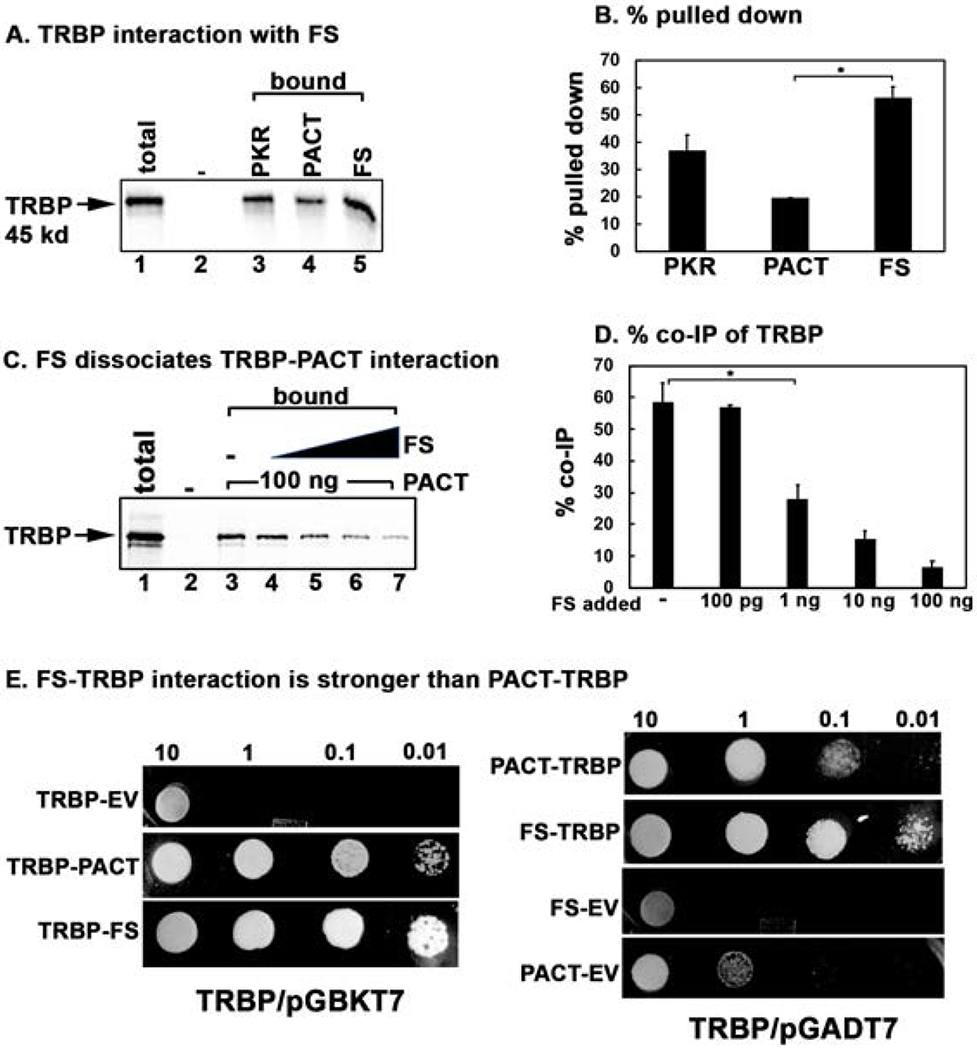
(A) Pull down assay of in vitro translated TRBP protein with pure recombinant PKR, PACT, and FS proteins. 5 μl of in vitro translated, 35S-labeled flag-tagged TRBP protein was mixed with 100 ng of purified, hexahistidine tagged recombinant PKR, PACT, and FS proteins immobilized in Ni-charged Sepharose beads. Pull down of 35S-labelled TRBP was analyzed by SDS-PAGE after washing the beads five times with wash buffer. Total lane: total input TRBP (50% of the bound samples); Bound lanes: pulled down TRBP. The “-” lane shows TRBP pull down with no recombinant protein on Ni-charged Sepharose beads as a negative control. (C) FS mutant protein can dissociate TRBP from PACT. 5 μl of the in vitro translated 35S-labeled TRBP protein was incubated with 100 ng of pure recombinant hexahistidine tagged PACT and anti-PACT rabbit monoclonal antibody bound to 10 μl of protein A sepharose beads. Increasing amounts of pure recombinant FS protein was added as indicated in lanes 4-7. Pull down of 35S-labelled TRBP was analyzed by SDS-PAGE after washing the beads five times with wash buffer. Total lane: total input TRBP (50% of the bound samples); Bound lanes: pulled down TRBP. The “-” lane shows TRBP pull down with no recombinant protein on Ni-charged Sepharose beads as a negative control. Lanes 4-7: pure recombinant FS mutant protein added in increasing amounts (100 pg, 1 ng, 10 ng, 20 ng). (B and D) Quantification of data in 6 A, and 6 C. The radioactivity present in the bands was measured by phosphorimager analysis and the % pull down was calculated as (radioactivity present in the pull down (bound) TRBP bands/the radioactivity present in the TRBP band in the total lane) X 100. Error bars: standard error of mean from 4 independent experiments. Student T-tests were performed, and p values are as follows (B) * = 0.00011, (D) * = 0.000012, n=4. E. TRBP-FS interaction is stronger than TRBP-PACT interaction in yeast two-hybrid assay. PACT/pGADT7, FS/pGADT7 or empty vector (EV) pGADT7 were co-transformed with TRBP/pGBKT7 into AH109 yeast cells and selected on SD double dropout media (-tryptophan, - leucine). Ten microliters of transformed yeast cells (OD600 = 10, 1, 0.1, 0.01) were spotted on SD quadruple dropout media (- tryptophan, - leucine, - histidine, -adenine) containing 10 mM 3-amino-1,2,4-triazole (3-AT). Plates were incubated for 3 days at 30 °C. The assay was also performed with either PACT/pGBKT7 or FS/pGBKT7 and TRBP/pGADT7 in a similar manner (TRBP/pGADT7 panels). Co-transformation of PACT or FS in pGBKT7 and empty vector pGADT7 served as negative controls.
