Figure 7. A schematic model of PKR activation in cells expressing FS mutant.
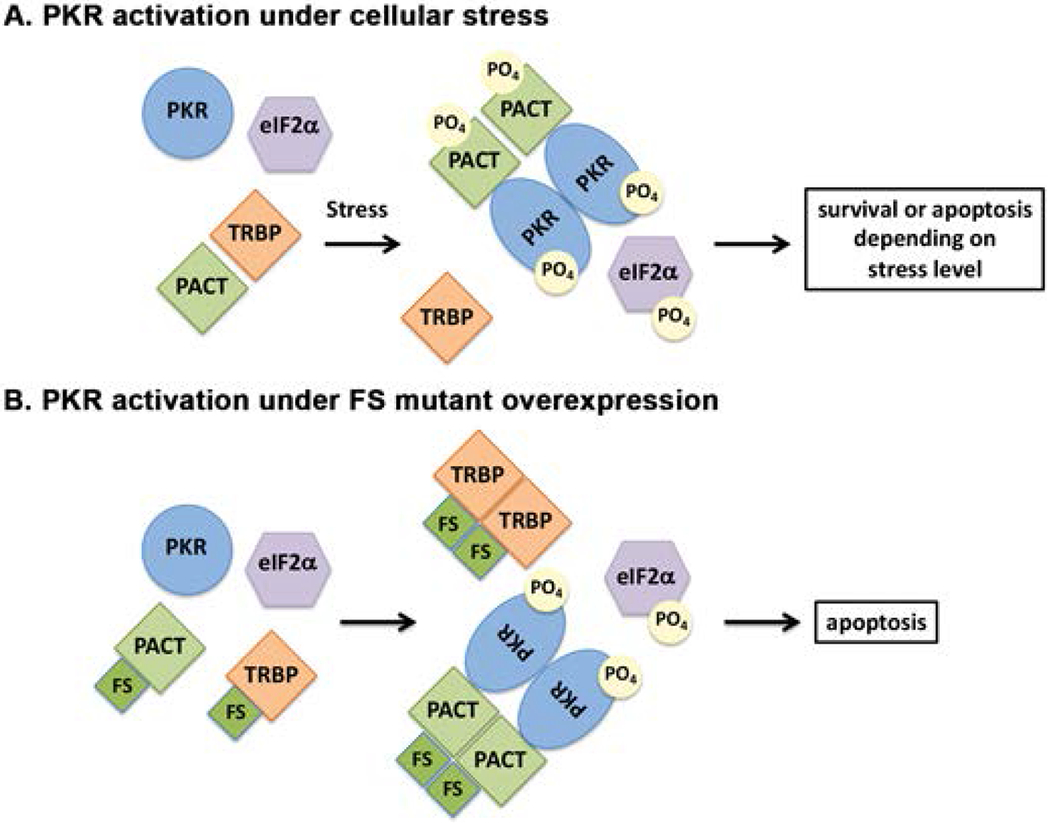
(A) wt cells. As previously established, in the absence of stress, PACT heterodimerizes with TRBP, PKR is catalytically inactive and eIF2α is not phosphorylated. In response to a stress signal, PACT dissociates from TRBP due to its phosphorylation, forms homodimers that bind to PKR with high affinity, activate its kinase activity leading to eIF2α phosphorylation. (B) Cells expressing FS mutant protein. In the absence of stress, FS mutant protein heterodimerizes with TRBP with high affinity (and possibly also with PACT) to displace PACT from TRBP-PACT heterodimers. Consequently, PACT homodimers form due to high affinity interactions between FS mutant protein molecules. Such homodimers bind to PKR with high affinity and activate its kinase activity leading to eIF2α phosphorylation leading to apoptosis via caspase activation.
