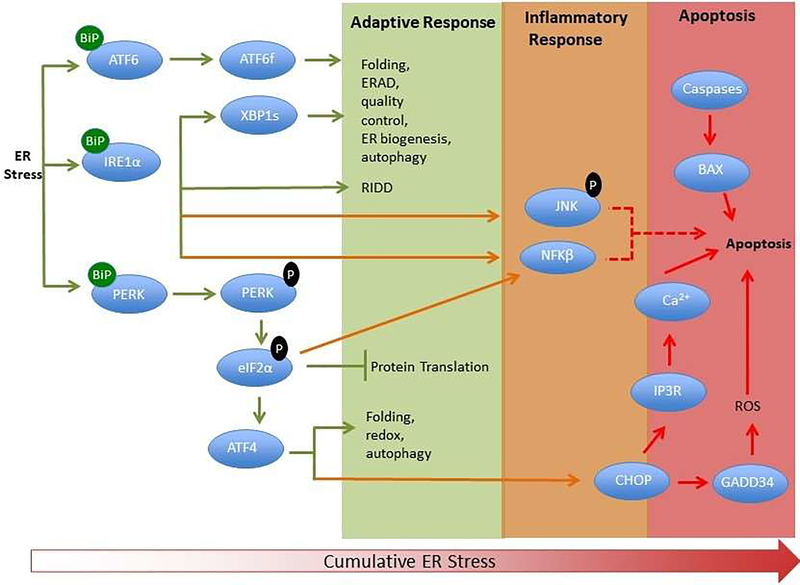
Figure 2:
Simplified scheme showing the adaptive, inflammatory and apoptotic phases of the UPR. The three ER stress sensors (PERK, IRE1 and ATF6) are activated upon dissociation from BiP initiating signaling events that increase protein-folding capacity and reduce protein load on the ER. Phosphorylation of eukaryotic Initiation Factor 2α (eIF2α) by PERK inhibits protein translation, while IRE1 activation leads to splicing of xbp1 and chaperone production as well as regulated IRE1-dependent decay (RIDD). Both ATF6 and IRE1 contribute to ER associated degradation (ERAD) of misfolded proteins.
These transcriptional and translational outputs tend to re-establish protein-folding homeostasis in the ER and promote cell survival. JNK activation downstream of IRE1 contributes to inflammatory signaling. Activated IRE1 acting on downstream factors activates JNK and caspases. ATF4 dependent transcription in mammals leads to increases in C/ebp homologous binding protein (CHOP), a pro-apoptotic transcription factor. CHOP and JNK also promote the translocation of Bax (BCl-2 associated X protein) to the mitochondria where it facilitates the release of cytochrome c required for caspase activation. ER specific caspases are thought to directly induce cell death. Translation attenuation leads to NFκB entry into the nucleus and transcription of inflammatory genes. Calcium release from the ER, exacerbated protein synthesis, and ROS production influences the induction of apoptosis.