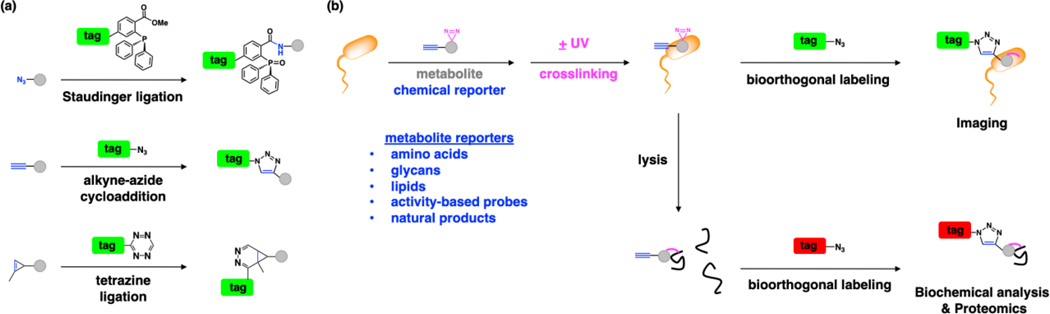
Figure 2.
Chemical strategy for exploring metabolite trafficking and protein interactions in bacteria. (a) Representative bioorthogonal labeling reactions; Staudinger ligation [1], click chemistry (copper(I)-catalyzed azide-alkyne cycloaddition) [2, 3] and tetrazine ligation [4], enable selective labeling of azide, alkyne and activated-alkene modified metabolites, respectively. (b) These advances in bioorthogonal labeling have inspired the development of diverse azide, alkyne and activated-alkene functionalized metabolites as chemical reporters. Chemical reporter-labeled targets can then be reacted with complementary-functionalized fluorescent dye or affinity tag for imaging and/or proteomic applications. For non-covalent metabolite-protein interactions, chemical reporters can be further modified with diazirines for photocrosslinking.