| Code/trivial namea | Chemical name/SMILES notationb | Structural formulac |
|---|---|---|
| Imidacloprid |
(E)‐1‐[(6‐chloro‐3‐pyridyl)methyl]‐N‐nitroimidazolidin‐2‐ylideneamine [O‐][N+](=O)/N=C1\NCCN1Cc1cnc(Cl)cc1 YWTYJOPNNQFBPC‐DLSJENCCNA‐N |
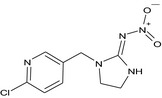
|
| imidacloprid‐5‐hydroxy (M01) |
(5RS)‐1‐[(6‐chloropyridin‐3‐yl)methyl]‐2‐(nitroamino)‐4,5‐dihydro‐1H‐imidazol‐5‐ol [O‐][N+](=O)NC1=NCC(O)N1Cc1cnc(Cl)cc1 MATMQDMQFSFQHB‐UHFFFAOYSA‐N |
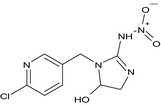
|
| imidacloprid olefin (M06) |
1‐[(6‐chloropyridin‐3‐yl)methyl]‐N‐nitro‐1H‐imidazol‐2‐amine [O‐][N+](=O)Nc1nccn1Cc1cnc(Cl)cc1 TYLCDJYHUVCRBH‐UHFFFAOYSA‐N |
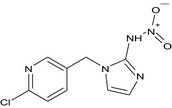
|
| imidacloprid‐desnitro (M09) |
1‐[(6‐chloropyridin‐3‐yl)methyl]‐4,5‐dihydro‐1H‐imidazol‐2‐amine Clc1ncc(CN2CCNC2=N)cc1 UEQZFAGVRGWPDK‐UHFFFAOYSA‐N |
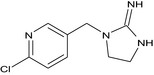
|
| imidacloprid‐6‐CNA (M14) |
6‐chloronicotinic acid OC(=O)c1cnc(Cl)cc1 UAWMVMPAYRWUFX‐UHFFFAOYSA‐N |
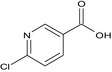
|
| imidacloprid‐CHMP (M28) |
(6‐chloropyridin‐3‐yl)methanol OCc1cnc(Cl)cc1 GOXYBEXWMJZLJB‐UHFFFAOYSA‐N |
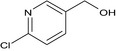
|
| imidacloprid‐CHMP‐glucoside (M29) |
(6‐chloropyridin‐3‐yl)methyl D‐glucopyranoside Clc1ccc(COC2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cn1 ZRRXFGLNJBNGQI‐AZMJIDJFSA‐N |
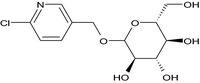
|
| glycine‐conjugate of 6‐chloropyridine‐3‐carboxylic acid |
N‐[(6‐chloropyridin‐3‐yl)carbonyl]glycine O=C(NCC(=O)O)c1cnc(Cl)cc1 VGSLNHSCEKVAIM‐UHFFFAOYSA‐N |
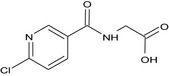
|
SMILES: simplified molecular‐input line‐entry system.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 6 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
