| Code/trivial name | Chemical name/SMILES notation/InChiKeya | Structural formulab |
|---|---|---|
| Lambda‐cyhalothrin |
A 1:1 mixture of: (R)‐α‐cyano‐3‐phenoxybenzyl (1S,3S)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or a 1:1 mixture of: (R)‐α‐cyano‐3‐phenoxybenzyl (1S)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate and (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@@H]3[C@H](C(=O)O[C@@H](C#N)c2cccc(Oc1ccccc1)c2)C3 (C)C)C(F)(F)F.N#C[C@@H](OC(=O)[C@@H]1[C@H](/C=C(\Cl)C(F)(F)F)C1(C)C)c3cccc(Oc2ccccc2)c3 BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
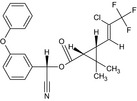
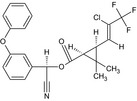
|
| Gamma‐cyhalothrin |
(S)‐α‐cyano‐3‐phenoxybenzyl (1R,3R)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or (S)‐α‐cyano‐3‐phenoxybenzyl (1R)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate CC1([C@H]([C@H]1C(=O)O[C@H](C#N)c2cccc(c2)Oc3ccccc3)/C=C(/C(F)(F)F)\Cl)C BFPGVJIMBRLFIR‐GUCBCRIZSA‐N |
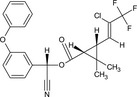
|
| Cyhalothrin |
(RS)‐α‐cyano‐3‐phenoxybenzyl (1RS,3RS)‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate or (RS)‐α‐cyano‐3‐phenoxybenzyl (1RS)‐cis‐3‐[(Z)‐2‐chloro‐3,3,3‐trifluoropropenyl]‐2,2‐dimethylcyclopropanecarboxylate Cl\C(=C/[C@H]3[C@@H](C(=O)OC(C#N)c2cccc(Oc1ccccc1)c2)C3(C)C)C(F)(F)F.FC(F)(F)C(/Cl)=C/[C@@H]3[C@H](C(=O)OC(C#N)c2cccc(Oc1ccccc1)c2)C3(C)C OOAOVGPMANECPJ‐RWEUCVCFSA‐N |
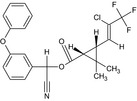
|
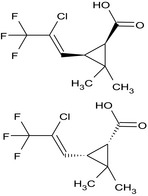
| Compound Ia |
(1RS,3RS)‐3‐[(1Z)‐2‐chloro‐3,3,3‐ trifluoro‐1‐propen‐1‐yl]‐2,2‐dimethylcyclopropanecarboxylic acid Cl\C(=C/[C@H]1[C@@H](C(=O)O)C1(C)C)C(F)(F)F.FC(F)(F)C(/Cl)=C/[C@@H]1[C@H](C(=O)O)C1(C)C DPUIEEBDWOJPHB‐OBDQHKNMSA‐N |
|
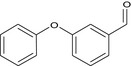
| Compound IV |
3‐phenoxybenzaldehyde O=Cc2cc(Oc1ccccc1)ccc2 MRLGCTNJRREZHZ‐UHFFFAOYSA‐N |
|
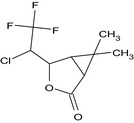
| Gamma‐lactone (R947650) |
(1RS,4RS,5SR)‐4‐[(1RS)‐1‐chloro‐2,2,2‐trifluoroethyl]‐6,6‐dimethyl‐3‐oxabicyclo[3.1.0]hexan‐2‐one (Unstated stereochemistry) CC2(C)C1C(=O)OC(C(Cl)C(F)(F)F)C12 ZSSZFVGRINYCPY‐UHFFFAOYSA‐N |
|
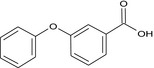
| Metabolite V (PBA) |
3‐phenoxybenzoic acid O=C(O)c2cc(Oc1ccccc1)ccc2 NXTDJHZGHOFSQG‐UHFFFAOYSA‐N |
|
| Metabolite XXIII (PBA(OH) |
3‐(4‐hydroxyphenoxy)benzoic acid O=C(O)c2cc(Oc1ccc(O)cc1)ccc2 OSGCDVKVZWMYBG‐UHFFFAOYSA‐N |
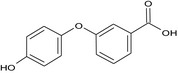
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2017.2.1 ACD/Labs 2017 Release (File version N40E41, Build 96719, 6 September 2017).
ACD/ChemSketch 2017.2.1 ACD/Labs 2017 Release (File version C40H41, Build 99535, 14 February 2018).
