Abstract
The data on antimicrobial resistance in zoonotic and indicator bacteria in 2017, submitted by 28 EU Member States (MSs), were jointly analysed by EFSA and ECDC. Resistance in zoonotic Salmonella and Campylobacter from humans, animals and food, and resistance in indicator Escherichia coli as well as meticillin‐resistant Staphylococcus aureus in animals and food were addressed, and temporal trends assessed. ‘Microbiological’ resistance was assessed using epidemiological cut‐off (ECOFF) values; for some countries, qualitative data on human isolates were interpreted in a way which corresponds closely to the ECOFF‐defined ‘microbiological’ resistance. In Salmonella from humans, as well as in Salmonella and E. coli isolates from fattening pigs and calves of less than 1 year of age, high proportions of isolates were resistant to ampicillin, sulfonamides and tetracyclines, whereas resistance to third‐generation cephalosporins was uncommon. Varying occurrence/prevalence rates of presumptive extended‐spectrum beta‐lactamase (ESBL)/AmpC producers in Salmonella and E. coli monitored in meat (pork and beef), fattening pigs and calves, and Salmonella monitored in humans, were observed between countries. Carbapenemase‐producing E. coli were detected in one single sample from fattening pigs in one MS. Resistance to colistin was observed at low levels in Salmonella and E. coli from fattening pigs and calves and meat thereof and in Salmonella from humans. In Campylobacter from humans, high to extremely high proportions of isolates were resistant to ciprofloxacin and tetracyclines, particularly in Campylobacter coli. In five countries, high to very high proportions of C. coli from humans were resistant also to erythromycin, leaving few options for treatment of severe Campylobacter infections. High resistance to ciprofloxacin and tetracyclines was observed in C. coli isolates from fattening pigs, whereas much lower levels were recorded for erythromycin. Combined resistance to critically important antimicrobials in both human and animal isolates was generally uncommon but very high to extremely high multidrug resistance levels were observed in S. Typhimurium and its monophasic variant in both humans and animals. S. Kentucky from humans exhibited high‐level resistance to ciprofloxacin, in addition to a high prevalence of ESBL.
Keywords: antimicrobial resistance, zoonotic bacteria, indicator bacteria, ESBL
Summary
1.
1.1.
Highlights
Zoonoses are infections that are transmissible between animals and humans. Infections can be acquired directly from animals, via environmental exposure or through the ingestion of contaminated foodstuffs. The severity of these diseases in humans can vary from mild symptoms to life‐threatening conditions. Zoonotic bacteria that are resistant to antimicrobials are of particular concern, as they might compromise the effective treatment of infections in humans. Data from the EU Member States (MSs) are collected and analysed in order to monitor the occurrence of antimicrobial resistance (AMR) in zoonotic bacteria isolated from humans, animals and food in the European Union (EU).
For 2017, 28 MSs reported data on AMR in zoonotic bacteria to the European Food Safety Authority (EFSA), and 24 MSs reported data to the European Centre for Disease Prevention and Control (ECDC). In addition, three other European countries reported data; Iceland and Norway reported to ECDC, while Iceland, Norway and Switzerland reported to EFSA. The enhanced monitoring of AMR in bacteria from food and food‐producing animals set out in the Commission Implementing Decision 2013/652/EU was successfully implemented in reporting MSs and non‐MSs in the EU during 2017. In accordance with the legislation, the 2017 AMR data on food and food‐producing animals specifically targeted fattening pigs and calves under 1 year of age and meat derived thereof. EFSA and ECDC performed the analyses of the data, the results of which are published in this EU Summary Report on AMR. Data on resistance were reported regarding Salmonella and Campylobacter isolates from humans and fattening pigs, whereas data on indicator Escherichia coli isolates were related only to fattening pigs and calves under 1 year of age and meat derived thereof. Some MSs also reported data on the occurrence of meticillin‐resistant Staphylococcus aureus (MRSA) in animals and food; the antimicrobial susceptibility of MRSA isolates was additionally reported by three countries.
MSs reported all AMR data on humans, fattening pigs and calves under 1 year of age and meat thereof at the isolate (or case) level. The information published in this report provides an overview of resistance in most MSs with detailed consideration of certain important aspects, such as multidrug resistance (MDR), combined resistance patterns to critically important antimicrobials (CIA) and levels of complete susceptibility in both human and animal isolates not only at the EU level but also at the country level. More specifically, reporting data at isolate level allowed characterisation of important patterns of resistance, enabling Salmonella serovars to be linked to particular resistance patterns and to identify high‐level resistance to fluoroquinolones and important resistance phenotypes in both Salmonella and indicator E. coli.
The continually evolving threat from emerging resistance underlines the need to review the data collected, interpret the findings and assess trends. This report has attempted to highlight some of the most important findings in 2017. This report highlights in particular include the continued monitoring of the spread of certain highly resistant Salmonella serovars. Two serovars in particular, S. Typhimurium and monophasic S. Typhimurium, contribute significantly to the overall numbers of multidrug‐resistant Salmonella in Europe.
The inclusion within the harmonised monitoring scheme of a supplementary panel of antimicrobials, to be tested when certain resistances to an initial panel of antimicrobials are detected, enabled detailed screening of resistance to three carbapenem compounds. Carbapenemase‐producing E. coli were detected in the mandatory, specific monitoring for ESBL/AmpC/carbapenemase‐producing E. coli in fattening pigs in Germany The isolate from fattening pigs in Germany produced the carbapenemase enzyme VIM‐1 and genes encoding for this enzyme have been previously detected in isolates from pigs in Germany (Irrgang et al., 2017). The detection of carbapenemase‐producing Enterobacteriaceae in the environment of a swine farrow‐to‐finish operation in the United States was also recently reported (Mollenkopf et al., 2017). These findings are important, because carbapenems are critically important in human medicine.
The supplementary testing also allowed, for the second time, detailed characterisation of the beta‐lactam resistance phenotypes occurring in Salmonella and indicator E. coli from fattening pigs and from calves under 1 year of age. It enabled further phenotypic characterisation of third‐generation cephalosporin and carbapenem resistance in Salmonella and indicator E. coli, by inferring presumptive genotypes of ESBL/AmpC/carbapenemase producers. The occurrence of presumptive ESBL/AmpC producers in Salmonella and indicator E. coli from fattening pigs and from calves under 1 year of age was assessed as being at low levels. ESBL‐ and AmpC‐producing Salmonella was detected at low levels also in humans, but in a significant proportion of some serovars, although this could be affected by selective sampling.
In 2017, specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli, which is able to detect very low numbers of resistant isolates present within a sample, was performed on caecal samples from fattening pigs, calves under 1 year of age and meat derived thereof from these animals. The occurrence and prevalence of E. coli showing a presumptive ESBL, AmpC and ESBL + AmpC profiles from these animal populations and kinds of meat were assessed at both the reporting MS‐group level and the individual MS level. Overall and in most but not all countries, the detection of ESBL‐producing E. coli exceeded that of AmpC‐producing E. coli in fattening pigs, calves and meat derived thereof. Prevalence figures observed for the two kinds of meat studied were remarkably similar in all reporting countries and overall much lower than those observed in animals. The prevalence of E. coli with a presumptive ESBL phenotype in the animals tested varied widely, from low to very high levels, between reporting countries.
Main findings on Salmonella spp.
The Salmonella data presented in this report comprise all reported non‐typhoidal Salmonella serovars and represent the overall occurrence of AMR in Salmonella spp. in humans, carcases of fattening pigs and calves (under 1 year of age), fattening pigs and cattle. Differences in the prevalence of particular serovars and phage types of Salmonella in different countries and animal populations, and their associated patterns of resistance, may explain some of the differences in the overall levels of AMR and MDR.1 The spread of resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. Other factors, such as foreign travel by humans, international food trade, animal movements, farming systems, animal husbandry and the pyramidal structure of some types of animal primary production, may also influence the spread of resistant clones.
In addition to the aggregated data for Salmonella spp., resistance data for the most common serovars from the different animal categories were analysed separately; as well as human Salmonella AMR data for selected serovars prevalent in pigs and cattle (S. Typhimurium, monophasic S. Typhimurium and S. Derby). From the different animal origins, resistance profiles of isolates belonging to these serovars were also considered when less than 10 isolates were recovered from a given animal origin in a country, to account for the low prevalence of certain serovars, to prevent exclusion of emerging serovars and to ensure that the analysis included all relevant data.
In humans
For 2017, 24 MSs and 2 non‐MSs reported data on AMR in Salmonella isolates from human cases of salmonellosis, which was one country more than in 2016. Seventeen countries provided data as measured values (quantitative data). The reported data represented 21.3% of the confirmed human salmonellosis cases reported in the EU/European Economic Area (EEA) in 2017.
High proportions of human Salmonella isolates were resistant to sulfonamides (32.8%), tetracyclines (30.2%) and ampicillin (27.5%). MDR was high overall (28.6%) in the EU (Figure 1). About 40% of S. Typhimurium isolates and 80% of monophasic S. Typhimurium 1,4,[5],12:i:‐ were MDR and two isolates of S. Typhimurium were resistant to eight of the nine tested substances, only susceptible to meropenem. Pigs are the main animal reservoir for monophasic S. Typhimurium while S. Typhimurium is commonly found in most food‐production animals, though most common in pigs and cattle (EFSA and ECDC, 2018b).
Figure 1.
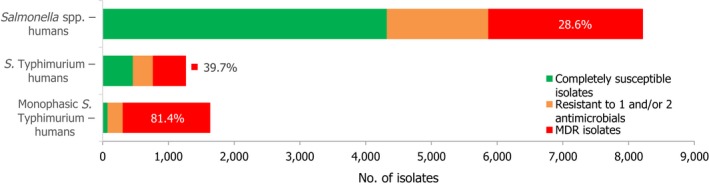
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobials and completely susceptible Salmonella isolates from humans in 2017
The proportions of Salmonella isolates resistant to either of the clinically important antimicrobials ciprofloxacin and cefotaxime were relatively low overall (13.0% resistant to ciprofloxacin and 1.9% to cefotaxime), although in S. Kentucky, the eighth most common serovar in humans in 2017, an extremely high proportion (92.6%) of the isolates were high‐level ciprofloxacin resistant. S. Kentucky is primarily found in poultry, in 2017 specifically common in laying hens, but generally common also in broilers and turkeys (EFSA and ECDC, 2018a). Both combined ‘microbiological’ and ‘clinical’ resistance to ciprofloxacin and cefotaxime was overall low in Salmonella spp. (0.9% and 0.6%, respectively).
More countries observed increasing trends in ciprofloxacin resistance in S. Typhimurium and monophasic S. Typhimurium in 2013–2017 than countries with decreasing trends in the same period. Cefotaxime resistance was more commonly decreasing than increasing by country in both serovars. In S. Typhimurium, also ampicillin and tetracycline resistance was more commonly decreasing while in monophasic S. Typhimurium, tetracycline increased in two countries.
No isolates resistant to meropenem were reported in 2017, although meropenem results were interpreted with clinical breakpoints (CBPs) in eight of the 22 countries testing this antimicrobial and the CBP is much less sensitive than the epidemiological cut‐off (ECOFF). Sequencing of ESBL‐producing S. Kentucky revealed that two isolates were also carrying a carbapenemase gene. Resistance to colistin was detected in 4.7% of isolates, although 88.9% of the resistant isolates were either S. Enteritidis or S. Dublin which have been reported to have higher natural tolerance to colistin (Agersø et al., 2012). Resistance to other last line antimicrobials, such as azithromycin and tigecycline, was relatively low at 2.5% and 0.8%, respectively.
Occurrence of antimicrobial resistance in carcases of pigs (fatteners) and calves (under 1 year of age), fattening pigs and cattle
In 2017, AMR data for Salmonella isolates recovered from carcase swabs of pigs (fatteners) and calves (under 1 year of age), and caecal contents of fattening pigs and cattle were reported by 25 MSs and 2 non‐MSs. Among the Salmonella isolates recovered from the mandatory carcase swabbing of pigs (fatteners), the highest levels of resistance were noted to ampicillin, sulfamethoxazole and tetracycline, where high to extremely high levels were recorded by most of the MSs included in the analysis (overall, 53%, 59.5% and 56.8%, respectively). In Salmonella isolates recovered from the mandatory carcase swabbing of calves (under 1 year of age), overall antimicrobial resistance levels were lower than those observed in pig carcases. This was with the exception of colistin resistance, where Denmark reported 3/5 colistin‐resistant isolates (all attributed to S. Dublin), resulting in an overall low level (3.7%) of resistance to this compound, considering all reporting countries. Although countries reporting data for calf carcases also reported results for pig carcases, the number of countries reporting data from pig carcases was considerably higher. Additionally, the number of isolates reported by countries varied and these factors introduce a source of variation to the overall results for all reporting countries. ‘Microbiological’ resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) in Salmonella spp. from pig carcases was either not discerned or detected at low levels in most of the reporting MSs; resistance to these compounds was not detected in isolates from calf carcases by any of the reporting countries. Combined resistance to ciprofloxacin and cefotaxime in Salmonella spp. from pig carcases was only detected by one MS at low levels of ‘microbiological’ resistance (1.1%); these isolates did not exhibit ‘clinical’ combined resistance to these compounds using CBPs. Resistance to azithromycin in Salmonella spp. from pig carcases was generally low or not detected, with the exception of Portugal (N = 34) which reported a moderate level of resistance at 11.8%. In calf carcases, resistance to azithromycin in Salmonella spp. was only reported by Denmark, in 1/5 isolates. Overall, MDR was considerably higher in Salmonella spp. recovered from pig carcases (47.4%) than calf carcases (22%), which will partly reflect the relative contribution of particular serovars and their associated resistance within these carcase categories.
Among Salmonella isolates recovered from the voluntary monitoring of caecal contents of fattening pigs, most MSs reported high to extremely high resistance to tetracycline and sulfamethoxazole, with similar or slightly lower levels of ampicillin resistance. Resistance levels to these antimicrobials were generally higher in isolates from fattening pigs than in those recovered from the voluntary monitoring of caecal contents of cattle. Overall, lower levels of resistance to (fluoro)quinolones (ciprofloxacin and nalidixic acid) were observed in Salmonella spp. from pigs compared with the levels recorded in those from cattle, although results for isolates from cattle were strongly influenced by the individual contribution from one MS. Resistance to third‐generation cephalosporins was not detected in cattle, consistent with the results obtained for Salmonella spp. recovered from calf carcases. In Salmonella isolates from pigs, cefotaxime and ceftazidime resistance was not detected by most MSs; Italy and Spain were the only countries to report resistance to third‐generation cephalosporins resulting in overall low/very low levels considering all reporting MSs. Additionally, Italy and Spain were the only countries to report combined resistance to ciprofloxacin and cefotaxime in Salmonella spp. from pigs at low levels of ‘microbiological’ resistance (1.7% and 0.6%, respectively); these isolates did not exhibit ‘clinical’ resistance to these compounds using CBPs. Generally resistance to azithromycin in Salmonella spp. from pigs and cattle was not detected or recorded at a low level. Overall, MDR was higher in Salmonella spp. recovered from pigs (51.3%) than in those recovered from cattle (29.5%). Although similar countries reported data on Salmonella isolates from pigs and cattle, the overall number of isolates recovered from pigs was considerably higher; greater variation can be associated with larger data sets.
The supplementary testing performed in 2017 allowed further phenotypic characterisation of those Salmonella isolates which were resistant to third‐generation cephalosporins (see further below: main findings on ESBL‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli). Resistance to carbapenems (meropenem) in Salmonella spp. recovered from carcases of pigs and calves, pigs and cattle was not observed in any of the reporting countries.
Occurrence of resistance at Salmonella serovar level
Salmonella isolates recovered from carcase swabs of fattening pigs and calves under 1 year of age were the main focus of the monitoring in 2017, in accordance with Commission Implementing Decision 2013/652/EU. The detailed reporting of results at serovar level clearly demonstrates the major contribution of a few serovars to the observed occurrence of resistance in Salmonella spp. In pig carcases, seven serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Infantis, London and Brandenburg) accounted for 88% of isolates, while in calf carcases, 11 serovars (monophasic Typhimurium, Meleagridis, Mbandaka, Derby, Dublin, Livingstone, Montevideo, Typhimurium, Braenderup, Muenchen and Rissen), accounted for 76.8% of isolates. Additionally, some MSs reported voluntary AMR data for Salmonella isolates recovered from caecal contents of fattening pigs and cattle. In fattening pigs, eight serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Brandenburg, London, Bredeney and Kapemba) accounted for 89.5% of Salmonella isolates, and among those from cattle, nine serovars (Typhimurium, monophasic Typhimurium, Dublin, Enteritidis, Derby, Mbandaka, Agona, Coeln and Rissen) accounted for 90.9% of Salmonella spp. Patterns of resistance associated with these different serovars may therefore have a marked influence on the overall resistance levels in Salmonella spp. recovered from the four animal origins.
Monophasic S . Typhimurium was the predominant serovar reported from pig carcases, pigs and calf carcases, accounting for 34.8%, 34% and 14.6% of Salmonella isolates recovered from these animal origins, respectively. Additionally, monophasic S. Typhimurium was the second most frequently reported serovar detected in cattle, accounting for 14.8% of Salmonella isolates recovered from this animal species. In both pig carcases and pigs, the proportion of all Salmonella isolates showing MDR, was greatly influenced by the occurrence of multiresistant monophasic S. Typhimurium, this serovar accounting for approximately 56.7% and 52.3% of the multiresistant isolates in pig carcases and pigs, respectively. While resistance to third‐generation cephalosporins was not detected in monophasic S. Typhimurium isolates reported from pig carcases (N = 334), calf carcases (N = 12) or cattle (N = 26), cefotaxime and ceftazidime resistance among isolates from fattening pigs (N = 161) were reported at overall levels of 1.2% and 0.6%, respectively (Figure 2).
Figure 2.
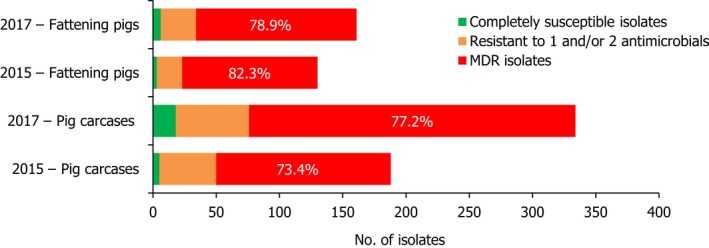
A comparison of the number of MDR and completely susceptible monophasic S. Typhimurium isolates recovered from pig carcases and fattening pigs in 2015 and 2017
S. Derby was the second most common serovar detected in pig carcases and fattening pigs, accounting for 26.5% and 20.7% of Salmonella isolates recovered from these origins, respectively. While MDR was not frequently observed among S. Derby isolates (11.8% in isolates from pig carcases and 15.3% in isolates from pigs), Figure 3 compares the relative frequencies of MDR and completely susceptible S. Derby isolates recovered from pig carcases and pigs in 2015 and 2017. Resistance to third‐generation cephalosporins was not detected in isolates from pigs, and only a single S. Derby isolate recovered from pig carcases by Germany (N = 7) was resistant to this antimicrobial class.
Figure 3.
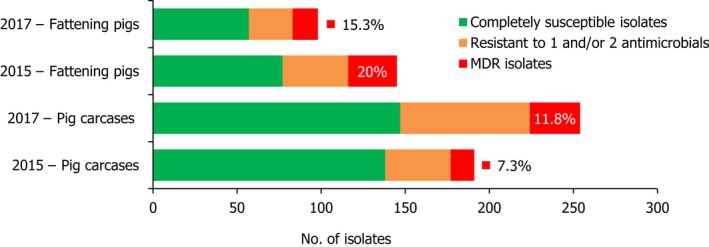
A comparison of the number of MDR and completely susceptible S. Derby isolates recovered from pig carcases and fattening pigs in 2015 and 2017
S . Typhimurium was the most frequent serovar reported in cattle and the third most commonly reported serovar in fattening pigs and pig carcases, accounting for 44.3%, 17.1% and 12.8% of Salmonella isolates recovered from these origins, respectively. Among S. Typhimurium isolates recovered from pig carcases and pigs, MDR was frequently observed; 64.2% (79/123) and 59.3% (48/81), respectively. While resistance to third‐generation cephalosporins was not detected among S. Typhimurium isolates reported from pig carcases (N = 123), calf carcases (N = 4) or cattle (N = 78), cefotaxime and ceftazidime resistance among isolates from fattening pigs (N = 81) were reported at overall levels of 1.2% (Figure 4).
Figure 4.
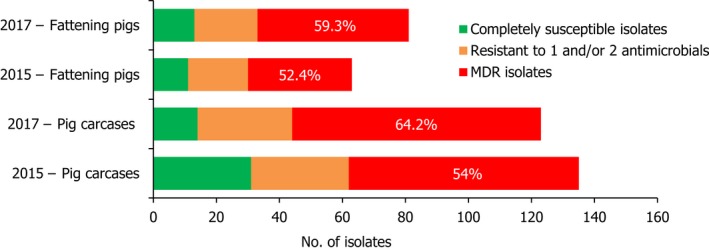
A comparison of the number of MDR and completely susceptible S. Typhimurium isolates recovered from pig carcases and fattening pigs in 2015 and 2017
S. Rissen isolates recovered from pig carcases were frequently multiresistant, with 46.5% of isolates displaying MDR. Figure 5 presents the numbers of MDR and completely susceptible S. Rissen isolates recovered from pig carcases in 2015 and 2017. Resistance to third‐generation cephalosporins was only detected in a single S. Rissen isolate recovered from pig carcases by Spain (N = 32).
Figure 5.
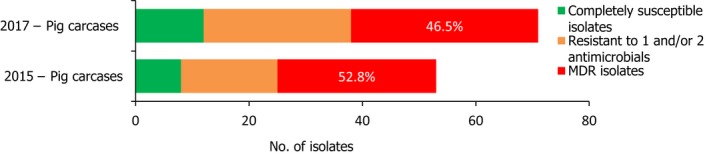
A comparison of the number of MDR and completely susceptible S. Rissen isolates recovered from pig carcases in 2015 and 2017
Conversely, S. Infantis isolates recovered from pig carcases were not frequently multiresistant, with 19.4% of isolates displaying MDR. Figure 6 presents the relative frequencies of MDR and completely susceptible S. Infantis isolates recovered from pig carcases in 2015 and 2017.
Figure 6.
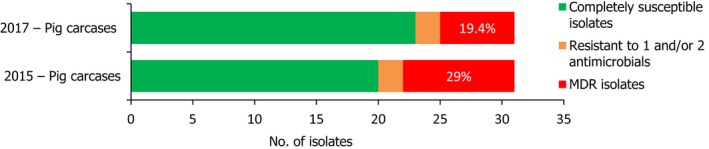
A comparison of the number of MDR and completely susceptible S. Infantis isolates recovered from pig carcases in 2015 and 2017
In 2017, no Salmonella isolates recovered from any of the four animal origins displayed high‐level resistance to ciprofloxacin (minimum inhibitory concentration (MIC) of ≥ 4 mg/L).
Considering tigecycline resistance, MSs reported resistance to this compound in 1.5% of 474 Salmonella spp. from fattening pigs, 1.4% of 954 Salmonella spp. from pig carcases and 0.9% of 110 Salmonella spp. from cattle; tigecycline resistance was not detected in Salmonella spp. from calf carcases under 1 year of age (N = 82). Notably, certain serovars displayed ‘microbiological’ resistance to tigecycline, which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars: 4/7 tigecycline‐resistant isolates recovered from pigs belonged to serovar S. Typhimurium, 11/13 isolates from pig carcases were S. Typhimurium (N = 4) and S. Rissen (N = 7), while the only tigecycline‐resistant isolate recovered from cattle was S. Typhimurium.
Additionally, colistin‐resistant Salmonella isolates were detected by several MSs originating from carcases of pigs and calves, fattening pigs and cattle. All bovine isolates were S. Dublin, a group D Salmonella. Group D Salmonella isolates tend to show elevated colistin MICs when compared to other serovars, a phenomenon considered to reflect slightly decreased susceptibility of wild‐type isolates belonging to Group D. Certain serovars among porcine isolates displayed colistin resistance, where monophasic S. Typhimurium accounted for 4/6 colistin‐resistant isolates from pig carcases and 4/9 isolates from pigs. High colistin MICs of ≥ 16 mg/L were observed in two S . Derby isolates, one from a pig carcase and one from a fattening pig, possibly reflecting the presence of multiple additive mechanisms of colistin resistance.
Main findings on Campylobacter
In humans
For 2017, 19 MSs and two non‐MSs reported data on AMR in Campylobacter isolates from human cases of campylobacteriosis which was two more countries than for 2016. Thirteen countries provided data as measured values (quantitative data). The reported data represented 22.2% and 24.1% of the confirmed human infections with Campylobacter jejuni and Campylobacter coli, respectively, reported in the EU/EEA in 2017.
Very high to extremely high resistance levels to ciprofloxacin were reported in C. jejuni isolates from humans by all countries except Denmark, Iceland, Ireland, Norway and the United Kingdom. Thirteen out of 18 MSs had extremely high levels of ciprofloxacin resistance in C. coli of 70–100% with increasing trends during 2013–2017 in four MSs. For C. jejuni, increasing trends of fluoroquinolone resistance was observed in seven MSs and Iceland. The level of acquired resistance to fluoroquinolones is so high in some MSs that this antimicrobial can no longer be considered appropriate for routine empirical treatment of Campylobacter infections in humans.
While the proportion of human C. jejuni isolates resistant to erythromycin was low overall (2.0%), it was markedly higher in C. coli (12.8%) with high to very high proportions (21.4–59.6%) of C. coli being resistant in four MSs. Increasing trends of erythromycin resistance in 2013–2017 were observed in two MSs and one non‐MS for C. jejuni from humans while decreasing trends were observed in two MSs for C. jejuni and one for C. coli. Tetracycline resistance increased in seven MSs for C. jejuni and four MSs for C. coli in the same period with only one country observing a decreasing trend for C. jejuni.
Combined clinical and microbiological resistance to ciprofloxacin and erythromycin, both of which are considered critically important for treatment of campylobacteriosis, was low (1.2%) in C. jejuni and moderate (10.2%) in C. coli. Three MSs and one non‐MS however reported high levels and one MS very high levels of combined clinical resistance in C. coli from humans. MDR in isolates tested to four antimicrobial classes was low in C. jejuni but moderate in C. coli (Figure 7).
Figure 7.
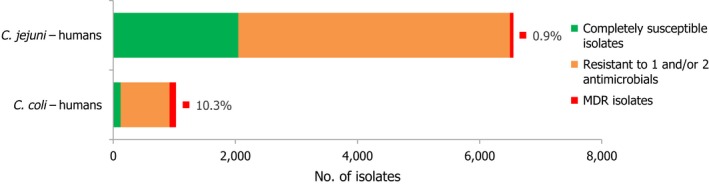
Number of MDR isolates, isolates resistant to 1 and/or 2 antimicrobials and completely susceptible Campylobacter isolates from humans in 2017
In fattening pigs and calves of less than 1 year of age
For 2017, quantitative isolate‐based MIC data on C. coli from fattening pigs were collected and reported by seven MSs and two non‐MS, on a voluntary basis. Five MSs also transmitted voluntarily AMR data on C. jejuni from calves.
Regarding C. coli from fattening pigs, resistance typically varied markedly between countries. For ciprofloxacin, a highest priority CIA, resistance ranged between 16.8% and 97.1% among the seven reporting MSs, with an overall resistance to fluoroquinolones of 52.3%. For the CIA erythromycin, 15.6% of the isolates were resistant, (range: 0–61.8%). For the other antibiotics tested, the overall levels of resistance to streptomycin (64.4%) and tetracycline (51.5%) were very high, whereas resistance to gentamicin was low (7.7%). Fully susceptible isolates were detected in all countries but one. They made up 33.0% of the 979 C. coli isolates of the MSs. The combined resistance to both ciprofloxacin and erythromycin, which is of public health relevance, was the most frequently reported in Spain, at 61.2%. It was detected at much lower levels (8.1%) in Germany and at less than 5% in the other reporting countries. MDR was rare in northern countries but extremely high in some southern MSs. An increase in high‐level resistance to erythromycin in C. coli from fattening pigs was detected in Spain between 2015 and 2017. This observation requests a further follow‐up over the coming years.
Concerning the 585 C. jejuni isolates from cattle, the overall resistance levels observed equalled 52.5% and 52.1% for ciprofloxacin and nalidixic acid, respectively. They were 39% for tetracycline, and 15.6% streptomycin. Resistance to gentamicin (8.2%) and erythromycin (1.2%) was less frequent in the reporting MSs. Whereas complete susceptibility was the most frequent profile detected in Denmark and the Netherlands, it was observed at low levels only in Italy and Spain, and was undetected in Croatia. The combined resistance to ciprofloxacin and erythromycin equalled 1.7% overall, corresponding to a maximum of two C. jejuni isolates exhibiting combined resistance to erythromycin and ciprofloxacin recovered per reporting MSs. MDR was undetected in Denmark, but present in up to 28.3% of isolates from Croatia.
Main findings regarding indicator commensal Escherichia coli
For 2017, all EU MSs and three non‐MSs reported quantitative data on AMR in indicator E. coli isolates from fattening pigs and 10 EU MSs and 2 non‐MSs reported in calves under one year age. One MS voluntary reported AMR data also for E. coli isolates from meat of pigs and bovine animals.
In fattening pigs
Regarding fattening pigs, the highest overall ‘microbiological’ resistance levels observed at the reporting MS group level were to tetracycline (52.1%), sulfamethoxazole (42.4%), ampicillin (38.5%) and trimethoprim (32.2%). There was substantial variation in resistance to these antimicrobials between reporting MSs (Figure 8). Resistance to the third‐generation cephalosporins, cefotaxime and ceftazidime, were similar at 1.4% and 1.3%, respectively, and the highest level reported by a single MS was 7.4%. At the MS level, resistance to single antimicrobials was generally similar or slightly lower in 2017 than in 2015. Interestingly, certain MSs, implementing national control programmes for the use of antimicrobials in food‐producing animals, registered decreasing trends over the period 2009 to 2017, whereas levels of resistance in other MSs were either relatively stable or increasing in this period.
Figure 8.
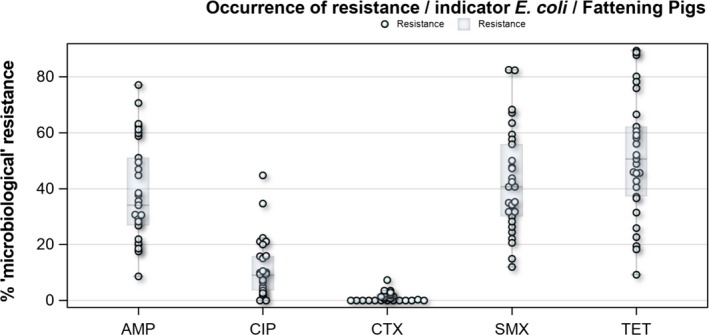
Distribution of the occurrence of resistance to ampicillin (AMP), ciprofloxacin (CIP), cefotaxime (CTX), sulfonamides (SMX) and tetracyclines (TET) in E. coli from fattening pigs, using ECOFFs, EU MSs, 2017
MDR levels (reduced susceptibility to at least three antimicrobial classes according to ECOFFs) were generally high in indicator E. coli isolates from fattening pigs. For all reporting countries, 34.9% of isolates displayed MDR, but with considerable variation in the occurrence between countries. The predominant MDR pattern was resistance to ampicillin, sulfamethoxazole, tetracyclines and trimethoprim and this was observed as a core resistance pattern in 48.5% of all MDR E. coli isolates from pigs. Combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was detected in 0.5% of E. coli isolates from fattening pigs and combined ‘clinical resistance’ in 0.2% of the isolates when resistance to ciprofloxacin and cefotaxime was interpreted using CBPs.
In calves under 1 year of age
In the reporting group of MSs, resistance levels in indicator E. coli isolates from calves under one year old were generally lower than among isolates from fattening pigs. The highest levels observed were to tetracyclines (43.8%), sulfamethoxazole (34.4%), ampicillin (29.0%) and trimethoprim (24.7%). The occurrence of resistance was variable between MSs for most of the antimicrobials (Figure 9). Overall, only a few isolates expressed resistance to cefotaxime (1.6%) or to ceftazidime (1.6%) and the highest level reported by a single MS was 5.3%. At the MS level, resistance to single antimicrobials was generally similar, or slightly lower, in 2017 than in 2015. Interestingly, one MS, implementing national control programmes for the use of antimicrobials in food‐producing animals, registered decreasing trends in resistance over the period 2009–2017, whereas levels of resistance in other MSs were either relatively stable or increasing in this period.
Figure 9.
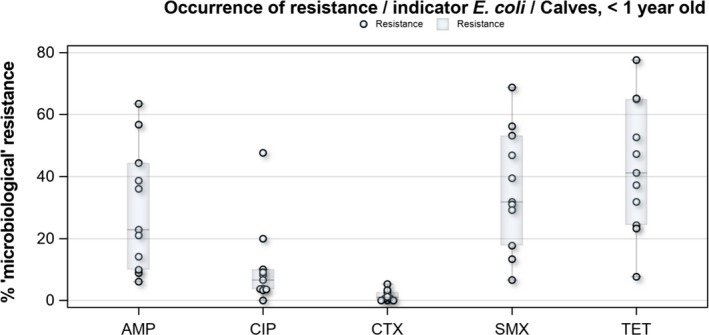
Distribution of the occurrence of resistance to ampicillin (AMP), ciprofloxacin (CIP), cefotaxime (CTX), sulfonamides (SMX) and tetracyclines (TET) in E. coli from calves under 1 year of age, using ECOFFs, EU MSs, 2017
MDR levels were generally high in indicator E. coli isolates from calves under 1 year of age. For all reporting countries, 27.7% of the isolates displayed MDR, but with wide variation in the occurrence between countries. The predominant MDR pattern in calves under 1 year of age was resistance to ampicillin, sulfamethoxazole, tetracyclines and trimethoprim and this was a core resistance pattern in 54.4% of all MDR E. coli isolates from calves. Combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime was detected in 0.7% of indicator E. coli isolates from calves and combined ‘clinical’ resistance in 0.3% of the isolates.
General observations on indicator E. coli from fattening pigs and calves under 1 year of age
Overall, 39.2% of the E. coli isolates from pigs and 56.7% of the isolates from calves under 1 year of age were fully susceptible to the range of antimicrobials tested, but for both animal species there were large differences between reporting countries. At the MS level, the proportions of fully susceptible isolates were similar to data for 2015 for both pigs and calves. In individual reporting countries, there are statistically significant increases as well as decreases in the proportion of fully susceptible isolates.
The high levels of resistance to tetracyclines, sulfamethoxazole, ampicillin and trimethoprim in E. coli from both fattening pigs and calves under one year, as well as the frequent occurrence of resistance to these compounds as a core component of MDR patterns in many reporting countries, most likely reflects extensive usage of these antimicrobials in these countries over many years. However, since the genes conferring resistance to these four compounds are also frequently linked together on mobile genetic elements, co‐selection of resistance is most likely an important factor.
Strains of E. coli are not separated on phenotypic characteristics (e.g. serotype) in the current monitoring programme and a less detailed analysis is therefore possible than for Salmonella where isolates can be subdivided by serovar. A common pattern of ’microbiological’ resistance to ampicillin, sulfamethoxazole, tetracycline and trimethoprim was observed in 19.6% of all E. coli isolates from fattening pigs and in 20.9% in calves under one year age, but a wide range of other patterns was also recorded, suggesting that a diverse range of strains was captured in the monitoring programme.
At the MS level, ciprofloxacin resistance was overall moderate at 10.6% in both pigs and calves under 1 year of age. This is considerably lower than reported for broilers (66.9%) and fattening turkeys (76.2%) in 2016 and indicate that there are differences in selection pressure in these food animal populations. Also, 35% of the E. coli isolates from pigs and 45% of the isolates from calves under 1 year of age were resistant to ciprofloxacin but not to nalidixic acid. This phenotype indicates the presence of transmissible genes conferring fluoroquinolone resistance in a large proportion of isolates. In contrast, only 7% of E. coli isolates from broilers and 20% from fattening turkeys reported in 2016 had this phenotype.
Colistin resistance in indicator E. coli isolates from fattening pigs and calves under year age were found by several MSs but at overall low levels of 0.4% and 0.8%, respectively. The highest level reported by a MS was 2.1% in pigs and 2.9% in calves under 1 year of age. The overall levels are lower than reported for broilers (1.7%) and fattening turkeys (5.7%) in 2016. Moreover, levels of colistin resistance in single MS was as high as 9.4% in broilers and 25.1% in fattening turkeys which is considerably higher than reported for pigs and calves.
Resistance to meropenem was not detected in indicator E. coli from pigs and calves under 1 year of age and resistance to tigecycline was detected in only one isolate from pigs but not in isolates from calves. A rare occurrence of meropenem and tigecycline resistance was reported also for broilers and fattening turkeys in 2016. This indicates that resistance to these antimicrobials is uncommon in E. coli from food‐producing animals in Europe.
Main findings on extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli
Presumptive ESBL/AmpC/CP producers in Salmonella spp. from humans (voluntary testing/reporting)
Twelve MSs and 1 non‐MS tested for the presence of ESBL and AmpC in Salmonella isolates from humans in 2017. Of these, all countries but one identified ESBL‐producing Salmonella with an average proportion of 0.8% (Table 1). Among the nine serovars reported with ESBL, the carriage was the highest in S. Kentucky (20.3%). In S. Typhimurium and monophasic S. Typhimurium 1,4,[5],12:i:‐, 1.4% and 0.6% of the tested isolates were, respectively, ESBL producers. AmpC was detected in six countries at an overall proportion of 0.1%. No carbapenemase producers were detected through the phenotypic screening but carbapenemase genes were identified in two S. Kentucky isolates reported as clinically susceptible to meropenem. Since these were also ESBL‐producing, high‐level ciprofloxacin resistant and MDR, it is of utmost importance that further spread of these bacteria, between humans and/or into the food chain, is prevented.
Table 1.
Summary of presumptive ESBL‐, and AmpC‐producing Salmonella spp. from meat and indicator E. coli isolates from caecal samples collected within the routine monitoring in 2017
| Matrix | Presumptive ESBL and/or AmpC producersa | Presumptive ESBL producersa | Presumptive AmpC producersc | Presumptive ESBL + AmpC producersd | Presumptive CP producers |
|---|---|---|---|---|---|
| n (%R) | n (%R)b | n (%R) | n (%R) | n (%R)e | |
| Salmonella | |||||
|
Humans (N = 8,020, 12 MSs) |
77 (1.0) | 62 (0.8) | 12 (0.1) | 3 (0.04) | 0f |
|
Pig meat (N = 954, 22 MSs) |
5 (0.5) | 2 (0.2) | 3 (0.3) | 0 | 0 |
|
Bovine meat (N = 82, 7 MSs) |
0 | 0 | 0 | 0 | 0 |
| E. coli | |||||
|
Fattening pigs (N = 4,205, 28 MSs) |
52 (1.2) | 38 (0.9) | 14 (0.3) | 0 | 0 |
|
Calves, < 1 y. old (N = 1,893, 10 MSs) |
26 (1.4) | 25 (1.3) | 5 (0.3) | 4 (0.2) | 0 |
N: Total of isolates reported for this monitoring by the MSs; n: number of the isolates resistant; % R: percentage of resistant isolates; ESBL: extended‐spectrum beta‐lactamase; MS: EU Member States.
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or ESBL + AmpC phenotype.
Isolates exhibiting an ESBL‐ and ESBL/AmpC phenotype.
Isolates exhibiting an AmpC‐ and ESBL/AmpC phenotype.
Isolates exhibiting only ESBL/AmpC phenotype.
Isolates exhibiting CP phenotype.
Two isolates reported as clinically susceptible to meropenem were later confirmed to carry carbapenemase genes.
Routine antimicrobial resistance monitoring in fattening pigs, meat from pigs, cattle and meat from cattle: presumptive ESBL/AmpC/CP producers
In 2017, third‐generation cephalosporin resistance was not detected or was reported at low levels in Salmonella isolates collected in accordance with Commission implementing Decision 2013/652/EU from pig and bovine carcases. Such resistance was only reported by 4 out of 23 countries (22 MSs) detecting and reporting Salmonella spp. from carcases of fattening pigs (0.5%, 5/954, Table 1) and in none of the 7 countries (7 MSs) detecting and reporting Salmonella spp. from carcases of cattle under one year (0/82, Table 1). Third‐generation cephalosporin resistance in indicator E. coli from pigs and cattle under one year was not detected or was reported at very low or low levels by most countries. The overall occurrence of cefotaxime resistance was 1.4% (58/4,205) in E. coli from fattening pigs among the MSs, and 1.6% (30/1,893) in E. coli from cattle under one year among the MSs (Table 1).
Specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli from fattening pigs, meat from pigs, bovine meat and cattle under 1 year of age and Presumptive ESBL/AmpC/CP producers
In 2017, the specific ESBL/AmpC/carbapenemase‐producing monitoring was performed on a mandatory basis on meat from pigs and bovines (fresh meat at retail) and in fattening pigs by all MSs, as well as by three non‐MSs and on a mandatory or voluntary basis in cattle under 1 year of age by 10 MSs as well as two non‐MSs. A summary of the occurrence and prevalence of E. coli with presumptive ESBL, AmpC or ESBL + AmpC as well as carbapenem‐resistant phenotypes from meat from pigs, meat from bovines, pigs, and cattle under one year deriving from specific monitoring in 2017 assessed at the reporting MS‐group level is presented in Table 2.
Table 2.
Summary of presumptive ESBL‐ and AmpC‐producing E. coli isolates from meat from pigs, fattening pigs, bovine meat and cattle under 1 year of age and collected by the EU MSs within the specific ESBLs/AmpC/carbapenemase‐producing monitoring and subjected to supplementary testing in 2017
| Presumptive ESBL and/or AmpC producersa | Presumptive ESBL producersb | Presumptive AmpC producers c | Presumptive ESBL + AmpC producersd | Presumptive CP producerse | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %O | %P | n | %O | %P | n | %O | %P | n | %O | %P | N | %O | %P | |
|
Pig Meat Ns = 6,803 N = 380 28 MSs |
378 | 99.5 | 6.0 | 298 | 78.4 | 4.7 | 99 | 26.1 | 1.6 | 19 | 5.0 | 0.3 | 0 | 0.0 | 0.0 |
|
Fattening pigs Ns = 6,836 N = 2,819 28 MSs |
2,783 | 98.3 | 43.8 | 2,180 | 77.0 | 34.3 | 703 | 24.8 | 11.1 | 100 | 3.5 | 1.6 | 1 | 0.04 | 0.01 |
|
Bovine meat Ns = 6,621 N = 304 28 MSs |
298 | 97.7 | 4.8 | 238 | 78.0 | 3.9 | 67 | 22.0 | 1.1 | 7 | 2.3 | 0.1 | 0 | 0.0 | 0.0 |
|
Cattle, < 1 year old Ns = 3,113 N = 1,326 10 MSs |
1,312 | 98.9 | 44.5 | 1,223 | 92.2 | 41.5 | 177 | 13.3 | 6.0 | 88 | 6.6 | 3.0 | 0 | 0.0 | 0.0 |
Ns: number of animal/meat samples; N: number of the isolates tested; n: number of the isolates resistant; %O: occurrence: percentage of cephalosporin‐resistant isolates presenting a presumptive phenotype; %P: prevalence: percentage of samples harbouring a presumptive ESBL‐/AmpC‐producing E. coli; MSs: EU Member States.
Isolates exhibiting only ESBL‐ and/or only AmpC‐ and/or ESBL + AmpC phenotype.
Isolates exhibiting an ESBL‐ and ESBL/AmpC phenotype.
Isolates exhibiting an AmpC‐ and ESBL/AmpC phenotype.
Isolates exhibiting only ESBL/AmpC phenotype.
Isolates exhibiting CP phenotype.
The overall prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates in caecal samples from both fattening pigs (43.8%) and cattle under 1 year (44.5%) was high. In samples of meat from pigs or bovines the overall prevalence was however low (6.0% and 4.8% respectively). The prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates in the caecal samples from fattening pigs (43.8%) and cattle under 1 year (44.5%) is comparable with the prevalence in these animal categories reported in 2015 (40.1% and 39.6% respectively). It is also comparable with the prevalence in broilers and turkey reported in 2016 (47.4% and 42.2%, respectively). The prevalence in samples of meat from pigs and bovines (6.0% and 4.8%, respectively) is comparable with the prevalence in these types of meat reported in 2015 (approximately 6% and 4%, respectively). It is however markedly lower compared to samples of broiler meat in 2016 (57.4%).
The difference in prevalence between caecal and meat samples in the pig and bovine samples indicates that many of the animals are carrying E. coli with resistance to third‐generation cephalosporins in their intestinal content, but that the bacteria do not contaminate the carcases during the slaughter process, alternatively that the bacteria do contaminate the carcases but are somehow removed later in the process.
Within the specific ESBL/AmpC/carbapenemase‐producing monitoring, one strain isolated from a pig with resistance to meropenem was detected in Germany. The isolate from Germany was confirmed by the MS to be positive for the production of VIM‐1 (information kindly shared by the MS).
Voluntary specific monitoring of carbapenemase‐producing E. coli
The specific monitoring of carbapenemase‐producing microorganisms in meat from pigs, meat from bovines and fattening pigs was performed and reported by 18 MSs and Switzerland on a voluntary basis in 2017, according to the Commission Implementing Decision 2013/652/EU. Eight of the MSs and Switzerland also reported monitoring of carbapenemase‐producing microorganisms in cattle under one year. All reporting countries focused on the isolation of E. coli.
Together, the 19 countries investigated 17,497 samples from meat from pigs (sampled at retail), fattening pigs (sampled at slaughter), meat from bovines (sampled at retail) and cattle under one year (sampled at slaughter) in accordance with Commission Implementing Decision 2013/652/EU. All these samples were negative for carbapenemase‐producing E. coli.
Some MSs reported voluntary data from additional national carbapenemase‐producing Enterobacteriaceae monitoring. Germany reported the presence of a VIM‐1‐producing E. coli isolated from fattening pigs (sampled in a farm). The Netherlands reported the detection of two different carbapenemase‐producing Enterobacter cloacae complex isolates from imported frozen shrimps (producing IMI‐1 and a novel carbapenemase, respectively).
Main findings on meticillin‐resistant Staphylococcus aureus
Periodic monitoring of food‐producing animals is carried out in conjunction with systematic surveillance of MRSA in humans, so that trends in the diffusion and evolution of zoonotically acquired MRSA in humans can be identified. The monitoring of MRSA in food‐producing animals and food is currently voluntary and only a limited number of countries reported MRSA data in 2017, with some countries additionally reporting data on spa‐type and antimicrobial susceptibility. Monitoring of other animal species, with which certain types of MRSA can be associated, provided additional useful information.
Monitoring of MRSA in food
A low number of countries (N = 5) reported data on the occurrence of MRSA in food. MRSA was detected in meat from cattle, pigs or rabbits by four countries. The occurrence of MRSA in meat can reflect colonisation of the animals from which the meat was derived with MRSA. MRSA is not generally considered to be transmitted by food, and detection often involves selective culture techniques which may detect very low levels of contamination. spa‐typing data were reported by two countries for 15 of the 80 MRSA isolates recovered from meat and considering the three broad categories of MRSA – community‐associated (CA), healthcare‐associated (HA) and livestock‐associated (LA) – most reported spa‐types (14/15) were those associated with LA‐MRSA (CC398). The remaining isolate, spa‐type t002, was recovered from fresh pig meat in Switzerland. spa‐type t002 has been associated with several multilocus sequence types within clonal complex (CC) 5, but is most commonly associated with sequence type (ST) 5. ST5 includes MRSA isolates considered as either community or healthcare‐associated MRSA. Although further molecular typing data (including Panton‐Valentine leukocidin (PVL) status) were not available, the isolate is likely to represent a HA‐MRSA lineage and was categorised as such.
Monitoring of MRSA in healthy food‐producing animals
Seven countries reported data on the occurrence of MRSA in healthy food‐producing animals; MRSA was detected in pigs, calves and broiler/laying hen flocks. There was a large degree of variation between reporting countries in the occurrence of MRSA in pigs, with 0.4–90.4% of animals/herds/slaughter batches testing positive. This variation highlights the success of Norwegian eradication programmes (0.4% prevalence), but is also likely to reflect in part the differences in sampling protocols, for example whether testing individual or batches of pigs and whether animals were sampled at slaughter or on farms. spa‐typing data were available for 530 MRSA isolates from pigs, calves and broiler/laying hen flocks, with additional multilocus sequence typing (MLST) data available for some of these isolates. While spa‐types associated with each type of MRSA (LA‐MRSA, HA‐MRSA and CA‐MRSA), as well as mecC‐MRSA, were reported from food‐producing animals, most spa‐types were those associated with CC398 (525/530 isolates) – see Figure 10.
Figure 10.
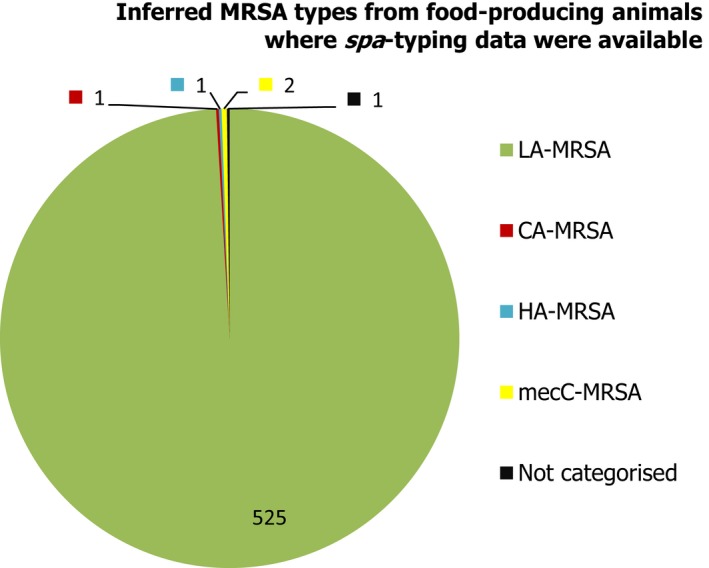
Inferred MRSA types in food‐producing animals, 2017. MRSA isolates were recovered from pigs, calves and Gallus gallus (broilers and laying hen flocks). 799 MRSA isolates were reported, of which 530 were subject to spa‐typing; some of these were subject to MLST. NB. In Finland, all MRSA isolates were subject to spa‐typing; furthermore, from a slaughter batch of pigs, up to three different spa‐types were detected.
In calves at slaughter (under 1 year of age), Switzerland reported spa‐type t127. This spa‐type has been associated with MRSA belonging to several sequence types within CC1, as well as to types in CC474, but is most frequently associated with ST1 (CC1) and considered a CA‐MRSA regardless of PVL status. The isolate was categorised as a CA‐MRSA.
Spain reported spa‐type t109 from a batch of fattening pigs at slaughter. This spa‐type has been associated with ST5 and ST228 (both members of CC5), but is generally associated with ST228 and was considered a HA‐MRSA lineage.
spa‐type t091 was reported from a multiplier pig herd in Norway; MLST confirmed the isolate belonged to CC7. Additionally, Norway reported that the t091 isolate was PVL negative, which could indicate a HA‐MRSA lineage; however, meticillin‐sensitive S. aureus (MSSA) belonging to this spa‐type have also been reported in pigs (Krupa et al., 2015) and therefore a category was not inferred.
mecC‐MRSA was reported in two Norwegian farrow‐to‐finish pig herds; spa‐types t843 and t6292. MLST confirmed them to belong to CC130 and CC425, respectively.
The novel spa‐types t17061, t17304 and t17627 were reported from batches of Finnish or Spanish fattening pigs at slaughter; MLST data were not available. Although these spa‐types appear not to have been previously reported or associated with particular MRSA sequence types, based upon similarities of their spa repeats to other spa‐types associated with CC398, they were inferred to belong to CC398. Additionally, Switzerland reported the novel spa‐type t17339 from two calves at slaughter, which was confirmed to belong to CC398.
Temporal trends in the occurrence of MRSA in food‐producing animals and meat
Generally, the temporal occurrence of MRSA in Swiss fattening pigs at slaughter showed a steady increase from 2009 to 2015, whereas a more marked increase was observed from 2015 to 2017 (25.7% to 44.0%); primarily reflecting the diffusion of spa‐types t011 and t034 within Swiss fattening pig populations. Statistical tests were performed on the Swiss longitudinal data and confirmed a statistically significant increasing trend over these years. Considering longitudinal data for other countries, a modest decline was evident in the occurrence of MRSA reported in fattening pig herds and slaughter calves in Germany, compared to the monitoring performed in previous years. The reasons for the observed declines were not apparent, with no statistically significant differences detected; however, findings are interesting because generally the occurrence of MRSA in animals and food has shown a progressive increase, where it has been investigated. For example, an increase was observed in batches of Finnish fattening pigs at slaughter from 2010 to 2017; illustrating the possible dissemination of spa‐types t034 and t2741 within Finnish fattening pig populations. Tests for statistical significance in relation to these changes confirmed a statistically significant increasing trend in the occurrence of MRSA in Finnish fattening pigs at slaughter from 2010 to 2017. Similarly, MRSA occurrence in Finnish pig meat was reported at a higher level in 2017 compared to that observed in 2015, with statistical analysis also detecting an increasing trend.
Although methods for the isolation of MRSA from food and animals are not harmonised at the EU level, changes to the recommended methods of isolation may impact future longitudinal studies, since interpretation of data would be problematic.
Monitoring of MRSA in clinical investigations
Several MSs reported results of clinical investigations which yielded MRSA in cattle, goats, sheep, horses or companion animals. Although corresponding spa‐typing data were not reported by the Netherlands, data were available for MRSA isolates reported by Sweden (denominator data were not available) – see Figure 11. spa‐Types associated with each type of MRSA (LA‐MRSA, HA‐MRSA and CA‐MRSA) were identified in companion animals, and both LA‐MRSA and HA‐MRSA were reported in domestic horses. CA‐MRSA and HA‐MRSA recovered from companion animals probably represent colonisation or infection of pets with human MRSA strains – from close contact with people or nosocomial infection at the veterinary clinic – rather than persistent establishment of these strains within companion animals. In addition, mecC‐MRSA was reported in 2 sheep at a zoo and 10 goats. Understanding of the epidemiology of mecC‐MRSA is incomplete but studies have indicated that direct animal contact and zoonotic transmission are likely to be important in human infections with this organism.
Figure 11.
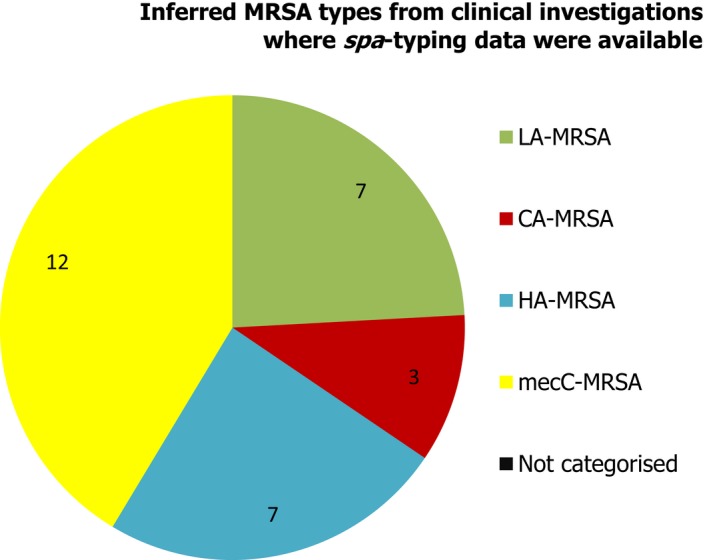
Inferred MRSA types from clinical investigations, 2017. MRSA isolates were recovered from cattle, goats, sheep, horses and companion animals (29/66 MRSA isolates were subject to spa‐typing; denominator data were not available for the isolates which were subject to spa‐typing).
Summary data on the occurrence and susceptibility of MRSA
Resistance to the important medical antimicrobials, vancomycin and linezolid, was not detected in MRSA isolates from meat, food‐producing animals or following clinical investigations.
Overall, where spa‐typing data were available, most isolates were those associated with LA‐MRSA (Figure 12).
Figure 12.
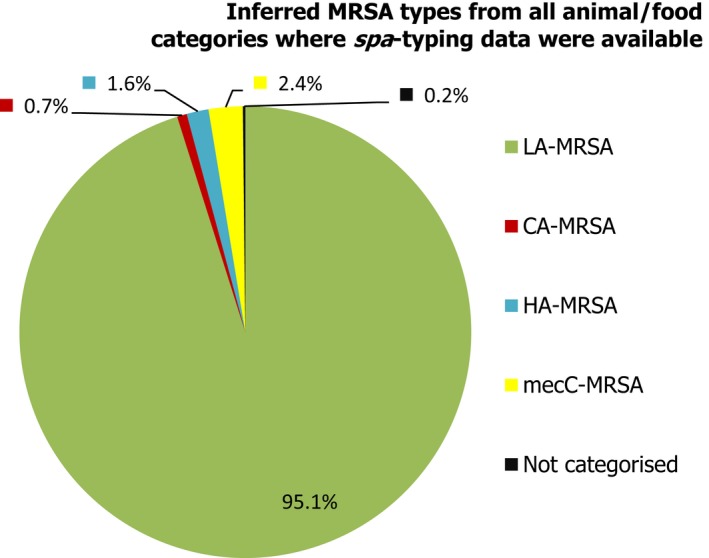
Percentage of MRSA types reported in 2017, inferred from spa‐typing data (574 MRSA isolates were spa‐typed) – from meat, food‐producing animals and following clinical investigations (goats, sheep, horses and companion animals)
The lineages and occurrence of the MRSA isolates which were detected can be summarised as follows (Figure 13): (1) LA‐MRSA was reported in Gallus gallus (broiler and laying hen flocks), fattening pigs at slaughter, calves at slaughter, pig meat, and following clinical investigations in dogs and horses; (2) CA‐MRSA was reported in a calf at slaughter, and following clinical investigations of a low number of companion animals; (3) HA‐MRSA was reported in a batch of fattening pigs at slaughter, pig meat, and following clinical investigations in a low number of animals; (4) mecC‐MRSA was recorded in two pig herds, and following clinical investigations of 2 sheep and 10 goats; (5) spa‐type t091 (from a pig herd) was not categorised as either LA‐MRSA or HA‐MRSA.
Figure 13.
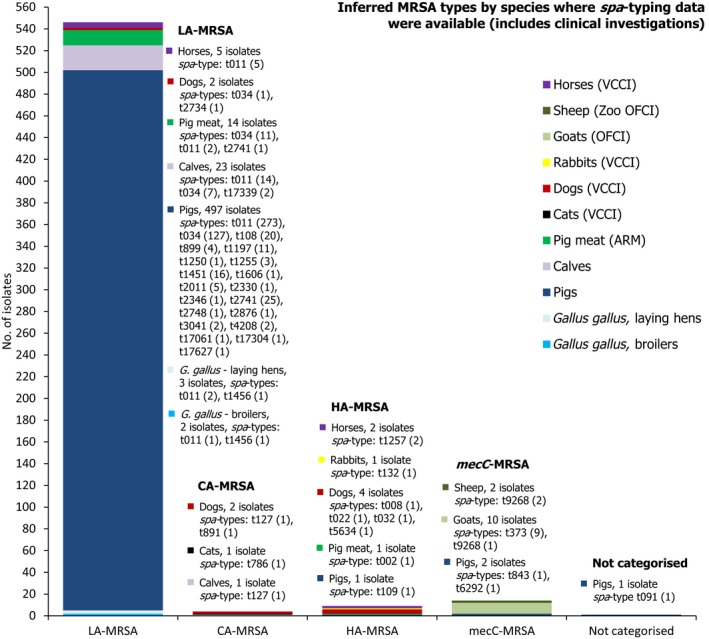
Overview of MRSA types by species reported in 2017, including healthy animals and clinical investigations
ST/CC and MRSA categories have mostly been inferred from spa‐typing data; MLST was only carried out on a few isolates. spa‐type t091 was not categorised as either HA‐MRSA or LA‐MRSA. In total, 574 MRSA isolates were spa‐typed VCCI: At‐veterinary‐clinic clinical investigation; OFCI: On‐farm clinical investigations; ARM: At‐retail monitoring.
LA‐MRSA is evidently widespread geographically and present in a variety of host species. The findings have underlined the requirement for continued monitoring and appropriate molecular characterisation of MRSA isolates. The isolation of novel spa‐types highlights that the situation is constantly evolving and, although the likelihood is that these types are associated with CC398, their detection underlines the limitations of spa‐typing as a single method of definitively assigning isolates to particular lineages, especially where MLST has not previously been undertaken. The presence or absence of certain virulence or other factors which tend to be associated with certain MRSA lineages is also assuming great importance when assessing the significance of MRSA isolates. However, the presence/absence of certain factors may not always be indicative, reflected by genotypes t786‐CC88 and t127‐CC1 which are predominantly community‐associated lineages yet lack PVL. The movement of live animals, as well as human travel, are important contributing factors to the spread of MRSA within and between countries, and therefore, the occurrence data pertained in this report may reflect such circumstances. Monitoring of MRSA is currently voluntary and although it provides a considerable amount of useful information, the picture obtained is incomplete.
Legal basis
According to Directive 2003/99/EC on the monitoring of zoonoses and zoonotic agents, Member States (MSs) are obliged to monitor and report antimicrobial resistance (AMR) in Salmonella and Campylobacter isolates obtained from healthy food‐producing animals and from food. Commission Implementing Decision 2013/652/EU of 12 November 20132 sets up priorities for the monitoring of AMR from a public health perspective, drafts a list of combinations of bacterial species, food‐producing animal populations and foodstuffs and lays down detailed requirements on the harmonised monitoring and reporting of AMR in food‐producing animals and food.
The data collection on human diseases from MSs is conducted in accordance with Decision 1082/2013/EU3 on serious cross‐border threats to health. The case definitions that were to be followed when reporting 2017 data on infectious diseases, including AMR, to the European Centre for Disease Prevention and Control (ECDC) are described in Decision 2012/506/EU.4 ECDC has provided data on zoonotic infections in humans, as well as their analyses, for the EU Summary Reports since 2005. Since 2007, data on human cases have been reported from The European Surveillance System (TESSy), maintained by ECDC.
1.
1.1.
About the European Food Safety Authority
The European Food Safety Authority (EFSA), located in Parma, Italy, and established and funded by the EU as an independent agency in 2002, provides objective scientific advice, in close collaboration with national authorities and in open consultation with its stakeholders, with a direct or indirect impact on food and feed safety, including animal health and welfare and plant protection. EFSA is also consulted on nutrition in relation to EU legislation. EFSA's risk assessments provide risk managers (the European Commission, the European Parliament and the Council) with a sound scientific basis for defining policy‐driven legislative or regulatory measures required to ensure a high level of consumer protection regarding food and feed safety. EFSA communicates to the public in an open and transparent way on all matters within its remit. Collection and analysis of scientific data, identification of emerging risks and scientific support to the European Commission, particularly during a food crisis, are also part of EFSA's mandate, as laid down in founding Regulation (EC) No. 178/20025 of 28 January 2002.
About the European Centre for Disease Prevention and Control
The European Centre for Disease Prevention and Control (ECDC), an EU agency based in Stockholm, Sweden, was set up in 2005. The objective of ECDC is to strengthen Europe's defences against infectious diseases. According to Article 3 of founding Regulation (EC) No. 851/20046 of 21 April 2004, ECDC's mission is to identify, assess and communicate current and emerging threats to human health posed by infectious diseases. To achieve this goal, ECDC works in partnership with national public health bodies across Europe to strengthen and develop EU‐wide disease surveillance and early warning systems. By working with experts throughout Europe, ECDC pools Europe's knowledge in health to develop authoritative scientific opinions about the risks posed by current and emerging infectious diseases.
Terms of Reference
The EU system for the monitoring and collection of information on zoonoses is based on the Zoonoses Directive 2003/99/EC, which obliges EU MSs to collect relevant and, where applicable, comparable data on zoonoses, zoonotic agents, AMR and food‐borne outbreaks. In addition, MSs are required to assess trends and sources of these agents, as well as outbreaks in their territory, submitting an annual report each year by the end of May to the European Commission covering the data collected. EFSA is assigned the tasks of examining these data and publishing the EU annual Summary Reports. In accordance with Article 9 of the Zoonoses Directive 2003/99/EC, EFSA shall examine the submitted national reports of the EU MSs and publish by the end of November a summary report on the trends and sources of zoonoses, zoonotic agents and AMR in the EU.
1. Introduction
The antimicrobial agents used in food‐producing animals in Europe are frequently the same, or belong to the same classes, as those used in human medicine. AMR is the main undesirable side effect of antimicrobial use in both humans and animals, and results from the continuous positive selection of resistant bacterial clones, whether these are pathogenic, commensal or even environmental bacteria. This will change the population structure of microbial communities, leading to accelerated evolutionary trends with unpredictable consequences for human and animal health. Both the route of administration and the administered quantities of antimicrobials may differ between humans and food‐producing animals; moreover, there are important variations between and within food‐producing animal populations, as well as between countries.
Antimicrobial resistance
Antimicrobial resistance is the ability of microorganisms, such as bacteria, to become increasingly resistant to an antimicrobial to which they were previously susceptible. AMR is a consequence of natural selection and genetic mutation. Such mutation is then passed on conferring resistance. This natural selection process is exacerbated by human factors such as inappropriate use of antimicrobials in human and veterinary medicine, poor hygiene conditions and practices in healthcare settings or in the food chain facilitating the transmission of resistant microorganisms. Over time, this makes antimicrobials less effective and ultimately useless.
Bacterial resistance to antimicrobials occurring in food‐producing animals can spread to people not only via food‐borne routes, but also by routes such as water or other environmental contamination, as well as through direct animal contact. Campylobacter, Salmonella and some strains of Escherichia coli are examples of zoonotic bacteria that can infect people by the food‐borne route. Infections with bacteria that are resistant to antimicrobials may result in treatment failures or necessitate the use of second‐line antimicrobials for therapy. The commensal bacterial flora can also form a reservoir of resistance genes, which may be transferred between bacterial species, including organisms capable of causing disease in both humans and animals (EFSA, 2008).
The monitoring of AMR in zoonotic and commensal bacteria in food‐producing animals and their food products is a prerequisite for understanding the development and diffusion of resistance, providing relevant risk assessment data, and evaluating targeted interventions. Resistance monitoring entails specific and continuous data collection, analysis and reporting and enables the following of temporal trends in the occurrence and distribution of resistance to antimicrobials. Resistance monitoring should also allow for the identification of emerging or specific patterns of resistance.
1.1. Monitoring and reporting of antimicrobial resistance at the EU level
Based on Article 33 in Regulation (EC) 178/2002, EFSA is responsible for examining data on AMR collected from the MSs in accordance with Directive 2003/99/EC and for preparing the EU Summary Report from the results. This EU Summary Report 2017 includes data related to the occurrence of AMR both in isolates from animals and foodstuffs and in isolates from human cases. The report is a joint collaboration between the EFSA and the ECDC with the assistance of EFSA's contractor. MSs, other reporting countries, the European Commission and the relevant EU Reference Laboratory (EURL‐AR) were consulted, while preparing the report. The efforts made by MSs, the reporting non‐MSs and the European Commission in the reporting of data on AMR and in the preparation of this report are gratefully acknowledged.
1.2. Further harmonised monitoring of antimicrobial resistance
The main issues when comparing AMR data originating from different countries are the use of different laboratory methods and different interpretive criteria of resistance. These issues have been addressed by the development of ECDC's protocol for harmonised monitoring and reporting of resistance in humans and recent legislation on harmonised monitoring in food‐producing animals and the food produced.
1.2.1. Legislation on antimicrobial resistance monitoring in animals and food
Commission Decision 2013/652/EU of 12 November 20137 drafts a list of combinations of bacterial species, food‐producing animal populations and food products and sets up priorities for the monitoring of AMR from a public health perspective. Monitoring of AMR in E. coli became mandatory, as it is for Salmonella and Campylobacter jejuni in the major food‐producing animal populations – broilers, laying hens, fattening turkeys, fattening pigs, calves – and their derived meat. The specific monitoring of extended‐spectrum beta‐lactamase (ESBL)‐, AmpC‐ and carbapenemase‐producing Salmonella and indicator commensal E. coli is also included. The collection and reporting of data are to be performed at the isolate level, to enable more in‐depth analyses to be conducted, in particular on the occurrence of multidrug resistance (MDR). Representative sampling should be performed according to general legislation and to detailed technical specifications issued by EFSA. Monitoring of AMR in food‐producing animals should be performed at the level of domestically produced animal populations, corresponding to different production types with the aim of collecting data that could be combined with those on exposure to antimicrobials (JIACRA I and II). Provisions have been taken where possible to exploit samples that would be collected under other existing control programmes. Commission Implementing Decision 2013/652/EU entered into force in 2014, as did Commission Implementing Decision 2013/653/EU of 12 November 2013 on financial aid towards a coordinated control plan for AMR monitoring in zoonotic agents in MSs in 2014.
Microdilution methods for testing should be used and results should be interpreted by the application of European Committee on Antimicrobial Susceptibility Testing (EUCAST) epidemiological cut‐off (ECOFF) values8 for the interpretation of ‘microbiological’ resistance. The harmonised panel of antimicrobials used for Salmonella, Campylobacter, E. coli and Enterococcus spp. is broadened with the inclusion of substances that either are important for human health or can provide clearer insight into the resistance mechanisms involved. The concentration ranges to be used ensure that both the ECOFF and the CBPs are included so that comparability of results with human data is made possible. Within the animal and food monitoring programmes, the new legislation has specified those types of animals that should be monitored in particular years. Ensuring that all MSs test the same species in a given year has simplified the presentation and increased the comparability of the results, because each annual report will now focus primarily on the target species for a given year.
A particular feature of the revised monitoring protocol for Salmonella and E. coli is the use of a supplementary panel of antimicrobials for testing isolates that show resistance to third‐generation cephalosporins or carbapenems in the first panel. The reporting of isolate‐based data, which was introduced several years ago, has facilitated this change, which allows in‐depth phenotypic characterisation of certain mechanisms of resistance, for example, third‐generation cephalosporin resistance and carbapenem resistance can be further characterised. It seems likely that this principle can be further developed and refined in time.
External quality assurance is provided by the EURL‐AR, which distribute panels of well characterised organisms to all MSs for susceptibility testing. MSs must test and obtain the correct results in such tests to ensure proficiency. The EURL‐AR also provides a source of reference for MSs in cases in which there are issues or problems with the susceptibility test methodology.
1.2.2. Developments in the harmonised monitoring of antimicrobial resistance in humans
Together with its Food‐ and Waterborne Diseases and Zoonoses (FWD) network, ECDC developed an EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2014, 2016). This document is intended for the National Public Health Reference Laboratories to guide the susceptibility testing required for EU surveillance and reporting to ECDC. Consultation was also sought from EFSA, EUCAST and the EU Reference Laboratory for Antimicrobial Resistance (EURL‐AR) to facilitate comparison of data between countries and with results from the AMR monitoring performed in isolates from animals and from food products. The protocol has been effective from 2014 and supports the implementation of the Commission Action Plan on AMR. One of the recommendations is that, for the purpose of the joint report with EFSA, human data should also be interpreted based on ECOFFs. As this requires quantitative data, ECDC introduced reporting of quantitative antimicrobial susceptibility testing (AST) results in the 2013 data collection and encourages countries to use it. As the EU protocol is not a legal document but a recommendation and joint agreement, it is for each National Public Health Reference Laboratory to decide whether to adapt their practices to the protocol. In 2017, most laboratories had adopted the priority panel of antimicrobials suggested in the protocol with the exception of the last‐line antimicrobials which were tested by fewer laboratories. The protocol also proposes a testing algorithm for screening and confirmation of ESBL‐producing Salmonella spp., including detection of AmpC. This has been implemented by some laboratories while others use a modification of the algorithm or test suspected isolates directly with polymerase chain reaction (PCR) or whole genome sequencing (WGS). About half of the countries are not doing any further testing for ESBL and AmpC in Salmonella isolated from humans.
As most laboratories use disk diffusion for AST, ECDC collaborates with EUCAST to set up inhibition zone diameter ECOFFs for C. jejuni, C. coli and Salmonella spp., when missing (Matuschek et al., 2015). External quality assessment to support laboratories in implementing the recommended test methods and antimicrobials and obtaining high‐quality AST results is provided by the Statens Serum Institute in Denmark through a contract with ECDC.
1.3. The 2017 EU Summary Report on AMR
Most data reported to EFSA by MSs comprise data collected in accordance with Commission Implementing Decision 2013/652/EU. The antimicrobial susceptibility data reported to EFSA for 2017 for Campylobacter, Salmonella, indicator E. coli isolates from animals and food were analysed and all quantitative data were interpreted using ECOFFs. This report also includes results of phenotypic monitoring of resistance to third‐generation cephalosporins and/or carbapenems caused by ESBLs, AmpC beta‐lactamases or carbapenemases in Salmonella and indicator E. coli, as well as the investigation at the EU level of the occurrence of complete susceptibility and MDR in data reported at the isolate level. A list of the antimicrobials included in this evaluation of MDR can be found in Section 2, ‘Materials and methods’.
The report also includes data on resistance in Salmonella and Campylobacter isolates from human cases of salmonellosis and campylobacteriosis, respectively. These data were reported by MSs to TESSy either as quantitative or categorical/qualitative data. The quantitative data were interpreted using EUCAST ECOFFs, where available. The qualitative data had been interpreted using CBPs to guide medical treatment of the patient. The breakpoints for ‘clinical’ resistance are, in many cases, less sensitive than the ECOFF for a specific bacterium–drug combination resulting in higher levels of ‘microbiological’ resistance than ‘clinical’ resistance. By combining the categories of ‘clinically’ resistant and intermediate resistant into a non‐susceptible category, however, close correspondence with the ECOFF was achieved.
CBPs enable clinicians to choose the appropriate treatment based on information relevant to the individual patient. ECOFFs recognise that epidemiologists need to be aware of small changes in bacterial susceptibility, which may indicate emerging resistance and allow for appropriate control measures to be considered. ECOFFs, CBPs and related concepts on AMR/susceptibility are presented in detail within the text.
A new EU action plan against antimicrobial resistance
The European Commission adopted a new Action Plan to tackle AMR on 29 June 2017. The Action Plan is underpinned by a One Health approach that addresses resistance in both humans and animals. The key objectives of this new plan are built on three main pillars:
Pillar 1: Making the EU a best practice region: as the evaluation of the 2011 action plan highlighted, this will require better evidence, better coordination and surveillance, and better control measures: EU action will focus on key areas and help Member States in establishing, implementing and monitoring their own One Health action plans on AMR, which they agreed to develop at the 2015 World Health Assembly.
Pillar 2: Boosting research, development and innovation by closing current knowledge gaps, providing novel solutions and tools to prevent and treat infectious diseases, and improving diagnosis in order to control the spread of AMR.
Pillar 3: Intensifying EU effort worldwide to shape the global agenda on AMR and the related risks in an increasingly interconnected world.
In particular, under the first pillar, EU actions will focus on the areas with the highest added value for MSs, e.g. promoting the prudent use of antimicrobials, enhancing cross‐sectorial work, improving infection prevention and consolidating surveillance of AMR and antimicrobial consumption. Examples of support include providing evidence‐based data with the support of EFSA, European Medicines Agency (EMA) and ECDC, updating EU implementing legislation on monitoring and reporting AMR in zoonotic and commensal bacteria in farm animals and food, to take into account new scientific development and monitoring needs, enabling mutual learning, exchange of innovative ideas and consensus building, and co‐fund activities in MSs to tackle AMR.
The new plan includes more than 75 concrete actions with EU added value that the EU Commission will develop and strengthen as appropriate in the coming years. All these actions are important in themselves, but they are also interdependent and need to be implemented in parallel to achieve the best outcome.
2. Materials and methods
All tables on resistance data used to produce this 2017 EUSR and cross‐referenced in the text are available on the EFSA Knowledge Junction at: https://doi.org/10.5281/zenodo.2562858
2.1. Antimicrobial susceptibility data from humans available in 2017
2.1.1. Data reported to The European Surveillance System (TESSy)
Member States report results from AST of Salmonella spp. and Campylobacter spp. isolated from clinical cases to ECDC on an annual basis. Data can be submitted to ECDC and TESSy either as measured values (inhibition zone diameters or minimum inhibitory concentrations (MICs)) through the isolate‐based reporting in TESSy or as results interpreted with CBPs via the case‐based reporting of Salmonella and Campylobacter infections. The reporting of quantitative data via the isolate‐based reporting is the preferred route, as stipulated in the EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2016).
Salmonella spp.
For 2017, 24 MSs, plus Iceland and Norway provided data on AMR in human Salmonella isolates, which was one more country than for 2016. Seventeen countries reported measured values and nine reported results interpreted as susceptible, intermediate or resistant (SIR) according to the CBPs applied (Table 3).
Table 3.
Antimicrobials reported, methods used, type of data reported and interpretive criteria applied by MSs for human Salmonella AST data in 2017
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | acid | Ciprofloxacin/pefloxacin | Azithromycin | Colistin | Sulfonamides | Trimethoprim | Trimethoprim‐sulfa | Tetracyclines | Method used | Quantitative (Q) or categorical (SIR) | Interpretive criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | ● | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | DD | Q | Interpreted by ECDC. EUCAST ECOFFs for all except CLSI CBP for SUL | |||
| Belgium | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | ||
| Cyprus | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL/DLG | Q | Interpreted by ECDC, as for Austria, except for CTX and MEM where EUCAST CBP were used | ||||||
| Denmark | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Estonia | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL, DD | Q | Interpreted by ECDC, as for Austria | |||
| Finland | ● | ● | ● | ● | ● | ● | ●a | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||||
| France | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria EFSA criteria for AZM MIC | |
| Germany | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | SIR | German DIN standard CBP. Only R included for GEN & TET to align with ECOFF | |||||
| Greece | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||
| Hungary | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | DD | SIR | No update provided. Earlier EUCAST CBP except CLSI CBP for NAL, SUL and TET | |||||
| Iceland | ● | ● | ● | ●a | ● | DD | SIR | EUCAST CBP | ||||||||||
| Ireland | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Italy | ● | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | ● | ● | ● | DL/DD | Q | Interpreted by ECDC, as for Austria. |
| Latvia | ● | ● | ● | ● | ● | DD | SIR | No recent information on guideline used | ||||||||||
| Lithuania | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | DL/DD | SIR | EUCAST CBP | ||||
| Luxembourg | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||
| Malta | ●b | ● | ● | ● | ● | ● | DL | SIR | Biomerieux Vitek II system; follows EUCAST CBP | |||||||||
| Netherlands | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria. EFSA criteria for AZM MIC | |
| Norway | ● | ● | ● | ● | ● | ● | ●a | ● | ● | DD | Q | Interpreted by ECDC, as for Austria. | ||||||
| Poland | ● | ● | ● | ● | ● | ● | ● | ● | SIR | No information provided | ||||||||
| Portugal | ● | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Romania | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | |||
| Slovakia | ●b | ● | ● | ● | ● | ● | ● | ● | ● | DD/DL | SIR | No update provided. Earlier EUCAST CBP except CLSI CBP for NAL, SUL and TET | ||||||
| Slovenia | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | ● | DD/DLG | Q | Interpreted by ECDC, as for Austria, except for MEM where EUCAST CBP were used | ||||
| Spain | ● | ● | ● | ● | ● | ● | ● | ●a | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||||
| United Kingdom | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | ● | DD/DL/DLG | SIR | Clinical breakpoints used varies depending on clinical microbiology laboratory |
AST: antimicrobial susceptibility testing; CBP: clinical breakpoint; DD: disk diffusion; DL: dilution; DLG: dilution with gradient strip; Q: quantitative data; SIR: susceptible, intermediate or resistant (categorical data); ECDC: European Centre for Disease Prevention and Control; ECOFF: epidemiological cut‐off; CLSI: Clinical and Laboratory Standards Institute; EUCAST: European Committee on Antimicrobial Susceptibility Testing; AZM: azithromycin; CTX: cefotaxime; GEN: gentamicin; MEM: meropenem; NAL: nalidixic acid; SUL: sulfonamides; TET: tetracycline.
Pefloxacin used in disk diffusion.
Test results in part or fully from VITEK system which seemingly apply CLSI criteria and report all aminoglycoside results as resistant. Results therefore excluded.
Campylobacter spp.
For Campylobacter, 19 MSs, plus Iceland and Norway provided AMR data from human isolates for 2017, which was two more countries than for 2016. Thirteen countries reported measured values and eight reported results interpreted as SIR according to the CBPs applied (Table 4).
Table 4.
Antimicrobials reported, method used, type of data reported and interpretive criteria applied by MSs for human Campylobacter AST data in 2017
| Country | Gentamicin | Co‐amoxiclav | Ciprofloxacin | Erythromycin | Tetracyclines | Method used | Quantitative (Q) or categorical (SIR) | Interpretive criteria |
|---|---|---|---|---|---|---|---|---|
| Austria | ● | ● | ● | ● | DL | Q | Interpreted by ECDC. EUCAST ECOFF (CIP, ERY, GEN, TET), CA‐SFM CBP 2017 (AMC) | |
| Cyprus | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Denmark | ● | ● | ● | ● | DL | Q | Interpreted by ECDC, as for Austria | |
| Estonia | ● | ● | ● | ● | DD/DL | Q | Interpreted by ECDC, as for Austria | |
| Finland | ● | ● | ● | DD/DLG | Q | Interpreted by ECDC, as for Austria | ||
| France | ● | ● | ● | ● | ● | DD | SIR | EUCAST CBP (CIP, ERY, TET), CA‐SFM CBP (AMC, GEN) |
| Iceland | ● | ● | DD/DLG | SIR | EUCAST CBP | |||
| Ireland | ● | ● | ● | DD | SIR | EUCAST CBP | ||
| Italy | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | |
| Lithuania | ● | ● | ● | DD | SIR | EUCAST CBP | ||
| Luxembourg | ● | ● | ● | ● | DD/DLG | Q | Interpreted by ECDC, as for Austria | |
| Malta | ● | ● | ● | ● | ● | DLG/DD | Q | Interpreted by ECDC, as for Austria |
| Netherlands | ● | ● | ● | DD/DL | SIR | Survey in 12 clinical labs in NL in 2009 (Ned Tijdschr Med Microbiol 2009;17:nr1) | ||
| Norway | ● | ● | ● | ● | DLG | Q | Interpreted by ECDC, as for Austria | |
| Poland | ● | ● | ● | No information | SIR | No information provided | ||
| Portugal | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | |
| Romania | ● | ● | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria |
| Slovakia | ● | ● | ● | ● | ● | No update | SIR | In 2013, CLSI CB. No update since |
| Slovenia | ● | ● | ● | DD | Q | Interpreted by ECDC, as for Austria | ||
| Spain | ● | ● | ● | ● | ● | DLG | Q | Interpreted by ECDC, as for Austria |
| United Kingdom | ● | ● | ● | ● | ● | DD/DL/DLG | SIR | EUCAST CBP |
AST: antimicrobial susceptibility testing; CA‐SFM: French Society for Microbiology; CBP: clinical breakpoint; DD: disk diffusion; DL: dilution; DLG: dilution with gradient strip; ECDC: European Centre for Disease Prevention and Control; ECOFF: epidemiological cut‐off; EUCAST: European Committee on Antimicrobial Susceptibility Testing; Q: quantitative data; SIR: susceptible, intermediate or resistant (categorical data); AMC: amoxicillin/clavulanate; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; TET: tetracycline.
2.1.2. Harmonised testing
Most laboratories follow the ‘EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates’ (ECDC, 2016) on the antimicrobial panel to be tested. The antimicrobials tested, the method used (dilution, disk diffusion, gradient strip), the type of data provided and the interpretive criteria applied are presented in Table 3 for Salmonella and in Table 4 for Campylobacter. For Salmonella, eight MSs, plus Iceland and Norway used only disk diffusion methods (DDs) for their AST, eight MSs used dilution methods (DLs) and another seven MSs used a combination of the two, mostly disk diffusion and gradient strip, depending on the situation and the antimicrobial (Table 3). For Campylobacter, eight MSs used only DDs for their AST, three MSs and Norway used DLs and another seven MSs used a combination of the two, mostly disk diffusion and gradient strip (Table 4).
All data on measured MIC or zone mm values were results of AST at the national public health reference laboratories, with the exception of Italy for Salmonella where two regional laboratories also contributed, and Finland for Campylobacter where the quantitative data had been collected from regional laboratories.
Data interpreted with CBPs were normally from local or regional laboratories and reported together with the information on the clinical case. In these cases, AST had primarily been performed with the purpose of treatment of the case rather than AMR monitoring. For this reason, the number of tests per antimicrobial varied.
Salmonella test panel
In 2013, the national public health laboratories within the FWD network agreed on a panel of priority antimicrobials and optional antimicrobials to test for and report to ECDC (2014). Two antimicrobials – ceftazidime and meropenem – were new in the priority panel compared with earlier recommendations. For 2017, all but two MSs reported results on meropenem and all but three for ceftazidime. It was also agreed that three last‐line antimicrobials – azithromycin, colistin and tigecycline – should be included in the priority list when interpretive criteria were available for disk diffusion, in addition to dilution. EUCAST developed such ECOFFs for azithromycin and tigecycline in 2015 (Matuschek et al., 2015). For colistin, however, the methodology is complicated due to chemical properties of the substance. A joint EUCAST and Clinical and Laboratory Standards Institute (CLSI) subcommittee confirmed that broth microdilution is so far the only valid method for colistin susceptibility testing (CLSI & EUCAST, 2016). Disk diffusion does not work because of poor diffusion of the large colistin molecule in the agar and tested gradient strips also underestimate colistin MIC values, again most likely due to poor diffusion in the agar (Matuschek et al., 2017). The three last‐line antimicrobials were added to the priority list in June 2016 (ECDC, 2016); however only countries performing broth microdilution should report on colistin resistance. Eight MSs were reporting on azithromycin and tigecycline and six on colistin for 2017.
Due to the problems in detecting low‐level fluoroquinolone resistance in Salmonella spp. using disk diffusion, nalidixic acid was, for a long time, used as a marker for fluoroquinolone resistance. After the discovery that plasmid‐mediated fluoroquinolone resistance is often not detected using nalidixic acid, EUCAST studied alternative disks and concluded that pefloxacin was an excellent surrogate marker (except for isolates having the aac(6′)‐Ib‐cr gene as the only resistance determinant) (Skov et al., 2015). Since 2014, EUCAST has recommend this agent for screening of low‐level fluoroquinolone resistance in Salmonella with disk diffusion (EUCAST, 2014) and, since June 2016, this is also reflected in the EU protocol. In 2017, all countries reporting measured values for disk diffusion tested with pefloxacin instead of ciprofloxacin.
Half of the countries reported the combination drug co‐trimoxazole (trimethoprim–sulfamethoxazole) in addition to, or instead of, testing the substances separately, partly because this combination is used for clinical treatment and partly because no EUCAST interpretive criterion exists for sulfamethoxazole for Salmonella.
Campylobacter test panel
The antimicrobials included in the 2017 report followed the panel of antimicrobials from the EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates (ECDC, 2016). The priority panel for Campylobacter includes ciprofloxacin, erythromycin, tetracyclines and, since June 2016, gentamicin. Gentamicin is recommended for screening of invasive isolates and was added to the priority panel after a EUCAST ECOFF became available for disk diffusion for C. jejuni. Co‐amoxiclav (amoxicillin and clavulanic acid) was included from the list of optional antimicrobials. In 2017, all countries except Iceland tested the three antimicrobials ciprofloxacin, erythromycin and tetracycline, 12 also tested gentamicin and seven tested co‐amoxiclav.
2.1.3. Analyses of antimicrobial resistance data
Harmonised interpretation of data with animal and food data
Data reported as measured values were interpreted by ECDC based on the EUCAST epidemiological cut‐off (ECOFF) values, when available. For MIC data, the same criteria were applied as used by EFSA (Tables 5 and 6) while for zone diameter data, corresponding EUCAST disk diffusion ECOFF values were applied with a few exceptions (Tables 3 and 4). Regarding data reported as SIR values, the categories of ‘clinically’ intermediate and ‘clinically’ resistant were combined in a ‘non‐susceptible’ group. Alignment of the susceptible category with the ‘wild type’ category based on epidemiological cut‐off values (ECOFFs) and of the non‐susceptible category with the ECOFF‐based ‘non‐wild type’ category provides better comparability and more straightforward interpretation of the data for most antimicrobial agents included (Figure 15 in Section 3.1.1; Figure 47 in Section 4.1.1).
Table 5.
Panel of antimicrobial substances included in AMR monitoring, EUCAST ECOFFs and concentration ranges tested in Salmonella spp. and indicator commensal E. coli (first panel) as laid down in Commission Implementing Decision 2013/652/EU
| Antimicrobial | Salmonella EUCAST ECOFFa | E. coli EUCAST ECOFFa | Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Ampicillin | > 8 | > 8 | 1–64 (7) |
| Cefotaxime | > 0.5 | > 0.25 | 0.25–4 (5) |
| Ceftazidime | > 2 | > 0.5 | 0.5–8 (5) |
| Meropenem | > 0.125 | > 0.125 | 0.03–16 (10) |
| Nalidixic acid | > 16 | > 16 | 4–128 (6) |
| Ciprofloxacin | > 0.064 | > 0.064 | 0.015–8 (10) |
| Tetracycline | > 8 | > 8 | 2–64 (6) |
| Colistin | > 2b | > 2 | 1–16 (5) |
| Gentamicin | > 2 | > 2 | 0.5–32 (7) |
| Trimethoprim | > 2 | > 2 | 0.25–32 (8) |
| Sulfamethoxazole | NAc | > 64 | 8–1,024 (8) |
| Chloramphenicol | > 16 | > 16 | 8–128 (5) |
| Azithromycin | NAd | NAd | 2–64 (6) |
| Tigecycline | > 1b | > 1b | 0.25–8 (6) |
AMR: antimicrobial resistance; ECOFFs: epidemiological cut‐off values; EUCAST: European Committee on Antimicrobial Susceptibility Testing; NA: not available.
EUCAST epidemiological cut‐off values available in Decision 2013/652/EU was drafted (2013). ‘>’ than the ECOFF, criteria used for determining microbiological resistance.
EUCAST ECOFFs not available any more (1.12.18)
> 256 mg/L was used.
> 16 mg/L was used.
Table 6.
Panel of antimicrobial substances included in AMR monitoring, EUCAST ECOFFs and concentration ranges tested in C. jejuni and C. coli
| Antimicrobial | C. jejuni EUCAST ECOFFa | C. coli EUCAST ECOFFa | Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Erythromycin | > 4 | > 8 | 1–128 (8) |
| Ciprofloxacin | > 0.5 | > 0.5 | 0.12–16 (8) |
| Tetracycline | > 1 | > 2 | 0.5–64 (8) |
| Gentamicin | > 2 | > 2 | 0.12–16 (8) |
| Nalidixic acid | > 16 | > 16 | 1–64 (7) |
| Streptomycin b | > 4 | > 4 | 0.25–16 (7) |
AMR: antimicrobial resistance; EUCAST: European Committee on Antimicrobial Susceptibility Testing; ECOFFs: epidemiological cut‐off values; NA: not available.
EUCAST epidemiological cut‐off values. ‘>’ than the ECOFF, criteria used for determining microbiological resistance.
On a voluntary basis.
Figure 15.
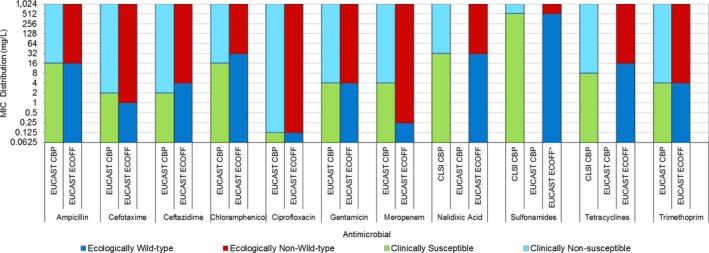
Comparison of CBPs for non‐susceptibility (intermediate and resistant categories combined) and ECOFFs used to interpret MIC data reported for Salmonella spp. from humans, animals or food
Figure 47.
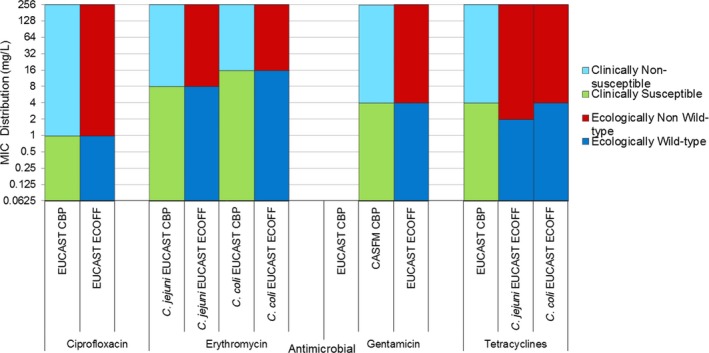
Comparison of clinical breakpoints (CBPs) and epidemiological cut‐off values (ECOFFs) used to interpret MIC data reported for Campylobacter spp. from humans, animals or food
For Salmonella, this procedure results in good concordance (±1 dilution) across categories with the exception of meropenem where the MIC for non‐susceptible category is substantially higher (+4 dilutions) than the ECOFF. For Campylobacter, there was total concordance across interpretive categories with this procedure, except for the EUCAST CBP for C. jejuni for tetracyclines, which is one dilution step higher than the EUCAST ECOFF.
Separation by species or serovar
As resistance levels differ substantially between Salmonella serovars, results are presented separately for selected serovars of importance, particularly those found in pigs and cattle due to the focus of the 2017 report. The serovars presented in the report are S. Typhimurium, monophasic S. Typhimurium and S. Derby, while data on additional serovars among the 10 most common in human cases in 2017 are available in appendices (S. Enteritidis, S. Infantis, S. Newport, S. Stanley, S. Derby, S. Kentucky, S. Virchow and S. Agona). Also, for Campylobacter, resistance levels differ quite substantially between the two most important Campylobacter species, C. jejuni and C. coli, and data are therefore presented by species. The proportion of resistant isolates is only shown when at least 10 isolates were reported from a MS.
Exclusion of travel‐associated cases
To better assess the impact from food consumed within each reporting country on the AMR levels found in human isolates, cases known to have travelled outside of the country during the incubation period was excluded from the analysis. However, as several countries had not provided any information on travel status of their cases, cases with unknown travel status were also included in addition to domestically acquired cases. The proportions of travel‐associated, domestic and unknown cases among the tested isolates are presented in Tables SALMTRAVHUM and CAMPTRAVHUM.9
Temporal trends in resistance
Trends in the proportion of resistant isolates to selected antimicrobials over the period 2013–2017 were analysed by country. The statistical significance was assessed with logistic regression in Stata 14.2 and a p‐value of < 0.05 was considered to be significant. Only countries testing at least 10 isolates per year and for at least 3 years in the 5‐year period were included. For Salmonella, the antimicrobials analysed were ciprofloxacin/pefloxacin/nalidixic acid, cefotaxime, ampicillin and tetracycline. For Campylobacter, the corresponding antimicrobials were ciprofloxacin, erythromycin and tetracycline.
Maps for critically important antimicrobials
For Salmonella, the proportions of human isolates resistant to the CIA for treatment of severe Salmonella infections (Urdahl et al., 2017), fluoroquinolones (ciprofloxacin/pefloxacin) and cephalosporins (cefotaxime), were presented in maps to provide an overview of the geographical distribution of resistance in the EU/EEA. Maps were provided for resistance to each of the two substances for the respective serovars. Combined resistance (resistance to both) of ciprofloxacin/pefloxacin and cefotaxime was determined both as ‘microbiological’ resistance (using EUCAST ECOFFs) and ‘clinical’ resistance (using EUCAST CBPs). Combined ‘microbiological resistance’ was presented in a map for Salmonella spp.
For Campylobacter, the proportions of human isolates resistant to the CIA for treatment of severe Campylobacter infections (Urdahl et al., 2017), fluoroquinolones (ciprofloxacin) and macrolides (erythromycin), were presented in maps to provide an overview of the geographical distribution of resistance in the EU/EEA. Combined resistance (resistance to both) of ciprofloxacin and erythromycin was determined both as ‘microbiological’ resistance (using EUCAST ECOFFs) and ‘clinical’ resistance (using EUCAST CBPs).
Analysis of multidrug resistance
Multidrug resistance of human Salmonella spp. to nine antimicrobial classes was analysed, these classes being harmonised between ECDC and EFSA for better comparison between the two sectors. MDR of an isolate was defined as resistance or non‐susceptibility to at least three different antimicrobial classes (Magiorakos et al., 2012). The antimicrobials included were ampicillin, cefotaxime/ceftazidime, chloramphenicol, ciprofloxacin/pefloxacin/nalidixic acid, gentamicin, meropenem, sulfonamides/sulfamethoxazole, tetracyclines and trimethoprim/trimethoprim‐sulfamethoxazole (co‐trimoxazole). Resistance to nalidixic acid, ciprofloxacin and pefloxacin were addressed together, as they belong to the same class of antimicrobials: quinolones. Isolates that were resistant or non‐susceptible to any of these antimicrobials were classified as resistant or non‐susceptible to the class of quinolones. The same method was applied to the two third‐generation cephalosporins cefotaxime and ceftazidime. Trimethoprim and co‐trimoxazole were also addressed together, as a few countries had only tested for susceptibility to the combination. This approach was considered appropriate because among the countries that provided data on both trimethoprim alone and the combination co‐trimoxazole, the proportion of resistant or non‐susceptibles corresponded closely between the two.
MDR of a C. jejuni or C. coli isolate was defined as resistance or non‐susceptibility to at least three different antimicrobial classes (Magiorakos et al., 2012). The antimicrobials in the MDR analysis were harmonised between EFSA and ECDC and included ciprofloxacin, erythromycin, gentamicin and tetracyclines.
Analysis of ESBL, AmpC and carbapenemase production in Salmonella
Of the 19 MSs plus Norway which had reported microbiological resistance to third‐generation cephalosporins, 12 MSs plus Norway could provide results on further phenotypic and/or genotypic testing for ESBL and/or AmpC. Four MSs did not test for ESBL/AmpC and three MSs did not respond to the request.
2.2. Antimicrobial susceptibility data from animals and food in 2017
2.2.1. Data reported under Directive 2003/99/EC and Commission Implementing Decision 2013/652/EU
For 2017, MSs reported mandatory data collected from AMR routine monitoring in Salmonella spp. and indicator commensal E. coli, as well as from the E. coli specific ESBL‐/AmpC‐/carbapenemase‐producing monitoring, according to Commission Implementing Decision 2013/652/EU.
For the routine monitoring of AMR in Salmonella spp., 22 MSs and 1 non‐MS reported data on meat from pigs (carcases) and 7 MSs on meat from bovine animals (carcases), 7 MSs reported data on fattening pigs, 6 MSs and 1 non‐MS in calves under 1 year of age. For the routine monitoring of AMR in indicator commensal E. coli, 28 MSs and 3 non‐MSs reported data on fattening pigs and 10 MSs and 2 non‐MS reported on calves under 1 year.
For the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli, all MSs and 3 non‐MSs, reported data on fresh meat from pigs and bovines gathered at retail, and fattening pigs, whereas 10 MSs and 1 non‐MS reported data on calves under one year.
Isolates were sampled through harmonised national schema. Microbroth dilution testing methods were used for susceptibility testing, and quantitative10 isolate‐based data were reported to EFSA and considered for this report. Resistance was interpreted using EUCAST ECOFF values (see following text box for further information). The antimicrobials incorporated in this summary analysis were selected based on their public health relevance and as representatives of different antimicrobial classes.
Data on C. coli in fattening pigs and data on meticillin‐resistant Staphylococcus aureus (MRSA) and on specific monitoring of carbapenemase‐producing microorganisms were reported on a voluntary basis.
Harmonised representative sampling and monitoring
Representative sampling should be performed according to general provisions of the legislation and to detailed technical specifications issued by EFSA (2014).
Salmonella spp.
In 2017, representative Salmonella isolates for monitoring AMR were collected by MSs from carcases of fattening pigs sampled for testing and verification of compliance, in accordance with point 2.1.4 of Chapter 2 of Annex I to Regulation (EC) No 2073/2005; as well as carcases of bovines under 1 year of age where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year sampled for testing and verification of compliance, in accordance with point 2.1.3 of Chapter 2 of Annex I to Regulation (EC) No 2073/2005. MSs sampled carcases of fattening pigs/carcases of bovines under 1 year of age of healthy slaughter at the slaughterhouse. A two‐stage stratified sampling design, with slaughterhouses as primary sampling units and carcases as secondary units, with proportional allocation of the number of samples to the annual throughput of the slaughterhouse, was applied in the reporting countries.
Not more than one isolate per Salmonella serovar from the same epidemiological unit (herd/holding) per year should be included in the AMR monitoring. In most MSs, the isolates tested for antimicrobial susceptibility constituted a representative subsample of the total Salmonella isolates available at the National Reference Laboratory (NRL) and/or other laboratories involved, obtained in a way that ensured geographical representativeness and even distribution over the year. Conversely, for low prevalence, all the Salmonella isolates available should be tested for susceptibility.
Campylobacter and indicator commensal E. coli11
Routine monitoring of indicator E. coli
MSs collected indicator E. coli isolates as part of their national monitoring programme of AMR according to the provisions of the Decision 2013/652/EU, based on random sampling of caecal samples gathered at slaughter from fattening pigs and calves under 1 year of age where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA's recommendations (EFSA, 2013, 2014). MSs shall test 170 isolates for AST for each of animal population listed above. However, in MSs with a production of less than 100,000 tonnes of pig meat slaughtered per year, they shall test 85 isolates instead of 170 isolates. The sample collection was approximately evenly distributed over the year 2017.
Specific monitoring of E. coli ESBL/AmpC/carbapenemase producers
Caecal samples gathered at slaughter from fattening pigs and bovines under 1 year of age where the production of meat of those bovines in the MSs is more than 10,000 tonnes slaughtered per year and samples of fresh pig meat and bovine meat gathered at retail were collected. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd) was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA's recommendations (EFSA, 2013, 2014). MSs shall analyse 300 samples of each of the animal population and food category, listed in above However, in MSs with a production of less than 100,000 tonnes of pig meat slaughtered per year and less than 50,000 tonnes bovine meat slaughtered per year, the MS shall analyse 150 samples instead of 300 samples for each corresponding specific combination. The sample collection was approximately evenly distributed over the year 2017.
Epidemiological cut‐off values (ECOFFs) and clinical breakpoints (CBPs)
A microorganism is defined as ‘clinically’ resistant when the degree of resistance shown is associated with a high likelihood of therapeutic failure. The microorganism is categorised as resistant by applying the appropriate CBP in a defined phenotypic test system, and this breakpoint may alter with legitimate changes in circumstances (e.g. alterations in dosing regimen, drug formulation, patient factors). A microorganism is defined as wild type for a bacterial species when no acquired or mutational resistance mechanisms are present to the antimicrobial in question. A microorganism is categorised as wild type for a given bacterial species presenting a lower MIC to the antimicrobial in question than the appropriate ECOFF in a defined phenotypic test system. This cut‐off value will not be altered by changing circumstances (such as alterations in frequency of antimicrobial administration). Wild‐type microorganisms may or may not respond clinically to antimicrobial treatment. A microorganism is defined as non‐wild type for a given bacterial species by the presence of an acquired or mutational resistance mechanism to the antimicrobial in question. A microorganism is categorised as non‐wild type for a given bacterial species by applying the appropriate ECOFF value in a defined phenotypic test system; non‐wild‐type organisms are considered to show ‘microbiological’ resistance (as opposed to ‘clinical’ resistance). CBPs and ECOFFs may be the same, although it is often the case that the ECOFF is lower than the CBP. EUCAST has defined CBPs and ECOFFs.
Clinical breakpoints (clinical resistance)
The clinician, or veterinarian, choosing an antimicrobial agent to treat humans or animals with a bacterial infection requires information that the antimicrobial selected is effective against the bacterial pathogen. Such information will be used, together with clinical details such as the site of infection, ability of the antimicrobial to reach the site of infection, formulations available and dosage regimes, when determining an appropriate therapeutic course of action. The in vitro susceptibility of the bacterial pathogen can be determined and CBPs used to ascertain whether the organism is likely to respond to treatment. CBPs will take into account the distribution of the drug in the tissues of the body following administration and assume that a clinical response will be obtained if the drug is given as recommended and there are no other adverse factors which affect the outcome. Conversely, if the CBP indicates resistance, then it is likely that treatment will be unsuccessful. Frequency of dosing is one factor that can affect the antimicrobial concentration achieved at the site of infection. Therefore, different dosing regimens can lead to the development of different CBPs, as occurs in some countries for certain antimicrobials where different therapeutic regimes are in place. Although the rationale for the selection of different CBPs may be clear, their use makes the interpretation of results from different countries in reports of this type problematic, as the results are not directly comparable between those different countries.
Epidemiological cut‐off values (microbiological resistance)
For a given bacterial species, the pattern of the MIC distribution (i.e. the frequency of occurrence of each given MIC plotted against the MIC value) can enable the separation of the wild‐type population of microorganisms from those populations that show a degree of acquired resistance. The wild‐type susceptible population is assumed to have no acquired or mutational resistance and commonly shows a normal distribution. When bacteria acquire resistance by a clearly defined and efficacious mechanism, such as the acquisition of a plasmid bearing a gene which produces an enzyme capable of destroying the antimicrobial, then the MIC commonly shows two major subpopulations, one a fully susceptible normal distribution of isolates and the other a fully resistant population which has acquired the resistance mechanism. Resistance may be achieved by a series of small steps, such as changes in the permeability of the bacterial cell wall to the antimicrobial or other mechanisms which confer a degree of resistance. In this case, there may be populations of organisms which occur lying between the fully susceptible population and more resistant populations. The ECOFF value indicates the MIC or zone diameter above which the pathogen has some detectable reduction in susceptibility. ECOFFs are derived by testing an adequate number of isolates to ensure that the wild‐type population can be confidently identified for a given antimicrobial. The CBP, which is set to determine the therapeutic effectiveness of the antimicrobial, may fail to detect emergent resistance. Conversely, the ECOFF detects any deviation in susceptibility from the wild‐type population, although it may not be appropriate for determining the likelihood of success or failure for clinical treatment.
Campylobacter coli
Caecal samples gathered at slaughter from fattening pigs were collected on a voluntary basis. Only one representative caecal sample (single or pooled) per epidemiological unit (batch of carcases deriving from the same herd), was gathered to account for clustering. Isolates were recovered from caecal contents samples (single or pooled), in accordance with EFSA's recommendations (EFSA, 2013). The sample collection was approximately evenly distributed over the year 2017.
MRSA
Isolates may have been collected by different monitoring approaches, either by active monitoring of animals and foods or, in some cases, by passive monitoring based on diagnostic submission of samples from clinical cases of disease in animals, or from foods sampled as part of investigatory work.
Harmonised antimicrobial susceptibility testing
Routine monitoring antimicrobial susceptibility
MSs tested antimicrobials and interpreted the results using the epidemiological cut‐off values and concentration ranges shown in Tables 5 and 6 to determine the susceptibility of Salmonella spp., C. coli, and indicator commensal E. coli. All E. coli isolates, randomly selected isolates of Salmonella spp. and E. coli that, after testing with the first panel of antimicrobials in accordance with Commission Implementing Decision 2013/652/EU were found to be resistant to cefotaxime, ceftazidime or meropenem, were further tested with a second panel of antimicrobial substances as shown in 0. This panel notably includes cefoxitin, cefepime and clavulanate in combination with cefotaxime and ceftazidime for the detection of presumptive ESBL and AmpC producers, as well as imipenem, meropenem and ertapenem to phenotypically identify presumptive carbapenemase producers.
Specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli
For the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli, the isolation method started with a non‐selective pre‐enrichment step, followed by inoculation on MacConkey agar containing a third‐generation cephalosporin in a selective concentration (cefotaxime 1 mg/L), in accordance with the most recent version of the detailed protocol for standardisation of the EURL‐AR.12 Using this protocol, also carbapenemase‐producing isolates can also be recovered.
If available, one presumptive ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolate obtained from each positive caecal sample and meat sample was tested for its antimicrobial susceptibility to the first panel of antimicrobials (Table 5) to confirm the microbiological resistance to cefotaxime (expected as the antimicrobial is present in the isolation medium at a concentration higher than the ECOFF), and identify possible resistance to ceftazidime and/or ceftazidime and/or meropenem. In a second step, the isolate should be tested using the second panel of antimicrobials (0) to infer the presumptive ESBL‐/AmpC‐/carbapenemase‐producing phenotype according to the β‐lactam resistance phenotype obtained (Figure 14).
Figure 14.
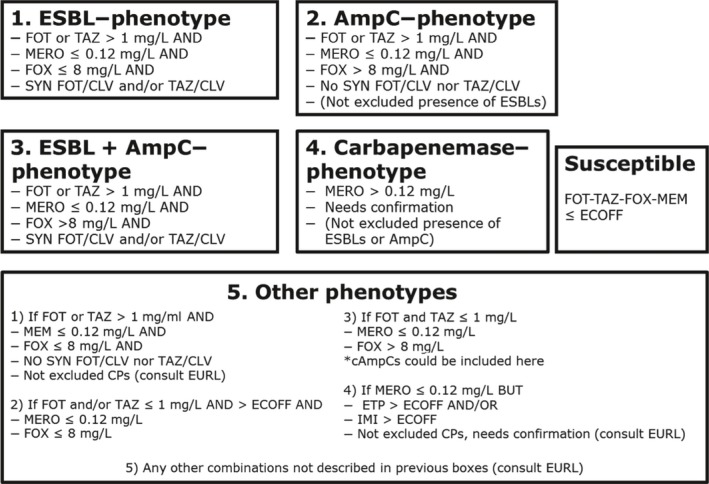
Phenotypes inferred based on the resistance to the β‐lactams included in Panel 2
- Presumptive ESBL producers include isolates exhibiting phenotype 1 or 3.
- Presumptive AmpC producers include isolates exhibiting phenotype 2 or 3.
Specific monitoring of carbapenemase‐producing microorganisms
This monitoring programme was performed and reported on a voluntary basis. For the specific monitoring of carbapenemase‐producing microorganisms, isolation required the use of non‐selective pre‐enrichment and subsequent selective plating on carbapenem‐containing media, in accordance with the most recent version of the detailed protocol of the EURL‐AR. The microbial species was identified using an appropriate method.
If available, one presumptive carbapenemase‐producing isolate (primarily E. coli, but also Salmonella) obtained from each positive caecal sample and meat sample should be tested for its antimicrobial susceptibility to the first panel of antimicrobials (Table 5) to confirm the microbiological resistance to meropenem, and identify possible resistance to cefotaxime and/or ceftazidime. In a second step, the isolate should be tested using the second panel of antimicrobials (to infer the presumptive carbapenemase‐producer phenotype according to the β‐lactam resistance phenotype obtained (Figure 14).
The EUCAST epidemiological cut‐off values applied for the AST (Tables 3, 4, 5) are the ones available during the drafting of the Decision 2013/652/EU (2013). For some antimicrobials, these values have been updated by EUCAST (http://www.eucast.org, last accessed 1.12.18). Currently, for Salmonella, there is no ECOFF available anymore for colistin and tigecycline. For E. coli, there is no tigecycline nor ertapenem ECOFFs available anymore. To allow comparison with the data collected in previous years, the ECOFFs laid down in the legislation are considered.
2.2.2. Data validation
Validation against business rules
The reported data were first checked for usability against a series of ‘business rules’, which were automatically applied in the EFSA data collection system once a file was sent. This automatic data validation process refers to the first validation of incoming data. Quality checks are related to a specific business only. The positive result of the automatic validation process places the file in a valid state and makes it available for further steps of validation performed by EFSA.
Scientific data validation
The scientific validation of the data collected by the MSs/non‐MSs and submitted to EFSA consisted on the revision of data and comparison between data reported for the same antimicrobials when tested by different panels. Special attention was given to carbapenems, colistin, azithromycin, tigecycline and to possible discrepancies between results for antimicrobials present in both panels (i.e. cefotaxime, ceftazidime, meropenem). MSs were contacted by EFSA asking for clarifications. If considered needed, MSs were asked to confirm the MIC results and the species identification of the reported isolates.
Reference testing
To ensure the quality of data submitted, a reference testing exercise was run by the EURL‐AR in close collaboration with the MSs. The exercise consisted in retesting the AST of the isolates received using both Panel 1 and Panel 2 of antimicrobials, as well as WGS analyses of the isolates (WGS analyses still ongoing by the time of drafting the present report). Based on the data submitted to EFSA, a selection of approximately 400 isolates was made. The selection of these isolates was based on different criteria:
The EURL‐AR had reported technical issues when testing azithromycin, tigecycline and colistin during the EURL workshop hold in Lyngby (Denmark) in 2016 (http://www.eurl-ar.eu). Resistant isolates from countries with outstanding prevalence for these antimicrobials were asked to provide selected isolates to the EURL‐AR. Most of the E. coli isolates chosen were selected among the ones reported mainly for the specific ESBL/AmpC/carbapenemase monitoring.
There was a discrepancy between MIC values reported for the antimicrobials present in both panels (impacting the categorisation of the isolate as resistant or susceptible).
If according to the criteria applied (1.2.5), the presence of carbapenemase producers was suspected.
Isolates representing the categorisations presumptive ESBLs, AmpC and ESBL + AmpC producers.
Isolates with odd phenotypes.
Multidrug‐resistant S. Rissen
Isolates microbiologically resistant to ciprofloxacin and susceptible to nalidixic acid (the presence of plasmid‐mediated quinolone resistance (PMQR) encoding genes, suspected) were included in the selection.
The MSs/non‐MSs sent the selected isolates to the EURL‐AR, where they were retested. EFSA, EURL‐AR and MSs liaised together to address possible discrepancies found.
2.2.3. Analyses of antimicrobial resistance data
Data are reported in separate sections dedicated to each microorganism. Clinical investigation data were not accounted for in this report.
Overview tables of the resistance data reported
Data generated from the AST and reported as quantitative at the isolate level by MSs have been described in the overview tables published on the EFSA website.
Minimum inhibitory concentration distributions
For each combination of microorganism, antimicrobial and food category/animal population were tested, MIC distributions were tabulated in frequency tables, giving the number of isolates tested that have a given MIC at each test dilution (mg/L) of the antimicrobial. Isolate‐based dilution results allowed MIC distributions reported:
for Salmonella for ampicillin, azithromycin, cefepime, cefotaxime, cefotaxime and clavulanic acid, ceftazidime, ceftazidime and clavulanic acid, cefoxitin, chloramphenicol, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, meropenem, nalidixic acid, sulfamethoxazole, temocillin, tetracycline, tigecycline and trimethoprim;
for Campylobacter for ciprofloxacin, erythromycin, gentamicin, nalidixic acid, streptomycin and tetracycline;
for indicator E. coli for ampicillin, azithromycin, cefepime, cefotaxime, cefotaxime and clavulanic acid, ceftazidime, ceftazidime and clavulanic acid, cefoxitin, chloramphenicol, ciprofloxacin, colistin, ertapenem, gentamicin, imipenem, meropenem, nalidixic acid, sulfamethoxazole, temocillin, tetracycline, tigecycline and trimethoprim;
for MRSA for cefoxitin, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, fusidic acid, gentamicin, kanamycin, linezolid, mupirocin, penicillin, quinupristin/dalfopristin, rifampicin, streptomycin, sulfamethoxazole, tetracycline, tiamulin, trimethoprim and vancomycin.
Epidemiological cut‐off values and the occurrence of resistance
ECOFFs, as listed in Decision 2013/652/EC, have been used in this report to interpret the isolate‐based reported MIC data and determine non‐wild‐type organisms also termed ‘microbiologically’ resistant organisms (i.e. displaying a decreased susceptibility), and to ensure that results from different MSs are comparable. From this point onwards in this report, ‘microbiologically’ antimicrobial‐resistant organisms are referred to as ‘resistant’ for brevity. This report also incorporates re‐evaluation of the historical data accounting for the revised EU legislation, which included the revised ECOFFs.
The occurrence of resistance13 to a number of antimicrobials was determined for Salmonella, Campylobacter, and indicator commensal E. coli isolates and are tabulated at the production‐type level in this report. The occurrence of resistance (i.e. resistance levels) in reporting MS groups was calculated as totals (the total number of resistant isolates out of the total number of tested isolates across reporting MSs) and not the weighted means.
Resistance in Salmonella serovars of public health importance
In this report, AMR in tested Salmonella isolates were aggregated to give a value for Salmonella spp. for each country and food/animal category. In addition, the most prevalent Salmonella serovars were also reported separately for particular food/animal category. Additional tables have been included in this report to describe the occurrence of AMR among selected Salmonella serovars of public health importance or of high prevalence in animals. To present a complete overview of the animal populations and food categories in which specific Salmonella serovars of public health importance have been recovered, all the data reported (derived even from fewer than 4 reporting countries and less than 10 isolates tested) have been included.
Data description
Throughout the report, level or occurrence of AMR means the percentage of resistant isolates as a proportion of the isolates tested of that microorganism. MSs reporting group means the MSs that provided data and were included in the relevant table of antimicrobial resistance for that bacterium–food or animal category–antimicrobial combination. Terms used to describe the levels or occurrence of antimicrobial resistance are ‘rare’: < 0.1%, ‘very low’: 0.1–1.0%, ‘low’: > 1–10.0%, ‘moderate’: > 10.0–20.0%, ‘high’: > 20.0–50.0%, ‘very high’: > 50.0–70.0%, ‘extremely high’: > 70.0%. Although these terms are applied to all antimicrobials, the significance of a given level of resistance depends on the particular antimicrobial and its importance in human and veterinary medicine.
Temporal trends in resistance
Where the minimum criteria12 for data inclusion in this report were met, temporal trend graphs were generated showing the resistance to different antimicrobials from 2009 to 2017, by plotting the level of resistance for each year of sampling. Graphs were created for those countries for which resistance data were available for four or more years in the 2009–2017 period for at least one of the two antimicrobials. MS‐specific resistance levels trend graphs use a unique scale and countries are shown in alphabetical order. For ampicillin, cefotaxime, ciprofloxacin, nalidixic acid and tetracyclines (Salmonella and indicator E. coli), ciprofloxacin, erythromycin, nalidixic acid, streptomycin and tetracycline (Campylobacter), resistance trends over time were visually explored by trellis graphs, using the lattice package in the R software (R version 2.14.2 (29/2/2012)).
To assess the statistical significance of temporal trends, the proportions of resistance were modelled against time in a logistic regression. This analysis was carried out using the PROC LOGISTIC of SAS 9.2 for each country where there were 5 years or more of available data to use in the model. The PROC LOGISTIC function uses a logit transform to model the proportion of prevalence against year, and provides estimates for both intercepts and slope. Models where the likelihood ratio test suggested it to be meaningful and resulting in a p‐value associated with slope of < 0.05 were considered to be significant.
Spatial analysis of resistance through maps
MS‐specific AMR levels for selected bacterium–food category/animal population combinations were plotted in maps for 2017, using ArcGIS 9.3. In the maps, resistance levels are presented with colours reflecting the continuous scale of resistance to the antimicrobial of interest among reporting MSs; so, there might be some apparent discrepancies between the colours and resistance levels between maps.
2.2.4. Analysis of multidrug resistance and co‐resistance data
As a consequence of the availability of AMR data at the isolate level in the MSs, the analysis of MDR and co‐resistance data becomes an important procedure in the light of the public health relevance of the emergence of multiresistant bacteria. The intention is to focus mainly on multi/co‐resistance patterns involving CIA (Collignon et al., 2016; Urdahl et al., 2016) according to the bacterial species, such as cephalosporins, fluoroquinolones and macrolides, and to summarise important information in the EU Summary Report. The occurrence of the isolates of a serotype/ resistance pattern of interest is studied at the MS level and at the reporting MS group/EU level, as the overall picture for all MSs might show a more definite pattern of emergence and spread. In addition, the analysis of data may reveal the existence of new or emerging patterns of MDR, particularly in Salmonella serotypes.
Definitions
For this analysis, a multiresistant isolate is one defined as resistant to at least three different antimicrobial substances, belonging to any three antimicrobial families listed in the harmonised set of antimicrobials included in the Commission Implementing Decision 2013/652/EU. Tables 3 and 4 list those recommended antimicrobials. Resistance to nalidixic acid and resistance to ciprofloxacin, as well as the resistance to cefotaxime and to ceftazidime are, respectively, addressed together.
In contrast, a fully susceptible isolate is one defined as non‐resistant to all of the antimicrobial substances included in the harmonised set of substances for Salmonella, Campylobacter and indicator E. coli.
The term combined resistance is used in this report to indicate phenotypic resistance to two or more different classes of antimicrobials, exhibited by the same bacterial isolate.
MDR patterns
The frequency and percentage of isolates exhibiting various MDR patterns considering the antimicrobials tested were determined for Salmonella (Salmonella spp. and for certain serovars of interest), Campylobacter species and indicator E. coli for each country and each animal population/food category. Isolates for which no susceptibility data were provided for some of the antimicrobial substances were disregarded.
Summary indicators’ and ‘diversity’ of MDR
The objective is first to give an overview of the situation on MDR through summary indicators: (1) the proportion of fully susceptible isolates; and (2) the proportion of multiresistant isolates. To illustrate the relative proportions of multiresistant isolates and the diversity of the resistance to multiple antimicrobials, graphical illustration was chosen. The percentage of isolates susceptible and resistant to one, two, three, etc., antimicrobials are shown using a composite bar graph displaying stacked bars, but only for certain combinations of bacterium–animal population or food category–MSs of particular interest.
The co‐resistance patterns of interest
In Salmonella and E. coli isolates, co‐resistance to cefotaxime (CTX) and ciprofloxacin (CIP) was estimated, as these two antimicrobials are of particular interest in human medicine. Co‐resistance was addressed using both ECOFFs (CTX > 0.25 mg/L and CIP > 0.064 mg/L) and CBPs (CTX > 2 mg/L and CIP > 1 mg/L) for E. coli. In C. jejuni and C. coli isolates, co‐resistance to ciprofloxacin and erythromycin (ERY) was estimated, as these two antimicrobials are of particular interest in human medicine in the treatment of severe campylobacteriosis. The interpretive ECOFFs used to address co‐resistance to ciprofloxacin and erythromycin were, for C. jejuni, CIP > 0.5 mg/L and ERY > 4 mg/L and, for C. coli, CIP > 0.5 mg/L and ERY > 8 mg/L. These values may be considered as very similar to CBPs.
2.2.5. Identification of presumptive ESBL, AmpC and/or carbapenemase producers
Definition of ESBL, AmpC, ESBL + AmpC, CP phenotypes:
The categorisation of isolates resistant to third‐generation cephalosporins and/or carbapenems in presumptive ESBL, AmpC or carbapenemase producers was carried out based on the EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance (EUCAST, 2013). In these expert guidelines, and based on other EUCAST and CLSI guidelines to detect ESBL/AmpC producers, a screening breakpoint of > 1 mg/L is recommended for cefotaxime and ceftazidime. This screening breakpoint is higher than the ECOFFs applied for antimicrobial susceptibility of both antimicrobials for E. coli, and to cefotaxime for Salmonella. For this report, a first condition for classifying isolates as presumptive ESBL/AmpC producers related to their MIC for either cefotaxime or ceftazidime, was to apply this screening breakpoint of MICs > 1 mg/L. Only isolates which presented MIC values accomplishing with this requisite (as expected for most of the ESBL/AmpC producers) were further considered. In total, for the third‐generation cephalosporin‐ and/or carbapenem‐resistant isolates, five main categorisations are made: (1) ESBL phenotype; (2) AmpC phenotype; (3) ESBL + AmpC phenotype; (4) CP phenotype; and (5) Other phenotypes (Figure 14).
To detect the production of ESBLs, a synergy test for cefotaxime and ceftazidime, in combination with clavulanic acid was performed. An eight‐fold reduction in the MIC for the cephalosporin combined with clavulanic acid compared with that obtained for the cephalosporin alone was interpreted as a positive synergy test. In all other cases, the synergy test was considered negative. For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and a synergy test positive for any of these antimicrobials, together with susceptibility to cefoxitin (≤ 8 mg/L) and meropenem (MEM ≤ 0.125 mg/L see CP phenotype) were classified as ESBL phenotype (Figure 14).
For the AmpC phenotype, the combination MIC > 8 mg/L (ECOFF) for cefoxitin together with MICs > 1 mg/L for cefotaxime and/or ceftazidime was used as phenotypic criteria to investigate the presence of AmpC production in E. coli. It should be also underlined that there are a few AmpC enzymes that do not confer resistance to cefoxitin (i.e. ACC‐1), and that there are other mechanisms (porin loss, the presence of carbapenemases, a few ESBLs like cefotaxime (CTX‐M)‐5 that could generate similar MIC values for the different antimicrobials (EFSA, 2012a; EUCAST, 2013). Phenotypic AmpC confirmation tests (i.e. cloxacillin synergy) were not required for the present monitoring. For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and cefoxitin MIC > 8 mg/L, together with negative synergy test for both cefotaxime and ceftazidime/clavulanic acid, together with susceptibility to meropenem (MEM ≤ 0.125 mg/L) were classified in the AmpC phenotype category. No distinction between acquired AmpC and natural AmpC was made (Figure 14).
For the present report, isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime, positive synergy tests for any of these antimicrobials with clavulanic acid and cefoxitin MIC > 8 mg/L, together with susceptibility to meropenem (MEM ≤ 0.12 mg/L) were classified under the ESBL + AmpC phenotype category (Figure 14). In some isolates, several mechanisms can be present at the same time, making it very difficult to differentiate the phenotypes. Also the high‐level expression of AmpC β‐lactamases can mask the presence of ESBLs. AmpC can also be present in isolates with positive ESBL tests (clavulanic acid synergy). In this case, the cefepime/clavulanic acid synergy test should be used to overturn/confirm the presence of ESBLs in these isolates (EUCAST, 2013) but, unfortunately, the combination cefepime/clavulanic acid was not included among the substances tested for monitoring. The inclusion of resistance to cefepime with a MIC value ≥ 4 mg/L as an additional criterion proposed elsewhere (EFSA, 2012a), could be useful to ascertain the presence of an ESBL producer.
For the classification of isolates into the putative carbapenem producers (CPs), a meropenem screening cut‐off of > 0.125 mg/L (which coincides with the harmonised ECOFF) was chosen. It is known that other mechanisms (i.e. hyperproduction or combination of ESBLs and/or AmpC and porin loss) can also affect to the MIC values generated for the different carbapenems, especially for ertapenem. The confirmation of the carbapenemase production recommended by the EUCAST guidelines cannot be inferred from the carbapenem susceptibility testing data reported, but needs further phenotypic or molecular testing. Those MSs that reported data suggesting the presence of putative CPs were recommended to validate the results by performing further confirmatory testing, and the EURL‐AR offered to apply WGS of the isolates. For the present report, isolates with MIC > 0.125 mg/L for meropenem would be considered as presumptive CP producers and were classified under the CP phenotype. The presence of other resistance mechanisms (ESBLs, AmpC, etc.) within the isolates placed in this group cannot be ruled out.
In this group, phenotypes not included in the categorisations defined above were included: isolates with a MIC > 0.125 for ertapenem and/or MIC > 1 mg/L for imipenem (EUCAST screening cut‐offs, one dilution step higher than the currently defined ECOFFs) but no resistance to meropenem (MIC < 125 mg/L) were classified under the category ‘other phenotype’. Finally, isolates with MICs ≤ 1 mg/L for cefotaxime and ceftazidime would be considered as not ESBL and/or AmpC producers. This implied that some isolates considered as microbiologically resistant (MICs over the ECOFFs) would not be further classified, as probably other mechanisms or technical issues in the MIC testing (i.e. MIC value close to the ECOFF) would be responsible for the MIC values obtained. For the present report, cefotaxime‐ and ceftazidime‐resistant isolates with MICs ≤ 1 mg/L for both antimicrobials were considered as putative non‐ESBL/AmpC producers and were classified under the category ‘other phenotype’.
We are aware that without a further molecular characterisation of the isolates, it will not be possible to know exactly which resistance mechanisms are present. For epidemiological purposes and based on the EUCAST guidelines, the classification of ‘presumptive’ producers for the different mechanism conferring resistance to third‐generation cephalosporins and/or carbapenems was considered. Molecular characterisation of these mechanisms is recommended.
For the occurrence and prevalence tables shown in Section ‘ESBL/AmpC/CP producers monitoring’, presumptive ESBL producers were considered as those exhibiting an ESBL and/or ESBL + AmpC phenotype, and presumptive AmpC producers, those with an AmpC and ESBL + AmpC phenotype (see below).
For the present report, the terms:
‘Presumptive ESBL/AmpC producers’ refers to those isolates who present an ESBL and/or and AmpC and/or an ESBL + AmpC phenotype (presumptive ESBL producers and/or presumptive AmpC producers).
‘Presumptive ESBL producers’ refers to those isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and a synergy test positive for any of these antimicrobials and susceptibility to meropenem (MEM ≤ 0.125 mg/L, see CP phenotype). These isolates may also harbour other resistance mechanisms (e.g. AmpC‐encoding genes).
‘Presumptive ESBL‐cefotaximase producers’ refers to those presumptive ESBL producers with MICs > 1 mg/L for cefotaxime and a synergy test positive for cefotaxime only. These isolates may also harbour other resistance mechanisms.
‘Presumptive ESBL‐ceftazidimase producers’ refers to those presumptive ESBL producers with MICs > 1 mg/L for ceftazidime and synergy test positive for ceftazidime only. These isolates may also harbour other resistance mechanisms.
‘Presumptive AmpC producers’ refers to isolates with MICs > 1 mg/L for cefotaxime and/or ceftazidime and cefoxitin MIC > 8 mg/L together with susceptibility to meropenem (MEM ≤ 0.125 mg/L, see CP phenotype). No distinction between acquired AmpC and natural AmpC was made. These isolates may also harbour other resistance mechanisms (e.g. ESBL‐encoding genes).
‘Presumptive ESBL + AmpC producers’ refers to isolates with the ESBL + AmpC phenotype described above.
‘Presumptive carbapenemase producers (CP producers)’ refers to those isolates with the CP phenotype described above.
2.2.6. Data on meticillin‐resistant Staphylococcus aureus (MRSA)
In 2017, Finland, Germany, Slovakia, Spain and Switzerland reported information on the occurrence/prevalence of MRSA in various categories of food. In 2017, Belgium, Finland, Germany, the Netherlands and Spain, and Norway and Switzerland reported data on the occurrence/prevalence of MRSA in food‐producing animals. The Netherlands and Slovakia reported data on MRSA in companion animals in 2017.
In 2017, data on the antimicrobial susceptibility of MRSA isolates were reported by Belgium, Finland, Switzerland and Sweden. Details of the antimicrobials selected by Belgium, Finland, Sweden and Switzerland are provided in the section on MRSA. For further information on reported MIC distributions and the number of resistant isolates, refer to the submitted and validated MS data published on the EFSA website.
The methods for collecting and testing samples for MRSA are not harmonised between MSs and, as a result, MSs may use differing procedures. Due to the variety of methods employed by MSs, these are explained in detail within the section on MRSA to enable readers to better follow the procedures carried out by individual countries.
3. Antimicrobial resistance in Salmonella 14
Human infections with Salmonella
Most Salmonella infections in humans result in mild, self‐limiting, gastrointestinal illness and usually do not require antimicrobial treatment. In some patients, the infection may be more serious as the bacteria may spread from the intestines to the bloodstream and then to other body sites, the consequences of which can be life threatening. Acute Salmonella infections may sometimes also result in long‐term sequelae affecting the joints (reactive arthritis). In cases of severe enteric disease or invasive infection, effective antimicrobials are essential for treatment. Fluoroquinolones are widely recommended for treating adults and third‐generation cephalosporins are recommended for treating children. Infection with Salmonella strains resistant to these antimicrobials may be associated with treatment failure, which in turn can lead to poor outcomes for patients. Therefore, recommended treatment should take account of up‐to‐date information on local patterns of resistance.
For 2017, 24 MSs, plus Iceland and Norway provided data on AMR in human Salmonella isolates. Seventeen countries reported isolate‐based AST results as measured values (inhibition zone diameters or MICs). Nine countries reported case‐based AST results interpreted as susceptible (S), intermediate (I) or resistant (R) according to the CBPs applied, which is one country more than for 2016 (Table SALMOVERVIEW).
3.1. Antimicrobial resistance in Salmonella isolates from humans
When referring to ‘Salmonella spp.’ in this chapter results for all non‐typhoidal Salmonella serovars from human cases with AST results are included. The resistance levels for Salmonella spp. are greatly influenced by the serovars included, with some serovars exhibiting higher levels of resistance to certain antimicrobials or expressing MDR to a higher degree than other serovars. Results are therefore presented separately for selected serovars prevalent in pigs and cattle (S. Typhimurium and monophasic S. Typhimurium) due to the legislative monitoring of isolates in this animal groups in 2017. Data on additional serovars among the 10 most common in human cases in 2017 are available in appendices (S. Enteritidis, S. Infantis, S. Newport, S. Stanley, S. Derby, S. Kentucky, S. Virchow and S. Agona). Findings of ESBL‐ and AmpC‐producing Salmonella in isolates from humans is available in Section 6 (ESBL‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli).
To better assess the impact of exposure within each reporting country on the AMR levels found in Salmonella isolates from humans, the analysis focused on domestically acquired infections. Travel information was however missing for a high proportion of cases in some countries (Table SALMTRAVHUM) and the impact of travel‐related infections on the occurrence of resistance can therefore not be ruled out in those countries.
Methods and interpretive criteria used for antimicrobial susceptibility testing of Salmonella isolates from humans
Most laboratories follow the ‘EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates’ (ECDC, 2016) on the antimicrobial panel to be tested. The type of method (dilution, disk diffusion, gradient strip) and the interpretive criteria used when providing interpreted results instead of measured values, are presented in Table 1 in the Section 2 Materials and methods.
Quantitative data were interpreted by ECDC based on the EUCAST ECOFF values, when available, in the same way as for the animal and food data. Where ECOFFs do not exist, EUCAST or Clinical and Laboratory Standards Institute (CLSI) CBPs were applied. For the qualitative SIR15 data, intermediate and resistant results were combined into a non‐susceptible category.
For 11 antimicrobials, for which results were reported both as quantitative and interpreted data, the commonly used interpretive criteria were aligned (Figure 15). For this purpose, susceptible isolates were aligned with wild‐type isolates based on ECOFFs and non‐susceptible isolates (intermediate and resistant) were aligned with non‐wild‐type isolates. When analysed in this way, there is generally good concordance (±1 dilution) across categories, also for ciprofloxacin after the CBP for Salmonella was lowered in 2014. A notable exception is the EUCAST CBP for meropenem, which is substantially higher (+4 dilutions) than the ECOFF.
3.1.1. Antimicrobial resistance in Salmonella spp. isolates from humans
In total, 19,734 Salmonella isolates from clinical samples, encompassing 320 different serovars and serogroups, were tested for resistance to one or more antimicrobials and reported by 24 MSs, plus Iceland and Norway. This number represents 21.3% of the 92,649 confirmed human salmonellosis cases reported in the EU/EEA in 2017. The serovar distribution within the Salmonella spp. varies by country depending on their frequency among human cases and/or specific sampling strategies for further typing and AST at the national public health reference laboratories. For this reason, comparisons between countries should be avoided at the level of Salmonella spp.
Resistance levels in Salmonella spp. isolates from humans
The highest proportions of resistance in Salmonella spp. isolates from humans in 2017 were reported for sulfonamides/sulfamethoxazole (32.8%), tetracyclines (30.2%) and ampicillin (27.5%) (Table 2).
Resistance to ciprofloxacin was reported in 13.0% of the isolates which was a slight increase compared with 2016 (when it was 11.0%). Resistance to cefotaxime or ceftazidime was observed in 1.9% and 1.1% of the isolates which was similar to the levels in 2016 (1.2% and 1.4% resistance, respectively). These antimicrobials represent the clinically most important antimicrobial classes (fluoroquinolones and third‐generation cephalosporins) for treatment of salmonellosis, and they are classified by WHO as the highest priority CIA (WHO, 2017). Ciprofloxacin resistance levels were the highest in Poland, Cyprus, Romania and Malta (24.8–30.3%) and cefotaxime resistance the highest in Malta, Belgium and Poland (6.6–13.5%). The higher proportion of ciprofloxacin resistance in Poland and Romania could be explained by a large proportion of S. Enteritidis among the isolates, as this serovar is more frequently ciprofloxacin resistant than many other serovars. Poland also reported 6.0% of their S. Enteritidis as resistant to cefotaxime. In Malta, a quarter (27 of 111) of the tested Salmonella isolates were S. Kentucky, with high‐level ciprofloxacin resistance and half of the isolates being ESBL‐producing (see further on ESBL in human Salmonella in Section 6).
No isolates were reported resistant to meropenem in 2017, although it should be noted that meropenem results were interpreted with CBPs in eight of the 22 countries testing this antimicrobial and the CBP for intermediate resistance for meropenem differs from the ECOFF by four dilutions. Sequencing of ESBL‐producing S. Kentucky as part of an FWD‐Net study revealed that two of the isolates from Malta were also carrying carbapenemase genes (see textbox in Section 6). Resistance to colistin was detected in 4.7% of isolates though 88.9% of the resistant isolates were either S. Enteritidis or S. Dublin. Serovars within serogroup O:9, such as S. Enteritidis and S. Dublin, have been reported to have inherent resistance to colistin (Agersø et al., 2012). Resistance to other last line antimicrobials such as azithromycin and tigecycline, was relatively low at 2.5% and 0.8%, respectively. The relatively high resistance to azithromycin (13.6%) observed in Ireland was due to a point source outbreak with an azithromycin‐resistant S. Brandenburg in a catering establishment. The outbreak involved 35 laboratory confirmed cases where poultry was the suspected source (N. Delappe, HSE, Ireland, personal communication 15 October 2018).
Combined resistance to critically important antimicrobials in Salmonella spp. isolates from humans
Combined ‘microbiological’ resistance to both of the CIA for treatment of human salmonellosis, ciprofloxacin and cefotaxime, was observed in 109 (0.9%) of 11,907 tested isolates (24 MSs; Table COMSALMHUM, Figure 16). The highest combined resistance was observed in Malta and Poland, with 12.6 and 8.6%, respectively, of the Salmonella isolates. Combined clinical resistance to both ciprofloxacin and cefotaxime was observed in 74 (0.6%) of the isolates. Of these, 27 were S. Kentucky, 12 S. Infantis, 11 S. Typhimurium, seven monophasic S. Typhimurium 1,4,[5],12:i:‐, four S. Enteritidis, two S. Agona and one each of S. Mikawasima, S. Newport, S. Paratyphi B var. Java, S. Tumodi and S. Virchow, and six with unknown or non‐defined serovar.
Figure 16.
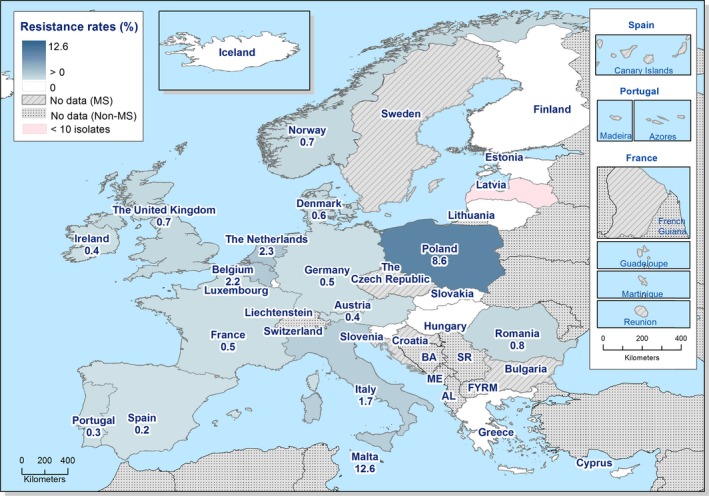
Spatial distribution of combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime among Salmonella spp. from human cases in reporting countries in 2017
High‐level ciprofloxacin resistance in Salmonella spp. isolates from humans
Of the 3,947 Salmonella spp. isolates with ciprofloxacin MIC data, 2.2% (87 isolates) had a MIC ≥ 4 mg/L (Table 8). Such isolates were reported from five of the eight countries which had provided quantitative data from dilution tests. High‐level ciprofloxacin resistance was most frequently reported in S. Kentucky (in 92.6% of tested S. Kentucky) among the eleven serovars reported with MIC ≥ 4 mg/L.
Table 8.
Occurrence of high‐level resistance to ciprofloxacin (MIC ≥ 4 mg/L) in Salmonella serovars isolated from humans in 2017, 5 MSs
| Serovar | N | High‐level resistance to ciprofloxacin(MIC ≥ 4 mg/L) | |
|---|---|---|---|
| n | % | ||
| S. Agona | 56 | 1 | 1.8 |
| S. Brandenburg | 66 | 1 | 1.5 |
| S. Derby | 89 | 1 | 1.1 |
| S. Dublin | 59 | 2 | 3.4 |
| S. Enteritidis | 866 | 4 | 0.5 |
| S. Infantis | 126 | 2 | 1.6 |
| S. Kentucky | 68 | 63 | 92.6 |
| Monophasic S. Typhimurium | 861 | 6 | 0.7 |
| S. Newport | 77 | 2 | 2.6 |
| S. Saintpaul | 26 | 1 | 3.8 |
| S. Typhimurium | 756 | 4 | 0.5 |
| Other | 897 | 0 | 0.0 |
| Total (5 MSs) | 3,947 | 87 | 2.2 |
MIC: minimum inhibitory concentration; MS: Member State.
Multidrug resistance in Salmonella spp. isolates from humans
Fourteen MSs tested at least 10 isolates for the nine antimicrobial classes included in the MDR analysis (Figure 17). On average 52.6% of Salmonella spp. isolates were susceptible to all nine antimicrobial classes (14 MSs, N = 8,218, Table MDRSALMHUM). MDR was on average observed in 28.6% of the isolates and highest in Greece (81.4%) and Belgium (43.3%). In the case of Greece, this was due to that the AST was focused on monophasic S. Typhimurium in 2017. Overall, 34 isolates (0.4% of the 8,218 isolates) were resistant to 7 or 8 antimicrobial classes, including 13 isolates of monophasic S. Typhimurium, 7 of S. Typhimurium, 4 of S. Saintpaul, 2 each of S. Infantis and S. Rissen and 1 each of S. Dublin, S. Enteritidis,, S. Kentucky, S. Livingstone, S. Mkamba and S. Virchow. No isolates were reported resistant to all nine classes.
Figure 17.
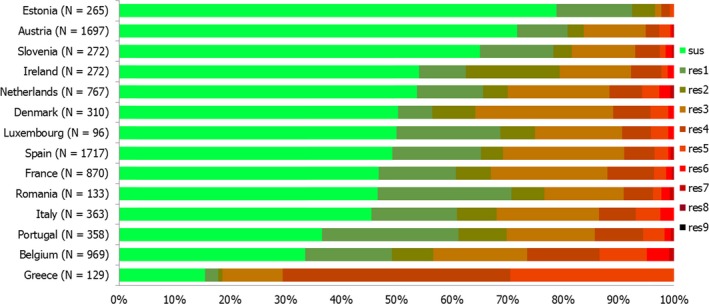
Frequency distribution of Salmonella spp. isolates from humans completely susceptible or resistant to one to nine antimicrobial classes in 2017
- N: total number of isolates tested for susceptibility against the whole common set of antimicrobials for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one up to nine antimicrobial classes of the common set for Salmonella.
Table 9.
Antimicrobial resistance in Salmonella spp. (all non‐typhoidal serovars) from humans per country in 2017
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 1,697 | 0.9 | 1,697 | 3.3 | 1,697 | 14.8 | 1,697 | 0.5 | 1,697 | 0.4 | 1,697 | 0 | 1,697 | 0.1 |
| Belgium | 980 | 3.4 | 980 | 12.8 | 980 | 51.8 | 980 | 7.7 | 980 | 0.8 | 980 | 0 | 969 | 2.9 |
| Cyprus | 74 | 13.5 | – | – | 76 | 27.6 | 29 | 0 | 76 | 0 | 76 | 0 | – | – |
| Denmark | 310 | 2.9 | 310 | 9.0 | 310 | 40.6 | 310 | 1.3 | 310 | 1.0 | 310 | 0 | 310 | 0.3 |
| Estonia | 265 | 0 | 265 | 0.8 | 265 | 6.4 | 265 | 0.8 | 265 | 0.8 | 265 | 0 | 10 | 0 |
| Finland | 214 | 0.9 | 214 | 4.2 | 214 | 24.8 | 214 | 0 | – | – | 214 | 0 | – | – |
| France | 870 | 1.8 | 870 | 7.2 | 870 | 35.3 | 870 | 0.8 | 870 | 0.3 | 870 | 0 | 870 | 0 |
| Germany a | 652 | 2.0 | 652 | 6.3 | 652 | 30.1 | 651 | 2.3 | 650 | 1.7 | 652 | 0 | – | – |
| Greece | 130 | 0 | 130 | 30.0 | 129 | 81.4 | 129 | 0.8 | 130 | 0 | 130 | 0 | – | – |
| Hungary a | 448 | 2.7 | 448 | 30.4 | 448 | 63.6 | 448 | 0.2 | 448 | 0.7 | 448 | 0 | – | – |
| Ireland | 272 | 2.6 | 272 | 17.6 | 272 | 33.5 | 272 | 0.4 | 272 | 0.4 | 272 | 0 | 272 | 0 |
| Italy | 422 | 1.7 | 422 | 8.1 | 423 | 37.4 | 423 | 2.1 | 422 | 1.7 | 364 | 0 | 244 | 0.8 |
| Latvia a | – | – | – | – | 22 | 27.3 | 2 | NA | – | – | – | – | – | – |
| Lithuania a | 382 | 0.8 | 442 | 2.7 | 946 | 18.9 | 801 | 0.4 | 444 | 0.5 | 374 | 0 | – | – |
| Luxembourg | 97 | 1.0 | 97 | 8.2 | 97 | 27.8 | 97 | 2.1 | 97 | 0 | 97 | 0 | – | – |
| Malta a | – | – | – | – | 111 | 43.2 | 111 | 13.5 | 111 | 14.4 | 111 | 0 | – | – |
| Netherlands | 767 | 4.0 | 767 | 6.3 | 767 | 34.3 | 767 | 2.5 | 767 | 2.0 | 767 | 0 | 767 | 1.4 |
| Poland a | 108 | 11.1 | 20 | 45.0 | 687 | 17.9 | 413 | 6.1 | – | – | – | – | – | – |
| Portugal | 358 | 2.2 | 358 | 8.4 | 358 | 34.4 | 358 | 0.3 | 358 | 0.3 | 358 | 0 | 357 | 0.3 |
| Romania | 133 | 2.3 | 133 | 7.5 | 133 | 24.1 | 133 | 2.3 | 133 | 1.5 | 133 | 0 | – | – |
| Slovakia a | – | – | 16 | 0 | 858 | 9.3 | 182 | 0.0 | 63 | 0 | 55 | 0 | – | – |
| Slovenia | 275 | 1.5 | 275 | 3.3 | 275 | 19.6 | 275 | 0.4 | 275 | 0 | 275 | 0 | – | – |
| Spain | 1,719 | 1.3 | 1,719 | 5.9 | 1,718 | 33.1 | 1,718 | 0.9 | 1,718 | 0.5 | 1,720 | 0 | – | – |
| United Kingdom a | 643 | 4.7 | 2,241 | 10.4 | 3,490 | 21.0 | 1,437 | 2.2 | 795 | 3.4 | 883 | 0 | – | – |
| Total (MSs 24) | 10,816 | 2.2 | 12,328 | 8.5 | 15,798 | 27.5 | 12,582 | 1.9 | 10,848 | 1.1 | 11,051 | 0 | 5,496 | 0.8 |
| Iceland a | – | – | 4 | NA | 31 | 45.2 | 30 | 0 | – | – | – | – | – | – |
| Norway | 297 | 2.0 | 115 | 10.4 | 297 | 15.8 | 297 | 1.0 | 297 | 0.7 | 297 | 0 | – | – |
| Country | Nalidixic acid | Ciprofloxacinb | Azithromycin | Colistin | Sulfamethoxazolec | Trimethoprim | Co‐trimoxazole | Tetracycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 1,697 | 14.9 | 1,697 | 15.9 | – | – | – | – | 1,697 | 16.9 | 1,697 | 2.5 | – | – | 1,697 | 17.5 |
| Belgium | 11 | 9.1 | 980 | 18.1 | 980 | 4.3 | – | – | 980 | 47.6 | 969 | 19.8 | – | – | 980 | 45.9 |
| Cyprus | – | – | 76 | 30.3 | – | – | 44 | 0 | – | – | – | – | 74 | 14.9 | 3 | NA |
| Denmark | 310 | 1.9 | 310 | 6.8 | 310 | 0.3 | 310 | 3.2 | 310 | 41.6 | 310 | 3.9 | – | – | 310 | 39.0 |
| Estonia | 265 | 9.8 | 265 | 9.8 | – | – | 265 | 0.4 | 265 | 7.2 | 265 | 1.5 | – | – | 265 | 8.7 |
| Finland | 214 | 4.7 | 214 | 5.6 | – | – | – | – | – | – | 214 | 2.8 | – | – | 214 | 24.3 |
| France | 870 | 10.3 | 870 | 12.2 | 870 | 0.3 | 870 | 4.8 | 870 | 40.5 | 870 | 6.4 | – | – | 870 | 38.5 |
| Germany a | 652 | 10.0 | 651 | 1.8 | – | – | – | – | – | – | – | – | 652 | 5.7 | 651 | 23.0 |
| Greece | 130 | 0 | 109 | 0 | – | – | – | – | 130 | 82.3 | 130 | 73.8 | – | – | 130 | 80.0 |
| Hungary a | – | – | 448 | 5.4 | – | – | – | – | – | – | 448 | 8 | 448 | 10.7 | 448 | 57.6 |
| Ireland | 272 | 8.8 | 272 | 11.8 | 272 | 13.6 | 272 | 2.2 | 272 | 21.3 | 272 | 3.3 | – | – | 272 | 24.6 |
| Italy | 423 | 9.2 | 423 | 9.2 | 244 | 0.8 | 346 | 4.0 | 399 | 44.9 | 399 | 12.8 | 16 | 12.5 | 423 | 40.4 |
| Latvia a | – | – | 22 | 4.5 | – | – | – | – | – | – | 5 | NA | 15 | 6.7 | – | – |
| Lithuania a | 358 | 9.2 | 746 | 8.8 | – | – | – | – | – | – | 345 | 3.8 | 944 | 2.6 | 380 | 13.7 |
| Luxembourg | – | – | 97 | 1.0 | – | – | – | – | 98 | 37.8 | 97 | 9.3 | 97 | 9.3 | 99 | 33.3 |
| Malta a | – | – | 111 | 25.2 | – | – | – | – | – | – | – | – | – | – | – | – |
| Netherlands | 767 | 12.0 | 767 | 14.1 | 767 | 0.5 | 767 | 8.1 | 767 | 31.9 | 767 | 7.3 | – | – | 767 | 31.0 |
| Poland a | 8 | NA | 343 | 30.0 | – | – | – | – | 57 | 17.5 | – | – | 678 | 5.2 | 17 | 41.2 |
| Portugal | 358 | 9.5 | 358 | 12.6 | 358 | 1.7 | – | – | 358 | 52.8 | 358 | 8.9 | – | – | 358 | 33.2 |
| Romania | 133 | 24.8 | 133 | 24.8 | – | – | – | – | 133 | 27.8 | 133 | 5.3 | 133 | 4.5 | 133 | 27.8 |
| Slovakia a | – | – | 497 | 8.9 | – | – | – | – | 2 | NA | – | – | 373 | 5.1 | 546 | 9.3 |
| Slovenia | – | – | 275 | 12.4 | – | – | – | – | 272 | 20.6 | 275 | 5.8 | 275 | 4.7 | 275 | 22.9 |
| Spain | 1,720 | 13.9 | 1,720 | 13.2 | – | – | – | – | 1,719 | 35.7 | 1,719 | 5.4 | – | – | 1,720 | 33.3 |
| United Kingdom a | 1,047 | 15.9 | 3,480 | 14.5 | – | – | – | – | 651 | 24.4 | 1,779 | 5.9 | 1,004 | 4.4 | 568 | 27.3 |
| Total (MSs 24) | 9,235 | 12.1 | 14,864 | 13.0 | 3,801 | 2.5 | 2,874 | 4.7 | 8,980 | 32.8 | 11,052 | 7.6 | 4,709 | 5.3 | 11,126 | 30.2 |
| Iceland a | – | – | 31 | 9.7 | – | – | – | – | – | – | – | – | 31 | 6.5 | – | – |
| Norway | – | – | 297 | 18.9 | 115 | 0.9 | – | – | – | – | – | – | – | – | 115 | 31.3 |
N: number of isolates tested; % Res: percentage of microbiologically resistant isolates (either interpreted as non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable – if less than 10 isolates were tested, the percentage of resistance was not calculated; MS: Member State.
Data interpreted with clinical breakpoints.
Countries doing disk diffusion have replaced ciprofloxacin with pefloxacin when screening for fluoroquinolone resistance, as recommended by EUCAST.
Combined data on the class of sulfonamides and the substance sulfamethoxazole within this group.
3.1.2. Antimicrobial resistance in Salmonella Typhimurium isolates from humans
Resistance levels in S. Typhimurium isolates from humans
S. Typhimurium was the second most common Salmonella serovar identified in 2017 with 10,675 cases reported in the EU/EEA (excluding monophasic S. Typhimurium 1,4,[5],12:i:). The highest proportion of resistance in S. Typhimurium was observed for ampicillin (53.3%), sulfonamides (48.1%) and tetracyclines (44.5%) (24 MSs, Table 10). The levels of resistance to these antimicrobials were high to extremely high in all reporting MSs, except in Estonia and Greece. The proportions of isolates resistant to either of the two clinically most critical antimicrobials were on average 8.0% for ciprofloxacin and 2.6% for cefotaxime. The highest proportion of isolates resistant to ciprofloxacin was reported from Austria, Poland and Slovakia (16.3%, 15.4% and 15.0%, respectively), whereas the highest proportion of cefotaxime resistance was reported from Belgium and Poland (10.4% and 10.0% respectively). It should be noted that the number of isolates tested in Slovakia and Poland was low (≤ 20 isolates).
Table 10.
Antimicrobial resistance in Salmonella Typhimurium from humans per country in 2017
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 208 | 1.4 | 208 | 13.9 | 208 | 42.8 | 208 | 1.4 | 208 | 1 | 208 | 0 | 208 | 0 |
| Belgium | 270 | 1.9 | 270 | 21.5 | 270 | 75.6 | 270 | 10.4 | 270 | 1.1 | 270 | 0 | 268 | 4.1 |
| Cyprus | 21 | 0 | – | – | 22 | 45.5 | 9 | NA | 22 | 0 | 22 | 0 | – | – |
| Denmark | 57 | 0 | 57 | 26.3 | 57 | 61.4 | 57 | 0 | 57 | 0 | 57 | 0 | 57 | 1.8 |
| Estonia | 72 | 0 | 72 | 1.4 | 72 | 4.2 | 72 | 0 | 72 | 0 | 72 | 0 | – | – |
| Finland | 35 | 0 | 35 | 20.0 | 35 | 20.0 | 35 | 0 | – | – | 35 | 0 | – | – |
| France | 106 | 1.9 | 106 | 41.5 | 106 | 55.7 | 106 | 1.9 | 106 | 0 | 106 | 0 | 106 | 0 |
| Germany a | 151 | 0.7 | 151 | 13.9 | 151 | 77.5 | 151 | 0.7 | 151 | 0.7 | 151 | 0 | – | – |
| Greece | 19 | 0 | 19 | 5.3 | 19 | 5.3 | 18 | 5.6 | 19 | 0 | 19 | 0 | – | – |
| Hungary | 248 | 4 | 248 | 44.4 | 248 | 50.4 | 248 | 0.4 | 248 | 1.2 | 248 | 0 | – | – |
| Ireland | 40 | 0 | 40 | 17.5 | 40 | 30.0 | 40 | 0 | 40 | 0 | 40 | 0 | 40 | 0 |
| Italy | 44 | 0 | 44 | 22.7 | 44 | 54.5 | 44 | 0 | 44 | 0 | 40 | 0 | 30 | 3.3 |
| Latvia a | – | – | – | – | 9 | 55.6 | 1 | 0 | – | – | – | – | – | – |
| Lithuania | 45 | 0 | 56 | 16.1 | 101 | 76.2 | 70 | 1.4 | 57 | 1.8 | 43 | 0 | – | – |
| Luxembourg | 13 | 0 | 13 | 30.8 | 13 | 38.5 | 13 | 7.7 | 13 | 0 | 13 | 0 | – | – |
| Malta a | – | – | – | – | 18 | 83.3 | 18 | 0 | 18 | 0 | 18 | 0 | – | – |
| Netherlands | 150 | 2 | 150 | 18.0 | 150 | 55.3 | 150 | 1.3 | 150 | 0 | 150 | 0 | 150 | 2.0 |
| Poland a | 5 | NA | 1 | NA | 25 | 60.0 | 20 | 10.0 | – | – | – | – | – | – |
| Portugal | 69 | 0 | 69 | 20.3 | 69 | 42.0 | 69 | 1.4 | 69 | 1.4 | 69 | 0 | 68 | 0 |
| Romania | 40 | 7.5 | 40 | 20.0 | 40 | 67.5 | 40 | 5.0 | 40 | 5.0 | 40 | 0 | – | – |
| Slovakia a | – | – | – | – | 38 | 57.9 | 11 | 0 | 2 | NA | 3 | NA | – | – |
| Slovenia | 38 | 5.3 | 38 | 10.5 | 38 | 28.9 | 38 | 2.6 | 38 | 0 | 38 | 0 | – | – |
| Spain | 147 | 0 | 147 | 23.8 | 147 | 57.1 | 147 | 0 | 147 | 0 | 147 | 0 | – | – |
| United Kingdom a | 141 | 0.7 | 444 | 19.6 | 685 | 48.2 | 273 | 2.9 | 119 | 5 | 133 | 0 | – | – |
| Total (MSs 24) | 1,919 | 1.7 | 2,208 | 22.3 | 2,605 | 53.3 | 2,108 | 2.6 | 1,890 | 1 | 1,922 | 0 | 927 | 1.7 |
| Iceland a | – | – | 2 | NA | 14 | 85.7 | 13 | 0 | – | – | – | – | – | – |
| Norway | 59 | 0.0 | 30 | 23.3 | 59 | 25.4 | 59 | 3.4 | 59 | 1.7 | 59 | 0 | – | – |
| Country | Nalidixic acid | Ciprofloxacinb | Azithromycin | Colistin | Sulfamethoxazolec | Trimethoprim | Co‐trimoxazole | Tetracycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 208 | 13 | 208 | 16.3 | – | – | – | – | 208 | 41.3 | 208 | 3.4 | – | – | 208 | 37.5 |
| Belgium | 2 | NA | 270 | 8.9 | 270 | 2.2 | – | – | 270 | 53.3 | 268 | 23.9 | – | – | 270 | 50.7 |
| Cyprus | – | – | 22 | 13.6 | – | – | 11 | 0 | – | – | – | – | 21 | 4.8 | 1 | NA |
| Denmark | 57 | 0 | 57 | 1.8 | 57 | 0.0 | 57 | 0 | 57 | 66.7 | 57 | 3.5 | – | – | 57 | 50.9 |
| Estonia | 72 | 1.4 | 72 | 1.4 | – | – | 72 | 0 | 72 | 5.6 | 72 | 1.4 | – | – | 72 | 6.9 |
| Finland | 35 | 2.9 | 35 | 5.7 | – | – | – | – | – | – | 35 | 2.9 | – | – | 35 | 20.0 |
| France | 106 | 9.4 | 106 | 12.3 | 106 | 0 | 106 | 0.9 | 106 | 60.4 | 106 | 13.2 | – | – | 106 | 54.7 |
| Germany a | 151 | 4.0 | 151 | 0 | – | – | – | – | – | – | – | – | 151 | 7.9 | 150 | 59.3 |
| Greece | 19 | 0 | 17 | 0 | – | – | – | – | 19 | 15.8 | 19 | 5.3 | – | – | 19 | 10.5 |
| Hungary | – | – | 248 | 4.4 | – | – | – | – | – | – | 248 | 4.0 | 248 | 4.0 | 248 | 44.4 |
| Ireland | 40 | 5.0 | 40 | 7.5 | 40 | 0 | 40 | 2.5 | 40 | 32.5 | 40 | 2.5 | – | – | 40 | 35.0 |
| Italy | 44 | 13.6 | 44 | 13.6 | 30 | 0 | 42 | 0 | 43 | 51.2 | 43 | 9.3 | – | – | 44 | 61.4 |
| Latvia a | – | – | 9 | NA | – | – | – | – | – | – | 4 | NA | 4 | NA | – | – |
| Lithuania a | 40 | 20.0 | 88 | 6.8 | – | – | – | – | – | – | 36 | 8.3 | 100 | 3.0 | 44 | 81.8 |
| Luxembourg | – | – | 13 | 0 | – | – | – | – | 13 | 53.8 | 13 | 23.1 | 13 | 23.1 | 13 | 46.2 |
| Malta a | – | – | 18 | 0 | – | – | – | – | – | – | – | – | – | – | – | – |
| Netherlands | 150 | 2.7 | 150 | 4.7 | 150 | 0.7 | 150 | 0 | 150 | 36.7 | 150 | 12.7 | – | – | 150 | 30.7 |
| Poland a | 1 | NA | 13 | 15.4 | – | – | – | – | 2 | NA | – | – | 24 | 12.5 | – | – |
| Portugal | 69 | 10.1 | 69 | 10.1 | 69 | 1.4 | – | – | 69 | 49.3 | 69 | 8.7 | – | – | 69 | 27.5 |
| Romania | 40 | 0 | 40 | 0 | – | – | – | – | 40 | 70.0 | 40 | 7.5 | 40 | 7.5 | 40 | 67.5 |
| Slovakia a | – | – | 20 | 15.0 | – | – | – | – | – | – | – | – | 12 | 33.3 | 26 | 50.0 |
| Slovenia | – | – | 38 | 7.9 | – | – | – | – | 37 | 29.7 | 38 | 10.5 | 38 | 10.5 | 38 | 34.2 |
| Spain | 147 | 6.1 | 147 | 5.4 | – | – | – | – | 147 | 58.5 | 147 | 7.5 | – | – | 147 | 57.1 |
| United Kingdom a | 217 | 5.1 | 674 | 10.4 | – | – | – | – | 155 | 59.4 | 355 | 7.9 | 197 | 4.6 | 119 | 36.1 |
| Total (MSs 22) | 1,398 | 6.7 | 2,551 | 8.0 | 722 | 1.1 | 478 | 0.4 | 1,428 | 48.1 | 1,948 | 9.3 | 848 | 6.3 | 1,896 | 44.5 |
| Iceland a | – | – | 14 | 0 | – | – | – | – | – | – | – | – | 14 | 14.3 | – | – |
| Norway | – | – | 59 | 11.9 | 30 | 0 | – | – | – | – | – | – | – | – | 30 | 40.0 |
N: number of isolates tested; % Res: percentage of microbiologically resistant isolates (either interpreted as non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable – if less than 10 isolates were tested, the percentage of resistance was not calculated; MS: Member State.
Data interpreted with clinical breakpoints.
Countries doing disk diffusion have replaced ciprofloxacin with pefloxacin when screening for fluoroquinolone resistance, as recommended by EUCAST.
Combined data on the class of sulfonamides and the substance sulfamethoxazole within this group.
Spatial distribution of resistance among S. Typhimurium isolates from humans
The highest proportions of ciprofloxacin resistance in S. Typhimurium isolates from human cases were observed in three countries in central and eastern Europe (Figure 18). No clear geographical pattern was observed for cefotaxime resistance where the highest proportions were reported in Poland and Belgium (Figure 18).
Figure 18.
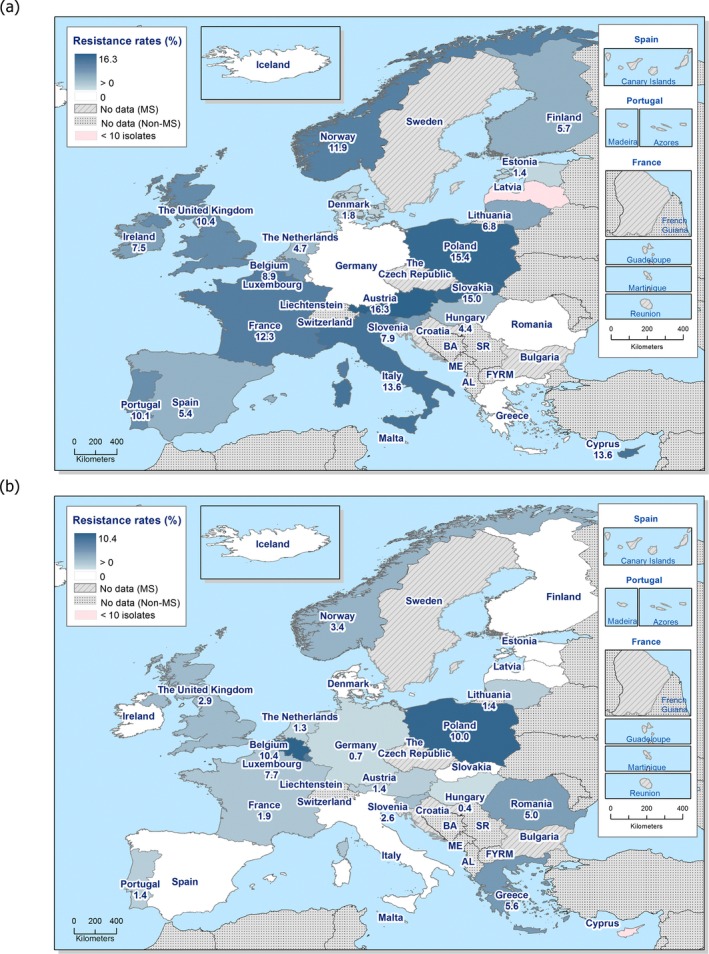
Spatial distribution of (a) ciprofloxacin and (b) cefotaxime resistance among S. Typhimurium from human cases in reporting countries in 2017
Combined resistance to critically important antimicrobials in S. Typhimurium isolates from humans
Combined ‘microbiological’ resistance to both of the CIA for treatment of human salmonellosis, ciprofloxacin and cefotaxime, was observed in 13 (0.6%) of 2.046 tested S. Typhimurium isolates (24 MSs, Table COMTYPHIHUM). Combined clinical resistance to both ciprofloxacin and cefotaxime was observed in 10 (0.5%) of the isolates.
Temporal trends in resistance among S. Typhimurium isolates from humans
Trend analysis was performed for the years 2013–2017 for resistance proportions of four antimicrobials. Twenty‐two MSs and Norway were included in the trend analysis as they had provided resistance data for a minimum of 3 years in this period and a minimum of 10 S. Typhimurium isolates (Figure 19). Resistance to (fluoro)quinolones was assessed as resistance to either ciprofloxacin, pefloxacin or nalidixic acid due to breakpoint changes during the period and methodological issues (see further in Section 2 Materials and methods). Statistically significant increases in (fluoro)quinolone resistance were observed in Austria, Finland, Slovakia and the United Kingdom while decreasing trends were observed in Greece and Spain. Resistance to ampicillin increased significantly in Belgium, Denmark and Slovakia while it decreased in Cyprus, Estonia, Germany, Greece, Lithuania, Luxembourg, Norway, Portugal and Spain. Tetracycline resistance increased significantly in Belgium, Denmark, Lithuania and Romania, while it decreased in Estonia, Finland, Germany, Greece, the Netherlands, Portugal and Spain. Cefotaxime resistance increased significantly in Belgium and decreased in Norway, Portugal and Slovenia.
Figure 19.
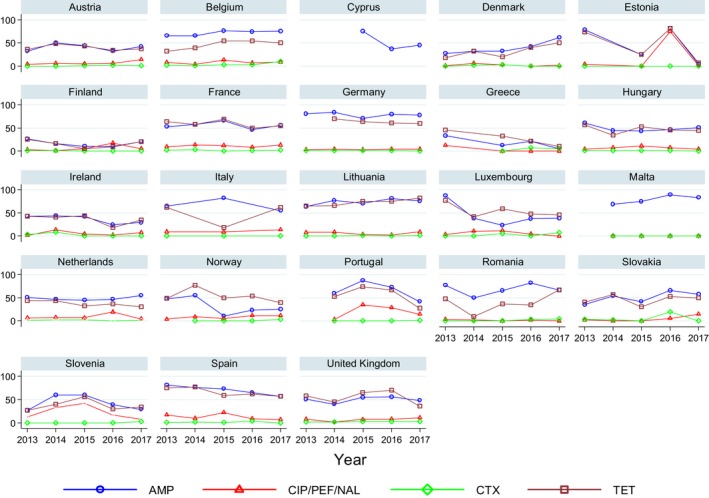
Trends in resistance to ampicillin, ciprofloxacin/pefloxacin, cefotaxime and tetracycline in Salmonella Typhimurium from humans in 23 reporting countries, 2013–2017
- Statistically significant increasing trends over 3–5 years, as tested by logistic regression (p ≤ 0.05), were observed for ciprofloxacin in Austria, Finland, Slovakia and the United Kingdom (↑), for ampicillin in Belgium, Denmark and Slovakia (↑), for tetracyclines in Belgium, Denmark, Lithuania and Romania (↑) and for cefotaxime in Belgium (↑). Statistically significant decreasing trends over 3–5 years were observed for ciprofloxacin in Greece and Spain (↓), for ampicillin in Cyprus, Estonia, Germany, Greece, Lithuania, Luxembourg, Norway, Portugal and Spain (↓), for tetracyclines in Estonia, Finland, Germany, Greece, the Netherlands, Portugal and Spain (↓), and for cefotaxime in Norway, Portugal and Slovenia (↓). Only countries testing at least 10 isolates per year were included in the analysis.
Multidrug resistance in S. Typhimurium isolates from humans
In humans, 39.7% (14 MSs, N = 1,266) of the S. Typhimurium isolates were multiresistant (Table MDRTYPHIHUM, Figure 20). Very high MDR was reported in Romania (65.0%), Spain (53.7%) and France (51.9%) in 2017. S. Typhimurium isolates resistant to 6, 7 or 8 antimicrobial classes were identified in 6 of 14 reporting MSs. The two isolates of S. Typhimurium resistant to eight of the nine tested substances were only susceptible to meropenem.
Figure 20.
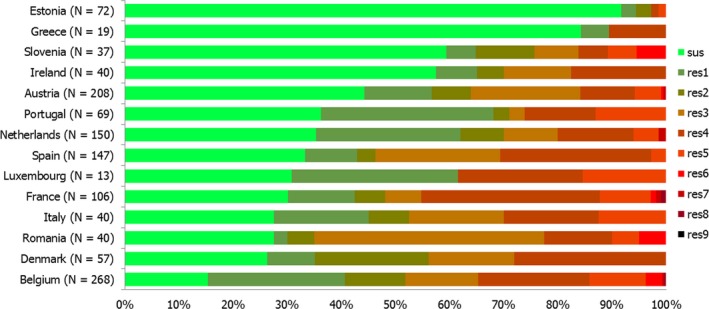
Frequency distribution of Salmonella Typhimurium isolates from humans completely susceptible or resistant to one to nine antimicrobial classes in 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one up to nine antimicrobial classes of the common set for Salmonella.
3.1.3. Antimicrobial resistance in monophasic Salmonella Typhimurium 1,4,[5],12:i: isolates from humans
Resistance levels in monophasic S. Typhimurium 1,4,[5],12:i:‐ isolates from humans
For the purpose of this report, monophasic S. Typhimurium 1,4,[5],12:i:‐ is treated as a separate serovar, and as such, it is currently the third most common serovar in Europe. For 2017, 6,322 cases were reported by the EU/EEA countries. Extremely high levels of resistance were observed for ampicillin (86.8%), sulfonamides (86.7%) and tetracyclines (87.9%) (13 MSs, Table 11). This resistance pattern, ASuT,16 is a well‐known characteristic of monophasic S. Typhimurium 1,4,[5],12:i:‐ and was observed at similar levels in all reporting MSs with the exception of Estonia which reported lower proportions of resistant isolates. The proportion of isolates resistant to either of the two clinically most important antimicrobials was 6.0% for ciprofloxacin and 2.2% for cefotaxime, with the highest levels of ciprofloxacin resistance in Norway (29.2%) and of cefotaxime resistance in Belgium (9.3%) and Estonia (8.7%). The number of isolates tested in Estonia and Norway was however low (N = 23 and 24).
Table 11.
Antimicrobial resistance in monophasic Salmonella Typhimurium 1,4,[5],12:i:‐ from humans per country in 2017
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 104 | 1.0 | 104 | 14.4 | 104 | 92.3 | 104 | 1.0 | 104 | 1.0 | 104 | 0 | 104 | 0 |
| Belgium | 194 | 2.6 | 194 | 13.9 | 194 | 88.7 | 194 | 9.3 | 194 | 0.5 | 194 | 0 | 194 | 3.1 |
| Denmark | 96 | 8.3 | 96 | 10.4 | 96 | 80.2 | 96 | 3.1 | 96 | 2.1 | 96 | 0 | 96 | 0 |
| Estonia | 23 | 0 | 23 | 4.3 | 23 | 52.2 | 23 | 8.7 | 23 | 8.7 | 23 | 0 | – | – |
| France | 250 | 2.0 | 250 | 4.8 | 250 | 84.4 | 250 | 0.4 | 250 | 0.4 | 250 | 0 | 250 | 0 |
| Greece | 106 | 0 | 106 | 35.8 | 105 | 98.1 | 106 | 0 | 106 | 0 | 106 | 0 | – | – |
| Ireland | 38 | 7.9 | 38 | 5.3 | 38 | 89.5 | 38 | 2.6 | 38 | 2.6 | 38 | 0 | 38 | 0 |
| Italy | 138 | 2.9 | 137 | 12.4 | 138 | 82.6 | 138 | 0.7 | 137 | 0 | 104 | 0 | 67 | 1.5 |
| Luxembourg | 20 | 0 | 20 | 5.0 | 20 | 85.0 | 20 | 0 | 20 | 0 | 20 | 0 | – | – |
| Netherlands | 143 | 2.8 | 143 | 7.0 | 143 | 89.5 | 143 | 0.7 | 143 | 0.7 | 143 | 0 | 143 | 0.7 |
| Portugal | 98 | 4.1 | 98 | 5.1 | 98 | 76.5 | 98 | 0 | 98 | 0 | 98 | 0 | 98 | 1.0 |
| Spain | 462 | 2.2 | 462 | 9.1 | 462 | 89.0 | 462 | 1.9 | 462 | 0.4 | 462 | 0 | – | – |
| United Kingdom a | 1 | NA | 74 | 10.8 | 141 | 86.5 | 37 | 2.7 | 39 | 5.1 | 42 | 0 | – | – |
| Total (MSs 13) | 1,673 | 2.7 | 1,745 | 10.8 | 1,812 | 86.8 | 1,709 | 2.2 | 1,710 | 0.8 | 1,680 | 0 | 991 | 0.9 |
| Norway | 24 | 4.2 | 19 | 21.1 | 24 | 70.8 | 24 | 0 | 24 | 0 | 24 | 0 | – | – |
| Country | Nalidixic acid | Ciprofloxacinb | Azithromycin | Colistin | Sulfamethoxazolec | Trimethoprim | Co‐trimoxazole | Tetracycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 104 | 3.8 | 104 | 5.8 | – | – | – | – | 104 | 89.4 | 104 | 11.5 | – | – | 104 | 87.5 |
| Belgium | – | – | 194 | 10.8 | 194 | 7.7 | – | – | 194 | 87.1 | 194 | 26.8 | – | – | 194 | 85.6 |
| Denmark | 96 | 1.0 | 96 | 3.1 | 96 | 0 | 96 | 1.0 | 96 | 78.1 | 96 | 0 | – | – | 96 | 78.1 |
| Estonia | 23 | 4.3 | 23 | 4.3 | – | – | 23 | 0 | 23 | 52.2 | 23 | 13.0 | – | – | 23 | 65.2 |
| France | 250 | 2.0 | 250 | 3.6 | 250 | 0.4 | 250 | 2.0 | 250 | 89.6 | 250 | 9.2 | – | – | 250 | 91.6 |
| Greece | 106 | 0 | 87 | 0 | – | – | – | – | 106 | 97.2 | 106 | 88.7 | – | – | 106 | 95.3 |
| Ireland | 38 | 2.6 | 38 | 5.3 | 38 | 0 | 38 | 0 | 38 | 86.8 | 38 | 10.5 | – | – | 38 | 100 |
| Italy | 138 | 5.1 | 138 | 3.6 | 67 | 1.5 | 112 | 0.9 | 122 | 83 | 121 | 19.0 | 1 | NA | 138 | 79.7 |
| Luxembourg | – | – | 20 | 0 | – | – | – | – | 19 | 89.5 | 20 | 20.0 | 20 | 20.0 | 20 | 75.0 |
| Netherlands | 143 | 4.2 | 143 | 6.3 | 143 | 0 | 143 | 0.7 | 143 | 90.2 | 143 | 5.6 | – | – | 143 | 90.9 |
| Portugal | 98 | 3.1 | 98 | 11.2 | 98 | 0 | – | – | 98 | 79.6 | 98 | 9.2 | – | – | 98 | 85.7 |
| Spain | 462 | 5.6 | 462 | 5.4 | – | – | – | – | 462 | 87.0 | 462 | 8.7 | – | – | 462 | 90.3 |
| United Kingdom a | 16 | 6.3 | 152 | 11.2 | – | – | – | – | 4 | NA | 65 | 3.1 | 58 | 3.4 | 3 | NA |
| Total (MSs 12) | 1,474 | 3.7 | 1,805 | 6.0 | 886 | 1.9 | 662 | 1.2 | 1,659 | 86.7 | 1,720 | 15.9 | 79 | 8.9 | 1,675 | 87.9 |
| Norway | – | – | 24 | 29.2 | 19 | 5.3 | – | – | – | – | – | – | 24 | 8.3 | 19 | 84.2 |
N: number of isolates tested; % Res: percentage of microbiologically resistant isolates (either interpreted as non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable – if fewer than 10 isolates were tested, the percentage of resistance was not calculated; MS: Member State.
Provided measured values. Data interpreted by ECDC.
Ciprofloxacin has in several countries been replaced by pefloxacin for screening of fluoroquinolone resistance with disc diffusion, as recommended by EUCAST.
Combined data on the class of sulfonamides and the substance sulfamethoxazole within this group.
Spatial distribution of resistance among monophasic S. Typhimurium 1,4,[5],12:i:‐ isolates from human cases
No clear geographical pattern was observed for ciprofloxacin or cefotaxime resistance in monophasic S. Typhimurium 1,4,[5],12:i:‐ (Figure 21). A few outliers were observed with higher resistance levels to ciprofloxacin in Norway and to cefotaxime in Belgium and Estonia.
Figure 21.
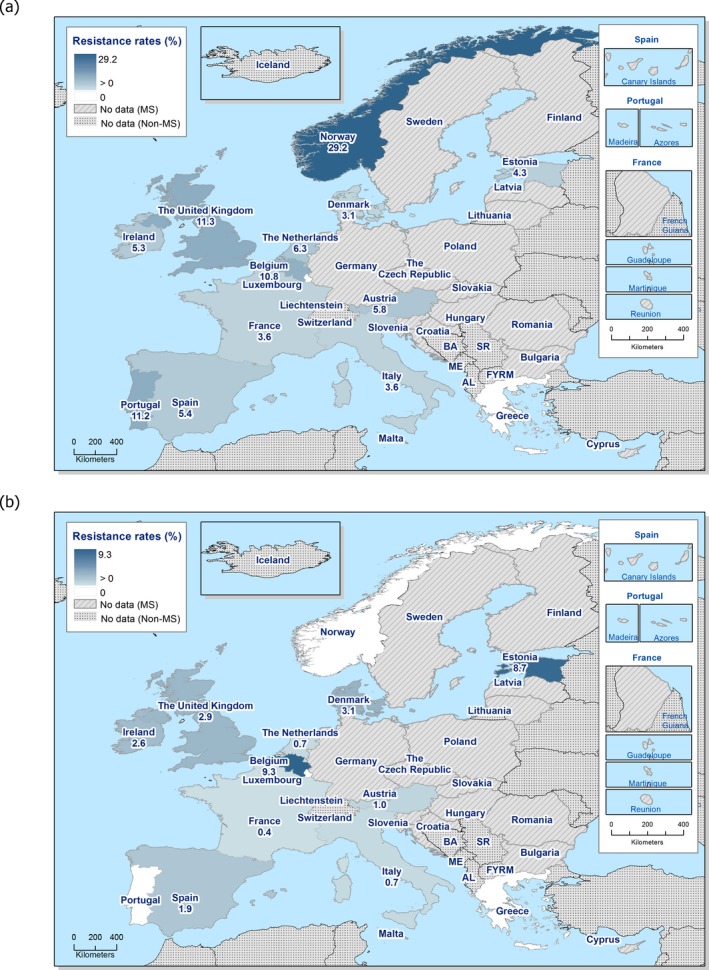
Spatial distribution of (a) ciprofloxacin and (b) cefotaxime resistance among monophasic S. Typhimurium from human cases in reporting countries in 2017
Combined resistance to critically important antimicrobials in monophasic S. Typhimurium 1,4,[5],12:i:‐ isolates from humans
Combined ‘microbiological’ resistance to both of the CIA for treatment of human salmonellosis, ciprofloxacin and cefotaxime, was observed in 11 (0.7%) of 1,685 tested monophasic S. Typhimurium isolates (13 MSs, Table COMMONTYPHIHUM). Combined clinical resistance to both ciprofloxacin and cefotaxime was observed in seven (0.4%) of the isolates.
Temporal trends in resistance among monophasic S. Typhimurium 1,4,[5],12:i:‐ isolates from humans
Twelve MSs were included in the analysis as they had provided resistance data for a minimum of 3 years in the period 2013–2017 and for a minimum of 10 monophasic S. Typhimurium isolates (Figure 22). Statistically significant increases in (fluoro)quinolone resistance were observed in Austria and Portugal while decreasing trends were observed in Greece. Resistance to ampicillin increased significantly in Greece and the Netherlands while it decreased in Estonia and Portugal. Tetracycline resistance increased significantly in Greece and Ireland while cefotaxime resistance decreased in Estonia, Italy and Luxembourg.
Figure 22.
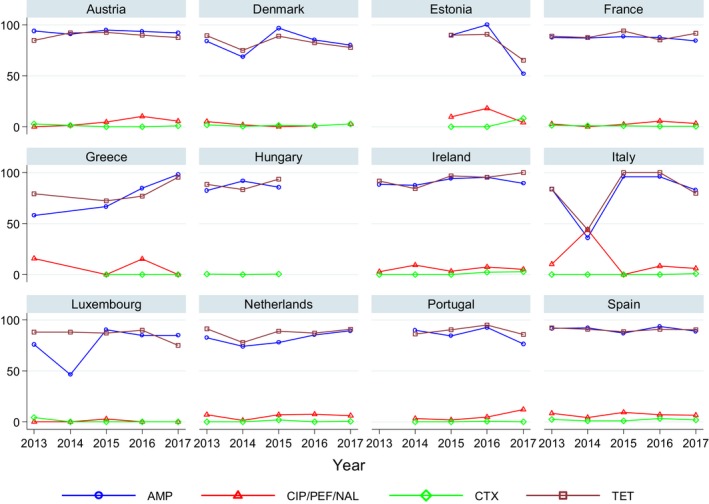
Trends in resistance to ampicillin, ciprofloxacin/pefloxacin/nalidixic acid, cefotaxime and tetracycline in monophasic Salmonella Typhimurium isolates from humans in 12 reporting countries, 2013–2017
- Statistically significant increasing trends over 3–5 years, as tested by logistic regression (p ≤ 0.05), were observed for ciprofloxacin in Austria and Portugal (↑), for ampicillin in Greece and the Netherlands (↑) and for tetracyclines in Greece and Ireland (↑). Statistically significant decreasing trends over 3–5 years were observed for ciprofloxacin in Greece (↓), for ampicillin in Estonia and Portugal (↓) and for cefotaxime in Estonia, Italy and Luxembourg (↓). Only countries testing at least 10 isolates per year were included in the analysis.
Multidrug resistance in monophasic S. Typhimurium 1,4,[5],12:i:‐ isolates from humans
In humans, 81.4% (12 MSs, N = 1,636) of the monophasic S. Typhimurium isolates were multiresistant (Table MDRMONTYPHIHUM, Figure 23). Extremely high MDR was observed in monophasic S. Typhimurium in 10 of 12 reporting MSs. Isolates resistant to six or seven antimicrobial classes were identified in eight of 12 reporting MSs. No country reported isolates resistant to eight or nine classes.
Figure 23.
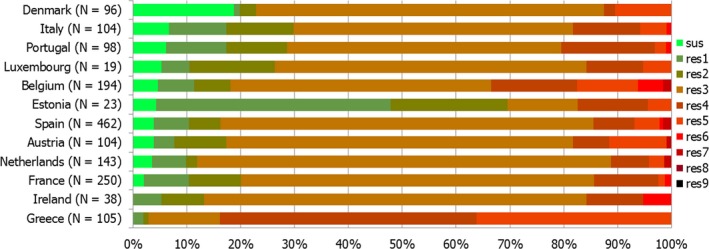
Frequency distribution of monophasic Salmonella Typhimurium isolates from humans completely susceptible or resistant to one to nine antimicrobial classes in 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one up to nine antimicrobial classes of the common set for Salmonella.
3.1.4. Antimicrobial resistance in Salmonella Derby isolates from humans
Resistance levels in S. Derby isolates from humans
S. Derby was the seventh most common serovar in 2017 with 612 cases reported by the EU/EEA countries. Resistance to sulfonamides and tetracycline was relatively common in S. Derby (30.0% and 26.2%, respectively) while ampicillin resistance was moderate (15.5%) (Table 12). The proportion of isolates resistant to either of the two clinically most important antimicrobials was on average 2.0% for ciprofloxacin and 3.4% for cefotaxime.
Table 12.
Antimicrobial resistance in Salmonella Derby from humans per country in 2017
| Country | Gentamicin | Chloramphenicol | Ampicillin | Cefotaxime | Ceftazidime | Meropenem | Tigecycline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA |
| Belgium | 46 | 0 | 46 | 8.7 | 46 | 17.4 | 46 | 4.3 | 46 | 0 | 46 | 0 | 45 | 0 |
| Cyprus | – | – | – | – | 1 | NA | 1 | NA | 1 | NA | 1 | NA | – | – |
| Denmark | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA |
| Estonia | 4 | NA | 4 | NA | 4 | NA | 4 | NA | 4 | NA | 4 | NA | – | – |
| France | 15 | 0 | 15 | 0 | 15 | 6.7 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 |
| Germany a | 14 | 0 | 14 | 0 | 14 | 21.4 | 14 | 14.3 | 14 | 7.1 | 14 | 0 | – | – |
| Italy | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 5 | NA | 5 | NA |
| Lithuania a | 16 | 0 | 16 | 0 | 18 | 16.7 | 18 | 0 | 16 | 0 | 16 | 0 | – | – |
| Luxembourg | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | – | – |
| Netherlands | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA |
| Poland a | – | – | – | – | 1 | NA | – | – | – | – | – | – | – | – |
| Portugal | 3 | NA | 3 | NA | 3 | NA | 3 | NA | 3 | NA | 3 | NA | 3 | NA |
| Romania | 3 | NA | 3 | NA | 3 | NA | 3 | NA | 3 | NA | 3 | NA | – | – |
| Slovenia | – | – | – | – | 3 | NA | 1 | NA | – | – | – | – | – | – |
| Spain | 11 | 0 | 11 | 0 | 11 | 36.4 | 11 | 0 | 11 | 0 | 11 | 0 | – | – |
| United Kingdom a | 2 | NA | 5 | NA | 8 | NA | 4 | NA | 2 | NA | 2 | NA | – | – |
| Total (MSs 17) | 142 | 0.7 | 145 | 6.2 | 155 | 15.5 | 148 | 3.4 | 143 | 1.4 | 140 | 0 | 86 | 0 |
| Norway | 1 | NA | – | – | 1 | NA | 1 | NA | 1 | NA | 1 | NA | – | – |
| Austria | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA | 2 | NA |
| Country | Nalidixic acid | Ciprofloxacinb | Azithromycin | Colistin | Sulfamethoxazolec | Trimethoprim | Co‐trimoxazole | Tetracycline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 2 | NA | 2 | NA | – | – | – | – | 2 | NA | 2 | NA | – | – | 2 | NA |
| Belgium | 1 | NA | 46 | 4.3 | 46 | 0 | – | – | 46 | 19.6 | 45 | 15.6 | – | – | 46 | 15.2 |
| Cyprus | – | NA | 1 | NA | – | – | – | – | – | – | – | – | 1 | NA | – | – |
| Denmark | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | – | – | 8 | NA |
| Estonia | 4 | NA | 4 | NA | – | – | 4 | NA | 4 | NA | 4 | NA | – | – | 4 | NA |
| France | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 0 | 15 | 46.7 | 15 | 6.7 | – | – | 15 | 33.3 |
| Germany a | 14 | 0 | 14 | 0 | – | – | – | – | – | – | – | – | 14 | 21.4 | 14 | 7.1 |
| Italy | 8 | NA | 8 | NA | 5 | NA | 8 | NA | 5 | NA | 6 | NA | – | – | 8 | NA |
| Lithuania a | 15 | 0 | 17 | 0 | – | – | – | – | – | – | 13 | 0 | 17 | 0 | 16 | 50.0 |
| NA Luxembourg | – | – | 2 | NA | – | – | – | – | 2 | NA | 2 | NA | 2 | NA | 2 | NA |
| Netherlands | 8 | 0 | 8 | NA | 8 | NA | 8 | NA | 8 | NA | 8 | NA | – | – | 8 | NA |
| Poland a | – | – | 1 | NA | – | – | – | – | 1 | NA | – | – | – | – | – | – |
| Portugal | 3 | 0 | 3 | NA | 3 | NA | – | – | 3 | NA | 3 | NA | – | – | 3 | NA |
| Romania | 3 | 0 | 3 | NA | – | – | – | – | 3 | NA | 3 | NA | 3 | NA | 3 | NA |
| Slovenia | – | – | 1 | NA | – | – | – | – | – | – | – | – | 2 | NA | 1 | NA |
| Spain | 11 | 0 | 11 | 0 | – | – | – | – | 11 | 36.4 | 11 | 9.1 | – | – | 11 | 63.6 |
| United Kingdom a | 2 | NA | 5 | NA | – | – | – | – | 2 | NA | 5 | NA | 4 | NA | – | – |
| Total (MSs 17) | 94 | 1.1 | 149 | 2.0 | 85 | 0 | 43 | 0 | 110 | 30.0 | 125 | 10.4 | 43 | 9.3 | 141 | 26.2 |
| Norway | – | – | 1 | NA | – | – | – | – | – | – | – | – | – | – | – | – |
| Austria | 2 | NA | 2 | NA | – | – | – | – | 2 | NA | 2 | NA | – | – | 2 | NA |
N: number of isolates tested; % Res: percentage of microbiologically resistant isolates (either interpreted as non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable – if fewer than 10 isolates were tested, the percentage of resistance was not calculated; MS: Member State.
Provided measured values. Data interpreted by ECDC.
Ciprofloxacin has in several countries been replaced by pefloxacin for screening of fluoroquinolone resistance with disc diffusion, as recommended by EUCAST.
Combined data on the class of sulfonamides and the substance sulfamethoxazole within this group.
Combined resistance to critically important antimicrobials in S. Derby isolates from humans
None of the 145 S. Derby isolates expressed either ‘microbiological’ or clinical resistance to both of the CIA for treatment of human salmonellosis, ciprofloxacin and cefotaxime (16 MSs).
Temporal trends in resistance among S. Derby isolates from humans
Six MSs were included in the trend analysis for 2013–2017 as they had provided resistance data for a minimum of 3 years in this period and a minimum of 10 S. Typhimurium isolates. Statistically significant increases in the proportion of resistant S. Derby isolates were observed for ampicillin and cefotaxime in Germany and for tetracyclines in Lithuania. Significant decreasing trends were observed for ampicillin and cefotaxime in Belgium, cefotaxime in the Netherlands and Spain and for (fluoro)quinolones in France.
3.2. Antimicrobial resistance in Salmonella spp. from animals and food17
In 2017, the monitoring of AMR in Salmonella isolates recovered from carcase swabs of fattening pigs and calves under 1 year of age at slaughter was mandatory, in accordance with Regulation (EC) No 2073/2005. Additionally, some MSs collected voluntary Salmonella AMR data from fattening pigs and cattle at slaughter, where one representative sample of caecal contents was collected per epidemiological unit (i.e. the holding) to account for clustering.
The Salmonella spp. data includes results for all serovars reported within the different animal origins, where no more than one isolate per Salmonella serovar from the same epidemiological unit per year was tested for AMR (Decision 2013/652/EU). As the potential for acquiring AMR markedly varies between serovars, the relative contribution of different serovars may significantly influence the general level of resistance presented in the data for Salmonella spp. Furthermore, trends in the dissemination of specific clones or resistance traits should ideally be considered individually for the different serovars and for that reason results are presented for selected serovars of importance (see related Appendices A, B, C and D).
The World Health Organization states that transmission of bacterial infection from non‐human sources to humans, with the ability to cause disease, is more evident in particular bacteria, which includes non‐typhoidal Salmonella; and comments that the potential for such transmission should be recognised (Urdahl et al., 2017). Additionally, a recent study inferred that multidrug‐resistant non‐typhoidal Salmonella infections may have more serious human health implications compared to those of pan‐susceptible strains (Parisi et al., 2016).
3.2.1. Antimicrobial resistance in Salmonella spp. recovered from carcase swabs of fattening pigs
Resistance levels in Salmonella spp. from fattening pig carcases
In 2017, 22 EU MSs reported data on isolates of Salmonella spp. recovered from the mandatory carcase swabbing of fattening pigs at slaughter, according to the provisions of Decision 2013/652/EU, as did one non‐MS (Table 13). Notably, six MSs did not obtain any positive Salmonella isolates from fattening pig carcases and, therefore, data are not presented for these countries. For nine countries, resistance levels were assessed on less than 10 isolates. The reported levels of resistance to ampicillin, sulfamethoxazole and tetracycline ranged from moderate to extremely high (10.5–100%) in Salmonella spp. from pig carcases in most reporting EU MSs; no resistance to these compounds was recorded by Latvia (N = 1), and additionally, Lithuania (N = 2) reported no resistance to sulfamethoxazole. Resistance to chloramphenicol ranged from low (4.8%) to high (50%), with the exception of 10 EU MSs registering no resistance to this compound. Generally, resistance to this compound was moderate considering all reporting EU MSs. Although six EU MSs did not report any resistance to trimethoprim, resistance to this compound ranged from low to high (4.9–35.2%) in the majority of reporting EU MSs. Notably in Greece, only one isolate was assessed resulting in an extremely high level of resistance to this compound (100%). Overall resistance to gentamicin remained at a low level (4.6%), although a high level (27.8%) of resistance was registered by Ireland (N = 54). Resistance to azithromycin was recorded at a moderate level in one EU MS, at low levels in three EU MSs, and was not detected in all other reporting countries. Nalidixic acid resistance was either not detected or reported at a low level in eight EU MS, whereas ciprofloxacin resistance ranged from low to moderate (2.9–16.7%), with 14 countries (including a non‐MS) recording no resistance to this compound. ‘Microbiological’ resistance to cefotaxime and ceftazidime was observed in four countries; three EU MSs reported a low level and Lithuania (N = 2) reported a high level (50%). Resistance to tigecycline was not detected in most EU MSs (16/22), while very low to low levels (0.6% to 3.7%) were reported by six MSs. Meropenem resistance was not detected in any of the Salmonella spp. isolates recovered by reporting countries. Overall, colistin resistance was very low considering all reporting EU MSs; Spain (N = 180) reported a low level of resistance to this compound (1.1%), while Germany (N = 31) recorded a moderate level of resistance (12.9%) – see Table SALMPIGMEATD.
Table 13.
Occurrence of resistance (%) to selected antimicrobials in Salmonella spp. from fattening pig carcases, using harmonised ECOFFs, 22 EU MSs and 1 non‐MS, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 98 | 0 | 8.2 | 65.3 | 0 | 0 | 0 | 1 | 6.1 | 7.1 | 2 | 0 | 61.2 | 27.6 | 49 |
| Bulgariaa | 6 | 0 | 0 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 16.7 | 33.3 |
| Croatia | 25 | 0 | 12 | 52 | 0 | 0 | 0 | 0 | 8 | 12 | 0 | 0 | 52 | 8 | 48 |
| Cyprusa | 4 | 0 | 50 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 25 |
| Czech Republic | 21 | 0 | 4.8 | 42.9 | 0 | 0 | 0 | 0 | 4.8 | 4.8 | 0 | 0 | 42.9 | 0 | 42.9 |
| Denmark | 69 | 7.2 | 14.5 | 55.1 | 0 | 0 | 0 | 0 | 0 | 0 | 1.4 | 0 | 59.4 | 14.5 | 47.8 |
| Estoniaa | 9 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 | 11.1 | 0 |
| France | 206 | 1.9 | 5.8 | 45.1 | 0 | 0 | 0 | 3.4 | 0 | 0 | 0 | 0 | 68.9 | 4.9 | 63.6 |
| Germany | 31 | 3.2 | 12.9 | 64.5 | 3.2 | 3.2 | 0 | 3.2 | 3.2 | 6.5 | 0 | 12.9 | 64.5 | 16.1 | 51.6 |
| Greecea | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 |
| Hungary | 19 | 0 | 0 | 26.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 21.1 | 10.5 | 26.3 |
| Ireland | 54 | 27.8 | 13 | 64.8 | 0 | 0 | 0 | 3.7 | 7.4 | 13 | 0 | 0 | 72.2 | 35.2 | 66.7 |
| Italy | 124 | 4 | 12.9 | 39.5 | 0 | 0 | 0 | 0 | 5.6 | 10.5 | 0 | 0 | 46 | 16.1 | 45.2 |
| Latviaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuaniaa | 2 | 0 | 0 | 100 | 50 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 |
| Malta | 17 | 0 | 0 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 70.6 | 5.9 | 41.2 |
| Poland | 24 | 0 | 0 | 33.3 | 0 | 0 | 0 | 0 | 4.2 | 4.2 | 0 | 0 | 54.2 | 16.7 | 33.3 |
| Portugal | 34 | 2.9 | 20.6 | 32.4 | 2.9 | 2.9 | 0 | 2.9 | 0 | 2.9 | 11.8 | 0 | 35.3 | 20.6 | 47.1 |
| Romaniaa | 6 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16.7 | 0 | 33.3 |
| Slovakia | 19 | 0 | 5.3 | 15.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10.5 | 5.3 | 15.8 |
| Spain | 180 | 7.2 | 21.7 | 79.4 | 1.1 | 1.1 | 0 | 0.6 | 8.9 | 16.7 | 3.3 | 1.1 | 75.6 | 20.6 | 85 |
| United Kingdoma | 4 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 |
| Total (22 MSs) | 954 | 4.6 | 11.5 | 53 | 0.5 | 0.5 | 0 | 1.4 | 4 | 6.8 | 1.4 | 0.6 | 59.5 | 15.5 | 56.8 |
| Icelanda | 6 | 0 | 0 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 33.3 | 0 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Spatial distribution of resistance in Salmonella spp. from fattening pig carcases
The spatial distributions of cefotaxime and ciprofloxacin resistance in Salmonella spp. from pig carcases are shown in Figure 24. Considering only countries reporting data on 10 or more isolates, cefotaxime resistance was observed at low levels by three MS from western and southern Europe (Germany, Portugal and Spain), and was not detected by other MSs (Figure 24a). Fluoroquinolone resistance was detected at moderate levels in MSs from southern and northern Europe (Spain, Croatia, Italy and Ireland), while low levels were detected in MSs from western and eastern Europe (Belgium, Germany, the Czech Republic and Poland). Additionally, one MS from southern Europe (Portugal) reported low level ciprofloxacin resistance. No resistance to ciprofloxacin was detected by other MSs.
Figure 24.
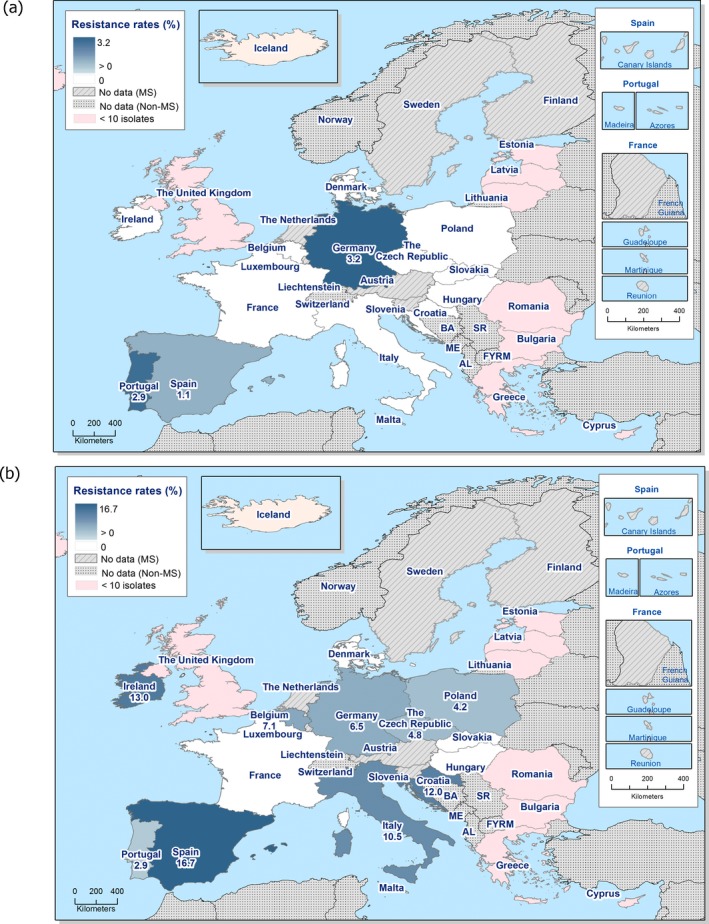
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella spp. from fattening pig carcases, 22 EU MSs and 1 non‐MS, 2017
Combined resistance to ciprofloxacin and cefotaxime in Salmonella spp. from fattening pig carcases
Spain was the only country to report combined resistance to ciprofloxacin and cefotaxime in 2/180 Salmonella isolates (Figure 25; Table COMSALMPIGMEAT). ‘Microbiological’ combined resistance was not recorded in any other isolates from reporting countries.
Figure 25.
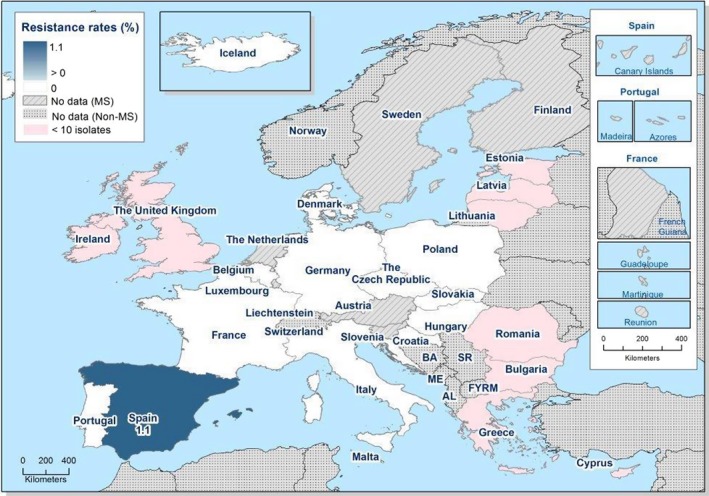
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in Salmonella spp. from fattening pig carcases, using harmonised ECOFFs, 22 EU MSs and 1 non‐MS, 2017
Multidrug resistance in Salmonella spp. from fattening pig carcases
Multidrug resistance is defined as resistance to three or more antimicrobial classes. In 2017, 23 reporting countries recorded data for individual isolates from pig carcases, which were included in the MDR analysis (N = 960). The proportion of Salmonella isolates which were multiresistant varied from 0% to 100%, as did the proportion of fully susceptible isolates (Figure 26). Overall, 47.4% of Salmonella isolates were multiresistant. Notably, where MDR was detected at 100%, only one Salmonella isolate was reported by the MS for assessment (Table COMSALMPIGMEAT).
Figure 26.
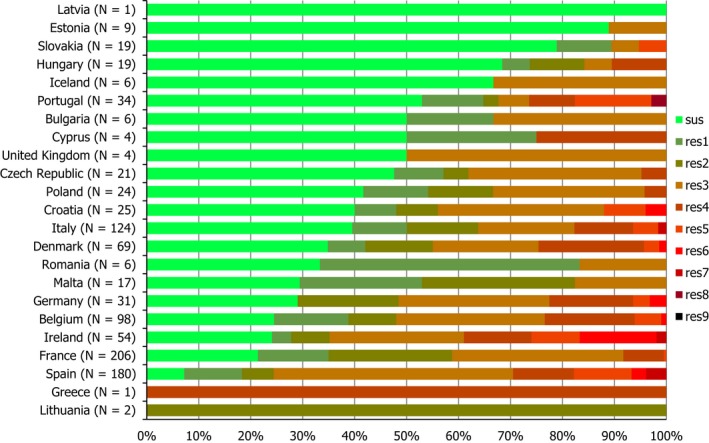
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella spp. from fattening pig carcases, 22 MSs and 1 non‐MS, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Spatial distribution of complete susceptibility in Salmonella spp. from fattening pig carcases
The susceptibility of isolates to individual antimicrobials was determined using ECOFFs; all isolates were tested against the same mandatory panel of antibiotics. Considering only countries where 10 or more isolates were assessed, the percentage of completely susceptible Salmonella spp. isolates ranged from low in Spain (7.2%) to extremely high in Slovakia (78.9%). Generally, spatial distributions (Figure 4) demonstrated that complete susceptibility ranged from high to extremely high in isolates reported by MSs from eastern Europe (Poland, the Czech Republic, Hungary and Slovakia; 41.7–78.9%). High levels were also reported in isolates from northern and western European MSs (Ireland, Denmark: 24.1–34.8%; France, Belgium, Germany: 21.4%‐29%). In MSs from southern Europe, complete susceptibility ranged from low to very high (Spain, Malta, Italy, Portugal and Croatia; 7.2–52.9%). Overall, 29.1% of Salmonella isolates were susceptible to all 11 antimicrobials used in the analysis (this figure includes countries which submitted data for 10 or less isolates), with complete susceptibility ranging from 0% to 100%. Notably, where complete susceptibility was detected at 100%, only one Salmonella spp. was reported by the MS for assessment; similarly, where complete susceptibility was not detected, only one Salmonella spp. was reported by the MS for assessment (Table COMSALMPIGMEAT).
Figure 27.
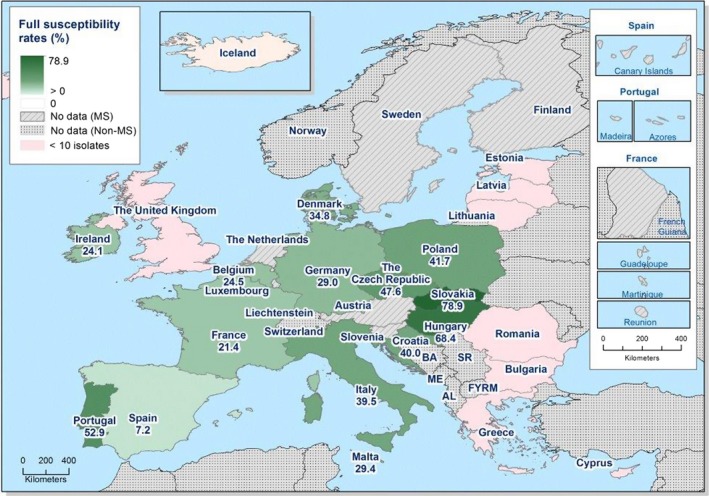
Spatial distribution of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from fattening pig carcases, using harmonised ECOFFs, 22 EU MSs and 1 non‐MS, 2017
Temporal trends in resistance in Salmonella spp. from pig meat and fattening pig carcases
Eleven MSs provided resistance data for four or more years to be included in the statistical analysis of temporal trends (Figure 28). Over the nine years of available data, most reporting countries registered a statistical significant trend in ampicillin resistance; four MSs observing an increasing trend and five MSs observing a decreasing trend. Within each MS, similar levels of resistance to ciprofloxacin and nalidixic acid were observed from 2009 to 2017. Although slight, but statistically significant, an increasing trend for ciprofloxacin and nalidixic acid resistance was observed in Belgium and Romania; a decreasing trend to these compounds was observed in the Czech Republic. Resistance to cefotaxime was generally very low and, although the trend was slight, a statistically significant increasing trend was observed in Germany. Tetracycline resistance showed some fluctuations, tending to parallel that of ampicillin. A statistically significant increasing trend to tetracycline was observed in two MSs and a decreasing trend was observed in five MSs.
Figure 28.
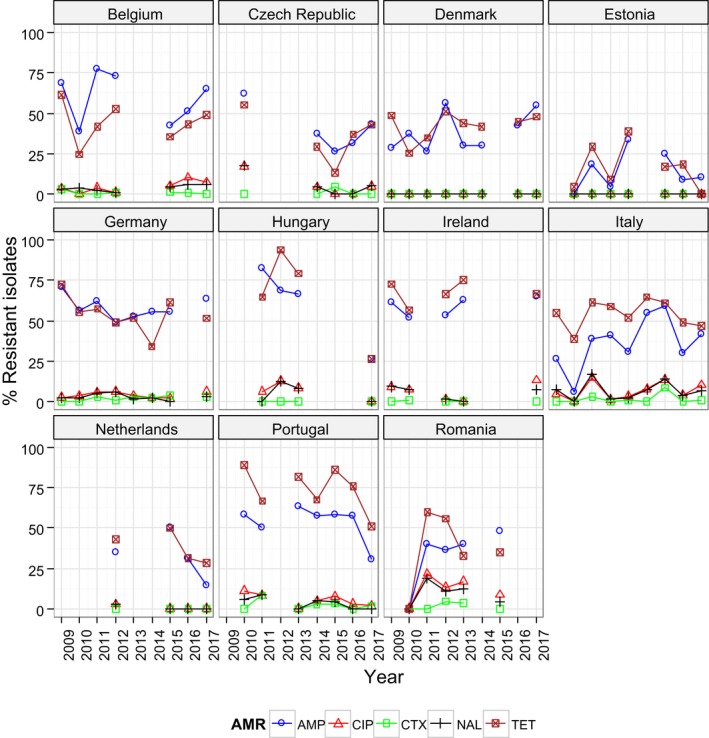
Trends in ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid (NAL) and tetracycline (TET) resistance in tested Salmonella spp. from pig meat and fattening pig carcases, 11 EU MSs, 2009–2017
- Statistical significant trends for 4 or more years were assessed using a logistic regression model (p ≤ 0.05). Statistically significant increasing trends were observed for ampicillin in Denmark (↑), Estonia (↑), Italy (↑) and Romania (↑), for cefotaxime in Germany (↑), for ciprofloxacin and nalidixic acid in Belgium (↑) and Romania (↑), as well as for tetracycline in the Czech Republic (↑) and Romania (↑).
- Statistically significant decreasing trends were observed for ampicillin in Belgium (↓), the Czech Republic (↓), Germany (↓), Hungary (↓) and Portugal (↓), for ciprofloxacin and nalidixic acid in the Czech Republic (↓), as well as for tetracycline in Belgium (↓), Estonia (↓), Germany (↓), Hungary (↓) and Portugal (↓).
As AMR is often associated with particular serovars or clones within serovars, fluctuations in the occurrence of resistance in Salmonella spp. within a country may be the result of changes in the proportions of different Salmonella serovars which contribute to the total numbers of Salmonella spp.
Information detailing serovar distribution reported from fattening pig carcases in 2017 is presented in Appendix A. Additionally, AMR data for S. Derby, monophasic S. Typhimurium and S. Typhimurium isolates recovered from fattening pig carcases are separately analysed in this corresponding appendix.
3.2.2. Antimicrobial resistance in Salmonella spp. recovered from caecal contents of fattening pigs
Resistance levels in Salmonella spp. from fattening pigs
In 2017, eight MSs reported voluntary data on isolates of Salmonella spp. recovered from caecal contents of fattening pigs at slaughter (Table 14). Notably, in 2 MSs, resistance levels were assessed on less than 10 isolates. The reported levels of resistance to ampicillin, sulfamethoxazole and tetracycline ranged from moderate to extremely high (14.3–76%) in Salmonella spp. from pigs in most reporting MSs; no resistance to these compounds was recorded by Sweden (N = 2). Overall resistance to chloramphenicol was moderate (14.6%), although resistance levels varied between reporting MSs from none to 24.5%. Similarly, trimethoprim resistance varied between reporting MSs from none to 36%, resulting in an overall high level (20.7%) considering all reporting countries. Gentamicin resistance was reported in Salmonella spp. from 4/7 countries, resulting in an overall low level of resistance; azithromycin resistance was detected by three MSs, again resulting in an overall low level of resistance. Overall, resistance to nalidixic acid and ciprofloxacin were observed at low (6.3%) and moderate levels (10.3%), respectively; an equivalent moderate level to both compounds was reported by Croatia (12.5%). Resistance to cefotaxime and ceftazidime were detected only by Spain and Italy at very low/low levels. No resistance to meropenem was reported in Salmonella isolates from pigs. Tigecycline resistance was detected in 4/7 reporting MSs, resulting in an overall low level of resistance. Although three MSs did not report colistin resistance, in the remaining MSs, resistance to this compound ranged from very low to moderate (0.6–14.3%) – see Table SALMFATPIG.
Table 14.
Occurrence of resistance (%) to selected antimicrobials in Salmonella spp. from fattening pigs, using harmonised ECOFFs, 8 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatia | 40 | 2.5 | 12.5 | 45 | 0 | 0 | 0 | 0 | 12.5 | 12.5 | 0 | 0 | 47.5 | 7.5 | 47.5 |
| Denmark | 44 | 2.3 | 2.3 | 29.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2.3 | 0 | 34.1 | 13.6 | 52.3 |
| Estoniaa | 7 | 0 | 0 | 14.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.3 | 14.3 | 28.6 | 14.3 |
| Germany | 49 | 0 | 24.5 | 71.4 | 0 | 0 | 0 | 8.2 | 2 | 6.1 | 0 | 2 | 73.5 | 20.4 | 71.4 |
| Italy | 118 | 8.5 | 9.3 | 38.1 | 2.5 | 2.5 | 0 | 0.8 | 2.5 | 5.9 | 0.8 | 2.5 | 48.3 | 8.5 | 49.2 |
| Netherlands | 50 | 0 | 10 | 76 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 6 | 68 | 16 | 58 |
| Spain | 164 | 9.8 | 21.3 | 67.1 | 1.2 | 0.6 | 0 | 0.6 | 12.8 | 20.7 | 6.1 | 0.6 | 72 | 36 | 75 |
| Swedena | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (8 MS) | 474 | 5.9 | 14.6 | 54.9 | 1.1 | 0.8 | 0 | 1.5 | 6.3 | 10.3 | 2.5 | 1.9 | 59.1 | 20.7 | 60.8 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates.
Spatial distribution of resistance in Salmonella spp. from fattening pigs
Considering MSs reporting data on 10 or more isolates (six MSs), the spatial distributions of cefotaxime and ciprofloxacin resistance in Salmonella isolates recovered from pigs are shown in Figure 6. Cefotaxime resistance was reported at a low level by two MSs from southern Europe (Italy and Spain; 2.5% and 1.2%, respectively). Ciprofloxacin resistance was reported at high to low levels by MSs from southern Europe (Spain: 20.7%; Croatia: 12.5%; Italy: 5.9%). A low level was also reported by one MS from western Europe (Germany: 6.1%).
Combined resistance to cefotaxime and ciprofloxacin in Salmonella spp. from fattening pigs
Italy and Spain were the only countries to report combined resistance to ciprofloxacin and cefotaxime in 2/118 and 1/164 Salmonella isolates, respectively (Figure 29; Table COMSALMFATPIG). ‘Microbiological’ combined resistance was not recorded in any other isolates from reporting countries (Figure 30).
Figure 29.
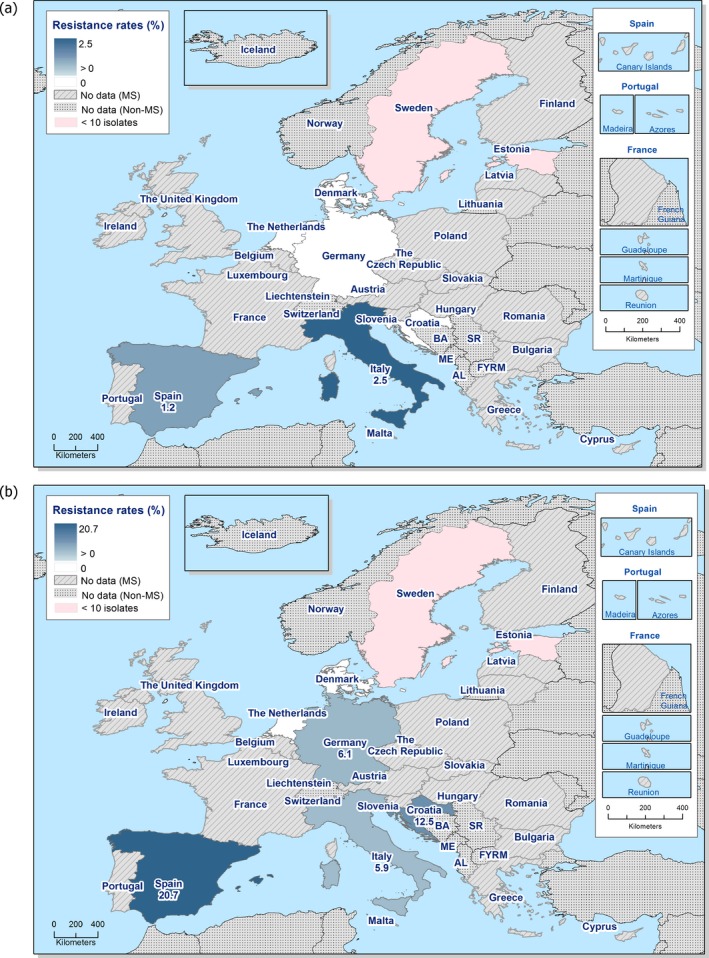
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella spp. from fattening pigs, using harmonised ECOFFs, 8 EU MSs, 2017
Figure 30.
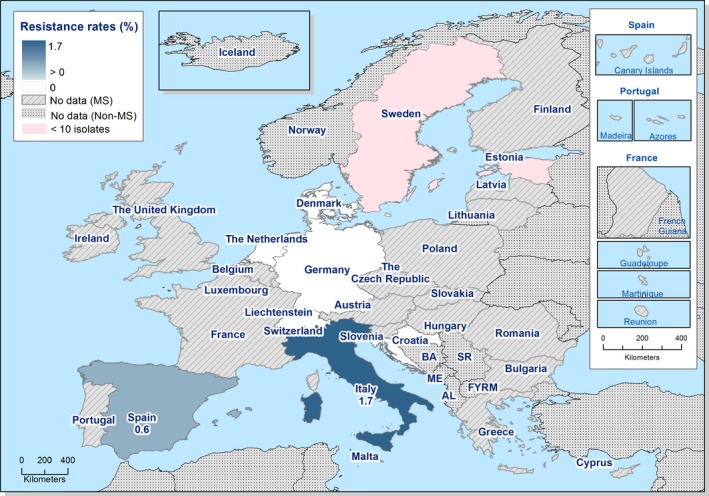
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in Salmonella spp. from fattening pigs, using harmonised ECOFFs, 8 EU MSs, 2017
Multidrug resistance in Salmonella spp. from fattening pigs
Eight reporting countries recorded data for individual isolates, which were included in the MDR analysis (N = 474). The proportion of Salmonella isolates which were multiresistant varied from 0% to 69.4% (Figure 31). Overall, 51.3% of Salmonella isolates were multiresistant. Notably, where MDR was not detected (0%), only two isolates were reported by the MS for assessment (Table COMSALMFATPIG).
Figure 31.
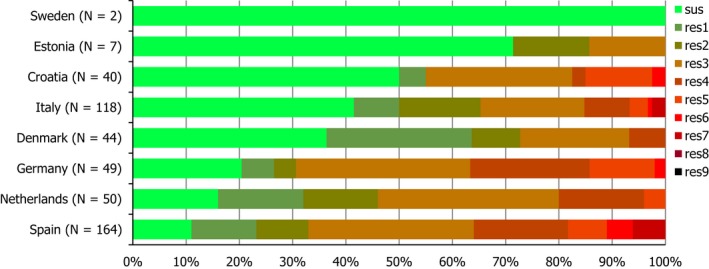
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella spp. from fattening pigs, 8 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Spatial distribution of complete susceptibility among Salmonella spp. from fattening pigs
The susceptibility of isolates to individual antimicrobials was determined using ECOFFs; all isolates were tested against the same mandatory panel of antibiotics. Considering only countries where 10 or more isolates were assessed, the percentage of completely susceptible Salmonella isolates ranged from moderate in Spain (11%) to high in Croatia (50%). Spatial distributions (Figure 32) demonstrated that complete susceptibility was higher in isolates reported by two MSs from southern Europe (Croatia and Italy) than in those reported by MSs from northern and western Europe (Denmark, Germany and the Netherlands). Overall, 27% of Salmonella isolates recovered from pigs were susceptible to all 11 antimicrobials used in the analysis (this figure includes countries which submitted data for 10 or less isolates), with complete susceptibility ranging from 11% to 100%. Notably, where complete susceptibility was detected at 100%, only two Salmonella isolates were reported by the MS for assessment (Table COMSALPIGMEAT).
Figure 32.
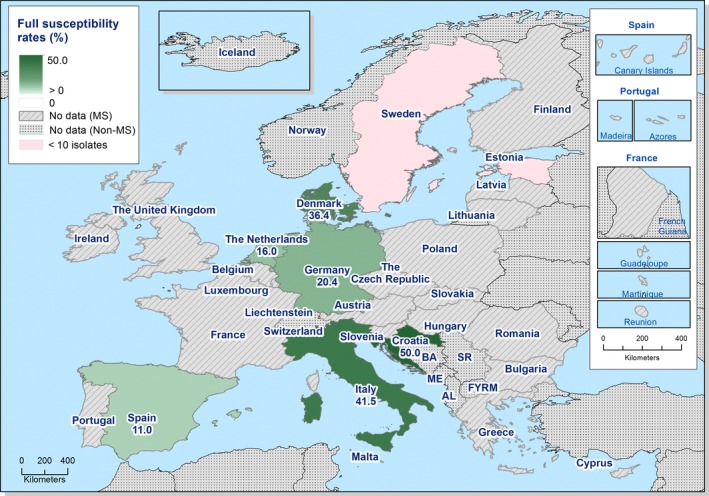
Spatial distribution of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from fattening pigs in reporting countries, 8 EU MSs, 2017, using harmonised ECOFFs
Temporal trends in resistance in Salmonella spp. from fattening pigs
Eight MSs provided resistance data for 4 or more years to be included in the statistical analysis of temporal trends (Figure 33). Over the 9 years of available data, the majority of reporting countries registered a statistical significant trend in ampicillin resistance; two MSs observing an increasing trend and three MSs observing a decreasing trend. Within each MSs, similar levels of resistance to ciprofloxacin and nalidixic acid were observed from 2009 to 2017. Although slight, but statistically significant, an increasing trend to these compounds was observed in Germany, and opposing trends to these compounds were registered by Ireland. Conversely, a decreasing trend to these compounds was observed in Italy. Additionally, Estonia registered an increasing trend to ciprofloxacin. Resistance to cefotaxime was generally very low. A statistically significant increasing trend to tetracycline was observed in the Netherlands and decreasing trends in Ireland and Italy. Tetracycline resistance exceeded ampicillin resistance in many MSs and, as observed in isolates from pig meat/pig carcases, fluctuations in resistance levels tended to parallel each other, maintaining the interval between tetracycline and ampicillin resistance.
Figure 33.
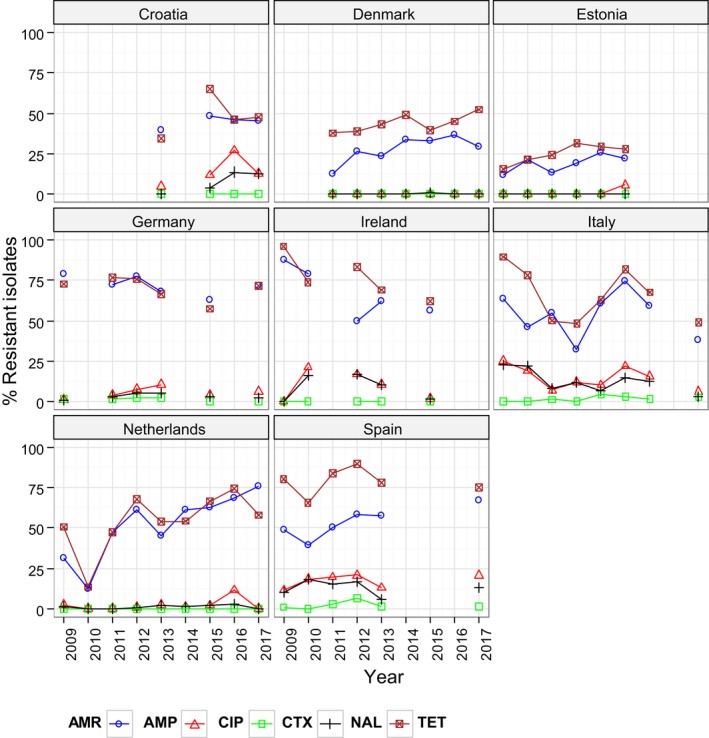
Trends in ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid (NAL) and tetracyclines (TET) resistance in Salmonella spp. from fattening pigs, using harmonised ECOFFs, 8 EU MSs, 2009–2017
- Statistical significant trends for 4 or more years were assessed using a logistic regression model (p ≤ 0.05). Statistically significant increasing trends were observed for ampicillin in Denmark (↑) and the Netherlands (↑), for ciprofloxacin in Estonia (↑), for nalidixic acid in Ireland (↑), for ciprofloxacin and nalidixic acid in Germany (↑), as well as for tetracycline in the Netherlands (↑).
- Statistically significant decreasing trends were observed for ampicillin in Germany (↓), Ireland (↓) and Italy (↓), for ciprofloxacin in Ireland (↓), for ciprofloxacin and nalidixic acid in Italy (↓), as well as for tetracycline in Ireland (↓) and Italy (↓).
As AMR is often associated with particular serovars or clones within serovars, fluctuations in the occurrence of resistance in Salmonella spp. within a country may be the result of changes in the proportions of different Salmonella serovars which contribute to the total numbers of Salmonella spp. isolates.
Information detailing serovar distribution reported from fattening pigs in 2017 is presented in Appendix B. Additionally, AMR data for S. Derby, monophasic S. Typhimurium and S. Typhimurium isolates recovered from fattening pigs are separately analysed in this corresponding appendix.
3.2.3. Antimicrobial resistance in Salmonella spp. recovered from carcase swabs of calves (less than 1 year of age)
Resistance levels in Salmonella spp. from carcases of calves of less than 1 year of age
In 2017, seven MSs reported data on isolates of Salmonella spp. recovered from the mandatory carcase swabbing of calves (under 1 year of age) at slaughter (Table 15). Notably, 21 MSs did not obtain any positive Salmonella isolates from calf carcases and, therefore, data are not presented for these countries. For five countries, resistance levels were assessed on less than 10 isolates. Generally, levels of resistance were lower than those observed in isolates recovered from fattening pig carcases. Overall, resistance to ampicillin, sulfamethoxazole and tetracycline were reported at high levels (24.4%, 30.5% and 28%, respectively); Croatia (N = 9) reported very high and extremely high levels of resistance to these compounds. A very high level of resistance to trimethoprim was also reported by Croatia, although overall resistance to this antimicrobial was observed at a moderate level (12.2%). Chloramphenicol resistance was reported by three MSs, resulting in an overall low level of resistance (6.1%), while gentamicin resistance was reported by only one MS, similarly resulting in a low level of resistance considering all reporting MSs (1.2%). Croatia and France were the only MSs to report resistance to ciprofloxacin and nalidixic acid, where resistance to these compounds were observed at equivalent levels within reporting countries; (11.1% in Croatia and 6.3% in France). Denmark was the only reporting MS to detect azithromycin resistance in a single Salmonella isolate, resulting in an overall low level of resistance to this compound. Similarly, Denmark was the only country to report colistin resistance in 3/5 isolates (all three isolates were attributed to S. Dublin), again resulting in an overall low level of resistance to this compound in view of all reporting MSs. No resistance to third‐generation cephalosporins, meropenem or tigecycline was reported in the Salmonella spp. isolated from calf carcases (Table SALMBOVCARCD).
Table 15.
Occurrence of resistance (%) to selected antimicrobials in Salmonella spp. from carcases of calves of less than 1 year of age, using harmonised ECOFFs, 7 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgiuma | 6 | 0 | 0 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 0 | 33.3 |
| Bulgariaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Croatiaa | 9 | 0 | 33.3 | 66.7 | 0 | 0 | 0 | 0 | 11.1 | 11.1 | 0 | 0 | 100 | 55.6 | 55.6 |
| Denmarka | 5 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 60 | 0 | 0 | 20 |
| France | 16 | 0 | 6.3 | 25 | 0 | 0 | 0 | 0 | 6.3 | 6.3 | 0 | 0 | 37.5 | 6.3 | 25 |
| Italya | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | 44 | 2.3 | 2.3 | 15.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 18.2 | 9.1 | 25 |
| Total (7 MSs) | 82 | 1.2 | 6.1 | 24.4 | 0 | 0 | 0 | 0 | 2.4 | 2.4 | 1.2 | 3.7 | 30.5 | 12.2 | 28 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Spatial distribution of resistance in Salmonella spp. from carcases of calves of less than 1 year of age
No resistance to cefotaxime was detected among Salmonella spp. isolated from calf carcases by seven MSs (Figure 34). Considering MSs reporting data on 10 or more isolates (two MSs), the spatial distributions of ciprofloxacin resistance in Salmonella isolates recovered from calf carcases are shown in Figure 34. Only one western European country (France) detected fluoroquinolone resistance (6.3%).
Figure 34.
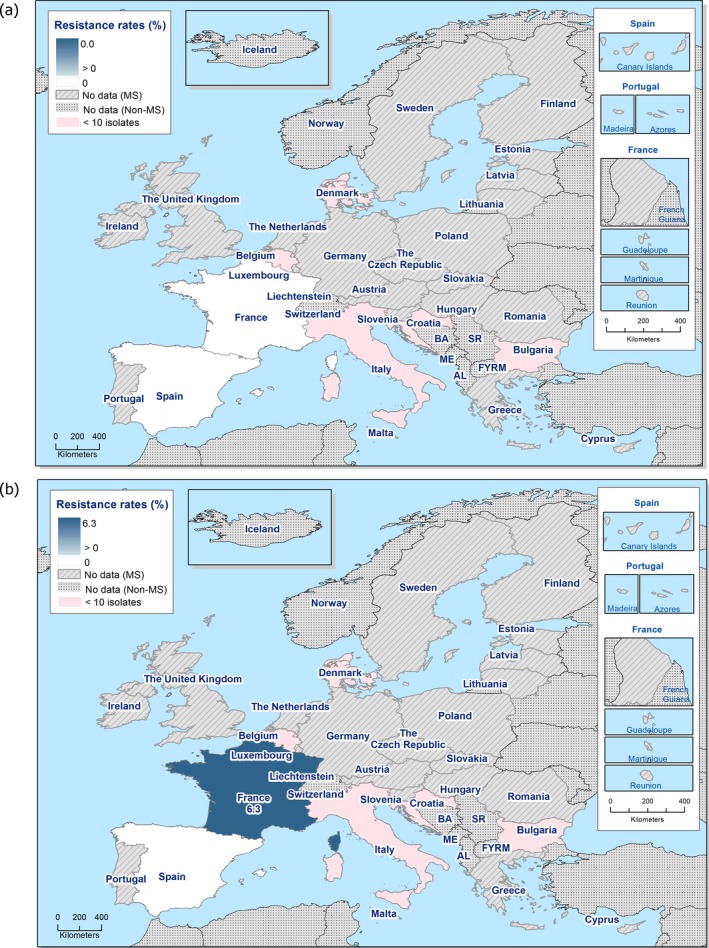
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella spp. from carcases of calves of less than 1 year of age, 7 EU MSs, 2017
Combined resistance to cefotaxime and ciprofloxacin in Salmonella spp. from carcases of calves of less than 1 year of age
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime in Salmonella isolates from calf carcases was not detected by any of the seven reporting MSs.
Multidrug resistance in Salmonella spp. from carcases of calves of less than 1 year of age
Seven reporting countries recorded data for individual isolates, which were included in the MDR analysis (N = 82). Levels of resistance were generally lower than those observed in carcases of fattening pigs. The proportion of Salmonella spp. which were multiresistant varied from 0% to 77.8% (Figure 35; Table COMSALMBOVMEAT). Overall, 22% of Salmonella isolates were multiresistant.
Figure 35.
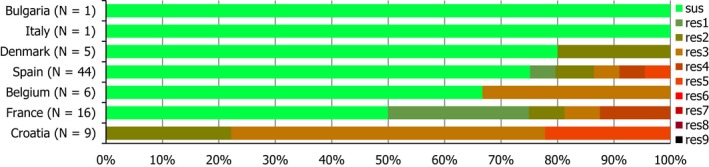
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella spp. from carcases of calves of less than 1 year of age, 7 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Spatial distribution of complete susceptibility in Salmonella spp. from carcases of calves of less than 1 year of age
The susceptibility of isolates to individual antimicrobials was determined using ECOFFs; all isolates were tested against the same mandatory panel of antibiotics. Considering only countries where 10 or more isolates were assessed (only two MSs), spatial distributions (Figure 36) demonstrated that complete susceptibility was higher in one MS from southern Europe (Spain) than in one MS from western Europe (France); where extremely high and high levels were observed (75% and 50%, respectively). Overall, 62.2% of Salmonella isolates recovered from calf carcases were susceptible to all 11 antimicrobials used in the analysis (this figure includes countries which submitted data for 10 or less isolates), with complete susceptibility ranging from 0% to 100%. Notably, where complete susceptibility was detected at 100%, only one Salmonella isolate was reported by MSs for assessment (Table COMSALMBOVMEAT).
Figure 36.
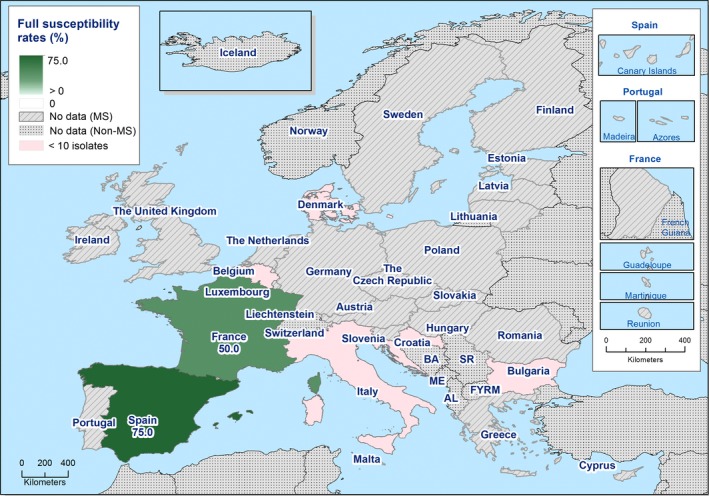
Spatial distribution of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from carcases calves of less than 1 year of age in reporting countries, 7 EU MSs, 2017, using harmonised ECOFFs
Information detailing serovar distribution reported from calf carcases in 2017 is presented in Appendix C. Additionally, AMR data for monophasic S. Typhimurium, S. Typhimurium, S. Meleagridis and S. Mbandaka isolates recovered from calf carcases are separately analysed in this corresponding appendix.
3.2.4. Antimicrobial resistance in Salmonella spp. recovered from caecal contents of cattle
Resistance levels in Salmonella spp. from cattle
In 2017, seven MSs and one non‐MS reported voluntary data on isolates of Salmonella spp. recovered from caecal contents of cattle at slaughter (Table 4). Notably, in three countries, resistance levels were assessed on less than 10 isolates. Considering all reporting MSs, resistance to ampicillin, chloramphenicol, sulfamethoxazole and tetracycline were observed at high levels (30%, 22.7%, 42.7% and 40.9%, respectively), with Italy (N = 26) reporting extremely high levels of resistance to sulfamethoxazole and tetracycline (84.6% and 88.5%, respectively). Resistance to trimethoprim and ciprofloxacin were observed at moderate levels considering all reporting MSs (14.5% and 12.7%, respectively), with Italy reporting high levels of resistance to these compounds. Similarly, Italy reported a high level of resistance to nalidixic acid, resulting in an overall low level of resistance to this antimicrobial considering all reporting MSs (10%). The Netherlands were the only country to report azithromycin resistance in two isolates, while Italy was the only country to report tigecycline resistance in a single isolate. Overall resistance to these antimicrobials were noted at low and very low levels, respectively. Similarly, Italy and the Netherlands were the only MSs to report resistance to gentamicin, resulting in an overall moderate level considering all reporting MSs. Three MSs reported resistance to colistin, resulting in an overall moderate level of resistance to this compound (14.5%); notably, the Netherlands reported 14 colistin‐resistant Salmonella spp. (all of which were attributed to S. Dublin). No resistance to cefotaxime, ceftazidime or meropenem was reported in the Salmonella spp. recovered from cattle (Table SALMCATT).
Spatial distribution of resistance in Salmonella spp. from cattle
Resistance to cefotaxime was not detected among Salmonella spp. recovered from cattle by the seven reporting MSs and one non‐MS (Figure 37). Considering countries reporting data on 10 or more isolates (four MSs and one non‐MS), the spatial distributions of ciprofloxacin resistance in Salmonella isolates recovered from cattle are shown in Figure 37b. Ciprofloxacin resistance was observed at a high to low level by MSs from southern Europe (Italy: 42.3%; Spain: 6.3%) at a low level in one MS from western Europe (the Netherlands: 2.5%) and was not detected by any other countries reporting data on 10 or more isolates (Table 16).
Figure 37.
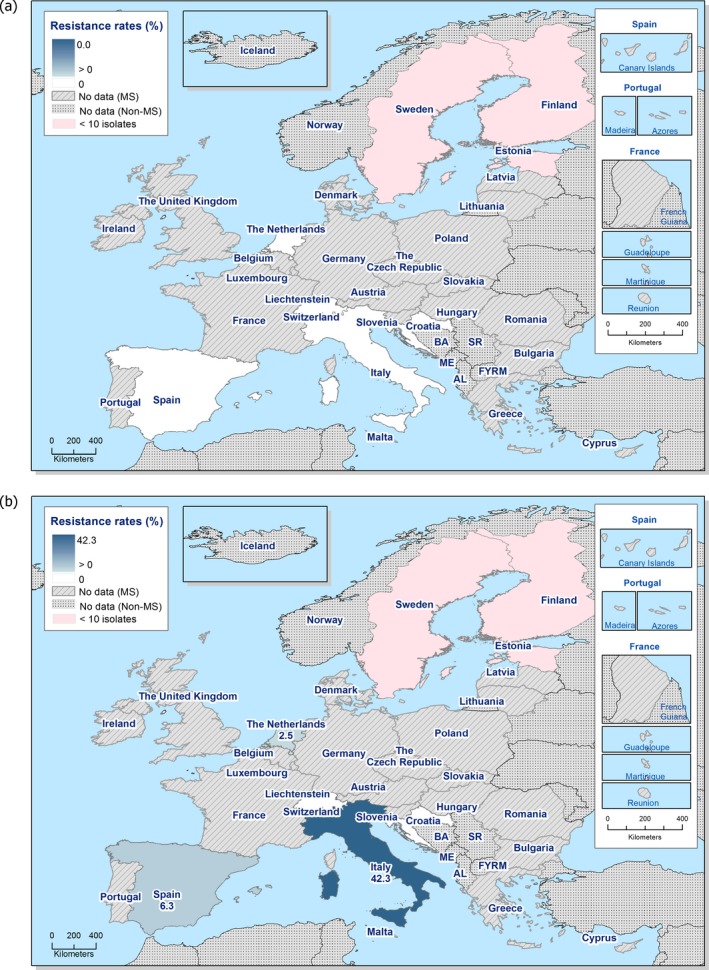
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance in Salmonella spp. from cattle, 7 EU MSs and 1 non‐EU MS, 2017
Table 16.
Occurrence of resistance (%) to selected antimicrobials in Salmonella spp. from cattle, using harmonised ECOFFs, 7 EU MSs and 1 non‐MS, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatia | 14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.3 | 14.3 | 0 |
| Estoniaa | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 25 | 0 | 0 | 0 |
| Finlanda | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | 26 | 11.5 | 38.5 | 57.7 | 0 | 0 | 0 | 3.8 | 34.6 | 42.3 | 0 | 0 | 84.6 | 23.1 | 88.5 |
| Netherlands | 40 | 22.5 | 35 | 37.5 | 0 | 0 | 0 | 0 | 2.5 | 2.5 | 5 | 35 | 47.5 | 17.5 | 45 |
| Spain | 16 | 0 | 6.3 | 18.8 | 0 | 0 | 0 | 0 | 6.3 | 6.3 | 0 | 0 | 25 | 6.3 | 25 |
| Swedena | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 25 | 0 | 0 | 0 |
| Total (7 MSs) | 110 | 10.9 | 22.7 | 30.0 | 0 | 0 | 0 | 0.9 | 10.0 | 12.7 | 1.8 | 14.5 | 42.7 | 14.5 | 40.9 |
| Switzerland | 66 | 0 | 3 | 30.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 31.8 | 0 | 34.8 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Combined resistance to ciprofloxacin and cefotaxime in Salmonella spp. from cattle
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among Salmonella spp. from cattle was not detected by any of the eight reporting countries (Figure 38). Clinical resistance was also therefore not detected.
Figure 38.
Spatial distribution of co‐resistance to cefotaxime and ciprofloxacin resistance in Salmonella spp. from cattle, 7 EU MSs and 1 non‐MS, 2017
Multidrug resistance and complete susceptibility in Salmonella spp. from cattle
Eight reporting countries recorded data for individual isolates, which were included in the MDR analysis (N = 176). The proportion of Salmonella isolates which were multiresistant varied between countries from 0% to 61.5% (Figure 39; Table COMSALMBOV). Overall, 29.5% of Salmonella isolates were multiresistant.
Figure 39.
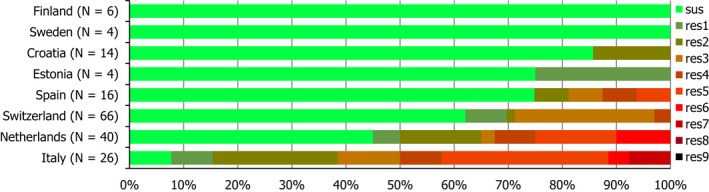
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella spp. from cattle, 7 EU MSs and 1 non‐EU MS, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Spatial distribution of complete susceptibility in Salmonella spp. from cattle
The susceptibility of isolates to individual antimicrobials was determined using ECOFFs; all isolates were tested against the same mandatory panel of antibiotics. Considering only countries where 10 or more isolates were assessed (four MSs and one non‐MS), spatial distributions (Figure 40) demonstrated that complete susceptibility was variable, ranging from low to extremely high in isolates reported by MSs from southern Europe (Italy: 7.7%, Spain: 75%, Croatia: 85.7%), and high to very high in isolates reported by countries from western Europe (the Netherlands: 45%, Switzerland: 62.1%). Overall, 55.7% of Salmonella isolates recovered from cattle were susceptible to all 11 antimicrobials used in the analysis (this figure includes countries which submitted data for 10 or less isolates), with complete susceptibility ranging from 7.7% to 100% – see Table COMSALMBOV.
Figure 40.
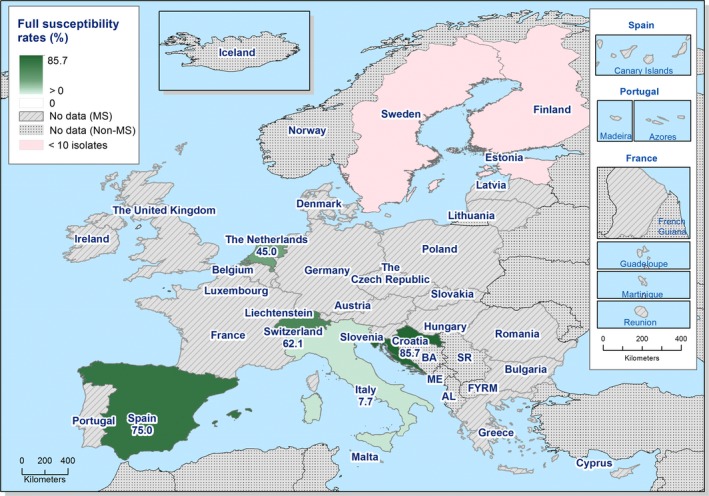
Spatial distribution of complete susceptibility to the panel of antimicrobials tested among Salmonella spp. from cattle, 7 EU MSs and 1 non‐EU MS, 2017, using harmonised ECOFFs
Temporal trends in resistance among Salmonella spp. from cattle
Four MSs provided resistance data for four or more years to be included in the statistical analysis of temporal trends (Figure 18). Over the nine years of available data, levels of cefotaxime resistance were generally very low in each MS. Within each MS, similar levels of resistance to ciprofloxacin and nalidixic acid were observed from 2009 to 2017. A statistically significant increasing trend of resistance to these compounds was observed in Italy. The levels of ampicillin resistance remained fairly constant in Sweden, while a statistically significant decreasing trend was observed in Finland and an increasing trend was observed in Italy and the Netherlands. A statistically significant increasing trend of resistance to tetracycline was also observed in Italy and the Netherlands. As observed in isolates from pig meat/pig carcases and fattening pigs, fluctuations in ampicillin and tetracycline resistance levels tended to parallel each other.
As AMR is often associated with particular serovars or clones within serovars, fluctuations in the occurrence of resistance in Salmonella spp. within a country may be the result of changes in the proportions of different Salmonella serovars which contribute to the total numbers of Salmonella spp. isolates.
Figure 41.
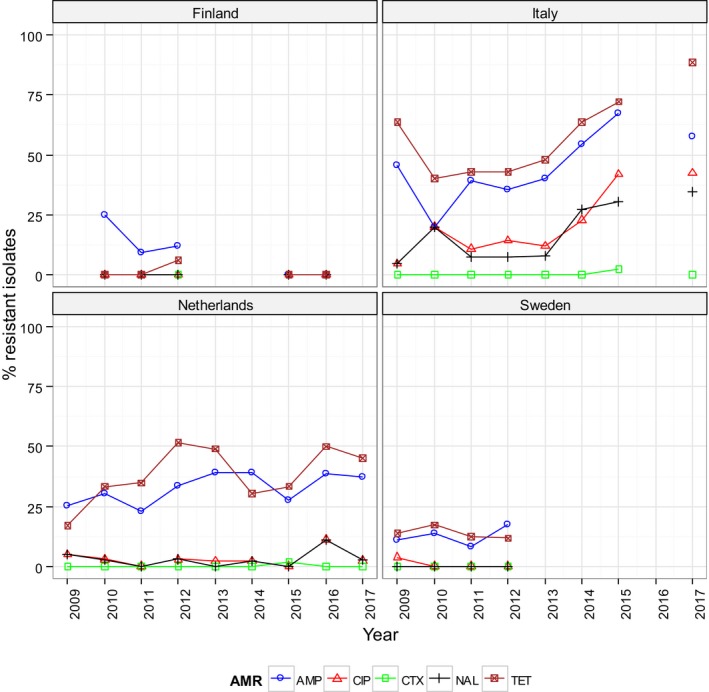
Trends in ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid (NAL) and tetracycline (TET) resistance in tested Salmonella spp. from cattle, 4 EU MSs, 2009–2017
- Statistical significant trends for 4 or more years were assessed using a logistic regression model (p ≤ 0.05). Statistically significant increasing trends were observed for ampicillin in Italy (↑) and the Netherlands (↑), for ciprofloxacin and nalidixic acid in Italy (↑), as well as for tetracycline in Italy (↑) and the Netherlands (↑).
- Statistically significant decreasing trends were observed for ampicillin in Finland (↓).
Information detailing serovar distribution reported from cattle in 2017 is presented in Appendix D. Additionally, AMR data for S. Typhimurium, monophasic S. Typhimurium and S. Dublin isolates recovered from cattle are separately analysed in this corresponding appendix.
3.2.5. Analyses of high‐level cefotaxime and ciprofloxacin resistance
Fluoroquinolones and third‐generation cephalosporins are internationally recognised as highest priority CIA in human medicine (Urdahl et al., 2017). These classes include ciprofloxacin and cefotaxime/ceftazidime; compounds which are specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp., as stipulated by Decision 2013/652/EU. Although fluoroquinolones are not recommended for use in children, these CIA often constitute first‐line treatment for invasive salmonellosis in humans; and as such, resistance to these compounds in Salmonella spp. originating from animals is of concern. Furthermore, fluoroquinolones and third/fourth‐generation cephalosporins may be used in veterinary medicine for the treatment of pigs and cattle in Europe, which underlines the importance of monitoring resistance to these compounds in zoonotic bacteria from animals.
Comparison of ‘clinical’ and ‘microbiological’ resistance to cefotaxime
Where an MIC value to a particular antimicrobial is above that of the EUCAST epidemiological cut‐off, the isolate is termed as exhibiting ‘microbiological’ resistance; ‘clinical’ resistance is noted when an MIC value to a particular antimicrobial is above that of the EUCAST CBP.
Overall, a very low level of ‘microbiological’ and ‘clinical’ resistance to cefotaxime was reported in Salmonella spp. from fattening pig carcases and fattening pigs (0.5% and 1.1%, respectively) – see Table 5. All Salmonella spp. exhibiting ‘microbiological’ resistance (MIC > 0.5 mg/L) from pig carcases and fattening pigs showed equivalent ‘clinical’ resistance (MIC > 2 mg/L) indicating that the isolates detected as cefotaxime‐resistant exhibited resistance above the CBP.
In Salmonella spp. from pig carcases, a low level of resistance was reported by Germany, Portugal and Spain, and a high level was reported by Lithuania (N = 2). In Salmonella spp. from pigs, Italy and Spain were the only countries to report resistance to cefotaxime (at low levels of 2.5% and 1.2%, respectively).
Neither ‘microbiological’ nor ‘clinical’ resistance to cefotaxime in Salmonella spp. from calf carcases or cattle was detected.
Quinolone/fluoroquinolone (i.e. nalidixic acid/ciprofloxacin) resistance in Salmonella usually arises due to point mutations within the DNA gyrase (gyrA and gyrB) and topoisomerase IV (parC and parE) genes, at locations comprising the quinolone resistance‐determining regions (QRDR) of the bacterial chromosome. Additionally, plasmid‐mediated quinolone resistance (PMQR) mechanisms have also been recognised, including the action of efflux pumps (qepA gene), enzymatic modifications (aac(6′)Ib‐cr gene – also conferring resistance to kanamycin), and protection of the DNA gyrase (qnrA, qnrB, qnrD and qnrS genes) (Cavaco et al., 2009; Dionisi et al., 2009).
The CBP for ciprofloxacin in Salmonella has been lowered by EUCAST from > 1 mg/L to > 0.06 mg/L, resulting in the CBP and ECOFF (microbiological breakpoint) for ciprofloxacin applying the same threshold (MIC > 0.064 mg/L). The presence of two single point mutations in the QRDR will usually confer resistance to ciprofloxacin, with isolates typically exhibiting MICs of > 1 mg/L, as well as conferring resistance to nalidixic acid. In contrast, isolates harbouring only one single point mutation in the QRDR will usually still display resistance to ciprofloxacin and nalidixic acid, but the degree of resistance to ciprofloxacin is reduced (MIC > 0.064 mg/L). Salmonella isolates causing systemic infections in humans and displaying MICs of > 0.064 mg/L but < 1 mg/L, have shown a poor response to treatment in some studies. This provides the rationale for setting the CBP at > 0.064 mg/L and it follows that monitoring of low‐level resistance to this compound is therefore indicated.
In the absence of other fluoroquinolone resistance mechanisms, the presence of PMQR determinants (i.e. primarily qnr genes) in a bacterium usually confers resistance to ciprofloxacin, with an MIC of > 0.064 mg/L, but the isolate will be susceptible to nalidixic acid. This contrasts with mutation in the QRDR regions of the bacterial chromosome, which confer resistance to both ciprofloxacin and nalidixic acid.
Analysis of high‐level ciprofloxacin resistance
In Salmonella, an isolate exhibiting a ciprofloxacin MIC of ≥ 4 mg/L is defined as demonstrating high‐level resistance to this compound. In 2017, no Salmonella isolates displayed high‐level resistance to ciprofloxacin, according to this distinction (Tables: HIGHSALMPIGMEAT; HIGHSALMBOVCARC; HIGHSALMFATPIG; HIGHSALMBOV) (Table 17).
Table 17.
Occurrence of resistance (%) to cefotaxime among Salmonella spp. from carcases of fattening pigs, fattening pigs, carcases of calves under one year age and cattle in 2017, using harmonised ECOFFs and EUCAST CBPs
| Countrya | Carcases of fattening pigs | Fattening pigs | Carcases of calves (< 1 year) | Cattle | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n res. ECOFF | % res. ECOFF | n res. CBP | % res. CBP | N | n res. ECOFF | % res. ECOFF | n res. CBP | % res. CBP | N | n res. ECOFF | % res. ECOFF | n res. CBP | % res. CBP | N | n res. ECOFF | % res. ECOFF | n res. CBP | % res. CBP | |
| Belgium | 98 | 0 | 0 | 0 | 0 | – | – | – | – | – | 6 | 0 | 0 | 0 | 0 | – | – | – | – | – |
| Bulgaria | 6 | 0 | 0 | 0 | 0 | – | – | – | – | – | 1 | 0 | 0 | 0 | 0 | – | – | – | – | – |
| Croatia | 25 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 14 | 0 | 0 | 0 | 0 |
| Cyprus | 4 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Czech Republic | 21 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Denmark | 69 | 0 | 0 | 0 | 0 | 44 | 0 | 0 | – | – | 5 | 0 | 0 | 0 | 0 | – | – | – | – | – |
| Estonia | 9 | 0 | 0 | 0 | 0 | 7 | 0 | 0 | – | – | – | – | – | – | – | 4 | 0 | 0 | 0 | 0 |
| Finland | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 6 | 0 | 0 | 0 | 0 |
| France | 206 | 0 | 0 | 0 | 0 | – | – | – | – | – | 16 | 0 | 0 | 0 | 0 | – | – | – | – | – |
| Germany | 31 | 1 | 3.2 | 1 | 3.2 | 49 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – |
| Greece | 1 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Hungary | 19 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ireland | 54 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Italy | 124 | 0 | 0 | 0 | 0 | 118 | 3 | 2.5 | 3 | 2.5 | 1 | 0 | 0 | 0 | 0 | 26 | 0 | 0 | 0 | 0 |
| Latvia | 1 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Lithuania | 2 | 1 | 50 | 1 | 50 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Malta | 17 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Netherlands | – | – | – | – | – | 50 | 0 | 0 | 0 | 0 | – | – | – | – | – | 40 | 0 | 0 | 0 | 0 |
| Poland | 24 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Portugal | 34 | 1 | 2.9 | 1 | 2.9 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Romania | 6 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Slovakia | 19 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Spain | 180 | 2 | 1.1 | 2 | 1.1 | 164 | 2 | 1.2 | 2 | 1.2 | 44 | 0 | 0 | 0 | 0 | 16 | 0 | 0 | 0 | 0 |
| Sweden | – | – | – | – | – | 2 | 0 | 0 | 0 | 0 | – | – | – | – | – | 4 | 0 | 0 | 0 | 0 |
| United Kingdom | 4 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Total | 954 | 5 | 0.5 | 5 | 0.5 | 474 | 5 | 1.1 | 5 | 1.1 | 82 | 0 | 0 | 0 | 0 | 110 | 0 | 0 | 0 | 0 |
| Iceland | 6 | 0 | 0 | 0 | 0 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Switzerland | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 66 | 0 | 0 | 0 | 0 |
CBP: clinical breakpoint; ECOFFs: epidemiological cut‐off values; EUCAST: European Committee on Antimicrobial Susceptibility Testing; N: number of isolates tested; n: number of isolates resistant; % res: percentage of resistant isolates.
Where the occurrence of resistance is assessed on less than 10 isolates and, should only be considered as part of the total from all MSs data.
3.2.6. Tigecycline resistance in Salmonella spp
The WHO also recognises tigecycline as a CIA, which constitutes a second‐line agent for the treatment of human salmonellosis infections. MSs reported tigecycline resistance in 1.5% of 474 Salmonella spp. from fattening pigs, 1.4% of 954 Salmonella spp. from fattening pig carcases, 0.9% of 110 Salmonella spp. from cattle and was not detected in Salmonella spp. from calf carcases under 1 year of age (N = 82). Considering individual countries reporting tigecycline resistance, certain features relating to resistance are evident. Considering the 7 resistant isolates recovered from pigs, Germany reported 4 of these, while France reported seven of the 13 resistant isolates recovered from pig carcases.
Additionally, certain serovars displayed ‘microbiological’ resistance to tigecycline, which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars. For instance, 4/7 tigecycline‐resistant isolates from pigs belonged to serovar S. Typhimurium, all of which were also resistant to ampicillin, sulfamethoxazole, trimethoprim and tetracycline. Those reported from pig carcases (N = 13) were mostly attributed to S. Typhimurium (N = 4) and S. Rissen (N = 7); with ampicillin, chloramphenicol, sulfamethoxazole, trimethoprim and tetracycline resistance a feature of all the S. Typhimurium isolates, as well as (fluoro)quinolone resistance in 2/4 S. Typhimurium isolates (one of which also showed resistance to gentamicin). Conversely, MDR among the tigecycline‐resistant S. Rissen isolates was not a common feature, whereby MDR was only reported in a single isolate from Spain and in 1/6 tigecycline‐resistant S. Rissen isolates from France; and although differing resistance patterns were observed in these two isolates, both exhibited sulfamethoxazole, trimethoprim and tetracycline resistance.
All countries detecting tigecycline resistance reported low or very low levels, and those reporting results for fewer than 40 isolates did not detect tigecycline resistance in Salmonella spp., with the exception of single isolates recovered from pig carcases by Germany and Portugal, and a single isolate recovered from cattle by Italy (all S. Typhimurium).
The tigecycline MIC distributions for Salmonella spp. from fattening pigs and cattle, in addition to carcases of fattening pigs and calves (under one year) are shown below. As depicted in Figure 19, the ECOFF for tigecycline is 1 mg/L, and therefore, ‘microbiological’ resistance is observed when an isolate has an MIC of > 1 mg/L. As shown below, a proportion of isolates displayed a tigecycline MIC of 1 mg/L; and given this distribution, ‘microbiological’ resistance may be exhibited in some of these isolates due to the inherent variation of the MIC method and the proximity of the MIC to the microbiological breakpoint.
Figure 42.
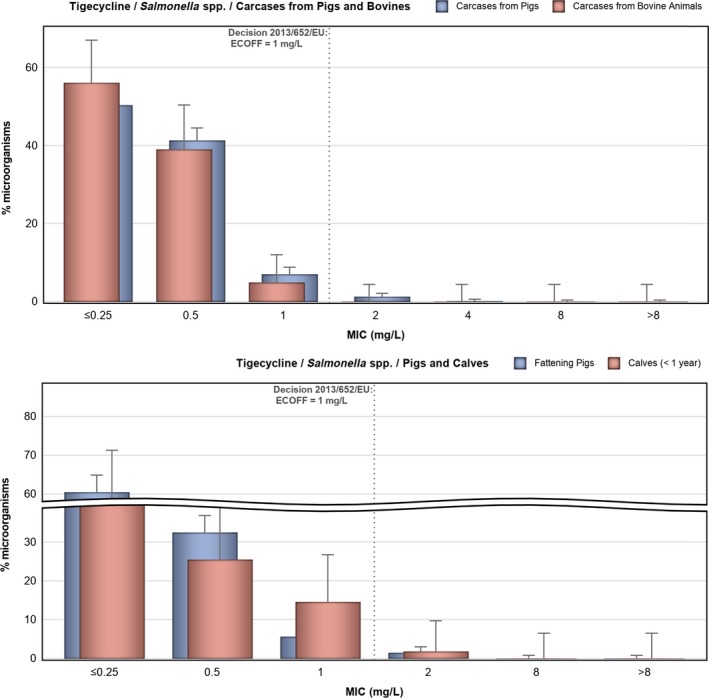
Tigecycline resistance in Salmonella spp. from carcases of pigs (fatteners) and calves (under one year), fattening pigs and cattle
3.2.7. Colistin resistance in Salmonella spp.
Colistin is a nephrotoxic antimicrobial compound, belonging to the polymyxin class and considered as a CIA for the treatment of serious human infections (Urdahl et al., 2017). Although not frequently used in human medicine due to its nephrotoxic effects, colistin has been widely used in veterinary medicine for prophylactic/metaphylactic treatment and formerly as a growth promoter in several countries (Kieffer et al., 2017). MSs reported colistin resistance in 14.5% of 110 Salmonella spp. recovered from cattle, 3.7% of 82 Salmonella spp. from calf carcases (under 1 year of age), 1.9% of 474 Salmonella spp. from fattening pigs and 0.6% of 954 Salmonella spp. from fattening pig carcases. Figure 20 shows the distribution of colistin MICs for isolates recovered from these animal origins against the clinical/microbiological threshold of > 2 mg/L. As is the case with tigecycline, isolates with MICs close to the threshold will be subject to inherent variation of the MIC method. Isolates resistant to colistin are categorised by serovar in Table 18.
Table 18.
Distribution of MICs of colistin by serovar in Salmonella spp. from carcases of pigs (fatteners) and calves (under 1 year of age), fattening pigs and cattle, 8 EU MSs, 2017
| Salmonella serovar | MIC | Total | |||
|---|---|---|---|---|---|
| 4 | 8 | 16 | > 16 | ||
| Pig carcases (fatteners) | |||||
| S. 1,4,[5],12:i:‐ (2 DE) | 1 | 1 | – | – | 2 |
| S. Derby (1 DE) | – | – | – | 1 | 1 |
| S. Ohio (1 DE) | – | 1 | – | – | 1 |
| Monophasic S. Typhimurium (2 ES) | 2 | – | – | – | 2 |
| Total | 3 | 2 | 0 | 1 | 6 |
| Calf carcases (< 1 year of age) | |||||
| S. Dublin (2 DK) | 2 | – | – | – | 2 |
| S. Dublin (1 DK) | – | 1 | – | – | 1 |
| Total | 2 | 1 | 0 | 0 | 3 |
| Fattening pigs | |||||
| S. Derby (1 EE) | – | – | 1 | – | 1 |
| S. Typhimurium (1 DE) | – | 1 | – | – | 1 |
| S. Bredeney (1 IT) | 1 | – | – | – | 1 |
| Monophasic S. Typhimurium (2 IT) | – | 2 | – | – | 2 |
| S. 4,12:i:‐ (1 NL) | 1 | – | – | – | 1 |
| S. Derby (1 NL) | 1 | – | – | – | 1 |
| S. Dublin (1 NL) | 1 | – | – | – | 1 |
| Monophasic S. Typhimurium (1 ES) | |||||
| Total | 4 | 3 | 1 | 0 | 9 |
| Cattle | |||||
| S. Dublin (1 EE) | 1 | – | – | – | 1 |
| S. Dublin (14 NL) | 14 | – | – | – | 14 |
| S. Dublin (1 SE) | 1 | – | – | – | 1 |
| Total | 16 | 0 | 0 | 0 | 16 |
DE: Germany; ES: Spain; DK: Denmark; EE: Estonia; IT: Italy; NL: Netherlands; SE: Sweden; MIC: minimum inhibitory concentration.
Notably, group D salmonellas (serogroup O9), which include Salmonella Dublin (antigenic formula: 1,9,12[Vi]:g,p:‐), are reported to show higher intrinsic levels of resistance to colistin than other serogroups; exemplified in 2017 by the proportion of colistin‐resistant isolates belonging to serovar S. Dublin. In particular, all colistin‐resistant isolates from calf carcases (N = 3) and cattle (N = 16) were serotyped as S. Dublin, with the Netherlands reporting 14 of these isolates from cattle. The other Salmonella serovars reported in Table 6 do not belong to serogroup O9 and while some display resistance only one dilution above the breakpoint (> 2 mg/L), others show higher levels of resistance.
Considering S. Derby and monophasic S. Typhimurium isolates reported from pig carcases by MSs (N = 254 and N = 332, respectively), only a single S. Derby isolate was resistant to colistin, while 4/6 colistin‐resistant isolates detected were monophasic S. Typhimurium, originating from Germany and Spain. Mechanisms of polymyxin resistance in Gram‐negative bacteria have been described (lipopolysaccharide modifications, efflux pumps, capsule formation and overexpression of membrane protein – Olaitan et al., 2014); and transferable mobile colistin resistance (mcr) genes have also been detected in Salmonella isolates (Campos et al., 2016; Carnevali et al., 2016). In an Italian study, Carnevali et al. (2016) detected mcr‐1 in a number of Salmonella serovars, of which monophasic S. Typhimurium was the most frequent (isolates from pigs, pork and man) and S. Derby was the second most frequently found (isolates from pigs). Interestingly, among the colistin‐resistant isolates recovered from pig carcases, 4/6 were reported by Germany (two monophasic S. Typhimurium, one S. Derby and one S. Ohio); Germany also reported that 0.4% of 227 indicator E. coli from pig carcases were resistant to colistin (Section 5).
Concerning individual serovars from pigs, 4/9 colistin‐resistant isolates detected were monophasic S. Typhimurium; the remaining colistin‐resistant Salmonella spp. were S. Derby (two isolates) with single isolates of S. Typhimurium, S. Bredeney and S. Dublin.
The S. Derby isolates in which the highest colistin MICs were observed, originated from a pig carcase in Germany (MIC > 16 mg/L) and a fattening pig in Estonia (MIC 16 mg/L). Estonia also reported that 0% of 67 indicator E. coli from fattening pigs were resistant to colistin (Section 5).
Figure 43.
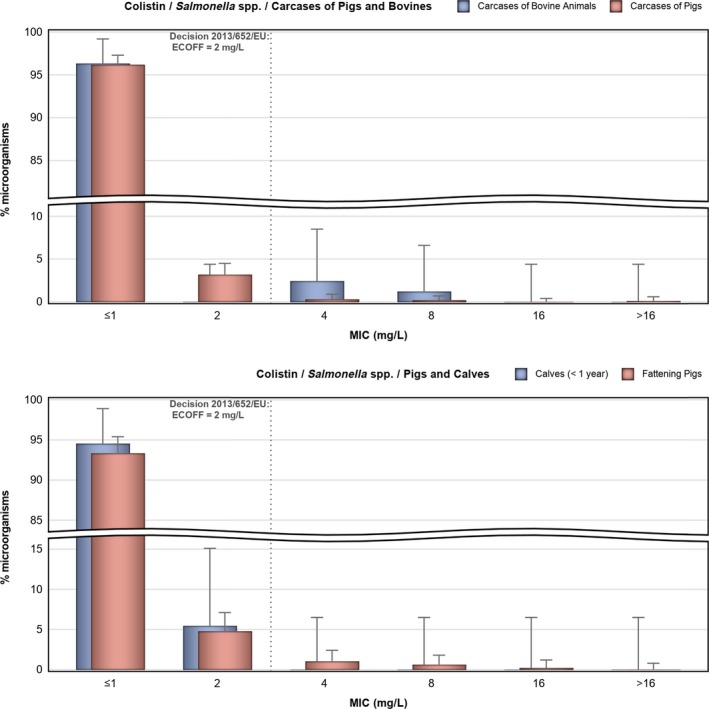
Colistin resistance in Salmonella spp. from carcases of pigs (fatteners) and calves (under one year), fattening pigs and cattle
3.2.8. Multidrug resistance patterns in certain Salmonella serovars
Considering Salmonella spp. data, AMR patterns are greatly reflected by serovar diversity, due to the propensity of different types to exhibit AMR. Therefore, levels of MDR among Salmonella spp. are subject to the serovars reported and interpretation should take this into account. For instance in 2017, S. Derby exhibited much lower MDR than monophasic S. Typhimurium and S. Typhimurium.
3.2.8.1.
Salmonella spp.
The patterns of AMR exhibited by all reported Salmonella isolates revealed numerous combinations of resistance to the different antimicrobial agents used in the analysis. The occurrence of specific MDR profiles reported by MSs in carcases of pigs and calves, fattening pigs and cattle (obtained from caecal contents) are presented in the MDR pattern tables. In pigs, eight serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Brandenburg, London, Bredeney and Kapemba) accounted for 89.5% of Salmonella spp. A further 25 serovars were also reported from pigs, with 13 serovars represented by single isolates (Table SERFATPIGS). In cattle, nine serovars (Typhimurium, monophasic Typhimurium, Dublin, Enteritidis, Derby, Mbandaka, Agona, Coeln and Rissen) accounted for 90.9% of Salmonella spp.; single isolates of a further 16 serovars were also reported (Table SERCATT).
In pig carcases, seven serovars (monophasic Typhimurium, Derby, Typhimurium, Rissen, Infantis, London and Brandenburg) accounted for 88% of Salmonella spp. (Table SERPIGCARCD); numerous other serovars were also reported. In carcases of calves of less than 1 year of age, 11 serovars (monophasic Typhimurium, Meleagridis, Mbandaka, Derby, Dublin, Livingstone, Montevideo, Typhimurium, Braenderup, Muenchen and Rissen), accounted for 76.8% of Salmonella spp. (Table SEROBOVMEATD), with a further 15 other reported serovars.
Detailed analysis of the specific patterns of resistance detected is most useful when performed at the serovar level. However, the overall data from all Salmonella spp. have also been examined to determine the pattern most common in highly prevalent sources per country. In pig carcases, where 455/960 (47.4%) isolates were MDR (Table MULTISALMPIGMEAT) and pigs where 243/474 (51.3%) isolates were MDR (Table MULTISALMFATPIG), the most common resistance profile was to ampicillin, sulfamethoxazole and tetracycline; followed by the same pattern with the addition of trimethoprim in both pig carcases and pigs. Where MDR was detected, this resistance profile (resistance to only ampicillin, sulfamethoxazole and tetracycline) was predominantly observed in pig carcases by France (75.3%), Croatia (72.7%), Spain (56.6%), Germany (56.3%) and Denmark (41.9%); and in pigs by Croatia (61.1%), the Netherlands (59.3%), Denmark (58.3%) and Italy (43.9%). Considering MDR among different serovars, the highest proportion of isolates exhibiting this resistance pattern (ampicillin, sulfamethoxazole and tetracycline) in both pig carcases and pigs were attributed to monophasic S. Typhimurium.
In carcases of calves under 1 year of age, where 18/82 (22%) Salmonella isolates were MDR (Table MULTISALMBOVMEAT), monophasic S. Typhimurium accounted for 61.1% of the MDR isolates from calf carcases. The most common pattern of resistance (in both Salmonella spp. and monophasic S. Typhimurium isolates) was again the combination: ampicillin, sulfamethoxazole and tetracycline. In cattle, where 52/176 (29.5%) Salmonella isolates were MDR (Table COMSALMBOV), monophasic S. Typhimurium and S. Typhimurium accounted for 42.3% and 46.2% of the MDR isolates from cattle, respectively.
Salmonella Typhimurium
Multidrug resistance S. Typhimurium isolates were reported in pig carcases, where 79/123 (64.2%) isolates were MDR (Table MULTITYPHIPIGMEAT); in fattening pigs, where 48/81 (59.3%) isolates were MDR (Table MULTITYPHIFATPIG); and in calf carcases of less than one year old, where 1/4 (25%) isolates were MDR (Table MULTITYPHIBOVMEAT). A wide range of different MDR patterns were reported among S. Typhimurium isolates from pig carcases and pigs. The most frequent MDR core pattern among isolates from pigs and calf carcases was resistance to ampicillin, chloramphenicol, sulfamethoxazole and tetracycline; although only one S. Typhimurium isolate exhibited MDR from calf carcases. Among MDR isolates from pig carcases, two core resistance patterns predominated: ampicillin, sulfamethoxazole and tetracycline, and the same pattern with the addition of chloramphenicol. Notably, all MDR S. Typhimurium isolates from all three animal origins exhibited resistance to ampicillin (100%). Additionally, resistance to five antimicrobial classes was observed among isolates from pig carcases and pigs, as well as resistance to six antimicrobial classes among a few isolates from pig carcases. Furthermore, resistance to seven antimicrobial classes was observed in a single isolate originating from pig carcases and two isolates originating from pigs; one isolate recovered from a pig carcase also exhibited resistance to eight antimicrobial classes. Resistance to cefotaxime/ceftazidime was only reported in a single MDR isolate from pigs and was absent in all other sources. Ciprofloxacin/nalidixic acid resistance among MDR isolates from pig carcases and pigs were observed at levels of 8.9% and 20.8%, respectively, while tigecycline resistance among MDR isolates from pig carcases and pigs were observed at levels of 5.1% and 8.3%, respectively.
Monophasic Salmonella Typhimurium
Multidrug resistance patterns for monophasic S. Typhimurium were reported in pig carcases, where 258/334 (77.2%) isolates were MDR (Table MULTIMOTYPHIPIGMEAT); in fattening pigs, where 127/161 (78.9%) isolates were MDR (Table MULTIMOTYPHIPIG); and in calf carcases of less than one year old, where 11/12 (91.7%) isolates were MDR (Table MULTIMONTYPHIBOVCARC). Among these animal origins, the most frequent pattern of resistance was to ampicillin, sulfamethoxazole and tetracycline; followed in pigs by the same pattern with the addition of ciprofloxacin/nalidixic acid, and in pig carcases by the pattern ampicillin, gentamicin, sulfamethoxazole and tetracycline. Notably, among the MDR isolates, sulfamethoxazole resistance was observed at 99.6%, 99.2% and 100% in pig carcases, pigs and calf carcases, respectively. Resistance to five antimicrobial classes was observed among isolates from all three animal origins; as well as resistance to six antimicrobial classes in six isolates from pig carcases and four isolates from pigs. Three isolates originating from pigs also exhibited resistance to seven antimicrobial classes. Resistance to cefotaxime/ceftazidime was only reported in two MDR isolates originating from pigs and was absent in all other sources. Ciprofloxacin/nalidixic acid resistance among MDR isolates from pig carcases, pigs and calf carcases were observed at levels of 5.4%, 10.2% and 9.1%, respectively. Tigecycline resistance was reported in two MDR isolates from pigs.
Salmonella Derby
In pig carcases, where 30/254 (11.8%) isolates were MDR (Table MULTIDERBYPIGMEAT), and in fattening pigs where 15/98 (15.3%) isolates were MDR (Table MULTIDERBYFATPIG), the most common resistance pattern was to sulfamethoxazole, trimethoprim and tetracycline. Resistance to five antimicrobial classes was observed in three isolates recovered from pig carcases and in two isolates from pigs. Ciprofloxacin/nalidixic acid resistance among MDR isolates was reported in two isolates recovered from pig carcases and one isolate from pigs; tigecycline resistance was only observed in a single MDR isolate recovered from a pig carcase. In calf carcases under 1 year of age, a single S. Derby isolate (1/7, 14.3%) exhibited MDR (to chloramphenicol, sulfamethoxazole and trimethoprim) – see Table MULTIDERBYBOVCARC.
Salmonella Rissen
Multidrug resistance patterns for S. Rissen isolates were reported in pig carcases. Overall, 33/71 (46.5%) isolates exhibited MDR and a wide range of different resistance patterns were evident (Table MULTIRISSENSPIGMEAT). The most common pattern of resistance (24.2%) among MDR isolates was to chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline. Approximately one‐fifth (18.2%) of MDR isolates exhibited the resistance profile: gentamicin, chloramphenicol, ampicillin, ciprofloxacin/nalidixic acid, sulfamethoxazole, trimethoprim and tetracycline. Notably, 90.9% and 97% of the MDR isolates exhibited ampicillin and tetracycline resistance, respectively, while sulfamethoxazole and trimethoprim resistance were observed at levels of 81.8% and 84.8%, respectively.
Salmonella Infantis
Multidrug resistance patterns for S. Infantis isolates recovered from pig carcases are shown in Table MULTIINFANTINSPIGMEAT (six isolates were MDR out of 31 isolates reported, 19.4%). Among the MDR isolates, all showed resistance to ampicillin, sulfamethoxazole and trimethoprim. The most common pattern of resistance (83.3%) among MDR isolates was to chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline; all isolates exhibiting this resistance pattern were reported by Spain.
Salmonella Dublin
The patterns of MDR for S. Dublin isolates were reported in cattle. Overall, 4/24 (16.7%) isolates exhibited MDR, all showing resistance to sulfamethoxazole and tetracycline (Table MULTIDUBLINBOV). Interestingly, no MDR S. Dublin isolates exhibited colistin resistance.
3.3. Discussion
3.3.1. Antimicrobial resistance in Salmonella isolates from humans
Salmonellosis is the second most commonly reported zoonotic disease in humans in the EU, exceeded only by campylobacteriosis. A decline in incidence has been observed since 2004, most likely attributable to the reduction in prevalence of Salmonella in flocks of laying hens and, to some extent, in broilers and turkeys. The number of cases of salmonellosis in the EU has however stabilised in the last 5 years (EFSA and ECDC, 2018b). While most salmonellosis infections cause mild disease, effective antimicrobials are essential for treatment of severe enteric disease or invasive infections.
In 2017, information on AMR in Salmonella isolates from human cases was reported by 24 MSs and two non‐MSs. Resistance in Salmonella isolates from humans was high to ampicillin, sulfamethoxazole and tetracycline. These antimicrobials or other agents of the same class are commonly used for treating infections in animals and humans (although usually not for treating Salmonella infections in humans).
Resistance to ciprofloxacin, a highest priority CIA for treating salmonellosis in adults (Urdahl et al., 2017), has stabilised somewhat at the EU level in Salmonella spp. isolates from humans after a few years of marked increasing resistance. Most of the increase could be explained by the lowering of the EUCAST threshold for clinical resistance to ciprofloxacin in Salmonella in 2014 (affecting results from countries reporting interpreted SIR data) and an increased sensitivity to detect low‐level fluoroquinolone resistance with disk diffusion by replacing ciprofloxacin disks with pefloxacin disks (EUCAST, 2014) (affecting quantitative data). In many countries, a higher proportion of Salmonella isolates were resistant to ciprofloxacin/pefloxacin than to nalidixic acid in 2017. This observation could be due to PMQR which is easier detected with ciprofloxacin/pefloxacin than with nalidixic acid. While PMQR genes usually only confer low‐level resistance to fluoroquinolones, bacteria carrying such genes, particularly qnr genes, may have a selective advantage in an environment with fluoroquinolone exposure and may subsequently also develop higher‐level chromosomal quinolone resistance (Hooper and Jacoby, 2015; Kuang et al., 2018). High‐level ciprofloxacin resistance was most commonly observed in S. Kentucky in 2017, where 93% of the tested isolates expressed high‐level resistance. This is consistent with S. Kentucky ST198, which is nowadays the dominant S. Kentucky strain in Europe. Fluoroquinolone resistance in ST198 is chromosomal with double substitutions in gyrA and a single substitution in parC, resulting in high‐level resistance (Le Hello et al., 2013).
Resistance to cefotaxime (a third‐generation cephalosporin), the second highest priority CIA drug for salmonellosis, used for treating infections in children (Urdahl et al., 2017), was relatively low in Salmonella isolates from humans. The highest proportion of resistant isolates was reported in Malta and related to ESBL‐producing S. Kentucky (for further details, see Section 6: ESBL‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli). Combined resistance to both of the two highest priority CIA (ciprofloxacin/pefloxacin and cefotaxime) was low overall in Salmonella isolates from humans but higher in some serovars, e.g. 24.0% in S. Kentucky, ranging from 0% to 52% depending on country (data not shown).
Based on a harmonised panel of antimicrobials used for analysis by both ECDC and EFSA, MDR was assessed in isolates tested for at least nine different antimicrobial classes. MDR was high overall in Salmonella from humans, but few isolates were clinically resistant to the two antimicrobials regarded as highest priority critically important for human treatment. Thirty‐three isolates (0.4% of the 8,220 isolates) were resistant to seven or eight antimicrobial classes, of which a third were monophasic S. Typhimurium.
The serovar distribution within the Salmonella spp. varies by country depending on their frequency among human cases and/or specific sampling strategies for further typing and susceptibility testing at the national public health reference laboratories. For these reasons, comparisons between countries should be avoided at the level of Salmonella spp. Because of the compulsory AST of isolates recovered from pig and calf (under one year) carcases in 2017, the analysis of human data for this report has focused on the most common serovars found in these animals. Similar levels of resistance were observed among S. Typhimurium, monophasic S. Typhimurium or S. Derby isolates from both humans and pig carcases across the range of tested antimicrobial substances.
S. Typhimurium was the second most common serovar among human Salmonella infections in 2017, and the third and eighth most common in fattening pig and calf carcases, respectively. The proportion of isolates resistant to ampicillin, sulfonamides and tetracycline was high to extremely high in S. Typhimurium from humans in all but two reporting Member States. This resulted in overall high MDR and isolates resistant to six, seven or eight antimicrobial classes were identified in six of 14 reporting MSs. Resistance to ciprofloxacin was overall relatively low but moderate levels were reported in a third of the Member States. Increasing trends of fluoroquinolone resistance in S. Typhimurium were observed in four MSs in the period 2013–2017 (and decreasing in two). To circumvent the impact of the changes in ciprofloxacin surveillance (see above), temporal trend analysis of fluoroquinolone resistance used results combined for the antimicrobials ciprofloxacin, nalidixic acid and pefloxacin. For ampicillin, tetracycline and cefotaxime, a greater number of countries reported decreasing trends compared to the number of countries reporting increasing trends. It has not been assessed whether this observation is related to any specific measures to reduce antimicrobial use in animals (and humans) implemented in these countries.
Monophasic S. Typhimurium 1,4,[5],12:i:‐ was the third most common Salmonella serovar among human Salmonella infections in 2017, while the most common serovar in fattening pigs and carcases of pigs and calves. In this variant of S. Typhimurium, even higher proportions of resistance were observed to ampicillin, sulfonamides and tetracycline than in S. Typhimurium, with close to 90% of isolates from humans displaying resistance to these antimicrobials. Extremely high proportions of MDR were observed in monophasic S. Typhimurium in 10 of eleven reporting MSs and isolates resistant to six or seven antimicrobial classes were identified in seven of these. Resistance to both of the highest priority CIA, ciprofloxacin and cefotaxime, was however very low (0.6%). Increasing trends of fluoroquinolone resistance in monophasic S. Typhimurium were observed in three MSs in the period 2013–2017, whereas decreasing trends were noted in one MS. While tetracycline resistance increased in three countries, cefotaxime resistance decreased in another three countries.
S. Derby was the seventh most common Salmonella serovar among human Salmonella infections in 2017, and the second and fourth most common serovar in fattening pig and calf carcases, respectively. In comparison to S. Typhimurium and its monophasic variant, much lower proportions (15–30%) of S. Derby were resistant to ampicillin, sulfonamides and tetracyclines. Resistance to the CIA ciprofloxacin/pefloxacin and cefotaxime, was low and no human isolates were resistant to both of these.
Considering the high proportion of MDR among some of the most common Salmonella serovars in humans, high ciprofloxacin resistance in some serovars and the findings of ESBL production, it is also important to monitor Salmonella for resistance to last line antimicrobials such as meropenem, colistin, azithromycin and tigecycline which may need to be considered for treatment of extremely drug‐resistant isolates. For 2017, 22 countries reported data on meropenem. No meropenem resistance was reported in the phenotypic test results; however, 8/22 reporting countries used the EUCAST CBP (MIC > 8 mg/L) to interpret data, which is substantially higher than the EUCAST ECOFF (MIC > 0.125 mg/L), and WGS later revealed that two isolates reported as susceptible were carrying carbapenemase genes (for further details, see Section 6: ESBL‐, AmpC‐ and/or carbapenemase‐producing Salmonella and E. coli). Five, seven and eight countries reported data on the other last‐resort drugs: colistin, azithromycin and tigecycline, respectively. Resistance to colistin was detected in 4.7% of Salmonella isolates, although the vast majority (88.9%) of the resistant isolates were either S. Enteritidis or S. Dublin. Serovars within serogroup O:9, such as S. Enteritidis and S. Dublin, have been reported to possess an inherent resistance to colistin (Agersø et al., 2012). Resistance to azithromycin and tigecycline was relatively low, although Ireland reported much higher levels of azithromycin resistance (13.6%) due to a point source outbreak with an azithromycin‐resistant S. Brandenburg. The outbreak occurred in a catering establishment where poultry of unknown origin was the suspected source (N. Delappe, HSE, Ireland, personal communication 15 October 2018).
In this report, isolates from cases reported as having been acquired while travelling abroad were excluded from the analysis. The rationale was to facilitate assessment of the relationship between AMR in Salmonella isolates from food and food‐producing animals with AMR in human isolates of Salmonella spp. However, as imported or traded food can constitute a large proportion of the food available in some countries, the relationship between resistance in food and food‐producing animals and in the human population remains complex. To better understand the sources of AMR in domestically acquired Salmonella isolates, it would be of value to collect AST data from structured sampling at retail of both animal and non‐animal foods, including information on country of origin.
The quality and completeness of the AMR data for Salmonella from humans continues to improve, as a result of the agreement on harmonised monitoring and reporting (ECDC, 2016) and related external quality assessment (EQA) schemes. Compared to 2016, one more country, Poland, reported AMR data from human isolates in 2017. Of 26 countries, 17 provided data as measured values to which ECOFFs could be applied. For the nine countries providing results interpreted with CBPs, the method to combine the categories of clinically ‘intermediate’ resistant and clinically ‘resistant’ into a ‘non‐susceptible’ category resulted in a close correspondence with the ECOFF‐based category of ‘non‐wild type’. Similarly, the ‘susceptible’ category corresponded closely to the ‘wild‐type’ category with only one dilution difference across all antimicrobials except meropenem (see above). Thus, this approach further improves the comparability of human and non‐human data.
3.3.2. Antimicrobial resistance in Salmonella isolates obtained from carcase swabs of fattening pigs and calves, and caecal contents of fattening pigs and cattle
In Salmonella isolates recovered from the mandatory carcase swabbing of fattening pigs and calves at slaughter, harmonised isolate‐based data were reported by 22 MSs and 1 non‐MS for fattening pigs and 7 MSs for calves. Notably, some MSs did not obtain any positive Salmonella isolates from fattening pig or calf carcases and, therefore, data are not presented for these countries in the corresponding results section. Eight MSs also reported voluntary data on Salmonella isolates recovered from caecal contents of fattening pigs and cattle at slaughter. The reporting of isolate‐based data enables the analysis of MDR patterns, detection of high‐level ciprofloxacin resistance, and co‐resistance to ciprofloxacin and cefotaxime; first‐line agents critically important for treating human salmonellosis. Resistance levels were also reported by serovar for the different animal origins (see Appendices A, B, C and D), which allows for more accurate analysis and, as required by Decision 2013/652/EU, all MSs included information on serovars and production type. In line with this decision, streptomycin is no longer included in the specified test panels for the monitoring and reporting of AMR in Salmonella, which has an impact on how MDR patterns are interpreted. The reporting of carcase data is mandatory in accordance with Decision 2013/652/EU, and in 2017 was primarily focused on Salmonella spp. from fattening pigs and calves less than 1 year of age. In addition, this chapter also includes Salmonella data from caecal contents of fattening pigs and cattle which was not mandatory to report, according to the Decision.
Antimicrobials such as ampicillin, sulfamethoxazole and tetracycline have been widely used for many years in veterinary medicine to treat infections in production animals. Generally, moderate to high levels of resistance to these antimicrobials are reported by MSs from producing animals. Overall in 2017, the highest levels of resistance to ampicillin, sulfamethoxazole and tetracycline were recorded in Salmonella isolates recovered from pig carcases and fattening pigs (caecal contents); the lowest levels were reported in Salmonella isolates recovered from calf carcases (less than 1 year of age). Considering individual serovars, monophasic S. Typhimurium generally showed the highest resistance to these compounds across all animal origins. The genes conferring resistance to these agents are commonly found in association together on various mobile genetic elements, such as class 1 integrons or in the variant Salmonella genomic islands which have been described, explaining both their frequent occurrence as well as their frequent occurrence together or in various combinations.
Colistin is a CIA, considered as a last‐resort for the treatment of serious human infections. In 2017, colistin‐resistant Salmonella isolates were detected by several MSs originating from carcases of pigs and calves, pigs and cattle. All isolates recovered from cattle and carcases of calves were serotyped as S. Dublin, a serovar which belongs to group D Salmonella (serogroup O9). Group D Salmonella isolates tend to show elevated colistin MICs compared to other serovars, a phenomenon which is considered to reflect slightly decreased intrinsic susceptibility to colistin of Group D serovars. Considering MSs reporting data for pig carcases and pigs, 6/954 and 9/474 Salmonella isolates were determined to be colistin‐resistant, with MICs ranging from 4 to > 16 mg/L. Monophasic S. Typhimurium was the most frequently reported serovar among the colistin‐resistant isolates recovered from pig carcases (4/6) and pigs (4/9). High colistin MICs of ≥16 mg/L were observed in two S. Derby isolates; one originating from a pig carcase in Germany (MIC > 16 mg/L) and the other originating from a fattening pig in Estonia (MIC 16 mg/L).
Third‐generation cephalosporins and fluoroquinolones are highest priority CIA for the treatment of human invasive salmonellosis. Combined resistance to cefotaxime and ciprofloxacin was not detected among isolates from most MSs and was only detected at a very low/low level in Salmonella isolates from two southern European countries. Spain reported combined resistance to these antimicrobials in two Salmonella isolates recovered from pig carcases (attributed to serovars S. Rissen and S. Bredeney), while Italy reported combined resistance in two Salmonella isolates recovered from pigs (attributed to serovars S. Typhimurium and S. Kapemba), as well as Spain in one isolate also recovered from fattening pigs. Considering reporting MSs, overall resistance to ciprofloxacin was registered at low levels in Salmonella isolates from pig and calf carcases. Italy registered a high level of resistance to ciprofloxacin (42.3%) in Salmonella isolates recovered from cattle (N = 26), contributing to an overall moderate level among cattle considering all reporting MSs. Similarly, Spain (N = 164) reported ciprofloxacin resistance at a high level (20.7%) in Salmonella isolates recovered from pigs, again contributing to an overall moderate level in pigs considering all reporting MSs. No Salmonella isolates from any of the animal origins exhibited high‐level ciprofloxacin resistance (MIC ≥ 4 mg/L).
Considering nalidixic acid and ciprofloxacin resistance in Salmonella spp., overall resistance to these compounds was generally very similar across isolates recovered from all four animal origins. However, Salmonella spp. isolates exhibiting ciprofloxacin resistance and nalidixic acid susceptibility were also evident, and probably indicate the occurrence of PMQR mechanisms (qepA, aac(6′)Ib‐cr, qnr genes); this was particularly apparent among S. Rissen isolates recovered from pig carcases in Spain, where 9/32 S. Rissen isolates displayed nalidixic acid resistance, yet 16/32 isolates displayed ciprofloxacin resistance.
S. Rissen was detected by nine MSs from pig carcases and was the fourth most common serovar, accounting for 7.4% of Salmonella isolates recovered from pig carcases. MDR, defined as resistance to three or more antimicrobial classes, was frequently observed (46.5%) among the S. Rissen isolates from pig carcases (N = 71), especially considering isolates from southern Europe, although MDR was also encountered in isolates from Belgium and France. Additionally, the majority of MDR S. Rissen isolates were resistant to ampicillin, sulfamethoxazole, trimethoprim and tetracycline; this resistance pattern (with or without additional resistance) accounting for 66.7% of the MDR isolates. García‐Fierro et al. (2016) previously identified a dominant S. Rissen clone in pigs, pork and man in Spain, which was shown to carry genes conferring resistance to ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline and trimethoprim at varying frequencies, mostly on integrons. S. Rissen is also a common serovar in pigs, chicken, pork and man in some parts of Asia. Pornsukarom et al. (2015) demonstrated that S. Rissen isolates originating from Thai pig farms were frequently multidrug resistant to most of the antimicrobials listed above. The potential for this serovar to spread into livestock, particularly pigs, and disseminate along the food chain to affect man was highlighted in the 2015 report (EFSA and ECDC, 2017), and although in 2015, S. Rissen isolates recovered from pig carcases also frequently exhibited MDR (28/53 isolates, 52.8%), this figure was slightly higher than that observed in 2017 (33/71 isolates, 46.5%) – see Figure 44. Considering longitudinal data for particular MSs, 16/20 S. Rissen isolates reported from pig carcases by Portugal in 2015 were MDR, while 4/4 isolates exhibited MDR in 2017. Additionally, 6/17 S. Rissen isolates reported from pig carcases by Spain were MDR in 2015, while 19/32 isolates displayed MDR in 2017.
Figure 44.
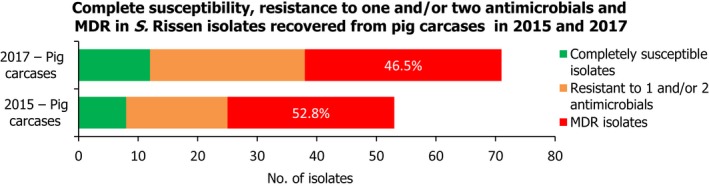
A comparison of the number of MDR and completely susceptible S. Rissen isolates recovered from pig carcases in 2015 and 2017 (MDR levels are also expressed as a percentage)
MDR was higher in Salmonella spp. from pig carcases (47.4% of isolates) and fattening pigs (51.3% of isolates) than in cattle (29.5% of isolates) and calf carcases of less than a year of age (22% of isolates). In both pig carcases and pigs, the proportion of all Salmonella isolates showing MDR, was greatly influenced by the occurrence of MDR monophasic S. Typhimurium, this serovar accounting for approximately 56.7% and 52.3% of the MDR isolates in pig carcases and pigs, respectively. This serovar has spread widely among European pig populations. Particular MDR patterns are associated with monophasic S. Typhimurium and because this serovar was prevalent in many countries, these patterns greatly influenced the overall resistance figures. This is exemplified by resistance to ampicillin, sulfamethoxazole and tetracycline which occurred as an MDR pattern without additional resistances in 199/334 (59.6%) monophasic S. Typhimurium isolates from pig carcases and 91/161 (56.5%) monophasic S. Typhimurium isolates from pigs. This resistance pattern (together with resistance to streptomycin) is typical of monophasic S. Typhimurium (Hopkins et al., 2010). Generally, resistance levels varied among serovars which may exhibit particular MDR patterns, so the relative contribution of individual serovars within the different animal origins and between MSs should be considered when comparing the situation between reporting countries.
Although S. Infantis is more frequently observed in isolates originating from poultry, this serovar was detected by 12 reporting countries from pig carcases and was the fifth most common serovar, accounting for 3.2% of Salmonella isolates from pig carcases. While MDR was not frequently observed among S. Infantis isolates from pig carcases (6/31, 19.4%), considering individual MSs, all isolates reported by Spain (N = 5) exhibited MDR, as did a single isolate from Poland. Additionally, the S. Infantis isolates reported by Spain all displayed the same core resistance pattern: chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline. Although genotypic data were not reported, mobile genetic elements which could account for this resistance pattern in S. Infantis isolates have previously been described. Salmonella genomic island 1 (SGI1), known to contain a MDR region located on a complex integron designated In104, confers pentavalent resistance (the ampicillin, chloramphenicol, streptomycin, sulfamethoxazole, tetracycline resistance phenotype) and has widely been documented in a range of Salmonella serovars. In Australia, an S. Infantis strain harbouring a SGI1 homologue with an integron related to In104 and conferring resistance to streptomycin, sulfamethoxazole and trimethoprim was identified (Levings et al., 2005). Additionally in Italy, Franco et al. (2015) identified S. Infantis isolates harbouring a pESI‐like megaplasmid (pESI = ‘plasmid for emerging S. Infantis’; Aviv et al., 2014; Tate et al., 2017), which carried the ESBL gene bla CTX‐M‐1 mediating cefotaxime resistance, as well as the resistance genes tet(A), sul1, dfrA1 and dfrA14, conferring resistance to tetracycline, sulfamethoxazole and trimethoprim, respectively. Figure 45 presents the numbers of MDR and completely susceptible S. Infantis isolates recovered from pig carcases in 2015 and 2017.
Figure 45.
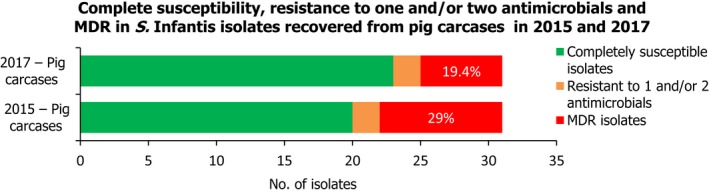
A comparison of the number of MDR and completely susceptible S. Infantis isolates recovered from pig carcases in 2015 and 2017 (MDR levels are also expressed as a percentage)
S. Derby was the second most common serovar detected in pig carcases and fattening pigs, accounting for 26.5% and 20.7% of Salmonella isolates from these origins, respectively. While MDR was again not frequently observed among S. Derby isolates (11.8% in isolates from pig carcases and 15.3% in isolates from pigs), where MDR was observed, the most common resistance pattern in both pig carcases and pigs was to sulfamethoxazole, trimethoprim and tetracycline; this core resistance pattern was also the most frequently observed in MDR S. Derby isolates from pig carcases and pigs in 2015. Figure 46 compares the numbers of MDR and completely susceptible S. Derby isolates recovered from pig carcases and pigs in 2015 and 2017. Interestingly in 2017, the proportion of multiresistant S. Derby isolates was higher in pig carcases and lower in pigs compared to the 2015 data.
Figure 46.
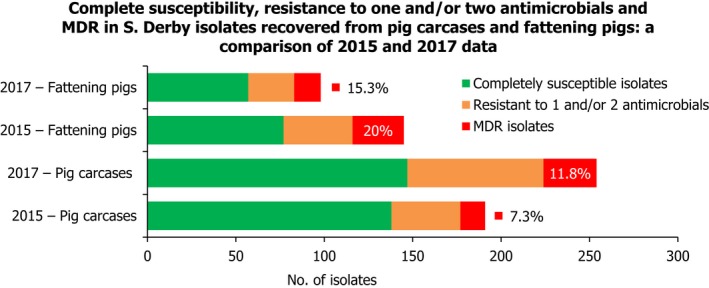
A comparison of the number of MDR and completely susceptible S. Derby isolates recovered from pig carcases and fattening pigs in 2015 and 2017 (MDR levels are also expressed as a percentage)
The analysis of MDR patterns in Salmonella spp. (as shown in Figures 3, 8, 12 and 16) broadly illustrates serovar diversity and associated typical MDR patterns, among MSs within the differing animal origins. The temporal trend analyses generally demonstrate that tetracycline resistance exceeds that of ampicillin in many MSs, and although tetracycline resistance may show fluctuations, ampicillin resistance tends to exhibit parallel fluctuations, maintaining the interval between resistance levels to these compounds. This may be related to the occurrence of underlying genetic structures and the proportion of Salmonella spp. carrying linked resistance genes to tetracycline and ampicillin.
Certain serovars displayed ‘microbiological’ resistance to tigecycline, which may suggest clonal expansion of microbiologically resistant strains belonging to these serovars: 4/7 tigecycline‐resistant isolates recovered from fattening pigs belonged to serovar S. Typhimurium, 11/13 isolates from pig carcases were S. Typhimurium (N = 4) and S. Rissen (N = 7), while the only tigecycline‐resistant isolate recovered from cattle was S. Typhimurium. There are however, some technically difficult aspects of susceptibility testing in relation to tigecycline and further testing can be useful. Determining the susceptibility of tigecycline is not straightforward as test reagents used in methods may be affected by oxidation, which may lead to falsely reported ‘microbiological’ resistance. Several mechanisms of resistance to tigecycline in Salmonella/Enterobacterales OR Enterobacteriaceae have previously been described: increased activity of efflux pumps (AcrAB), mutation of the ribosomal protein S10 and modification of the Mla system involved in phospholipid transport in cell membranes (He et al., 2016). The mechanisms of development of microbiological resistance, which may involve upregulation of normal cell pathways or processes, probably also contribute to the occurrence of a ‘tail’ of isolates on the MIC distribution with values just above the ECOFF.
There were no Salmonella isolates recovered from carcases of pigs and calves, fattening pigs and cattle which were resistant to carbapenems, a class of antimicrobials which are not used therapeutically in food‐producing animals, but are reserved for use in humans. Carbapenems are also recognised as CIA (Urdahl et al., 2017) and include meropenem, a compound which is specified in the antimicrobial panels for the monitoring and reporting of AMR in Salmonella spp., as stipulated by Decision 2013/652/EU.
In summary, the prevalence of particular Salmonella serovars within countries and animal populations, and their associated patterns of resistance, may explain some of the differences in the levels of AMR and MDR. The spread of resistant clones and the occurrence of resistance genes within these clones can be exacerbated by the use of antimicrobials in human and animal populations and the associated selective pressure. Within a given MS, any attempt to relate the occurrence of AMR in human Salmonella isolates to that in isolates from food/food‐producing animals is complicated, as much of the food consumed in a MS may have originated from other MSs or non‐member countries. Salmonella infections can also be associated with foreign travel, other types of animal contact (such as pet reptiles) or the environment. Additionally, some human infections may result from human to human transmission. To improve investigation of these relationships, human isolates from cases notified as having been acquired during travel outside of the reporting country were excluded from the analysis, except with respect to the analysis of resistance in different geographical regions.
4. Antimicrobial resistance in Campylobacter
Human infections with Campylobacter
Campylobacter is a common cause of gastroenteritis in humans, and despite considerable underreporting (Haagsma et al., 2013; Havelaar et al., 2013; Gibbons et al., 2014), campylobacteriosis has been the most frequently reported cause of human food‐borne zoonoses in the EU since 2004 (EFSA and ECDC, 2018a,b). In 2017, more than 250,000 laboratory‐confirmed cases of campylobacteriosis were reported in the EU/EEA. C. jejuni and C. coli accounted for 99.7% of cases for which species information was provided. Patients infected with Campylobacter may experience mild to severe illness. Symptoms may include (bloody) diarrhoea, abdominal pain, fever, headache and nausea. The mean duration of illness is 2–5 days but can be up to 10 days. Most campylobacteriosis enteric infections are self‐limiting; however, infection can be associated with serious complications. Campylobacteriosis is a significant trigger for autoimmune inflammatory conditions of the central nervous system, heart and joints, which can result in prolonged and debilitating illness (e.g. Guillain–Barré syndrome, acute transverse myelitis and reactive arthritis). Blood stream infection with Campylobacter spp. is very rare, except for infections with Campylobacter fetus.
Antimicrobial treatment is usually not required, but effective treatment may shorten the duration of illness. Resistance to antimicrobials in Campylobacter is of concern because of the large number of human infections and the fact that some cases require treatment. Treatment of enteric infections in humans may involve administration of macrolides, such as erythromycin, or fluoroquinolones (e.g. ciprofloxacin) as the first‐ and second‐choice drugs (ECDC, EFSA, EMEA and SCENIHR, 2009). Due to high occurrence of acquired ciprofloxacin resistance in Campylobacter however, this drug will not be effective for treatment of Campylobacter infections in many countries.
4.1. Antimicrobial resistance in Campylobacter isolates from humans18
Nineteen MSs, plus Iceland and Norway provided AMR data from human Campylobacter isolates for 2017. Thirteen countries reported quantitative isolate‐based AST results as measured values of either inhibition zone diameters or MICs. Eight countries reported case‐based or isolate‐based AST results interpreted as susceptible (S), intermediate (I) or resistant (R) according to the CBPs applied. Countries reporting resistance in Campylobacter from humans in 2017 are presented in Tables CAMPJEOVERVIEW and CAMPCOOVERVIEW.
Interpretation of data should take into account the wide variation in the numbers of Campylobacter isolates reported by MSs. While this may in part be related to true differences in the incidence of campylobacteriosis, it is also likely to be greatly influenced by practices related to referral of isolates from primary clinical laboratories to the national public health reference laboratory/ies or by reporting AST data from the primary laboratories to the national public health institutes.
Methods and interpretive criteria used for antimicrobial susceptibility testing of Campylobacter isolates from humans
Most laboratories follow the ‘EU protocol for harmonised monitoring of AMR in human Salmonella and Campylobacter isolates’ (ECDC, 2016) on the antimicrobial panel to be tested. The type of method (dilution, disk diffusion, gradient strip) and the interpretive criteria used when providing interpreted results for Campylobacter are presented in Table 2 in the Section 2 Materials and methods.
Quantitative data were interpreted by ECDC based on the EUCAST ECOFF values, when available. In the absence of EUCAST CBP for gentamicin, CBPs from the French Society for Microbiology (CA‐SFM/EUCAST, 2017a) were applied. For the qualitative SIR data, the intermediate and resistant results were combined into a ‘non‐susceptible’ category. For the four antimicrobials reported for both human and animal/food isolates, the commonly used interpretive criteria were aligned (Figure 47). For this purpose, ‘susceptible’ isolates were aligned with wild‐type isolates based on ECOFFs, and ‘non‐susceptible’ isolates (‘intermediate’ and ‘resistant’) were aligned with non‐wild‐type isolates. This resulted in total concordance across interpretive categories, except for the EUCAST CBP for C. jejuni for tetracyclines, which is one dilution step higher than the EUCAST ECOFF.
4.1.1. Antimicrobial resistance in Campylobacter jejuni isolates from humans
As in previous years, C. jejuni was the most common Campylobacter species identified in 2017, with 114,458 cases reported in the EU/EEA. AST data were reported for 22.2% of these cases by 19 MSs, plus Iceland and Norway. A very high proportion (57.7%) of human isolates was resistant to ciprofloxacin in 2017 (19 MSs, Table 19) with extremely high proportions observed in several countries, most noticeably in Portugal (96.5%), Lithuania (91.5%), Spain (88.6%), Estonia (84.0%) and Cyprus (80.0%). The lowest proportions of isolates resistant to ciprofloxacin were reported by Norway (24.5%), Iceland (27.1%) and Denmark (37.3%). Similar observations were made on the levels of resistance to tetracyclines which were high overall (45.4%) with the highest proportion of resistance reported by Cyprus, Malta, Portugal and Spain, all with > 80% resistant isolates. The lowest proportions were reported by Norway (16.4%), Denmark (21.8%) and Ireland (22.2%). The level of resistance to erythromycin was overall relatively low (2.0%) but varied between countries (Table 19). The highest proportion of erythromycin‐resistant isolates was reported by Portugal (6.3%) and Malta (5.7%). Resistance to gentamicin was overall very low (0.5%) but higher in Malta (12.5%) and Italy (5.9%). Among the countries testing susceptibility to co‐amoxiclav, resistance ranged from 0.2% in Luxembourg to 69.2% in Malta.
Table 19.
Antimicrobial resistance in Campylobacter jejuni from humans per country in 2017
| Country | Gentamicin | Co‐amoxiclav | Ciprofloxacin | Erythromycin | Tetracyclines | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 372 | 0 | – | – | 372 | 71.8 | 372 | 0 | 372 | 45.7 |
| Cyprus | – | – | – | – | 25 | 80.0 | 25 | 0 | 25 | 80.0 |
| Denmark | 252 | 1.2 | – | – | 252 | 37.3 | 252 | 1.2 | 252 | 21.8 |
| Estonia | 200 | 0 | – | – | 207 | 84.1 | 207 | 0.5 | 207 | 45.9 |
| Finlanda | – | – | – | – | 3,080 | 64.6 | 2,915 | 2.4 | 1,700 | 44.4 |
| Franceb | 5,017 | 0.4 | 5,422 | 0.4 | 5,425 | 58.0 | 5,422 | 0.6 | 5,386 | 48.4 |
| Irelandb | – | – | – | – | 54 | 40.7 | 54 | 3.7 | 54 | 22.2 |
| Italy | 34 | 5.9 | – | – | 34 | 70.6 | 34 | 0 | 34 | 55.9 |
| Lithuaniab | – | – | – | – | 553 | 91.5 | 547 | 0.7 | 372 | 62.6 |
| Luxembourg | – | – | 529 | 0.2 | 529 | 66.2 | 529 | 0 | 529 | 47.6 |
| Malta | 16 | 12.5 | 13 | 69.2 | 194 | 62.4 | 193 | 5.7 | 16 | 81.3 |
| Netherlandsb | – | – | – | – | 2,100 | 59.9 | 1,818 | 2.5 | 1,712 | 45.2 |
| Polandb | 1 | NA | – | – | 7 | NA | 77 | 0 | 8 | NA |
| Portugal | 254 | 0 | – | – | 254 | 96.5 | 254 | 6.3 | 254 | 81.9 |
| Romania | 4 | NA | 4 | NA | 4 | NA | 4 | NA | 4 | NA |
| Slovakiab | 10 | 0 | 200 | 3.0 | 654 | 72.8 | 524 | 3.2 | 678 | 51.0 |
| Slovenia | – | – | – | – | 1,021 | 75.2 | 1,021 | 0.5 | 1,021 | 38.3 |
| Spain | 281 | 1.4 | 277 | 0.4 | 281 | 88.6 | 280 | 2.5 | 281 | 80.4 |
| United Kingdomb | 176 | 0.0 | – | – | 8,668 | 45.8 | 8,138 | 3.0 | 4,194 | 37.6 |
| Total (19 MSs) | 6,617 | 0.5 | 6,445 | 0.6 | 23,714 | 57.7 | 22,666 | 2.0 | 17,099 | 45.4 |
| Icelandb | – | – | – | – | 48 | 27.1 | 48 | 0 | 1 | NA |
| Norway | 269 | 1.1 | – | – | 269 | 24.5 | 269 | 3.7 | 269 | 16.4 |
N: number of isolates tested; % Res: percentage of resistant isolates (either non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable – if fewer than 10 isolates were tested resistance was not calculated.
Travel‐associated cases, accounting for 79% of Campylobacter infections in Finland in 2017, could not be excluded from the Finnish AST data.
Data interpreted with clinical breakpoints.
Spatial distribution of resistance among Campylobacter jejuni isolates from human cases
The spatial distribution of ciprofloxacin resistance in C. jejuni isolates from human cases (Figure 48a) shows that the highest proportion of resistance was reported by countries in southern and eastern Europe and in the Baltic, whereas countries in northern and central parts of Europe reported lower resistance levels. Travel information was not available for the Finnish AST data and since 79% of Campylobacter infections in Finland were related to travel in 2017, the observed resistance levels cannot be considered representative for domestically acquired infections. Erythromycin resistance did not show any distinct geographical trend (Figure 48b).
Figure 48.
Spatial distribution of ciprofloxacin (a) and erythromycin (b) resistance among Campylobacter jejuni from human cases in reporting countries in 2017
Combined resistance to ciprofloxacin and erythromycin in C. jejuni from humans
A low proportion of C. jejuni isolates (1.2%) exhibited ‘microbiological’ as well as ‘clinical’ resistance to both ciprofloxacin and erythromycin with the highest proportions observed in Portugal (6.3%), Malta (5.2%) and Ireland (3.7%) (19 MSs, Table COMCAMPJEHUM; Figure 49]
Figure 49.
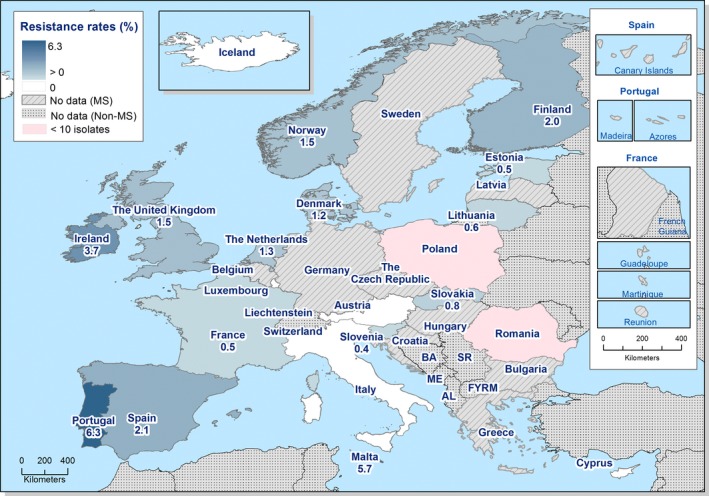
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter jejuni from human cases in reporting countries in 2017
Temporal trends in resistance among C. jejuni isolates from humans
Trend analysis was performed for the years 2013–2017 for ciprofloxacin, erythromycin and tetracycline. Fifteen MSs and two non‐MS were included in the analysis as they had provided resistance data for a minimum of 3 years in this period and a minimum of 10 C. jejuni isolates (Figure 50). For ciprofloxacin resistance, significant increases were observed in Austria, Estonia, Finland, France, Iceland, Italy, Slovakia and Slovenia. Resistance to erythromycin in C. jejuni remained stable at low levels in many countries during the period 2013–2017, with increasing resistance observed in France, Norway and Slovakia and decreasing resistance observed in Luxembourg and Malta. Tetracycline resistance increased in Austria, Cyprus, Estonia, the Netherlands, Slovakia, Slovenia and the United Kingdom in the same period and decreased in France. Note that Estonia, Italy and Slovenia changed from reporting interpreted SIR data for 2013 to measured values for 2014 and onwards. Since the CBP for non‐susceptible to tetracycline is less sensitive than the ECOFF for C. jejuni, this change may have affected the proportion of resistant isolates to tetracycline.
Figure 50.
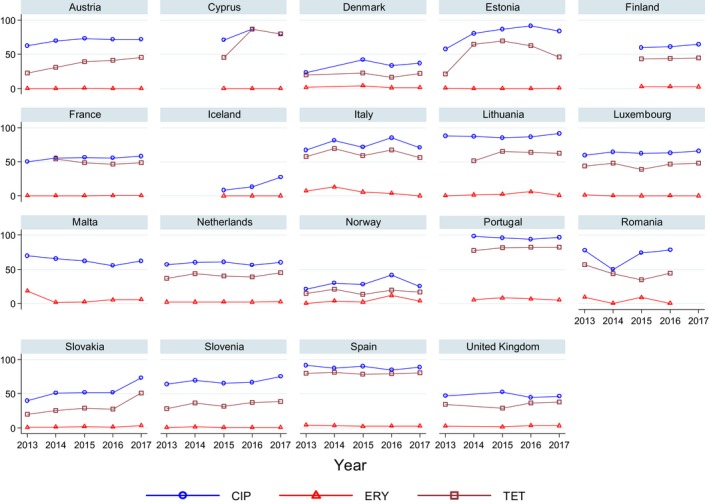
Trends in ciprofloxacin, erythromycin and tetracycline resistance in Campylobacter jejuni from humans in reporting countries, 2013–2017
- Statistically significant increasing trends over 3–5 years, as tested by logistic regression (p ≤ 0.05), were observed for ciprofloxacin in Austria, Estonia, Finland, France, Iceland, Italy, Slovakia and Slovenia (↑), for erythromycin France, Norway and Slovakia (↑) and for tetracycline in Austria, Cyprus, Estonia, the Netherlands, Slovakia, Slovenia and the United Kingdom (↑). Statistically significant decreasing trends were observed for erythromycin in Luxembourg and Malta (↓) and for tetracycline in France. Only countries testing at least 10 isolates per year were included in the analysis.
High‐level erythromycin resistance in Campylobacter jejuni
To assess if transferrable erythromycin resistance due to the presence of the erm(B) gene was present in C. jejuni isolates from humans in the EU/EEA, quantitative data were analysed for high‐level erythromycin MICs (> 128 mg/L) (see further text box on mechanisms of high‐level erythromycin resistance in Campylobacter spp. below). Of the C. jejuni isolates with MIC data, 1.3% (28 isolates) had a MIC > 128 mg/L (Figure 51). Such isolates were reported from four of nine countries which had provided quantitative data from dilution tests (Table 20). Similarly, for 50 of 3,939 isolates (1.3%) tested with disk diffusion no inhibition zone could be observed (6 mm zone equals the disk size), which corresponds to a MIC of ≥ 128 mg/L (EUCAST, 2017a). Note, however, that high‐level erythromycin resistance also could be due to mutations in the genome and not necessarily transferrable resistance.
Figure 51.
Erythromycin MIC distribution in C. jejuni from humans, 2017 (n = 2,485)
Table 20.
Occurrence of high‐level resistance to erythromycin (MIC > 128 mg/L) in Campylobacter jejuni from humans in 2017
| Country | N | High‐level resistance to erythromycin (MIC > 128 mg/L) | |
|---|---|---|---|
| n | % | ||
| Austria | 372 | 0 | 0 |
| Denmark | 317 | 3 | 0.9 |
| Estonia | 11 | 0 | 0 |
| Finland | 968 | 15 | 1.5 |
| Luxembourg | 148 | 0 | 0 |
| Malta | 118 | 6 | 5.1 |
| Portugal | 4 | 0 | NA |
| Spain | 280 | 4 | 1.4 |
| Total (8 MSs) | 2,218 | 28 | 1.3 |
| Norway | 269 | 2 | 0.7 |
MIC: minimum inhibitory concentration; MS: Member State; NA: Not applicable – if fewer than 10 isolates were tested, the percentage of resistance was not calculated.
MDR among Campylobacter jejuni isolates from humans
Nine MSs and Norway tested at least 10 isolates of C. jejuni for resistance to the four antimicrobial classes included in the MDR analysis. Overall, 31.3% of human C. jejuni isolates were susceptible to all four antimicrobial classes (9 MSs, Table MDRCAMPJEHUM). The highest levels of susceptibility were reported from Norway (71.4%), Denmark (58.7%) and the United Kingdom (46.6%). Particularly low levels of susceptibility were reported from Malta (0%), Portugal (2.8%) and Spain (7.1%) (Figure 52). MDR was very low overall (0.9%). The highest proportions of MDR were observed in Malta (62.5%), Portugal (5.5%) and Spain (2.1%). France reported three isolates, Malta two and Spain one isolate resistant to all four antimicrobial classes.
Figure 52.
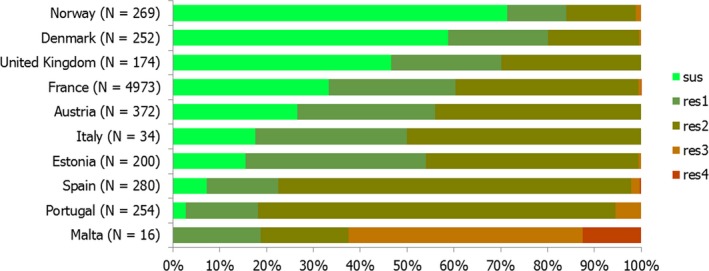
Frequency distribution of Campylobacter jejuni isolates from humans completely susceptible or resistant to one to four antimicrobial classes in 2017
4.1.2. Antimicrobial resistance in Campylobacter coli isolates from humans
With 12,365 human cases, C. coli was the second most common Campylobacter species reported in the EU/EEA in 2017. AST data were reported for 24.1% of these cases by 18 MSs, plus Iceland and Norway. Very high proportions of resistance were observed for ciprofloxacin (63.5%) and tetracyclines (68.3%), with extremely high proportions (70.5–100.0%) resistant to ciprofloxacin in 13 of the 18 reporting EU countries (Table 21, some proportions not presented due to low number of isolates tested). Proportions of isolates resistant to erythromycin and gentamicin were markedly higher in C. coli than in C. jejuni (12.8% vs 2.0% and 1.8% vs 0.5%, respectively). Portugal, Malta and Spain reported the highest levels of resistance to erythromycin (59.5%, 31.9% and 27.7%, respectively).
Table 21.
Antimicrobial resistance in Campylobacter coli from humans per country in 2017
| Country | Gentamicin | Co‐amoxiclav | Ciprofloxacin | Erythromycin | Tetracyclines | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % Res | N | % Res | N | % Res | N | % Res | N | % Res | |
| Austria | 45 | 0 | – | – | 45 | 77.8 | 45 | 4.4 | 45 | 55.6 |
| Cyprus | – | – | – | – | 4 | NA | 4 | NA | 4 | NA |
| Estonia | 27 | 0 | – | – | 27 | 100 | 27 | 14.8 | 27 | 85.2 |
| Finlanda | – | – | – | – | 351 | 89.7 | 332 | 21.4 | 219 | 74 |
| Franceb | 841 | 1.1 | 894 | 1.5 | 896 | 63.5 | 895 | 8.0 | 893 | 78.1 |
| Irelandb | – | – | – | – | 7 | NA | 7 | NA | 7 | NA |
| Italy | 7 | NA | – | – | 7 | NA | 7 | NA | 7 | NA |
| Lithuaniab | – | – | – | – | 34 | 97.1 | 35 | 17.1 | 34 | 88.2 |
| Luxembourg | – | – | 67 | 6.0 | 67 | 76.1 | 67 | 11.9 | 67 | 79.1 |
| Malta | 14 | 7.1 | 12 | 33.3 | 47 | 76.6 | 47 | 31.9 | 15 | 73.3 |
| Netherlandsb | – | – | – | – | 149 | 66.4 | 117 | 14.5 | 110 | 70.9 |
| Polandb | – | – | – | – | 1 | NA | 13 | 0 | 1 | NA |
| Portugal | 37 | 2.7 | – | – | 37 | 100 | 37 | 59.5 | 37 | 91.9 |
| Romania | 5 | NA | 5 | NA | 5 | NA | 5 | NA | 5 | NA |
| Slovakiab | – | – | 30 | 16.7 | 95 | 76.8 | 70 | 11.4 | 105 | 58.1 |
| Slovenia | – | – | – | – | 83 | 89.2 | 83 | 3.6 | 83 | 66.3 |
| Spain | 47 | 10.6 | 47 | 6.4 | 47 | 95.7 | 47 | 27.7 | 47 | 95.7 |
| United Kingdomb | 20 | 10.0 | – | – | 874 | 40.8 | 838 | 11.8 | 397 | 37.8 |
| Total (18 MSs) | 1,043 | 1.8 | 1,055 | 2.7 | 2,776 | 63.5 | 2,676 | 12.8 | 2,103 | 68.3 |
| Icelandb | – | – | – | – | 3 | NA | 3 | NA | – | – |
| Norway | 10 | 0 | – | – | 10 | 40.0 | 10 | 20.0 | 10 | 50.0 |
N: number of isolates tested; % Res: percentage of resistant isolates (either non‐wild type by ECOFFs or clinically non‐susceptible by combining resistant and intermediate categories); –: no data reported; NA: not applicable (if less than 10 isolates were tested, the percentage of resistance was not calculated); MS: Member State.
Travel‐associated cases, accounting for 79% of Campylobacter infections in Finland in 2017, could not be excluded from the Finnish AST data.
Data interpreted with clinical breakpoints.
Spatial distribution of resistance among Campylobacter coli isolates from humans
Ciprofloxacin resistance was very common in C. coli from humans in most reporting countries, with lower proportions reported only from Norway and the United Kingdom (Figure 53a). In Estonia and Portugal, all tested isolates were resistant to ciprofloxacin. The number of isolates tested by country was however often low. The proportions of erythromycin resistance was notably high in Portugal (Figure 53b). Note that data from Finland also include isolates from travel‐associated cases, accounting for 79% of Campylobacter infections in the country in 2017 as these could not be separated in the Finnish AST data provided by local laboratories. The observed resistance levels in Finland can therefore not be considered representative for domestically acquired infections.
Figure 53.
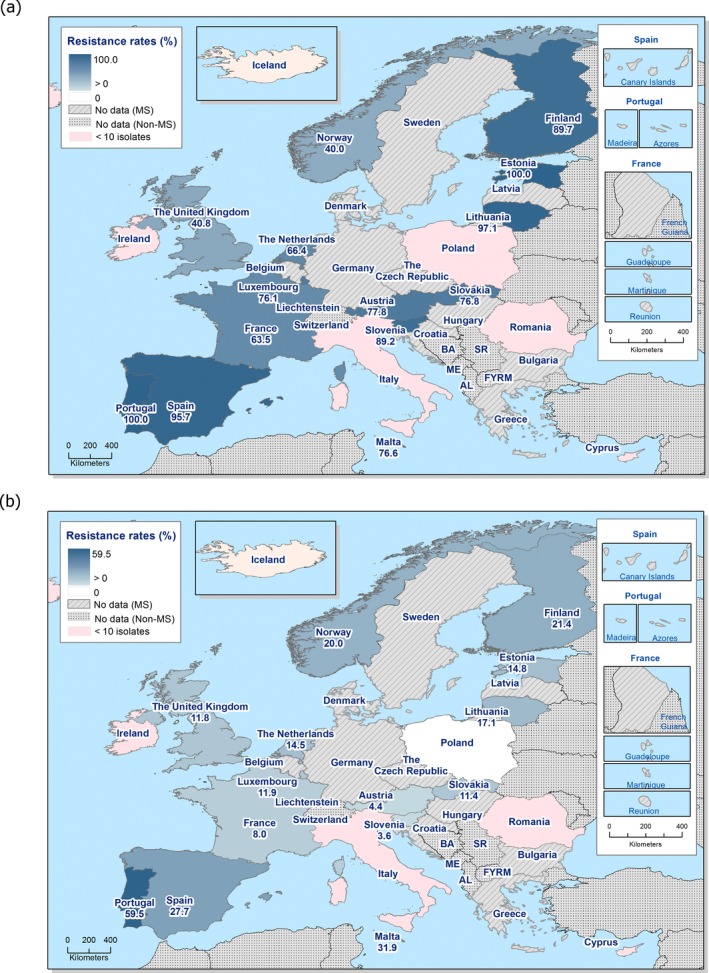
Spatial distribution of ciprofloxacin (a) and erythromycin (b) resistance among Campylobacter coli from human cases in reporting countries in 2017
Combined resistance to ciprofloxacin and erythromycin in C. coli from humans
Every tenth C. coli isolate (10.2%) exhibited ‘microbiological’ as well as ‘clinical’ resistance to both ciprofloxacin and erythromycin with the highest proportions observed in Portugal (59.5%), Spain (27.7), Malta (25.5%) and Finland (20.1%) (18 MSs, Table COMCAMPCOHUM; Figure 54)
Figure 54.
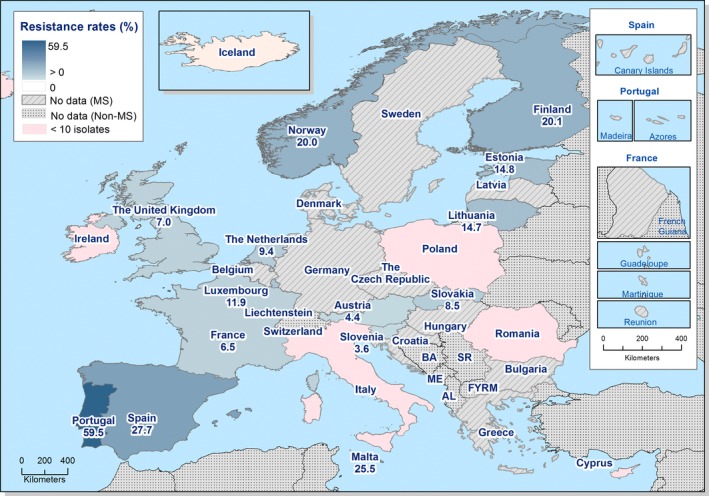
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter coli from human cases in reporting countries in 2017
Temporal trends in resistance among C. coli isolates from humans
Trend analysis was performed for the years 2013–2017 and for the three antimicrobials ciprofloxacin, erythromycin and tetracyclines. Fourteen MSs were included in the analysis as they had provided resistance data for a minimum of 3 years in this period and a minimum of 10 C. coli isolates (Figure 55). For ciprofloxacin resistance in C. coli, significant increasing trends were observed in Estonia, Finland, Slovakia and Slovenia and a decreasing trend in France. Resistance to erythromycin also decreased in France. Increasing trends were observed for tetracycline resistance in France, the Netherlands, Slovakia and Slovenia.
Figure 55.
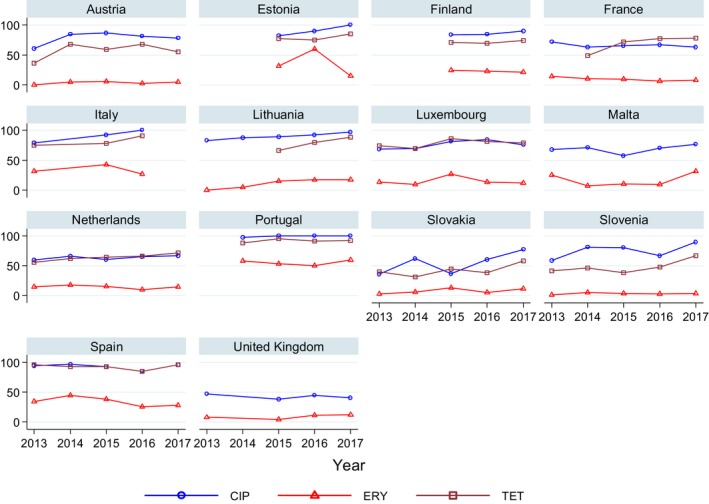
Trends in ciprofloxacin, erythromycin and tetracycline resistance in Campylobacter coli from humans in reporting countries, 2013–2017
- Statistically significant increasing trends over 3–5 years, as tested by logistic regression (p ≤ 0.05), were observed for ciprofloxacin in Estonia, Finland, Slovakia and Slovenia (↑), for tetracycline in France, the Netherlands, Slovakia and Slovenia (↑). Statistically significant decreasing trends were observed for ciprofloxacin and erythromycin in France (↓). Only countries reporting at least 10 isolates per year were included in the analysis.
High‐level erythromycin resistance in Campylobacter coli
Of the 274 C. coli isolates with MIC data, 15.0% (41 isolates) had a MIC > 128 mg/L (Figure 56). Such isolates were reported from five of eight countries which had provided quantitative data from dilution tests (Table 22). Similarly, for 63 of 436 isolates (14.5%) tested with disc diffusion no inhibition zone could be observed (6 mm zone equals the disc size), which in 95% of isolates corresponds to a MIC of ≥ 128 mg/L and in 5% a MIC of 64 mg/L (EUCAST, 2017a).
Figure 56.
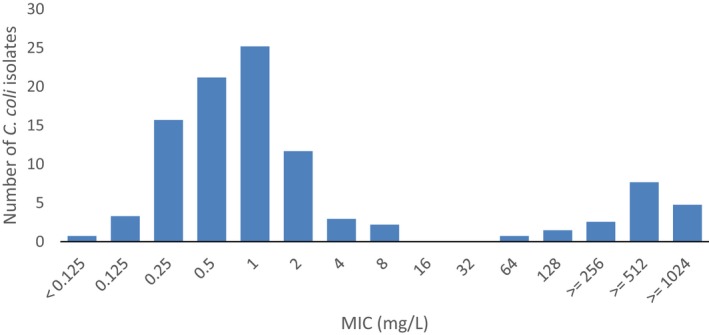
Erythromycin MIC distribution in C. coli from humans, 2017 (n = 274)
Table 22.
Occurrence of high‐level resistance to erythromycin (MIC > 128 mg/L) in Campylobacter coli from humans in 2017
| Country | N | High‐level resistance to erythromycin (MIC > 128 mg/L) | |
|---|---|---|---|
| n | % | ||
| Austria | 45 | 1 | 2.2 |
| Estonia | 2 | 0 | NA |
| Finland | 127 | 19 | 15.0 |
| Luxembourg | 15 | 2 | 13.3 |
| Malta | 28 | 6 | 21.4 |
| Portugal | 1 | 0 | NA |
| Spain | 47 | 13 | 27.7 |
| Total (7 MSs) | 265 | 41 | 15.5 |
| Norway | 10 | 2 | 20.0 |
NA: not applicable (if less than 10 isolates were tested, the percentage of resistance was not calculated).
MDR in Campylobacter coli isolates from humans
Overall, 11.9% of the human C. coli isolates were susceptible to all four antimicrobial classes, with no susceptible isolates reported by Estonia, Malta, Portugal and Spain (Figure 57, 7 MSs, Table MDRCAMPCOHUM). The level of MDR was moderate overall (10.3%) and ranged from 4.4% in Austria to 61.6% in Malta. France reported six isolates, Spain five and Portugal and the United Kingdom one isolate each resistant to all four antimicrobial classes (Figure 57).
Figure 57.
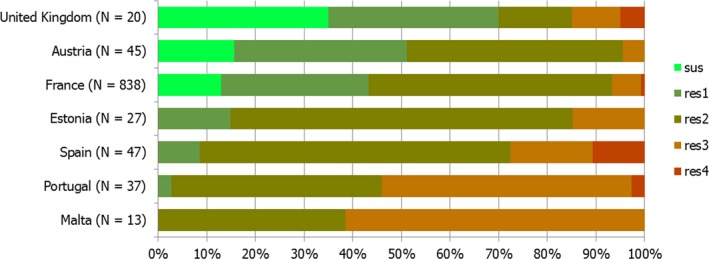
Frequency distribution of Campylobacter coli isolates from humans completely susceptible or resistant to one to four antimicrobial classes in 2017
4.2. Antimicrobial resistance in Campylobacter spp. from animals and food19
Under the framework of Commission Implementing Decision 2013/652/EU, for the year 2017, quantitative isolate‐based MIC data on Campylobacter were primarily collected and reported on C. coli from fattening pigs by seven MSs and two non‐MS and meat derived thereof (only one MS) on a voluntary basis. In addition, limited data on C. jejuni from fattening pigs (11 isolates) were also reported on a voluntary basis by 3 MSs and Switzerland. Few AMR data on C. jejuni isolates from meat of bovine animals were also reported by Croatia (n = 6 isolates) for 2016 and the Netherlands (n = 3 isolates) for 2017, respectively, and five MSs transmitted AMR data on C. jejuni from calves.
4.2.1. Antimicrobial resistance in Campylobacter spp. from pig and bovine meat
Representative monitoring
In 2016, Croatia and Portugal reported data on C. coli from pig meat, with, respectively, 31 and 3 isolates tested. In 2017, Portugal also reported limited data on 5 C. coli isolates from pig meat.
Resistance levels in C. jejuni and C. coli from pig meat
Considering the 34 C. coli isolates from pig meat tested for susceptibility in 2016, resistance to gentamicin (2.9%) and to erythromycin (2.9%) was low, whereas very high to extremely high resistance to streptomycin (88.2%), nalidixic acid (76.5%), ciprofloxacin (76.5%) and tetracycline (85.3%) were observed.
Regarding the five C. coli from pig meat in Portugal isolated in 2017, resistance levels were high (gentamicin: 40%), very high (nalidixic acid, ciprofloxacin and erythromycin: 60% each) and all isolates were found resistant to streptomycin and tetracycline (Table 23).
Table 23.
Occurrence of resistance (%) to selected antimicrobials in Campylobacter coli from pig meat, using harmonised epidemiological cut‐off values (ECOFFs), 2 EU MSs, 2016 and 2017
| Country | N | GEN | STR | NAL | CIP | ERY | TET |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| Croatia | 31 | 3.2 | 87.1 | 77.4 | 77.4 | 0 | 87.1 |
| Portugala | 3 | 0 | 100 | 66.7 | 66.7 | 33.3 | 66.7 |
| Total (2 MSs) | 34 | 2.9 | 88.2 | 76.5 | 76.5 | 2.9 | 85.3 |
| 2017 | |||||||
| Portugala | 5 | 40 | 100 | 60 | 60 | 60 | 100 |
MSs: Member States; N: number of isolates tested; %Res: percentage of resistant isolates per category of susceptibility; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; NAL: nalidixic acid; STR: streptomycin; TET: tetracycline.
Occurrence of resistance assessed on less than 10 isolates.
4.2.2. Antimicrobial resistance among Campylobacter spp. from fattening pigs
Representative monitoring
In 2016 and 2017, susceptibility data on 59 and 979 C. coli isolates from fattening pigs were, respectively, reported by one (Croatia) and seven MSs (Croatia, the Czech Republic, Estonia, Finland, Germany, Spain and Sweden). Two non‐MSs countries (Norway and Switzerland) also reported data for 2017 on a total of 411 isolates. It is of note that all these data were reported on a voluntary basis with a minimum of 20 isolates tested in Estonia and a maximum of 255 in Norway.
Resistance levels in C. coli from pigs
The occurrence of resistance to the harmonised set of antimicrobials in the C. coli isolates from fattening pigs studied varied greatly between the reporting countries. In 2017, the overall levels of resistance observed in C. coli to streptomycin (64.4%), ciprofloxacin (52.3%), nalidixic acid (52.3%) and tetracyclines (51.5%) were very high, whereas those to the remaining substances to the harmonised panel were moderate (erythromycin, 15.6%) or low (gentamicin, 7.7%) (Table 24).
Table 24.
Occurrence of resistance (%) to selected antimicrobials in Campylobacter coli isolates from fattening pigs, using harmonised ECOFFs, 7 EU/EEA MSs, 2016 and 2017
| Country | N | GEN | STR | NAL | CIP | ERY | TET |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| Croatia | 59 | 3.4 | 94.9 | 89.8 | 89.8 | 5.1 | 88.1 |
| 2017 | |||||||
| Croatia | 79 | 65.8 | 97.5 | 93.7 | 89.9 | 3.8 | 69.6 |
| Czech Republic | 130 | 0.8 | 78.5 | 47.7 | 46.9 | 7.7 | 66.2 |
| Estonia | 20 | 0 | 85 | 20 | 30 | 0 | 40 |
| Finland | 196 | 0 | 11.2 | 17.3 | 16.8 | 1.5 | 0 |
| Germany | 247 | 0 | 74.9 | 52.6 | 53.8 | 12.6 | 74.1 |
| Spain | 170 | 12.9 | 91.2 | 95.9 | 97.1 | 61.8 | 100 |
| Sweden | 137 | 0 | 52.6 | 32.8 | 31.4 | 0.7 | 1.5 |
| Total (7 MSs) | 979 | 7.7 | 64.4 | 52.3 | 52.3 | 15.6 | 51.5 |
| Netherlands a | 83 | 0 | 73.5 | 15.7 | 15.7 | 6.0 | 88.0 |
| Norway | 255 | 0 | 31 | 19.2 | 18.8 | 0 | 0 |
| Switzerland | 161 | 1.2 | 81.4 | 52.2 | 50.3 | 1.9 | 62.1 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; %Res: percentage of resistant isolates per category of susceptibility; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; NAL: nalidixic acid; STR: streptomycin; TET: tetracycline.
The sample origin was not mentioned in reporting.
Considering ciprofloxacin (overall 52.3%), the lowest levels of resistance were found in Finland (16.8% out of 196 strains tested) and Norway (18.8% out of 255 strains tested), whereas much higher levels of resistance were observed in Croatia (89.9% out of 79 strains tested) and Spain (97.1% out of 170 strains tested). A similar variability in nalidixic acid resistance was observed among reporting countries, ranging from 17.3% in Finland and 19.2% in Norway to 93.7% in Croatia and 95.9% in Spain.
Fluoroquinolone resistance in Campylobacter
Resistance to quinolones and fluoroquinolones is usually due to mutations in the gyrase gene; the C257T mutation on gyrA gene (Thr86Ile in the GyrA protein) being the major mechanism for ciprofloxacin resistance. The recent use of WGS to characterise the genomic changes of 589 C. jejuni or C. coli isolates, collected as part of the National Antimicrobial Resistance Monitoring System (NARMS) in the USA has showed a 100% correlation between genotype (Thr86Ile) and phenotype (resistance to nalidixic acid and ciprofloxacin, n = 109) (Whitehouse et al., 2018). Interestingly, in certain strains, this mutation may not result in a biological cost, and those fluoroquinolone‐resistant strains can still outcompete susceptible ones in chickens in the absence of a selective pressure (Luo et al., 2005). The rapid emergence of fluoroquinolone‐resistant Campylobacter might be partly attributable to this phenomenon. Nevertheless, quinolone‐ and/or fluoroquinolone‐resistant C. jejuni strains of human and chicken origins without the C257T (Thr86Ile) mutation in gyrA and without any other amino acid mutations detected in the GyrA protein were also recently described in Japan (Ohishi et al., 2017). Still, the same authors also detected 13 C. jejuni isolates of human origin with the C257T mutation which still appeared susceptible to fluoroquinolones. Further characterisation of these strains is required.
For erythromycin, an important therapeutic compound for human infections, the overall resistance was moderate (15.6% for 7 MSs). Erythromycin resistance was undetected in Estonia (20 isolates tested) and in Norway (255 isolates tested) and very low in Sweden (0.7% out of 137 strains tested). Conversely, C. coli resistance to macrolides was very frequent in Spain (61.8% out of 170 strains tested).
Resistance to gentamicin was undetected in Estonia, Finland, Germany and Sweden as well as Norway, representing together a total of 855 isolates, and was very low in the Czech Republic and low in Switzerland. Conversely, isolates from Croatia were very frequently resistant to gentamicin (65.8% out of 79 isolates tested). A moderate percentage of resistance was reported for Spain (12.9% out of 170 strains tested). In Finland the percentage of resistance to streptomycin only reached 11.2% out of 196 strains tested whereas most countries reported very high to extremely high percentages (overall percentage for the 7 MSs: 64.4%).
For tetracycline (overall resistance 51.5%), no resistant isolate could be detected in Finland (196 strains tested) and Norway (255 isolates tested), and the percentage was only 1.5% in Sweden (137 strains tested), whereas all 170 isolates from Spain were resistant.
Comparison of resistance in C. coli from pigs and pig meat
As very few isolates from pig meat were reported for 2017, a comparison between overall resistance levels of isolates from pigs in 2017 (7 MSs, 979 isolates) and pig meat in 2016 (2 MSs, including Croatia (31/34 isolates and Portugal (3/34 isolates)) was first conducted. The percentages of resistance were significantly different for streptomycin (p = 0.003), nalidixic acid (p = 0.005), ciprofloxacin (p = 0.005), erythromycin (p = 0.048) and tetracycline (p < 0.001), with higher percentages in meat in 2016 for streptomycin, nalidixic acid, ciprofloxacin and tetracycline, but lower percentage for erythromycin.
As most isolates from meat originated from Croatia, a second comparison was performed between isolates from pigs from Croatia reported for 2017 and isolates from pig meat from the same MS in 2016. Significant differences (p < 0.05) were detected for gentamicin and nalidixic acid with higher percentages in pigs in 2017.
Resistance levels in C. jejuni from pigs
A few C. jejuni isolates (n = 11) from fattening pigs were also reported on a voluntary basis for 2017, originating from the Czech Republic (2 isolates), Germany (8 isolates) and Luxembourg (1 isolate). Whereas all isolates were susceptible to gentamicin and erythromycin and resistance to streptomycin was reported only in Germany, resistance to nalidixic acid, ciprofloxacin and tetracycline was reported at high levels. Additionally, Switzerland reported nine isolates; all being susceptible to gentamicin and erythromycin, and two of them being resistant to nalidixic acid, ciprofloxacin or tetracycline.
Spatial distribution of ciprofloxacin and erythromycin resistance in C. coli from pigs
The spatial distribution of ciprofloxacin resistance in C. coli from fattening pigs showed that the levels of resistance were the highest in southern Europe (Spain, 97.1% and Croatia, 89.9%), whereas they were closed to 50% in central Europe (Germany, Switzerland and the Czech Republic) and ranged from 16.8% to 31.4% in the northern European reporting countries (Finland, Norway, Estonia and Sweden). Regarding erythromycin, resistance was detected in Spain at 61.8%, close to 10% in central Europe (Germany and the Czech Republic) and lower than 5% in the other northern European reporting countries (Figure 58).
Figure 58.
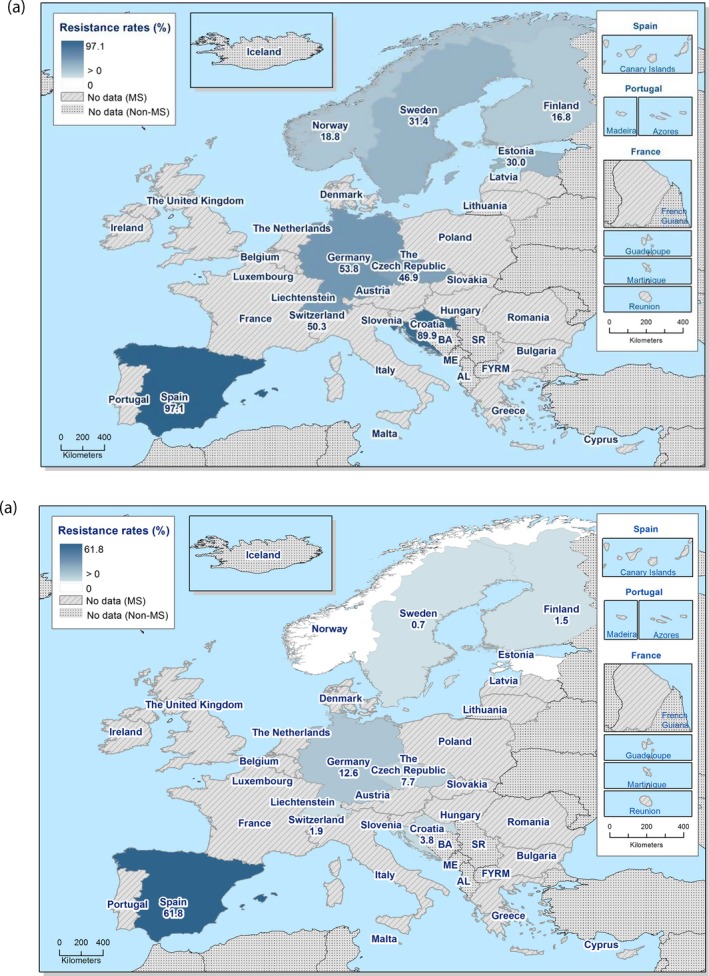
Spatial distribution of ciprofloxacin (a) and erythromycin (b) resistance in Campylobacter coli isolates from fattening pigs, 7 EU/EEA MSs, 2017
Combined resistance to ciprofloxacin and erythromycin in C. coli from pigs
The important combined resistance for public health to both ciprofloxacin and erythromycin was more frequently reported in Spain (61.2%). Levels of combined resistance were much lower in Germany (8.1%) and in other countries (less than 5% of reported isolates) in 2017 (Figure 59).
Figure 59.
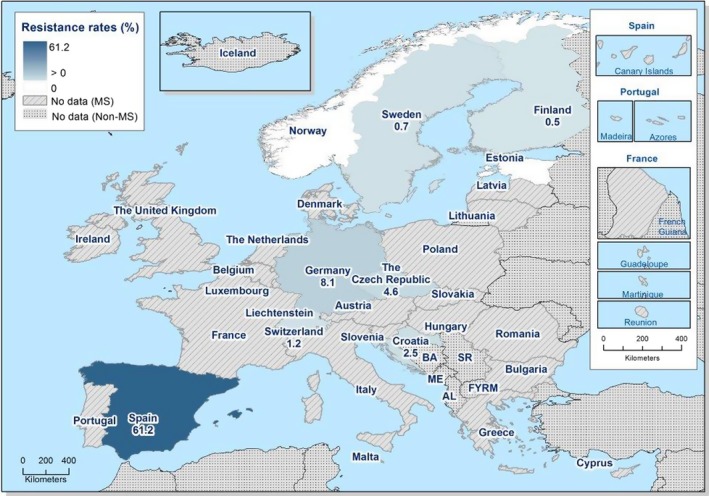
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter coli isolates from fattening pigs, 7 EU/EEA MSs, 2017
Temporal trends in resistance in C. coli from pigs
Longitudinal temporal trends can be assessed on the medium term for only a limited subset of reporting countries. The statistical analysis (Figure 60) shows that, in Switzerland, increasing trends have been observed for most of the substances monitored, namely ciprofloxacin, gentamicin, streptomycin and tetracycline, over 2009–2017 period. Interestingly, in the three reporting countries considered, the level of erythromycin resistance has significantly decreased over the study period, although it has remained very high in Spain, whereas in both the Netherlands and Switzerland, erythromycin resistance has reached low levels.
Figure 60.
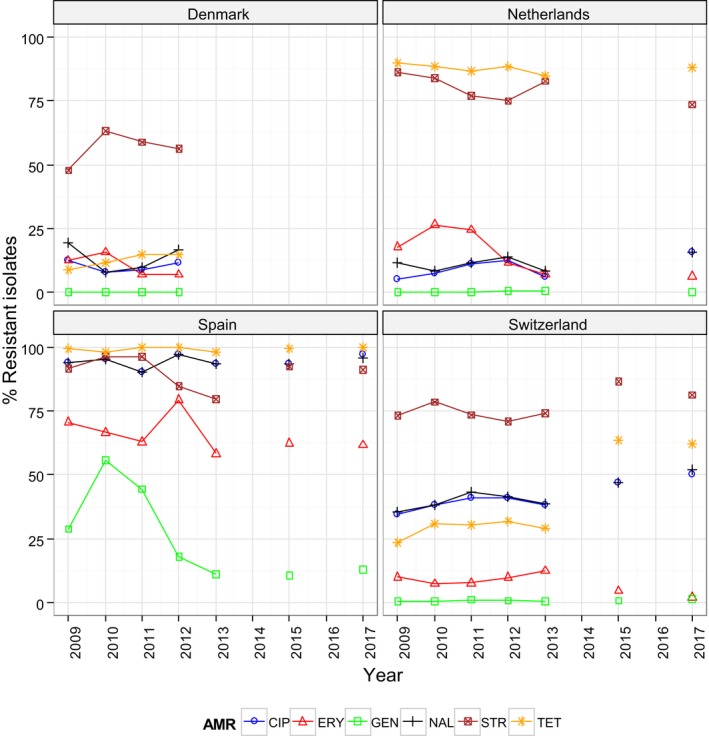
Trends in ciprofloxacin (CIP), erythromycin (ERY), gentamicin (GEN), nalidixic acid (NAL), streptomycin (STR) and tetracycline (TET) resistance in Campylobacter coli isolates from pigs, 3 reporting countries, 2008–2017
- Statistically significance of trends over 4/5 or more years was tested by a logistic regression model (p ≤ 0.05).
- Statistically significant increasing trends were observed for ciprofloxacin, nalidixic acid, streptomycin and tetracycline in Switzerland.
- Statistically significant decreasing trends were observed for erythromycin in the Netherlands, Spain and Switzerland, for gentamicin in Spain, for streptomycin in Spain.
Complete susceptibility and multidrug resistance patterns in C. coli from pigs
Resistance profiles to the harmonised set of antimicrobials (nalidixic acid/ciprofloxacin, erythromycin, gentamicin and tetracycline) were studied for the 1395 isolates tested in the nine reporting countries. Marked variations in both levels of complete susceptibility and MDR were observed in the isolates recovered in the reporting countries (Figure 61).
Figure 61.
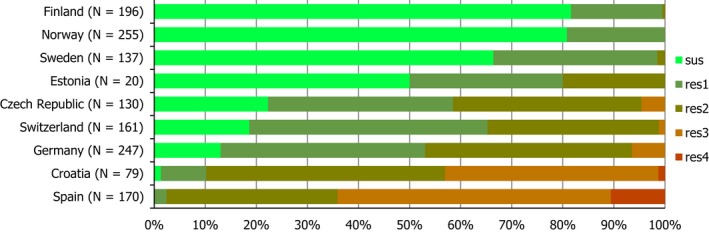
Frequency distribution of Campylobacter coli isolates completely susceptible and resistant to one to four antimicrobials, from fattening pigs, 9 reporting countries, 2017
- N: total number of isolates tested for susceptibility against the whole harmonised set of antimicrobials for Campylobacter; sus: susceptible to all antimicrobial classes of the harmonised set for Campylobacter; res1–res4: resistance to one up to four antimicrobial classes of the harmonised set for Campylobacter.
The isolates exhibiting complete susceptibility to nalidixic acid/ciprofloxacin, erythromycin, gentamicin and tetracycline were detected in all reporting countries, with the exception of Spain, accounting overall for 33.0% of the 979 isolates from the MSs and 40.1% of the 1,395 isolates from all reporting countries. Those isolates represented the major profile observed in Finland, Norway, Sweden and Estonia, with, respectively, 81.6%, 80.8%, 66.4% and 50.0% of the isolates tested. Their frequencies were lower in the Czech Republic, Switzerland and Germany (respectively, at 22.3%, 18.6% and 13.0%) and much lower in Croatia (at 1.3%).
In Switzerland, most isolates were resistant to one antimicrobial class, whereas, in Croatia, the Czech Republic and Germany, most isolates exhibited resistance to two classes. In Spain, the predominant profile of MDR was resistance to nalidixic acid/ciprofloxacin, erythromycin and tetracycline (51.2% of the isolates tested) and 53.5% and 10.6% of isolates were, respectively, resistant to three and four classes(Table 5). MDR was observed in all countries, with the exception of Norway. The rate of MDR was very low in Finland, low in Sweden and Estonia, but high in the Czech Republic, Germany and Switzerland, and extremely high in Croatia and Spain. Overall, profiles of MDR always included ciprofloxacin, the most frequent one being resistance to nalidixic acid/ciprofloxacin, erythromycin and tetracycline (109/1,395 isolates, 7.8%) (Table 25).
Table 25.
Multiresistance patterns of selected antimicrobials in Campylobacter coli from fattening pigs, 2017
| Multiresistance pattern | MS Group (N = 787) | Number of MDR isolates by country | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GEN | CIP/NAL | ERY | TET | n | %r | %MS group | HR (79) | CZ (130) | DE (247) | ES (170) | CH (161) |
| . | R | R | R | 109 | 65.3 | 13.9 | . | 5 | 16 | 87 | 1 |
| R | R | . | R | 37 | 22.2 | 4.7 | 31 | 1 | . | 4 | 1 |
| R | R | R | R | 19 | 11.4 | 2.4 | 1 | . | . | 18 | . |
| R | R | R | . | 2 | 1.2 | 0.3 | 2 | . | . | . | . |
| 167 | 100 | 21.2 | 34 | 6 | 16 | 109 | 2 | ||||
MS: Member State; GEN: gentamicin; ERY: erythromycin; TET: tetracycline; CIP: ciprofloxacin; NAL: nalidixic acid; HR: Croatia; CZ: Czech Republic; DE: Germany; ES: Spain; CH: Switzerland; MDR: multidrug‐resistant pattern.
4.2.3. High‐level erythromycin resistance in Campylobacter spp. from fattening pigs
Macrolide resistance in Campylobacter spp.: mutations and multidrug resistance genomic island (MDRGI)
Up to 2014, resistance to erythromycin was mainly thought to result from mutations in the ribosomal proteins L4 and L22 or in one or several copies of the ribosomal RNA genes, such as A2074G, A2074C, and A2075G (Luangtongkum et al., 2009). The WGS study recently performed on isolates from poultry at retail in the USA showed that the 30 erythromycin‐resistant strains studied carried either a 23S rRNA A2075G (n = 29) or a A2074T (n = 1) mutation, which were absent in erythromycin‐susceptible strains (Whitehouse et al., 2018). In addition, all 45 erythromycin‐resistant isolates of turkey origin studied in the USA, harboured A2075G substitutions in the 23S rRNA genes (Bolinger et al., 2018). The rare A2074T mutation was also discovered in a strain isolated from a diarrheic patient, and it was shown that, when present in the three copies of the 23S rRNA gene, it conferred high‐level macrolide resistance (Mossong et al., 2016). Conversely to the gyrA mutations in fluoroquinolone‐resistant isolates, these mutations usually result, at least in C. jejuni, in a biological cost (Wang et al., 2014), probably explaining the relatively low prevalence of macrolide‐resistant C. jejuni.
In 2014, the erm(B) gene, was first detected in a multidrug‐resistant C. coli of porcine origin in China, and was associated with a chromosomal MDR genomic island (MDRGI), probably originated from Gram‐positive bacteria (Qin et al., 2014). The erm(B)‐containing MDRGI could be transferred from C. coli to C. jejuni by natural transformation. It contained, besides erm(B), several other resistance determinants (aac, aadE, aacA‐aphD, the aadE‐sat4‐ aphA3 cluster and a truncated tet(O)) and could confer resistance to macrolides, lincosamides and aminoglycosides to a recipient strain. As this MDRGI was present in a fluoroquinolone‐ and tetracycline‐resistant C. coli strain, this strain was resistant to all drugs used for the treatment of Campylobacter infections in humans. Wang et al. (2014) subsequently identified 58 isolates (57 C. coli and one C. jejuni) harbouring the erm(B) gene among a total of 1,157 isolates from human patients, swine and poultry origins. All these isolates were resistant to erythromycin (with mainly but not only high‐level MIC), clindamycin, ciprofloxacin, tetracycline, and 67% of them were also resistant to gentamicin. The erm(B) gene was carried on the chromosome and on plasmids of various sizes in, respectively, 57% and 41% of the isolates. Plasmids carrying erm(B) were only found in swine isolates. Six different chromosomal MDRGI (types I to VI) were characterised, the type III being the most common and present in both human and animal isolates. All chromosomal MDRGI types could be transferred by transformation but assays of transfer of resistance plasmids by natural transformation or conjugation were unsuccessful. According to Zhang et al. (2016), among 154 erythromycin‐resistant C. coli from diarrheal patients, chickens and pigs, nine (isolated from chickens) and eight (seven from patients and one from chicken) belonged to ST6322 and ST1145, respectively, and all of them had the erm(B) determinant. In the study of Zhang et al. (2016) regarding 9 erythromycin‐non susceptible C. jejuni isolates from human diarrhoea in Beijing, China, obtained from 1994 to 2010, four strains harboured the erm(B) gene. Interestingly, the earliest erm(B)‐positive strain cj94473 was detected in a strain isolated in 1994, and the erm(B) gene was also detected in two intermediately resistant isolates, (erythromycin MIC = 16 mg/L). Deng et al. (Deng et al., 2015) highlighted constitutive and inducible expression of the erm(B) gene, the constitutive expression of erm(B) gene being more prevalent and associated with insertions and deletions in the regulatory region of the gene. The two inducible erm(B)‐positive isolates had low erythromycin MIC (2–4 mg/L) prior to induction and the erythromycin MIC only reached 16–32 mg/L after induction.
Li et al. (2017) showed that the prevalence of erm(B)‐positive Campylobacter increased remarkably from 2013 to 2015 in one region of China. Most erm(B) strains were isolated from chickens (83) and one was from a pig. The erm(B) gene was located most often in MDRGI of types V and VII and erm(B)‐positive isolates of chicken origin were found to share PFGE profiles and MLST sequences with human strains, suggesting again the transmission between animals and humans.
In Campylobacter isolates obtained from broilers in live bird markets in Shanghai, China, resistances to erythromycin and to azithromycin reached very high levels, respectively 84.0% and 80.8% among 125 C. coli, but remained low (6.0% for erythromycin) for the 84 C. jejuni tested (Li et al., 2017). Analysis evidenced the A2075G mutation in the 23S rRNA gene and the erm(B) gene in, respectively, 75.7% and 20.4% of the 103 azithromycin‐resistant Campylobacter spp. The authors conclude that the part played by these live bird markets in Asia in transmission of antimicrobial‐resistant bacteria between flocks and from poultry to humans should be better controlled.
Up to now, only two reports relative to erm(B)‐positive Campylobacter in Europe have been published (Florez‐Cuadrado et al., 2016, 2017). First, a C. coli strain which was also resistant to fluoroquinolones, tetracycline and streptomycin but susceptible to gentamicin was isolated from a broiler in Spain. The erm(B) gene was carried on a MDRGI of a new type (type VIII), which contained also tetracycline and aminoglycoside resistance genes. Then, two turkey C. coli isolates positive for the erm(B) gene, clustering with genes conferring resistance to aminoglycosides (aad9, aadE, aph(2’’)‐IIIa, aph(3’)‐IIIa), and tetracycline (tet(O)) were described. The NCBI sequences of the erm(B) genes of Campylobacter from China and Spain presented four different allelic variations. The identification of identical erm(B) sequences among Campylobacter from turkeys, Streptococcus suis from pigs, and Enterococcus faecium and Clostridium difficile from humans suggested, however, the horizontal spread of the erm(B) gene among different bacterial hosts and animal species.
Finally, a IXth type of erm(B) MDRGI was identified in a clinical isolate in the USA resistant to all tested compounds except florfenicol; an acquisition of the isolate from Malaysia was suspected (Chen et al., 2018). Thus, the recent emergence of transferable macrolide resistance in Campylobacter with the erm(B) gene borne on plasmids or chromosomal MDRGI may provide a means of dissemination and/or co‐selection of macrolide‐ or multidrug resistance.
A MIC distribution can be used to assess the proportion of isolates exhibiting higher levels of resistance to the substance in question. The MIC distribution for erythromycin for C. coli isolates from fattening pigs in 2017 (Figure 5) shows that isolates of C. coli from fattening pigs with MICs > 128 mg/L have been detected. The distribution by reporting country of isolates that have an erythromycin MIC higher than the highest erythromycin concentration tested (MIC > 128 mg/L) – under the harmonised method set out in Decision 2013/652/EU – is shown in Table 26. Spain was the MS which detected most high‐level resistance to erythromycin; Spain and Germany together accounted for 87.9% of high‐level erythromycin resistant C. coli isolates which were detected. It is also of note that, among the countries reporting data on a voluntary basis, no isolates recovered from Estonia and Norway exhibited an MIC > 128 mg/L, although the number of isolates tested in Estonia is much lower than that tested in Norway (Figure 62).
Table 26.
Occurrence (%) of high‐level resistance to erythromycin (MIC > 128 mg/L) in Campylobacter coli isolates from fattening pigs, 6 reporting EU MSs, 2017
| Country | N | High‐level resistance to ERY (MIC > 128 mg/L) | |
|---|---|---|---|
| n | % | ||
| Croatia | 79 | 2 | 2.5 |
| Czech Republic | 130 | 3 | 2.3 |
| Estonia | 20 | 0 | 0.0 |
| Finland | 196 | 3 | 1.5 |
| Germany | 247 | 28 | 11.3 |
| Spain | 170 | 95 | 55.9 |
| Sweden | 137 | 1 | 0.73 |
| Total (7 MSs) | 979 | 132 | 13.5 |
| Netherlandsa | 83 | 5 | 6.0 |
| Norway | 255 | 0 | 0.0 |
| Switzerland | 161 | 3 | 1.9 |
ERY: erythromycin; MIC: minimum inhibitory concentration; MS: Member State.
The sample origin was not mentioned in reporting.
Figure 62.
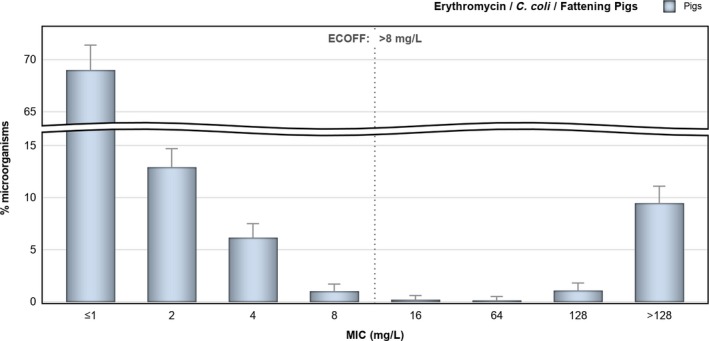
MIC distribution to erythromycin in Campylobacter coli from fattening pigs ‐ 1,478 isolates, 10 reporting countries, 2017
Although transferable erythromycin resistance conferred by erm(B) generally results in high‐level resistance to erythromycin, mutational resistance can also result in high‐level resistance to erythromycin, but may equally result in lower MICs, though still above the ECOFF, dependent on the particular mutations having occurred (see text box above). Those isolates exhibiting MICs > 128 mg/L therefore have an erythromycin resistance phenotype consistent with either possession of transferable – erm(B) – or mutational resistance. Genetic investigation of isolates will be necessary for definitive characterisation of the resistance mechanisms which are present. Any significant fluctuation observed in the MIC proportions may nevertheless provide an early indication of a possible change in the occurrence of high‐level macrolide resistance in Campylobacter. This was observed notably in Spain where the proportion of C. coli isolates from fattening pigs exhibiting high‐level resistance to erythromycin detected in 2017 (55.9%) was statistically significantly greater than that observed in 2015 (44.1%) (one‐tailed z‐test to compare proportions, p‐value = 0.0148). A greater proportion of high‐level erythromycin resistant C. coli isolates was also observed in Germany in 2017 compared with 2015, although not at a statistically significant level. Further investigation of the concerned strains may be valuable in Spain and in a lesser extent, in Germany.
4.2.4. Prevalence of antimicrobial resistance of Campylobacter coli in fattening pigs
Attempt at assessing the prevalence of antimicrobial resistance in C. coli from pigs
The occurrence of resistance in C. coli in fattening pigs describes the proportion of all C. coli isolates tested showing microbiological resistance to each antimicrobial 0). The prevalence of resistant C. coli in fattening pigs describes the proportion of C. coli showing microbiological resistance to each antimicrobial as a percentage of all caecal samples cultured for C. coli. This prevalence of C. coli resistant is the product of the prevalence of C. coli in caecal samples from fattening pigs (Table 27) and the occurrence of resistance in the C. coli isolates tested for susceptibility (Table 24).
Table 27.
Number and proportions (%) of Campylobacter coli‐positive caecal samples of fattening pigs, 7 reporting EU MSs, 2017
| Country | Total number of caecal samples tested | Number of C. coli‐positive | % |
|---|---|---|---|
| caecal samples | |||
| Croatia | 370 | 216 | 58.4 |
| Czech Republic | 255 | 142 | 55.7 |
| Estonia | 68 | 20 | 29.4 |
| Finland | 279 | 203 | 72.8 |
| Germany | 380 | 278 | 73.2 |
| Spain | 384 | 176 | 45.8 |
| Sweden | 181 | 137 | 75.7 |
| Total (7 MSs) | 1,547 | 956 | 61.8 |
| Netherlandsa | 108 | 83 | 76.8 |
| Norway | 300 | 255 | 85 |
| Switzerland | 296 | 161 | 54.4 |
MS: Member State.
The sample origin was not mentioned in reporting.
The estimates of the prevalence of C. coli in caecal samples of fattening pigs are presented in Table 27. Various factors, such as rearing conditions, feed, climate, etc., may affect the C. coli true prevalence. Additionally, the number of colonies tested may have affected the C. coli prevalence estimates, because testing multiple colonies, increases the likelihood of detecting a positive C. coli sample. The prevalence of C. coli resistant to particular antimicrobials in fattening pigs at slaughter is shown in Table 6 and discussed below.
Methodological consideration on assessing the prevalence of resistance: isolation and speciation of C. coli from pigs
Although the overarching principle of the monitoring is that only one C. coli isolate from each epidemiological unit should be included in the sampling frame, variations in methods used for isolation and speciation of C. coli from pigs have occurred, as they are not fully harmonised between MSs, conversely to the susceptibility testing method. When primary culture plates are examined for suspect Campylobacter colonies, either one or several suspect C. coli isolates can be selected for further examination and confirmation of bacterial identification. Three of the countries submitting results (Germany, Norway and Spain) selected a single suspect Campylobacter colony from primary culture plates, whereas the remaining countries selected between two and five colonies. Conversely, the culture methods performed by reporting countries tended to be similar.
The methodology applied may have affected C. coli prevalence estimates and, subsequently, the estimates of prevalence of resistant C. coli. In general, it may be assumed that MSs using methods with increased intensity of effort to detect C. coli will report a higher relative prevalence. Table 27 presents results obtained using the available data, which should be interpreted with the caveat that the intensity of sampling effort is not equal between MSs. Further refinement and harmonisation of the methods and procedures is required and the figures in Tables 27 and 28 should be considered in this context, keeping in mind these methodological differences between MSs.
Table 28.
Prevalence of resistance to selected antimicrobials in Campylobacter coli from fattening pigs, using harmonised ECOFFs, EU MSs, 2017
| Country | Nsp | GEN | STR | CIP | NAL | ERY | TET | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prev. | 95%CI | Prev. | 95%CI | Prev. | 95%CI | Prev. | 95%CI | Prev. | 95%CI | Prev. | 95%CI | ||
| Croatia | 370 | 38.4 | 33.4, 43.6 | 56.9 | 51.8, 62.1 | 52.5 | 47.2, 57.6 | 54.7 | 49.4, 59.8 | 2.2 | 0.9, 4.2 | 40.6 | 35.5, 45.7 |
| Czech Republic | 255 | 0.4 | 0, 2.2 | 43.7 | 33.9, 46.3 | 26.1 | 18.8, 29.6 | 26.6 | 19.2, 30.1 | 4.3 | 1.9, 7.1 | 36.9 | 27.9, 39.9 |
| Estonia | 68 | 0 | 0, 5.3 | 25 | 15.3, 37 | 8.8 | 3.3, 18.2 | 5.9 | 1.6, 14.4 | 0 | 0, 5.3 | 11.8 | 5.2, 21.9 |
| Finland | 279 | 0 | 0, 1.3 | 8.1 | 5, 11.7 | 12.2 | 8.3, 16.2 | 12.6 | 8.6, 16.6 | 1.1 | 0.2, 3.1 | 0 | 0, 1.3 |
| Germany | 380 | 0 | 0, 1 | 54.8 | 43.6, 53.8 | 39.4 | 30.2, 40 | 38.5 | 29.4, 39.2 | 9.2 | 5.6, 11.4 | 54.2 | 43, 53.3 |
| Spain | 384 | 5.9 | 3.6, 8.5 | 41.8 | 35.4, 45.5 | 44.5 | 38, 48.1 | 44 | 37.4, 47.6 | 28.3 | 22.9, 32.1 | 45.8 | 39.2, 49.4 |
| Sweden | 181 | 0 | 0, 2 | 39.8 | 32.6, 47.3 | 23.8 | 17.8, 30.6 | 24.8 | 18.7, 31.8 | 0.5 | 0, 3 | 1.1 | 0.1, 3.9 |
| Total (7 MSs) | 1,547 | 4.8 | 3.8, 6 | 39.8 | 38.3, 43.2 | 32.3 | 30.8, 35.5 | 32.3 | 30.8, 35.5 | 9.6 | 8.4, 11.5 | 31.8 | 30.2, 35 |
| Netherlandsa | 108 | 0 | 0, 3.4 | 56.5 | 46.6, 66 | 12.1 | 6.6, 19.7 | 12.1 | 6.6, 19.7 | 4.6 | 1.5, 10.5 | 67.6 | 57.9, 76.3 |
| Norway | 300 | 0 | 0, 1.2 | 26.4 | 21.4, 31.7 | 16 | 12, 20.6 | 16.3 | 12.3, 21 | 0 | 0, 1.2 | 0 | 0, 1.2 |
| Switzerland | 296 | 0.7 | 0.1, 2.4 | 44.3 | 38.5, 50.1 | 27.4 | 22.4, 32.8 | 28.4 | 23.3, 33.9 | 1 | 0.2, 2.9 | 33.8 | 28.4, 39.5 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; Nsp: total number of caecal samples tested; Prev.: prevalence of resistant bacteria (percentage of slaughtered fattening pigs (caecal samples) harbouring resistant isolates per category of susceptibility; 95% CI: 95% confidence interval of % Prev.; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; NAL: nalidixic acid; STR: streptomycin; TET: tetracycline.
The sample origin was not mentioned in reporting.
4.2.5. Antimicrobial resistance among Campylobacter spp. from calves under 1 year of age
Representative monitoring
Five MSs reported AMR data on C. jejuni from cattle, on a voluntary basis. The numbers of tested isolates ranged from 53, in Croatia, to 236, in Denmark, and reached 585, considering together the five reporting MSs. The C. jejuni isolates tested in 2017 typically originated from calves under 1 year of age, with the exception of the Netherlands, where they derived from fattening steers.
Resistance levels in Campylobacter jejuni from calves
The occurence of resistance varied greatly between MSs. For gentamicin, whereas no resistant isolate was detected in Denmark and the Netherlands, resistance to this aminoglycoside was observed in 77.4% of the 53 isolates tested in Croatia. The overall resistance level for the five MSs was 8.2%. The percentages of resistance to streptomycin, another aminoglycoside, paralleled that to gentamicin. Resistance to nalidixic acid or ciprofloxacin was high to extremely high and the overall level for ciprofloxacin was 52.5%. All isolates from Croatia were resistant to fluoroquinolones. Resistance to erythromycin, another important antibiotic for public health, was detected in all reporting MSs, but at low to very low levels; the overall resistance equalling 1.2%. Tetracycline resistance was more frequently observed with an overall percentage of 39.0%, but differences between MSs were reported with levels of resistance ranging from 6.8% (Denmark) to 91.9% (Italy).
Table 29.
Occurrence of resistance to selected antimicrobials in Campylobacter jejuni from calves under 1 year of age, using harmonised ECOFFs, 5 EU MSs, 2017
| Country | N | GEN | STR | NAL | CIP | ERY | TET |
|---|---|---|---|---|---|---|---|
| Croatia | 53 | 77.4 | 81.1 | 100 | 100 | 3.8 | 34.0 |
| Denmark | 236 | 0 | 0.4 | 30.1 | 30.1 | 0.4 | 6.8 |
| Italy | 74 | 5.4 | 13.5 | 77.0 | 78.4 | 1.4 | 91.9 |
| Netherlandsa | 90 | 0 | 1.1 | 25.6 | 23.3 | 1.1 | 17.8 |
| Spain | 132 | 2.3 | 27.3 | 76.5 | 78.8 | 1.5 | 83.3 |
| Total (5 MSs) | 585 | 8.2 | 15.6 | 52.1 | 52.5 | 1.2 | 39.0 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; CIP: ciprofloxacin; ERY: erythromycin; GEN: gentamicin; NAL: nalidixic acid; STR: streptomycin; TET: tetracycline
C. jejuni isolates from cattle tested in the Netherlands in 2017 derive from fattening steers.
Spatial distribution of ciprofloxacin and erythromycin resistance in C. jejuni from calves
The spatial distribution of ciprofloxacin resistance in cattle shows that the highest levels of resistance were reported in the three southern European reporting MSs (Croatia, Spain and Italy), whereas lower resistance levels were reported in northern countries (Denmark and the Netherlands). The resistance levels for erythromycin were not significantly different with one or two resistant isolates in each country (Figure 63).
Figure 63.
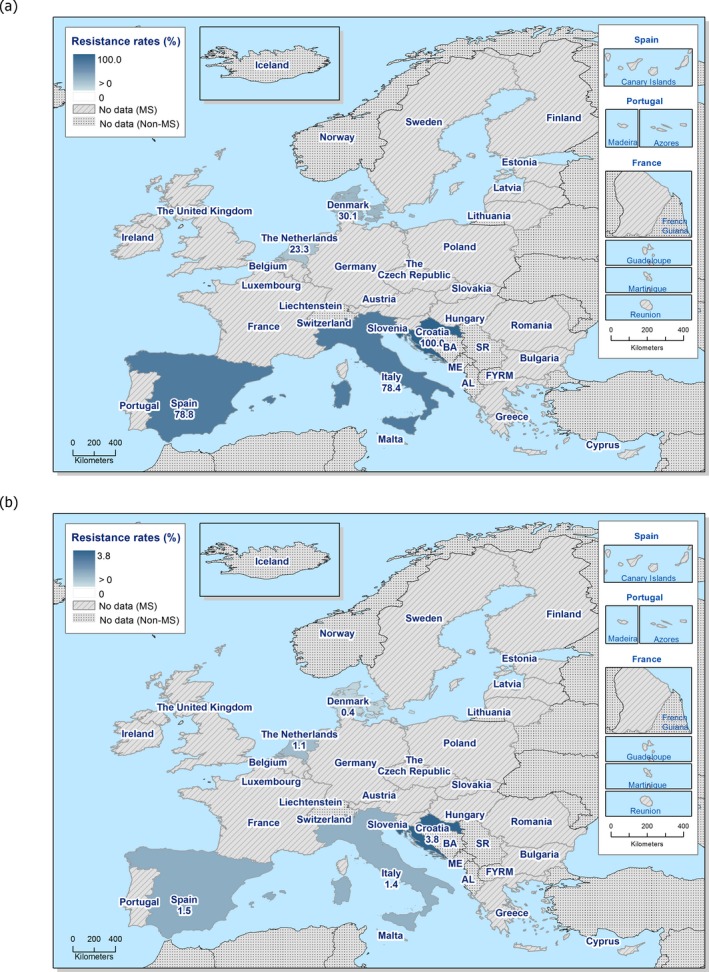
Spatial distribution of ciprofloxacin (a) and erythromycin (b) resistance in Campylobacter jejuni isolates from calves under 1 year of age, 5 EU MSs, 2017
- C. jejuni isolates from cattle tested in the Netherlands in 2017 derive from fattening steers.
Combined resistance to ciprofloxacin and erythromycin in C. jejuni from calves
No C. jejuni isolate from Denmark was found to be resistant to the two important therapeutic compounds, erythromycin and ciprofloxacin. In the other reporting countries, only one (Italy, the Netherlands) or two isolates (Croatia and Spain) were resistant to ciprofloxacin and erythromycin. The overall level of combined resistance to ciprofloxacin and erythromycin was 1.7% (Figure 64).
Figure 64.
Spatial distribution of combined resistance to ciprofloxacin and erythromycin in Campylobacter jejuni isolates from calves under 1 year of age, 5 EU MSs, 2017
- C. jejuni isolates from cattle tested in the Netherlands in 2017 derive from fattening steers.
Complete susceptibility and multidrug resistance patterns in C. jejuni from calves
Two thirds of the C. jejuni isolates from calves from Denmark, as well as two thirds of those from fattening steers from the Netherlands were susceptible to the four antimicrobial classes considered (gentamicin, erythromycin, tetracycline and ciprofloxacin/nalidixic acid). Conversely, in both Italy and Spain, complete susceptibility was observed in approximately 5% of isolates only, and this profile was undetected in Croatia. Most strains from Croatia, Spain and Italy had two resistances (respectively 58.5%, 66.7% and 68.9%). Resistance to three antimicrobial classes was undetected in Denmark and was very low in the Netherlands and Spain. In this latter MS, two isolates (1.5%) also exhibited resistance to the four classes considered. The percentage of isolates with resistance to three classes was low in Italy (6.8%) but high in Croatia (28.3%) (Figure 65).
Figure 65.
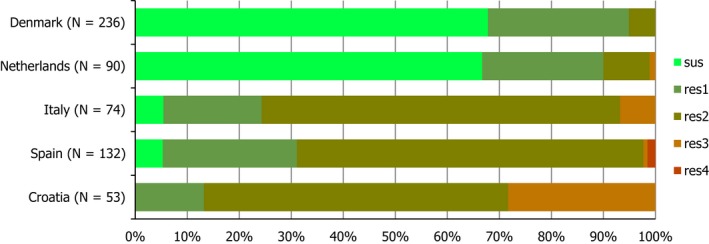
Frequency distribution of Campylobacter jejuni isolates completely susceptible and resistant to one to four antimicrobial classes, from calves under 1 year of age, 5 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole harmonised set of antimicrobials for Campylobacter; sus: susceptible to all antimicrobial classes of the harmonised set for Campylobacter; res1–res4: resistance to one up to four antimicrobial classes of the harmonised set for Campylobacter.
Resistance to gentamicin, ciprofloxacin/nalidixic acid and tetracycline was detected in most MDR isolates from Croatia (13 out of 15), and Italy (4 out of 5) and in one isolate from Spain. The two other MDR isolates from Spain were resistant to the four classes of antimicrobials. The other profiles of MDR always included resistance to ciprofloxacin/nalidixic acid, with either gentamicin and erythromycin (two isolates in Croatia) or erythromycin and tetracycline (one isolate in Italy and one isolate in the Netherlands).
Table 30.
Multidrug resistance patterns of selected antimicrobials in Campylobacter jejuni from calves under 1 year of age, 2017
| MDR Pattern | MS Group (N = 349) | Number of MDR isolates by country | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GEN | CIP/NAL | ERY | TET | n | %r | %MS group | HR (53) | IT (74) | NL (90) | ES (132) |
| R | R | R | 18 | 75.0 | 5.2 | 13 | 4 | . | 1 | |
| R | R | R | 2 | 8.33 | 0.6 | 2 | . | . | . | |
| R | R | R | R | 2 | 8.33 | 0.6 | . | . | . | 2 |
| R | R | R | 2 | 8.33 | 0.6 | . | 1 | 1 | . | |
| 24 | 100 | 6.9 | 15 | 5 | 1 | 3 | ||||
GEN: gentamicin; ERY: erythromycin; TET: tetracycline; CIP: ciprofloxacin; NAL: nalidixic acid; ES: Spain; HR: Croatia; IT: Italy; NL: Netherlands; MDR: multidrug resistance/resistant pattern; MS: Member State.
4.3. Discussion
4.3.1. Antimicrobial resistance in Campylobacter spp. in humans
Information on AMR in Campylobacter isolates from human cases of campylobacteriosis was available from 19 MSs, Iceland and Norway in 2017. For ciprofloxacin, which is one of the two antimicrobials regarded as critically important for treatment of Campylobacter infections in humans (Urdahl et al., 2017), very high (> 50%) to extremely high (> 70%) resistance levels were reported in C. jejuni isolates from all MSs except Denmark, Ireland, Iceland and Norway. Of the 13 countries testing more than 10 C. coli isolates, 6 had levels of ciprofloxacin resistance in C. coli of 80–100%. The level of acquired resistance to fluoroquinolones was so high in some MSs that this antimicrobial agent can no longer be considered appropriate for routine empirical treatment of Campylobacter infections in humans. Increasing trends of fluoroquinolone resistance were observed from 2013 to 2017 in eight countries regarding C. jejuni and in four countries regarding C. coli. Fluoroquinolone resistance in Campylobacter is mainly mediated by point mutations in the gyrA gene and these mutations seems to be stable and even constitute enhanced fitness, also after the antibiotic pressure has been removed (Luangtongkum et al., 2009). It may therefore be anticipated that ciprofloxacin resistance levels will remain high even after reduction of antimicrobial consumption.
The second antimicrobial regarded as critically important for treatment of campylobacteriosis in humans is erythromycin, within the class of macrolides (Urdahl et al., 2017). The proportion of human C. jejuni isolates resistant to erythromycin was overall low (2.0%) but markedly higher in C. coli (12.8%) with high to very high (21.4–59.5%) proportions of C. coli being resistant to erythromycin in four of fourteen countries testing more than 10 isolates. Increasing trends of erythromycin resistance was observed in three countries for C. jejuni during the period 2013–2017 and a decreasing trend was observed in two countries for C. jejuni and one for C. coli. In contrast to fluoroquinolone resistance, macrolide resistance, normally caused by mutations in the ribosomal target and/or by active efflux via the CmeABC efflux pump, implies a fitness cost for Campylobacter and removal of the selective pressure will reduce the prevalence of resistance (Luangtongkum et al., 2009). Recently, transferrable macrolide resistance via erm(B) was detected in Campylobacter from animals and humans in China (Wang et al., 2014) and more recently in a broiler isolate and two turkey isolates in Spain (Florez‐Cuadrado et al., 2016, 2017) (see further the textbox in Section 4.2.3). Since this gene, which confers high‐level resistance to erythromycin, is often found together with other resistance genes on a multidrug‐resistant genomic island, it is likely that the carriage of this gene can be sustained by antibiotic pressure from other antimicrobials, e.g. tetracycline and aminoglycosides. One percent of C. jejuni from humans and 15% of C. coli expressed high‐level erythromycin resistance (MIC > 128 mg/L) in 2017 but genotyping would be necessary to determine if this was due to the erm(B) gene.
Combined resistance to ciprofloxacin and erythromycin was low in C. jejuni but moderate in C. coli with four countries reporting high to very high proportions of isolates being resistant to both of these critical antimicrobials. In Malta and Portugal, 62% and 54% respectively, of C. coli isolates were multidrug resistant, i.e. resistant to at least three antimicrobial classes. In Malta, however, testing of the additional antimicrobials included in the MDR analysis is mainly done on isolates found to be resistant to the primary/critical antimicrobials, thus most likely resulting in an overestimation of the MDR, as can also be observed for C. jejuni (63% MDR in Malta compared to the EU average 0.9%).
Recent source attribution studies in Europe, often combined with case‐control studies, have shown that poultry accounts for about 60–70% of campylobacter infections while the second most common source is ruminants, accounting for from 19% of campylobacter infections in Denmark to 47% in France (Rosner et al., 2017; McCrackin et al., 2016; Tang et al., 2017; Anonymous, 2018). Pigs only accounted for 2% and 0.6% of the infections in Denmark and Luxembourg, respectively. In Luxembourg and Germany, 82% and 56% respectively, of the C. coli infections were attributable to poultry while the majority of ruminant‐associated campylobacter infections in Luxembourg and Denmark were with C. jejuni. C. coli has previously mostly been associated with the pig reservoir but in some EU countries, C. coli is now as prevalent, or even more prevalent, in poultry than C. jejuni (Stella et al., 2016; Wieczorek et al., 2013; Torralbo et al., 2015). With all this in mind, it would be important to sample poultry also for C. coli, not only C. jejuni, and cattle for C. jejuni to direct the AST to the most important sources for human campylobacter infections. Sampling of pigs seems to be of less relevance due to the low proportion of campylobacter cases attributed to this source. The ongoing revision of the Commission Implementing Decision 2013/652/EU is therefore welcomed and will hopefully take these needs into consideration.
In this report, isolates from cases notified as having been acquired while travelling abroad were excluded from the analysis. This was done to better assess the relationship between AMR in Campylobacter isolates from food and food‐producing animals with AMR in human isolates of Campylobacter spp. However, as imported or traded food can constitute a large proportion of the food available in some countries, the relationship between resistance in food and food‐producing animals and in the human population is complex.
The quality of the AMR data for Campylobacter from humans has improved as the result of harmonised monitoring and reporting (ECDC, 2014, 2016). The data completeness also continues to increase when more countries report data. In 2017, 19 MSs and two EEA countries reported data, with Ireland and Poland reporting AMR data for Campylobacter to ECDC for the first time. Still, only a few countries reported data from both the public health and the food sector. This severely hampers the possibility to draw conclusions on the occurrence of AMR in a one‐health‐perspective.
4.3.2. Antimicrobial resistance in Campylobacter spp. in food and animals
Scope of the monitoring
A recent systematic review of literature concluded that, on the farm, antibiotic selection pressure could increase colonisation of animals with drug‐resistant Campylobacter spp. (McCrackin et al., 2016). The second JIACRA report stated that the use of macrolides in animals was significantly associated with macrolide resistance in C. coli in humans and that consumption of fluoroquinolones in animals was related to resistance to fluoroquinolones in Campylobacter spp. from humans. C. jejuni is by far the predominant species involved in human infections (EFSA and ECDC, 2018a), and poultry has been pointed out many times as an important source of human campylobacteriosis. Given the prevalence of C. jejuni in poultry and to a lesser extent in cattle, and its nearly total absence in pigs, the Commission Implementing Decision 2013/652/EU has targeted as a priority the monitoring of AMR in C. jejuni from poultry.
Nevertheless, C. coli infections still constitute 9.2% of human Campylobacter infections in Europe (EFSA and ECDC, 2018b). C. coli is the predominant species in pigs, and recent reports have shown that C. coli may also be quite prevalent in broilers or in turkeys in some countries (EFSA and ECDC, 2016, 2018a,b). This justifies the specific interest of addressing data collection related to AMR in C. coli from animals. The Commission Implementing Decision 2013/652/EU sets out requirements for monitoring AMR in C. coli from fattening pigs in 2017, on a voluntary basis. Seven MSs and two non‐MSs transmitted data on pigs for 2017, whereas resistance in pig meat was studied in only two MSs in 2016 and one in 2017, with limited numbers of isolates. Also on a voluntary basis, five MSs monitored resistance in C. jejuni from cattle.
Comparison of resistance between Campylobacter species and animal species
Although the numbers of reporting MSs vary importantly between the different animal categories, it is of specific interest to compare the levels of resistance to the antimicrobials tested in C. jejuni from broilers, turkeys and calves and in C. coli from broilers, turkeys and fattening pigs for 2016 and 2017.
For streptomycin, the percentages were below 10% in C. jejuni from poultry, reached levels of 15–30% for C. jejuni from cattle and C. coli from poultry, the highest percentage being detected in C. coli from pigs (64.4%).
For ciprofloxacin, percentages close to 52% were registered in C. coli from fattening pigs and in C. jejuni from cattle, but significantly higher percentages (66.8% for broilers and 73.8% for turkeys) were detected in C. jejuni from poultry and percentages in C. coli were even higher for broilers and turkeys.
The gentamicin percentages in C. coli from pigs and in C. jejuni from cattle in 2017 were significantly higher compared to data for poultry productions in 2016.
For erythromycin, resistances percentages were not significantly different for C. jejuni from cattle, broilers and turkeys and C. coli from broilers, with percentages lower than 1.3%, but they differed significantly from the percentages reported for C. coli from pigs and turkeys, both close to 15.5%
C. jejuni isolates from cattle were significantly less frequently resistant to tetracycline than isolates from other productions. Inversely the significantly higher percentage of resistance to tetracycline was reported for C. coli isolates from turkeys.
All these differences may reflect differences in usage in the different animal productions, and in the different countries, but such data are difficult to obtain (JIACRA report), and also probably different biological fitness of resistant C. jejuni and C. coli (Luangtongkum et al., 2009).
Other sources of human contamination
The discrepancies between resistance levels in C. coli in humans and those observed in pig and pig meat may result from humans being contaminated by C. coli from other sources. Indeed as previously said, C. coli seems more and more prevalent in poultry. Source attribution studies have been conducted to evaluate the role of pigs in human Campylobacter cases. Results indicated that the part played by pigs was limited to a few percent (McCrackin et al., 2016; Dearlove et al., 2016) or remained unclear (Nohra et al., 2016; Whitehouse et al., 2018 review). It is also important to mention that human cases may also be due to imported or traded food.
Temporal trends
The most noteworthy result was the tremendous increase in gentamicin resistance in C. jejuni and C. coli in Croatia. In 2016, in this MS, gentamicin resistance was lower than 6% in C. jejuni from broilers, cattle and their products and lower than 4% in C. coli from broilers, pigs and their products (EFSA report). One year later, percentages for C. jejuni from cattle and C. coli from pigs were higher than 65%, with most (50/52 gentamicin resistant) of the C. coli from pigs being also resistant to nalidixic acid/ciprofloxacin.
Resistance profiles and MDR
In the seven countries (Norway, Estonia, Sweden, Switzerland, Germany, Croatia and Spain) reporting data on resistance profiles in C. coli from pigs for 2015 and 2017, complete susceptibility varied by less than 10% between 2015 and 2017. In 2015, the most common profile of MDR in C. coli from pigs was resistance to nalidixic acid/ciprofloxacin, erythromycin and tetracycline (83.6% of MDR isolates) in all reporting countries. This MDR profile was still predominant in 2017, but accounted only for 65.3%, with other MDR profiles including resistance to gentamicin being detected in 34.7% of MDR isolates.
Mechanisms of resistance to quinolones and fluoroquinolones
In 2017, more than half of the tested Campylobacter isolates from either pigs or cattle exhibited resistance to quinolones and fluoroquinolones, and increasing trends were also observed in isolates from pigs in a number of MSs. Resistance to quinolones and fluoroquinolones is usually due to mutations in the gyrase gene; the C257T mutation on gyrA gene (Thr86Ile in the GyrA protein) being the major mechanism for ciprofloxacin resistance (see the corresponding text box).
Mechanisms of resistance to macrolides
Whereas resistance to erythromycin was low in C. jejuni from cattle in 2017, as in C. jejuni from broilers in 2016 (EFSA report on 2016), 15.6% of C. coli isolates from pigs exhibited such resistance, with great disparities between the reporting MSs. Investigations on the molecular mechanisms of macrolide resistance, and in priority in isolates resistant to high concentrations of erythromycin, should be carried out to detect chromosomal mutations or the presence of the transferable erm(B) gene (see the corresponding text box).
Resistance driven by efflux pump
Efflux pumps encoded by bacteria can protect them from natural substances produced by the host such as bile, hormones and host‐defence molecules, and they are able to confer some resistance against structurally diverse antimicrobials. In C. jejuni, mutations in the CmeABC efflux pump cause a decrease in the MICs of various molecules such as ciprofloxacin, erythromycin, cefotaxime, rifampin, gentamicin and tetracycline (Guo et al., 2010). Efflux pump inhibitors could be promising solutions to reverse resistance, although Wei et al. showed that the effects of PAβN on the MICs of azithromycin, a potent macrolide, were limited compared to those on erythromycin (Wei and Kang, 2018). The expression of the CmeABC efflux pump is negatively regulated by CmeR which binds to an inverted repeat (IR) on the cmeR‐cmeA intergenic region of C. jejuni. Two recent publications (Yao et al., 2017; Yang et al., 2017) point out the impact of substitutions, insertions or deletions in the IR: strains with such polymorphisms, sometimes in addition to the gyrA C257T mutation, are more often resistant to tetracycline, doxycycline, florfenicol, chloramphenicol and gentamicin and their ciprofloxacin MICs are higher (4–128 mg/L according to (Yao et al., 2017) than the ones of strains without modifications of the IR (4–8 mg/L according to (Yao et al., 2017). The explanation lies in the lower binding of CmeR to CmeABC, resulting in an overexpression of the efflux pump. Besides these modifications in the expression of the CmeABC pump, Yao et al. (Yao et al., 2016) identified in China, isolates with a ‘super’ efflux pump variant of CmeABC (named RE‐CmeABC). The ciprofloxacin MICs of the isolates bearing the RE‐CmeABC and the C257T mutation in gyrA were excessively high (256–512 mg/L), and the isolates were also resistant to tetracycline and florfenicol, whereas erythromycin and chloramphenicol MICs varied, respectively, from 4 to 256 mg/L (erythromycin ECOFF: 4 mg/L for C. jejuni) and from 16 mg/L to 128 mg/L (chloramphenicol ECOFF: 16 mg/L for C. jejuni). The RE‐CmeABC coding region could be transferred between Campylobacter isolates by natural transformation and the MICs of florfenicol, chloramphenicol, ciprofloxacin, erythromycin and tetracycline were increased in the transformants. The authors could evidence modifications of the protein sequence in the drug‐binding pocket of CmeB which may possibly contribute to the enhanced efflux function. The prevalence of the RE‐CmeABC increased in the swine and broilers isolates from 2012 to 2015, mainly in C. jejuni, as a result of both clonal expansion and horizontal transmission, probably in link with the various antimicrobial selection pressures in these animal productions. Acquisition of such ‘super’ efflux pumps is quite worrying as it gives isolates the capacity to gain simultaneously resistance or decreased susceptibility to diverse classes of antimicrobials, including molecules with a therapeutic interest in humans. It is worth mentioning that Campylobacter sequences found in the gene databases indicate that such ‘super’ efflux pumps were already present in Europe more than 10 years ago. The current harmonised monitoring programme does not allow for detecting the precise ciprofloxacin MIC of isolates not inhibited by 16 mg/L and florfenicol is not included in the list of molecules to test; thus, it is not possible to phenotypically detect Campylobacter isolates producing these ‘super’ efflux pumps. Alterations of the monitoring programme to include a larger range of ciprofloxacin concentrations and the addition of florfenicol or chloramphenicol to the harmonised set of antimicrobial substances could be a valuable option in order to identify isolates in which modifications of the sequence of the CmeABC pump should be further investigated via molecular methods.
Levels and mechanisms of resistance to gentamicin
As in 2015, the occurrence of resistance to streptomycin was very high in C. coli from pigs, and significantly more frequently detected than in C. jejuni or C. coli from poultry in 2016 or from cattle in 2017.
Importantly from a public health perspective, gentamicin is used in some instances to treat Campylobacter spp. systemic infections in humans, justifying the epidemiological monitoring of resistance to this substance in food‐producing animals and food. Resistance to gentamicin was very low in C. jejuni from poultry (0.1% and 0.2% in isolates from broilers and fattening turkeys, respectively) in 2016, but reached up to 8.2% in C. jejuni isolates from cattle in 2017. For C. coli, resistance levels to gentamicin in poultry (0.6% in isolates from broilers and 2% in isolates from fattening turkeys) were lower than those of C. coli from pigs in 2017 (7.7%). As previously mentioned, the increase was mainly due to the very high levels of gentamicin resistance reported in Croatia. The molecular mechanisms of resistance would need to be investigated further.
Campylobacter spp. can resist to aminoglycosides by production of three types of aminoglycosides modifying enzymes (AME) including aminoglycoside acetyltransferases (AAC), aminoglycoside nucleotidyltransferases (ANT) and aminoglycoside phosphotransferases (APH). Thus, aadA and aadE can confer resistance to streptomycin, aacA4 resistance to gentamicin and tobramycin and aph(3’) resistance to kanamycin and neomycin. In 2012, increase in gentamicin resistance up to more than 20% of C. coli from broilers and swine was described in China (Qin et al., 2012). Several AME genes (the aadE‐sat4‐aphA‐3 cluster, aacA‐aphD, aac and aadE) could be identified borne on a chromosomal genomic island, which was particularly present in isolates belonging to the same C. coli clone of sequence type (ST) 1625. In vitro, the genomic island could be transferred to a C. jejuni recipient strain by natural transformation. Only a few years later, in 2017, gentamicin resistance had reached the alarming percentages of 15.6% in C. jejuni and 79.9% in C. coli of poultry and swine origins (Yao et al., 2017). The AME aph(2’’)‐If gene was at that time more prevalent than the previously reported aacA/aphD gene. The aph(2’’)‐If gene was located on a chromosomal segment of 10.5 kbp containing seven other AME and the cat (mediating chloramphenicol resistance) genes. This chromosomal segment could be transferred in vitro by natural transformation and the transformants were resistant to gentamicin, amikacin, kanamycin and neomycin. Molecular typing of isolates originating from different regions suggested that the high level of gentamicin resistance resulted from both the diffusion of a particular C. coli clone and the horizontal transfer of the aph(2’’)‐If gene‐containing chromosomal segment.
In the USA, resistance to gentamicin increased rapidly from rare in 2000–2006 to 18.1% of retail isolates and 12.2% of human isolates in 2011 (Zhao et al., 2015). According to PCR and WGS of human and poultry strains, nine variants of gentamicin resistance genes aph(2’’)‐Ib, Ic, Ig, If, If1, If3, Ih, aac(6’)‐Ie/aph(2’’)‐Ia and aac(6’)‐Ie/aph(2’’)‐If were evidenced; some of them (aph(2’’)‐Ib, Ic, If1, If3, Ih and aac(6’)‐Ie/aph(2’’)‐If2) being described for the first time in Campylobacter. The aph(2’’)‐Ig gene was the only AME gene found in both C. coli isolates from humans and chickens at retail; those isolates sharing the same PFGE and resistance profiles. The aph(2’’)‐Ig was carried by a self‐transmissible plasmid bearing other resistance genes (aad9, aadE, sat4, aph‐3 and tet(O)). Thus, expansion of the clone and dissemination of the plasmid may ensure a rapid diffusion of the gentamicin resistance. In the WGS study of Whitehouse et al. (2018), out of 589 isolates, only 12 were resistant to gentamicin, and they carried aph(2”)If, aph(2”)Ig or aph(2”)Ic genes which were absent in all gentamicin‐susceptible isolates. Fabre et al. (2018) studied 12 gentamicin–resistant strains isolated from humans in France or from Portuguese pork. WGS enabled the authors to identify for the first time, in the pork isolate, the acetyltransferase apmA gene, first described in Staphylococcus aureus of bovine and porcine samples, encoding an enzyme associated with apramycin and gentamicin hydrolysis. Various aph(2”) genes were found in the other gentamicin‐resistant strains, sometimes parts of clusters located at the chromosome site or at a plasmid site.
Those different epidemiological and WGS studies have shown that gentamicin resistance genes, often borne on chromosomal genomic islands or on plasmids may spread very rapidly, either by horizontal transfer of AME genes or clonal diffusion, and may be co‐selected by use of other families of antimicrobials. A better knowledge of the mechanisms of resistance of the emerging gentamicin‐resistant Campylobacter strains isolated from animals in Europe is urgently needed.
Campylobacter spp. strain harbouring cfr gene
Additionally to the different MDRGI previously described, plasmids carrying genes conferring resistance to different families of antimicrobials (Yao et al., 2017; Crespo et al., 2016), efflux pumps extruding structurally diverse molecules, a new resistance determinant, cfr(C), was recently reported in Campylobacter (Tang et al., 2017). The cfr gene has already been described in various Gram‐positive and Gram‐negative bacteria and shown to confer resistance to phenicols, lincosamides, streptogramin A, pleuromutilins and oxazolidinones. In the studied C. coli isolates obtained from feedlot cattle in the USA, the florfenicol resistance was transferable by conjugation to a susceptible C. jejuni recipient strain, and molecular analyses revealed the presence of a conjugative plasmid of approximately 48 kbp, containing several virulence genes, the tet(O) and the aphA‐3 resistance genes, and the new cfr(C) gene, which was confirmed to increase the MICs of chloramphenicol, florfenicol, clindamycin, tiamulin and linezolid of the recipient strain (Campylobacter are intrinsically resistant to streptogramins). The cfr(C) gene was detected in 10% of 344 C. coli from cattle samples collected in five US states but not in 1886 C. jejuni. All cfr(C)‐positive isolates were resistant to chloramphenicol and shared the same PFGE and MLST profiles, suggesting clonal dispersion. Thus, although phenicols, lincosamides, streptogramins, pleuromutilins and oxazolidinones are not used for the treatment of human Campylobacter infections, the cfr(C) gene should be monitored as its presence in Campylobacter widens the possibilities of selection or co‐selection of antimicrobial‐resistant Campylobacter isolates in animals as phenicols, lincosamides and pleuromutilins can be used in animals.
Possible further assessment
Methods for determination of the susceptibility of Campylobacter isolates are harmonised in the EU MSs, contrary to the methods used for Campylobacter isolation. As mentioned in the previous EFSA report (report on 2016), discrepancies in the isolation methods, and particularly the enrichment, isolation media and the number of identified isolates probably impact the prevalence of Campylobacter and thus the calculated prevalence of resistance and maybe the diversity of isolates including their antimicrobial susceptibility. Harmonisation of detection methods is needed.
For a better phenotypic detection of isolates possibly carrying erm(B) gene, it could be suggested to increase the upper range of erythromycin concentrations. Moreover, a better detection of isolates with modifications of the sequence of the CmeABC pump and its regulating region would be facilitated by increasing the range of concentrations of ciprofloxacin and including a phenicole molecule. Finally, as carbapenems‐non‐susceptible or ‐resistant isolates have now been detected in human infections, the inclusion of this class of antimicrobials seems to be considered.
New mechanisms of AMR in Campylobacter have emerged or have been evidenced in the last few years. Some of these, such as erm(B), ‘super’ efflux pumps, gentamicin resistance genes borne on chromosomal genomic islands or on self‐transmissible plasmids or cfr(C), seem to spread very rapidly, either by clonal diffusion or horizontal gene transfer. Molecular basis of resistance in all resistant‐isolates or in those resistant to critical antimicrobials, especially when they exhibit MDR or resistance to high concentrations, should be investigated. Such molecular studies should also be rapidly undertaken in case of sudden increase of occurrence of resistance in a MS (like for gentamicin in Croatia).
5. Antimicrobial resistance in indicator Escherichia coli
Rationale for monitoring antimicrobial resistance in indicator E. coli in food‐producing animals and food
Commensal E. coli is typically chosen as the representative indicator of AMR in Gram‐negative bacteria, as it is commonly present in animal faeces, may be relevant to human medicine and can often acquire conjugative plasmids, which can carry resistance determinants and are transferable between enteric bacteria. Commensal E. coli that are resistant and present in the intestines of food‐producing animals constitute a reservoir of resistance genes that can spread horizontally to zoonotic and other bacteria present in the food chain or in the intestinal tract of humans. The monitoring of AMR in indicator E. coli, isolated from either randomly selected healthy animals or carcases and their meat, and chosen to be representative of the general population, provides valuable data on resistance occurring in that population. Determining the occurrence of resistance to antimicrobials in a representative sample of indicator E. coli provides useful data for investigating the relationship between the occurrence of resistance and the selective pressure exerted by the use of antimicrobials on the intestinal population of bacteria in food‐producing animals. Indicator E. coli is also helpful as a representative of the Enterobacteriaceae to monitor the emergence of and changes in the proportion of bacteria producing ESBLs. Since 2014, monitoring of AMR in indicator E. coli from food‐producing animals and their food products has been mandatory under the EU legislation.
5.1. Antimicrobial resistance in indicator E. coli from animals20
5.1.1. Antimicrobial resistance in indicator E. coli from fattening pigs
Representative monitoring
For 2017, all EU MSs and three non‐MSs reported susceptibility data on indicator (commensal) E. coli from caecal content of fattening pigs. In addition, four MSs and one non‐MS provided similar data for 2016 on a voluntary basis (Appendix D). Data were obtained according to the requirements laid down in Commission Implementing Decision 2013/652/EU. The ‘microbiological resistance’ to the harmonised set of antimicrobials (as opposed to clinical resistance) was interpreted using ECOFFs laid down in the Decision.21 Italy also provided on a voluntary basis data on E. coli from pig meat in 2017 (Appendix D).
Resistance levels in indicator E. coli from fattening pigs
The occurrence of resistance in indicator E. coli isolated from caecal content of fattening pigs varied markedly between reporting countries (Table 1). Generally, resistance was higher in southern and eastern Europe than in northern Europe. Notably, Norway reported the overall lowest occurrence of resistance with rare or very low occurrence for 10 of the 14 substances tested and low occurrence for 4 substances (sulfamethoxazole, ampicillin, tetracycline, trimethoprim).
The overall tetracycline resistance in the MSs was very high at 52.1% and was the most common trait of resistance in 14 of the 31 reporting countries. In Greece, Italy, Bulgaria, Cyprus, Spain and Portugal, tetracycline resistance was extremely high but in contrast, low in Norway and Sweden. The overall resistance to sulfamethoxazole, ampicillin and trimethoprim in MSs was also high at 42.4%, 38.5% and 32.2%, respectively. In Norway, Sweden, and Switzerland, sulfamethoxazole resistance was the single most common trait, whereas in Slovenia, Belgium and Romania, the most common trait of resistance was to ampicillin.
Chloramphenicol resistance was detected in all the 31 reporting countries and was overall at the moderate level of 18.0% in the MS group. The occurrence varied greatly between countries, from low to very low in 13 countries to moderate or high in 9 countries. Gentamicin resistance was overall low in MSs, 2.7%, but varied between 9.4% in Greece and Italy to 0% in Slovakia, Slovenia, Estonia, Luxembourg, Iceland, Sweden and Finland.
Quinolone resistance in MSs was overall low for nalidixic acid, at 5.7%, and moderate for ciprofloxacin, at 10.6%. There were, however, large variations between reporting countries. Thus, in the Nordic countries Norway, Sweden, Finland and Denmark resistance to quinolones was not detected or detected in occasional isolates. In contrast, ciprofloxacin resistance was high in Italy, Cyprus, Portugal, Slovenia, Romania and Spain. In Spain, 44.7% of the isolates were resistant to ciprofloxacin. At the MS level, the ratio of nalidixic acid resistance to ciprofloxacin resistance was 0.54 but there were large variations between countries. For example, in Greece, Italy and Latvia the ratio was 0.29–0.36, whereas in most countries the ratio was higher and in Iceland, Estonia, Denmark, France and Luxembourg the ratio was 1.
Resistance to the third‐generation cephalosporins was overall low in MSs at 1.4% and 1.3% for cefotaxime and ceftazidime, respectively. In 18 countries, resistance to cefotaxime and ceftazidime was not detected (rare) or detected in occasional isolates only (very low) and in 12 countries resistance ranged between 1.2% and 3.5% (low). Belgium deviated from this general situation and although classified as low, occurrence was 7.4% and 7.4% for cefotaxime and ceftazidime, respectively. Generally, resistance to cefotaxime and ceftazidime were of similar magnitude.
Azithromycin resistance was overall low, 1.5% at the MSs level, and 16 countries did not report resistance and 7 countries reported only one or two resistant isolates. However, Cyprus and Portugal deviated from the general situation and reported azithromycin resistance at 12.3% and 16.2%, respectively.
Colistin resistance was overall very low, 0.3% at the MSs level, and 22 countries did not report any isolates resistant to this antimicrobial. However, in 4 countries colistin resistance was low, ranging from about 1% in Lithuania and Romania to around 2% in Hungary and Portugal.
Meropenem resistance was not detected in any of the reporting countries and tigecycline resistance in only one isolate from Belgium.
Spatial distribution of resistance in indicator E. coli from fattening pigs
The occurrence of resistance to several antimicrobials in indicator E. coli from caecal content of fattening pigs showed substantial difference between European countries in 2017 (Table 31). Generally, resistance was higher in southern and eastern Europe than in northern Europe. Detailed information on the spatial distribution of resistance to the third‐generation cephalosporin cefotaxime and to the (fluoro)quinolone ciprofloxacin are provided in Figure 66.
Table 31.
Occurrence of resistance (%) to selected antimicrobials in indicator Escherichia coli from fattening pigs, using harmonised ECOFFs, 28 EU MSs and 3 non‐MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 180 | 1.7 | 3.3 | 20 | 1.7 | 1.7 | 0 | 0 | 2.8 | 3.9 | 0.6 | 0 | 24.4 | 12.8 | 40.6 |
| Belgium | 176 | 1.1 | 26.1 | 51.1 | 7.4 | 7.4 | 0 | 0.6 | 4 | 9.1 | 1.7 | 0.6 | 47.7 | 44.9 | 46 |
| Bulgaria | 101 | 1 | 36.6 | 62.4 | 0 | 0 | 0 | 0 | 10.9 | 15.8 | 0 | 0 | 59.4 | 36.6 | 80.2 |
| Croatia | 86 | 2.3 | 11.6 | 30.2 | 0 | 0 | 0 | 0 | 11.6 | 15.1 | 0 | 0 | 40.7 | 23.3 | 48.8 |
| Cyprus | 57 | 8.8 | 54.4 | 63.2 | 0 | 0 | 0 | 0 | 8.8 | 21.1 | 12.3 | 0 | 82.5 | 70.2 | 87.7 |
| Czech Republic | 180 | 0.6 | 3.3 | 37.8 | 2.8 | 2.8 | 0 | 0 | 3.3 | 5 | 1.7 | 0 | 33.9 | 26.7 | 45.6 |
| Denmark | 172 | 2.3 | 5.8 | 35.5 | 0 | 0 | 0 | 0 | 0.6 | 0.6 | 1.2 | 0 | 34.9 | 30.2 | 37.2 |
| Estonia | 67 | 0 | 4.5 | 17.9 | 0 | 0 | 0 | 0 | 1.5 | 1.5 | 0 | 0 | 14.9 | 16.4 | 19.4 |
| Finland | 175 | 0 | 0.6 | 8.6 | 0 | 0 | 0 | 0 | 0.6 | 0 | 0 | 0 | 12 | 11.4 | 18.3 |
| France | 214 | 1.4 | 13.1 | 30.8 | 0 | 0 | 0 | 0 | 4.7 | 4.7 | 0.5 | 0 | 40.7 | 34.1 | 56.1 |
| Germany | 227 | 1.3 | 9.7 | 29.1 | 2.2 | 1.3 | 0 | 0 | 4.4 | 6.6 | 0.9 | 0.4 | 30 | 23.8 | 36.6 |
| Greece | 170 | 9.4 | 40.6 | 58.8 | 0.6 | 0.6 | 0 | 0 | 2.9 | 10 | 1.8 | 0.6 | 82.4 | 50 | 75.9 |
| Hungary | 170 | 1.8 | 17.1 | 44.7 | 1.2 | 1.2 | 0 | 0 | 5.3 | 7.6 | 2.4 | 1.8 | 31.8 | 25.9 | 66.5 |
| Ireland | 203 | 3.4 | 13.8 | 27.6 | 1.5 | 1.5 | 0 | 0 | 6.4 | 7.4 | 0 | 0 | 43.8 | 35.5 | 62.1 |
| Italy | 170 | 9.4 | 47.6 | 70.6 | 1.2 | 0.6 | 0 | 0 | 7.6 | 22.4 | 1.2 | 0.6 | 67.1 | 60 | 78.2 |
| Latvia | 149 | 2 | 4 | 26.8 | 0.7 | 0.7 | 0 | 0 | 3.4 | 9.4 | 0 | 0 | 22.1 | 13.4 | 31.5 |
| Lithuania | 99 | 4 | 8.1 | 28.3 | 0 | 0 | 0 | 0 | 4 | 10.1 | 0 | 1 | 26.3 | 21.2 | 45.5 |
| Luxembourg | 44 | 0 | 13.6 | 20.5 | 2.3 | 2.3 | 0 | 0 | 2.3 | 2.3 | 0 | 0 | 31.8 | 22.7 | 22.7 |
| Malta | 74 | 1.4 | 6.8 | 17.6 | 0 | 1.4 | 0 | 0 | 5.4 | 9.5 | 0 | 0 | 50 | 33.8 | 62.2 |
| Netherlands | 300 | 0.7 | 12.3 | 22 | 0.3 | 0.3 | 0 | 0 | 1.3 | 2 | 0.7 | 0 | 34.3 | 30.7 | 42.7 |
| Poland | 213 | 3.3 | 13.6 | 46.9 | 1.9 | 1.9 | 0 | 0 | 8 | 16 | 0 | 0 | 57.7 | 28.2 | 58.2 |
| Portugal | 142 | 2.8 | 44.4 | 59.9 | 3.5 | 3.5 | 0 | 0 | 12 | 21.1 | 16.2 | 2.1 | 68.3 | 56.3 | 89.4 |
| Romania | 170 | 5.9 | 38.8 | 61.2 | 2.4 | 2.4 | 0 | 0 | 18.2 | 34.7 | 4.1 | 1.2 | 55.9 | 35.3 | 60.6 |
| Slovakia | 85 | 0 | 12.9 | 49.4 | 0 | 0 | 0 | 0 | 2.4 | 3.5 | 0 | 0 | 35.3 | 28.2 | 50.6 |
| Slovenia | 85 | 0 | 16.5 | 34.1 | 3.5 | 2.4 | 0 | 0 | 14.1 | 20 | 0 | 0 | 28.2 | 14.1 | 25.9 |
| Spain | 170 | 4.7 | 35.3 | 77.1 | 2.9 | 2.9 | 0 | 0 | 19.4 | 44.7 | 1.8 | 0.6 | 63.5 | 60.6 | 88.8 |
| Sweden | 140 | 0 | 5.7 | 18.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20.7 | 15 | 9.3 |
| United Kingdom | 186 | 3.8 | 20.4 | 30.6 | 0 | 0 | 0 | 0 | 2.2 | 2.7 | 0 | 0 | 47.3 | 36.6 | 59.1 |
| Total (28 MSs) | 4205 | 2.7 | 18 | 38.5 | 1.4 | 1.3 | 0 | 0 | 5.7 | 10.6 | 1.5 | 0.3 | 42.4 | 32.2 | 52.1 |
| Iceland | 68 | 0 | 1.5 | 14.7 | 0 | 0 | 0 | 0 | 4.4 | 4.4 | 0 | 0 | 14.7 | 13.2 | 19.1 |
| Norway | 304 | 0.3 | 0.3 | 6.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8.9 | 5.6 | 6.6 |
| Switzerland | 197 | 3 | 5.1 | 14.2 | 0 | 0 | 0 | 0 | 2 | 2.5 | 0.5 | 0 | 36 | 15.2 | 20.8 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
Figure 66.
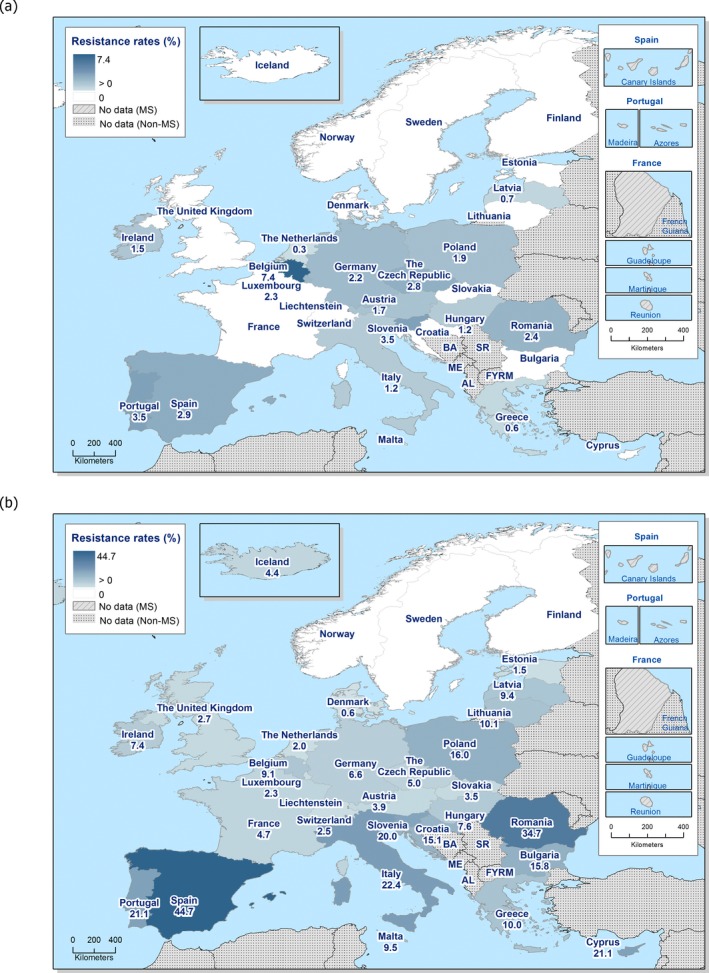
Spatial distribution of resistance to cefotaxime (a) and ciprofloxacin (b) in indicator Escherichia coli isolates from fattening pigs, using harmonised ECOFFs, 28 EU MSs and 3 non‐MSs, 2017
Cefotaxime resistance was overall low in the EU MSs (1.4%) and expectedly, variation between countries was limited with occurrence ranging from rare (0%) in 14 countries to at most 7.4% in Belgium. There was no obvious spatial pattern of cefotaxime resistance, as rare to low resistance was reported from all parts of Europe (Figure 66a).
Ciprofloxacin resistance in the EU MSs was moderate at 10.6% but marked disparities between countries were recorded. Thus, quinolone resistance was either not detected or only detected in occasional isolates in the Nordic countries, Norway, Sweden, Finland and Denmark whereas the observed levels were high in Italy, Cyprus, Portugal, Slovenia, Romania and Spain. Overall, ciprofloxacin resistance was lowest in the Nordic countries and highest in certain countries of southern and eastern Europe (Figure 66).
Combined resistance to cefotaxime and ciprofloxacin in indicator E. coli from fattening pigs
Resistance to CIA of highest priority in human medicine, and in particular the occurrence of isolates exhibiting combined resistance to such antimicrobials, is of specific public health relevance. The harmonised monitoring provides valuable insights on combined resistance to the third‐generation cephalosporin cefotaxime and the (fluoro)quinolone ciprofloxacin in indicator E. coli from fattening pigs. In 2017, 0.5% (24/4,774) of the isolates were resistant to both cefotaxime and ciprofloxacin when MICs were evaluated by ECOFFs and 7 of those isolates (0.2%; 7/4,774) were also resistant, when evaluated by CBPs (Table 3).
Isolates exhibiting combined resistance to both cefotaxime and ciprofloxacin, after evaluation by ECOFFs, were detected in 12 MSs but not in the remaining 16 MSs and 3 non‐MSs (Table 3; Figure 2). In the MSs reporting combined resistance, the occurrence was very low in 6 MSs and low in 6 MSs varying between 0.6% in the Czech Republic and Italy to at most 2.8% in Belgium (Figure 2). Of the 25 isolates with combined resistance, 17 were resistant also to sulfamethoxazole, 17 to trimethoprim, 16 to tetracycline and 9 to chloramphenicol. Five isolates were resistant to all these antimicrobials in addition to cefotaxime and ciprofloxacin and nine isolates were resistant to sulfamethoxazole, trimethoprim and tetracycline in addition to cefotaxime and ciprofloxacin.
In six MSs, some isolates exhibited combined resistance also after evaluation of MICs by CBPs (Table 3). Such resistance was mainly detected in single isolates within a country, but Germany reports two isolates with clinical resistance to both cefotaxime and ciprofloxacin.
Notably, none of the isolates with combined resistance to cefotaxime and ciprofloxacin were resistant to the other CIA of highest priority tested, i.e. meropenem and colistin. It is to be noted that azithromycin was not included in the evaluation of resistance patterns of isolates with combined resistance to cefotaxime and ciprofloxacin (Figure 67).
Figure 67.
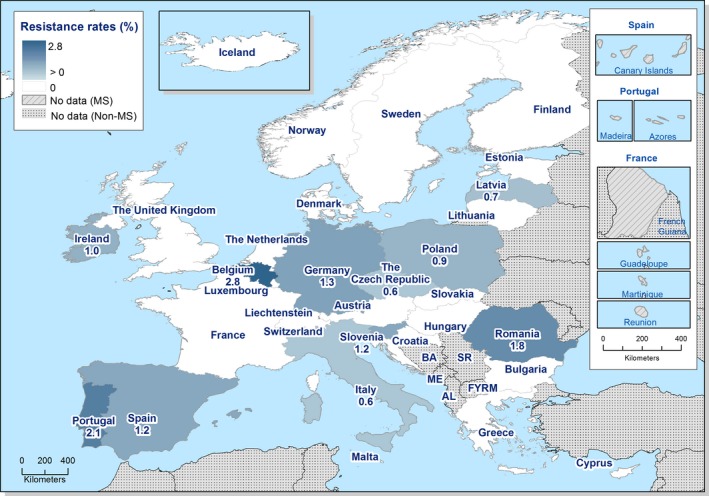
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in indicator Escherichia coli from fattening pigs, using harmonised ECOFFs, 28 EU MSs and 3 non‐MSs, 2017
Table 32.
Combined resistance to fluoroquinolones and third‐generation cephalosporins in indicator Escherichia coli from fattening pigs, 11 EU MSs, 2017
| Country | N | MDR patterns of isolates exhibiting combined resistance to CIP and CTX (number of isolates) | Microbiological Combined Resistance to CIP and CTXa | Clinical Combined Resistance to CIP and CTXb | ||
|---|---|---|---|---|---|---|
| n | %r | n | %r | |||
| Belgium | 176 | AMP‐CTX‐CAZ‐CHL‐CIP‐SMX‐TMP (1) | 5 | 2.8 | 1 | 0.6 |
| AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TET‐TMP (2) | ||||||
| AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TMP (1) | ||||||
| AMP‐CTX‐CAZ‐CIP‐TET (1) | ||||||
| Czech Republic | 180 | AMP‐CTX‐CAZ‐CIP‐NAL‐TMP (1) | 1 | 0.6 | 1 | 0.6 |
| Germany | 227 | AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TMP (2) | 3 | 1.3 | 2 | 0.9 |
| AMP‐CTX‐CAZ‐CIP‐TET (1) | ||||||
| Ireland | 203 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET (1) | 2 | 1 | ||
| AMP‐CTX‐CAZ‐CIP‐GEN‐NAL‐TMP (1) | ||||||
| Italy | 170 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET (1) | 1 | 0.6 | ||
| Latvia | 149 | AMP‐CTX‐CAZ‐CIP‐GEN‐SMX‐TET‐TMP (1) | 1 | 0.7 | ||
| Poland | 213 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP (1) | 2 | 0.9 | ||
| AMP‐CTX‐CAZ‐CIP‐SMX‐TMP (1) | ||||||
| Portugal | 142 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP (1) | 3 | 2.1 | 1 | 0.7 |
| AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TET‐TMP (1) | ||||||
| AMP‐CTX‐CAZ‐CIP‐TET (1) | ||||||
| Romania | 170 | AMP‐CTX‐CAZ‐CHL‐CIP‐GEN‐NAL‐SMX‐TET‐TMP (1) | 3 | 1.8 | 1 | 0.6 |
| AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP (1) | ||||||
| AMP‐CTX‐CAZ‐CHL‐CIP‐SMX‐TET (1) | ||||||
| Slovenia | 85 | AMP‐CTX‐CAZ‐CIP‐NAL (1) | 1 | 1.2 | 1 | 1.2 |
| Spain | 170 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP (1) | 2 | 1.2 | ||
| AMP‐CTX‐CAZ‐CIP‐NAL‐TET‐TMP (1) | ||||||
| Total (11 MSs) | 1,885 | – | 24 | 1.3 | 7 | 0.4 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
applying ECOFFs.
applying CBPs.
Temporal trends in resistance in indicator E. coli from fattening pigs
Due to the lack of longitudinal data, evaluation of temporal trends in resistance cannot yet be made for all the countries participating in the harmonised monitoring. For 11 countries (10 MSs and 1 non‐MS) that have provided data on indicator E. coli from caecal content of fattening pigs for 4 years or more in the period 2009–2017, trends in resistance to ampicillin, ciprofloxacin, cefotaxime, nalidixic acid and tetracycline are presented in Figure 3. Additionally, for these countries, the statistical significance (p ≤ 0.05) of the trends was tested by logistic regression (see Section 2 Materials and methods for further details).
The statistical analysis shows that resistance to ampicillin has decreased in three countries (Hungary, the Netherlands and Switzerland) and increased in five countries (Belgium, Denmark, France, Poland and Spain). Resistance to cefotaxime has decreased in three countries (France, Hungary and the Netherlands) and increased in one country (Belgium). Ciprofloxacin resistance has decreased in two countries (Belgium and the Netherlands) and increased in two countries (Poland and Spain). Nalidixic acid resistance has decreased in three countries (Belgium, Hungary and the Netherlands) and increased in none. Tetracycline resistance has decreased in five countries (Austria, Belgium, France, the Netherlands and Switzerland) and increased in three countries (Estonia, Hungary and Poland).
Overall, in the 11 countries, there are 20 decreasing and 8 increasing trends over the period 2009–2017. In Estonia, France, the Netherlands and Switzerland, only decreasing trends are registered over the period. Notably in the Netherlands, resistance is decreasing for all five antimicrobials considered and in France, resistance to two of the substances is decreasing. In contrast, in two countries, there are only increasing trends, in Spain for ampicillin and ciprofloxacin and in Denmark for ampicillin. In Belgium, France, Hungary and Poland, both decreasing and increasing trends are detected and in Finland resistance is stable at low levels.
Longitudinal trends in resistance can be assessed on the medium term as above for only a subset of the reporting countries. The comparison of resistance in indicator E. coli from caecal content of fattening pigs in 2015 and 2017 can, however, be made at the EU‐level because in both years data were reported by most MSs (n = 27) and were obtained according to the harmonised methodology laid down in Commission Implementing Decision 2013/652/EU. Thus, at the EU level for all antimicrobials, the occurrence of resistance in isolates from pigs in 2017 is numerically similar, or lower by up to 3 percent points, compared to occurrence in 2015 (Figure 68).
Figure 68.
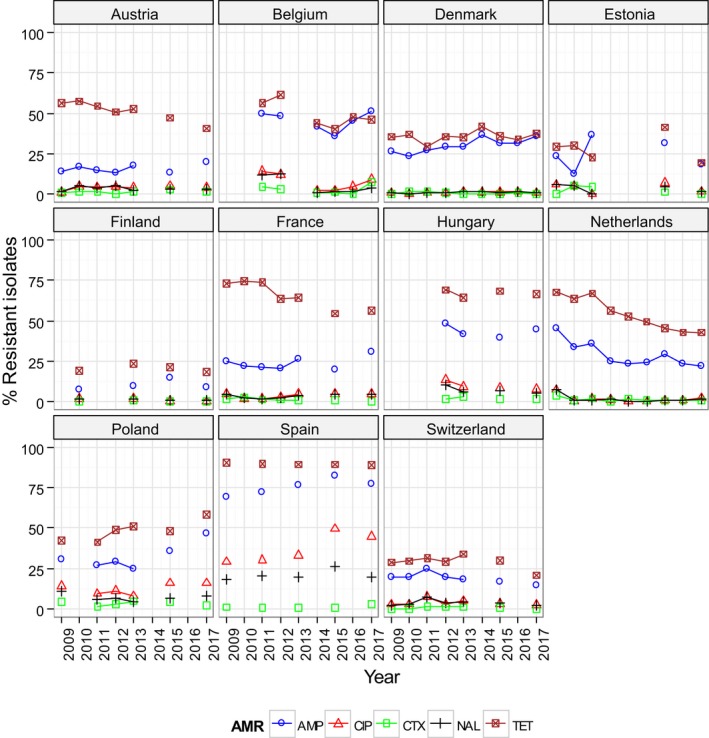
Trends in ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid (NAL) and tetracyclines (TET) resistance in indicator commensal Escherichia coli from pigs in reporting countries, 2009–2017
Resistance to tigecycline
Tigecycline is an antimicrobial of the glycylcycline group belonging to the third‐generation tetracyclines and is listed as critically important of highest priority in human medicine by WHO. In human medicine, tigecycline is clinically useful against staphylococci, β‐haemolytic streptococci, enterococci and several Enterobacteriaceae, including E. coli and Klebsiella spp., and is active against some strains resistant to older generation tetracyclines. Tigecycline is not authorised for use in animals in the EU.
In Enterobacteriaceae, resistance to tigecycline is mainly due to chromosomal mutations that upregulate AcrAB efflux pumps (Pournaras et al., 2016) but mutations causing alterations in the ribosomal S10 protein (Beabout et al., 2015) or the Mla system (He et al., 2016) have also been proposed. Recently, it was shown that mutated tetA genes, coding for efflux pumps, can confer resistance to tigecycline and that the mechanism was transferable between bacteria (Du et al., 2018). The ECOFF for tigecycline in E. coli, separating the wild type isolates from those exhibiting a reduced susceptibility, was set at ≤ 1 mg/L, when the Commission Implementing Decision 2013/622/EU was published in 2013. Since then, this ECOFF has been used to interpret the data in the EU Summary Reports, including this report. However, the ECOFF for tigecycline is under revision by EUCAST and currently (August 2018) no ECOFF is defined for E. coli. Nevertheless, the CBP to interpret clinical resistance is set at > 2 mg/L by EUCAST (http://www.eucast.org).
In 2017, resistance to tigecycline, after evaluation of MIC by the tentative ECOFF (≤ 1 mg/L), was detected in one of the 4,774 isolates of indicator E. coli from caecal content of fattening pigs reported by 28 MSs and 3 non‐MSs (Table 1). The isolate was reported by Belgium and exhibited a MIC value of 2 mg/L and accordingly were not considered clinically resistant. Tigecyline resistance was not detected in any of the 2,383 isolates of indicator E. coli from caecal content of calves under 1 year of age reported by 10 MSs and 2 non‐MSs (Table 4). Likewise, tigecycline resistance was not detected in E. coli from meat of pigs or meat of bovines. In the 2015 monitoring, tigecycline resistance was detected in 12 of 4,720 tested isolates of indicator E. coli from caecal content of pigs. The resistant isolates had MICs 2–4 mg/L and were reported by three countries (Cyprus, Malta and Norway). Also, in 2015, none of 2,187 isolates of indicator E. coli from calves under 1 year of age reported by 10 MSs and 2 non‐MSs was resistant to tigecycline. The distribution of tigecycline MICs for E. coli isolated from pigs and calves in 2017 are presented in Figure 69.
Figure 69.
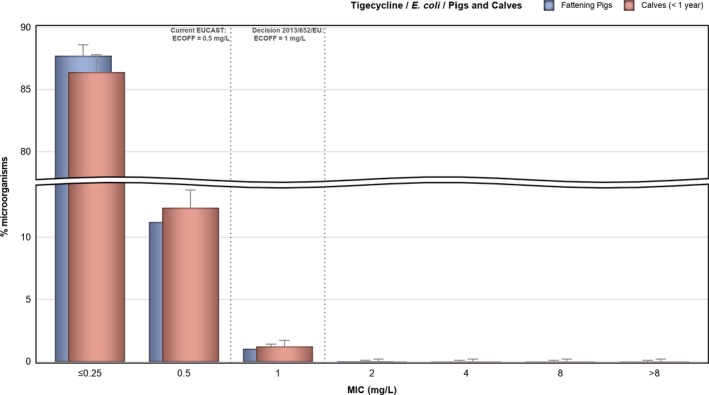
Distribution of MICs of tigecycline in indicator Escherichia coli from fattening pigs (4,774 isolates from 28 EU MSs and 3 non‐MSs) and calves under 1 year of age (2,383 isolates from 10 EU MSs and 2 non‐MSs), 2017
Figure 69.

Distribution of MICs of tigecycline in indicator Escherichia coli from fattening pigs (4,774 isolates from 28 EU MSs and 3 non‐MSs) and calves under 1 year of age (2,383 isolates from 10 EU MSs and 2 non‐MSs), 2017
Multidrug resistance in indicator E. coli from fattening pigs
For 2017, out of the 4,774 isolates of indicator E. coli from caecal content of fattening pigs reported 2,899 isolates (60.7%) exhibited resistance to one or more of the antimicrobial classes tested (Figure 5). Azithromycin was not considered in this calculation and cefotaxime and ceftazidime were evaluated together, as were nalidixic acid and ciprofloxacin. Considering the design of the panel used in the harmonised monitoring, the highest possible resistance count for an isolate is 11. Overall, 25.8% (1,231/4,774) of the isolates showed resistance to 1 or 2 classes, and 34.9% (1,668/4,774) exhibited MDR to 3 or more antimicrobial classes. Multidrug‐resistant isolates with resistance count 3 accounted for 10.9% (519/4,774), isolates with count 4 for 12.1% (577/4,774) and isolates with count 5 for 8.4% (399/4,774) of all isolates. Higher resistance counts were less common and 3.1% (150/4,774) had count 6 and 0.5% (22/4,774) count 7. One isolate had 8 antimicrobial classes in their phenotype, which was the highest resistance count detected in 2017.
There were, however, marked variations in the occurrence of MDR between countries (Figure 5). In some countries in southern Europe (Cyprus, Spain, Portugal, Italy, Greece, Bulgaria), 60% or more of the isolates tested were resistant to three or more antimicrobial classes. Also, in these countries up to 17% of the isolates were resistant to 6 or more classes. In contrast, in some countries in northern and central Europe (Norway, Finland, Iceland, Estonia, Sweden, Latvia, Austria, Switzerland), resistance to 3 or more antimicrobial classes was detected in less than 17% of the isolates and only occasional isolates were resistant to 6 or more classes. Notably, in Norway, only 3.3% of the isolates were resistant to 3 or more antimicrobial classes and no isolate was resistant to more than 4 classes (Figure 70).
Figure 70.
Frequency distribution of Escherichia coli isolates completely susceptible or resistant to 1–11 antimicrobials, from fattening pigs, 31 EU/EEA MSs, 2017
- N: total number of isolates tested for susceptibility against the whole harmonised set of antimicrobials for Escherichia coli; sus: susceptible to all antimicrobial classes of the harmonised set for E. coli; res1–res9: resistance to 1 up to 11 antimicrobial classes of the harmonised set for E. coli.
Multidrug resistance patterns in indicator E. coli isolates from fattening pigs
Multidrug resistance in indicator E. coli from pigs was detected by all reporting countries (28 MSs and 3 non‐MSs) for 2017. Overall, 34.9% (1,668/4,774) of all isolates were MDR but occurrence varied markedly between countries, ranging from 3.3% in Norway up to 82.5% in Cyprus (Figure 5). Occurrence of MDR was generally higher in southern than in northern Europe. Azithromycin was not considered in this calculation and cefotaxime and ceftazidime were evaluated together, as were nalidixic acid and ciprofloxacin.
Of the MDR isolates, 31.1% (519/1,668) were resistant to 3 antimicrobial classes only but most MDR isolates (68.9%, 1,149/1,668) were resistant to 4 or more substances. Among the MDR isolates, 98 different resistance patterns were detected. Tetracycline, sulfamethoxazole, ampicillin and trimethoprim were included in the patterns of almost half of the MDR isolates (48.5%, 809/1,668), often in combination with other substances. Chloramphenicol was also often included in the resistance patterns and 23.3% (388/1,668) of the MDR isolates were resistant to this antimicrobial in combination with resistance to tetracycline, sulfamethoxazole, ampicillin, trimethoprim and often also to other substances.
About one fourth of the MDR isolates (24.8%, 414/1,668) were resistant to quinolones (nalidixic acid and/or ciprofloxacin) and almost half of these isolates (199) were resistant also to tetracycline, sulfamethoxazole, ampicillin and trimethoprim, often in combination with other antimicrobials. A smaller proportion of the MDR isolates were resistant to colistin (0.5%, 9/1,668) or third‐generation cephalosporins (3.3%, 54/1,668). The overall most common single resistance pattern was tetracycline, sulfamethoxazole, ampicillin and trimethoprim and was detected in 19.6% (327/1,668) of the MDR isolates and found in all reporting countries except Slovenia. The second most common pattern was resistance to tetracycline, sulfamethoxazole, ampicillin, trimethoprim and chloramphenicol detected in 14.1% (236/1,668) of the MDR isolates. Other resistance patterns were less common with no single pattern accounting for more than 8% of the MDR isolates.
Spatial distribution of complete susceptibility in indicator E. coli from pigs
Another way of addressing the phenomenon of multiple resistance is to consider the proportion of isolates exhibiting a complete susceptibility to the antimicrobial classes tested in the harmonised panel. In 2017, all reporting countries (28 MSs and 3 non‐MSs) detected indicator E. coli isolates completely susceptible in fattening pigs and overall, 39.2% (1,875/4,774) of the isolates were susceptible to all antimicrobial classes tested. There were marked disparities between countries in the levels of complete susceptibility, ranging from 5.3% in Spain and Cyprus to 84.5% in Norway (Figure 71). In countries in northern Europe (Norway, Finland, Sweden, Iceland, Estonia), 60% or more of the isolates were susceptible to all antimicrobial classes tested, whereas in some countries in southern Europe (Cyprus, Spain, Portugal, Greece, Italy, Bulgaria), only about 10% or less of the isolates exhibited complete susceptibility.
Figure 71.
Spatial distribution of complete susceptibility to the panel of antimicrobials tested in indicator Escherichia coli isolates from fattening pigs, using harmonised ECOFFs, 28 EU MSs, 2017
Changes in complete susceptibility among E. coli from fattening pigs between 2015 and 2017
The comparison of levels of complete susceptibility in E. coli isolates from fattening pigs between 2015 and 2017 showed statistically significant changes in proportions of resistant isolates (Figure 72). Positive differences in complete susceptibility between 2017 and 2015 were notably detected in Bulgaria, Estonia, Germany, whereas a significant negative difference was recorded in Belgium, Greece and Poland. At the overall level (27 MSs reporting for both 2015 and 2017), no significant difference was registered between 2015 and 2017.
Figure 72.
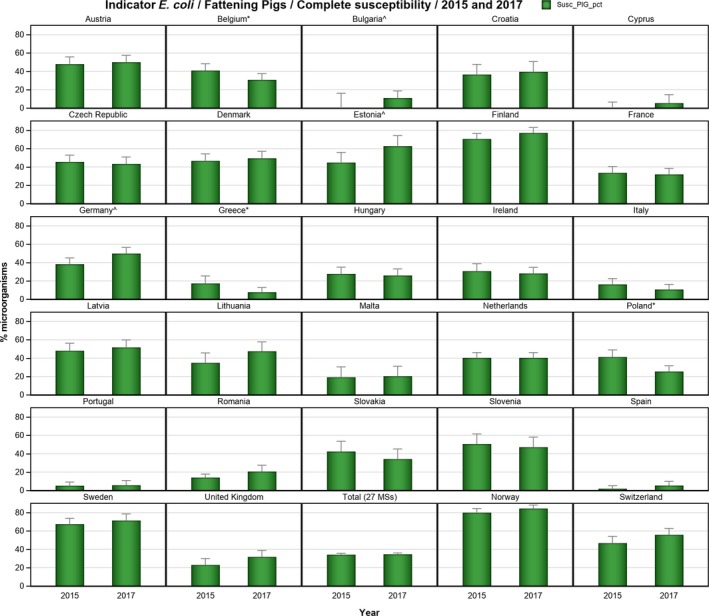
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator Escherichia coli isolates from fattening pigs, using harmonised ECOFFs, 28 EU MSs, 2015 and 2017
- ^indicates statistically significant positive difference in the occurrence of complete susceptibility between 2017 and 2015.
- *indicates statistically significant negative difference in the occurrence of complete susceptibility between 2017 and 2015.
5.1.2. Antimicrobial resistance in indicator Escherichia coli from calves under 1 year of age
Representative monitoring
For 2017, 10 EU MSs and 2 non‐MSs reported susceptibility data on indicator (commensal) E. coli from caecal content of calves under 1 year of age (Table 4). In addition, on a voluntary basis 4 MSs also provided data on indicator E. coli from caecal content of calves under 1 year of age for 2016 (Appendix E). Data were obtained according to the requirements laid down in Commission Implementing Decision 2013/652/EU. And ‘microbiological resistance’ to the harmonised set of antimicrobials (as opposed to clinical resistance) was interpreted using ECOFFs laid down in the Decision.17 Since not all countries reported data for calves under 1 year of age in 2017, information on the spatial distribution of resistance is not complete. Notably, only Croatia in the eastern part of Europe reported data. On a voluntary basis, 1 MS (Italy) also provided data on E. coli from meat of bovines (Appendix E).
Resistance levels in indicator E. coli from calves
The occurrence of resistance in indicator E. coli isolated from caecal content of calves under 1 year of age varied markedly between the reporting countries (Table 4). Norway reported notably the overall lowest occurrence of resistance with rare or very low occurrence for 11 of the 14 substances tested and low occurrence for 3 substances (sulfamethoxazole, ampicillin, tetracycline).
Tetracycline resistance in the reporting MSs was overall high, at 43.8%, and the most common trait in 10 of the 12 reporting countries. In Croatia, the levels of tetracycline resistance equalled those of sulfamethoxazole resistance. In Spain, Belgium and France, tetracycline resistance was very high and extremely high in Italy. In contrast, low levels of tetracycline resistance were recorded in Norway and Denmark.
Sulfamethoxazole, ampicillin and trimethoprim resistance was overall high in MSs at 34.4%, 29.0% and 24.7% for sulfamethoxazole, ampicillin and trimethoprim, respectively. In Switzerland, sulfamethoxazole resistance was the single most common trait, and in Croatia it was equally high as tetracycline resistance. Resistance to these three antimicrobials varied greatly between countries from very high for all three substance in Italy to rare (trimetoprim) and low (sulfamethoxazole, ampicillin) in Norway.
Chloramphenicol resistance was detected in all the 12 reporting countries and was overall moderate, at 14.5%, in the MS group. However, resistance varied greatly between countries, from moderate or high in 5 reporting countries to very low in Norway.
Quinolone resistance in MSs was overall low for nalidixic acid, 6.7%, and moderate for ciprofloxacin, 10.6%. There were however large variations between reporting countries and in Norway and Denmark resistance to quinolones was not detected (rare), whereas resistance to both antimicrobials was high in Italy. At the MSs level, the ratio of nalidixic acid resistance to ciprofloxacin resistance was 0.64 but there were large variations between countries. For example, in Belgium and Italy the ratio was 0.52 and 0.49, respectively, but in the other countries reporting quinolone resistance the ratio ranged from 0.72 to 1.0.
Gentamicin resistance was overall low in the MS group, at 3.9%, but varied greatly from very low in Austria, Denmark and Norway to moderate (13.5%) in Italy.
Resistance to the third‐generation cephalosporins was overall low in the reporting MSs at 1.6% and 1.4% for cefotaxime and ceftazidime, respectively. However, occurrence varied between countries and in Croatia, the Netherlands and Switzerland resistance to cefotaxime and ceftazidime was not detected (rare) whereas Italy reported an occurrence of 5.9% (low) for both cefotaxime and ceftazidime resistance. In most countries resistance to cefotaxime and ceftazidime were of similar magnitude.
Azithromycin resistance was overall low, 2% at the MSs level, and 8 countries did not report resistance (rare) or single isolates only (very low). However, 4 countries reported low occurrence ranging from 1.2% up to at most 7.0% in France.
Colistin resistance was overall very low, 0.8% at the MSs level, and 8 countries did not report any isolates resistant to this antimicrobial. In the 4 countries reporting colistin resistance, occurrence ranged from 1.0% (very low) to at most 2.9% (low) in Italy.
Meropenem and tigecycline resistance was not detected (rare) in any of the reporting countries.
Spatial distribution of resistance in indicator E. coli from calves
For several antimicrobials, the occurrence of resistance in indicator E. coli from caecal content of calves under 1 year of age differed substantially between countries in 2017 (Table 33). The spatial distribution of resistance to the third‐generation cephalosporin cefotaxime and to the (fluoro)quinolone ciprofloxacin are shown in Figure 73. Since not all countries reported data for calves under 1 year of age in 2017 information on the spatial distribution of resistance is not complete. Notably only Croatia in the eastern part of Europe reported data.
Table 33.
Occurrence of resistance (%) to selected antimicrobials in indicator Escherichia coli from calves under 1 year of age, using harmonised ECOFFs, 10 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 181 | 0.6 | 6.1 | 8.8 | 1.1 | 0 | 0 | 0 | 2.8 | 3.9 | 0 | 0 | 17.7 | 7.2 | 24.3 |
| Belgium | 185 | 4.9 | 27.6 | 56.8 | 2.7 | 2.2 | 0 | 0 | 10.3 | 20 | 4.3 | 1.1 | 56.2 | 53.5 | 64.9 |
| Croatia | 85 | 1.2 | 5.9 | 14.1 | 0 | 0 | 0 | 0 | 7.1 | 8.2 | 0 | 0 | 31.8 | 8.2 | 31.8 |
| Denmark | 181 | 0.6 | 5.5 | 6.1 | 0.6 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 6.6 | 2.2 | 7.7 |
| France | 200 | 4 | 19 | 44 | 0 | 0 | 0 | 0 | 8 | 9.5 | 7 | 1 | 53 | 33 | 65.5 |
| Germany | 242 | 3.3 | 7.4 | 36 | 2.1 | 2.1 | 0 | 0 | 7 | 9.1 | 5 | 2.5 | 31 | 26.4 | 37.2 |
| Italy | 170 | 13.5 | 31.8 | 63.5 | 5.3 | 5.3 | 0 | 0 | 22.9 | 47.6 | 1.8 | 2.9 | 68.8 | 63.5 | 77.6 |
| Netherlands | 301 | 3.3 | 16.6 | 22.9 | 0 | 0 | 0 | 0 | 3 | 3.7 | 0 | 0 | 29.2 | 21.3 | 47.2 |
| Portugal | 181 | 1.7 | 4.4 | 9.9 | 3.3 | 3.3 | 0 | 0 | 3.3 | 3.3 | 0 | 0 | 13.3 | 7.7 | 23.2 |
| Spain | 167 | 5.4 | 16.8 | 21 | 1.2 | 1.2 | 0 | 0 | 5.4 | 6.6 | 0.6 | 0 | 39.5 | 17.4 | 52.7 |
| Total (10 MSs) | 1893 | 3.9 | 14.4 | 29 | 1.6 | 1.4 | 0 | 0 | 6.7 | 10.6 | 2 | 0.8 | 34.4 | 24.7 | 43.8 |
| Norway | 296 | 0.3 | 0.7 | 1.7 | 0.7 | 0.7 | 0 | 0 | 0 | 0 | 0 | 0 | 4.4 | 0 | 3.4 |
| Switzerland | 194 | 4.6 | 9.8 | 38.7 | 0 | 0 | 0 | 0 | 3.6 | 3.6 | 0 | 0 | 46.9 | 19.1 | 41.2 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
Figure 73.
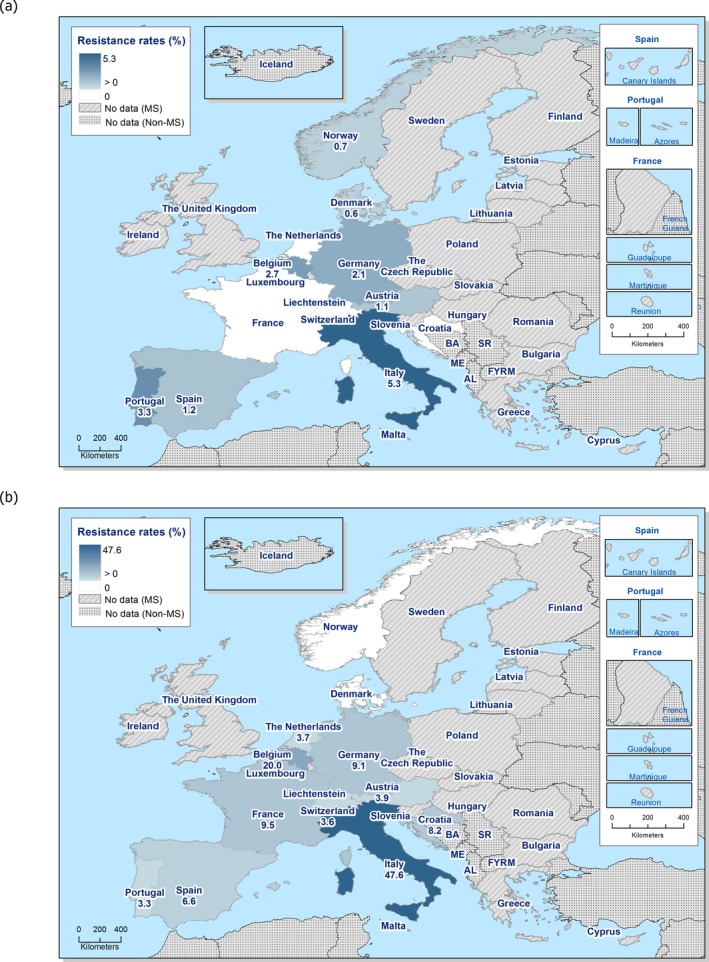
Spatial distribution of resistance to cefotaxime (a) and ciprofloxacin (b) in indicator Escherichia coli isolates from calves under 1 year of age, 10 EU MSs and 2 non‐MSs, 2017
Cefotaxime resistance was overall low in MSs (1.6%) and the variation between countries was small with occurrence ranging from 0% (rare) in Croatia, the Netherlands and Switzerland to at most 5.9% (low) in Italy. There was no obvious spatial pattern for cefotaxime resistance in isolates from calves under 1 year of age among the countries reporting data in 2017 (Figure 73).
Ciprofloxacin resistance was overall moderate in MSs (10.6%) but there were large variations between countries. Thus, in the Nordic countries Norway and Denmark resistance to quinolones was not detected, but occurrence was high in Belgium (20%) and Italy (47.6%). There was no obvious spatial pattern for ciprofloxacin resistance among the countries reporting data in 2017; however, it is notable that the such resistance was not detected in the Nordic countries (Figure 73).
Combined resistance to cefotaxime and ciprofloxacin in indicator E. coli from calves
The monitoring of resistance to CIA of highest priority in human medicine, and in particular that of the occurrence of isolates exhibiting combined resistance to such antimicrobials, is of specific public health relevance. In 2017, the combined resistance to the third‐generation cephalosporin, cefotaxime, and the (fluoro)quinolone, ciprofloxacin, in indicator E. coli from calves under 1 year of age was assessed at 0.7% (16/2,383 of the isolates), when resistance was interpreted using ECOFFs, and at 0.3% (6/2,383 of the isolates), when resistance was evaluated using CBPs.
Isolates exhibiting combined resistance to cefotaxime and ciprofloxacin, applying ECOFFs, were reported by six MSs and not detected in four MSs and two non‐MSs (Table 34 and Figure 74). In the MSs detecting this combined resistance, the occurrence was very low in three MSs and low in three MSs, varying from 0.4% in Germany to at most 4.7% in Italy (Figure 9). Out of the 16 isolates exhibiting combined resistance, 15 were also resistant to sulfamethoxazole, tetracycline or trimethoprim and 13 were resistant to all those antimicrobials.
Table 34.
Combined resistance to fluoroquinolones and third‐generation cephalosporins in indicator Escherichia coli isolates from calves under 1 year of age, 6 EU MSs, 2017
| Country | N | MDR patterns of isolates exhibiting combined resistance to CIP and CTX (number of isolates) | Microbiological combined resistance to CIP and CTX(a) | Clinical combined resistance to CIP and CTX(b) | ||
|---|---|---|---|---|---|---|
| n | %r | n | %r | |||
| Austria | 181 | AMP‐CTX‐CHL‐CIP‐NAL‐SMX‐TET‐TMP(1) | 1 | 0.6 | ||
| Belgium | 185 | AMP‐AZM‐CTX‐CAZ‐CHL‐CIP‐COL‐GEN‐NAL‐SMX‐TET‐TMP(1) | 3 | 1.6 | 1 | 0.5 |
| AMP‐CTX‐CAZ‐CHL‐CIP‐TET‐TMP(1) | ||||||
| AMP‐CTX‐CHL‐CIP‐NAL‐SMX‐TET‐TMP(1) | ||||||
| Germany | 242 | AMP‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP(1) | 1 | 0.4 | 1 | 0.4 |
| Italy | 170 | AMP‐CTX‐CAZ‐CHL‐CIP‐GEN‐NAL‐SMX‐TET‐TMP(4) | 8 | 4.7 | 3 | 1.8 |
| AMP–CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP(1) | ||||||
| AMP‐CTX‐CAZ‐CHL‐CIP‐SMX‐TET‐TMP(3) | ||||||
| AMP‐CTX‐CAZ‐CIP‐SMX‐TET(1) | ||||||
| Portugal | 181 | AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TET‐TMP(1) | 1 | 0.6 | ||
| Spain | 167 | AMP‐AZM‐CTX‐CAZ‐CHL‐CIP‐NAL‐SMX‐TET‐TMP(1) | 2 | 1.2 | 1 | 0.6 |
| AMP‐CTX‐CAZ‐CIP‐NAL‐SMX‐TET(1) | ||||||
| Total (6 MSs) | 1,126 | – | 16 | 1.4 | 6 | 0.5 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; NAL: nalidixic acid; CIP: ciprofloxacin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
Figure 74.
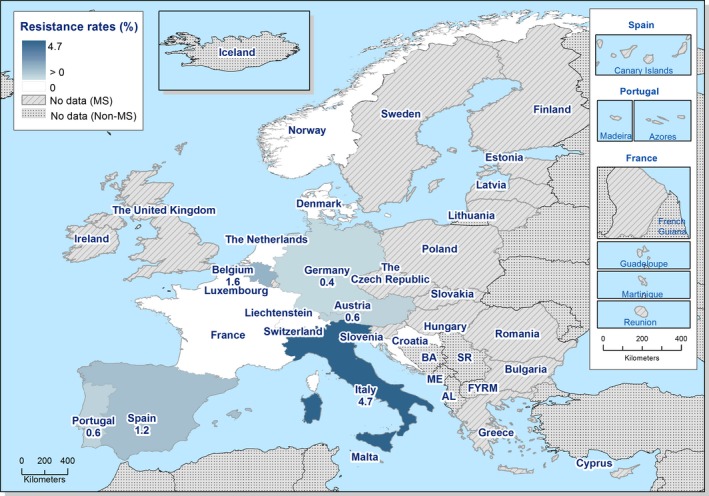
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in indicator Escherichia coli isolates from calves under 1 year of age, 10 EU MSs and 2 non‐MSs, 2017
In four MSs, some isolates exhibited ‘clinical’ combined resistance, applying CBPs. Such resistance was mainly detected in single isolates within a country, but Italy reported three isolates with clinical resistance to both cefotaxime and ciprofloxacin. It is also of note that one isolate, from Belgium, with combined clinical resistance to cefotaxime and ciprofloxacin also was resistant to colistin, another CIA of highest priority (Table 6). It is to be noted that azithromycin was not included in the evaluation of resistance patterns of isolates with combined resistance to cefotaxime and ciprofloxacin.
Temporal trends in resistance in indicator E. coli from bovine animals
Due to the lack of longitudinal data, evaluation of temporal trends in resistance cannot yet be made for all the countries participating in the harmonised monitoring. For eight countries (seven MSs and one non‐MS) that have provided data on indicator E. coli from caecal content of calves under 1 year of age for 4 years or more in the period 2009–2017, occurrences of resistance to ampicillin, ciprofloxacin, cefotaxime, nalidixic acid and tetracycline are presented in Figure 10. Additionally, for these countries, statistical significance (p ≤ 0.05) of trends was tested by logistic regression (see Section 2 Materials and methods for details).
The statistical analysis shows that resistance to ampicillin has decreased in two countries (Germany and the Netherlands) and increased in two countries (Austria and Switzerland). Resistance to cefotaxime has decreased in three countries (Belgium, Germany and the Netherlands) and increased in one country (Poland). Ciprofloxacin resistance has decreased in three countries (Belgium, Germany and the Netherlands) and increased in two countries (Austria and Switzerland). Nalidixic acid resistance has decreased in three countries (Belgium, Germany and the Netherlands) and increased in one country (Switzerland). Tetracycline resistance has decreased in two countries (Germany and the Netherlands) and increased in three countries (Austria, Belgium and Switzerland).
Overall, in the 8 countries, there are 13 decreasing and 7 increasing trends over the period 2009–2017. In two countries (Germany and the Netherlands), there are only decreasing trends and notably in both countries, levels of resistance are decreasing for all five antimicrobials analysed. In contrast in three countries, there are only increasing trends, in Switzerland for three antimicrobials, in Austria for two antimicrobials and in Poland for one antimicrobial. For two countries (Denmark and Spain), there are no statistical trend in resistance and levels of resistance are stable at low levels in Denmark and high levels in Spain.
Longitudinal trends in resistance can be assessed on the medium term as above for only a subset of the reporting countries. The comparison of resistance in indicator E. coli from caecal content of calves under 1 year of age in 2015 and 2017 can, however, be made at the EU level for nine MSs reporting data obtained according to the harmonised methodology laid down in Commission Implementing Decision 2013/652/EU in both years. In these nine MSs, for all antimicrobials the occurrence of resistance in isolates from calves in 2017 is numerically about the same as in 2015.
Figure 75.
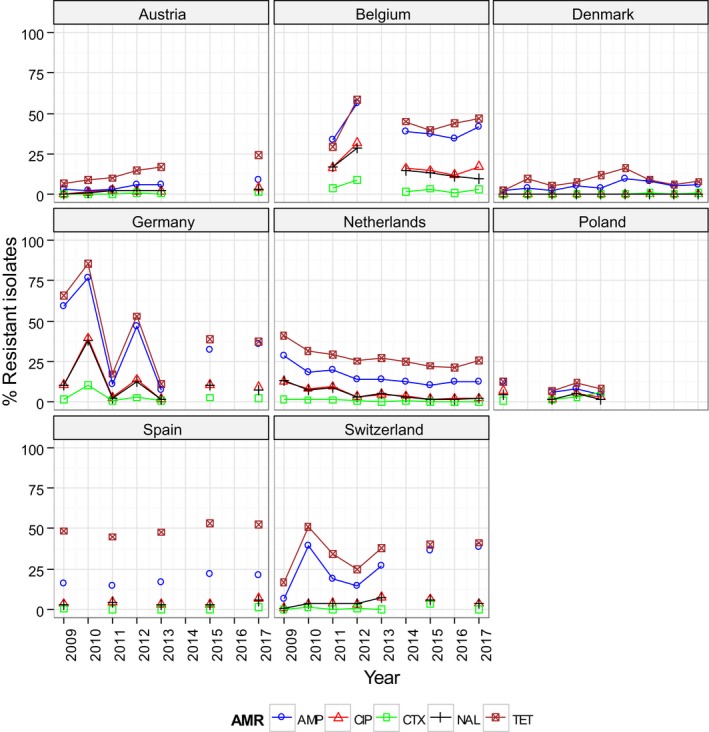
Trends in ampicillin (AMP), cefotaxime (CTX), ciprofloxacin (CIP), nalidixic acid (NAL) and tetracyclines (TET) resistance in indicator commensal Escherichia coli from bovine animals in reporting countries, 2009–2017
Multidrug resistance in indicator E. coli from calves
For 2017, susceptibility data for 2,383 isolates of indicator E. coli from caecal content of calves under 1 year of age were reported and of these 1,033 isolates (43.3%) were resistant to one or more of the antimicrobial classes tested (Figure 76). Azithromycin was not considered in this calculation and cefotaxime and ceftazidime were evaluated together, as were nalidixic acid and ciprofloxacin. Thus, considering the design of the panel used in the harmonised monitoring, the highest possible resistance count for an isolate is 11.
Figure 76.
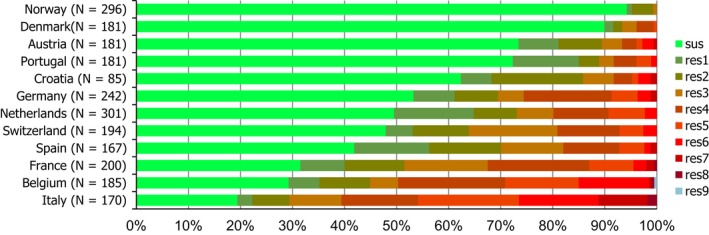
Frequency distribution of Escherichia coli isolates completely susceptible and resistant to 1–11 antimicrobials, from calves under 1 year of age, 10 EU MSs and 2 non‐MSs, 2017
- N: total number of isolates tested for susceptibility against the whole harmonised set of antimicrobials for Escherichia coli; sus: susceptible to all antimicrobial classes of the harmonised set for Escherichia coli; res1–res9: resistance to one up to 11 antimicrobial classes of the harmonised set for Escherichia coli.
Overall, 15.7% (374/2,383) of the isolates had resistance counts of 1 or 2, but 27.7% (659/2,383) were MDR with 3 or more antimicrobial classes in the resistance phenotype. MDR isolates with resistance count 3 accounted for 7.1% (169/2,383), isolates with count 4 for 10.0% (238/2,383) and isolates with count 5 for 5.7% (135/2,383) of all isolates. Higher resistance counts were less common and 3.5% (84/2,383) had count 6 and 1.1% (27/2,383) count 7. Five isolates had 8 antimicrobial classes in their phenotype and 1 isolate 9 classes, which was the highest resistance count detected in 2017.
There were however large differences in occurrence of multiple resistance between countries (Figure 76). In Italy, Belgium and France about 50% or more of the isolates tested were resistant to 3 or more antimicrobial classes. Also, in these countries up to 26.5% of the isolates were resistant to 6 or more classes. In contrast, in Norway and Denmark resistance to 3 or more antimicrobial classes was detected in less than 10% of the isolates and no isolate was resistant to 6 or more classes. Notably, in Norway, only 0.7% of the isolates were resistant to 3 or more antimicrobial classes and no isolate was resistant to 4 or more classes.
Multidrug resistance patterns in indicator E. coli isolates from calves
Multidrug resistance in indicator E. coli from calves under 1 year of age, i.e. resistance to 3 or more antimicrobial classes, was detected by all reporting countries (10 MSs and 2 non‐MSs) providing data for 2017. Azithromycin was not considered in this calculation and cefotaxime and ceftazidime were evaluated together, as were nalidixic acid and ciprofloxacin.
Overall, 27.7% (659/2,383) of all isolates were MDR but occurrence varied markedly between countries, ranging from 0.7% in Norway to 70.6% in Italy (Figure 11). A low occurrence of MDR was detected not only in the reporting Nordic countries (Norway and Denmark) but also in countries in southern and central Europe (Austria, Portugal and Croatia). Thus, there was no obvious spatial pattern for occurrence of MDR in 2017.
Of the MDR isolates, 25.6% (169/659) were resistant to 3 antimicrobial classes only but most MDR isolates (74.4%, 490/659) were resistant to 4 or more substances. Among the MDR isolates 59 different resistance patterns were detected. The antimicrobials most often included in the resistance patterns of MDR isolates were tetracycline and sulfamethoxazole and 90.6% (597/659) of the MDR isolates were resistant to both these antimicrobials in combination with other substances. Ampicillin and trimethoprim were also common in the resistance pattern of MDR isolates and overall, more than half of the MDR isolates (54.5%, 359/659) were co‐resistant to tetracycline, sulfamethoxazole, ampicillin and trimethoprim, often in combination with other substances. Chloramphenicol was also often included in the resistance patterns and 22.8% (150/659) of the MDR isolates were resistant to this antimicrobial in combination with resistance to tetracycline, sulfamethoxazole, ampicillin, trimethoprim and often also to other antimicrobials.
About one third of the MDR isolates (28.9%, 191/659) were resistant to quinolones (nalidixic acid and/or ciprofloxacin) and most of these isolates (n = 139) were resistant also to tetracycline, sulfamethoxazole, ampicillin and trimethoprim. Isolates with this resistance pattern comprised 21.1% of the MDR isolates. A smaller proportion of the MDR isolates were resistant to colistin (2.3%, 15/659) or third‐generation cephalosporins (3.6%, 24/659).
The overall most common single resistance pattern was co‐resistance to tetracycline, sulfamethoxazole, ampicillin and trimethoprim and was detected in 20.9% (138/659) of the MDR isolates and detected in all reporting countries except Denmark and Norway. Other resistance patterns were less common with no single pattern accounting for more than 10% of the MDR isolates.
Spatial distribution of complete susceptibility in indicator E. coli from calves
Another way of addressing the phenomenon of multiple resistance is to consider the proportion of isolates exhibiting a complete susceptibility to the antimicrobial classes tested in the harmonised panel. In 2017, all countries providing data (10 MSs and 2 non‐MSs) detected completely susceptible isolates in indicator E. coli from calves under 1 year of age and overall, 56.7% (1,350/2,383) of the isolates were susceptible to all antimicrobial classes tested.
There were, however, marked differences between countries in the occurrence of complete susceptibility, which ranged between 19.4% in Italy and more than 90% in Norway and Denmark (Figure 77). The highest occurrence of complete susceptibility was detected in the reporting Nordic countries (Norway and Denmark) but occurrence of complete susceptibility was high also some countries in central and southern Europe (Austria, Portugal and Croatia). Thus, there was no obvious spatial pattern for complete susceptibility among the countries reporting data in 2017.
Figure 77.
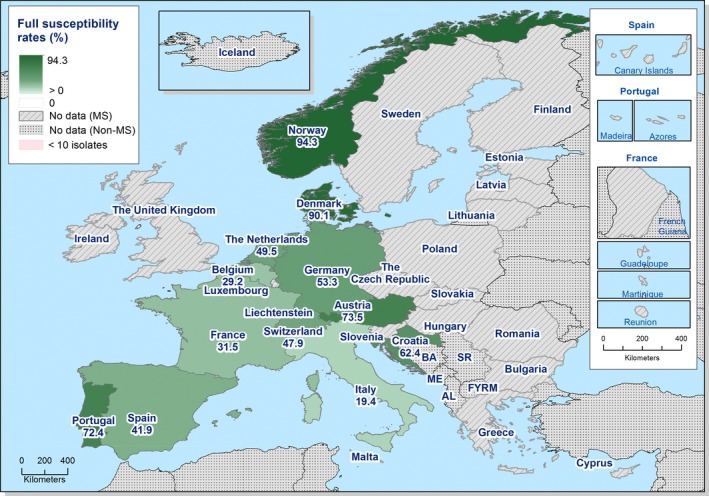
Spatial distribution of complete susceptibility to the panel of antimicrobials tested among indicator Escherichia coli isolates from calves under 1 year of age, using harmonised ECOFFs, 10 EU MSs, 2017
Changes in complete susceptibility among E. coli from calves under 1 year of age between 2015 and 2017
The comparison of the levels of complete susceptibility in indicator E. coli isolates from calves under 1 year of age between 2015 and 2017 showed that complete susceptibility has remained globally stable (Figure 78). The notable exception is the Netherlands, where the level of complete susceptibility observed in 2017 is statistically significantly lower than that reported for 2015.
Figure 78.
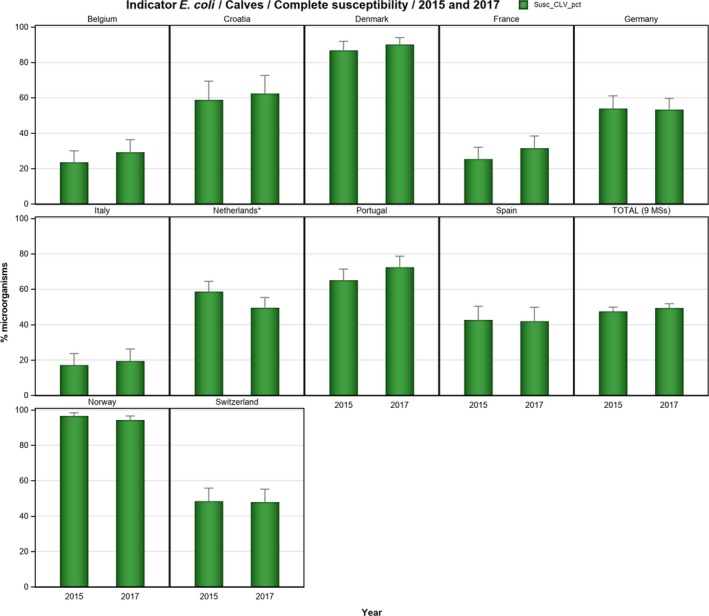
Changes in the occurrence of complete susceptibility to the panel of antimicrobials tested in indicator Escherichia coli isolates from calves under 1 year of age, using harmonised ECOFFs, 10 EU MSs, 2015 and 2017
- Stars indicate statistically significant negative difference in occurrence of complete susceptibility between 2017 and 2015.
5.2. Resistance to colistin in indicator E. coli from fattening pigs and calves under 1 year of age
Colistin (polymyxin E) is an antimicrobial of the polymyxin group that has been used extensively in farm animals all over the world, including Europe. In human medicine, use of colistin has historically been very limited. However, in recent years, there has been an increased usage in human medicine due to the urgent need for last resort antimicrobials to treat infections caused by multidrug‐resistant Gram‐negative bacteria. Consequently, of the antimicrobials listed by WHO as critically important of highest priority for human medicine, polymyxins are now among the five substances considered to be of highest priority.
Colistin resistance in bacteria from animals has therefore attracted increasing interest in recent years. Not the least when in 2015 transferable colistin resistance, conferred by the mcr‐1 gene, was reported for the first time in E. coli from animals and humans in China (Liu et al., 2015). Such genes, and variants, were subsequently found in bacteria from animals and humans around the world including Europe (Kempf et al., 2016; Schwarz and Johnson, 2016). The presence of transmissible genetic elements conferring colistin resistance likely increases the potential for spread of such resistance which fuelled the interest in colistin resistance.
In the light of this, the European Commission requested EMA to update the previous advice on the impact of, and need for, colistin use for human and animal health (EMA, 2013). In the updated advice, EMA concludes that findings of the mcr‐1 gene in similar plasmids in the same bacteria species from food‐producing animals, food, humans, and environment indicate a possible transmission between these compartments (EMA, 2016). EMA further concludes that transmission from animals to humans is likely to have taken place in Europe but at low frequency. As a risk mitigation strategy, EMA recommends that the use of colistin in animals should be reduced in the EU and that the monitoring of colistin resistance, and specifically transmissible resistance genes, in bacteria from food‐producing animals should be enhanced. Since 2014, colistin is included in the mandatory monitoring in EU performed under Decision 2013/652/EU.
In 2017, occurrence of phenotypic colistin resistance in E. coli from pigs was very low (0.3%) at the EU level and detected by 8 MSs but not by the 3 reporting non‐MSs (Table 1). Likewise, colistin resistance was very low at the EU level (0.8%) in E. coli from calves under 1 year of age and detected by 4 of the 10 reporting MSs but not by the 2 reporting non‐MSs (Table 4). Occurrence of colistin resistance at the EU level in 2017 was similar as in 2015, both in pigs (0.4%) and in calves under 1 year of age (0.9%). In one MSs (Italy), reporting data for E. coli from both meat and caecal content of pigs in 2017, colistin resistance was higher in isolates from meat than in isolates from caecal content (5.3% vs 0.6%) whereas occurrence was similar in both matrices for bovines (2.1% vs 2.9%).
Considering all reporting countries in 2017, 14 of 4,774 E. coli isolates from caecal content of pigs and 15 of 2,383 isolates from calves under 1 year of age exhibited colistin MIC values above the ECOFF (> 2 mg/L) for microbiological resistance to colistin. Out of the 14 isolates from pigs, 11 had MIC 4 mg/L, 2 had MIC 8 mg/L and 1 had MIC > 16 mg/L (Figure 79). The 15 colistin‐resistant isolates from calves under 1 year of age all had MIC 4 mg/L.
Figure 79.
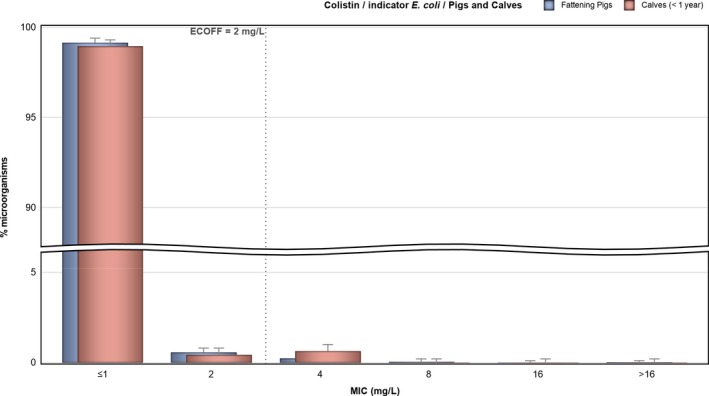
Distribution of MICs of colistin in indicator E. coli from fattening pigs (4,774 isolates from 28 EU MSs and 3 non‐MSs) and calves under 1 year of age (2,383 isolates from 10 EU MSs and 2 non‐MSs), 2017
The mandatory monitoring according to Decision 2013/652/EU is based on phenotypic susceptibility and does not discriminate between different resistance mechanisms. Therefore, inference regarding the presence of, e.g. mcr‐genes cannot be made from the available data. For such evaluation, isolates must be further investigated by molecular methods which currently are out of the scope of the mandatory monitoring. However, in a recent study from France, all 22 isolates of E. coli from pigs, turkeys and broilers collected within the framework of the mandatory monitoring in years 2011–2014, and with colistin MIC > 2 mg/L, were found to harbour the mcr‐1 gene (Perrin‐Guyomard et al., 2016). This indicates that a substantial proportion of the phenotypically resistant isolates reported by other countries participating in the mandatory monitoring also harbour mcr‐genes. Although the overall occurrence of colistin resistance in food‐producing animals at the EU level is low, knowledge of the proportion of resistant isolates harbouring transmissible genes conferring colistin resistance is urgently needed for the assessment of the potential impact on human healthcare.
Figure 79.

Distribution of MICs of colistin in indicator E. coli from fattening pigs (4,774 isolates from 28 EU MSs and 3 non‐MSs) and calves under 1 year of age (2,383 isolates from 10 EU MSs and 2 non‐MSs), 2017
5.3. Discussion
Studying phenotypic AMR of commensal ‘indicator’ E‐ coli from intestinal content of healthy food‐producing animals and food thereof provides valuable information on the reservoirs of resistant bacteria that could potentially be transferred between animals and between animals and humans. Monitoring, therefore, has relevance to both public and animal health. The occurrence of resistance to antimicrobials in indicator E. coli is likely to depend on several factors, such as the selective pressure exerted by the use of antimicrobials in populations of food‐producing animals, the co‐selection of bacteria with multiple resistance, the clonal spread of resistant bacteria and the dissemination of genetic elements, such as plasmids, between bacteria.
5.3.1.
Representative monitoring
In 2017, data on resistance in indicator E. coli from food‐producing animals were for the fourth time collected and reported in accordance with the methodology for harmonised AMR monitoring laid down in Commission Implementing Decision 2013/652/EU. Data reported were harmonised with respect to sampling design, laboratory methodology, reporting and interpretation of resistance. The first year in which data on fattening pigs and calves under 1 year of age and meat thereof were reported according to the Decision was 2015 and the data collected in previous years may have been impacted by differences in methodology.
Resistance data on indicator E. coli from caecal content of fattening pigs were reported by 28 MSs in 2017 and by 27 MSs in 2015. The available data can therefore be considered representative at the EU level and an evaluation of differences in resistance between years 2015 and 2017 is valid. Data on resistance in E. coli from meat of pigs obtained according to the harmonised methodology have only been reported by 1 MS (Italy).
Resistance data on indicator E. coli from caecal content of calves under 1 year of age were reported by 10 MSs for 2015 and for 2017. Among the nine MSs that reported data for both 2015 and 2017 are the 8 main producers of meat from calves and young cattle in EU (Eurostat). Together, these 8 MSs produced 95% of the meat from calves and young cattle in EU in 2017. The available data can be considered representative at the EU level and an evaluation of differences in resistance between years 2015 and 2017 is valid. Data on resistance in E. coli from meat of calves under 1 year of age obtained according to the harmonised methodology have only been reported by 1 MS (Italy).
General observations
It can be observed that, at the EU level, resistance to some antimicrobials was common in indicator E. coli from caecal content in both fattening pigs and calves under 1 year of age. Notably, in both animal species, tetracycline resistance was the most common trait and was overall very high in pigs (52.1%) and high in calves (43.8%). Resistance to sulfamethoxazole, ampicillin and trimethoprim was overall high in both animal species ranging from 24.7% to 42.4%. The high levels of resistance to these antimicrobials in both pigs and calves probably reflect that these antimicrobials are commonly used in both pigs and calves in many countries.
Ciprofloxacin resistance was moderate at the EU level in at 10.6% in both pigs and calves whereas resistance to nalidixic acid was low at 5.8% in pigs and 6.7% in calves. The overall higher occurrence of resistance to ciprofloxacin than to nalidixic acid shows that a substantial proportion of the isolates have a resistance phenotype with reduced susceptibility to ciprofloxacin but not to nalidixic acid. This phenotype could indicate the presence of transmissible genes conferring reduced susceptibility to fluoroquinolones (ciprofloxacin) but not to quinolones (nalidixic acid), e.g. qnr‐genes.
Chloramphenicol resistance was moderate in both pigs (18.0%) and calves (14.4%) and resistance to the other antimicrobials tested was in both pigs and calves either low (gentamicin, cefotaxime, ceftazidime, azithromycin), very low (colistin) or not detected (meropenem, tigecycline).
At the EU level, resistance to tetracycline, trimethoprim, sulfamethoxazole and ampicillin in E. coli was higher in pigs than in calves under 1 year of age but for the other antimicrobials tested, resistance was typically of a similar magnitude in both animal species.
There were notable spatial differences in the occurrence of resistance in E. coli from pigs as, for most antimicrobials, resistance was lower in northern than in southern and eastern Europe. For resistance in calves under 1 year of age, the limited number of reporting countries (n = 12) precludes valid conclusions on spatial differences but the available data gives a more complex picture than in pigs. Thus, resistance was lowest in the reporting Nordic countries (Norway, Denmark) but countries in southern (Portugal, Croatia) and central Europe (Austria) also reported low levels in comparison to neighbouring countries in these regions.
Comparison of resistance in fattening pigs, calves under 1 year of age, broilers and fattening turkeys
At the EU level, resistance in E. coli from caecal content of fattening pigs, calves under 1 year of age, broilers and fattening turkeys can be compared using data for 2017 for pigs and calves and data from 2016 for broilers and turkeys. Among the 12,541 isolates tested for antimicrobial susceptibility in these years, meropenem resistance was detected in one isolate from broilers and tigecycline resistance in altogether 15 isolates from pigs, broilers and turkeys. This shows that in EU resistance to these antimicrobials in E. coli from the intestinal flora of major food‐producing animals is infrequent.
Resistance to colistin, azithromycin, gentamicin and the third‐generation cephalosporins cefotaxime and ceftazidime was low in all animal species and resistance to chloramphenicol was moderate in broilers, pigs, and calves but high in turkeys. Resistance to sulfamethoxazole, trimethoprim or tetracycline was high in all the animal species except for tetracycline resistance which was very high in pigs and fattening turkeys. Notably, resistance levels to all these antimicrobials were of similar magnitude in the animal species, but in general levels were numerically higher in isolates from broilers and fattening turkeys than in isolates from pigs and calves under 1 year of age. In contrast, there were large differences between the animal species in (fluoro)quinolone resistance (nalidixic acid and ciprofloxacin) which was low or moderate in pigs and calves, but very high in broilers and high in turkeys. There were also noticeable differences for ampicillin resistance with a very high occurrence in broilers and turkeys and a high occurrence in pigs and calves. These differences between animal species could reflect a difference in use of antimicrobials with respect to quantity, but perhaps also the mode of administration influences selection for resistance. In poultry, flock treatment is almost exclusively practised, whereas pigs and calves also are treated individually.
It is to be observed that when four food‐producing animal species were considered, spatial differences in occurrence of resistance were observed between MSs, with generally lower resistance in northern Europe compared with southern and eastern Europe.
Trends in resistance
Due to the lack of harmonised data over a longer period, evaluation of trends is difficult because data collected before 2014, when harmonised provisions according to Decision 2013/652/EU were implemented, may be biased by differences in methodology and sampling scheme. However, trends in resistance to ampicillin, ciprofloxacin, nalidixic acid, cefotaxime and tetracycline were statistically assessed by logistic regression for 11 countries that have provided relevant data for pigs and 8 countries for cattle over the period 2009–2017.
Regarding pigs, there are 20 decreasing and 8 increasing trends in resistance in the 11 countries evaluated. Overall, resistance to ampicillin has decreased in four and increased in five countries. Cefotaxime resistance has decreased in three and increased in one country. Resistance to ciprofloxacin has decreased in three and increased in one country. Nalidixic acid resistance has decreased in three countries. Tetracycline resistance has decreased in seven and increased in one country. In four countries, there are only decreasing trends and notably in the Netherlands resistance has decreased to all five antimicrobials and in France to three substances. In contrast, in three countries, there are only increasing trends.
Regarding calves, there are 13 decreasing and 7 increasing trends in the 8 countries evaluated. Overall, resistance to ampicillin has decreased in two and increased in two countries. Cefotaxime resistance has decreased in three and increased in one country. Resistance to ciprofloxacin has decreased in three and increased in two countries. Nalidixic acid resistance has decreased in three and increased in one country. Tetracycline resistance has decreased in two and increased in three countries. Notably, in Germany and the Netherlands, levels of resistance are decreasing for all five antimicrobials analysed. In contrast, there are only increasing trends in two countries.
Complete susceptibility and multidrug resistance
Considering all reporting countries, the occurrence of E. coli isolates susceptible to all antimicrobial classes tested was lower in pigs than in calves under 1 year of age at 39.2% and 56.7%, respectively. However, these levels are considerably higher than reported for broilers (22.2%) and fattening turkeys (23.4%) in 2016. Also, the occurrence of MDR isolates reported 2017 for pigs and calves at 34.9% and 27.7%, respectively, was more favourable than in isolates from broilers and turkeys reported in 2016, at 50.2% and 48.7%, respectively.
There were, however, marked differences between countries with respect to the proportion of completely susceptible as well as MDR isolates for both pigs and calves. Generally, for pigs completely susceptible isolates were more common in northern than in southern and eastern Europe whereas the converse situation was observed for MDR. These observations agree with the situation for broilers and fattening turkeys reported in 2016. However, for calves there was no obvious spatial pattern and a favourable situation were reported from the Nordic countries (Norway, Denmark) as well as in countries in southern and central Europe (Austria, Portugal, Croatia).
The antimicrobials most often represented in the pattern of MDR isolates in both pigs and calves under 1 year of age were tetracycline, ampicillin, sulfamethoxazole and trimethoprim. About half of the MDR isolates from both pigs and calves were resistant to all these four antimicrobials and often also to other substances tested. Ampicillin, sulfamethoxazole, tetracyclines and trimethoprim were also included in the core pattern of MDR isolates from pigs and calves reported in 2015. These antimicrobials were also common in resistance patterns of MDR isolates from poultry reported in 2016. Additionally, quinolone resistance was common in MDR isolates from poultry at 82.1% and 74.0% for broilers and turkeys, respectively. In contrast, this antimicrobial is less often included in the pattern of MDR isolates from pigs and calves, at 24.8% and 28.9%, respectively.
The common resistance to these antimicrobials in MDR profiles could be linked to the current and past usage of these substances in the respective animal species. Selection pressure for MDR isolates by this use is probably augmented by co‐selection due to the presence in poultry microbiota of genetic elements (plasmids), carrying resistance genes to several antimicrobials and transmissible between bacteria, but also by the presence of specific MDR clones that are clonally spread.
Resistance to critically important antimicrobials
Among the antimicrobials tested in the mandatory monitoring, ciprofloxacin (fluoroquinolones), cefotaxime and ceftazidime (third‐generation cephalosporins), meropenem (carbapenems), colistin (polymyxin E) and azithromycin (macrolides) have been categorised by the WHO as CIA and among substances of the highest priority (Urdahl et al., 2016; WHO, 2017). In monitoring resistance in food‐producing animals these antimicrobials are of particular interest because of the potential risk that possible reservoirs among animals of bacteria resistant to these substances could spread to humans along the food chain.
In 2017, the occurrence of phenotypic resistance to third‐generation cephalosporins (cefotaxime and ceftazidime) at the EU level was overall low in E. coli from caecal content in both pigs and calves under 1 year of age. In pigs, 1.4% and 1.3% of the isolates were resistant to cefotaxime and ceftazidime, respectively. In calves under 1 year of age, the corresponding figures were 1.6% and 1.4%. The occurrences in pigs and calves are slightly lower than those in broilers and fattening turkeys reported in 2016 where the occurrence ranged between 2.6% and 4.0%. Within the mandatory monitoring, samples of caecal content are also cultured on selective media to specifically detect the presence of E. coli resistant to third‐generation cephalosporins. The results of these analyses are presented in Section 7.2.
Resistance to carbapenems (meropenem) was not detected in indicator E. coli tested by panel 1 in 2017. Further information on carbapenem resistance is presented in Section 7. Taken together, the monitoring provides strong indications that carbapenem resistance is most infrequent in E. coli from pigs and calves under 1 year of age.
At the EU level, resistance to ciprofloxacin was overall moderate in both pigs and calves under 1 year of age and nalidixic acid resistance was low in both species. This contrast to the situation in poultry where quinolone resistance in 2016 was very high in broilers and high in fattening turkeys although with large differences between countries. Of the quinolone‐resistant isolates, 64% from calves and 55% from pigs showed resistance to both nalidixic acid and ciprofloxacin. This indicates that resistance is mediated by chromosomal mutations and therefore spread mainly by clonal dissemination of resistant clones. However, a large proportion of isolates from both calves and pigs were resistant to ciprofloxacin only. This finding indicates that, apart from mutational resistance, there is also, among E. coli from food‐producing animals in Europe, quinolone resistance mediated by transmissible genes that can spread between bacteria. Interestingly this contrast to the situation in poultry in 2016 when 93% of the quinolone‐resistant isolates from broilers and 80% from fattening turkeys were resistant to both ciprofloxacin and nalidixic acid.
In 2017, 0.5% (24/4,774) of indicator E. coli isolates from pigs and 0.7% (16/2,383) from calves under 1 year of age were resistant to both ciprofloxacin and third‐generation cephalosporins when MICs were interpreted by ECOFFs. This percentage indicates a lower occurrence of co‐resistance to ciprofloxacin and cefotaxime (ECOFF) than in isolates from broilers (3.1%) and fattening turkeys (2.2%) reported in 2016. When evaluated by CBPs, 0.2% (7/4,774) of the isolates from pigs and 0.3% (6/2,383) from calves under 1 year of age showed clinical co‐resistance to ciprofloxacin and cefotaxime. This is a smaller proportion than in broilers (1.2%) and fattening turkeys. (1.3%). Notably, of the isolates co‐resistant to ciprofloxacin and ceftazidime, one isolate from calves under 1 year of age was also resistant to colistin.
At the EU level, the occurrence of resistance to azithromycin in E. coli from pigs and calves under 1 year of age was overall low at 1.5% and 2%, respectively. This is about the same occurrence as reported for pigs (2.8%) and calves (2.4%) in 2015. Notably, 19 countries did not report azithromycin resistance in pigs or single isolates only in 2017, but in Cyprus and Portugal the occurrence was moderate at 12.3% and 16.2%, respectively. Likewise, in calves under 1 year of age, 8 of the 12 reporting countries did not detect azithromycin resistance whereas occurrence was low in 3 countries (4.3%–7%). In 2016, azithromycin resistance at the EU‐level was low also in broilers (4%) and fattening turkeys (2.6%) although for both species moderate levels were reported in individual countries. Azithromycin is not used in animals in Europe and the cause of the rather high levels of resistance to this antimicrobial in some countries is not known. However, azithromycin is an azalide which is a subgroup of the macrolides. Possibly, selection pressure exerted by use of macrolides, e.g. tylosin, in food‐producing animals may have favoured emergence of azithromycin resistance.
In 2017, occurrence of colistin resistance at the EU level was overall very low at 0.3% in pigs and 0.8% in calves under 1 year of age. These figures very similar to data reported for these animal species in 2015 and slightly lower than those reported for broilers (1.7%) and fattening turkeys (5.7%) in 2016. In 2017, there were no large differences between countries in occurrence of colistin resistance in pigs. Of the 31 countries providing data on pigs, 27 reported no colistin‐resistant isolates or single isolates only and the highest occurrence in a single country (Portugal) was 2.1%. Likewise, 8 of the 12 countries providing data on calves did not report colistin resistance or single isolates only and the highest occurrence in a single country (Italy) was 2.9%. In contrast, in countries reporting data for poultry in 2016 the occurrence ranged from 0% to 9.4% in broilers and from 0% to 25.1% in fattening turkeys. Colistin resistance is likely due to selection from use of colistin in animal production and the uneven spatial distribution of colistin resistance in poultry and the high occurrence in some countries indicate that there are large differences in the usage of colistin in poultry production in Europe.
6. Extended‐spectrum β‐lactamase (ESBL)‐, AmpC‐ and/or carbapenemase‐producing Salmonella and Escherichia coli
Resistance to third‐generation cephalosporins: the importance of extended‐spectrum β‐lactamases (ESBLs), AmpC enzymes and carbapenemases
Enterobacteria may become resistant to extended‐spectrum cephalosporins (ESCs) due to several different mechanisms, the most common being the production of β‐lactamases. ESBLs and AmpC β‐lactamases are enzymes that hydrolyse extended spectrum β‐lactam antimicrobials. Bacteria which produce ESBL/AmpC enzymes are usually resistant to many or all third‐generation cephalosporins, which are highest priority critically important antimicrobials (Collignon et al., 2016; Urdahl et al., 2016; WHO, 2017) for the treatment of systemic or invasive Gram‐negative bacterial infections in humans. Apart from their widespread use to treat E. coli infections, these drugs play a critical role in the treatment of certain invasive Salmonella infections, particularly in children and immunosuppressed patients. Occurrence of ESBLs and acquired AmpC (aAmpC) β‐lactamases in Gram‐negative bacteria is considered a public health concern (EFSA, 2011).
β‐Lactamases are encoded by genes that can be located on plasmids (mobile genetic elements) or on the bacterial chromosome. Based on structural similarities (amino acid content) they are subdivided into four classes, designated A to D in the Ambler's classification: ESBL enzymes of the TEM, SHV and CTX‐M families belong to class A, ESBL enzymes of the OXA‐family are included in class D, while class C includes the AmpC β‐lactamases. Some bacterial species have naturally intrinsic β‐lactamase encoding genes on their chromosome (often referred as chromosomal, ‘c’). Acquired (‘a’) β‐lactamases are gained by gene transfer between bacteria. Genes located on the chromosome or plasmids will be usually maintained within the bacterial generation – clonal spread – whereas genes located on mobile genetic elements (plasmids, transposons, integrons) can be further spread to other bacteria by horizontal gene transfer.
The occurrence of β‐lactamases in Salmonella and E. coli (both pathogens and commensals) is mostly due to the acquisition of genes usually from other Enterobacteriaceae by conjugation and to a lesser extent, transduction. The clonal spread of ESBL‐ or AmpC‐carrier bacteria is also important (i.e. international high‐risk clones like E. coli ST131 carrying the ESBL enzyme CTX‐M‐15 in humans; Rogers et al., 2011; Mathers et al., 2015). E. coli also possesses intrinsic AmpC β‐lactamase encoding genes that, in some circumstances can be activated (i.e. through mutations in the promotor regions), and also confer resistance to third‐generation cephalosporins. In contrast, wild‐type Salmonella does not possess endogenous β‐lactamase‐encoding genes. Although all four different types of β‐lactamase classes have been found in Salmonella and E. coli, within the EU, the most important mechanism of resistance to third‐generation cephalosporins in these bacterial species is the production of ESBLs followed by the production of aAmpC, although fluctuations in the level of occurrence or differences between countries and sectors may be expected. Commensal bacteria, such as indicator E. coli, may contribute to the dissemination of ESBLs/aAmpC, as these resistance mechanisms are usually transferable.
The emergence during the last years of resistance to carbapenems, which are regarded as last‐line antimicrobials in human medicine, is also considered as an important public health concern. Carbapenems are used for the treatment of highly resistant infections in humans, including, the treatment of infections with Gram‐negative bacteria producing ESBLs. Resistance to carbapenems in Gram‐negative bacteria is mainly related to the production of carbapenemases (β‐lactamases) and the acquisition of carbapenemase‐encoding genes, although other mechanisms (i.e. related to cell permeability) also exist. The most frequent β‐lactamases with carbapenemase activity can be found in class A (KPC), class D (OXA‐type carbapenemases) and class B (metallo‐β‐lactamases like NDM, VIM and IMI) of Ambler's classification. Although carbapenem antimicrobials are not used in food‐producing animals in the EU, resistance has occasionally been detected in bacteria carried by animals (EFSA BIOHAZ Panel, 2013; Guerra et al., 2014; Madec et al., 2017; Woodford et al., 2013), and dissemination from humans to animals directly or through environmental routes is suspected.
Considering the public health relevance of resistance to third‐generation cephalosporins, and carbapenem compounds, European legislation on harmonised monitoring of AMR in food‐producing animals and food (Commission Implementing Decision 2013/652/EU) has laid down mandatory monitoring of resistance to representative substances of these antimicrobial classes in Salmonella and indicator E. coli from 2014 onwards. All Salmonella and indicator E. coli isolates exhibiting microbiological resistance to cefotaxime, ceftazidime or meropenem are subsequently subjected to further testing using a supplementary panel of substances to obtain more detailed phenotypic characterisation of any resistance detected to third‐generation cephalosporins and/or the carbapenem compound meropenem (Table 5, Material and Methods).
6.1.
6.1.1.
Rationale for the supplementary panel of antimicrobial substances (panel 2)
Cefotaxime and ceftazidime have been included in the supplementary panel because, although most ESBL confer resistance to both compounds, some ESBL enzymes primarily confer resistance to one or the other compound.
Confirmatory synergy testing has been also included so that an ESBL phenotype may be identified.
Cefoxitin has been also included so that an AmpC phenotype may be identified.
Meropenem, imipenem and ertapenem have been included so that putative carbapenemase producers may be identified.
Temocillin (6‐α‐methoxy‐ticarcillin) efficacy is unaffected by most ESBL and AmpC enzymes and this substance may be particularly useful in human medicine to treat urinary tract infections caused by ESBL‐producing Gram‐negative organisms (Giske, 2015). Susceptibility to temocillin enables further phenotypic characterisation of carbapenemases.
The results of such further testing allow the inference of the presumptive class of β‐lactamase enzyme which are responsible for conferring the phenotypic profile of resistance to third‐generation cephalosporins or meropenem detected, providing additional epidemiological information.
The routine monitoring of indicator E. coli and Salmonella spp. performed by all MSs, as well as three non‐MSs did not use selective primary isolation media containing cephalosporins, so the results on occurrence and prevalence of resistance to these antimicrobials relate to organisms selected at random from primary culture media. In 2017 the ‘specific’ monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli (by using selective media containing cephalosporins) was also performed on a mandatory basis by all MSs, as well as three non‐MSs. The corresponding results are presented below. Furthermore, 18 MSs and 1 non‐MS also reported results of a ‘specific’ monitoring of carbapenemase‐producing microorganisms (by using selective media containing carbapenems), performed voluntarily. The Netherlands also reported data for this monitoring performed using different isolation methods.
All resistance occurrence tables (panel 1 and panel 2) mentioned in this chapter can be found in the Salmonella spp. or E. coli Microsoft Excel® documents available on Zenodo at: http://doi.org/10.5281/zenodo.2562858
Identification of presumptive ESBL, AmpC and/or carbapenemase producers (see also Materials and methods section)
To infer the class of β‐lactamase enzyme responsible for conferring the phenotypic profile of resistance to third‐generation cephalosporins or meropenem detected, the EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance (EUCAST, 2013) were applied. A screening breakpoint for cefotaxime and/or ceftazidime (> 1 mg/L) was applied to screen for ESBL and AmpC producers, as these isolates typically (with only a few exceptions) show MICs for cefotaxime and/or ceftazidime > 1 mg/L, whereas different resistance mechanisms are expected in the microbiologically resistant isolates (MIC > ECOFFs) exhibiting MICs lower than the screening breakpoint. Some of the countries also voluntarily reported results from the detection of ESBL‐/AmpC‐resistance genes in the third‐generation cephalosporin‐resistant isolates. These data were included with the classifications made on the basis of resistance phenotype. For the occurrence and prevalence tables shown in this section, presumptive ESBL producers were considered as those exhibiting an ESBL and/or ESBL/AmpC phenotype, and presumptive AmpC producers, those with an AmpC and AmpC/ESBL phenotype.
6.2. Presumptive ESBL/AmpC/CP producers in Salmonella spp. from humans (voluntary testing and reporting)
6.2.1. Distribution of ESBL, AmpC and CP phenotypes in Salmonella by country
In 2017, 218 of the 12,973 Salmonella isolates from humans (1.0%, 24 MSs, plus 2 non‐MSs), tested for ampicillin and either cefotaxime and/or ceftazidime, were ‘microbiologically’ resistant to either or both third‐generation cephalosporins while also being microbiologically resistant to the β‐lactam ampicillin. Twelve MSs and one non‐MS (of 20 countries reporting any resistance to cephalosporins) further tested all or some of their suspected isolates for the presence of ESBL and/or AmpC. ESBL‐producing Salmonella were identified in 0.8% of the tested isolates in the EU MSs with the highest occurrence in Malta (10.8%), the Netherlands (2.3%) and Romania (2.3%) (Table 35). AmpC was less frequent, identified in 0.1% of tested isolates. Three isolates were reported to be both AmpC and ESBL producing. No isolates were reported resistant to carbapenems, although it should be noted that meropenem resistance was interpreted with CBPs in eight of 22 countries reporting meropenem results. Sequencing of ESBL‐producing S. Kentucky as part of an ECDC/FWD‐Net study revealed that two of the isolates from Malta were also carrying carbapenemases (Table 36).
Table 35.
ESBL and AmpC phenotypes in Salmonella spp. isolates from humans by country, 2017
| Country | Total Salmonella tested for CTX and/or CAZ | Res to CTX and/or CAZ | Resistance Phenotype | Serovars | |||||
|---|---|---|---|---|---|---|---|---|---|
| ESBL | AmpC | AmpC + ESBL | |||||||
| N | N | N | % | N | % | N | % | ||
| Austria | 1,697 | 8 | 7 | 0.4 | 1 | 0.1 | Typhimurium (4), Virchow (2), Agona (1), monophasic Typhimurium (1), Paratyphi B var. Java (1) | ||
| Belgium | 980 | 60 | 8 | 0.8 | 3 | 0.3 | Typhimurium (6), monophasic Typhimurium (2), Agona (1), Infantis (1), Newport (1) | ||
| Denmark | 310 | 4 | 2 | 0.6 | 1 | 0.3 | monophasic Typhimurium (2), Derby (1) | ||
| Estonia | 265 | 2 | 2 | 0.8 | monophasic Typhimurium (2) | ||||
| France | 870 | 6 | 3 | 0.3 | 2 | 0.2 | Typhimurium (2), Enteritidis (1), monophasic Typhimurium (1), Miami (1) | ||
| Ireland | 272 | 1 | 1 | 0.4 | monophasic Typhimurium (1) | ||||
| Lithuania | 798 | 3 | 1 | 0.1 | Typhimurium (1) | ||||
| Luxembourg | 97 | 2 | 2 | 2.1 | Infantis (1), Typhimurium (1) | ||||
| Malta | 111 | 16 | 12 | 10.8 | 3 | 2.7 | Kentucky (13), Infantis (1), Newport (1) | ||
| Netherlands | 767 | 18 | 18 | 2.3 | Kentucky (13), Infantis (2), Typhimurium (2), monophasic Typhimurium (1) | ||||
| Romania | 133 | 3 | 3 | 2.3 | Typhimurium (2), Infantis (1) | ||||
| Spain | 1,718 | 15 | 5 | 0.3 | 3 | 0.2 | Bredeney (3), Enteritidis (2), monophasic Typhimurium (2), Mikawasima (1) | ||
| Total (12 MSs) | 8,018 | 138 | 62 | 0.8 | 12 | 0.1 | 3 | 0.0 | |
| Norway | 297 | 3 | 2 | 0.7 | 1 | 0.3 | Typhimurium (2), Kentucky (1) | ||
ESBL: extended‐spectrum β‐lactamase; N: isolates with this phenotype; %: percentage of isolates with this phenotype from the total tested; CTX: cefotaxime; CAZ: ceftazidime; MSs: Member States.
Table 36.
ESBL and AmpC phenotypes and genotypes in Salmonella spp. isolates from humans by serovar, 2017
| Serovar | Tested for CTX and/or CAZ | Res to CTX and/or CAZ | Resistance Phenotype | Genotype | |||||
|---|---|---|---|---|---|---|---|---|---|
| ESBL | AmpC | AmpC + ESBL | |||||||
| N | N | N | % | N | % | N | % | ||
| Agona | 96 | 2 | 2 | 2.1 | CMY‐2, CIT | ||||
| Bredeney | 32 | 3 | 3 | 9.4 | CMY‐2 (3) | ||||
| Derby | 118 | 2 | 1 | 0.8 | CMY‐2 | ||||
| Enteritidis | 3,180 | 7 | 3 | 0.1 | TEM‐52 (2) | ||||
| Infantis | 298 | 9 | 6 | 2.0 | CTX‐M‐1 (2), CTX‐M‐65 (2) | ||||
| Kentucky | 118 | 32 | 24 | 20.3 | 3 | 2.5 | CTX‐M‐14b (23), OXA‐48, OXA‐505 (OXA‐48 like) | ||
| Miami | 2 | 1 | 1 | 50.0 | CMY‐2 | ||||
| Mikawasima | 45 | 1 | 1 | 2.2 | CTX‐M‐55 | ||||
| Monophasic Typhimurium 1,4,[5],12:i:‐ | 1,250 | 34 | 8 | 0.6 | 4 | 0.3 | CTX‐M‐55 (4), CTX‐M‐9 (3), CTX‐M, CMY‐2 | ||
| Newport | 111 | 2 | 2 | 1.8 | CTX‐M‐1 | ||||
| Paratyphi B var. Java | 34 | 1 | 1 | 2.9 | CTX‐M | ||||
| Typhimurium | 1,250 | 37 | 17 | 1.4 | 2 | 0.2 | CTX‐M‐9 (7), CTX‐M (2), CTX‐M‐3, CTM‐M‐15, DHA, OXA‐1, SHV‐64 | ||
| Virchow | 63 | 2 | 2 | 3.2 | SHV‐64, TEM | ||||
ESBL: extended‐spectrum β‐lactamase; N: isolates with this phenotype; %: percentage of isolates with this phenotype from the total tested; CTX: cefotaxime; CAZ: ceftazidime; MSs: Member States.
6.2.2. Distribution of ESBL, AmpC and CP phenotypes in Salmonella by serovar
ESBL was reported in nine different serovars in 2017, most commonly in S. Kentucky (20.3%) where the dominating genotype was bla CTX‐M‐14b (Table 36). ESBL‐production was more frequent in S. Typhimurium (1.4%) and monophasic S. Typhimurium 1,4,[5],12:i:‐ (0.6%) than in S. Enteritidis (0.1%). In S. Typhimurium and its monophasic variant, mainly different types of bla CTX‐M were found while in S. Enteritidis, bla TEM‐52 was reported. AmpC‐type β‐lactamases were reported in seven different serovars, S. Agona, S. Bredeney, S. Derby, S. Kentucky, S. Miami, monophasic S. Typhimurium 1,4,[5],12:i:‐ and S. Typhimurium where the S. Kentucky also expressed an ESBL phenotype. Whole genome sequencing also identified OXA‐48/OXA‐48‐like carbapenemase genes in two S. Kentucky isolates.
6.3. Routine antimicrobial resistance monitoring in fattening pigs, meat from pigs, cattle under 1 year and bovine meat: presumptive ESBL/AmpC/CP producers
In 2017, third‐generation cephalosporin resistance was identified in Salmonella spp. from carcases (meat) of pigs and cattle under 1 year (bovine) as well as in indicator E. coli isolates from fattening pigs and cattle under 1 year tested with the panel 1 of antimicrobials (Table 5). Resistant isolates were also subjected to supplementary β‐lactams susceptibility testing (Panel 2, Table 7).
Table 7.
Panel of antimicrobial substances, EUCAST ECOFFs and concentration ranges used for testing only Salmonella spp. and indicator commensal E. coli isolates resistant to cefotaxime, ceftazidime or meropenem (second panel)
| Antimicrobial | Salmonella EUCAST ECOFFa | E. coli EUCAST ECOFFa | Concentration range, mg/L (no. of wells) |
|---|---|---|---|
| Cefoxitin | > 8 | > 8 | 0.5–64 (8) |
| Cefepime | NAb | > 0.125 | 0.06–32 (10) |
| Cefotaxime + clavulanic acid | NA | NAc | 0.06–64 (11) |
| Ceftazidime + clavulanic acid | NA | NAc | 0.125–128 (11) |
| Meropenem | > 0.125 | > 0.125 | 0.03–16 (10) |
| Temocillin | NAd | NAd | 0.5–64 (8) |
| Imipenem | > 1 | > 0.5 | 0.12–16 (8) |
| Ertapenem | > 0.06 | > 0.06 | 0.015–2 (8) |
| Cefotaxime | > 0.5 | > 0.25 | 0.25–64 (9) |
| Ceftazidime | > 2 | > 0.5 | 0.25–128 (10) |
ECOFFs: epidemiological cut‐off values; EUCAST: European Committee on Antimicrobial Susceptibility Testing; NA: not available.
EUCAST epidemiological cut‐off values available as the Decision 2013/652/EU was drafted (2013). For some antimicrobials, these values have been updated (see below). ‘>’ than the ECOFF, criteria used for determining microbiological resistance.
Current ECOFFs 0.25 and 0.5 mg/L.
For cefepime, the cut‐off value used in the analysis for Salmonella spp. was > 0.125 mg/L.
For temocillin, the cut‐off value used in the analysis was > 32 mg/L.
Cefotaxime and ceftazidime resistance in Salmonella spp. from carcases of pigs was detected in four countries, but no countries reported such isolates from carcases of cattle under 1 year (Table 37; Tables SALMPIGMEATD and SALMBOVMEATD).
Table 37.
Summary of presumptive ESBL‐/AmpC‐producing Salmonella spp. from humans and meat (carcases) and indicator E. coli isolates from caecal samples collected within the routine monitoring, 2017
| Matrix | Presumptive ESBL and/or AmpC producersa n (%R) | Presumptive ESBL producersa n (%R)b | Presumptive AmpC producersc n (%R) | Presumptive ESBL + AmpC producersd n (%R) | Presumptive CP producerse n (%R) |
|---|---|---|---|---|---|
| Salmonella | |||||
|
Humans (N = 8,020, 12 MSs) |
77 (1.0) | 62 (0.8) | 12 (0.1) | 3 (0.04) | 0f |
|
Pig meat (N = 954, 22 MSs) |
5 (0.5) | 2 (0.2) | 3 (0.3) | 0 | 0 |
|
Cattle meat (N = 82, 7 MS) |
0 | 0 | 0 | 0 | 0 |
| E. coli | |||||
|
Fattening pigs (N = 4,205, 28 MSs) |
52 (1.2) | 38 (0.9) | 14 (0.3) | 0 | 0 |
|
Calves, < 1 year old (N = 1,893, 10 MSs) |
26 (1.4) | 25 (1.3) | 5 (0.3) | 4 (0.2) | 0 |
N: total of isolates reported for this monitoring by the MSs; n: number of the isolates resistant; %R: percentage of resistant isolates; ESBL: extended‐ spectrum β‐lactamase; MSs: EU Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Two isolates reported as clinically susceptible to meropenem were later confirmed to carry carbapenemase genes.
Cefotaxime and ceftazidime resistance in indicator E. coli isolates from fattening pigs was detected in 18 out of the 31 reporting countries (28 MSs and 3 non‐MSs), and in indicator E. coli from cattle under 1 year of age, in 9 out of the 12 reporting countries (10 MSs and 2 non‐MSs). Overall, resistance to third‐generation cephalosporins was either not detected or was reported at low levels (Tables 1 and 37; Tables ESCHEPIGD and ESCHECALVD).
Detailed information from the findings for each matrix is provided below.
6.3.1. Presumptive ESBL‐/AmpC‐/CP‐producing Salmonella isolates from carcases of fattening pigs (meat from pigs) and carcases of cattle under 1 year (meat from bovines) collected within the routine AMR monitoring
In 2017, third‐generation cephalosporin resistance was not detected or was reported at low levels in Salmonella isolates collected according to Decision 2013/652/EU from pig or bovine carcases (see Section 3). In fact, such resistance was only reported by 4 out of 23 (22 MSs) countries detecting and reporting Salmonella spp. from carcases of fattening pigs. The overall occurrence of cefotaxime resistance among the reporting MSs was 0.5% (5/954; Table SALMPIGMEATD). Third‐generation cephalosporin resistance was not detected by any of the 7 (7 MSs) countries detecting and reporting Salmonella spp. from carcases of cattle under 1 year (0/82; Table SALMBOVMEATD).
The resistant isolates were subjected to supplementary β‐lactams susceptibility testing (panel 2; Table SALMPIGMEATD2). From the results obtained when testing the isolates with Panel 2, ESBLs, AmpC and ESBLs + AmpC and CP phenotypes were inferred. In total, the proportion of Salmonella spp. isolates from pig and bovine carcases collected within the routine monitoring in the MSs and considered as presumptive ESBL, AmpC or ESBL + AmpC producers was very low as only 5 isolates (out of 1042 tested by all reporting countries, 0.5%) presented any of these phenotypes (Table 37).
Of the five reported isolates from pig carcases (Table 38), two displayed an ESBL phenotype (one S. Derby from Germany and one S. Rissen from Spain), and three displayed an AmpC phenotype (three S. Bredeney from Lithuania, Portugal and Spain).
Table 38.
Presumptive ESBL‐ and AmpC‐producing Salmonella spp. isolates from carcases of fattening pigs (meat from pigs) collected within the routine monitoring and subjected to supplementary testing (panel 2) in 2017a
| Country | NP1 | NP2 | ESBL and/or AmpCa | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | |||
| Germany | 31 | 1 | 1 | 3.2 | 1 | 3.2 | – | – | – | – | – | – | – | – | – | – |
| Lithuania | 2 | 1 | 1 | 50 | – | – | – | – | – | – | 1 | 50 | –– | – | – | – |
| Portugal | 34 | 1 | 1 | 2.9 | – | – | – | – | – | – | 1 | 2.9 | – | – | – | – |
| Spain | 180 | 2 | 2 | 1.1 | 1 | 0.6 | – | – | – | – | 1 | 0.6 | – | – | – | – |
| Total (4 MSs) | 247 | 5 | 5 | 2.0 | 2 | 0.8 | – | – | – | – | 3 | 1.2 | – | – | – | – |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates with this phenotype from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States; NP1: Total number of isolates tested by panel 1; NP2: Total number of isolates tested by panel 2.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of Salmonella spp. isolates tested (with panel 1).
None of the Salmonella spp. isolates from pig or bovine carcases collected within the routine monitoring was reported as microbiologically resistant to carbapenems (Tables SALMPIGMEATD and SALMBOVMEATD).
6.3.2. Presumptive ESBL‐/AmpC‐producing E. coli isolates from caecal samples of slaughter pigs (= fattening pigs) within the routine AMR monitoring
In 2017, third‐generation cephalosporin resistance was not detected or was reported at low to very low levels in indicator E. coli isolates from fattening pigs (Section 5). Such resistance was reported by 18 of the reporting countries (18 MSs) and the overall occurrence of cefotaxime resistance among the MSs was 1.4% (58/4,205; Table ESCHEPIGD).
The resistant isolates were subjected to supplementary β‐lactams susceptibility testing (panel 2, Table ESCHEPIGD2). From the results obtained when testing the isolates with Panel 2, ESBLs, AmpC, ESBLs + AmpC and CP phenotypes were inferred. The proportion of indicator E. coli isolates from fattening pigs collected within the routine monitoring by the MSs with such resistance was low to very low. In total, 52 isolates (out of 2,837 isolates tested on Panel 1 by these countries, 1.9%, Table 39; and out of 4,205 isolates tested on Panel 1 by all MSs, 1.2%, Tables 1 and 37) from fattening pigs presented any of these phenotypes (Tables 37 and 39). In addition, one of the isolates reported by one MS (Poland) also displayed elevated MIC for ertapenem when analysed on Panel 2 (MIC = 0.12). Further investigation (WGS) of this isolate is ongoing.
Table 39.
Presumptive ESBL‐ and AmpC‐producing indicator E. coli isolates from fattening pigs collected within the routine monitoring and subjected to supplementary testing (panel 2) in 2017a
| Country | NP1 | NP2 | ESBL and/or AmpC | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | |||
| Austria | 180 | 3 | 3 | 1.7 | 2 | 1.1 | – | – | – | – | 1 | 0.6 | – | – | – | – |
| Belgium | 176 | 15i | 11 | 6.3 | 11 | 6.3 | 6 | 3.4 | – | – | – | – | – | – | – | – |
| Cyprus | 57 | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Czech Republic | 180 | 5 | 5 | 2.8 | 2 | 1.1 | – | – | – | – | 3 | 1.7 | – | – | – | – |
| Germany | 227 | 5 | 5 | 2.2 | 5 | 2.2 | 2 | 0.9 | – | – | – | – | – | – | – | – |
| Greece | 170 | 1 | 1 | 0.6 | 1 | 0.6 | – | – | – | – | – | – | – | – | – | – |
| Hungary | 170 | 2 | 2 | 1.2 | 1 | 0.6 | – | – | – | – | 1 | 0.6 | – | – | – | – |
| Ireland | 203 | 3 | 2 | 1.0 | 1 | 0.5 | 1 | 0.5 | – | – | 1 | 0.5 | – | – | – | – |
| Italyj | 170 | 2 | 2 | 1.2 | 1 | 0.6 | 1 | 0.6 | – | – | 1 | 0.6 | – | – | – | – |
| Latvia | 149 | 1 | 1 | 0.7 | 1 | 0.7 | – | – | – | – | – | – | – | – | – | – |
| Malta | 74 | 1i | – | – | – | 0.0 | – | – | – | – | – | – | – | – | – | – |
| Netherlands | 300 | 1i | – | – | – | 0.0 | – | – | – | – | – | – | – | – | – | – |
| Poland | 213 | 4i | 3 | 1.4 | 2 | 0.9 | 1 | 0.5 | 1 | 0.5 | 1 | 0.5 | – | – | – | – |
| Portugal | 142 | 5 | 5 | 3.5 | 5 | 3.5 | – | – | – | – | – | – | – | – | – | – |
| Romania | 170 | 4 | 4 | 2.4 | 1 | 0.6 | – | – | – | – | 3 | 1.8 | – | – | – | – |
| Slovenia | 85 | 3 | 3 | 3.5 | 1 | 1.2 | 1 | 1.2 | – | – | 2 | 2.4 | – | – | – | – |
| Spain | 170 | 5 | 5 | 2.9 | 4 | 2.4 | – | – | – | – | 1 | 0.6 | – | – | – | – |
| Total (17 MS) | 2,837 | 61 i | 52 | 1.9 | 38 | 1.4 | 12 | 0.4 | 1 | 0.04 | 14 | 0.5 | 0 | 0.0 | 0 | 0.0 |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States; NP1: Total number of isolates tested by panel 1; NP2: Total number of isolates tested by panel 2.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of E. coli. isolates tested (with panel 1).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
Molecular data were reported by Italy for one isolate: ESBLs: 1 CTX‐M.
6.3.3. Presumptive ESBL‐/AmpC‐/CP‐producing E. coli isolates from caecal samples of calves under 1 year of age gathered at slaughter within the routine AMR monitoring
In 2017, third‐generation cephalosporin resistance was not detected or was reported at very low or low levels in indicator E. coli isolates from cattle under 1 year (see Section 5). Such resistance was reported by 7 out of 12 (10 MSs) reporting countries and the overall occurrence of cefotaxime resistance among the MSs was 1.6% (30/1,893; Table ESCHECALVD).
The resistant isolates were subjected to supplementary β‐lactams susceptibility testing (panel 2, Table ESCHECALVD2). From the results obtained when testing the isolates with Panel 2, ESBLs, AmpC, ESBLs + AmpC and CP phenotypes were inferred. The proportion of indicator E. coli isolates from cattle under 1 year collected within the routine monitoring by the MSs with such resistance was low to very low. In total, 26 isolates (out of 1,307 isolates tested on Panel 1 by the 7 MSs with resistant isolates, 2%, Table 40; and out of 1,893 tested on Panel 1 by the 10 testing MSs, 1.4%, Tables 1 and 37) countries, 2.0%) from cattle under 1 year presented any of these phenotypes (Tables 37 and 40).
Table 40.
Presumptive ESBL and AmpC‐producing indicator E. coli isolates from calves under 1 year of age collected within the routine monitoring and subjected to supplementary testing (panel 2) in 2017a
| Country | NP1 | NP2 | ESBL and/or AmpC | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | |||
| Austria | 181 | 2i | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Belgium | 185 | 5i | 4 | 2.2 | 4 | 2.2 | 1 | 0.5 | – | – | – | – | – | – | – | – |
| Denmark | 181 | 1i | – | – | – | – | – | – | – | – | – | – | – | – | – | –– |
| Germany | 242 | 5 | 5 | 2.1 | 5 | 2.1 | 1 | 0.4 | – | – | 2 | 0.8 | 2 | 0.8 | – | –– |
| Italyj | 170 | 9 | 9 | 5.3 | 9 | 5.3 | 3 | 1.8 | – | – | 1 | 0.6 | 1 | 0.6 | – | – |
| Portugal | 181 | 6 | 6 | 3.3 | 5 | 2.8 | – | – | – | – | 1 | 0.6 | – | – | – | – |
| Spain | 167 | 2 | 2 | 1.2 | 2 | 1.2 | – | – | – | – | 1 | 0.6 | 1 | 0.6 | – | – |
| Total (7 MS) | 1,307 | 30 | 26 | 2.0 | 25 | 1.9 | 5 | 0.4 | – | 0.0 | 5 | 0.4 | 4 | 0.3 | – | 0.0 |
| Norway | 296 | 2 | 2 | 0.7 | 1 | 0.3 | – | – | 1 | 0.3 | 2 | 0.7 | 1 | 0.3 | – | – |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of E. coli. isolates tested (with panel 1).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
Molecular data were reported by Italy: ESBLs: 9 CTX‐M.
None of the isolates displayed elevated MIC any carbapenems.
6.4. Specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli in fattening pigs, pig meat, bovine meat and cattle under 1 year of age and presumptive ESBL/AmpC/CP producers
In certain types of monitoring, selective media containing cephalosporins may be used to investigate the presence of cephalosporin‐resistant organisms in a sample. This type of monitoring (which is referred to as ‘specific monitoring’ in this report) provides a different type of result from that of non‐selective culture. The selective media used (containing cefotaxime at 1 mg/L) in specific monitoring provides a greater sensitivity for detecting resistant organisms in a sample. Thereby the specific monitoring gives an estimate of the proportion of samples in which cephalosporin‐resistant E. coli are present, whereas the routine monitoring gives an estimate of the occurrence of third‐generation cephalosporin resistance among indicator E. coli in the same samples.
In 2017, the specific ESBL/AmpC/carbapenemase‐producing monitoring was performed on a mandatory basis on meat from pigs and bovines (fresh meat at retail) and in fattening pigs by all MSs, and 3 non‐MSs (Tables ESCHEPIGMEATESBL, ESCHEBOVMEATESBL, ESCHEPIGESBL, ESCHEPIGMEATESBL2, ESCHEBOVMEATESBL2 and ESCHEPIGESBL2) and on a mandatory or voluntary basis in cattle under 1 year by 10 MSs as well as by 2 non‐MSs (Tables ESCHECALVESBL and ESCHECALVESBL2). A summary of the occurrence and prevalence of E. coli with presumptive ESBL, AmpC or ESBL + AmpC as well as carbapenem‐resistant phenotypes from meat from pigs, meat from bovines, fattening pigs, and cattle under 1 year deriving from specific monitoring in 2017 assessed at the reporting MS group level is presented in Table 41. Detailed information from the findings for each matrix is provided below.
Table 41.
Summary of presumptive ESBL‐ and AmpC‐producing E. coli isolates from meat from pigs, fattening pigs, bovine meat and cattle under 1 year of age and collected by the EU MSs within the specific ESBLs/AmpC/carbapenemase‐producing monitoring and subjected to supplementary testing in 2017
| Presumptive ESBL and/or AmpC producersa | Presumptive ESBL producersb | Presumptive AmpC producers c | Presumptive ESBL + AmpC producersd | Presumptive CP producerse | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %O | %P | n | %O | %P | n | %O | %P | n | %O | %P | N | %O | %P | |
|
Pig Meat Ns = 6,803 N = 380 28 MSs |
378 | 99.5 | 6.0 | 298 | 78.4 | 4.4 | 99 | 26.1 | 1.6 | 19 | 5.0 | 0.3 | 0 | 0.0 | 0.0 |
|
Fattening pigs Ns = 6,836 N = 2,819 28 MSs |
2,783 | 98.3 | 43.8 | 2,180 | 77.0 | 34.4 | 703 | 24.8 | 11.1 | 100 | 3.5 | 1.6 | 1 | 0.04 | 0.01 |
|
Bovine meat Ns = 6,621 N = 304 28 MSs |
298 | 97.7 | 4.8 | 238 | 78.0 | 3.9 | 67 | 22.0 | 1.1 | 7 | 2.3 | 0.1 | 0 | 0.0 | 0.0 |
|
Cattle, < 1 year old Ns = 3,113 N = 1,326 10 MSs |
1,312 | 98.9 | 44.5 | 1,223 | 92.2 | 41.5 | 177 | 13.3 | 6.0 | 88 | 6.6 | 3.0 | 0 | 0.0 | 0.0 |
Ns: number of animal/meat samples; N: number of the isolates tested; n: number of the isolates resistant; %O: occurrence percentage of cephalosporin‐resistant isolates presenting a presumptive phenotype; %P: percentage of samples harbouring a presumptive ESBL‐/AmpC‐producing E. coli; MSs: EU Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
6.4.1. Specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring in meat from pigs
All 28 MSs as well as 3 non‐MS reported data for the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolated from meat from pigs (Tables ESCHEPIGMEATESBL and ESCHEPIGMEATESBL2).
Presumptive ESBL‐/AmpC‐producing E. coli from pig meat
The MSs tested 6,803 retail samples of pig meat and, following culture on selective media, 6.0% yielded presumptive ESBL, AmpC or ESBL + AmpC producing E. coli (4.7% with ESBL, 1.6% with AmpC, and 0.3% with ESBL + AmpC phenotype, Table 42). The result from 742 additional samples were reported by the non‐MSs.
Table 42.
Prevalence of presumptive ESBL‐ and/or AmpC‐producing E. coli in meat from pigs, 28 EU MSs and 3 non‐MSs, 2017a
| Country | Ns | ESBL and/or AmpCa | ESBLb | ESBL only CTX/CLA SYNc | ESBL only CAZ/CLA SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | ||
| Austria | 309 | 10.4 | 7.2–14.3 | 9.4 | 6.4–13.2 | 1.9 | 0.7–4.2 | 0.0 | 0.0–1.2 | 1.9 | 0.7–4.2 | 1.0 | 0.2–2.8 | 0.0 | 0.0–1.2 |
| Belgium | 299 | 4.3 | 2.3–7.3 | 4.3 | 2.3–7.3 | 1.3 | 0.4–3.4 | 0.3 | 0.0–1.8 | 1.0 | 0.2–2.9 | 1.0 | 0.2–2.9 | 0.0 | 0.0–1.2 |
| Bulgaria | 150 | 12.0 | 7.3–18.3 | 10.0 | 5.7–16.0 | 2.7 | 0.7–6.7 | 0.0 | 0.0–2.4 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Croatia | 156 | 5.8 | 2.7–10.7 | 5.1 | 2.2–9.9 | 2.6 | 0.7–6.4 | 0.0 | 0.0–2.3 | 0.6 | 0.0–3.5 | 0.0 | 0.0–2.3 | 0.0 | 0.0–2.3 |
| Cyprus | 139 | 2.9 | 0.8–7.2 | 1.4 | 0.2–5.1 | 0.7 | 0.0–3.9 | 0.0 | 0.0–2.6 | 1.4 | 0.2–5.1 | 0.0 | 0.0–2.6 | 0.0 | 0.0–2.6 |
| Czech Republic | 298 | 13.8 | 10.1–18.2 | 10.1 | 6.9–14.1 | 6.4 | 3.9–9.8 | 0.3 | 0.0–1.9 | 3.7 | 1.9–6.5 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Denmark | 281 | 2.5 | 1.0–5.1 | 2.1 | 0.8–4.6 | 1.4 | 0.4–3.6 | 0.0 | 0.0–1.3 | 0.4 | 0.0–2.0 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 |
| Estonia | 150 | 2.0 | 0.4–5.7 | 1.3 | 0.2–4.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.7 | 0.0–3.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Finland | 301 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| France | 324 | 0.3 | 0.0–1.7 | 0.3 | 0.0–1.7 | 0.3 | 0.0–1.7 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 |
| Germany | 458 | 5.5 | 2.5–6.4 | 4.9 | 2.2–5.9 | 2.0 | 0.6–3.1 | 0.0 | 0.0–0.8 | 1.2 | 0.2–2.2 | 0.6 | 0.1–1.6 | 0.0 | 0.0–0.8 |
| Greece | 133 | 7.5 | 3.7–13.4 | 6.8 | 3.1–12.5 | 1.5 | 0.2–5.3 | 0.0 | 0.0–2.7 | 0.8 | 0.0–4.1 | 0.0 | 0.0–2.7 | 0.0 | 0.0–2.7 |
| Hungary | 300 | 5.0 | 2.8–8.1 | 3.7 | 1.8–6.5 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 | 1.7 | 0.5–3.8 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 |
| Ireland | 300 | 3.7 | 1.8–6.5 | 2.3 | 0.9–4.7 | 1.7 | 0.5–3.8 | 0.0 | 0.0–1.2 | 1.3 | 0.4–3.4 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Italy | 272 | 7.7 | 4.8–11.6 | 7.0 | 4.3–10.7 | 3.3 | 1.5–6.2 | 0.0 | 0.0–1.3 | 0.7 | 0.1–2.6 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 |
| Latvia | 149 | 8.7 | 4.7–14.5 | 8.1 | 4.2–13.6 | 2.7 | 0.7–6.7 | 0.0 | 0.0–2.4 | 1.3 | 0.2–4.8 | 0.7 | 0.0–3.7 | 0.0 | 0.0–2.4 |
| Lithuania | 150 | 2.7 | 0.7–6.7 | 2.7 | 0.7–6.7 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Luxembourg | 37 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 | 0.0 | 0.0–9.5 |
| Malta | 300 | 13.7 | 9.9–18.1 | 11.1 | 7.7–15.1 | 4.3 | 2.3–7.3 | 0.0 | 0.0–1.2 | 3.4 | 1.6–6.0 | 0.9 | 0.2–2.9 | 0.0 | 0.0–1.2 |
| Netherlands | 273 | 1.1 | 0.2–3.2 | 0.7 | 0.1–2.6 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.4 | 0.0–2.0 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 |
| Poland | 300 | 5.7 | 3.3–8.9 | 3.7 | 1.8–6.5 | 2.0 | 0.7–4.3 | 0.0 | 0.0–1.2 | 2.3 | 0.9–4.7 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 |
| Portugal | 220 | 10.5 | 6.7–15.3 | 8.2 | 4.9–12.6 | 3.2 | 1.3–6.4 | 0.0 | 0.0–1.7 | 3.2 | 1.3–6.4 | 0.9 | 0.1–3.2 | 0.0 | 0.0–1.7 |
| Romania | 298 | 14.4 | 10.6–18.9 | 9.7 | 6.6–13.7 | 3.4 | 1.6–6.1 | 0.0 | 0.0–1.2 | 4.7 | 2.6–7.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Slovakia | 150 | 7.3 | 3.7–12.7 | 5.3 | 2.3–10.2 | 1.3 | 0.2–4.7 | 0.0 | 0.0–2.4 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Slovenia | 151 | 4.6 | 1.9–9.3 | 2.0 | 0.4–5.7 | 0.7 | 0.0–3.6 | 0.0 | 0.0–2.4 | 2.6 | 0.7–6.6 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Spain | 300 | 12.0 | 8.5–16.2 | 9.7 | 6.6–13.6 | 3.7 | 1.8–6.5 | 0.0 | 0.0–1.2 | 4.0 | 2.1–6.9 | 1.7 | 0.5–3.8 | 0.0 | 0.0–1.2 |
| Sweden | 295 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| United Kingdom | 310 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Total (28 MSs) | 6,803 | 6.0 | 5.0–6.1 | 4.7 | 3.9–4.9 | 1.9 | 1.4–2.1 | 0.0 | 0.0–0.1 | 1.6 | 1.2–1.8 | 0.3 | 0.2–0.4 | 0.0 | 0.0–0.1 |
| Iceland | 100 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 | 0.0 | 0.0–3.6 |
| Norway | 340 | 0.3 | 0.0–1.6 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.3 | 0.0–1.6 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 |
| Switzerland | 302 | 0.3 | 0.0–1.8 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
ESBL: extended‐spectrum β‐lactamase; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States; Ns: total number of samples tested.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for CTX and/or CAZ (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with CTX or CAZ or synergy with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with CTX only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with CAZ only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to FOX, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with CTX or CAZ and microbiological resistance to FOX, suggesting ESBL and AmpC enzymes in the same isolate. ESBL and AmpC columns include those isolates.
Isolates with microbiological meropenem resistance.
Four countries (Finland, Luxembourg, Sweden and Iceland) did not detect presumptive ESBL, AmpC or ESBL + AmpC producing E. coli in any samples. In the majority of the remaining countries the prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates was very low (France, the United Kingdom, Norway and Switzerland) or low (16 MSs). In seven MSs (Austria, Bulgaria, the Czech Republic, Malta, Portugal, Romania and Spain), the prevalence was moderate. The generally low prevalence makes the variations among countries small also when the prevalence of the ESBL and AmpC phenotypes are assessed separately (Figure 80).
Figure 80.
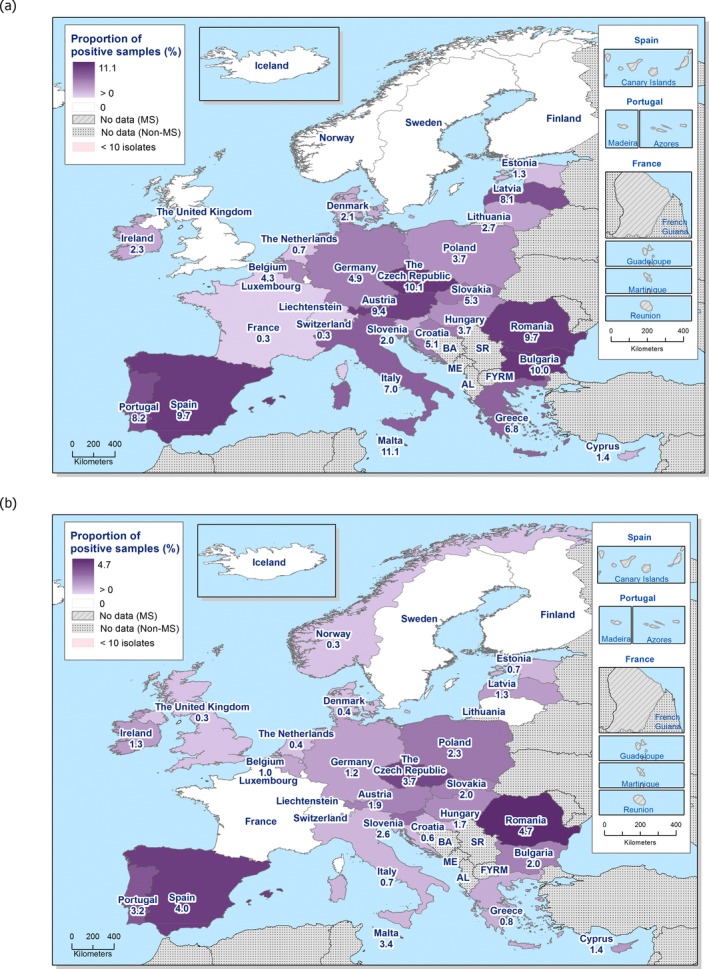
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli isolates in pig meat, assessed by the specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring, 28 EU MSs and 3 non‐MSs, 2017
The occurrence of presumptive ESBL‐producing E. coli among the third‐generation cephalosporin‐resistant isolates (380 isolates tested with Panel 2 by the MSs) from meat from pigs collected within this specific monitoring by the MSs was 78.4% (Table 43). Such isolates were detected in samples from all countries reporting third‐generation cephalosporin‐resistant isolates except the United Kingdom (and the non‐MS Norway). The occurrence of presumptive AmpC‐producing E. coli among the same isolates from meat from pigs collected within this specific monitoring was 26.1%. Such isolates were detected in samples from all countries reporting cephalosporin‐resistant isolates except France and Lithuania (and the non‐MS Switzerland). Here, 5.0% of the E. coli isolates from meat from pigs mentioned above, reported by 11 MSs, presented an ‘ESBL + AmpC’ phenotype. One (0.3%) of the isolates from the specific monitoring tested on Panel 2 did not show ESBL, AmpC or ESBL + AmpC phenotype according to the EUCAST guidelines.
Table 43.
Occurrence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates in meat from pigs collected within the specific ESBLs‐/AmpC‐/carbapenemase‐producing monitoring and subjected to supplementary testing in 2017a
| Country | NP2 | ESBL and/or AmpCa | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | ||
| Austria | 32 | 32 | 100 | 29 | 90.6 | 6 | 18.8 | 0 | 0 | 6 | 18.8 | 3 | 9.4 | 0 | 0 |
| Belgium | 13 | 13 | 100 | 13 | 100 | 4 | 30.8 | 1 | 7.7 | 3 | 23.1 | 3 | 23.1 | 0 | 0 |
| Bulgaria | 18 | 18 | 100 | 15 | 83.3 | 4 | 22.2 | 0 | 0 | 3 | 16.7 | 0 | 0 | 0 | 0 |
| Croatia | 9 | 9 | 100 | 8 | 88.9 | 4 | 44.4 | 0 | 0 | 1 | 11.1 | 0 | 0 | 0 | 0 |
| Cyprus | 4 | 4 | 100 | 2 | 50 | 1 | 25 | 0 | 0 | 2 | 50 | 0 | 0 | 0 | 0 |
| Czech Republic | 42 | 41 | 97.6 | 30 | 71.4 | 19 | 45.2 | 1 | 2.4 | 11 | 26.2 | 0 | 0 | 0 | 0 |
| Denmarki | 7 | 7 | 100 | 6 | 85.7 | 4 | 57.1 | 0 | 0 | 1 | 14.3 | 0 | 0 | 0 | 0 |
| Estonia | 3 | 3 | 100 | 2 | 66.7 | 0 | 0 | 0 | 0 | 1 | 33.3 | 0 | 0 | 0 | 0 |
| France | 1 | 1 | 100 | 1 | 100 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Germany | 19 | 19 | 100 | 17 | 89.5 | 7 | 36.8 | 0 | 0 | 4 | 21.1 | 2 | 10.5 | 0 | 0 |
| Greece | 10 | 10 | 100 | 9 | 90 | 2 | 20 | 0 | 0 | 1 | 10 | 0 | 0 | 0 | 0 |
| Hungary | 15 | 15 | 100 | 11 | 73.3 | 2 | 13.3 | 0 | 0 | 5 | 33.3 | 1 | 6.7 | 0 | 0 |
| Ireland | 11 | 11 | 100 | 7 | 63.6 | 5 | 45.5 | 0 | 0 | 4 | 36.4 | 0 | 0 | 0 | 0 |
| Italyi | 21 | 21 | 100 | 19 | 90.5 | 9 | 42.9 | 0 | 0 | 2 | 9.5 | 0 | 0 | 0 | 0 |
| Latviai | 13 | 13 | 100 | 12 | 92.3 | 4 | 30.8 | 0 | 0 | 2 | 15.4 | 1 | 7.7 | 0 | 0 |
| Lithuania | 4 | 4 | 100 | 4 | 100 | 3 | 75 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malta | 16 | 16 | 100 | 13 | 81.3 | 5 | 31.3 | 0 | 0 | 4 | 25 | 1 | 6.3 | 0 | 0 |
| Netherlandsi | 3 | 3 | 100 | 2 | 66.7 | 0 | 0 | 0 | 0 | 1 | 33.3 | 0 | 0 | 0 | 0 |
| Poland | 17 | 17 | 100 | 11 | 64.7 | 6 | 35.3 | 0 | 0 | 7 | 41.2 | 1 | 5.9 | 0 | 0 |
| Portugal | 23 | 23 | 100 | 18 | 78.3 | 7 | 30.4 | 0 | 0 | 7 | 30.4 | 2 | 8.7 | 0 | 0 |
| Romaniaj | 44 | 43 | 97.7 | 29 | 65.9 | 10 | 22.7 | 0 | 0 | 14 | 31.8 | 0 | 0 | 0 | 0 |
| Slovakia | 11 | 11 | 100 | 8 | 72.7 | 2 | 18.2 | 0 | 0 | 3 | 27.3 | 0 | 0 | 0 | 0 |
| Slovenia | 7 | 7 | 100 | 3 | 42.9 | 1 | 14.3 | 0 | 0 | 4 | 57.1 | 0 | 0 | 0 | 0 |
| Spain | 36 | 36 | 100 | 29 | 80.6 | 11 | 30.6 | 0 | 0 | 12 | 33.3 | 5 | 13.9 | 0 | 0 |
| United Kingdom | 1 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Total (25 MSs) | 380 | 378 | 99.5 | 298 | 78.4 | 117 | 30.8 | 2 | 0.5 | 99 | 26.1 | 19 | 5 | 0 | 0 |
| Norway | 1 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 100 | 0 | 0 | 0 | 0 |
| Switzerland | 1 | 1 | 100 | 1 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of E. coli isolates tested (with panel 2).
Molecular data were provided by
Denmark, 6 ESBLs: CTX‐M (1 CTX‐M‐14, 5 CTX‐M‐1)
Italy, 19 ESBLs: 16 CTX‐M, 2 SHV, 1 TEM‐52
Netherlands: 2 ESBL: 2 CTX‐M (1 CTX‐M‐1, 2 CTX‐M‐15); AmpC: 1 AmpC mutations.
Latvia reported 12 isolates with ESBL pheno‐/genotype, 1 isolate with an ESBL+ AmpC pheno‐/genotype (no genes reported).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
The detection of ESBL‐producing E. coli exceeded that of AmpC‐producing E. coli in most of the reporting countries. However, as many countries only report a low number of isolates the differences should be assessed with caution.
Concurrent resistance among the third‐generation cephalosporin‐resistant isolates varied markedly among substances and countries. The overall (lowest–highest) occurrences among the 28 reporting MSs for the different substances were: azithromycin 8.4% (0–30%), chloramphenicol 30.3% (0–80%), ciprofloxacin 46.1% (0–100%), colistin 1.3% (0–20%), gentamicin 18.4% (0–46.2%), meropenem 0%, nalidixic acid 36.3% (0–68.8%), sulfamethoxazole 75.5% (0–100%), tetracycline 68.4% (38.5–100%), tigecycline 0% and trimethoprim 56.8% (0–100%). However, as many countries only report a low number of isolates, the variations should be assessed with caution.
Presumptive carbapenemase‐producing E. coli from pig meat
None of the isolates from the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli displayed elevated MIC for meropenem. Two isolates (from Portugal and Spain) did however, when tested on Panel 2, display elevated MIC for ertapenem and one (from Malta) displayed elevated MIC for both ertapenem and imipenem. Further investigation of these isolates is ongoing.
6.4.2. Specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring in fattening pigs
All 28 MSs as well as 3 non‐MSs reported data for the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolated from fattening pigs (Tables ESCHEPIGESBL and ESCHEPIGESBL2).
Presumptive ESBL‐/AmpC‐producing E. coli from fattening pigs
The MSs tested 6,836 samples from fattening pigs and, following culture on selective media, 43.8% yielded presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli (34.3% with ESBL, 11.1% with AmpC, and 1.6% with ESBL + AmpC phenotype, Table 44). The result from 753 additional samples were reported by the 3 non‐MSs.
Table 44.
Prevalence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates in fattening pigs, 28 EU MSs and 3 non‐MSs, 2017a
| Country | Ns | ESBL and/or AmpC | ESBLb | ESBL only CTX/CLA SYNc | ESBL only CAZ/CLA SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | ||
| Austria | 291 | 62.2 | 56.4–67.8 | 58.8 | 52.9–64.5 | 11.3 | 7.9–15.6 | 0.0 | 0.0–1.3 | 4.1 | 2.1–7.1 | 0.7 | 0.1–2.5 | 0.0 | 0.0–1.3 |
| Belgium | 300 | 65.4 | 49.5–61.0 | 60.7 | 45.5–57.1 | 26.4 | 17.7–27.5 | 0.8 | 0.1–2.4 | 5.9 | 2.8–8.1 | 1.2 | 0.2–2.9 | 0.0 | 0.0–1.2 |
| Bulgaria | 150 | 45.3 | 37.2–53.7 | 42.0 | 34.0–50.3 | 10.0 | 5.7–16.0 | 0.0 | 0.0–2.4 | 3.4 | 1.1–7.6 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Croatia | 369 | 46.7 | 18.1–26.8 | 30.2 | 10.9–18.4 | 16.5 | 5.3–11.1 | 1.1 | 0.1–1.9 | 17.1 | 5.6–11.4 | 0.6 | 0.0–1.5 | 0.0 | 0.0–1.0 |
| Cyprus | 120 | 0.8 | 0.0–4.6 | 0.8 | 0.0–4.6 | 0.8 | 0.0–4.6 | 0.0 | 0.0–3.0 | 0.0 | 0.0–3.0 | 0.0 | 0.0–3.0 | 0.0 | 0.0–3.0 |
| Czech Republic | 301 | 33.2 | 27.9–38.9 | 17.6 | 13.5–22.4 | 10.0 | 6.8–13.9 | 0.3 | 0.0–1.8 | 15.6 | 11.7–20.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Denmark | 295 | 24.7 | 19.9–30.1 | 6.8 | 4.2–10.3 | 3.4 | 1.6–6.1 | 0.0 | 0.0–1.2 | 18.0 | 13.8–22.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Estonia | 68 | 35.3 | 24.1–47.8 | 29.4 | 19.0–41.7 | 1.5 | 0.0–7.9 | 0.0 | 0.0–5.3 | 5.9 | 1.6–14.4 | 0.0 | 0.0–5.3 | 0.0 | 0.0–5.3 |
| Finland | 299 | 2.7 | 1.2–5.2 | 0.3 | 0.0–1.8 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 2.3 | 0.9–4.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| France | 327 | 27.2 | 22.5–32.4 | 23.8 | 19.3–28.9 | 9.2 | 6.3–12.8 | 0.0 | 0.0–1.1 | 3.4 | 1.7–5.9 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 |
| Germany | 351 | 46.7 | 40.6–51.2 | 41.8 | 35.8–46.4 | 16.3 | 12.3–20.2 | 0.0 | 0.0–1.0 | 8.1 | 5.4–11.3 | 3.2 | 1.6–5.5 | 0.3 | 0.0–1.6 |
| Greece | 170 | 39.4 | 32.0–47.2 | 33.5 | 26.5–41.2 | 4.7 | 2.1–9.1 | 0.0 | 0.0–2.1 | 7.1 | 3.7–12 | 1.2 | 0.1–4.2 | 0.0 | 0.0–2.1 |
| Hungary | 249 | 70.3 | 64.2–75.9 | 56.2 | 49.8–62.5 | 17.3 | 12.8–22.5 | 1.2 | 0.2–3.5 | 15.3 | 11.0–20.3 | 1.2 | 0.2–3.5 | 0.0 | 0.0–1.5 |
| Ireland | 303 | 36.0 | 30.6–41.7 | 18.5 | 14.3–23.3 | 8.6 | 5.7–12.3 | 0.0 | 0.0–1.2 | 18.2 | 14.0–23.0 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 |
| Italy | 302 | 87.4 | 83.1–90.9 | 69.2 | 63.7–74.4 | 25.5 | 20.7–30.8 | 2.0 | 0.7–4.3 | 21.8 | 17.3–26.9 | 3.7 | 1.8–6.4 | 0.0 | 0.0–1.2 |
| Latvia | 149 | 46.3 | 38.1–54.7 | 42.3 | 34.2–50.6 | 20.1 | 14.0–27.5 | 0.6 | 0.0–3.7 | 6.7 | 3.3–12.0 | 2.7 | 0.7–6.7 | 0.0 | 0.0–2.4 |
| Lithuania | 150 | 48.7 | 39.8–56.3 | 37.9 | 29.6–45.6 | 10.1 | 5.7–16.0 | 0.0 | 0.0–2.4 | 11.5 | 6.7–17.5 | 0.7 | 0.0–3.7 | 0.0 | 0.0–2.4 |
| Luxembourg | 225 | 40.8 | 34.4–47.2 | 38.1 | 31.7–44.5 | 20.4 | 15.1–25.7 | 0.0 | 0.0–1.6 | 4.1 | 1.5–6.7 | 1.3 | 0.0–2.5 | 0.0 | 0.0–1.6 |
| Malta | 112 | 17.9 | 11.3–26.2 | 17.9 | 11.3–26.2 | 1.8 | 0.2–6.3 | 0.9 | 0.0–4.9 | 0.9 | 0.0–4.9 | 0.9 | 0.0–4.9 | 0.0 | 0.0–3.2 |
| Netherlands | 300 | 15.7 | 11.7–20.3 | 11.0 | 7.7–15.1 | 3.3 | 1.6–6.0 | 0.3 | 0.0–1.8 | 5.0 | 2.8–8.1 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 |
| Poland | 306 | 48.7 | 42.6–54.1 | 31.9 | 26.5–37.2 | 11.5 | 8.1–15.5 | 2.3 | 0.9–4.7 | 17.4 | 13.3–22 | 0.7 | 0.1–2.3 | 0.0 | 0.0–1.2 |
| Portugal | 254 | 63.8 | 53.1–65.5 | 54.9 | 44.9–57.5 | 11.0 | 6.8–14.6 | 0.0 | 0.0–1.4 | 13.5 | 8.8–17.3 | 4.7 | 2.2–7.6 | 0.0 | 0.0–1.4 |
| Romania | 255 | 66.3 | 60.1–72.1 | 53.7 | 47.4–60.0 | 18.0 | 13.5–23.3 | 2.0 | 0.6–4.5 | 14.5 | 10.4–19.4 | 2.0 | 0.6–4.5 | 0.0 | 0.0–1.4 |
| Slovakia | 150 | 48.7 | 40.4–57.0 | 34.0 | 26.5–42.2 | 14.0 | 8.9–20.6 | 0.0 | 0.0–2.4 | 17.3 | 11.6–24.4 | 2.7 | 0.7–6.7 | 0.0 | 0.0–2.4 |
| Slovenia | 152 | 50.0 | 41.8–58.2 | 25.7 | 18.9–33.4 | 10.6 | 6.1–16.5 | 0.0 | 0.0–2.4 | 24.4 | 17.8–32.0 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Spain | 300 | 85.7 | 81.2–89.4 | 80.3 | 75.4–84.7 | 11.7 | 8.3–15.9 | 0.0 | 0.0–1.2 | 15.7 | 11.7–20.3 | 10.3 | 7.1–14.3 | 0.0 | 0.0–1.2 |
| Sweden | 241 | 11.6 | 7.9–16.4 | 3.7 | 1.7–7.0 | 2.1 | 0.7–4.8 | 0.0 | 0.0–1.5 | 7.9 | 4.8–12.0 | 0.0 | 0.0–1.5 | 0.0 | 0.0–1.5 |
| United Kingdom | 347 | 21.6 | 17.4–26.3 | 16.1 | 12.4–20.4 | 4.9 | 2.9–7.7 | 0.0 | 0.0–1.1 | 6.6 | 4.2–9.8 | 1.1 | 0.3–2.9 | 0.0 | 0.0–1.1 |
| Total (28 MSs) | 6,836 | 43.8 | 39.5–41.9 | 34.3 | 30.8–33.0 | 11.0 | 9.5–11.0 | 0.4 | 0.3–0.6 | 11.1 | 9.6–11.0 | 1.6 | 1.2–1.8 | 0.01 | 0–0.1 |
| Iceland | 151 | 7.3 | 3.7–12.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 7.3 | 3.7–12.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Norway | 306 | 14.1 | 10.4–18.5 | 0.7 | 0.1–2.3 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 13.4 | 9.8–17.7 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Switzerland | 296 | 15.9 | 11.9–20.5 | 11.5 | 8.1–15.7 | 5.1 | 2.9–8.2 | 0.0 | 0.0–1.2 | 5.1 | 2.9–8.2 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 |
ESBL: extended‐spectrum β‐lactamase; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; P: prevalence; CI: confidence interval; MSs: Member States; Ns: total number of samples tested.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for FOX and/or CAZ (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with CTX or CAZ or synergy with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to FOX, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with CTX or CAZ and microbiological resistance to FOX, suggesting ESBL and AmpC enzymes in the same isolate. ESBL and AmpC columns include those isolates.
Isolates with microbiological meropenem resistance.
Isolates with ESBL, AmpC or ESBL + AmpC phenotype were reported from all countries (Table 44). Nevertheless, marked variations were observed in the prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates. It ranged from 0.8 in Cyprus, 2.7% in Finland, and 7.3% in Iceland, up to 70.3% in Hungary, 85.7% in Spain, and 87.4% in Italy. These variations are seen also if the prevalence of the ESBL phenotype is assessed separately (Figure 81). There are also indications of these variations when the AmpC phenotype is assessed separately although less marked (Figure 81).
Figure 81.
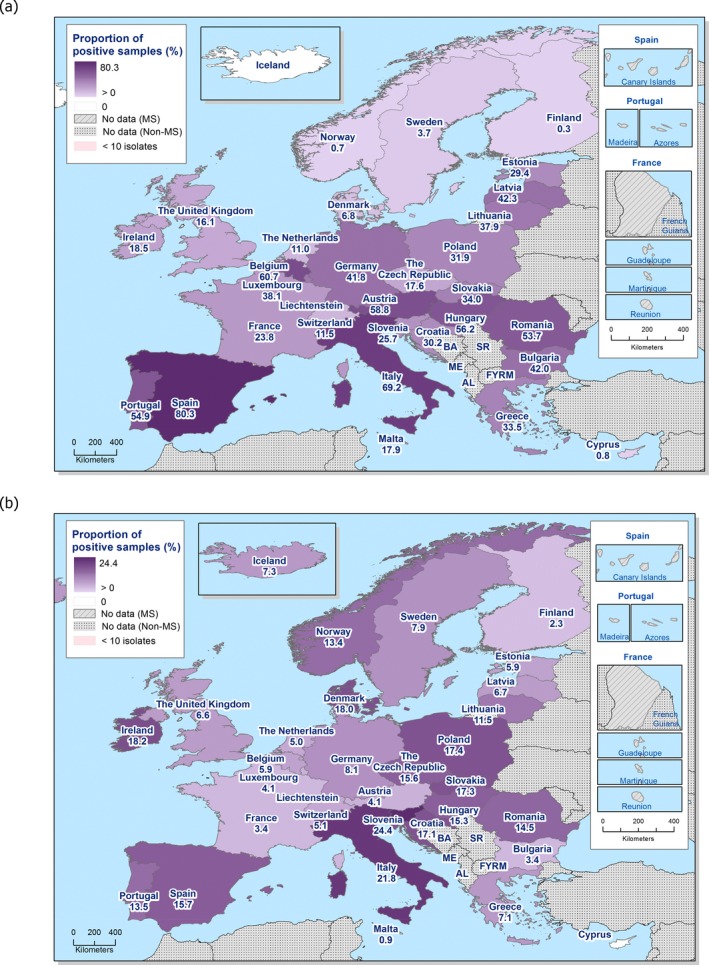
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli isolates in fattening pigs, assessed by the specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring, 28 EU MSs and 3 non‐MSs, 2017
The occurrence of presumptive ESBL‐producing E. coli among the third‐generation cephalosporin‐resistant isolates (2,819 isolates tested with Panel 2 by the MSs) from fattening pigs collected within this specific monitoring by the MSs was 77.0% (Table 45). Such isolates were detected in samples from all countries except the non‐MS Iceland. The occurrence of presumptive AmpC‐producing E. coli isolates from fattening pigs collected within this specific monitoring by the MS was 24.8%. Such isolates were detected in samples from all countries. Here, 3.5% of the E. coli isolates from fattening pigs mentioned above, reported by 20 MSs as well as Switzerland, presented an ‘ESBL + AmpC’ phenotype. Thirty‐five (1.2%) of the isolates tested on Panel 2 did not show ESBL, AmpC or ESBL + AmpC phenotype according to the EUCAST guidelines.
Table 45.
Occurrence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates from fattening pigs collected within the specific ESBLs‐/AmpC‐/carbapenemase‐producing monitoring and subjected to supplementary testing in 2017a
| Country | NP2 | ESBL and/or AmpCa | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | ||
| Austria | 181 | 181 | 100.0 | 171 | 94.5 | 33 | 18.2 | 0 | 0.0 | 12 | 6.6 | 2 | 1.1 | 0 | 0.0 |
| Belgiumi | 171 | 166 | 97.1 | 154 | 90.1 | 67 | 39.2 | 2 | 1.2 | 15 | 8.8 | 3 | 1.8 | 0 | 0.0 |
| Bulgaria | 68 | 68 | 100.0 | 63 | 92.6 | 15 | 22.1 | 0 | 0.0 | 5 | 7.4 | 0 | 0.0 | 0 | 0.0 |
| Croatia | 90 | 82 | 91.1 | 53 | 58.9 | 29 | 32.2 | 2 | 2.2 | 30 | 33.3 | 1 | 1.1 | 0 | 0.0 |
| Cyprus | 3 | 1 | 33.3 | 1 | 33.3 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Czech Republici | 104 | 100 | 96.2 | 53 | 51.0 | 30 | 28.8 | 1 | 1.0 | 47 | 45.2 | 0 | 0.0 | 0 | 0.0 |
| Denmark | 73 | 73 | 100.0 | 20 | 27.4 | 10 | 13.7 | 0 | 0.0 | 53 | 72.6 | 0 | 0.0 | 0 | 0.0 |
| Estonia | 24 | 24 | 100.0 | 20 | 83.3 | 1 | 4.2 | 0 | 0.0 | 4 | 16.7 | 0 | 0.0 | 0 | 0.0 |
| Finland | 8 | 8 | 100.0 | 1 | 12.5 | 1 | 12.5 | 0 | 0.0 | 7 | 87.5 | 0 | 0.0 | 0 | 0.0 |
| Francei | 91 | 89 | 97.8 | 78 | 85.7 | 30 | 33.0 | 0 | 0.0 | 11 | 12.1 | 0 | 0.0 | 0 | 0.0 |
| Germany | 162 | 161 | 99.4 | 144 | 88.9 | 56 | 34.6 | 0 | 0.0 | 28 | 17.3 | 11 | 6.8 | 1 | 0.62 |
| Greece | 67 | 67 | 100.0 | 57 | 85.1 | 8 | 11.9 | 0 | 0.0 | 12 | 17.9 | 2 | 3.0 | 0 | 0.0 |
| Hungary | 175 | 175 | 100.0 | 140 | 80.0 | 43 | 24.6 | 3 | 1.7 | 38 | 21.7 | 3 | 1.7 | 0 | 0.0 |
| Irelandi | 110 | 109 | 99.1 | 56 | 50.9 | 26 | 23.6 | 0 | 0.0 | 55 | 50.0 | 2 | 1.8 | 0 | 0.0 |
| Italyj | 265 | 264 | 99.6 | 209 | 78.9 | 77 | 29.1 | 6 | 2.3 | 66 | 24.9 | 11 | 4.2 | 0 | 0.0 |
| Latviaj | 69 | 69 | 100.0 | 63 | 91.3 | 30 | 43.5 | 1 | 1.4 | 10 | 14.5 | 4 | 5.8 | 0 | 0.0 |
| Lithuania | 72 | 72 | 100.0 | 56 | 77.8 | 15 | 20.8 | 0 | 0.0 | 17 | 23.6 | 1 | 1.4 | 0 | 0.0 |
| Luxembourgi | 34 | 30 | 88.2 | 28 | 82.4 | 15 | 44.1 | 0 | 0.0 | 3 | 8.8 | 1 | 2.9 | 0 | 0.0 |
| Malta | 20 | 20 | 100.0 | 20 | 100.0 | 2 | 10.0 | 1 | 5.0 | 1 | 5.0 | 1 | 5.0 | 0 | 0.0 |
| Netherlandsj | 47 | 47 | 100.0 | 33 | 70.2 | 10 | 21.3 | 1 | 2.1 | 15 | 31.9 | 1 | 2.1 | 0 | 0.0 |
| Polandi | 154 | 148 | 96.1 | 97 | 63.0 | 35 | 22.7 | 7 | 4.5 | 53 | 34.4 | 2 | 1.3 | 0 | 0.0 |
| Portugal | 151 | 151 | 93.2 | 130 | 80.2 | 26 | 16.0 | 0 | 0.0 | 32 | 19.8 | 11 | 6.8 | 0 | 0.0 |
| Romania | 169 | 169 | 100.0 | 137 | 81.1 | 46 | 27.2 | 5 | 3.0 | 37 | 21.9 | 5 | 3.0 | 0 | 0.0 |
| Slovakia | 73 | 73 | 100.0 | 51 | 69.9 | 21 | 28.8 | 0 | 0.0 | 26 | 35.6 | 4 | 5.5 | 0 | 0.0 |
| Slovenia | 76 | 76 | 100.0 | 39 | 51.3 | 16 | 21.1 | 0 | 0.0 | 37 | 48.7 | 0 | 0.0 | 0 | 0.0 |
| Spain | 258 | 257 | 99.6 | 241 | 93.4 | 35 | 13.6 | 0 | 0.0 | 47 | 18.2 | 31 | 12.0 | 0 | 0.0 |
| Swedeni , j | 29 | 28 | 96.6 | 9 | 31.0 | 5 | 17.2 | 0 | 0.0 | 19 | 65.5 | 0 | 0.0 | 0 | 0.0 |
| United Kingdom | 75 | 75 | 100.0 | 56 | 74.7 | 17 | 22.7 | 0 | 0.0 | 23 | 30.7 | 4 | 5.3 | 0 | 0.0 |
| Total (28 MSs) | 2,819 | 2,783 | 98.3 | 2,180 | 77.0 | 700 | 24.7 | 29 | 1.0 | 703 | 24.8 | 100 | 3.5 | 1 | 0.04 |
| Iceland | 11 | 11 | 100.0 | 0.0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 11 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Norway | 43 | 43 | 100.0 | 2 | 4.7 | 0 | 0.0 | 0 | 0.0 | 41 | 95.3 | 0 | 0.0 | 0 | 0.0 |
| Switzerlandi | 52 | 47 | 90.4 | 34 | 65.4 | 15 | 28.8 | 0 | 0.0 | 15 | 28.8 | 2 | 3.8 | 0 | 0.0 |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of E. coli isolates tested (with panel 2).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
Molecular data were provided by
Italy, ESBLs: 191 CTX‐M (1 CTX‐M‐14), 16 SHV‐12, 3 TEM‐52; AmpC: 3 CMY‐2. ESBL + AmpC: 1 SHV‐12 + CMY‐2, 1 CTX‐M + CMY‐2
Netherlands: ESBL: 27 CTX‐M (23 CTX‐M‐1, 3 CTX‐M‐14, 1 CTX‐M‐3), 1 SHV‐2, 5 TEM‐52; AmpC: 6 AmpC pheno/genotype (no genes reported).
Sweden, ESBLs: 9 CTX‐M (6 CTX‐M‐14, 2 CTX‐M15, 1 CTX‐M‐55)
Latvia reported 62 isolates ESBL pheno/genotype, 2 isolates with ESBL+ AmpC pheno/genotype (no genes reported).
The detection of ESBL‐producing E. coli exceeded that of AmpC‐producing E. coli in most of the reporting countries except for the Nordic countries (Denmark, Finland, Iceland, Norway and Sweden), where the AmpC phenotype dominated, and in the Czech Republic, Ireland, and Slovenia where the prevalence of the ESBL and the AmpC phenotypes were comparable.
Concurrent resistance among the third‐generation cephalosporin‐resistant isolates varied markedly among substances and countries. The overall (lowest–highest) occurrences among the 28 reporting MSs for the different substances were: azithromycin 8.8% (0–29.1%), chloramphenicol 28.2% (0–66.7%), ciprofloxacin 41.9% (0–85%), colistin 1.8% (0–9.9%), gentamicin 14.7% (0–36.1%), meropenem < 0.1% (0–0.6%), nalidixic acid 29.8% (0–70.9%), sulfamethoxazole 71.1% (29.2–88.2%), tetracycline 67.3% (25–91.2%), tigecycline 0.1% (0–0.7%) and trimethoprim 57.1% (34.6–80%).
Presumptive carbapenemase‐producing E. coli from fattening pigs
One isolate (from Germany) from the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli displayed elevated MIC (=0.5 mg/L) for meropenem both on Panel 1 and Panel 2. This isolate did also display elevated MIC for ertapenem and imipenem (0.5 and 2 mg/L, respectively). The isolate was confirmed by the MS to be positive for the production of VIM‐1 (information kindly shared by the MS). Apart from this German isolate, when tested on panel 2, two isolates (from France and Spain) displayed elevated MIC for imipenem and 51 isolates (from 14 MSs and Iceland) displayed elevated MIC for ertapenem. Further investigation of these isolates is ongoing.
6.4.3. Specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring in bovine meat
All MSs as well as 3 non‐MSs reported data for the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolated from meat from bovines (Tables ESCHEBOVMEATESBL and ESCHEBOVMEATESBL2).
Presumptive ESBL‐/AmpC‐producing E. coli from bovine meat
The MSs tested 6,621 retail samples of bovine meat and, following culture on selective media, 4.8% yielded presumptive ESBL, AmpC or ESBL + AmpC producing E. coli (3.9% with ESBL, 1.1% with AmpC, and 0.1% with ESBL + AmpC phenotype, Table 46). The result from 737 additional samples were reported by the 3 non‐MSs.
Table 46.
Prevalence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates in bovine meat, 28 MSs and 3 non‐MSs, 2017a
| Country | Ns | ESBL and/or AmpCa | ESBLb | ESBL only CTX/CLA SYNc | ESBL only CAZ/CLA SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | ||
| Austria | 300 | 1.7 | 0.5–3.8 | 1.7 | 0.5–3.8 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Belgium | 300 | 4.6 | 2.3–7.3 | 4.6 | 2.3–7.3 | 1.1 | 0.2–2.9 | 0.0 | 0.0–1.2 | 0.4 | 0.0–1.8 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 |
| Bulgaria | 150 | 12.7 | 7.8–19.1 | 10.0 | 5.7–16.0 | 5.3 | 2.3–10.2 | 0.0 | 0.0–2.4 | 2.7 | 0.7–6.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Croatia | 161 | 2.5 | 0.7–6.2 | 2.5 | 0.7–6.2 | 0.6 | 0.0–3.4 | 0.0 | 0.0–2.3 | 0.0 | 0.0–2.3 | 0.0 | 0.0–2.3 | 0.0 | 0.0–2.3 |
| Cyprus | 139 | 3.6 | 1.2–8.2 | 1.4 | 0.2–5.1 | 0.0 | 0.0–2.6 | 0.0 | 0.0–2.6 | 2.2 | 0.4–6.2 | 0.0 | 0.0–2.6 | 0.0 | 0.0–2.6 |
| Czech Republic | 301 | 11.3 | 8.0–15.4 | 6.3 | 3.8–9.7 | 4.0 | 2.1–6.9 | 0.0 | 0.0–1.2 | 5.0 | 2.8–8.1 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Denmark | 292 | 3.4 | 1.7–6.2 | 3.4 | 1.7–6.2 | 1.4 | 0.4–3.5 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 |
| Estonia | 150 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Finland | 302 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| France | 324 | 0.3 | 0.0–1.7 | 0.3 | 0.0–1.7 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 |
| Germany | 406 | 4.4 | 1.9–5.7 | 4.1 | 1.7–5.4 | 1.6 | 0.4–2.9 | 0.0 | 0.0–0.9 | 0.3 | 0.0–1.4 | 0.0 | 0.0–0.9 | 0.0 | 0.0–0.9 |
| Greece | 62 | 4.8 | 1.0–13.5 | 4.8 | 1.0–13.5 | 1.6 | 0.0–8.7 | 0.0 | 0.0–5.8 | 0.0 | 0.0–5.8 | 0.0 | 0.0–5.8 | 0.0 | 0.0–5.8 |
| Hungary | 184 | 9.8 | 5.9–15.0 | 8.7 | 5.1–13.7 | 3.8 | 1.5–7.7 | 0.0 | 0.0–2.0 | 1.1 | 0.1–3.9 | 0.0 | 0.0–2.0 | 0.0 | 0.0–2.0 |
| Ireland | 300 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Italy | 272 | 8.8 | 5.7–12.8 | 8.1 | 5.1–12.0 | 3.7 | 1.8–6.7 | 0.0 | 0.0–1.3 | 1.1 | 0.2–3.2 | 0.4 | 0.0–2.0 | 0.0 | 0.0–1.3 |
| Latvia | 149 | 8.1 | 4.2–13.6 | 6.7 | 3.3–12.0 | 3.4 | 1.1–7.7 | 0.0 | 0.0–2.4 | 2.0 | 0.4–5.8 | 0.7 | 0.0–3.7 | 0.0 | 0.0–2.4 |
| Lithuania | 150 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Luxembourg | 26 | 3.8 | 0.1–19.6 | 0.0 | 0.0–13.2 | 0.0 | 0.0–13.2 | 0.0 | 0.0–13.2 | 3.8 | 0.1–19.6 | 0.0 | 0.0–13.2 | 0.0 | 0.0–13.2 |
| Malta | 300 | 13.1 | 5.2–11.7 | 11.5 | 4.4–10.5 | 2.7 | 0.5–3.8 | 0.5 | 0.0–1.8 | 2.2 | 0.4–3.4 | 0.5 | 0.0–1.8 | 0.0 | 0.0–1.2 |
| Netherlands | 486 | 4.1 | 2.2–5.8 | 4.1 | 2.2–5.8 | 0.0 | 0.0–0.8 | 0.0 | 0.0–0.8 | 0.0 | 0.0–0.8 | 0.0 | 0.0–0.8 | 0.0 | 0.0–0.8 |
| Poland | 300 | 5.7 | 3.3–8.9 | 3.7 | 1.8–6.5 | 1.7 | 0.5–3.8 | 0.0 | 0.0–1.2 | 2.0 | 0.7–4.3 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Portugal | 220 | 11.8 | 7.9–16.8 | 10.5 | 6.7–15.3 | 3.6 | 1.6–7.0 | 0.0 | 0.0–1.7 | 1.8 | 0.5–4.6 | 0.4 | 0.0–2.5 | 0.0 | 0.0–1.7 |
| Romania | 146 | 3.4 | 1.1–7.8 | 3.4 | 1.1–7.8 | 1.4 | 0.2–4.9 | 0.0 | 0.0–2.5 | 0.0 | 0.0–2.5 | 0.0 | 0.0–2.5 | 0.0 | 0.0–2.5 |
| Slovakia | 150 | 4.0 | 1.5–8.5 | 4.0 | 1.5–8.5 | 2.0 | 0.4–5.7 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Slovenia | 151 | 6.0 | 2.8–11.0 | 2.6 | 0.7–6.6 | 0.7 | 0.0–3.6 | 0.0 | 0.0–2.4 | 3.3 | 1.1–7.6 | 0.0 | 0.0–2.4 | 0.0 | 0.0–2.4 |
| Spain | 300 | 7.0 | 4.4–10.5 | 4.7 | 2.6–7.7 | 1.0 | 0.2–2.9 | 0.0 | 0.0–1.2 | 3.0 | 1.4–5.6 | 0.7 | 0.1–2.4 | 0.0 | 0.0–1.2 |
| Sweden | 286 | 0.7 | 0.1–2.5 | 0.7 | 0.1–2.5 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 | 0.0 | 0.0–1.3 |
| United Kingdom | 314 | 0.6 | 0.1–2.3 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Total (28 MSs) | 6,621 | 4.8 | 4.0–5.0 | 3.9 | 3.2–4.1 | 1.4 | 0.1–1.6 | 0.02 | 0.0–0.1 | 1.1 | 0.8–1.3 | 0.1 | 0.0–0.2 | 0.0 | 0.0–0.1 |
| Iceland | 95 | 0.0 | 0.0–3.8 | 0.0 | 0.0–3.8 | 0 | 0.0–3.8 | 0.0 | 0.0–3.8 | 0.0 | 0.0–3.8 | 0.0 | 0.0–3.8 | 0.0 | 0.0–3.8 |
| Norway | 343 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 | 0.0 | 0.0–1.1 |
| Switzerland | 299 | 0.7 | 0.1–2.4 | 0.3 | 0.0–1.8 | 0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.3 | 0.0–1.8 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
ESBL: extended‐spectrum β‐lactamase; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; P: prevalence; CI: confidence interval; Ns: total number of samples.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing MIC > 1 mg/L for CTX and/or CAZ (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with CTX or CAZ or synergy with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to FOX, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with FOX or CAZ and microbiological resistance to FOX, suggesting ESBL and AmpC enzymes in the same isolate. ESBL and AmpC columns include those isolates.
Isolates with microbiological MEM resistance.
Four countries (Estonia, Finland, Iceland and Norway) did not detect presumptive ESBL, AmpC or ESBL + AmpC producing E. coli in any samples. In the majority of the remaining countries the prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates was very low (France, Ireland, Sweden, the United Kingdom and Switzerland) or low (18 MSs). In four MSs (Bulgaria, the Czech Republic, Malta and Portugal) the prevalence was moderate. The generally low prevalence makes the variations among countries small also when the prevalence of the ESBL and AmpC phenotypes are assessed separately (Figure 82).
Figure 82.
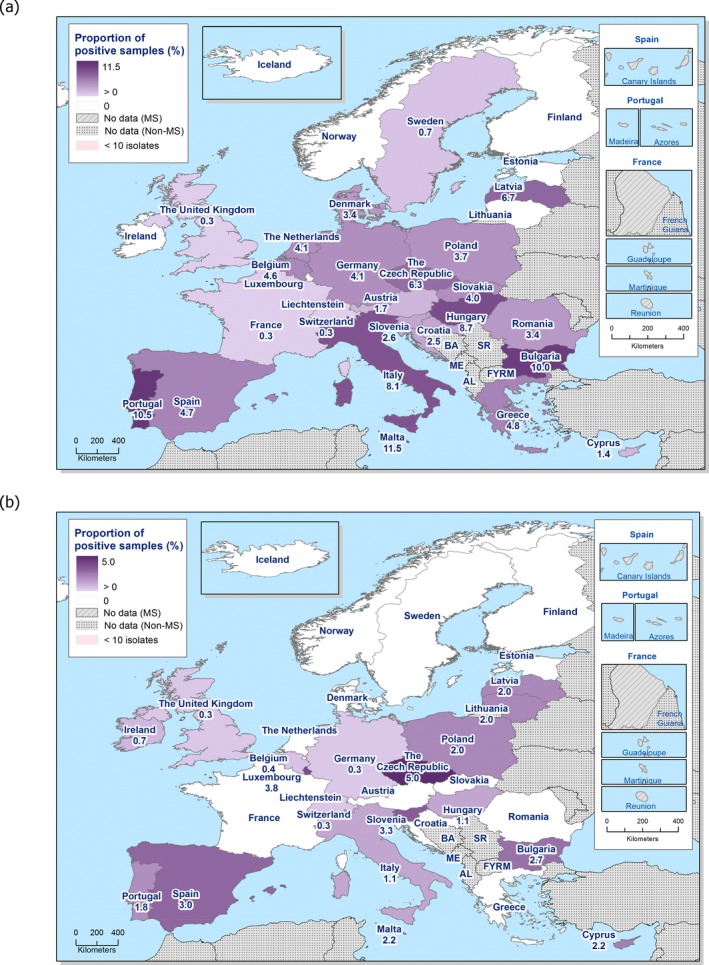
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli isolates in bovine meat, assessed by the specific ESBL‐/AmpC‐/carbapenemase‐producing monitoring, 28 EU MSs and 3‐non MSs, 2017
The occurrence of presumptive ESBL‐producing E. coli among the third‐generation cephalosporin‐resistant isolates (304 isolates tested with Panel 2 by the MSs) from meat from bovines collected within this specific monitoring by the MSs was 78.0% (Table 47). Such isolates were detected in samples from all countries reporting third‐generation cephalosporin‐resistant isolates except Ireland, Lithuania and Luxembourg. The occurrence of presumptive AmpC‐producing E. coli isolates from meat from bovines collected within this specific monitoring by the MS was 22.0%. These isolates were detected in samples from all countries reporting cephalosporin‐resistant isolates except Austria, Croatia, Denmark, France, Greece, the Netherlands, Romania, Slovakia and Sweden. Here, 2.3% of the E. coli isolates from meat from bovines mentioned above, reported by six MSs, presented an ‘ESBL + AmpC’ phenotype. Six (2.0%) of the isolates tested on Panel 2 did not show ESBL, AmpC or ESBL + AmpC phenotype according to the EUCAST guidelines.
Table 47.
Occurrence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates from bovine meat collected within the specific ESBLs/AmpC/Carbapenemase‐producing monitoring and subjected to supplementary testing in 2017a
| Country | NP2 | ESBL and/or AmpCa | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | ||
| Austria | 5 | 5 | 100.0 | 5 | 100.0 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Belgiumi | 15 | 13 | 81.3 | 13 | 81.3 | 3 | 18.8 | 0 | 0.0 | 1 | 6.3 | 1 | 6.3 | 0 | 0.0 |
| Bulgaria | 19 | 19 | 100.0 | 15 | 78.9 | 8 | 42.1 | 0 | 0.0 | 4 | 21.1 | 0 | 0.0 | 0 | 0.0 |
| Croatia | 4 | 4 | 100.0 | 4 | 100.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Cyprus | 5 | 5 | 100.0 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 3 | 60.0 | 0 | 0.0 | 0 | 0.0 |
| Czech Republic | 35 | 34 | 97.1 | 19 | 54.3 | 12 | 34.3 | 0 | 0.0 | 15 | 42.9 | 0 | 0.0 | 0 | 0.0 |
| Denmark | 10 | 10 | 100.0 | 10 | 100.0 | 4 | 40.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| France | 1 | 1 | 100.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Germany | 14 | 14 | 100.0 | 13 | 92.9 | 5 | 35.7 | 0 | 0.0 | 1 | 7.1 | 0 | 0.0 | 0 | 0.0 |
| Greece | 3 | 3 | 100.0 | 3 | 100.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hungary | 18 | 18 | 100.0 | 16 | 88.9 | 7 | 38.9 | 0 | 0.0 | 2 | 11.1 | 0 | 0.0 | 0 | 0.0 |
| Ireland | 2 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Italy | 24 | 24 | 100.0 | 22 | 91.7 | 10 | 41.7 | 0 | 0.0 | 3 | 12.5 | 1 | 4.2 | 0 | 0.0 |
| Latvia | 12 | 12 | 100.0 | 10 | 83.3 | 5 | 41.7 | 0 | 0.0 | 3 | 25.0 | 1 | 8.3 | 0 | 0.0 |
| Lithuania | 3 | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Luxembourg | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Maltai | 25 | 24 | 96.0 | 21 | 84.0 | 5 | 20.0 | 1 | 4.0 | 4 | 16.0 | 1 | 4.0 | 0 | 0.0 |
| Netherlands | 18 | 18 | 100.0 | 18 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Poland | 17 | 17 | 100.0 | 11 | 64.7 | 5 | 29.4 | 0 | 0.0 | 6 | 35.3 | 0 | 0.0 | 0 | 0.0 |
| Portugal | 26 | 26 | 100.0 | 23 | 88.5 | 8 | 30.8 | 0 | 0.0 | 4 | 15.4 | 1 | 3.8 | 0 | 0.0 |
| Romania | 5 | 5 | 100.0 | 5 | 100.0 | 2 | 40.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Slovakia | 6 | 6 | 100.0 | 6 | 100.0 | 3 | 50.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Sloveniai | 10 | 9 | 90.0 | 4 | 40.0 | 1 | 10.0 | 0 | 0.0 | 5 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Spain | 21 | 21 | 100.0 | 14 | 66.7 | 3 | 14.3 | 0 | 0.0 | 9 | 42.9 | 2 | 9.5 | 0 | 0.0 |
| Swedeni | 3 | 2 | 66.7 | 2 | 66.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| United Kingdom | 2 | 2 | 100.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
| Total (26 MSs) | 304 | 298 | 97.7 | 238 | 78.0 | 85 | 27.9 | 1 | 0.3 | 67 | 22.0 | 7 | 2.3 | 0 | 0.0 |
| Switzerland | 2 | 2 | 100.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 | 1 | 50.0 | 0 | 0.0 | 0 | 0.0 |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States.
Several countries reported only a few isolates. For countries reporting less than 10 isolates, occurrence data should be carefully considered.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Percentage of the total number of E. coli isolates tested (with panel 2).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
Molecular data were provided by
Denmark, ESBLs: 9 CTX‐M (4 CTX‐M‐1, 3 CTX‐M‐14, 2 CTX‐M‐15), 1 TEM‐52
Italy, ESBLs: 22 CTX‐M
Netherlands: ESBL: 19 CTX‐M (3 CTX‐M‐1, 2 CTX‐M‐2, 6 CTX‐M‐15, 4 CTX‐M‐32, 3 CTX‐M‐55, 1 CTX‐M‐65), 1 TEM‐52.
Sweden, ESBLs: 2 CTX‐M (1 CTX‐M‐15, 1 CTX‐M27)
Latvia reported 10 isolates ESBL pheno/genotype, 1 isolate with ESBL+ AmpC pheno/genotype (no genes reported).
The detection of ESBL‐producing E. coli exceeded that of AmpC‐producing E. coli in most of the reporting countries. However, as many countries only report a low number of isolates the differences should be assessed with caution.
Concurrent resistance among the third‐generation cephalosporin‐resistant isolates varied markedly among substances and countries. The overall (lowest–highest) occurrences among the 28 reporting MSs for the different substances were: azithromycin 5.6% (0–26.9%), chloramphenicol 38.0% (0–81.3%), ciprofloxacin 52.1% (0–100%), colistin 2.6% (0–33.3%), gentamicin 15.4% (0–33.3%), meropenem 0%, nalidixic acid 36.7% (0–100%), sulfamethoxazole 74.4% (50–100%), tetracycline 68.9% (33.3–100%), tigecycline 0% and trimethoprim 50.8% (0–100%). However, as many countries only report a low number of isolates the variations should be assessed with caution.
Presumptive carbapenemase‐producing E. coli from bovine meat
None of the isolates from the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli displayed elevated MIC for meropenem. When tested on Panel 2, one isolate (from Germany) displayed elevated MIC for imipenem (MIC = 1 mg/L), one isolate (from Poland) displayed elevated MIC for imipenem and ertapenem and eight isolates (from 5 MSs) displayed elevated MIC for ertapenem. Further investigation of these isolates is ongoing.
6.4.4. Specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring in cattle under 1 year of age (calves)
Ten MSs (Austria,22 Belgium, Croatia, Denmark, France, Germany, Italy, the Netherlands, Portugal and Spain) as well as Norway and Switzerland reported data for the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli isolated from cattle under 1 year (Tables ESCHECALVESBL and ESCHECALVESBL2).
Presumptive ESBL‐/AmpC‐producing E. coli from cattle under 1 year
The 10 MSs reporting data together tested 3,113 samples from cattle under 1 year and, following culture on selective media, 44.5% yielded presumptive ESBL, AmpC or ESBL + AmpC producing E. coli (41.5% with ESBL, 6.0% with AmpC, and 3.0% with ESBL + AmpC phenotype, Table 48). The results from 607 additional samples were reported by the two non‐MSs.
Table 48.
Prevalence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates in cattle under 1 year of age, 10 EU MSs and 2 non‐MSs, 2017a
| Country | Ns | ESBL and/or AmpCa | ESBLb | ESBL only CTX/CLA SYNc | ESBL only CAZ/CLA SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | %P | 95% CI | ||
| Austria | 303 | 22.4 | 17.9–27.6 | 20.5 | 16.1–25.4 | 2.3 | 0.9–4.7 | 0.0 | 0.0–1.2 | 2 | 0.7–4.3 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Belgium | 300 | 67.3 | 61.8–72.4 | 64.8 | 59.1–69.9 | 15.5 | 9.1–17.0 | 0.0 | 0.0–1.2 | 6.9 | 3.3–8.9 | 4.5 | 1.8–6.5 | 0.0 | 0.0–1.2 |
| Croatia | 354 | 18.6 | 14.7–23.1 | 10.5 | 7.5–14.1 | 3.3 | 0.8–4.0 | 0.5 | 0.0–1.6 | 8.1 | 2.8–7.6 | 0.0 | 0.0–1.0 | 0.0 | 0.0–1.0 |
| Denmark | 297 | 7.1 | 4.4–10.6 | 3.7 | 1.9–6.5 | 2.7 | 1.2–5.2 | 0.0 | 0.0–1.2 | 3.4 | 1.6–6.1 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| France | 299 | 39.5 | 33.9–45.3 | 32.4 | 27.2–38.1 | 7.7 | 4.9–11.3 | 0.3 | 0.0–1.8 | 8.7 | 5.8–12.5 | 1.7 | 0.5–3.9 | 0.0 | 0.0–1.2 |
| Germany | 350 | 67.7 | 60.5–70.7 | 66.8 | 59.6–69.9 | 15.0 | 11.0–18.7 | 0.0 | 0.0–1.0 | 7.1 | 4.4–10.0 | 6.2 | 3.8–9.0 | 0.0 | 0.0–1.0 |
| Italy | 319 | 89.0 | 85.1–92.2 | 86.8 | 82.6–90.3 | 22.6 | 18.1–27.6 | 1.6 | 0.5–3.6 | 7.9 | 5.1–11.4 | 5.6 | 3.4–8.8 | 0.0 | 0.0–1.1 |
| Netherlands | 302 | 37.7 | 32.3–43.5 | 36.4 | 31.0–42.1 | 8.3 | 5.4–12.0 | 0.0 | 0.0–1.2 | 4.6 | 2.6–7.7 | 3.3 | 1.6–6.0 | 0.0 | 0.0–1.2 |
| Portugal | 289 | 37.0 | 31.4–42.9 | 36.7 | 31.1–42.5 | 1.4 | 0.4–3.5 | 0.0 | 0.0–1.3 | 1.4 | 0.4–3.5 | 1.0 | 0.2–3.0 | 0.0 | 0.0–1.3 |
| Spain | 300 | 55.3 | 49.5–61 | 50.7 | 44.9–56.5 | 9.4 | 6.3–13.2 | 0.0 | 0.0–1.2 | 11.3 | 8.0–15.5 | 6.7 | 4.1–10.1 | 0.0 | 0.0–1.2 |
| TOTAL (10 MSs) | 3,113 | 44.5 | 42.7–46.3 | 41.5 | 39.9–43.4 | 8.9 | 7.5–9.5 | 0.2 | 0.1–0.5 | 6.0 | 4.9–6.6 | 3.0 | 2.3–3.5 | 0.0 | 0.0–0.12 |
| Norway | 303 | 4.6 | 2.5–7.6 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 | 4.6 | 2.5–7.6 | 0.0 | 0.0–1.2 | 0.0 | 0.0–1.2 |
| Switzerland | 304 | 30.3 | 25.1–35.8 | 19.1 | 14.8–24 | 5.9 | 3.5–9.2 | 0.0 | 0.0–1.2 | 13.2 | 9.6–17.5 | 2.0 | 0.7–4.2 | 0.0 | 0.0–1.2 |
ESBL: extended‐spectrum β‐lactamase; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; P: prevalence; CI: confidence interval; MSs: Member States; Ns: total number of samples.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing MIC > 1 mg/L for CTX and/or CAZ (screening breakpoint) were considered (see Section 2 Materials and methods.
All isolates showing clavulanate synergy with CTX or CAZ or synergy with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with CTX or CAZ and microbiological resistance to FOX, suggesting ESBL and AmpC enzymes in the same isolate. ESBL and AmpC columns include those isolates.
Isolates with microbiological meropenem resistance.
Isolates with ESBL, AmpC or ESBL + AmpC phenotype were reported from all countries. Nevertheless, marked variations were observed in the prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates. It ranged from 4.6% in Norway and 7.1% in Denmark, up to 89.0% in Italy. The variations among countries is mainly due to differences in prevalence of ESBL, as seen when the prevalence of the ESBL and AmpC phenotypes are assessed separately (Figure 83).
Figure 83.
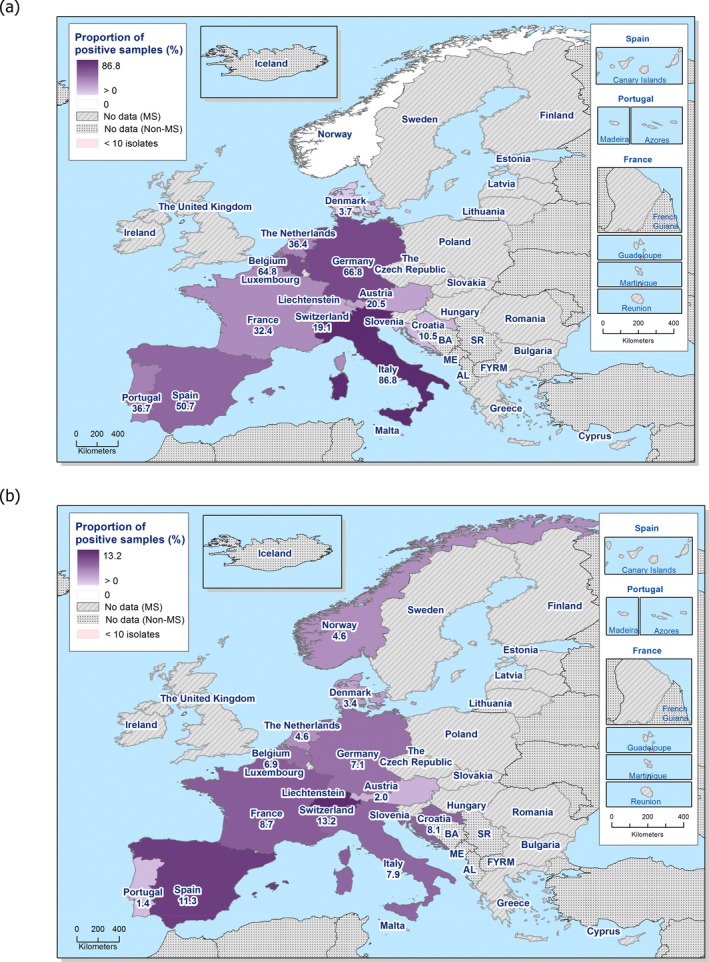
Prevalence of presumptive ESBL‐producing (a) and AmpC‐producing (b) E. coli isolates in cattle under 1 year of age, assessed by the specific ESBL‐/AmpC‐/carbapenemase‐producing E. coli monitoring, 10 EU MSs and 2 non‐MSs, 2017
The occurrence of presumptive ESBL‐producing E. coli among the third‐generation cephalosporin‐resistant isolates (1,326 isolates tested with Panel 2 by the MSs) from cattle under 1 year collected within this specific monitoring by the MSs was 92.2% (Table 49). Such isolates were detected in samples from all countries except the non‐MS Norway. The occurrence of presumptive AmpC‐producing E. coli isolates from cattle under 1 year collected within this specific monitoring by the MS was 13.3%. Such isolates were detected in samples from all countries. Here, 6.6% of the E. coli isolates from cattle under 1 year mentioned above, reported by 7 MSs as well as Switzerland, presented an ‘ESBL + AmpC’ phenotype. Fifteen (1.1%) of the isolates tested on Panel 2 did not show ESBL, AmpC or ESBL + AmpC phenotype according to the EUCAST guidelines.
Table 49.
Occurrence of presumptive ESBL‐ and/or AmpC‐producing E. coli isolates from cattle under 1 year of age collected within the specific ESBLs/AmpC/Carbapenemase‐producing monitoring and subjected to supplementary testing in 2017a
| Country | NP2 | ESBL and/or AmpCa | ESBLb | ESBL only CLA/CTX SYNc | ESBL only CLA/CAZ SYNd | AmpCe | AmpC + ESBLf | CPsg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | n | %h | ||
| Austria | 68 | 68 | 100 | 62 | 91.2 | 7 | 10.3 | 0 | 0 | 6 | 8.8 | 0 | 0 | 0 | 0 |
| Belgiumi | 170 | 165 | 97.1 | 159 | 93.5 | 38 | 22.4 | 0 | 0 | 17 | 10 | 11 | 6.5 | 0 | 0 |
| Croatia | 42 | 39 | 92.9 | 22 | 52.4 | 7 | 16.7 | 1 | 2.4 | 17 | 40.5 | 0 | 0 | 0 | 0 |
| Denmarki, j | 22 | 21 | 95.5 | 11 | 50 | 8 | 36.4 | 0 | 0 | 10 | 45.5 | 0 | 0 | 0 | 0 |
| France | 119 | 118 | 99.2 | 97 | 81.5 | 23 | 19.3 | 1 | 0.8 | 26 | 21.8 | 5 | 4.2 | 0 | 0 |
| Germanyi | 231 | 230 | 99.6 | 227 | 98.3 | 51 | 22.1 | 0 | 0 | 24 | 10.4 | 21 | 9.1 | 0 | 0 |
| Italyi, j | 285 | 284 | 99.6 | 277 | 97.2 | 72 | 25.3 | 5 | 1.8 | 25 | 8.8 | 18 | 6.3 | 0 | 0 |
| Netherlandsj | 114 | 114 | 100 | 110 | 96.5 | 25 | 21.9 | 0 | 0 | 14 | 12.3 | 10 | 8.8 | 0 | 0 |
| Portugal | 107 | 107 | 100 | 106 | 99.1 | 4 | 3.7 | 0 | 0 | 4 | 3.7 | 3 | 2.8 | 0 | 0 |
| Spaini | 168 | 166 | 98.8 | 152 | 90.5 | 28 | 16.7 | 0 | 0 | 34 | 20.2 | 20 | 11.9 | 0 | 0 |
| Total (10 MSs) | 1,326 | 1,312 | 98.9 | 1,223 | 92.2 | 263 | 19.8 | 7 | 0.5 | 177 | 13.3 | 88 | 6.6 | 0 | 0 |
| Norway | 16 | 14 | 87.5 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 87.5 | 0 | 0 | 0 | 0 |
| Switzerlandi | 101 | 92 | 91.1 | 58 | 57.4 | 18 | 17.8 | 0 | 0 | 40 | 39.6 | 6 | 5.9 | 0 | 0 |
ESBL: extended‐spectrum β‐lactamase; n: isolates with this phenotype; %: percentage of isolates from the total tested; SYN: synergy; CTX: cefotaxime; CAZ: ceftazidime; CLA: clavulanate; MSs: Member States.
According to EUCAST Guidelines (EUCAST, 2013), only isolates showing an MIC > 1 mg/L for cefotaxime and/or ceftazidime (screening breakpoint) were considered (see Section 2 Materials and methods).
All isolates showing clavulanate synergy with cefotaxime, ceftazidime or with both compounds, suggesting the presence of an ESBL (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime only, suggesting the presence of an ESBL with cefotaximase activity.
Isolates showing synergy with ceftazidime only, suggesting the presence of an ESBL with ceftazidimase activity.
Isolates with microbiological resistance to cefoxitin, suggesting the presence of an AmpC enzyme (independently of the presence of other mechanisms).
Isolates showing synergy with cefotaxime or ceftazidime and with microbiological resistance to cefoxitin, suggesting the presence of ESBL and AmpC enzymes in the same isolate. These isolates are also included in the ESBL and AmpC columns.
Isolates with microbiological meropenem resistance.
Percentage of the total number of Salmonella spp. isolates tested (with panel 1).
It includes isolates microbiologically resistant to cefotaxime and/or ceftazidime but with MIC ≤ 1 mg/L for both antimicrobials, suggesting the presence of other mechanisms (as stated above, they were not further classified).
Molecular data were provided by
Denmark, ESBLs: 9 CTX‐M (4 CTX‐M‐1, 3 CTX‐M‐14, 2 CTX‐M‐15), 1 TEM‐52
Italy, ESBLs: 266 CTX‐M, 10 SHV‐12, ESBL + AmpC: 1 CTX‐M + CMY‐2
Netherlands: ESBL: 110 CTX‐M (49 CTX‐M‐1, 5 CTX‐M‐14, 34 CTX‐M‐15, 1 CTX‐M‐2, 6 CTX‐M‐32, 2 CTX‐M‐55, 4 CTX‐M‐65, 1 CTX‐M‐9), 3 SHV‐12, 5 TEM‐52.
The detection of ESBL‐producing E. coli exceeded that of AmpC‐producing E. coli in most of the reporting countries except for Norway and where the AmpC phenotype dominated, and in Denmark where the prevalence of the ESBL and the AmpC phenotypes were comparable. However, as some countries only report a low number of isolates the differences should be assessed with caution.
Concurrent resistance among the third‐generation cephalosporin‐resistant isolates varied markedly among substances and countries. The overall (lowest–highest) occurrences among the 10 reporting MSs for the different substances were: azithromycin 9.1% (0–15.9%), chloramphenicol 40.6% (4.5–61.3%), ciprofloxacin 57.2% (0–82.5%), colistin 1.7% (0–3.0%), gentamicin 23.4% (9.1–38.1%), meropenem 0%, nalidixic acid 30.3% (0–61.3%), sulfamethoxazole 70.7% (22.7–89.5%), tetracycline 79.9% (22.7–94.7%), tigecycline 0.1% (0–0.9%) and trimethoprim 60.9% (9.1–85.3%). However, as some countries only report a low number of isolates, the variations should be assessed with caution.
Presumptive carbapenemase‐producing E. coli from cattle under 1 year of age
None of the isolates from the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli displayed elevated MIC for meropenem. When tested on panel 2, two isolates (from Belgium and Italy) displayed elevated MIC for imipenem and 23 isolates (from 5 MSs) displayed elevated MIC for ertapenem. Further investigation of these isolates is ongoing.
6.5. Voluntary specific monitoring of carbapenemase‐producing E. coli
The specific monitoring of carbapenemase‐producing microorganisms in fattening pigs, meat from pigs and meat from bovines and fattening pigs was performed and reported by 18 (fattening pigs) or 17 MSs (meat from pigs and meat from bovines) and Switzerland on a voluntary basis in 2017, according to the Commission Implementing Decision 2013/652/EU (Table 50). Eight of the MSs and Switzerland also reported monitoring of carbapenemase‐producing microorganisms in cattle under 1 year. Furthermore, the Netherlands also reported data from their national monitoring performed using different isolations protocols (see text box below). All reporting countries focused on the isolation of E. coli.
Table 50.
Prevalence of presumptive carbapenemase‐producing E. coli in meat from pigs, fattening pigs, bovine meat and cattle under 1 year of age collected – voluntary specific carbapenemase‐producing monitoring, 18 EU MSs and 1 non‐MS, 2017
| Countrya | Animal population / Meat | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pig Meat – fresh | Fattening Pigs | Bovine Meat ‐ fresh | Cattle – calves, < 1 year old | |||||||||||||
| N | nCP+ | %P | 95% CI | N | nCP+ | %P | 95% CI | N | nCP+ | %P | 95% CI | N | nCP+ | %P | 95% CI | |
| Austria | 302 | 0 | 0 | 0–1.2 | 283 | 0 | 0 | 0–1.3 | 297 | 0 | 0 | 0–1.2 | 300 | 0 | 0 | 0–1.2 |
| Belgium | 299 | 0 | 0 | 0–1.2 | 297 | 0 | 0 | 0–1.2 | 300 | 0 | 0 | 0–1.2 | 300 | 0 | 0 | 0–1.2 |
| Croatia | 156 | 0 | 0 | 0–2.3 | 369 | 0 | 0 | 0–1.0 | 161 | 0 | 0 | 0–2.3 | 354 | 0 | 0 | 0–1.0 |
| Czech Republic | 298 | 0 | 0 | 0–1.2 | 301 | 0 | 0 | 0–1.2 | 301 | 0 | 0 | 0–1.2 | – | 0 | – | – |
| Denmark | 281 | 0 | 0 | 0–1.3 | 295 | 0 | 0 | 0–1.2 | 292 | 0 | 0 | 0–1.3 | 297 | 0 | 0 | 0–1.2 |
| Estonia | 150 | 0 | 0 | 0–2.4 | 68 | 0 | 0 | 0–5.3 | 150 | 0 | 0 | 0–2.4 | – | 0 | – | – |
| Finland | 301 | 0 | 0 | 0–1.2 | 299 | 0 | 0 | 0–1.2 | 302 | 0 | 0 | 0–1.2 | – | 0 | – | – |
| France | 324 | 0 | 0 | 0–1.1 | 327 | 0 | 0 | 0–1.1 | 324 | 0 | 0 | 0–1.1 | 315 | 0 | 0 | 0–1.2 |
| Germany | 454 | 0 | 0 | 0–0.8 | 354 | 0 | 0 | 0–1.1 | 399 | 0 | 0 | 0–0.9 | 349 | 0 | 0 | 0–1.1 |
| Greece | 133 | 0 | 0 | 0–2.7 | 170 | 0 | 0 | 0–2.1 | 62 | 0 | 0 | 0–5.8 | – | 0 | – | – |
| Hungary | 298 | 0 | 0 | 0–1.2 | 249 | 0 | 0 | 0–1.5 | 184 | 0 | 0 | 0–2.0 | – | 0 | – | – |
| Ireland | 300 | 0 | 0 | 0–1.2 | 300 | 0 | 0 | 0–1.2 | 300 | 0 | 0 | 0–1.2 | – | 0 | – | – |
| Italy | 272 | 0 | 0 | 0–1.3 | 302 | 0 | 0 | 0–1.2 | 272 | 0 | 0 | 0–1.3 | 319 | 0 | 0 | 0–1.1 |
| Portugal | 220 | 0 | 0 | 0–1.7 | 254 | 0 | 0 | 0–1.4 | 220 | 0 | 0 | 0–1.7 | 289 | 0 | 0 | 0–1.3 |
| Slovenia | 151 | 0 | 0 | 0–2.4 | 152 | 0 | 0 | 0–2.4 | 151 | 0 | 0 | 0–2.4 | – | 0 | – | – |
| Poland | – | – | – | – | 306 | 0 | 0 | 0–1.2 | – | – | – | – | – | – | – | – |
| Sweden | 295 | 0 | 0 | 0–1.2 | 241 | 0 | 0 | 0–1.5 | 286 | 0 | 0 | 0–1.3 | – | 0 | – | – |
| United Kingdom | 310 | 0 | 0 | 0–1.2 | 347 | 0 | 0 | 0–1.1 | 314 | 0 | 0 | 0–1.2 | – | 0 | – | – |
| Total (18 MSs) | 4,544 | 0 | 0 | 0–0.1 | 4,914 | 0 | 0 | 0–0.1 | 4,315 | 0 | 0 | 0–0.1 | 2,523 | 0 | 0 | 0–0.1 |
| Switzerland | 302 | 0 | 0 | 0–1.2 | 296 | 0 | 0 | 0–1.2 | 299 | 0 | 0 | 0–1.2 | 304 | 0 | 0 | 0–1.2 |
N: Number of samples tested on selective culture media; nCP+: number of samples positive for presumptive carbapenemase‐producing E. coli; –: not reported; P: prevalence; CI: confidence interval.
The Netherlands also reported data from their specific carbapenemase monitoring performed with different protocol. These data are shown in a separate text box below.
Together, the 19 countries investigated 4,846 samples from meat from pigs, 5,210 samples from fattening pigs, 4,614 samples from meat from bovines, and 2,827 samples from cattle under 1 year. This gives a grand total of 17,497 samples, all of which were negative for carbapenemase‐producing E. coli.
Carbapenemase‐producing Enterobacteriaceae in the Netherlands (kindly provided by the Netherlands)
Monitoring in farm animals in the Netherlands
Since 2015, a sensitive molecular method is applied to screen for producers, ESBLs that can also hydrolyse carbapenems. This screening was described in more detail in the EUSR‐AR for 2016 (EFSA and ECDC, 2018a) and in MARAN 2016 (MARAN, 2016).
In 2017, this screening was performed on 1,200 samples (Table 51) and resulted in six bla OXA‐48‐like positive faecal samples in the RT‐PCR (three broilers, two slaughter pigs and one dairy cow). bla OXA ‐48‐like genes are known to be chromosomally associated with Shewanella spp. In three samples the presence of bla OXA‐48‐carrying Shewanella was confirmed by bacterial culturing followed by PCR and sequencing: bla OXA‐48b (n = 2) and bla OXA‐199 (n = 1). These results confirm the findings of previous years, as no carbapenemase‐producing Enterobacteriaceae were isolated from livestock in the Netherlands. bla OXA‐48‐like genes have also been found in faecal samples in 2013, 2014, 2015 and 2016 (MARAN 2016 and 2017). Given the role of Shewanella spp. as a natural progenitor of this carbapenemase family (Zong, 2012), these genes were considered of environmental origin and not a public health risk. Screening for carbapenemase‐producing isolates in faecal samples of food‐producing animals will continue in 2018.
Table 51.
Meticillin‐resistant Staphylococcus aureus in food, 2017
| Country | Production type/monitoring description (where specified)) | Sample unit | Number | |
|---|---|---|---|---|
| Units tested | Positive for MRSA (%) | |||
| Confectionery products and pastes | ||||
| Slovakia | Retail Surveillance | Batch | 4 | 0 |
| Single | 24 | 0 | ||
| Dairy products (excluding cheeses) | ||||
| Slovakia | Ice‐cream – Retail Surveillance | Single | 50 | 0 |
| Infant formula | ||||
| Slovakia | Dried – Retail Surveillance | Batch | 14 | 0 |
| Single | 10 | 0 | ||
| Ready‐to‐eat – Hospital or medical care Facility Surveillance | Batch | 2 | 0 | |
| Meat from bovine animals | ||||
| Germany | Fresh veal – Retail Monitoring ‐ active | Single | 354 | 40 (11.3%)* |
| Minced meat – Retail Monitoring ‐ active | Single | 289 | 20 (6.9%)* | |
| Slovakia | Meat preparation – Catering Surveillance | Single | 12 | 0 |
| Switzerland | Retail Monitoring | Single | 299 | 0 |
| Meat from pigs | ||||
| Finland | Fresh – Retail Survey – National Survey | Batch | 220 | 13 (5.9%)a |
| Slovakia | Meat preparation – Catering Surveillance | Single | 39 | 0 |
| Meat preparation – Retail Surveillance | Single | 6 | 0 | |
| Meat products – Catering Surveillance | Single | 7 | 0 | |
| Spain | Fresh – Retail Surveillance | Single | 60 | 2 (3.3%)* |
| Switzerland | Fresh – Retail Monitoring | Single | 301 | 2 (0.7%) b |
| Meat from poultry, unspecified | ||||
| Slovakia | Meat preparation – Catering Surveillance | Single | 69 | 0 |
| Meat from rabbit | ||||
| Spain | Fresh – Retail Surveillance | Single | 75 | 3 (4.0%)* |
| Other processed food products and prepared dishes | ||||
| Slovakia | Catering Surveillance | Batch | 5 | 0 |
| Single | 125 | 0 | ||
| Hospital or medical care facility Surveillance | Single | 9 | 0 | |
| Ices and similar frozen desserts – Retail Surveillance | Batch | 5 | 0 | |
| Single | 32 | 0 | ||
| Noodles – Catering Surveillance | Single | 11 | 0 | |
| Noodles – Hospital or medical care facility Surveillance | Single | 2 | 0 | |
| Sandwiches – Catering Surveillance | Batch | 12 | 0 | |
| Single | 8 | 0 | ||
| Vegetables | ||||
| Slovakia | Precut – Catering Surveillance | Single | 48 | 0 |
spa‐types: t034 (11 isolates), t011 (1), t2741 (1).
spa‐types: t011 (1 isolate), t002 (1). PVL status of the t002 isolate was not reported.
spa‐types not reported.
Overview of results using RT‐PCR screening for carbapenemase‐resistant Enterobacteriaceae in faecal samples of livestock in 2017 in the Netherlands
| Animal species | Number of samples screened for CPE | Number of samples positive for CPE | Prevalence (%) (95% CI) |
| Broilers | 301 | 0 | 0 (0–1.22) |
| Dairy cattle | 300 | 0 | 0 (0–1.22) |
| Veal calves | 302 | 0 | 0 (0–1.21) |
| Fattening pigs | 297 | 0 | 0 (0–1.23) |
CPE: carbapenemase‐producing Enterobacteriaceae.
Table 51.
Meticillin‐resistant Staphylococcus aureus in food, 2017
| Country | Production type/monitoring description (where specified)) | Sample unit | Number | |
|---|---|---|---|---|
| Units tested | Positive for MRSA (%) | |||
| Confectionery products and pastes | ||||
| Slovakia | Retail Surveillance | Batch | 4 | 0 |
| Single | 24 | 0 | ||
| Dairy products (excluding cheeses) | ||||
| Slovakia | Ice‐cream – Retail Surveillance | Single | 50 | 0 |
| Infant formula | ||||
| Slovakia | Dried – Retail Surveillance | Batch | 14 | 0 |
| Single | 10 | 0 | ||
| Ready‐to‐eat – Hospital or medical care Facility Surveillance | Batch | 2 | 0 | |
| Meat from bovine animals | ||||
| Germany | Fresh veal – Retail Monitoring ‐ active | Single | 354 | 40 (11.3%)* |
| Minced meat – Retail Monitoring ‐ active | Single | 289 | 20 (6.9%)* | |
| Slovakia | Meat preparation – Catering Surveillance | Single | 12 | 0 |
| Switzerland | Retail Monitoring | Single | 299 | 0 |
| Meat from pigs | ||||
| Finland | Fresh – Retail Survey – National Survey | Batch | 220 | 13 (5.9%)a |
| Slovakia | Meat preparation – Catering Surveillance | Single | 39 | 0 |
| Meat preparation – Retail Surveillance | Single | 6 | 0 | |
| Meat products – Catering Surveillance | Single | 7 | 0 | |
| Spain | Fresh – Retail Surveillance | Single | 60 | 2 (3.3%)* |
| Switzerland | Fresh – Retail Monitoring | Single | 301 | 2 (0.7%) b |
| Meat from poultry, unspecified | ||||
| Slovakia | Meat preparation – Catering Surveillance | Single | 69 | 0 |
| Meat from rabbit | ||||
| Spain | Fresh – Retail Surveillance | Single | 75 | 3 (4.0%)* |
| Other processed food products and prepared dishes | ||||
| Slovakia | Catering Surveillance | Batch | 5 | 0 |
| Single | 125 | 0 | ||
| Hospital or medical care facility Surveillance | Single | 9 | 0 | |
| Ices and similar frozen desserts – Retail Surveillance | Batch | 5 | 0 | |
| Single | 32 | 0 | ||
| Noodles – Catering Surveillance | Single | 11 | 0 | |
| Noodles – Hospital or medical care facility Surveillance | Single | 2 | 0 | |
| Sandwiches – Catering Surveillance | Batch | 12 | 0 | |
| Single | 8 | 0 | ||
| Vegetables | ||||
| Slovakia | Precut – Catering Surveillance | Single | 48 | 0 |
spa‐types: t034 (11 isolates), t011 (1), t2741 (1).
spa‐types: t011 (1 isolate), t002 (1). PVL status of the t002 isolate was not reported.
spa‐types not reported.
6.6. Additional information on carbapenemase producers provided voluntarily by MSs
CPE in German pig production (kindly provided by A. Irrgang, M. Grobbel, J‐A Hammerl and B‐A Tenhaguen)
In recent years, carbapenemase‐producing Enterobacteriaceae (CPE) were recovered only sporadically from German livestock. The first detection dates back to 2011, where different CPE were found in samples from pigs and their farm environments within a national research project (RESET, http://www.reset-verbund.de), which focused on the occurrence and transmission of ESBL‐ and pAmpC‐producing enterobacteria (Fischer et al., 2017b). At this time, this was the first detection of CPE in livestock globally. The carbapenemase‐producing S. Infantis and E. coli isolates recovered from the same pig fattening farm carried bla VIM‐1 on closely related IncHI2 plasmids (Falgenhauer et al., 2017). However, follow‐up studies 4 years later on the farm revealed no further occurrence of CPE at this farm (Roschanski et al., 2017). Due to the importance of the findings, the EURL‐AR and the German National Reference Laboratory for Antimicrobial Resistances (NRL‐AR) developed a method for CPE's detection for monitoring of food and livestock. The screening on CPE was implemented in the monitoring on AMR in zoonotic and commensal bacteria (Decision 2013/652/EU). Currently, the monitoring on CPE for EU Member States is voluntary. In Germany, the monitoring was introduced in 2014. Since 2016, it is part of the national monitoring on AMR.
In 2015, a carbapenemase‐producing E. coli was detected among isolates of the national monitoring on ESBL. The isolate originated from caecal content of a pig at slaughter and produced a VIM‐1 carbapenemase. Molecular dissection of the genetic basis of the isolate revealed a close relationship to the carbapenemase‐producing E. coli described in 2011 (Irrgang et al., 2017). Based on the available data, this isolate could be traced back to a farm, from which faecal and environmental samples were taken and investigated on CPE (especially E. coli and Salmonella) in spring 2016. There, further carbapenemase‐producing E. coli could be detected in several samples from different stables. The farmer was advised to apply proper cleaning and disinfection procedures between slaughter batches and to increase internal biosecurity to minimise cross‐contamination between stables. A follow‐up study on the farm 6 months after the first visit, revealed no further occurrence of CPE, which might be a result of a changing the piglet producer for other reasons. In the same period of time, a VIM‐1‐producing S. Infantis recovered from a diseased piglet, fattened at the same farm, was send to the German reference laboratory for Salmonella. In 2015, another VIM‐1 producing S. Infantis was isolated from pork minced meat. Both Salmonella strains showed high similarities to the isolates detected in 2011 (Borowiak et al., 2017).
In 2017, two novel VIM‐1‐producing isolates were found within the monitoring on AMR. One isolate was detected in the national monitoring program on CPE conducted in herds of fattening pigs. The second isolate was detected in the monitoring on ESBL according to CID 2013/652/EU in caecal contents of a pig at slaughter. In the specific monitoring on CPE, the sample of the same pig was negative. Both isolates differed substantially in their genetic basis but exhibited plasmid sequences that are closely related to the previously described bla VIM‐1 carrying plasmids from 2011 and 2015. In contrast to the E. coli VIM‐1 plasmids described in 2011, the plasmids from the isolates in 2017 were self‐transmissible as known from VIM‐1 plasmids harboured by the Salmonella isolates. Currently, follow‐up investigations on the two affected farms are performed.
In conclusion, previous and current microbiological investigations on fattening pig farms showed that detection of CPE from faecal samples with a low load of CPE is challenging. Within the One Health European Joint Program (EJP) project IMPART, the German NRL‐AR works in collaboration with other institutes on an optimisation of the CPE detection method in faecal samples.
Monitoring in imported seafood in the Netherlands (kindly provided by Kees Veldman)
In 2017, 56 batches of frozen fish and shrimps originating from fish farms in South‐East Asia were screened for the presence of CPE. Two isolates of carbapenemase‐producing Enterobacter cloacae complex were detected in different batches of frozen shrimps, both exhibiting resistance to carbapenems but not to third‐generation cephalosporins. The first isolate originated from India (April 2017) and preliminary analysis suggests the presence of a new plasmid located carbapenemase gene (M. Brouwer, submitted for publication). The second isolate originated from Vietnam (August 2017) and harboured a chromosomally located bla IMI‐1 embedded in an insertion element (EcloIMEX) (Brouwer et al., 2018). Consumption of antimicrobials is high in South‐East Asia both in humans and in animals, and aquaculture represents an environment with high selective pressure for resistant bacteria, including CPE and potential for faecal contamination. Therefore, detection of CPE in imported food products from this area is not surprising.
6.7. Discussion
Third‐generation cephalosporins are antimicrobials of particular importance as they are frequently used as the first‐line treatment in invasive Gram‐negative infections in humans, for example infections caused by E. coli or Salmonella. In 2017, as in the previous years, resistance to third‐generation cephalosporins was generally detected at low levels in Salmonella and indicator E. coli isolates recovered from meat from pigs or bovines, fattening pigs, or cattle under 1 year using non‐selective detection methods. Using selective detection methods, E. coli with resistance to third‐generation cephalosporins could however readily be detected in caecal samples from both fattening pigs and cattle under 1 year, and the overall prevalence was high. The prevalence in samples of meat from pigs or bovines was however low also when using selective detection methods. This indicates that many of the animals carry E. coli with resistance to third‐generation cephalosporins in low numbers in their intestinal content, but the bacteria do not contaminate the carcases during the slaughter process, alternatively the bacteria do contaminate the carcases but are somehow removed later in the process.
When assessed using selective media, the prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates in the intestinal content of fattening pigs (43.8%) and cattle under 1 year (44.5%) is comparable with the prevalence in these animal categories reported in 2015 (40.1% and 39.6% respectively). It is also comparable with the prevalence in broilers and turkey reported in 2016 (47.4% and 42.2% respectively).
The fact that the prevalence of E. coli with resistance to third‐generation cephalosporins is low in both meat from pigs and meat from cattle under 1 year, also when assessed using selective media, indicate that this type of food is probably not an important source of such bacteria to humans. A lowered prevalence of E. coli with resistance to third‐generation cephalosporins in fattening pigs and cattle under 1 year would however still be preferable in order to mitigate the risk for animal and public health from this type of resistance as well as spread of this type of resistant bacteria to the environment.
The emergence and spread of microorganisms with acquired carbapenemases is of public health concern. Among all isolates investigated in 2017, only one E. coli with elevated MIC to meropenem (from a caecal sample from a fattening pig in Germany) were detected. That no more isolates were detected indicates that carbapenemase‐producing E. coli is still rare among fattening pigs and cattle under 1 year in Europe and hopefully incentives to preserve this situation can still be effective. It should however be kept in mind that the absolute sensitivity of the method has not been determined, although the detection limits, when validation of the method was performed, were low (between 1 and 100 cfu/g depending on the matrix and isolates used; see San José et al., 2014; Hasman et al., 2015b).
6.7.1.
Third‐generation cephalosporin and carbapenem resistance in Salmonella spp. from humans (voluntary testing and reporting)
Data on ESBL‐ and AmpC‐producing Salmonella in humans were reported from the national public health reference laboratories. While the monitoring of these enzymes is voluntary, ECDC recommends screening following a phenotypical testing algorithm based on the ‘EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance’ (ECDC, 2016; EUCAST, 2017b).
Of the 20 countries reporting microbiological resistance to either or both of the third‐generation cephalosporins included in the panel, 12 MSs and 1 non‐MS had performed testing for presence of ESBL and AmpC. ESBL‐producing Salmonella were identified in all but one of these countries at an average rate of 0.8%. Among the nine serovars reported with ESBL, the carriage was the highest in S. Kentucky, with 20% of tested isolates being ESBL producing. After a similar finding in the EFSA‐ECDC AMR report 2016, ECDC initiated an urgent inquiry in its Epidemic Intelligence System for Food‐ and Waterborne Diseases (EPIS‐FWD) in March 2018 to assess the situation in the EU. Nine countries had identified such isolates in recent years in either domestically acquired or travel‐associated cases and a phylodynamic study was initiated with the FWD‐Network based on WGS of the isolates. The study is ongoing and will try to reconstruct the time and place of emergence of this strain, carrying bla CTX‐M‐14b, which seems to have evolved from the high‐level ciprofloxacin resistant, multidrug‐resistant clone S. Kentucky ST198. The latter has spread rapidly throughout Europe and elsewhere in the world, both in humans (see e.g. Table 1 in Section 3.1 – Salmonella) and in the food chain and is considered to originally have been imported to Europe via travellers to North Africa (Le Hello et al., 2013). No ESBL‐producing S. Kentucky has to date been reported to EFSA or the EURL for AMR. Besides that the ESBL S. Kentucky strain is highly resistant to both critically antimicrobials for treatment of severe salmonellosis (and in addition resistant to amoxicillin, gentamicin, sulfonamides and tetracycline), WGS revealed that two of the Maltese isolates also carried carbapenemases, leaving few options for treatment of severe infections. Prolonged carriage was reported in several of the Maltese cases and in some, the gastrointestinal symptoms returned when treatment was stopped. This development is worrying, and it is of utmost importance that further spread between humans and into the food chain, if not already present, is prevented.
Two MSs reported ESBL in S. Enteritidis in 2017 but the proportion of ESBL producers was small in comparison to the total number of S. Enteritidis isolates. ESBL was more frequent in S. Typhimurium and monophasic S. Typhimurium 1,4,[5],12:i:‐ and a few isolates of AmpC‐producing S. Typhimurium and monophasic S. Typhimurium were also reported by four MSs. AmpC was generally less frequently reported in human isolates than ESBL but bla CMY‐2 was observed in S. Bredeney from both pig carcases (Lithuania, Portugal and Spain) and humans (Spain) in 2017. bla CMY‐2 have been reported in this serovar also previously (Liebana et al. 2004, González‐Sanz et al., 2009).
The fluoroquinolone‐resistant and ESBL‐producing S. Infantis clone with bla CTX‐M‐1 circulating in Italy since 2011 (see textbox EFSA and ECDC, 2018a,b) seemed to still be present in 2017 (personal communication C. Lucarelli, Istituto Superiore di Sanità, 20 September 2018). While no ESBL testing was performed on the human isolates reported to ECDC, 8 of the 26 (30.8%) Italian S. Infantis isolates were clinically resistant to both ciprofloxacin and third‐generation cephalosporins. This clone with MDR carried on a conjugative mosaic megaplasmid, emerged in Italian poultry in 2011 with human infections being reported in 2012 and then increasing (Franco et al., 2015; Dionisi et al., 2016). Belgium and the Netherlands also reported S. Infantis with bla CTX‐M‐1 from humans in 2017, as in 2016 (when also Austria reported cases). Further typing would be required to assess if these were of the same lineage as the Italian clone.
No meropenem resistance was reported in Salmonella isolates from humans in 2017 but as demonstrated above for S. Kentucky, there is a possibility that carbapenemase‐producing isolates are missed in the eight countries reporting meropenem data to ECDC interpreted with CBPs. As recommended by EUCAST, ECOFFs should be used as the screening breakpoint to detect carbapenemase‐producing Enterobacteriaceae (EUCAST, 2017b). The CBP for non‐susceptibility to meropenem (resistant and intermediate‐resistant categories) differs from the ECOFF by four dilutions for Salmonella. Low‐grade meropenem resistance would thus not be detected in countries applying CBPs.
Third‐generation cephalosporin and carbapenem resistance in Salmonella spp. from food and animals (routine monitoring)
In 2017, as in the previous years, third‐generation cephalosporin resistance in Salmonella spp. was very low or absent for most of the MSs. In fact, only four MSs reported Salmonella spp. with resistance to third‐generation cephalosporins from carcases of fattening pigs and no countries reported such isolates from carcases of bovines.
Resistance to carbapenems was not detected in any of the reported Salmonella spp. isolates.
The analysis of Salmonella spp. serovars with similar characteristics detected in humans, food, and food‐ producing animals can assist in source attribution and epidemiological investigations, as well as suggesting areas for investigation at the molecular level, which may confirm that isolates are closely related. In relation to this, it is interesting that S. Bredeney with AmpC phenotype were isolated from both pig carcases (in Lithuania, Portugal and Spain) and from humans (Spain) in 2017. bla CMY‐2 have been reported in this serovar also previously (Liebana et al. 2004; González‐Sanz et al., 2009).
Third‐generation cephalosporin and carbapenem resistance in indicator E. coli from food and animals (routine monitoring)
One randomly selected E. coli isolate from each non‐selective culture plate is examined for the routine AMR monitoring in commensal indicator E. coli. This approach enables the assessment of the proportion of randomly selected E. coli that is resistant to third‐generation cephalosporins, and their categorisation as presumptive ESBL/AmpC/carbapenemase producers. It provides a lower degree of sensitivity than that obtained using specific monitoring based on selective media, particularly where third‐generation cephalosporin‐resistant E. coli constitutes a small proportion of the total E. coli flora. The approach is still useful for consumers risk assessment, as it is considered that E. coli will be transferred along the food chain in a random fashion (EFSA, 2012a).
In 2017, the proportion of indicator E. coli isolates from fattening pigs and cattle under 1 year collected within the routine monitoring by the MSs considered as presumptive ESBL, AmpC or ESBL + AmpC producers was in general low to very low. In total, only 52 (1.2%) out of all 4,205 isolates from fattening pigs and 26 (1.4%) of all 1,893 isolates from cattle under 1 year were presumptive ESBL, AmpC or ESBL + AmpC producers. No countries reported a prevalence of presumptive ESBL, AmpC or ESBL + AmpC producers above 10%.
In general, the occurrence was comparable to the one reported for isolates collected within the 2015 routine monitoring (EFSA and ECDC, 2017a).
Only one country (Italy) reported data on indicator E. coli from food on a voluntary basis (1 isolate with third‐generation cephalosporins‐resistance among 169 isolates investigated). Hence, no overall assessment of the occurrence among the MSs can be made.
Specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli
For specific ESBL/AmpC/carbapenemase monitoring, culture methods using a non‐selective enrichment and a selective medium containing a third‐generation cephalosporin for the detection of ESBL‐/AmpC‐/carbapenemase‐producing E. coli were used, in accordance with the protocol proposed by the EURL‐AR (www. eurl‐ar.eu; San José et al., 2014; Hasman et al., 2015a; Zhao et al., 2015; Cavaco et al., 2016). The selective medium contains 1 mg/L of cefotaxime, the screening breakpoint recommended by EUCAST to maximise sensitivity and specificity of the detection of AmpC‐ and ESBL‐producing E. coli. The specific monitoring therefore employs culture of samples on selective media, which is able to detect very low numbers of resistant isolates present within a sample. The method enables the determination of the proportion of the total number of samples tested containing ESBL‐/AmpC‐/carbapenemase‐producing E. coli even when low numbers of such resistant E. coli are present. The sensitivity to detect the ESBL‐/AmpC‐/carbapenemase‐producing E. coli by this approach is higher than that obtained when performing the routine monitoring in which E. coli are randomly selected from the total E. coli population present, especially when investigating populations with a low prevalence of ESBL‐producing E. coli. If large numbers of AmpC‐producing E. coli are present in samples, they may obscure the concurrent presence of ESBL‐producing E. coli in the same samples and vice versa, because only one confirmed E. coli is subjected to further testing per sample. The proportion of AmpC‐producing vs ESBL‐producing E. coli present within a sample can therefore influence the culture result obtained. Within this monitoring, carbapenemase‐producing isolates resistant to third‐generation cephalosporins could also be identified, although the probability to identify them similarly depends on the number of ESBL/AmpC producers which may concurrently be present in the sample.
In 2017, specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli was performed on caecal contents from fattening pigs and cattle under 1 year and fresh meat (retail) from pigs and bovines. All MSs and three non‐MSs reported data for fattening pigs and meat from pigs and bovines. The specific monitoring in cattle under 1 year is only mandatory for countries where the production of meat from that animal category is more than 10,000 tonnes slaughtered per year. Ten MSs and two non‐MS reported data for this monitoring. In this specific monitoring, the overall prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates in caecal samples from both fattening pigs (43.8%) and cattle under 1 year (44.5%) was high. In samples of meat from pigs or bovines, the overall prevalence was however low (6.0% and 4.8% respectively). The prevalence of presumptive ESBL, AmpC and/or ESBL + AmpC producing E. coli isolates in the caecal samples from fattening pigs (43.8%) and cattle under 1 year (44.6%) is comparable with the prevalence in these animal categories reported in 2015 (40.1% and 39.6% respectively). It is also comparable with the prevalence in broilers and turkey reported in 2016 (47.4% and 42.2%, respectively). The prevalence in samples of meat from pigs and bovines (6.0% and 4.8%, respectively) is comparable with the prevalence in these types of meat reported in 2015 (approximately 6% and 4%, respectively). It is, however, markedly lower compared to samples of broiler meat in 2016 (57.4%). The difference in prevalence between caecal and meat samples in the pig and bovine samples indicates that many of the animals are carrying E. coli with resistance to third‐generation cephalosporins in their intestinal content, but that the bacteria do not contaminate the carcases during the slaughter process, alternatively that the bacteria do contaminate the carcases but are somehow removed later in the process.
In general, and in most but not all countries, prevalence of presumptive ESBL producers in caecal samples was higher than the one for presumptive AmpC producers (34% vs 11% in isolates from fattening pigs, and 42% vs 6% in cattle under 1 year, respectively). This trend can also be seen for samples of meat but as the overall prevalence is low, it is less marked (5% vs 2% from isolates from meat from pigs, and 4% vs 1% from isolates from meat from bovines, respectively). The difference is, however, clearer when assessing the occurrence of E. coli with ESBL or AmpC phenotype among the isolates resistant to third‐generation cephalosporins (78% vs 26% of isolates from meat from pigs, and 78% vs 22% of isolates from meat from bovines, respectively). For all matrices investigated, there are however countries where the occurrence of isolates with an AmpC phenotype is higher than the occurrence of isolates with an ESBL phenotype.
As described above, there were marked geographical variations in the prevalence of ESBL, AmpC, and/or ESBL + AmpC producing E. coli with the highest prevalence in eastern and southern Europe, depending on the matrices.
Carbapenemase‐producing E. coli in 2017
The emergence and spread of bacteria with acquired resistance to carbapenems is of public health concern. One concern is that animals and food could constitute a reservoir of such bacteria for humans. There are a number of scientific reports of carbapenemase‐producing bacteria isolated from farm animals and food derived thereof as well as from companion animals from different parts of the world, and the matter has recently been reviewed (Köck et al., 2018; Fernández et al., 2018). Many of these reports do however only describe coincidental findings, for example in clinical isolates. Only a few studies report prevalence of carbapenem‐resistant Enterobacteriaceae and the indications from these is that the occurrence among animals is highest in countries where such bacteria are common among humans (Köck et al., 2018; Fernández et al., 2018).
In Europe, findings of carbapenemase‐producing Enterobacteriaceae in farm animals and food derived thereof described in the literature include E. coli and Salmonella with VIM‐1 from pigs and poultry from Germany, E. coli with OXA‐181 from pigs and E. coli with VIM‐1 from seafood from Italy, as well as Enterobacter cloacae with IMI‐1 from imported frozen seafood sampled in the Netherlands (Köck et al., 2018; Brouwer et al., 2018). One of the studies from Germany also describes long term persistence of carbapenemase‐producing E. coli carrying VIM‐1 at a pig farm (Irrgang et al., 2017). Furthermore, Pseudomonas fluorescens with VIM‐1 and IMP‐1 have been isolated from ready to eat salads in Italy (Iseppi et al., 2018). Salad was also the suspected source of Citrobacter freundii with VIM‐1 causing an outbreak at a hospital in Germany (Pletz et al., 2018).
Findings of carbapenemase‐producing Enterobacteriaceae in companion animals from Europe includes E. coli with OXA‐48 from a dog in France, E. coli and Klebsiella pneumoniae with OXA‐48 from cats, dogs and horses in Germany and K. pneumoniae with VIM‐1 from a dog in Spain (Köck et al., 2018). One of the studies from Germany also describes likely nosocomial spread of the resistant bacteria at a veterinary clinic (Stolle et al., 2013).
Following the adoption of the Commission Implementing Decision 2013/652/EU, carbapenems (meropenem, ertapenem and imipenem) were included in the AST both for the routine and the specific monitoring programmes. Thereby surveillance of carbapenem resistance in both Salmonella spp. and E. coli was implemented. The specific monitoring of ESBL‐/AmpC‐/CP‐producing E. coli, in which isolation is performed using a selective medium with extended‐spectrum cephalosporins, became mandatory from 2015 and onwards.
To increase the probability of detecting carbapenem‐resistant microorganisms, performing specific monitoring of carbapenemase‐producing microorganisms (mainly E. coli, but with the option to report other enterobacteria such as Salmonella) has been recommended within the harmonised monitoring (2013/652/EU). The recommended method for this specific monitoring comprises of a non‐selective enrichment and selective media containing carbapenems for the detection of carbapenemase‐producing E. coli (protocol recommended by the EURL‐AR, http://www.eurl-ar.eu; San José et al., 2014; Hasman et al., 2015b). In this monitoring, which is performed on a voluntary basis, bacteria that produced carbapenemases that do not confer resistance to cephalosporins (i.e. OXA‐48) can also be identified.
Since the implementation of the harmonised monitoring, only a few isolates of carbapenemase‐producing Enterobacteriaceae have been reported. In 2015, Germany reported the detection of a VIM‐1‐producing E. coli isolated from a fattening pig sample and Belgium reported the detection of a VIM‐1‐producing E. coli isolated from a pig meat sample. Furthermore, in 2016, Romania reported three isolates of OXA‐162‐producing E. coli isolated from broilers (n = 2) and broiler meat (n = 1), all of them from the specific monitoring of carbapenemase‐producing microorganisms. Furthermore, Cyprus reported eight isolates from broiler meat from the specific monitoring of ESBL‐/AmpC‐/CP‐producing E. coli, one isolate from the specific monitoring of carbapenemase‐producing microorganisms from broiler meat, one isolate from the specific monitoring of carbapenemase‐producing microorganisms from broilers and one isolate of indicator E. coli from the routine AMR monitoring from broilers (EFSA and ECDC, 2017a, 2018a). The underlying mechanism in the eleven isolates from Cyprus has however not been investigated.
In 2017, no isolates with carbapenemase phenotype were detected within the routine monitoring of Salmonella or indicator E. coli. However, in the specific monitoring of ESBL‐/AmpC‐/CP‐producing E. coli, one isolate with carbapenemase phenotype from a caecal sample collected at slaughter from a pig in Germany was detected. The German EURL‐AR confirmed the production of VIM‐1 by this isolate, who belonged to a different genetic type than the German isolates detected in previous years (kindly communicated by the MSs), supporting the spread of the bla‐VIM‐1 plasmid.
In 2017, no isolates with carbapenemase phenotype were detected by the 17 MSs (18 MSs for fattening pigs) and 1 non‐MSs that reported voluntary specific monitoring of carbapenemase‐producing E. coli performed according to Decision 2013/652/EU (carbapenem‐containing plates). This is the same result as for the specific carbapenemase‐producing monitoring in 2015, even if a considerably lower number of samples were analysed then (17,191 and 7,433 samples, respectively). Interestingly, the carbapenem‐resistant isolate from a pig in Germany detected within the specific monitoring of E. coli with resistance to third‐generation cephalosporins (isolated in plates with cefotaxime) was not detected with this method. The reasons for this remain to be clarified. However, following the same methodology, Germany reported voluntarily the results of additional national CP‐producing E. coli monitoring (different samples types), and among the samples analysed, they detected the presence of another VIM‐1‐producer E. coli isolated from pig faeces collected in one of the farms sampled. The genetic background of these isolates detected in 2017 was different, and also differed from the VIM‐1 producing E. coli of animal origin previously described. The VIM‐1 plasmid sequences are closely related to the previously described bla VIM‐1 carrying plasmids from 2011 and 2015, suggesting and described above the spread of the VIM‐1 plasmid.
Within the different parts of the harmonised monitoring, additional isolates resistant to ertapenem and/or imipenem were observed. The resistance mechanisms responsible for these phenotypes have not been determined yet. In most of the cases, ertapenem resistance alone is usually related to the presence of concomitant resistance mechanisms (i.e. ESBL or AmpC production in conjunction with loss of porins).
The Netherlands also reported voluntarily data from their national CP producers monitoring on samples from dairy cattle (300 samples), veal calves (302) and fattening pigs (297), as well as in additional matrices, using a different isolation method (Ceccarelli et al., 2017; Wang et al., 2017). No positive isolates were found among these samples. Interestingly, they also performed a voluntary CP‐monitoring, of frozen fish and shrimps originating from fish farms in South‐East Asia. For this monitoring, they used the same isolation method as all the other MSs for the specific carbapenemase‐producing Enterobacteriaceae monitoring. The MSs informed EFSA about the presence of two carbapenemase‐producing Enterobacter cloacae complex in different batches of imported frozen shrimps, both exhibiting resistance to carbapenems but not to third‐generation cephalosporins. The isolates were positive for bla IMI‐1 (Brouwer et al., 2018) and for a novel plasmid‐located carbapenemase gene (personal communication of the MS).
Among all samples and isolates investigated within the harmonised monitoring in 2017, only one E. coli with elevated MIC to meropenem were detected. That no more isolates were detected, together with the fact that only a few isolates have been detected in the previous years, indicates that carbapenemase‐producing E. coli is still rare among the investigated animal species in Europe. Thereby, potential incentives to preserve this situation can hopefully still be effective, ensuring that farm animals do not become an important source of such bacteria for humans.
Due to the public health importance of carbapenemase‐producing E. coli and/or Salmonella, both as pathogens or as vectors for resistance mechanisms there is a need to follow further developments in this area for farm animals and food derived thereof. It should also be kept in mind that there are several potential sources for the carbapenemase‐producing bacteria detected on food samples, including the animals from which the meat was derived, the environment in which the meat was produced, cross‐contamination with other items during production, as well as people involved in handling and preparing the meat. Even if the origin of the isolates does not affect the risk for public health, it is of importance when elaborating on effective risk management strategies.
Summary
Summarising, in 2017, as in the previous years, resistance to third‐generation cephalosporins was generally detected at low levels in Salmonella and indicator E. coli from all investigated matrices using non‐selective detection methods. Using selective detection methods, E. coli with resistance to third‐generation cephalosporins could however readily be detected in caecal samples from both fattening pigs and cattle under 1 year and the overall prevalence was high. The prevalence in samples of meat from pigs or bovines was however low also when using selective detection methods. This indicates that many of the animals are carrying E. coli with resistance to third‐generation cephalosporins in low numbers in their intestinal content, but the bacteria do not contaminate the carcases during the slaughter process, alternatively that the bacteria do contaminate the carcases but are somehow removed later in the process.
The fact that the prevalence of E. coli with resistance to third‐generation cephalosporins is low in both meat from pigs and meat from cattle indicates that this type of food is probably not an important source of such bacteria to humans. A lowered prevalence of E. coli with resistance to third‐generation cephalosporins in fattening pigs and cattle under 1 year would, however, still be preferable in order to mitigate the risk for animal and public health from this type of resistance as well as spread of this type of resistant bacteria to the environment.
Among all isolates investigated in 2017, only one E. coli with elevated MIC to meropenem (from a caecal sample from a fattening pig in Germany) was detected. That no more isolates were detected indicates that carbapenemase‐producing E. coli is still rare among fattening pigs and cattle under 1 year in Europe and hopefully incentives to preserve this situation can still be effective.
7. Meticillin‐resistant Staphylococcus aureus 23
Monitoring of food‐producing animals, in particular intensively reared animals, carried out periodically in conjunction with systematic surveillance of MRSA in humans, allows trends in the diffusion and evolution of zoonotically acquired MRSA in humans to be identified (EFSA, 2009a,b, 2012b). Isolates representative of various animal and food origins should therefore optimally be analysed for determination of lineage, antimicrobial susceptibility and virulence‐associated traits. Such monitoring may also provide an early indication of the occurrence of types of MRSA in animals which have previously not been recognised in animal populations. Furthermore, monitoring of other animal species, with which certain types of MRSA can be associated, provides additional useful information.
Meticillin‐resistant Staphylococcus aureus (MRSA)
MRSA has been recognised for decades as a serious cause of infections in humans. Strains of MRSA that cause infections in humans can be divided into three broad categories: community‐associated (CA‐), healthcare‐associated (HA‐) and livestock‐associated (LA‐)MRSA. Strains assigned to these different categories of MRSA differ in their epidemiology, although distinctions between types can be blurred. LA‐MRSA has been detected in pigs, poultry and veal calves, as well as in other farm animal species, companion animals and horses in many countries worldwide. HA‐MRSA and CA‐MRSA include strains that predominantly affect humans, and these are much less frequently reported from food‐producing animals. LA‐MRSA may also be carried by humans, especially those persons who have repeated occupational contact with affected livestock and their derived carcases. The severity of LA‐MRSA infection has been shown to be similar to that of other MRSA strains. Indeed, public health surveillance in the Netherlands and Denmark in 1999‐2014 detected that LA‐MRSA strains were developing more human‐adapted traits, with distinct strains establishing transmission in the community in the absence of livestock contact (Kinross et al., 2017).
The EFSA's assessment of the public health significance of MRSA in animals and food (EFSA, 2009b) and the Joint Scientific Report of ECDC, EFSA and EMA on MRSA in livestock, companion animals and food (EFSA, 2009a) provide more background information and recommendations on MRSA. These issues were also reviewed in the EFSA Scientific Report on proposed technical specifications to improve the harmonisation of the monitoring and reporting of the prevalence, genetic diversity and multidrug resistance profile of MRSA in food‐producing animals and food (EFSA, 2012b).
Antimicrobial susceptibility in European invasive Staphylococcus aureus isolates from humans is reported by the MSs to the European Antimicrobial Resistance Surveillance Network (EARS‐Net) hosted by ECDC. MRSA typing data are not reported and, therefore, when there may be possible links to the animal reservoir of LA‐MRSA, these cannot be detected easily with current monitoring procedures, at least at the European level. The EU/EEA population‐weighted mean proportion of MRSA among invasive S. aureus infections reported to EARS‐Net decreased significantly from 19.6% in 2014 to 16.9% in 2017, with similar decreasing trends reported from more than one fourth of the individual EU/EEA countries. Nevertheless, MRSA remains an important human pathogen in the EU/EEA, as the levels of MRSA were still high in several countries and combined resistance to other antimicrobial groups was common (ECDC, 2018).
Recent reports have highlighted the occurrence of MRSA in farmers and those repeatedly exposed to livestock. Fischer et al. (2017a) report that nasal MRSA carriage was detected in 84.7% of German pig farmers (72/85 pig farmers from 51 pig farms), while Kieffer et al. (2017) summarised recent studies that LA‐MRSA persistently colonises between 24 and 86% of pig farmers, 37% of cattle farmers and 9–37% of poultry farmers, as well as a proportion of slaughterhouse workers (3–6%) and veterinarians (3–45%). The risk of carriage in pig slaughterhouse workers was found to be related to the degree of occupational exposure to live pigs (Gilbert et al., 2012). These reports underline the usefulness of monitoring the MRSA status of livestock populations. The situation is dynamic, exemplified by a recent longitudinal study (Köck et al., 2017), as well as the detection of MRSA CC398 among an Australian pig farm, contributing to ongoing clinical MRSA infections among farm workers (Roschanski et al., 2017).
mecC‐meticillin‐resistant Staphylococcus aureus
A variant of the meticillin resistance gene mecA, termed mecC, was identified in 2011 in MRSA from humans and cattle in Europe (García‐Álvarez et al., 2011; Shore et al., 2011), and has subsequently been detected in ruminants, pigs and companion animals, with increasing reports from wild animals (Paterson et al., 2014; Bengtsson et al., 2017). Although first identified in 2011, mecC‐MRSA isolates have now been found dating back to 1975 (Petersen et al., 2013), and it is reported that the mecC gene shares 70% identity with mecA at the DNA level (García‐Álvarez et al., 2011).
Petersen et al. (2013) demonstrated that mecC‐MRSA infections in humans were primarily community acquired, typically affecting people living in rural areas and those older than typical for CA‐mecA‐MRSA patients. Although our understanding of the epidemiology of mecC‐MRSA is incomplete, studies have indicated that animal contact and zoonotic transmission are likely to be important. Petersen et al. (2013) also demonstrated that mecC‐MRSA can be transmitted between humans and ruminants: for two people with cattle/sheep contact, mecC‐MRSA was detected in both the animals and their owners, and isolates were of the same spa‐type and multiple‐locus variable number tandem repeat analysis (MLVA) profile. Paterson et al. (2014) reported that when tested, mecC‐MRSA strains have been negative for Panton‐Valentine leukocidin (PVL) toxin – a virulence feature typically associated with CA‐MRSA – and negative for human immune evasion cluster (IEC) genes, chp (chemotaxis inhibitor protein), sak (staphylokinase) and scn (encoding the staphylococcal complement protein inhibitor). Carriage of these IEC genes is considered an adaptation to enable S. aureus colonisation and infection of humans, and is not usually a feature of animal S. aureus strains (Cuny et al., 2015).
Determination of the susceptibility of MRSA isolates to antimicrobials, including those of particular medical importance, such as linezolid and vancomycin, also provides valuable information on the MRSA situation. Furthermore, the importance of monitoring AMR patterns among different lineages is underlined by the potential for multiple resistance genes harboured by less virulent strains to spread to other S. aureus strains (Roschanski et al., 2017). Monitoring of MRSA in animals and food is currently performed voluntarily by MSs and the findings presented in this report underline the value of such monitoring.
7.1. Meticillin‐resistant Staphylococcus aureus in food and animals
LA‐MRSA isolates are the main focus of this chapter, which summarises the occurrence of MRSA and AMR patterns in various food categories (including meat samples from various species) and food‐producing animals reported by six MSs and two non‐MSs to EFSA in 2017 (excluding clinical investigations; Table MRSAOVERVIEW). Finland and Switzerland were the only countries to report data on AMR of MRSA isolates from meat samples (both countries also reporting molecular typing data); Belgium and Switzerland were the only countries to report AMR data of MRSA isolates from food‐producing animals (both countries also reported molecular typing data, as did Finland, Norway and Spain). This chapter also includes occurrence data on MRSA reported from clinical investigations of food‐producing and companion animals. AMR and molecular typing data of isolates from dogs, goats, sheep, horses, a cat and a rabbit were also provided by Sweden and are shown in Table 7. Methods for the isolation of MRSA from food and animals have not been harmonised at the EU level and, therefore, the methods used by individual reporting MSs may differ in sensitivity. Further information on MRSA isolation methods is available in the text box below. Similarly, the sampling strategies used by reporting MSs are not harmonised at the EU level and these may also influence the results obtained.
Isolation of MRSA from food‐producing animals and the farm environment
Prior to June 2018, the EURL‐AR recommended method for the detection of MRSA from dust samples comprised a pre‐enrichment step and a selective enrichment step; known as the 2‐S method. The 2‐S method is briefly described below (EURL‐AR, 2009; EFSA, 2012b):
Pre‐enrichment step: Mueller–Hinton broth (MHB) containing 6.5% sodium chloride (NaCl).
Selective enrichment step: The enriched MHB is inoculated (after incubation) into Tryptone Soya Broth (TSB), which contains cefoxitin and aztreonam.
Selective plating: TSB is inoculated (after incubation) onto chromogenic MRSA agar. Suspect MRSA colonies are then subcultured onto blood agar, and presumptive colonies are confirmed by the detecting presence of mecA/mecC genes using PCR.
A recent study (Larsen et al., 2017) reported a high ratio of false‐negative results using the 2‐S method. During this investigation, sensitivity of the 2‐S method was evaluated by comparison with an alternative 1‐S method, whereby the selective enrichment step was bypassed. From 2014 to 2016, LA‐MRSA samples were collected from Danish and Norwegian pigs and their environment and examined by each method. Results confirmed that the 1‐S method resulted in a lower proportion of false‐negative results than the 2‐S method; the 1‐S method and the 2‐S method detecting MRSA in 82% and 74% of the Danish samples, and in 5.6% and 3.8% of the Norwegian samples, respectively. The authors urge caution in extrapolating the results to animals other than pigs and comment that previous studies in Belgium in poultry and cattle did not find significant differences between the performance of the methods.
Accordingly, in June 2018, the EURL‐AR published revised recommendations for the isolation of MRSA from food‐producing animals and the farm environment, which omits the use of a second enrichment step with cefoxitin and aztreonam (EURL‐AR, 2018). Methods for the isolation of MRSA from food and animals are not harmonised at the EU level and in 2017 the recommended method by the EURL‐AR and EFSA was the 2‐S method. Notably, changes to the recommended method of isolation may impact future longitudinal studies in pigs, since comparison of the data obtained using the different methods should be performed with caution.
7.1.1. Monitoring of meticillin‐resistant Staphylococcus aureus in food
In 2017, Finland, Germany, Slovakia, Spain and Switzerland reported information on the occurrence of MRSA in various categories of food (Table 51). Slovakia examined a range of food products (including meat samples from cattle, pigs and poultry) with no samples testing positive for MRSA. Germany investigated both fresh veal (354 samples in total) and minced bovine meat (289 samples in total), among which 11.3% and 6.9% tested positive for MRSA, respectively. Switzerland examined a large number of meat samples of bovine (N = 299) and porcine (N = 301) origin for MRSA, with no samples testing positive from bovine animals and two samples testing positive (0.7%) from porcine animals. Under a national survey, Finland examined 220 batches of fresh pig meat and 5.9% tested positive for MRSA. Spain examined both fresh pig meat (N = 60) and fresh rabbit meat (N = 75); with 2 positive samples (3.3%) detected in fresh pig meat and 3 positive samples (4.0%) detected in fresh rabbit meat.
The corresponding spa‐typing data were not reported by Germany and Spain, as positive isolates were recorded without specifying spa‐type. All spa‐types reported by Finland in batches of pig meat were those associated with CC398; spa‐types t011, t034 and t2741. Of the two positive isolates recovered from Swiss pig meat, one was reported as spa‐type t011 (associated with CC398) and the other as spa‐type t002. spa‐type t002 has been associated with several sequence types within CC5, but is most commonly associated with ST5 (CC5), a sequence type which can be considered as either a community or healthcare associated MRSA. Although further molecular typing data (including PVL status) were not available, the isolate is considered most likely to represent a HA‐MRSA lineage.
In summary, meat from cattle, pigs and rabbits proved positive for MRSA, although the prevalence varied between meats of different origins.
Temporal trends in the prevalence of MRSA in food
Germany reported annual results on MRSA prevalence in fresh veal in 2012 and 2017 (Table 2) at moderate levels of around 10% in both years). Interestingly, in 2011 and 2013, Germany performed similar monitoring on bovine meat from older animals; MRSA prevalence was reported at lower levels in meat from older animals to that in veal (8.1% and 5.5%, respectively). In all years, more than 350 individual samples were tested (the 2011 and 2013 data obtained from bovine meat of older animals is not included in Table 52). Although Finland did not report on MRSA prevalence in batches of fresh pig meat in 2016, data are available for 2015 and 2017. Although, in both years, prevalence was low, interestingly, it nearly doubled from 2015 to 2017 (3.0% in 2015 and 5.9% in 2017), corresponding to a statistically significant increase (Cochran–Armitage trend test, one‐sided p‐values = 0.0492). In both years, sample size examined remained high and common spa‐types associated with CC398 were reported. Spain reported annual results on MRSA prevalence in fresh pig meat in 2011, 2012, 2013, 2014 and 2017, testing a relatively low number of samples each year (no more than 60). With the exception of 2013 (8.3%), a similar level was recorded: 2.4% (2011), 1.7% (2012), 3.2% (2014) and 3.3% (2017). Spain also reported data on the annual prevalence of MRSA in fresh rabbit meat from 2015 to 2017. In all years, a relatively low number of samples were tested. Prevalence remained at similar levels in 2015 and 2016 (8.3% and 8.0%, respectively), decreasing to 4.0% in 2017.
Table 52.
Temporal trends in prevalence of meticillin‐resistant Staphylococcus aureus in various kinds of meat, 4 EU MSs
| Country | Year | Production type/description | Sample unit | Number | |
|---|---|---|---|---|---|
| Units tested | Positive for MRSA (%) (95% CI) | ||||
| Meat from bovine animals | |||||
| Germanya | 2012 | Veal – Retail Monitoring | Single | 421 | 44 (10.5%) (7.7%, 13.8%)g |
| 2017 | Veal – Fresh – Retail Monitoring | Single | 354 | 40 (11.3%) (8.2%, 15.1%)g | |
| Meat from pigs | |||||
| Finlandb | 2015 | Fresh – National Retail Survey | Batch | 303 | 9 (3.0%) (1.4%, 5.6%)c |
| 2017 | Fresh – National Retail Survey | Batch | 220 | 13 (5.9%) (3.2%, 9.9%)d | |
| Spaina | 2011 | Fresh | Single | 42 | 1 (2.4%) (0.06%, 12.6%)e |
| 2012 | Fresh | Single | 60 | 1 (1.7%) (0.04%, 8.9%)g | |
| 2013 | Fresh | Single | 60 | 5 (8.3%) (2.8%, 18.4%)g | |
| 2014 | Fresh – Surveillance | Single | 31 | 1 (3.2%) (0.08%, 16.2%)g | |
| 2017 | Fresh – Retail Surveillance | Single | 60 | 2 (3.3%) (0.4%, 11.5%)g | |
| Meat from rabbit | |||||
| Spaina | 2015 | Fresh – Retail Monitoring | Single | 60 | 5 (8.3%) (2.8%, 18.4%)g |
| 2016 | Fresh – Retail Monitoring | Single | 50 | 4 (8.0%) (3.2%, 18.9%)f | |
| 2017 | Fresh – Retail Monitoring | Single | 75 | 3 (4.0%) (0.8%, 11.3%)g | |
no statistically significant differences or temporal trends among years.
statistically significant increase between 2015 and 2017.
In 2015, spa‐types : t034 (6), t2741 (3).
In 2017, spa‐types: t034 (11 isolates), t011 (1), t2741 (1).
In 2011, spa‐type: t011 (1).
In 2016, spa‐types: t011 (3 isolates), t1190 (1). PVL status of the t1190 isolate was not reported.
spa‐types not reported.
Considering further statistical analyses of MRSA prevalence within countries/matrices, prevalence results are rather stable and no statistically significant differences or temporal trends among years were detected, with the exception of Finland.
7.1.2. Meticillin‐resistant Staphylococcus aureus in animals
Monitoring of MRSA in food‐producing animals
In 2017, Belgium, Finland, Germany, the Netherlands, Norway, Spain and Switzerland reported data on the occurrence of MRSA in food‐producing animals (Table 53). In calves at slaughter, MRSA prevalence was assessed at the high level of 39.7% in Germany and at the low level of 8.1% in Switzerland; both countries testing a large number of slaughter animals (N = 348 and N = 297, respectively).
Table 53.
Meticillin‐resistant Staphylococcus aureus in food‐producing animals (excluding clinical investigations), 2017
| Country | Production type/monitoring description (where specified)) | Sample unit | Number | |
|---|---|---|---|---|
| Units tested | Positive for MRSA (%) | |||
| Cattle (bovine animals) | ||||
| Germany | Calves (under or around 1 year), nasal swabs – Slaughterhouse Monitoring – active | Animal | 348 | 138 (39.7%)h |
| Switzerland | Calves (under 1 year), nasal swabs ‐ Slaughterhouse Monitoring | Animal | 297 | 24 (8.1%)a |
| Gallus gallus (fowl) | ||||
| Belgium | Broilers – Farm Surveillance | Herd/flock | 80 | 2 (2.5%)b |
| Laying hens – Farm Surveillance | Herd/flock | 236 | 3 (1.3%)c | |
| Pigs | ||||
| Finland | Fattening pigs, nasal swabs – Slaughterhouse Survey – National Survey | Slaughter batch | 61 | 47 (77.0%)d |
| Germany | Fattening pigs, boot swabs ‐ OFM – active | Herd/flock | 341 | 130 (38.1%)h |
| Netherlands | Breeding animals – OFM – active | Animal | 70 | 1 (1.4%)h |
| Norway | OFCEP, hide sample | Herd/flock | 826 | 3 (0.4%)e |
| Spain | Fattening pigs, nasal swabs – Slaughterhouse Monitoring – EFSA specifications | Slaughter batch | 323 | 292 (90.4%)f |
| Switzerland | Fattening pigs, nasal swabs – Slaughterhouse Monitoring | Animal | 298 | 131 (44.0%)g |
OFM: On‐farm monitoring; OFCEP: On‐farm control and eradication programme.
spa‐types: t011 (14 isolates), t034 (7), t127 (1), t17339 (2). PVL status of the t127 isolate was not reported.
spa‐types: t011 CC398 (1 isolate), t1456 CC398 (1).
spa‐types: t011 CC398 (2 isolates), t1456 CC398 (1).
spa‐types: t034 (32 isolates), t2741 (25), t011 (9), t108 (6), t1250 (1), t1255 (1), t17061 (1). NB. All MRSA isolates were subject to spa‐typing; from one slaughter batch, up to three different spa‐types were detected. Therefore, the total number of individual spa‐types exceeds the number of positive batches.
spa‐types: t091 CC7 (1 isolate), t843 CC130 (1), t6292 CC425 (1). The t091 isolate was PVL negative.
spa‐types: t011 (203 isolates), t034 (32), t108 (14), t109 (1), t899 (2), t1197 (11), t1255 (2), t1451 (13), t1606 (1), t2011 (5), t2346 (1), t2748 (1), t3041 (2), t4208 (2), t17304 (1), t17627 (1).
spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1).
spa‐types not reported.
As part of farm surveillance, Belgium tested broiler (N = 80) and laying hen flocks (N = 236), with both production types recording low MRSA prevalence (2.5% and 1.3%, respectively).
In pigs, the Netherlands reported a low MRSA prevalence (1.4%) in breeding animals during on‐farm monitoring. Norway tested a large number of pig herds in 2017, as part of an on‐farm control and eradication programme. In total, 826 herds were included in the survey, of which 85 were genetic nucleus or multiplier herds. Twelve herds were the central units of sow pool herds, and 729 were herds with more than 10 sows. MRSA was detected in three pig herds (one multiplier herd and in two farrow to finish herds), resulting in a very low prevalence of 0.4%. Finland and Spain reported an extremely high MRSA prevalence in batches of fattening pigs at slaughter (77.0% and 90.4%, respectively); Switzerland also reported a high prevalence (44.0%) in fattening pigs at slaughter, detected through monitoring one animal per herd at slaughter. Germany reported a MRSA prevalence of 38.1% in fattening pig herds as part of on‐farm monitoring.
Although Germany and the Netherlands did not report corresponding spa‐types, a number of different spa‐types were reported by other reporting MSs and non‐MSs (Table 3). In calves, most isolates (23/24) reported by Switzerland were livestock‐associated (CC398), comprising spa‐types t011, t034 and t17339. spa‐types t011 and t034 are common spa‐types associated with CC398; and although spa‐type t17339 has apparently not been previously subjected to multilocus sequence typing (MLST), Switzerland confirmed that the isolates also belonged to CC398. The remaining bovine isolate reported by Switzerland was spa‐type t127; this spa‐type has been associated with MRSA belonging to several sequence types within CC1, as well as to types in CC474. Nevertheless, spa‐type t127 is most commonly associated with ST1 (CC1) and this spa/sequence type combination was considered to represent a CA‐MRSA.
In broiler and laying hen flocks, Belgium reported spa‐types t011 and t1456, which were subject to multilocus sequence typing and confirmed to belong to CC398.
Generally in pigs, where spa‐typing data were available, most isolates were those associated with CC398. In Finland, most isolates recovered from batches of slaughter pigs were spa‐types t034 and t2741 (both associated with CC398); other spa‐types detected in this country and production type included t011, t108, t1250, t1255 (all associated with CC398) and t17061. spa‐type t17061 has not been previously sequenced typed, but based on the similarity of spa repeats to other known CC398 spa‐types, an association to CC398 was inferred. Notably, all Finnish MRSA isolates were subject to spa‐typing; from one slaughter batch, up to three different spa‐types were detected. Therefore, the total number of individual spa‐types exceeds the number of positive batches.
Considering the very low MRSA prevalence among Norwegian pig herds, spa‐types associated with CC398 were not detected. Of only three MRSA isolates reported by Norway, one was spa‐type t091 and MLST confirmed the isolate to belong to CC7. Additionally, Norway reported that the t091 isolate was PVL negative, which could indicate a HA‐MRSA lineage; however, there is evidence to suggest this spa‐type of meticillin‐sensitive S. aureus (MSSA) may occur rather frequently in pigs (Krupa et al., 2015) and therefore a category was not inferred. The two remaining Norwegian isolates, spa‐types t843 and t6292, were reported to carry the mecC gene and MLST confirmed them to belong to CC130 and CC425, respectively.
The majority of MRSA isolates recovered from batches of Spanish fattening pigs at slaughter were spa‐type t011 (associated with CC398), with lower numbers of t034, t108, t1197, t1451 and t2011, followed by two isolates of each t899, t1255, t3041 and t4208 (all associated with CC398). Although spa‐type t899 is generally associated with CC398, this spa‐type has also been observed in MRSA isolates with a mosaic genome and can therefore be associated with different clonal lineages, namely CC398 and ST9 (CC9); two unrelated LA‐MRSA clonal lineages. Genotype ST9‐t899 is the mosaic strain, consisting of a CC398 chromosomal backbone having acquired the CC9 region containing the staphylococcal protein A gene (Guardabassi et al., 2009; Larsen et al., 2016). The other spa‐types detected in this country and production type were single isolates of t1606, t2346 and t2748 (all associated with CC398), t109, t17304 and t17627. For spa‐types t17304 and t17627, an association with CC398 was inferred; although these spa‐types have not been previously sequenced typed, they show similar spa repeats to other known CC398 spa‐types. Most spa‐types detected in this country and production type were those associated with CC398, with the exception of a single isolate of t109. spa‐type t109 has previously been associated with ST5 and ST228 (both members of clonal complex 5), but is more commonly associated with ST228 and was therefore considered to belong to a HA‐MRSA lineage.
In Switzerland, most MRSA isolates recovered from fattening pigs at slaughter were spa‐types t011 and t034 (both associated with CC398). The other spa‐types detected in Switzerland in fattening pigs were t899, t1451, t2330 and t2876 (all associated with CC398).
Clinical investigations for MRSA in food‐producing animals
Typically, clinical investigations differ from monitoring studies in food‐producing animals; as selective culture methods may not be used, the number of units tested may be low and the sample may involve a biased sample population. Although these data do not allow prevalence to be inferred and cannot be extrapolated at the population level, it is still considered relevant to report the range of animal species/populations which were affected. In 2017, Ireland, the Netherlands and Slovakia reported data on clinical investigations for MRSA in various food‐producing animals (Table 4). Slovakia tested dairy cows, milk goats and milk ewes, as part of on‐farm clinical investigations. No animals tested positive for MRSA; however, in all cases, sample size was small. As part of on‐farm clinical investigations, Ireland tested two adult cattle (over two years of age) with neither proving positive for MRSA. The Netherlands tested dairy cows (N = 1,062) during veterinary‐clinic clinical investigations, with MRSA prevalence reported at 0.9%; the corresponding spa‐typing data were not available (Table 54).
Table 54.
Meticillin‐resistant Staphylococcus aureus in food‐producing animals, clinical investigations, 2017
| Country | Production type/monitoring description (where specified)) | Sample unit | Number | |
|---|---|---|---|---|
| Units tested | (%) positive for MRSA | |||
| Cattle (bovine animals) | ||||
| Ireland | Adult cattle over 2 years – OFCI | Animal | 2 | 0 |
| Netherlands | Dairy cows – VCCI | Animal | 1062 | 10 (0.9%)* |
| Slovakia | Dairy cows – OFCI | Animal | 13 | 0 |
| Goats | ||||
| Slovakia | Milk goats – OFCI | Animal | 8 | 0 |
| Sheep | ||||
| Slovakia | Milk ewes – OFCI | Animal | 4 | 0 |
VCCI: At‐veterinary‐clinic clinical investigations; OFCI: On‐farm clinical investigations.
spa‐types not reported.
Clinical investigations for MRSA in companion animals
The Netherlands and Slovakia reported data on MRSA in companion animals in 2017 (Table 5). Slovakia tested cats and dogs during veterinary‐clinic clinical investigations, with no animals testing positive for MRSA; however, a small number of cats were tested (N = 9). As part of veterinary‐clinic clinical investigations and on‐farm clinical investigations, the Netherlands tested a large number of cats (N = 572), dogs (N = 388) and horses (N = 268), resulting in 0.9%, 1.3% and 6.3% occurrence of MRSA, respectively. The corresponding spa‐typing data were not reported (Table 55).
Table 55.
Meticillin‐resistant Staphylococcus aureus in companion animals, clinical investigations, 2017
| Country | Production type/monitoring description (where specified)) | Sample unit | Number | |
|---|---|---|---|---|
| Units tested | (%) positive for MRSA | |||
| Cats | ||||
| Netherlands | VCCI | Animal | 572 | 5 (0.9%)a |
| Slovakia | Pet animals – VCCI | Animal | 9 | 0 |
| Dogs | ||||
| Netherlands | VCCI | Animal | 388 | 5 (1.3%)a |
| Slovakia | Pet animals – VCCI | Animal | 80 | 0 |
| Solipeds, domestic | ||||
| Netherlands | Horses – OFCI | Animal | 268 | 17 (6.3%)a |
VCCI: At‐veterinary‐clinic clinical investigations; OFCI: On‐farm clinical investigations.
spa‐types not reported.
Temporal trends in the prevalence of MRSA in food‐producing animals
Germany reported annual results on the prevalence of MRSA in calves at the slaughterhouse in 2012 and 2017 (Table 6). In both years, a similar number of calves were tested (320 in 2012 and 348 in 2017). Prevalence remained at a high level, although declined slightly from 2012 to 2017 (45% to 39.7%, respectively). In 2015 and 2017, Switzerland also monitored MRSA prevalence in calves at the slaughterhouse. Again, a similar number of animals were tested (2015: N = 292, 2017: N = 297). Although prevalence remained low, this increased slightly from 2015 to 2017 (6.5% to 8.1%, respectively). Most isolates were spa‐types associated with CC398 (LA‐MRSA), with the exception of spa‐type t008 in 2015 and spa‐type t127 in 2017; both categorised as CA‐MRSA.
In 2017, Finland reported MRSA prevalence at 77% in batches of fattening pigs at slaughter. Although in previous years, comparable data have not been submitted for inclusion in this report, Finland state that in 2009‐2010 an equivalent study was performed, reporting MRSA prevalence at 22% (Finnish Food Safety Authority Evira ‐ EVIRA, online). In both years, nasal swab samples were taken from five animals per slaughter batch. Notably in 2010, the most common spa‐types reported were t108 and t127, while in 2017, spa‐types t034 and t2741 predominated, and all spa‐types in 2017 were associated with CC398.
Although in 2016, Switzerland did not report data on the prevalence of MRSA in fattening pigs at slaughter, data are available for 2009–2015 and 2017. Throughout all years, a large number of nasal swabs were tested. Generally, prevalence has increased annually, rising from 2.2% in 2009 to 44.0% in 2017; and from 2015 to 2017, a marked increase was observed from 25.7% to 44.0%, respectively. Notably, spa‐types associated with CC398 exhibited a steady increase in prevalence, and in 2017, all reported isolates were those associated with CC398, with most belonging to spa‐types t011 and t034.
Spain reported data on MRSA prevalence in batches of fattening pigs at slaughter in 2011, 2015 and 2017. In all years, nasal swabs were tested. Although MRSA prevalence remained extremely high throughout, a slight increase was noted from 2011 to 2015 (84.1% in 2011 to 91.4% in 2015), while a very modest decline was noted from 2015 to 2017 (91.4% in 2015 to 90.4% in 2017). spa‐typing data were available for 123 isolates in 2011 and for all isolates in 2017; all spa‐types were those associated with CC398, with the exception of a single isolate of spa‐type t109 in 2017, a HA‐MRSA. Germany reported data on MRSA prevalence in fattening pig herds in 2015 and 2017. Although prevalence remained high in both years, this decreased slightly from 2015 to 2017 (41.3–38.1%, respectively). In both years, over 300 pig herds were monitored.
Norway reported data on the yearly prevalence of MRSA in pig herds from 2014 to 2017, as part of their on‐farm control and eradication programme. The herds sampled during this monitoring comprised different production types (further details on the Norwegian eradication programme are available in the text box at the end of the chapter). In all years, similar very low levels of prevalence were recorded at 0.1%, 0.5%, 0.1% and 0.4%; highlighting the favourable impact of the Norwegian programme in eradicating and maintaining freedom of MRSA from most pig herds.
Considering results of statistical analyses using the Cochran–Armitage trend test, statistically significant increasing trends in MRSA prevalence in fattening pigs were observed in Finland from 2010 to 2017 (one‐sided p‐values < .0001); and in Spain (one‐sided p‐values = 0.0062) and Switzerland (one‐sided p‐values < .0001), over the last years. For the other countries/animal populations, prevalence results are rather stable and no statistically significant differences or temporal trends among years were detected (Table 56).
Table 56.
Temporal trends in prevalence of meticillin‐resistant Staphylococcus aureus in various food‐producing animals, 5 reporting countries
| Country | Year | Production type/ description | Sample unit | Number | |
|---|---|---|---|---|---|
| Units tested | Positive for MRSA (%) (95% CI) | ||||
| Cattle (bovine animals) | |||||
| Germany 1 | 2012 | Calves (<1 year), SHM, NS | Animal | 320 | 144 (45%) (39.5%, 50.6%)* |
| 2017 | Calves (<1 year), SHM, NS | Animal | 348 | 138 (39.7%) (34.5%, 45.0%)* | |
| Switzerland 1 | 2015 | Calves (<1 year), SHM, NS | Animal | 292 | 19 (6.5%) (4%, 10%)a |
| 2017 | Calves (<1 year), SHM, NS | Animal | 297 | 24 (8.1%) (5.5%, 11.7%)b | |
| Pigs | |||||
| Finland 2 | 2010 | Fattening pigs, SHM, NS | Batch | 59 | 13 (22%) (12.3%, 34.7%)c |
| 2017 | Fattening pigs, SHM, NS | Batch | 61 | 47 (77%) (64.5, 86.9)d | |
| Switzerland 2 | 2009 | Fattening pigs, SHM, NS | Animal | 405 | 8 (2.2%) (0.9%, 3.9%)* |
| 2010 | Fattening pigs, SHM, NS | Animal | 392 | 23 (5.9%) (3.8%, 8.7%)e | |
| 2011 | Fattening pigs, SHM, NS | Animal | 392 | 22 (5.6%) (3.6%, 8.4%)f | |
| 2012 | Fattening pigs, SHM, NS | Animal | 397 | 72 (18.1%) (14.5%, 22.3%)g | |
| 2013 | Fattening pigs, SHM, NS | Animal | 351 | 73 (20.8%) (16.7%, 25.4%)h | |
| 2014 | Fattening pigs, SHM, NS | Animal | 298 | 79 (26.5%) (21.6%, 32%)i | |
| 2015 | Fattening pigs, SHM, NS | Animal | 300 | 77 (25.7%) (20.8%, 31.0%)j | |
| 2017 | Fattening pigs, SHM, NS | Animal | 298 | 131 (44.0%) (38.2%, 49.8%)k | |
| Spain 2 | 2011 | Fattening pigs, SHM, NS | Batch | 227 | 191 (84.1%) (78.7%, 88.6%)l |
| 2015 | Fattening pigs, SHM, NS | Batch | 383 | 350 (91.4%) (88.1%, 94%)* | |
| 2017 | Fattening pigs, SHM, NS | Batch | 323 | 292 (90.4%) (86.7%, 93.4%)m | |
| Germany 1 | 2015 | Fattening pigs, FM, BS – active | Herd | 332 | 137 (41.3%) (35.9%, 46.8%)* |
| 2017 | Fattening pigs, FM, BS – active | Herd | 341 | 130 (38.1%) (32.9%, 42.5%)* | |
| Norway 1 | 2014 | Pigs: NFCEP | Herd | 986 | 1 (0.1%) (0+%, 0.6%)n |
| 2015 | Pigs: NFCEP | Herd | 821 | 4 (0.5%) (0.13%, 1.2%)o | |
| 2016 | Pigs: NFCEP, HS | Herd | 872 | 1 (0.1%) (0+%, 0.6%)p | |
| 2017 | Pigs: NFCEP, HS | Herd | 826 | 3 (0.4%) (0.07%, 1.1%)q | |
SHM: at slaughterhouse monitoring; FM: at‐farm monitoring; NFCEP: National Farm Control and Eradication Programme; NS: nasal swabs; BS: boot swabs; HS: hide sample.
No statistically significant differences or temporal trends among years.
Statistically significant increase between 2010 and 2017 for Finland and between 2009 and 2017 for Switzerland.
In 2015, spa‐types: t011 (11 isolates), t034 (6) and t008 (2). The t008 isolates were PVL positive.
In 2017, spa‐types: t011 (14 isolates), t034 (7), t127 (1), t17339 (2). PVL status of the t127 isolate was not reported.
In 2010, spa‐types: t108 (6 isolates) and t127 (5) were the most commonly detected.
In 2017, spa‐types: t034 (32 isolates), t2741 (25), t011 (9), t108 (6), t1250 (1), t1255 (1), t17061 (1). NB. All MRSA isolates were subject to spa‐typing; from one slaughter batch, up to three different spa‐types were detected. Therefore, the total number of spa‐types exceeds the number of positive batches.
In 2010, spa‐types: t034 ST398 (17 isolates), t011 ST398 (1), t208 ST49 (5).
In 2011, spa‐types: t034 ST398 (19 isolates), t011 ST398 (1), t208 ST49 (1), t2279 ST1 (1).
In 2012, spa‐types: t034 CC398 (61 isolates), t011 CC398 (9), t208 ST49 (2).
In 2013, spa‐types: t034 (63 isolates), t011 (10).
In 2014, spa‐types: t034 (57 isolates), t011 (19), t208 (1), t899 (1), t2741 (1).
In 2015, spa‐types: t034 (48 isolates), t011 (23), t032 (1), t571 (1), t899 (1), t1145 (1), t1250 (1), t4475 (1).
In 2017, spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1).
In 2011, spa‐types : t011 (97 isolates), t034 (8), t108 (3), t1197 (7), t1451 (3), t2346 (3), unspecified (68).
In 2017, spa‐types: t011 (203 isolates), t034 (32), t108 (14), t109 (1), t899 (2), t1197 (11), t1255 (2), t1451 (13), t1606 (1), t2011 (5), t2346 (1), t2748 (1), t3041 (2), t4208 (2), t17304 (1), t17627 (1).
In 2014, spa‐type: t011 (1).
In 2015, spa‐type: t011.
In 2016, spa‐type: t034 CC398 (1).
In 2017, spa‐types: t091 CC7 (1 isolate), t843 CC130 (1), t6292 CC425 (1). The t091 isolate was PVL negative.
spa‐types not reported.
7.2. Susceptibility testing of meticillin‐resistant Staphylococcus aureus isolates
In 2017, data on the antimicrobial susceptibility of MRSA isolates were reported by Belgium, Finland, Switzerland and Sweden (Table 7). All countries used a broth dilution method and applied EUCAST ECOFFs to determine the susceptibility of isolates. As expected, all MRSA isolates were resistant to penicillin and cefoxitin. Tetracycline resistance was extremely high in MRSA isolates from Swiss calves (100%), Belgian broiler and laying hen flocks (100%), Swiss fattening pigs (100%), Finnish pig meat (100%); and all but one single isolate (spa‐type t127) were spa‐types associated with CC398. This was expected as livestock‐associated MRSA isolates belonging to CC398 are usually tetracycline resistant (Crombé et al., 2013).
The extremely high level of MRSA isolates showing resistance to trimethoprim and tiamulin in fresh pig meat from Finland (92.3% and 100%, respectively) presumably reflects the relatively common usage of this compound in pig medicine in Finland and many European countries. Resistance levels to these compounds were reported to be much lower in Swiss fattening pigs at slaughter (51.9% and 50.4%, respectively). Considering the isolates from Finnish pig meat, resistance to quinupristin/dalfopristin was also reported to be extremely high (100%); lincosamide and macrolide resistance were reported to be extremely high (100%) and high (30.8%), respectively. Isolates from Swiss fattening pigs at slaughter showed resistance in one or more isolates to all antimicrobials tested (with the exception of vancomycin and linezolid – see below). Again clindamycin resistance was observed at a higher prevalence when compared to erythromycin. Among the MRSA isolates reported in calves from Switzerland, lincosamide and macrolide resistance was reported at an equal extremely high level (70.8%). Similarly, clindamycin and erythromycin resistance was observed at an equal high level (60%) in Belgian broiler and laying hen flocks; chloramphenicol resistance was reported at 20%.
Linezolid and vancomycin are antimicrobials of last resort for treating S. aureus infections in humans. Where tested, all isolates were susceptible to vancomycin; which was as expected, since resistance to vancomycin is currently extremely rare in S. aureus. Although linezolid resistance was detected in two Belgian breeding pig LA‐MRSA isolates harbouring the plasmidic cfr gene in 2016, all countries reporting susceptibility data in 2017 tested isolates for linezolid susceptibility and all isolates proved susceptible.
Sweden reported one MRSA isolate from a pet cat, spa‐type t786, which only showed resistance to trimethoprim and the combination of trimethoprim + sulfonamide (in addition to cefoxitin and penicillin resistance, as expected). Eight MRSA isolates from pet dogs were reported by Sweden; spa‐types t008, t022, t032, t034, t127, t891, t2734 and t5634 and 5/8 were resistant to ciprofloxacin. Additionally, the canine isolates showed varied resistance patterns to gentamicin, erythromycin, clindamycin, fusidic acid, trimethoprim, tetracycline and the combination of trimethoprim + sulfonamide; illustrating the diversity of spa‐types reported. Fusidic acid resistance was reported in a MRSA isolate from a Swedish pet rabbit; no other resistance was observed in the lapine isolate (with the exception of cefoxitin and penicillin). This isolate was spa‐type t132. Among the low number (N = 7) of MRSA isolates from domestic horses reported by Sweden, all showed resistance to gentamicin, tetracycline and trimethoprim. Resistance to ciprofloxacin, erythromycin, clindamycin and the combination of trimethoprim + sulfonamide ranged from extremely high to high (71.4%, 57.1%, 28.6% and 28.6%, respectively). These isolates were spa‐types t011 and t1257. Notably, one t011 isolate was susceptible to oxacillin, with the MIC at the ECOFF.
Sweden reported 10 mecC‐MRSA isolates, spa‐types t373 and t9268, from goats following on‐farm clinical investigations; no resistance was recorded to antimicrobials with the exception as expected of cefoxitin (10/10) and penicillin (10/10), oxacillin was not tested. mecC‐MRSA was also detected in two sheep at a zoo. The isolates were spa‐type t9268 and had a similar resistance pattern, only showing resistance to β‐lactams (cefoxitin and penicillin; oxacillin was not tested) (Table 57).
Table 57.
Occurrence of resistance (%) to selected antimicrobials in MRSA from food and animals, 2017
| Country | N | GEN | KAN | STR | CHL | RIF | CIP | ERY | CLI | Q/D | TIA | MUP | FUS | SMX | TMP | T+S | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cats – pet animals | |||||||||||||||||
| Sweden | 1a | 0 | – | – | 0 | – | 0 | 0 | 0 | – | – | – | 0 | – | 100 | 100 | 0 |
| Cattle (bovine animals) – calves (under 1 year) | |||||||||||||||||
| Switzerland | 24b | 20.8 | 25 | 62.5 | 0 | 0 | 41.7 | 70.8 | 70.8 | 37.5 | 37.5 | 0 | 0 | 0 | 50 | – | 100 |
| Dogs – pet animals | |||||||||||||||||
| Sweden | 8c | 12.5 | – | – | 0 | – | 62.5 | 25 | 25 | – | – | – | 12.5 | – | 37.5 | 12.5 | 12.5 |
| Gallus gallus (fowl) – broiler and laying hen flocks | |||||||||||||||||
| Belgium | 5d | 20 | 40 | 20 | 20 | 20 | 40 | 60 | 60 | 40 | 20 | 0 | 0 | 60 | 60 | – | 100 |
| Goats | |||||||||||||||||
| Sweden | 10e | 0 | – | – | 0 | – | 0 | 0 | 0 | – | – | – | 0 | – | 0 | 0 | 0 |
| Meat from pigs – fresh | |||||||||||||||||
| Finland | 13f | 0 | 0 | 30.8 | 0 | 0 | 0 | 30.8 | 100 | 100 | 100 | 0 | 0 | 0 | 92.3 | –– | 100 |
| Switzerland | 2g | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | 50 |
| Pigs – fattening animals | |||||||||||||||||
| Switzerland | 131h | 11.5 | 12.2 | 51.1 | 1.5 | 2.3 | 11.5 | 45 | 51.1 | 50.4 | 50.4 | 2.3 | 3.1 | 6.1 | 51.9 | – | 100 |
| Rabbits – pet animals | |||||||||||||||||
| Sweden | 1i | 0 | – | – | 0 | – | 0 | 0 | 0 | – | – | – | 100 | – | 0 | 0 | 0 |
| Sheep | |||||||||||||||||
| Sweden | 2j | 0 | – | – | 0 | – | 0 | 0 | 0 | – | – | – | 0 | – | 0 | 0 | 0 |
| Solipeds, domestic horses | |||||||||||||||||
| Sweden | 7k | 100 | – | – | 0 | – | 71.4 | 57.1 | 28.6 | – | – | – | 0 | – | 100 | 28.6 | 100 |
N: Number of isolates tested; GEN: gentamicin; KAN: kanamycin; STR: streptomycin; CHL: chloramphenicol; RIF: rifampicin; CIP: ciprofloxacin; ERY: erythromycin; CLI: clindamycin;
Q/D: quinupristin/dalfopristin; TIA: tiamulin; MUP: mupirocin; FUS: fusidic acid; SMX: sulfamethoxazole; TMP: trimethoprim; T+S; trimethoprim + sulfonamide; TET: tetracycline;
–: No data reported.
All MRSA isolates were resistant to penicillin and cefoxitin, as expected. All isolates were susceptible to vancomycin (where tested) and linezolid.
spa‐type: t786 (1 isolate). The t786 isolate was PVL negative.
spa‐types: t011 (14 isolates), t034 (7), t127 (1), t17339 (2). PVL status of the t127 isolate was not reported.
spa‐types: t008 (1), t022 (1), t032 (1), t034 (1), t127 (1), t891 (1), t2734 (1), t5634 (1). t891 isolate was PVL positive; spa‐types t008, t022, t032, t127, t2734, t5634 were PVL negative.
spa‐types: t011 CC398 (3 isolates), t1456 CC398 (2).
spa‐types: t373 (9 isolates), t9268 (1).
spa‐types: t034 (11 isolates), t011 (1), t2741 (1).
spa‐types: t011 (1 isolate), t002 (1). PVL status of the t002 isolate was not reported.
spa‐types: t034 (63 isolates), t011 (61), t899 (2), t1451 (3), t2330 (1), t2876 (1).
spa‐type: t132 (1 isolate). The t132 isolate was PVL negative.
spa‐types: t9268 CC130 (1 isolate), t9268 (1).
spa‐types: t011 (5 isolates), t1257 (2). The t1257 isolates were PVL negative.
7.3. Discussion
The monitoring of MRSA in animals and food was voluntary in 2017 and only a limited number of countries reported data on the occurrence of MRSA, with some countries additionally reporting data on spa‐type and antimicrobial susceptibility. Where typing data were available, most MRSA isolates detected were those associated with LA‐MRSA (95.1%); Figure 1 provides an overview of the types of MRSA detected.
In 2017, monitoring of food comprised investigations of various food products including meat derived from different animal sources. The monitoring of MRSA in various food products performed by MSs consistently indicates that MRSA can be detected, quite frequently, in different types of food. Such food included meat from cattle, pigs and rabbits in 2017. It should be underlined that the laboratory techniques used to detect MRSA employ selective bacterial culture and therefore, very low levels of contamination can be detected. Cross‐contamination between carcases on slaughterhouse lines or during production processes may also result in a higher prevalence in meat produced from animals than in the animals themselves. LA‐MRSA is considered a poor coloniser of humans and occurs uncommonly in persons without direct or indirect contact with livestock or their carcases (Graveland et al., 2010). Although a previous report has cautiously suggested that some strains of LA‐MRSA may be adapted to colonise and infect humans and implicate poultry meat as a possible source for humans (Larsen et al., 2016), food is not generally considered to be a significant source of MRSA infection or colonisation of humans (EFSA, 2009b). A recent risk assessment published by the UK Food Standards Agency, reached the same conclusion (FSA, 2017).
The spa‐typing and susceptibility data reported in 2017 provided useful information in categorising MRSA isolates. Further typing data would in many cases provide extremely useful additional information to aid classification and help assess the origin and significance of the MRSA isolates. For example, possession of the IEC genes (chp, sak and scn) is considered an adaptation facilitating colonisation and infection of humans and is not usually a feature of animal strains (Cuny et al., 2015; Larsen et al., 2016). Similarly, the presence of the PVL toxin is a virulence feature typically associated with most CA‐MRSA strains; other genetic factors can be associated with particular strains or may suggest a particular host preference (e.g. lukM has been associated with certain animal strains).
spa‐typing data were available for 15/80 MRSA isolates from meat and most spa‐types detected were associated with CC398 (14/15). The remaining isolate, spa‐type t002 was recovered from fresh pig meat in Switzerland. spa‐type t002 has been associated with several sequence types within CC5, but is most commonly associated with ST5 (CC5), a sequence type which can be considered as either a community or healthcare associated MRSA. Although further molecular typing data (including PVL status) were not available, the isolate was considered likely to represent a HA‐MRSA lineage. In 2011, Monecke et al. documented that ST5‐MRSA‐II was the most frequently isolated strain from intensive care units in Dresden/Saxony (Monecke et al., 2011). In addition to β‐lactams (cefoxitin and penicillin), the t002 isolate was resistant to the aminoglycosides, gentamicin and kanamycin and susceptible to all other tested antimicrobials including streptomycin. Interestingly, a study carried out in the USA suggests that t002‐ST5 may also represent a livestock‐associated MRSA lineage, whereby this genotype was most frequently recovered during investigations focused on the short‐term exposures experienced by veterinary students conducting diagnostic enquiries on pig farms. The t002‐ST5 genotype accounted for 75% of MRSA isolates recovered from pigs, 83.8% of MRSA isolates from the farm environment, and 76.9% of MRSA isolates from veterinary students visiting these corresponding farms (Frana et al., 2013).
Considering food‐producing animals, spa‐types associated with each type of MRSA (LA‐MRSA, HA‐MRSA and CA‐MRSA), as well as mecC‐MRSA, were reported. In total, spa‐typing data were available for 530 MRSA isolates and most spa‐types detected were those associated with CC398 (525/530 isolates). Of the 525 categorised CC398 isolates, four novel spa‐types were reported (see text box below). The remaining five isolates which were not categorised as LA‐MRSA were spa‐types t091, t109, t127, t843 and t6292; the latter two spa‐types were confirmed to carry the mecC gene (see text box below). Concerning porcine isolates, spa‐type t091 was recovered from a Norwegian multiplier pig herd; and although additional molecular data was available (clonal complex 7, PVL‐negative), a MRSA category was not inferred. The PVL status of this isolate would suggest a HA‐MRSA lineage – as possession of the PVL toxin is typical of CA‐MRSA strains – however, meticillin‐sensitive S. aureus (MSSA) t091 isolates have been frequently reported in pigs/pork meat from south west Poland (Krupa et al., 2015). Therefore, the possibility that this MRSA genotype has emerged, through meticillin‐sensitive S. aureus in pigs acquiring the SCCmec cassette, cannot be discounted. Additionally, spa‐type t109 was recovered from a slaughter batch of Spanish fattening pigs; this spa‐type has been associated with ST5 and ST228 (both members of CC5), but is generally associated with ST228 and considered as a HA‐MRSA lineage. Concerning bovine isolates from food‐producing animals, a single t127 isolate was recovered from a Swiss calf at slaughter. Although spa‐type t127 has been associated with MRSA belonging to several sequence types within CC1, as well as to types in CC474, it is most frequently associated with ST1 (CC1); whereby this spa/sequence type combination represents a CA‐MRSA regardless of PVL status. The t127 isolate was therefore categorised as a CA‐MRSA, although the possible establishment of spa‐type t127 within livestock has also been reported. In a European harmonised baseline survey of breeding pig holdings, the potential clonal spread of spa‐type t127 (ST1) among Italian pig populations was documented (EFSA, 2009c; Franco et al., 2011). Subsequently, t127‐ST1 has frequently been detected among ruminants and/or their produce in Italy (Luini et al., 2015; Carfora et al., 2016; Ohno et al., 2016; Macori et al., 2017), suggesting that this genotype may also be circulating within livestock populations, and that its occurrence may not solely be due to transmission of human CA‐MRSA t127‐ST1 strains to animals. In addition, spa‐type t127 has also been reported in horses from Austria (Loncaric et al., 2014).
Detection of ‘new’ spa‐types in 2017
Principally, spa‐typing is a sequence‐based technique which analyses variable number tandem repeats (VNTR) in the 3’ coding region of the staphylococcal protein A gene (spa). Base sequences are assigned unique repeat codes, which comprise the repeat succession (spa repeats) for a given strain and determine spa‐type. Therefore, alterations to spa repeats may give rise to ‘new’ spa‐types, as a consequence of slipped strand mispairing during DNA synthesis (van Belkum, 1999).
Unlike spa‐typing, multilocus sequence typing (MLST) is a technique which types multiple loci; namely seven S. aureus housekeeping genes. The DNA sequences within each housekeeping gene are assigned as distinct alleles, and a sequence type/clonal lineage is allocated by comparing the set of alleles to other isolate profiles. Although some spa‐types can belong to several sequence types (some rarely possessing mosaic or hybrid genomes), generally most spa‐types are associated with a particular sequence type (Table 58).
Table 58.
VNTR compositions of spa‐types t17061, t17304, t17339 and t17627, and of common spa‐types associated with CC398
| spa‐type | VNTR/repeat succession a | spa repeat similarities |
|---|---|---|
| t2741b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 ‐ 16 | t17061 differs from t2741 by only one repeat |
| t17061 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ ‐ ‐ ‐ 25 ‐ 16 | |
| t1456b | 08 ‐ 16 ‐ 02 ‐ 25 | t17304 differs from t1456 by only one repeat |
| t17304 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 13 | |
| t034b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ ‐ ‐ ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17339 differs from t034 by only one repeat |
| t17339 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 51 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | |
| t011b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17627 differs from t011 by only one repeat |
| t17627 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 24 |
VNTR: variable number tandem repeats.
spa repeats as published on Ridom Spa Server (https://spa.ridom.de/spatypes.shtml).
Common spa‐types associated with CC398.
In 2017, spa‐types t17061, t17304 and t17627 were reported; MLST data were not available. Although these spa‐types appear not to have been previously sequenced typed, based upon similarities of spa repeats to other spa‐types associated with CC398, they were inferred to belong to CC398 – see Table 58. Additionally, Switzerland reported the novel spa‐type t17339 from two calves, which was confirmed to belong to CC398; spa repeats of t17339 are also shown in Table 58.
Considering the origins of these novel spa‐types – Finnish fattening pigs, Spanish fattening pigs and Swiss calves – their detection illustrates how rapidly S. aureus is able to evolve through repeat deletion, duplication and point mutation. In a study carried out by Boye and Westh (2010), the most frequent alteration observed was repeat deletion, followed by repeat duplication and point mutation. While ancestry cannot be surmised in this instance, the BURP (based upon repeat pattern) algorithm would provide a useful tool for determining the relationship between these closely‐related isolates.
Although the likelihood is that spa‐types t17061, t17304 and t17627 are also associated with CC398, the possibility that they possess mosaic or hybrid genomes cannot be definitively excluded, and EFSA recommend that novel spa‐types be sequence typed to confirm concordance between spa‐typing and assignment of a given isolate to a sequence type or lineage (EFSA, 2012b).
Table 58.
VNTR compositions of spa‐types t17061, t17304, t17339 and t17627, and of common spa‐types associated with CC398
| spa‐type | VNTR/repeat succession a | spa repeat similarities |
|---|---|---|
| t2741b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 ‐ 16 | t17061 differs from t2741 by only one repeat |
| t17061 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ ‐ ‐ ‐ 25 ‐ 16 | |
| t1456b | 08 ‐ 16 ‐ 02 ‐ 25 | t17304 differs from t1456 by only one repeat |
| t17304 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 13 | |
| t034b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ ‐ ‐ ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17339 differs from t034 by only one repeat |
| t17339 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 51 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | |
| t011b | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 25 | t17627 differs from t011 by only one repeat |
| t17627 | 08 ‐ 16 ‐ 02 ‐ 25 ‐ 34 ‐ 24 ‐ 24 |
VNTR: variable number tandem repeats.
spa repeats as published on Ridom Spa Server (https://spa.ridom.de/spatypes.shtml).
Common spa‐types associated with CC398.
Considering that the temporal prevalence of MRSA in Swiss fattening pigs at slaughter has shown a steady increase from 2009 to 2015, a more marked increase from 2015 to 2017 was observed. This marked increase represents the diffusion of spa‐types t011 and t034 within Swiss fattening pig populations; and in 2017, all reported isolates were those associated with CC398, with most belonging to spa‐types t011 and t034. Moreover, statistical tests performed on the Swiss longitudinal data revealed a statistically significant increasing trend over these years. A longitudinal study carried out by Köck et al. (2017) also supports these trends, in which MRSA prevalence of pig farms in western Switzerland were reported to increase from 7.3% in 2008 to 31% in 2015. The complete epidemiological data should however be considered when evaluating trends apparent in this chapter, because the summary data reported to EFSA may not include full details of any methodological or other changes to monitoring procedures. A detailed longitudinal study illustrated that pigs are often intermittently and repeatedly colonised, and that colonisation may also occur during transportation and while in the lairage (Bangerter et al., 2016). The detection of intermittent, repeated colonisation suggests that the number of animals sampled as part of a batch, including whether individual animals are sampled to represent a herd or batch, are likely to influence the batch or herd prevalence obtained. These factors should therefore be taken into consideration with regard to the statistical analyses, as the Swiss annual MRSA monitoring examines a single pig from a herd at slaughter. Regarding longitudinal data available for other countries, MRSA prevalence in German calves at slaughter declined from 2012 to 2017. A decline was also noted in German fattening pig herds from 2015 to 2017. The reasons for these modest declines were not apparent and are likely to reflect sampling variability, with no statistically significant differences detected; however findings are interesting because, generally, MRSA prevalence in animals and food has shown a progressive increase, where it has been investigated. For example, an increase was observed in batches of Finnish fattening pigs at slaughter from 2010 to 2017; illustrating the possible dissemination of spa‐types t034 and t2741 within Finnish fattening pig populations. Furthermore, a study conducted in 2015, identified spa‐type t2741 as a new dominant clone among Finnish fattening pigs at slaughter (Heikinheimo et al., 2016). Tests for statistical significance in relation to the changes in MRSA prevalence in Finnish fattening pigs at slaughter confirmed a statistically significant increasing trend from 2010 to 2017. Similarly, MRSA prevalence in Finnish pig meat was reported at a higher level in 2017 compared to that observed in 2015, with statistical analysis also detecting an increasing trend.
Lincosamide resistance and macrolide susceptibility is an unusual phenotype which may be conferred by lnu genes. In a study of Finnish fattening pigs at slaughter, this unusual phenotype was observed among some CC398 isolates, and was associated with isolates lacking ermB, but harbouring lnuB (Heikinheimo et al., 2016). Considering the susceptibility of MRSA isolates to clindamycin and erythromycin, there was an equal occurrence of resistance to both compounds in Swiss calves and Belgian chicken flocks, whereas clindamycin resistance exceeded that of erythromycin in Swiss pigs and Finnish pork; the latter phenotype suggesting the possible presence of lnu genes.
spa‐types associated with each type of MRSA (LA‐MRSA, HA‐MRSA and CA‐MRSA), as well as mecC‐MRSA, were also reported following clinical examinations carried out by Sweden in 2017; denominator data were not provided. mecC‐MRSA was reported in 10 goats and two sheep, discussed further in the text box below. Following veterinary‐clinic clinical investigations, Sweden reported spa‐type t1257 from two horses. This spa‐type has been associated with sequence types within CC8 and CC5, but appears to be more frequently associated with sequence type ST612 (CC8). Although the t1257‐ST612 genotype may be regarded as either a CA‐ or HA‐MRSA, Sweden confirmed that the isolates were PVL‐negative which is indicative of a probable healthcare‐associated lineage. Susceptibility testing revealed that both isolates were resistant to ciprofloxacin, erythromycin, tetracycline, trimethoprim, gentamicin, and the combination of trimethoprim + sulfonamide (in addition to β‐lactams); which reflects the fact that these horses were in the same animal hospital so transmission between animals is a possibility. Additionally, livestock‐associated MRSA was reported in five Swedish horses; these were spa‐type t011 (associated with CC398) and all were tetracycline‐resistant. Kieffer et al. (2017) documented that LA‐MRSA CC398 has recently emerged as a significant cause of primarily nosocomial infections in horses. Considering Swedish companion animals, spa‐type t132 was reported from a pet rabbit. While MLST was not reported, this spa‐type is associated with ST45 (CC45), and the isolate was inferred to represent a healthcare‐associated MRSA due to its PVL‐negative status. With the exception of β‐lactams (penicillin and cefoxitin; oxacillin not tested), the isolate only showed resistance to fusidic acid. Conversely, ST45‐MRSA community‐associated lineages have also been recognised in humans. In particular, a ST45‐MRSA‐N1 clone was found among intravenous drug users and their contacts in Switzerland; whereby this clone was reported to be similar to the epidemic Berlin MRSA clone (Qi et al., 2005). Considering MRSA cases reported in dogs, Sweden reported the isolation of spa‐types t008, t022, t032, t034, t127, t891, t2734 and t5634; representing all three MRSA categories. spa‐types t034 and t2734 were attributed to LA‐MRSA, whereby these types are associated with CC398 and CC97, respectively. spa‐type t2734 has, however, also been recognised as a CA‐MRSA in Argentina (Egea et al., 2014). spa‐types t891 and t127 were attributed to CA‐MRSA, whereby these types are associated with ST22 (CC22) and most frequently ST1 (CC1), respectively. Both CA‐ and HA‐MRSA have been reported among ST22 isolates, however, spa‐type t891 was reported to be PVL‐positive suggesting a community‐associated lineage as CA‐MRSA frequently possess the PVL toxin, which may confer an increase in virulence, although the exact role of the PVL toxin has been debated (Chadwick et al., 2013). Conversely, spa‐type t127 was reported to be PVL‐negative, yet this genotype still possibly represents a CA‐MRSA regardless of PVL status. Considering the remaining canine isolates, spa‐types t008, t022, t032 and t5634, all were considered to represent HA‐MRSA. spa‐type t008 has been associated with many sequence types within CC8 (ST8, ST247, ST250 and ST254), but is most commonly associated with ST8; Sweden confirmed that the isolate belonged to ST8 from whole genome sequence data. This spa‐type and sequence type combination is seen in isolates of the globally significant CA‐MRSA USA300 strain, which is PVL positive and frequently possesses arginine catabolic mobile element (ACME) genes. The CA‐MRSA USA300 strain can cause severe infections in humans and has a markedly different epidemiology from HA‐MRSA strains (Tenover and Goering, 2009). However, Sweden confirmed that the isolate was PVL‐negative and ACME genes (arcA) were not detected. Therefore the isolate is likely to represent a HA‐MRSA. spa‐types t022, t032 and t5634 are all associated with ST22 (CC22) and both CA‐ and HA‐MRSA have been reported within this sequence type. All three spa‐types were however reported to be PVL‐negative and were therefore categorised as HA‐MRSA. The final isolate reported by Sweden was spa‐type t786 from a pet cat and although this isolate was not sequence typed, spa‐type t786 is associated with CC88 (sequence types ST78 and ST88). While the t786 isolate was reported to be PVL‐negative, the CC88 lineage is predominantly regarded as a community‐associated MRSA and was categorised as such. Detection of CA‐MRSA and HA‐MRSA within these companion animals most likely represents colonisation with human MRSA strains rather than persistent establishment within these species. This is supported by the common occurrence of some of these spa‐types within the Swedish human population.
mecC‐meticillin‐resistant Staphylococcus aureus reported in 2017
In 2017, a total of 14 mecC‐MRSA isolates were reported. These included two mecC‐MRSA isolates recovered from two Norwegian farrow‐to‐finish pig herds; spa‐types t843 and t6292, MLST confirming them to belong to CC130 and CC425, respectively. Antimicrobial resistance patterns were not reported. In a recent study, the occurrence of mecC‐MRSA in wild hedgehogs from three regions of Sweden was investigated (Bengtsson et al., 2017); whereby mecC‐MRSA was isolated from 64% of 55 wild hedgehogs and spa‐type t843 was most commonly found (49%).
In addition to the porcine isolates, ovine and caprine mecC‐MRSA isolates were also reported following zoo/on‐farm clinical investigations by Sweden. spa‐type t9268 was recovered from two sheep at a zoo, and sequence typing of one t9268 isolate confirmed it to belong to CC130. Considering the caprine isolates, spa‐types t373 (nine isolates) and t9268 (one isolate) were reported; both of which are associated with CC130 (SWEDRES, 2011; Petersen et al., 2013). From 2011 to mid‐2017, spa‐type t373 was the second (20/92 cases) most common domestically‐acquired mecC‐MRSA spa‐type reported in humans in Sweden (Swedres‐Svarm, 2017).
Resistance to non‐β‐lactam antibiotics is currently uncommon among mecC ‐MRSA isolates (Paterson et al., 2014) and, typically, the t373 and t9268 (caprine/ovine) isolates from clinical investigations were susceptible to non‐β‐lactams. Although Sweden did not report oxacillin susceptibility for the caprine/ovine isolates, all were resistant to penicillin and cefoxitin; oxacillin has been demonstrated to be a less reliable marker than cefoxitin for detection of mecC‐MRSA (Paterson et al., 2014; Bengtsson et al., 2017).
Considering the four different mecC‐MRSA spa‐types (t843, t6292, t373 and t9268) reported in 2017, all have previously been observed in humans (Swedres‐Svarm, 2017; Paterson et al., 2014; Stella et al., 2016) and possible transmission between humans and animals is well documented (Harrison et al., 2013; Petersen et al., 2013; Angen et al., 2017). Angen et al. (2017) identified the first case of mecC‐MRSA in domesticated pigs and findings strongly indicated transmission between farmers and pigs. Additionally, the study carried out by Bengtsson et al. (2017) supports the hypothesis that wildlife may constitute a reservoir of mecC‐MRSA.
Notably, in the study carried out by Bengtsson et al. (2017), only 4/35 mecC‐MRSA were detected using a TSB with 4% salt, 3.5 mg/L cefoxitin and 50 mg/L aztreonam; while 35/35 mecC‐MRSA were detected using a TSB with 4% salt and 10 mg/mL aztreonam. Therefore, it will be interesting to note future mecC‐MRSA prevalence in accordance with changes to recommended isolation methods.
Figure 84.
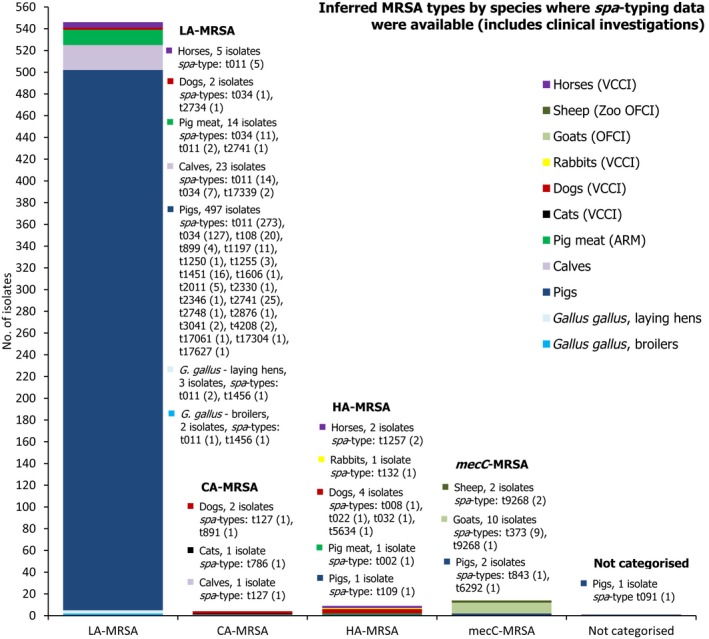
Overview of MRSA types by species reported in 2017, including healthy animals and clinical investigations
ST/CC and MRSA categories have mostly been inferred from spa‐typing data; MLST was only carried out on a few isolates. spa‐type t091 was not categorised as either HA‐MRSA or LA‐MRSA. In total, 574 MRSA isolates were spa‐typed. VCCI: At‐veterinary‐clinic clinical investigation; OFCI: On‐farm clinical investigations; ARM: At‐retail monitoring.
In summary, the monitoring of MRSA in 2017 has provided extremely useful information on the occurrence of MRSA in livestock and food. The situation continues to develop and evolve and there is a clear requirement for the continued monitoring and appropriate molecular characterisation of MRSA isolates recovered from livestock and food. Molecular characterisation is becoming increasingly necessary to fully evaluate the significance of MRSA isolates and there are limitations to the analyses which can be performed when spa‐typing is used as the only technique to characterise isolates. Conversely, the presence of the PVL toxin may not always be indicative of CA‐MRSA, highlighted by genotypes t786‐CC88 and t127‐CC1 which are predominantly community‐associated lineages yet lack PVL. Notably, the movement of live animals, as well as human travel, are important contributing factors to the spread of MRSA between countries, and therefore the occurrence data contained in this report may reflect such circumstances. Most reporting countries did not report susceptibility data for MRSA isolates, which also provides useful information for characterising isolates. The monitoring also includes some new findings: spa‐types t17061, t17304, t17339 and t17627 were reported from food‐producing animals, and these spa‐types appear not to have been reported previously. Although the likelihood is that t17061, t17304 and t17627 are associated with CC398, the findings once again illustrate the limitations of spa‐typing as a single method of definitively assigning novel isolates to particular lineages, where MLST has not previously been undertaken.
Surveillance and control of LA‐MRSA in the Norwegian pig population
Carl Andreas Grøntvedt and Anne Margrete Urdahl, Norwegian Veterinary Institute, P.O. Box 750 Sentrum, N‐0106 Oslo, Norway
There are three broad categories of MRSA, one of which is associated with animals (especially swine), collectively referred to as LA‐MRSA (livestock‐associated MRSA). Within a few years, LA‐MRSA became widespread in swine populations around the world, thereby representing a risk for dissemination to the human population. LA‐MRSA in European swine has mainly been attributed to clonal complex (CC) 398, though other MRSA belonging to other CCs may also show evidence of persistence and transmissibility in livestock holdings and thereby be classified as an LA‐MRSA.
Norway is the only country in the world to have implemented a control strategy from 2013 including measures to eradicate LA‐MRSA in swine (Grøntvedt et al., 2016). The rationale behind this strategy was to prevent the swine population from becoming a domestic reservoir of MRSA with the potential of zoonotic transmission, as MRSA is not a significant cause of disease in swine. Norway has adopted an epidemiologically based definition of LA‐MRSA that includes types of MRSA with evidence of persistence and transmissibility in livestock holdings. The LA‐MRSA surveillance and control strategy includes annual pig population screening, restrictions on trade of live animals upon suspicion, depopulation of pigs within LA‐MRSA positive pig holdings, and thorough cleaning and disinfection of premises before restocking with pigs from MRSA‐negative holdings (Grøntvedt et al., 2016). After restocking, samples are collected from animals and the environment to assess the effectiveness of the MRSA eradication. Results from follow‐up testing after restocking demonstrate that LA‐MRSA eradication has been successful in the first attempt in more than 90% of the pig farms, and that only a few farms need to go through more than one eradication process.
A yearly surveillance programme on MRSA in the swine population was implemented from 2014 in Norway. In the first year, all sow herds with more than 10 sows were examined (n = 986 herds) and a single positive herd with MRSA CC398, t011 was identified (Urdahl et al., 2015). In 2015, a total of 821 herds were included, of which 86 were nucleus or multiplier herds and 735 were finishing herds (Urdahl et al., 2016). MRSA was identified in four herds; three finishing herds and one multiplier herd. The isolates from two finishing herds were typed as CC1, t177 and further outbreak tracing showed that the two herds belonged to the same cluster of positive herds. The last two herds were not linked, but both were positive for MRSA CC398, t034 (Urdahl et al., 2016). In 2016, a total of 872 herds were investigated, of which 87 were genetic nucleus or multiplier herds, 12 sow pool herds and 773 herds with more than 10 sows (Urdahl et al., 2017). MRSA was not detected in any of the genetic nucleus, multiplier or sow pool herds. LA‐MRSA CC398, t034 was, however, identified in one herd that had recently converted to a specialised finisher herd. Follow‐up testing of contact herds revealed two additional herds positive for the same CC and spa‐type, and eradication was initiated. The surveillance programme in 2017 did not detect any herds with LA‐MRSA CC398 (Urdahl et al., 2017). However, single MRSA isolates belonging to spa‐types t091 (CC7), t843 (CC130) and t6292 (CC425) were detected in one multiplier herd and in two farrow to finish herds, respectively; the latter two isolates of spa‐types t843 and t6292 were confirmed to carry the mecC gene. MRSA was not detected in any of the genetic nucleus herds, nor in the central units of the sow pool herds. In total, 826 herds were included in the survey, of which 85 were genetic nucleus or multiplier herds. 12 herds were the central units of sow pool herds, and 729 were herds with more than 10 sows.
Population surveillance, outbreak investigations and measures to eradicate LA‐MRSA from pig farms are costly and labour‐intensive strategies. However, the imposed strategy has probably contributed substantially in preventing further dissemination and increased prevalence of LA‐MRSA among pig farms and humans in Norway (Grøntvedt et al., 2016). The strategy is therefore considered relevant under Norwegian conditions, presently characterised by a low overall prevalence of MRSA (including LA‐MRSA) in humans; few primary introductions of LA‐MRSA to the pig population; effective eradication of MRSA from positive pig farms, thereby preventing further transmission among pig farms; and an essentially closed pig population. Changes in these conditions may influence the authorities’ choice of strategy regarding LA‐MRSA in the future.
Abbreviations
- %
percentage of resistant isolates per category of susceptibility or multiple resistance
- % f
percentage frequency of isolates tested
- % Res
percentage of resistant isolates
- –
no data reported
- AAC
aminoglycoside acetyltransferases
- AME
aminoglycosides modifying enzyme
- AMR
antimicrobial resistance
- ANT
aminoglycoside nucleotidyltransferase
- APH
aminoglycoside phosphotransferases
- APHA
Animal and Plant Health Agency
- ARM
At‐retail monitoring
- AST
antimicrobial susceptibility testing
- BIOHAZ
EFSA Panel on Biological Hazards
- BS
boot swab
- CA
community‐associated
- CA‐SFM
French Society for Microbiology
- CBP
clinical breakpoints
- CC
clonal complex
- CIA
critically important antimicrobials
- CLSI
Clinical and Laboratory Standards Institute
- CP
carbapenemase producer
- CPE
carbapenemase‐producing Enterobacteriaceae
- CTX‐M
cefotaxime
- DD
disc diffusion method
- DIN
Deutsches Institut für Normung
- DL
dilution/dilution method
- DLG
dilution with gradient step
- EARS‐Net
European Antimicrobial Resistance Surveillance Network
- ECDC
European Centre for Disease Prevention and Control
- ECOFF
epidemiological cut‐off value
- EEA
European Economic Area
- EFSA
European Food Safety Authority
- EJP
European Joint Program
- EPIS‐FWD
Epidemic Intelligence System for Food‐ and Waterborne Diseases
- EQA
external quality assessment
- ESBL
extended‐spectrum beta‐lactamase
- ESC
extended‐spectrum cephalosporins
- EU
European Union
- EUCAST
European Committee on Antimicrobial Susceptibility Testing
- EURL‐AR
EU Reference Laboratory for Antimicrobial Resistance (http://www.crl-ar.eu)
- FM
at‐farm monitoring
- FWD
food‐ and waterborne diseases and zoonoses
- HA
healthcare‐associated
- HS
hide sample
- I
intermediate
- IEC
immune evasion cluster
- IR
inverted repeat
- LA
livestock‐associated
- MDR
multidrug resistance
- MDRGI
multidrug‐resistant genomic island
- MHB
Mueller–Hinton broth
- MIC
minimum inhibitory concentration
- MLST
multilocus sequence typing
- MLVA
multiple‐locus variable number tandem repeat analysis
- MRSA
meticillin‐resistant Staphylococcus aureus
- MS
Member State
- MSSA
meticillin‐susceptible Staphylococcus aureus
- NA
not applicable/not available
- NARMS
National Antimicrobial Resistance Monitoring System
- NCP
National Control Programme
- NFCEP
National Farm Control and Eradication Programme,
- NRL
National Reference Laboratory
- NS
nasal swab
- OFCEP
On‐farm control and eradication programme
- OFCI
On‐farm clinical investigations
- OFM
on‐farm monitoring
- PMQR
plasmid‐mediated quinolone resistance
- Q
quantitative
- QRDR
quinolone resistance‐determining regions
- PCR
polymerase chain reaction
- pESI
plasmid for emerging S. Infantis
- PVL
Panton‐Valentine leukocidin
- R
resistant
- res1–res9
resistance to one antimicrobial substance/resistance to nine antimicrobial substances of the common set for Salmonella
- S
susceptible
- SHM
at slaughterhouse monitoring
- SIR
susceptible, intermediate or resistant
- ST
sequence type
- TESSy
The European Surveillance System
- TSB
Tryptone Soya Broth
- VCCI
at‐veterinary‐clinic clinical investigation
- VNTR
variable number tandem repeats
- WGS
whole genome sequencing
- WHO
World Health Organization
Antimicrobial substances
- AMC
amoxicillin/clavulanate
- AMP
ampicillin
- AZM
azithromycin
- CAZ
ceftazidime
- CHL
chloramphenicol
- CIP
ciprofloxacin
- CLA
clavulanate
- CLI
clindamycin
- CST
colistin
- CTX
cefotaxime
- ERY
erythromycin
- FUS
fusidic acid
- GEN
gentamicin
- KAN
kanamycin
- LZD
linezolid
- MEM/MER
meropenem
- MUP
mupirocin
- NAL
nalidixic acid
- QD
quinupristin/dalfopristin
- RIF
rifampicin
- SUL
sulfonamides
- STR
streptomycin
- SXT
sulfamethoxazole
- TGC
tigecycline
- TIA
tiamulin
- TET
tetracycline
- TMP
trimethoprim
MSs of the EU and other reporting countries in 2015
- Austria
AT
- Belgium
BE
- Bulgaria
BG
- Croatia
HR
- Cyprus
CY
- Czech Republic
CZ
- Denmark
DK
- Estonia
EE
- Finland
FI
- France
FR
- Germany
DE
- Greece
GR
- Hungary
HU
- Ireland
IE
- Italy
IT
- Latvia
LV
- Lithuania
LT
- Luxembourg
LU
- Malta
MT
- Netherlands
NL
- Poland
PL
- Portugal
PT
- Romania
RO
- Slovakia
SK
- Slovenia
SI
- Spain
ES
- Sweden
SE
- United Kingdom
UK
Non‐MSs reporting, 2016
- Iceland
IS
- Norway
NO
- Switzerland
CH
Definitions
- Antimicrobial‐resistant isolate
In the case of quantitative data, an isolate was defined as ‘resistant’ to a selected antimicrobial when its minimum inhibitory concentration (MIC) value (in mg/L) was above the cut‐off value or the disc diffusion diameter (in mm) was below the cut‐off value. The cut‐off values, used to interpret MIC distributions (mg/L) for bacteria from animals and food, are shown in Material and methods, and Tables 5, 6 and 7. In the case of qualitative data, an isolate was regarded as resistant when the country reported it as resistant using its own cut‐off value or break point
- Level of antimicrobial resistance
The percentage of resistant isolates among the tested isolates
- Reporting MS group
MSs that provided data and were included in the relevant table for antimicrobial resistance data for the bacteria–food/animal category–antimicrobial combination
- Terms used to describe the antimicrobial resistance levels
Rare: < 0.1% Very low: 0.1% to 1.0% Low: > 1.0% to 10.0% Moderate: > 10.0% to 20.0% High: > 20.0% to 50.0% Very high: > 50.0% to 70.0% Extremely high: > 70.0%
Appendix A – Antimicrobial resistance in Salmonella serovars recovered from the mandatory carcase swabbing of fattening pig s at slaughter
Distribution of Salmonella serovars in fattening pig carcases
Serovar information was provided for all Salmonella spp. (N = 960); the most common serovars detected in fattening pig carcases (Table SERPIGCARCD) were monophasic S. Typhimurium (15 MSs and one non‐MS, 34.8%), S. Derby (19 MSs, 26.5%) and S. Typhimurium (17 MSs, 12.8%) – see Figure A.1. Resistance and MDR levels in S. Derby were much lower than those reported in S. Typhimurium, monophasic S. Typhimurium and Salmonella spp. Additionally, overall MDR was higher in monophasic S. Typhimurium than in S. Typhimurium isolates.
Figure A.1.
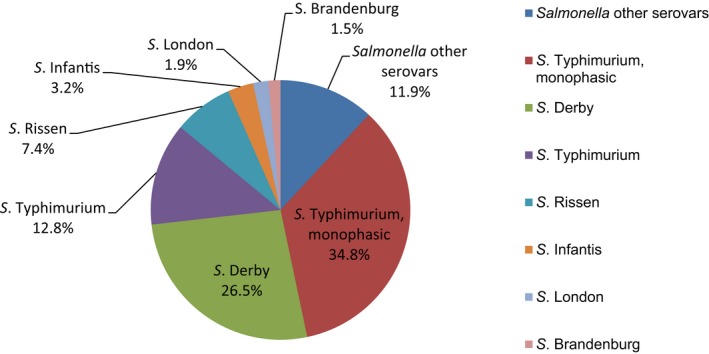
Breakdown of Salmonella serovars in fattening pig carcases, 22 EU MSs and one non‐EU MS, 2017 (N = 960)
Resistance in S. Derby from fattening pig carcases
Resistance levels in S. Derby from fattening pig carcases
S. Derby was the second most frequently reported serovar recovered from fattening pig carcases in 2017, accounting for 26.5% of Salmonella isolates serotyped (N = 960). Notably in 10 MSs, resistance levels were assessed on less than 10 isolates. Among a total of 254 isolates reported by 19 MSs (Table A.1), ampicillin and chloramphenicol resistance were observed at low levels (9.8% and 3.5%, respectively). Resistance to cefotaxime and ceftazidime was only reported by Germany, resulting in an overall very low level of resistance (0.4%), while overall resistance to nalidixic acid and ciprofloxacin were reported at low levels (1.2% and 2.4%, respectively). Resistance to sulfamethoxazole and tetracycline ranged from not detected to extremely high (0–100%), with high overall resistance (33.9% and 29.9%, respectively). Trimethoprim resistance was reported in isolates from 11/19 MSs, resulting in an overall moderate level of resistance, while gentamicin resistance was detected by only one MS. Resistance to meropenem or azithromycin was not detected by any reporting MSs. France was the only country to detect tigecycline resistance in a single S. Derby isolate, resulting in an overall very low level of resistance to this compound considering all reporting MSs. Similarly, a single S. Derby isolate reported by German y (N =7) was resistant to colistin; resulting in an overall very low level of resistance to this compound considering all reporting MSs (Table DERBYPIGMEATD).
Table A.1.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Derby from fattening pig carcases, using harmonised ECOFFs, 19 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 17 | 0 | 5.9 | 17.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 47.1 | 23.5 | 17.6 |
| Bulgariaa | 2 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 50 |
| Croatiaa | 3 | 0 | 33.3 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 33.3 | 33.3 |
| Czech Republic | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9.1 |
| Denmark | 22 | 0 | 9.1 | 18.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22.7 | 22.7 | 22.7 |
| Estoniaa | 9 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 | 11.1 | 0 |
| France | 62 | 3.2 | 1.6 | 4.8 | 0 | 0 | 0 | 1.6 | 0 | 0 | 0 | 0 | 62.9 | 1.6 | 58.1 |
| Germanya | 7 | 0 | 14.3 | 14.3 | 14.3 | 14.3 | 0 | 0 | 14.3 | 14.3 | 0 | 14.3 | 0 | 0 | 0 |
| Hungary | 10 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 20 | 40 |
| Irelanda | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 37.5 | 37.5 | 37.5 |
| Italy | 50 | 0 | 6 | 12 | 0 | 0 | 0 | 0 | 4 | 6.0 | 0 | 0 | 30 | 12 | 28 |
| Latviaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lithuaniaa | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 |
| Maltaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Polanda | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 62.5 | 25 | 25 |
| Portugal | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.6 | 0 | 5.6 |
| Slovakia | 13 | 0 | 0 | 7.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20.0 | 0 | 0 | 30 | 20 | 30 |
| United Kingdoma | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (19 MSs) | 254 | 0.8 | 3.5 | 9.8 | 0.4 | 0.4 | 0 | 0.4 | 1.2 | 2.4 | 0 | 0.4 | 33.9 | 11 | 29.9 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance among S. Derby from fattening pig carcases
The spatial distributions of cefotaxime and ciprofloxacin resistance in S. Derby isolates recovered from pig carcases are shown in Figure A.2 (and consider MSs reporting data on less than 10 isolates). Cefotaxime resistance was only detected by Germany (N = 7), with resistance to this compound reported at a moderate level (14.3%). Fluoroquinolone resistance was also observed in Germany at a moderate level (14.3%), as well as in southern Europe, where Spain (N = 10) and Italy (N = 50) reported moderate (20%) and low (6%) levels, respectively.
Figure A.2.
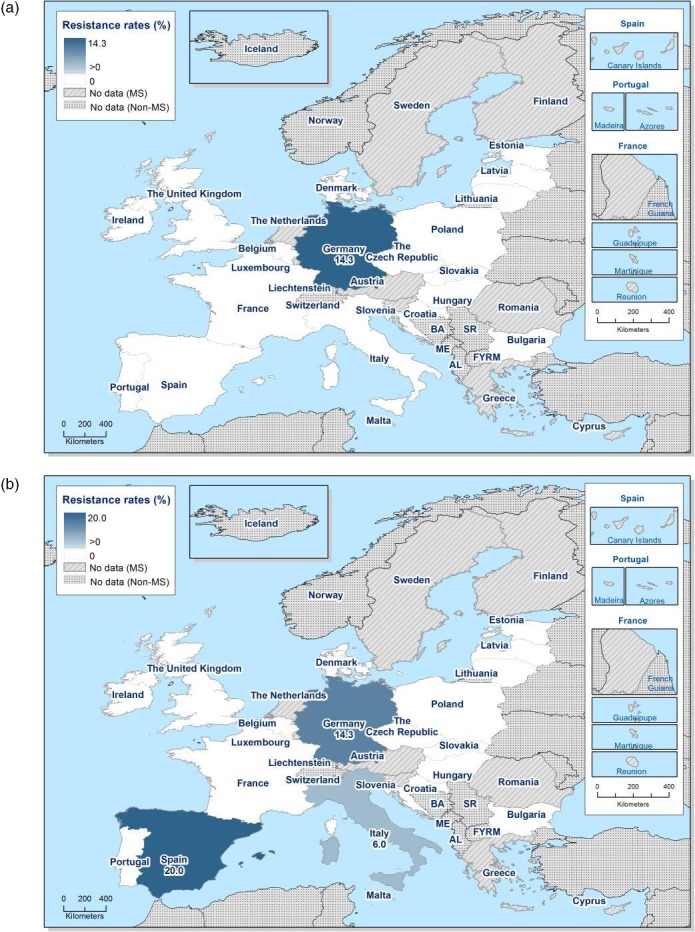
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella Derby from fattening pig carcases in countries reporting MIC data in 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Derby from fattening pig carcases
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among S. Derby isolates from pig carcases was not detected by any of the reporting MSs (Figure A.3).
Figure A.3.
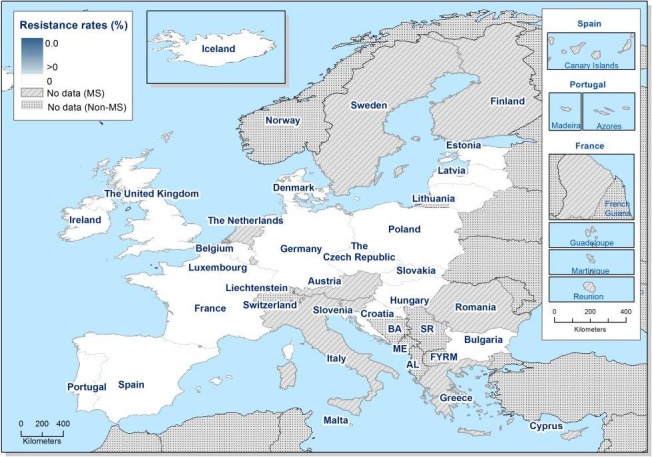
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in Salmonella Derby from fattening pig carcases, using harmonised ECOFFs, EU MSs, 2017.
Multidrug resistance and complete susceptibility in S. Derby from fattening pig carcases
Nineteen MSs reported data on 254 individual S. Derby isolates, which were included in the MDR analysis (Figure A.4). Overall, 57.9% of S. Derby isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 100%), while 11.8% of S. Derby isolates were multiresistant (MDR ranging from 0% to 50%) – see Table COMDERBYPIGMEAT. Notably, where complete susceptibility was detected in 100% of isolates, only one S. Derby isolate was reported by the MSs for assessment.
Figure A.4.
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Derby from fattening pig carcases, 19 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Resistance in monophasic S. Typhimurium from fattening pig carcases
Resistance levels in monophasic S. Typhimurium from fattening pig carcases
Monophasic S. Typhimurium was the most frequently reported serovar recovered from pig carcases in 2017, accounting for 34.8% of the Salmonella isolates serotyped (N = 960). Notably, in eight reporting countries, resistance levels were assessed on less than 10 isolates. Among a total of 332 isolates reported by 15 MSs (Table A.2), ampicillin, sulfamethoxazole and tetracycline resistance were observed at overall extremely high levels (86.4%, 87.7% and 81%, respectively). Trimethoprim resistance was reported in isolates from 8/15 MSs, resulting in an overall low level of resistance, while azithromycin resistance was detected by only two MSs. Overall, resistance to gentamicin, chloramphenicol, nalidixic acid and ciprofloxacin was low. No resistance to cefotaxime, ceftazidime, meropenem or tigecycline was reported in monophasic S. Typhimurium isolates recovered from pig carcases. Germany and Spain were the only countries to detect colistin resistance, with both countries reporting two colistin‐resistant isolates (Table MOTYPHIPIGMEATD). Additionally, Iceland (non‐MS) reported data on two monophasic S. Typhimurium isolates.
Table A.2.
Occurrence of resistance (%) to selected antimicrobials in monophasic Salmonella Typhimurium from fattening pig carcases, using harmonised ECOFFs, 15 EU MSs and 1 non‐MS, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 25 | 0 | 8 | 96 | 0 | 0 | 0 | 0 | 8 | 8 | 0 | 0 | 96 | 16 | 76 |
| Croatia | 12 | 0 | 8.3 | 83.3 | 0 | 0 | 0 | 0 | 8.3 | 8.3 | 0 | 0 | 83.3 | 0 | 83.3 |
| Czech Republica | 3 | 0 | 0 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66.7 | 0 | 100 |
| Denmark | 29 | 10.3 | 10.3 | 79.3 | 0 | 0 | 0 | 0 | 0 | 0 | 3.4 | 0 | 86.2 | 10.3 | 65.5 |
| France | 89 | 1.1 | 0 | 79.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 88.8 | 5.6 | 74.2 |
| Germany | 10 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 20 | 100 | 0 | 80 |
| Greecea | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 |
| Ireland | 21 | 38.1 | 19 | 90.5 | 0 | 0 | 0 | 0 | 9.5 | 9.5 | 0 | 0 | 90.5 | 23.8 | 95.2 |
| Italy | 30 | 6.7 | 20 | 80 | 0 | 0 | 0 | 0 | 3.3 | 13.3 | 0 | 0 | 80 | 10 | 80 |
| Maltaa | 2 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 |
| Polanda | 5 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 80 |
| Portugala | 5 | 0 | 40 | 80 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 40 | 100 |
| Slovakiaa | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Spain | 97 | 3.1 | 8.2 | 93.8 | 0 | 0 | 0 | 0 | 3.1 | 4.1 | 1 | 2.1 | 87.6 | 4.1 | 89.7 |
| United Kingdoma | 2 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 |
| Total (15 MSs) | 332 | 5.1 | 7.8 | 86.4 | 0 | 0 | 0 | 0 | 2.7 | 4.2 | 0.6 | 1.2 | 87.7 | 8.1 | 81 |
| Icelanda | 2 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 50 | 0 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates.
Spatial distribution of resistance in monophasic S. Typhimurium from fattening pig carcases
No resistance to cefotaxime was detected in monophasic S. Typhimurium isolates recovered from pig carcases by 15 MSs and 1 non‐MS (Figure A.5). Spatial distributions of ciprofloxacin resistance revealed no distinct geographical patterns; resistance to this compound was reported at a low level in five MSs (Belgium, Croatia, Germany, Ireland and Spain) and at a moderate level in Italy.
Figure A.5.
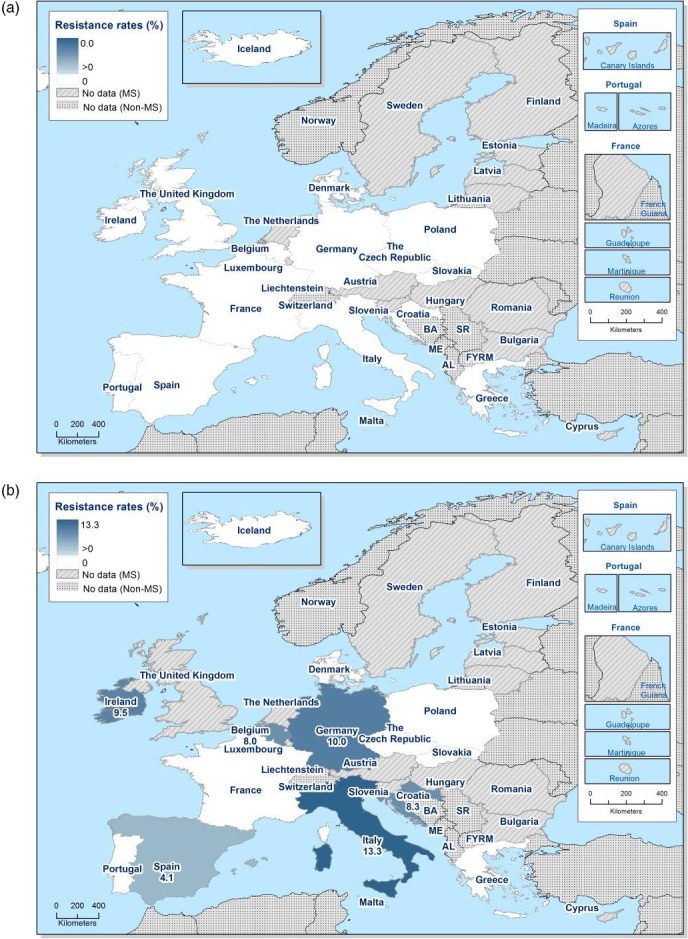
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance in monophasic Salmonella Typhimurium from fattening pig carcases in countries reporting MIC data, 2017
Combined resistance to ciprofloxacin and cefotaxime in monophasic S. Typhimurium from fattening pig carcases
Combined ‘microbiological’ resistance to ciprofloxacin and cefotaxime among monophasic S. Typhimurium isolates from pig carcases was not detected by any reporting country.
Multidrug resistance and complete susceptibility in monophasic S. Typhimurium from fattening pig carcases
In 2017, 15 MSs and 1 non‐MS reported quantitative MIC data for 334 monophasic S. Typhimurium isolates recovered from pig carcases (Figure A.6). In contrast to S. Derby isolates from pig carcases, an extremely high proportion of isolates were multiresistant; MDR was also reported at a higher level than that in S. Typhimurium isolates (64.2%). Overall, 5.4% of monophasic S. Typhimurium isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 50%), while 77.2% of isolates were multiresistant (MDR ranging from 50% to 100%) – see Table COMMOTYPHIPIGMEAT. Notably, where MDR was detected at 100%, only one monophasic S. Typhimurium isolate was reported by MSs for assessment.
Figure A.6.
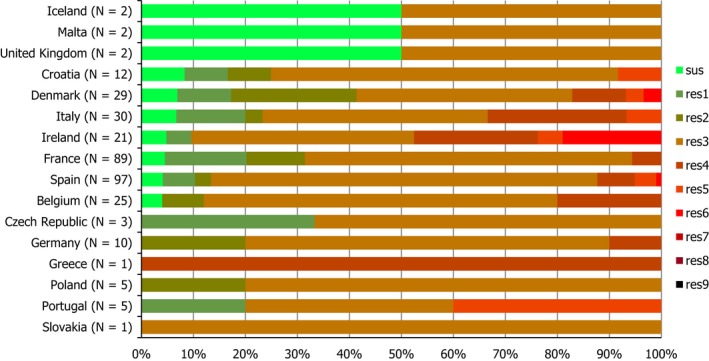
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in monophasic S. Typhimurium from fattening pig carcases, 15 MSs and 1 non‐EU MS, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eight antimicrobial classes of the common set for Salmonella.
Resistance in S. Typhimurium isolates from fattening pig carcases
Resistance levels in S. Typhimurium isolates from fattening pig carcases
S. Typhimurium was the third most frequently reported serovar recovered from pig carcases in 2017, accounting for 12.8% of Salmonella isolates serotyped (N = 960). Notably, in most reporting MSs, resistance levels were assessed on less than 10 isolates. Among a total of 123 isolates reported by 17 MSs (Table A.3), overall resistance to ampicillin and sulfamethoxazole were observed at extremely high levels (83.7% and 73.2%, respectively); tetracycline resistance was reported at a very high level (65%). Trimethoprim and chloramphenicol resistance were reported at high levels (22% and 27.6%, respectively), while gentamicin, nalidixic acid and ciprofloxacin were reported at low levels (8.1%, 4.1% and 7.3%, respectively). No resistance to cefotaxime, ceftazidime, meropenem, azithromycin or colistin was reported in S. Typhimurium isolates. Tigecycline resistance was reported by three MSs, resulting in an overall low level of resistance considering all MSs (3.3%) – see Table TYPHIPIGMEATD.
Table A.3.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Typhimurium from fattening pig carcases, using harmonised ECOFFs, 17 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 24 | 0 | 12.5 | 83.3 | 0 | 0 | 0 | 0 | 4.2 | 4.2 | 0 | 0 | 41.7 | 33.3 | 41.7 |
| Bulgariaa | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Croatiaa | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 |
| Cyprusa | 1 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Czech Republica | 5 | 0 | 20 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 80 |
| Denmark | 14 | 14.3 | 35.7 | 71.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71.4 | 14.3 | 57.1 |
| France | 24 | 0 | 37.5 | 62.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75 | 0 | 62.5 |
| Germanya | 6 | 0 | 50 | 83.3 | 0 | 0 | 0 | 16.7 | 0 | 0 | 0 | 0 | 83.3 | 33.3 | 83.3 |
| Hungarya | 3 | 0 | 0 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 0 | 33.3 |
| Ireland | 15 | 40 | 13.3 | 93.3 | 0 | 0 | 0 | 13.3 | 6.7 | 26.7 | 0 | 0 | 100 | 60 | 73.3 |
| Italya | 3 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66.7 | 0 | 66.7 |
| Maltaa | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Polanda | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Portugala | 2 | 50 | 100 | 100 | 0 | 0 | 0 | 50 | 0 | 50 | 0 | 0 | 100 | 50 | 100 |
| Romaniaa | 3 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33.3 | 0 | 33.3 |
| Slovakiaa | 1 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 100 |
| Spain | 18 | 5.6 | 38.9 | 100 | 0 | 0 | 0 | 0 | 16.7 | 16.7 | 0 | 0 | 83.3 | 22.2 | 88.9 |
| Total (17 MSs) | 123 | 8.1 | 27.6 | 83.7 | 0 | 0 | 0 | 3.3 | 4.1 | 7.3 | 0 | 0 | 73.2 | 22 | 65 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates.
Spatial distribution of resistance in S. Typhimurium from fattening pig carcases
No resistance to cefotaxime was detected in S. Typhimurium isolates recovered from pig carcases by 17 MSs (Figure A.7). The spatial distribution of ciprofloxacin resistance is shown in Figure A.7 (and considers MSs reporting data on less than 10 isolates). Resistance to this compound was reported at a high level in Ireland (26.7%), at a moderate level in Spain (16.7%) and at a low level in Belgium (4.2%). Fluoroquinolone resistance was also observed at a high level in Portugal (50%); however, only two S. Typhimurium isolates were recovered from pig carcases in this MS.
Figure A.7.
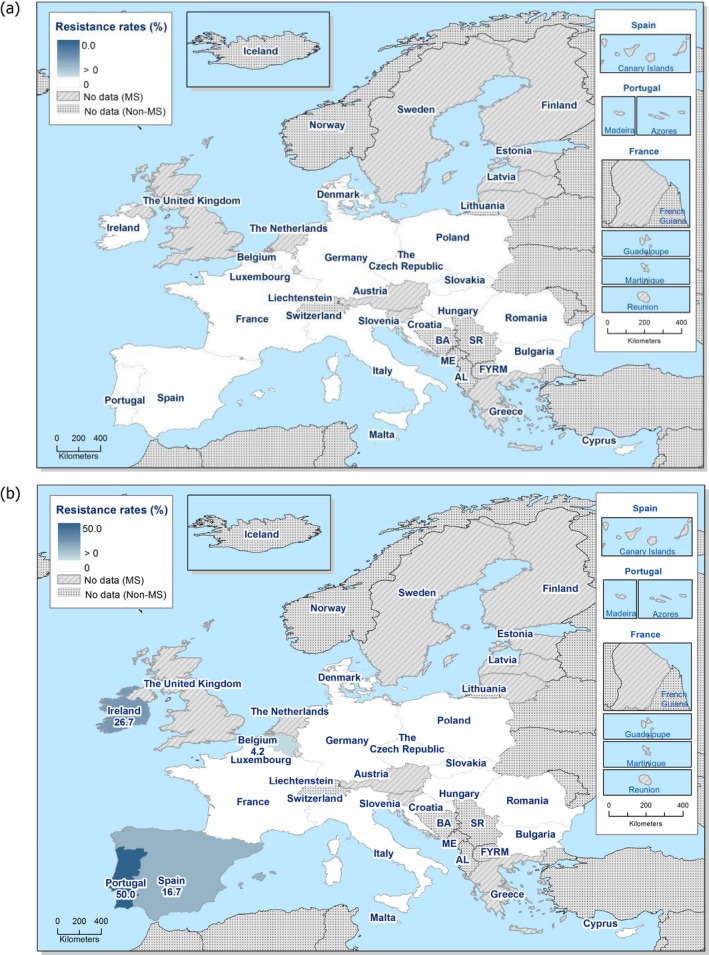
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella Typhimurium from fattening pig carcases, 17 MSs, 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Typhimurium from fattening pig carcases
Combined resistance to ciprofloxacin and cefotaxime (using microbiological breakpoints) among S. Typhimurium isolates recovered from pig carcases was not detected by any reporting MS.
Multidrug resistance and complete susceptibility in S. Typhimurium isolates from fattening pig carcases
17 MSs reported data on 123 individual S. Typhimurium isolates, which were included in the MDR analysis (Figure A.8). In contrast to S. Derby isolates recovered from pig carcases, the majority of S. Typhimurium isolates were multiresistant. Overall, 11.4% of S. Typhimurium isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 33.3%), while overall, 64.2% of S. Typhimurium isolates were multiresistant (MDR ranging from 33.3% to 100%) – see Table COMTYPHIPIGMEAT. Notably, where MDR was detected at 100%, only one or two S. Typhimurium isolates were reported by MSs for assessment. Overall, MDR in S. Typhimurium isolates was lower than that in monophasic S. Typhimurium isolates (77.2%).
Figure A.8.
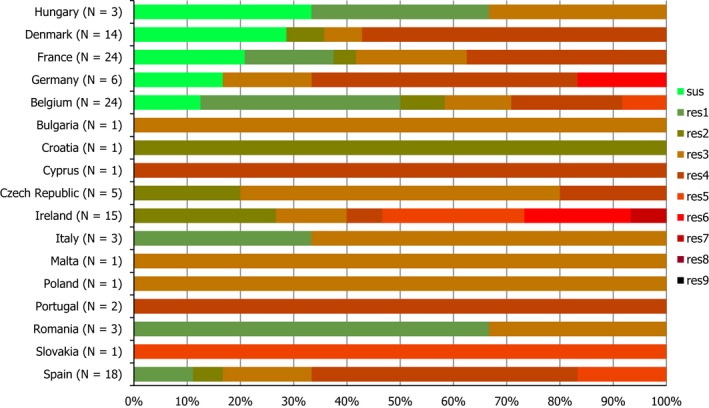
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Typhimurium from fattening pig carcases, 17 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Appendix B – Antimicrobial resistance in Salmonella serovars recovered from the voluntary monitoring of caecal contents of fattening pigs at slaughter
Distribution of Salmonella serovars in fattening pigs
Serovar information was provided for all 474 Salmonella isolates recovered from fattening pigs; the most common serovars detected (Table SERFATPIGS) were monophasic S. Typhimurium, including the antigenic formulas (six MSs, 34%), S. Derby (seven MSs, 20.7%) and S. Typhimurium (seven MSs, 17.1%) – see Figure B.1. Resistance and MDR levels in S. Derby were much lower than those reported in S. Typhimurium, monophasic S. Typhimurium and Salmonella spp. Additionally, overall MDR was higher in monophasic S. Typhimurium than in S. Typhimurium isolates.
Figure B.1.
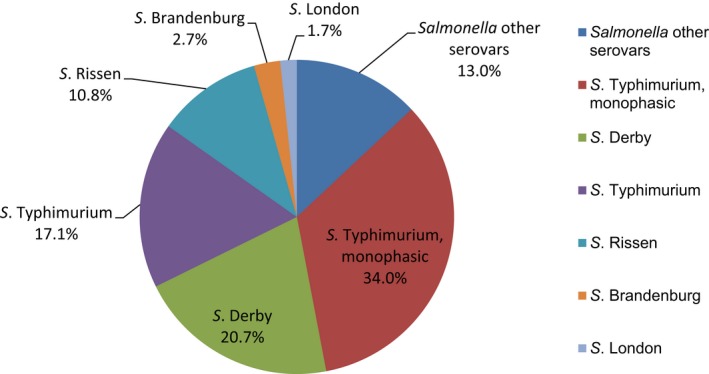
Breakdown of Salmonella serovars in fattening pigs, EU MSs, 2017 (N = 474)
Resistance in S. Derby from fattening pigs
Resistance levels in S. Derby from fattening pigs
S. Derby was the second most frequently reported serovar recovered from fattening pigs in 2017, accounting for 20.7% of Salmonella spp. serotyped. Notably, for 4 MSs, resistance levels were assessed on less than 10 isolates. Among a total of 98 isolates (seven MSs, Table B.1), resistance to sulfamethoxazole and tetracycline ranged from moderate to high in most of the reporting MSs, and overall high levels were recorded (25.5% and 34.7%, respectively); no resistance to these compounds was recorded by the Netherlands (N = 2). Similarly, the Netherlands reported no resistance to ampicillin, and considering all reporting MSs, resistance to this compound was overall low (8.2%). Trimethoprim resistance was reported in isolates from six MSs, resulting in an overall moderate level of resistance (17.3%). Again, the Netherlands reported no resistance to this compound. Only three MSs reported resistance to chloramphenicol, resulting in an overall low level of resistance to this antimicrobial (3.1%). Italy was the only country to report resistance to ciprofloxacin and nalidixic acid, where equivalent low levels of 2.5% were observed. Denmark was the only reporting MS to detect azithromycin resistance in a single S. Derby isolate, resulting in an overall very low level of resistance to this compound. Similarly, both Estonia (N = 5) and the Netherlands (N = 2) reported one colistin‐resistant S. Derby isolate; resulting in an overall very low level of resistance to this compound in view of all reporting MSs. No resistance to third‐generation cephalosporins, gentamicin, meropenem or tigecycline was detected in any reporting countries (Table DERBYPIG).
Table B.1.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Derby from fattening pigs, using harmonised ECOFFs, 7 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatiaa | 7 | 0 | 14.3 | 14.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.3 | 14.3 | 28.6 |
| Denmark | 21 | 0 | 0 | 14.3 | 0 | 0 | 0 | 0 | 0 | 0 | 4.8 | 0 | 19 | 19 | 38.1 |
| Estoniaa | 5 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 20 | 40 | 20 |
| Germanya | 8 | 0 | 12.5 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 | 12.5 | 12.5 |
| Italy | 40 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 2.5 | 2.5 | 0 | 0 | 27.5 | 5 | 30 |
| Netherlandsa | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 |
| Spain | 15 | 0 | 6.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 46.7 | 46.7 | 66.7 |
| Total (MSs 7) | 98 | 0 | 3.1 | 8.2 | 0 | 0 | 0 | 0 | 1 | 1.21 | 1 | 2 | 25.5 | 17.3 | 34.7 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance among S. Derby from fattening pigs
The spatial distributions of cefotaxime and ciprofloxacin resistance in S. Derby isolates recovered pigs are shown in Figure 2. No resistance to cefotaxime was detected among isolates reported by seven MSs. Ciprofloxacin resistance was only reported by Italy, at a low level.
Figure B.2.
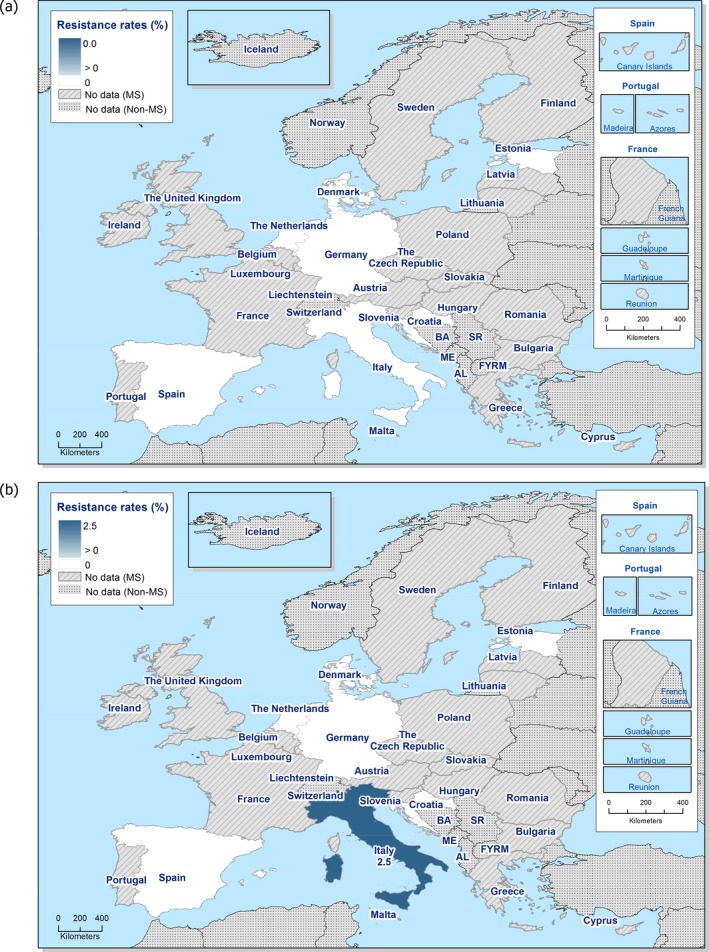
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella Derby from fattening pigs in countries reporting MIC data in 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Derby from fattening pigs
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among S. Derby isolates from pigs was not detected by any of the seven reporting MS (Figure B.3).
Figure B.3.
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin in Salmonella Derby from fattening pigs, using harmonised ECOFFs, 7 EU MSs, 2017
Multidrug resistance and complete susceptibility in S. Derby from fattening pigs
Seven MSs submitted isolate‐based data included in the MDR analysis (N = 98). Overall, 58.2% of S. Derby isolates were susceptible to all 11 antimicrobials used in the analysis, with complete susceptibility ranging from 26.7% to 100%. Overall, only 15.3% of S. Derby isolates were multiresistant, with MDR ranging from 0% to 40% (Figure B.4; Table COMDERBYFATPIG). Notably, where complete susceptibility was detected at 100% (and MDR was not detected), only two S. Derby isolates were reported by the MS for assessment.
Figure B.4.
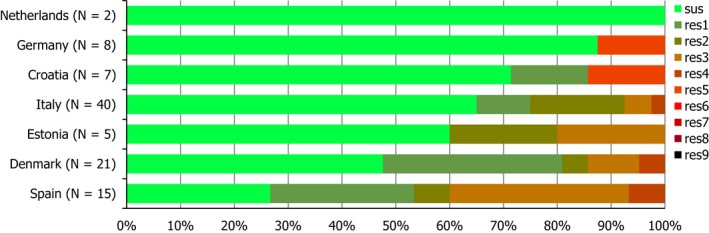
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Derby from fattening pigs, 7 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Resistance in monophasic S. Typhimurium from fattening pigs
Resistance levels in monophasic S. Typhimurium from fattening pigs
Monophasic S. Typhimurium was the most frequently reported serovar recovered from fattening pigs in 2017, accounting for 34% of Salmonella spp. serotyped (N = 474). Among a total of 161 isolates reported by six MSs (Table B.2), ampicillin, sulfamethoxazole and tetracycline resistance were observed at extremely high levels (90.1%, 87.6% and 82.6%, respectively). In most reporting countries, resistance to trimethoprim was observed at moderate levels; the Netherlands (N = 27) reported no resistance to this compound. Chloramphenicol resistance was reported by five MSs resulting in an overall low level, while azithromycin resistance was only detected in a single isolate from Italy and two isolates from Spain. Resistance to third‐generation cephalosporins was observed by two MSs at low levels; Italy reported cefotaxime and ceftazidime resistance at equivalent levels of 2.9%, while Spain only detected cefotaxime resistance at 1.8%. Overall, a low level of resistance to gentamicin, ciprofloxacin and nalidixic acid was reported (8.1%, 8.1% and 3.7%, respectively). Two MSs reported resistance to tigecycline, resulting in an overall low level of resistance, while, three MSs reported colistin resistance, again resulting in an overall low level of resistance. No MSs reported resistance to meropenem in monophasic S. Typhimurium isolates recovered from pigs.
Table B.2.
Occurrence of resistance (%) to selected antimicrobials in monophasic S. Typhimurium from fattening pigs, using harmonised ECOFFs, 6 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatiaa | 9 | 11.1 | 11.1 | 88.9 | 0 | 0 | 0 | 0 | 11.1 | 11.1 | 0 | 0 | 88.9 | 11.1 | 88.9 |
| Denmark | 14 | 7.1 | 0 | 71.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 71.4 | 14.3 | 85.7 |
| Germany | 19 | 0 | 15.8 | 89.5 | 0 | 0 | 0 | 5.3 | 0 | 10.5 | 0 | 0 | 94.7 | 15.8 | 89.5 |
| Italy | 35 | 20 | 14.3 | 94.3 | 2.9 | 2.9 | 0 | 2.9 | 2.9 | 8.6 | 2.9 | 5.7 | 91.4 | 14.3 | 82.9 |
| Netherlands | 27 | 0 | 7.4 | 85.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.7 | 81.5 | 0 | 66.7 |
| Spain | 57 | 7 | 8.8 | 94.7 | 1.8 | 0 | 0 | 0 | 7 | 12.3 | 3.5 | 1.8 | 89.5 | 12.3 | 86 |
| Total (MSs 6) | 161 | 8.1 | 9.9 | 90.1 | 1.2 | 0.6 | 0 | 1.2 | 3.7 | 8.1 | 1.9 | 2.5 | 87.6 | 11.2 | 82.6 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance in monophasic S. Typhimurium from fattening pigs
Cefotaxime resistance was reported by two MSs from southern Europe (Italy, N = 35; Spain, N = 57), where low levels of resistance were observed (2.9% and 1.8%, respectively) – see Figure B.5a. Considering all reporting countries (six MSs including Croatia which reported data for less than 10 isolates), the spatial distributions of ciprofloxacin resistance in monophasic Salmonella Typhimurium isolates recovered from pigs are shown in Figure B.5b. Fluoroquinolone resistance was observed in countries from western and southern Europe; with Spain (N = 57), Germany (N = 19) and Croatia (N = 9) reporting moderate levels of resistance (12.3%, 10.5% and 11.1%, respectively) and Italy (N = 35) reporting a low level of resistance to this antimicrobial (8.6%).
Figure B.5.
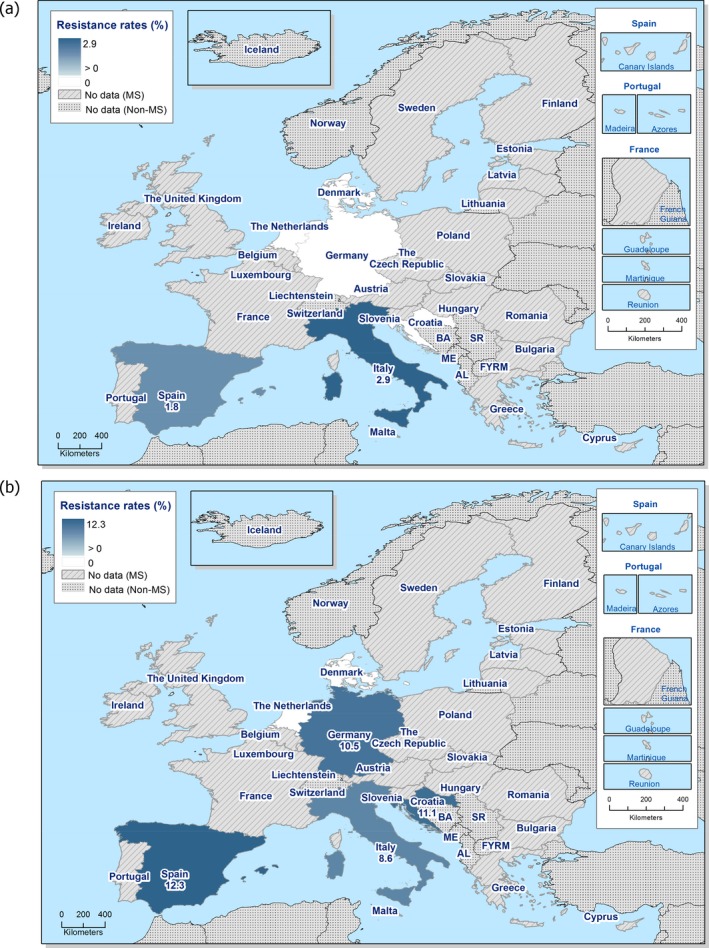
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among monophasic S. Typhimurium from fattening pigs in countries reporting MIC data in 2017
Combined resistance to ciprofloxacin and cefotaxime in monophasic S. Typhimurium from fattening pigs
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among monophasic Salmonella Typhimurium isolates from pigs was not detected by any of the six reporting MSs.
Multidrug resistance and complete susceptibility in monophasic S. Typhimurium from fattening pigs
In 2017, six MSs reported quantitative MIC data for 161 monophasic S. Typhimurium isolates recovered from pigs (Figure B.6). In contrast to S. Derby isolates from pigs, an extremely high proportion of isolates were multiresistant; MDR was also reported at a higher level than that in S. Typhimurium isolates (59.3%). Overall, only 3.7% of monophasic S. Typhimurium isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 11.1%), while 78.9% of isolates were multiresistant (MDR ranging from 63% to 89.5%) – see Table COMMOTYPHIPIG. Additionally, the observed level of complete susceptibility in monophasic S. Typhimurium was much lower than that reported in S. Typhimurium isolates (16%).
Figure B.6.
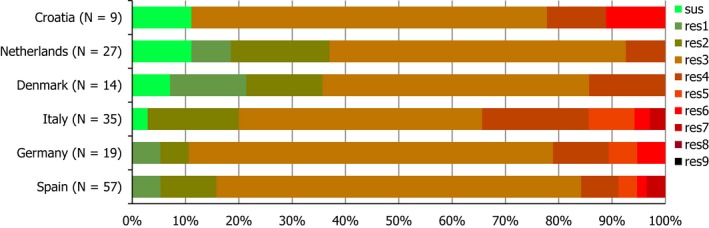
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in monophasic S. Typhimurium from fattening pigs in 6 MSs in 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eight antimicrobial classes of the common set for Salmonella.
Resistance in S. Typhimurium isolates from fattening pigs
Resistance levels in S. Typhimurium isolates from fattening pigs
S. Typhimurium was the third most frequently reported serovar recovered from fattening pigs in 2017, accounting for 17.1% of Salmonella spp. serotyped (N = 474). Notably for three MSs, resistance levels were assessed on less than 10 isolates. Among a total of 81 isolates reported by seven MSs (Table B.3), overall resistance to ampicillin and tetracycline was noted at an extremely high level (70.4%); resistance to sulfamethoxazole was observed at a slightly lower level of 65.4%. No resistance to these three compounds was reported by Sweden (N = 2), and additionally, Denmark (N = 7) did not report ampicillin resistance. Overall, trimethoprim and chloramphenicol resistance were reported at moderate (17.3%) and high levels (32.1%), respectively (), while gentamicin resistance was observed at a low level (2.5%). Italy (N = 5) was the only country to report resistance to cefotaxime and ceftazidime. Four MSs reported resistance to nalidixic acid and ciprofloxacin, resulting in overall moderate levels of resistance; Croatia (N = 13) reported equivalent high levels of resistance to these compounds (23.1%), while Spain (N = 20) reported equivalent moderate levels of resistance (20%). No resistance to meropenem or azithromycin was reported in monophasic S. Typhimurium isolates recovered from pigs. Tigecycline resistance was reported by two MSs, resulting in an overall low level of resistance (4.9%), and Germany was the only country to report colistin resistance, again resulting in an overall low level of resistance (1.2%) – see Table TYPHIFATPIG.
Table B.3.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Typhimurium from fattening pigs, using harmonised ECOFFs, 7 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatia | 13 | 0 | 23.1 | 61.5 | 0 | 0 | 0 | 0 | 23.1 | 23.1 | 0 | 0 | 61.5 | 0 | 61.5 |
| Denmarka | 7 | 0 | 14.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14.3 | 0 | 42.9 |
| Germany | 16 | 0 | 50 | 93.8 | 0 | 0 | 0 | 18.8 | 6.3 | 6.3 | 0 | 6.3 | 87.5 | 31.3 | 87.5 |
| Italya | 5 | 40 | 60 | 80 | 20 | 20 | 0 | 0 | 20 | 40 | 0 | 0 | 80 | 20 | 80 |
| Netherlands | 18 | 0 | 16.7 | 83.3 | 0 | 0 | 0 | 5.6 | 0 | 0 | 0 | 0 | 61.1 | 38.9 | 61.1 |
| Swedena | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spain | 20 | 0 | 40 | 75 | 0 | 0 | 0 | 0 | 20 | 20 | 0 | 0 | 75 | 5 | 85 |
| Total (MSs 7) | 81 | 2.5 | 32.1 | 70.4 | 1.2 | 1.2 | 0 | 4.9 | 11.1 | 12.3 | 0 | 1.2 | 65.4 | 17.3 | 70.4 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance in S. Typhimurium from fattening pigs
Cefotaxime resistance was only reported by one MS from southern Europe (Italy, N = 5), where a moderate level of resistance was detected (20%) – see Figure B.7a. Considering all reporting countries (including MSs reporting data for less than 10 isolates), the spatial distributions of ciprofloxacin resistance in Salmonella Typhimurium isolates recovered from pigs are shown in Figure B.7b. Fluoroquinolone resistance was detected at a low level in one western European country (Germany: 6.3%) and at a moderate/high level in three southern European countries (Spain: 20%; Croatia: 23.1%; Italy: 40%), although Italy only reported data on five S. Typhimurium isolates recovered from pigs.
Figure B.7.
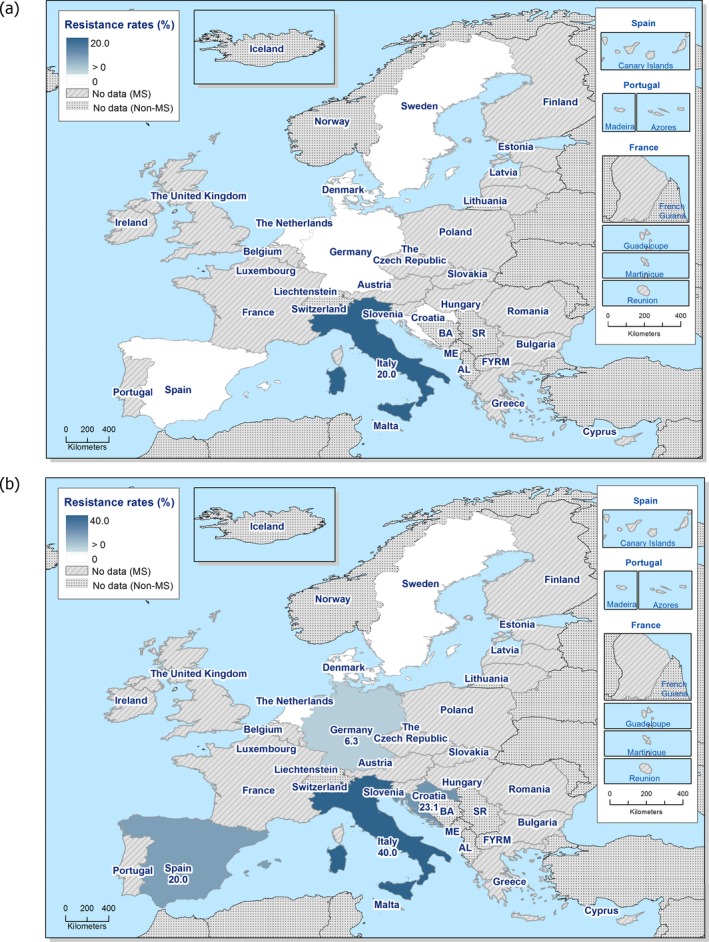
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among S. Typhimurium from fattening pigs in countries reporting MIC data in 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Typhimurium from fattening pigs
Italy was the only country to report combined resistance to ciprofloxacin and cefotaxime in 1/5 Salmonella Typhimurium isolates (Table COMTYPHIFATPIG). ‘Microbiological’ combined resistance to these antimicrobials was not detected in any other isolates from reporting countries (Figure B.8).
Figure B.8.
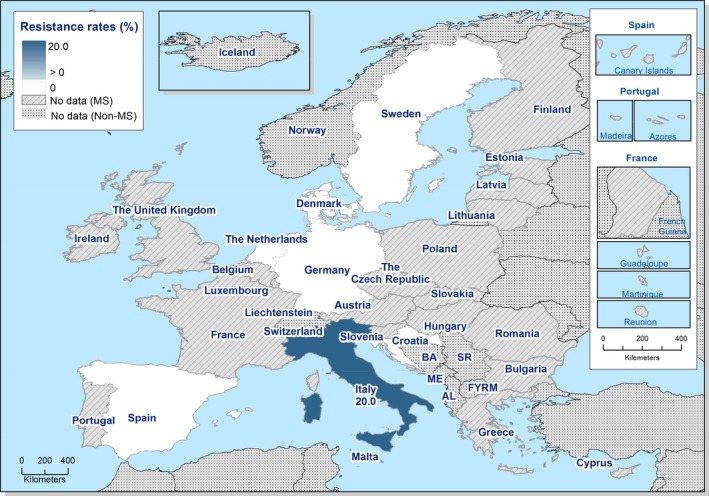
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin resistance in S. Typhimurium from fattening pigs, 7 EU MSs, 2017
Multidrug resistance and complete susceptibility in S. Typhimurium isolates from fattening pigs
Seven MSs reported data on 81 individual S. Typhimurium isolates, which were included in the MDR analysis (Figure B.9). In contrast to S. Derby isolates recovered from pigs, a higher proportion of S. Typhimurium isolates were multiresistant; this figure was lower than that reported in monophasic S. Typhimurium (78.9%). Overall, 16% of S. Typhimurium isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 100%), while overall, 59.3% of S. Typhimurium isolates were multiresistant (MDR ranging from 0% to 81.3%) – see Table COMTYPHIFATPIG. Notably, where complete susceptibility was detected at 100%, only two S. Typhimurium isolates were reported by the MS for assessment.
Figure B.9.
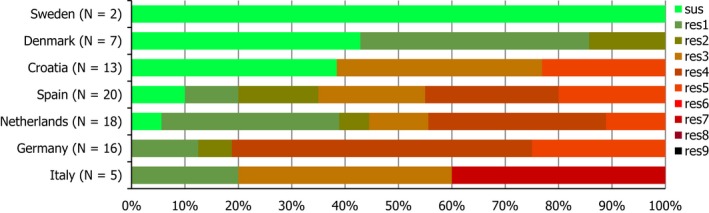
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in S. Typhimurium from fattening pigs, 7 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res7: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Appendix C – Antimicrobial resistance in Salmonella serovars recovered from the mandatory carcase swabbing of calves (of less than a year of age) at slaughter
Distribution of Salmonella serovars in carcases of calves of less than 1 year of age
Serovar information was provided for all 82 Salmonella isolates recovered from carcases of calves under 1 year of age; the most common serovars detected (Table SERBOVMEATD) were monophasic S. Typhimurium (four MSs, 14.6%), S. Meleagridis (two MSs, 12.2%) and S. Mbandaka (three MSs, 9.8%) – see Figure C.1. MDR was not detected in S. Meleagridis and S. Mbandaka isolates, and overall MDR was higher in monophasic S. Typhimurium (91.7%) than in S. Typhimurium (25%) isolates; however very low numbers of isolates were tested and so results may be subject to variation.
Figure C.1.
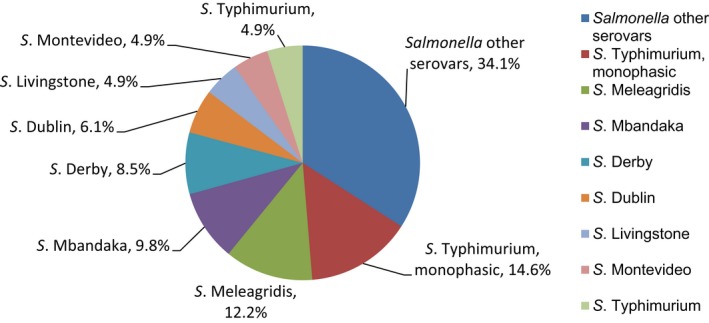
Breakdown of Salmonella serovars in carcases of calves of less than 1 year of age, EU MSs, 2017 (N = 82)
Resistance in monophasic S. Typhimurium from carcases of calves of less than 1 year of age
Resistance levels in monophasic S. Typhimurium from carcases of calves of less than 1 year of age
Monophasic S. Typhimurium was the most frequently reported serovar recovered from calf carcases in 2017, accounting for 14.6% of Salmonella spp. serotyped (N = 82). However, in total only 12 monophasic S. Typhimurium isolates were reported by four MSs (Table C.1) and so results may be subject to inherent variation associated with small sample sizes. Resistance to ampicillin, sulfamethoxazole and tetracycline was observed at extremely high levels in most reporting MSs. Three countries recorded trimethoprim resistance, resulting in an overall high level of resistance (25%). Croatia was the only country to report nalidixic acid and ciprofloxacin resistance in a single isolate, resulting in an overall low level of resistance (8.3%) considering all reporting MSs. Spain was the only MS to report gentamicin resistance, while Croatia was the only MS to report chloramphenicol resistance; both resulting in an overall low level of resistance in view of all reporting MSs. No resistance to cefotaxime, ceftazidime, meropenem, tigecycline, azithromycin or colistin was observed in any of the 12 isolates reported by MSs ‐ see Table MONOTYPHIBOVCARCD.
Table C.1.
Occurrence of resistance (%) to selected antimicrobials in monophasic Salmonella Typhimurium from carcases of calves of less than 1 year of age, using harmonised ECOFFs, 4 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgiuma | 2 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Croatiaa | 5 | 0 | 20 | 100 | 0 | 0 | 0 | 0 | 20 | 20 | 0 | 0 | 100 | 20 | 80 |
| Francea | 3 | 0 | 0 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 66.7 | 33.3 | 100 |
| Spaina | 2 | 50 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 50 | 100 |
| Total (4 MSs) | 12 | 8.3 | 8.3 | 91.7 | 0 | 0 | 0 | 0 | 8.3 | 8.3 | 0 | 0 | 91.7 | 25 | 91.7 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Spatial distribution of resistance in monophasic S. Typhimurium from carcases of calves of less than 1 year of age
Although extremely low numbers of monophasic S. Typhimurium isolates were recovered from calf carcases, the spatial distributions of cefotaxime and ciprofloxacin resistance are shown in Figure C.2. No resistance to cefotaxime was detected among isolates reported by the four MSs. Fluoroquinolone resistance was only detected by Croatia (N = 5) at a moderate level of 20%.
Figure C.2.
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among monophasic Salmonella Typhimurium from carcases of calves of less than 1 year of age in countries reporting MIC data in 2017
Combined resistance to ciprofloxacin and cefotaxime in monophasic S. Typhimurium from carcases of calves of less than 1 year of age
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among monophasic S. Typhimurium isolates recovered from calf carcases was not detected by any of the four reporting MSs.
Multidrug resistance and complete susceptibility in monophasic S. Typhimurium from carcases of calves of less than 1 year of age
In 2017, four MSs reported quantitative MIC data for 12 monophasic S. Typhimurium isolates recovered from calf carcases (Figure C.3). An extremely high proportion of monophasic S. Typhimurium were multiresistant, in contrast to S. Meleagridis and S. Mbandaka isolates where MDR was not detected. MDR was also reported at a higher level in monophasic S. Typhimurium than in S. Typhimurium isolates (25%) recovered from calf carcases. None of the reported monophasic S. Typhimurium isolates were susceptible to the full panel of antimicrobials used in the analysis, while 91.7% of isolates were multiresistant (MDR ranging from 66.7% to 100%) – see Table COMMONTYPHIBOVCARC. Notably, due to the low number of isolates reported by each MS, results may be subject to inherent variation associated with small sample sizes.
Figure C.3.
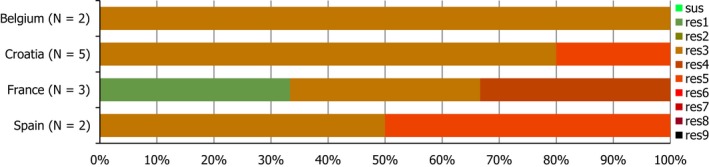
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in monophasic S. Typhimurium from carcases of calves of less than 1 year of age in MSs in 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eight antimicrobial classes of the common set for Salmonella.
Resistance in other serovar isolates from carcases of calves of less than 1 year of age
Resistance levels in S. Typhimurium, S. Meleagridis and S. Mbandaka isolates from carcases of calves of less than 1 year of age
In 2017, the second and third most commonly reported serovars recovered from calf carcases were S. Meleagridis and S. Mbandaka, accounting for 12.2% and 9.8% of Salmonella spp. serotyped (N = 82), respectively. S. Typhimurium was the sixth most commonly reported serovar (as were S. Livingstone and S. Montevideo), accounting for 4.9% of Salmonella spp. serotyped.
Considering S. Typhimurium, only four isolates were reported by two MSs (Table C.2); no resistance to the panel of antimicrobials was detected in a single isolate reported by Belgium. In France (N = 3), all isolates were resistant to sulfamethoxazole; ampicillin resistance was detected at a very high level, while tetracycline and chloramphenicol resistance were reported at high levels. Notably, as few isolates were reported, results may be subject to inherent variation.
Table C.2.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Typhimurium from carcases of calves of less than 1 year of age, using harmonised ECOFFs, 2 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgiuma | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Francea | 3 | 0 | 33.3 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 33.3 |
| Total (2 MSs) | 4 | 0 | 25 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 75 | 0 | 25 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
In S. Meleagridis isolates from calf carcases, sulfamethoxazole and tetracycline resistance was only reported by Spain, resulting in overall low levels of resistance to these compounds (10%). Resistance to other antimicrobials was not reported by Spain; Bulgaria reported no resistance to the panel of antimicrobials in a single S. Meleagridis isolate (Table 3).
In S. Mbandaka isolates, Spain (N = 6) was the only MS to report antimicrobial resistance, with resistance to ampicillin and tetracycline observed at moderate levels (16.7%) – see Table 4.
Table C.3.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Meleagridis from carcases of calves of less than 1 year of age, using harmonised ECOFFs, 2 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bulgariaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spaina | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11.1 | 0 | 11.1 |
| Total (2 MSs) | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 10 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Table C.4.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Mbandaka from carcases of calves of less than 1 year of age, using harmonised ECOFFs, 3 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgiuma | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Francea | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Spaina | 6 | 0 | 0 | 16.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 16.7 |
| Total (3 MSs) | 8 | 0 | 0 | 12.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12.5 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Multidrug resistance and complete susceptibility in S. Typhimurium, Mbandaka and Meleagridis isolates from carcases of calves of less than 1 year of age
A quarter (25%) of the S. Typhimurium isolates included in the MDR analysis (2 MSs, N = 4) were multiresistant (Figure C.4; Table COMTYPHIBOVMEAT). Similarly, 25% of isolates were susceptible to the full panel of antimicrobials used in the analysis. Notably, a very low number of S. Typhimurium isolates were reported and therefore, results may be subject to inherent variation. In S. Meleagridis, MDR was not detected among the 10 isolates (Figure C.5; Table COMMELEBOVCARC). The majority of isolates (90%) were fully susceptible to the panel of antimicrobials included in the analysis, with susceptibility ranging from 88.9% to 100%. Similarly, in S. Mbandaka isolates from calf carcases, MDR was not detected (Figure C.6; Table COMMBANBOVCARC). Again, the majority of isolates (87.5%) were fully susceptible to the panel of antimicrobials, with susceptibility ranging from 83.3% to 100%. The very low number of S. Meleagridis and S. Mbandaka isolates, may subject results to inherent variation.
Figure C.4.
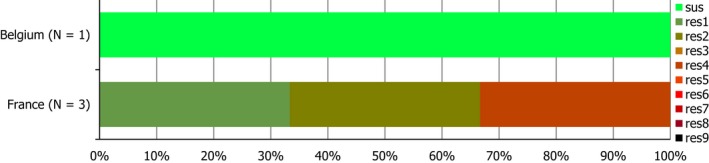
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Typhimurium from carcases of calves of less than 1 year of age, 2 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Figure C.5.
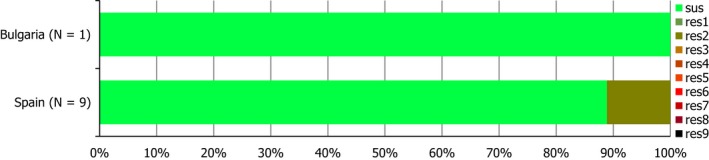
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Meleagridis from carcases of calves of less than 1 year of age, 2 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Figure C.6.
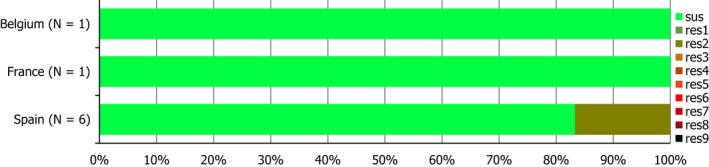
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Mbandaka from carcases of calves of less than 1 year of age, 3 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Spatial distribution of resistance in Salmonella Typhimurium, Mbandaka and Meleagridis from carcases of calves of less than 1 year of age
The spatial distributions of cefotaxime and ciprofloxacin resistance in S. Typhimurium, S. Mbandaka and S. Meleagridis isolates recovered from calf carcases are shown in Figures C.7, C.8 and C.9. No resistance to cefotaxime or ciprofloxacin was detected in isolates of these serovars. In all reporting countries, the total number of isolates examined was extremely low.
Figure C.7.
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin among Salmonella Typhimurium from carcases of calves of less than 1 year of age, 2 EU MSs, 2017
Figure C.8.
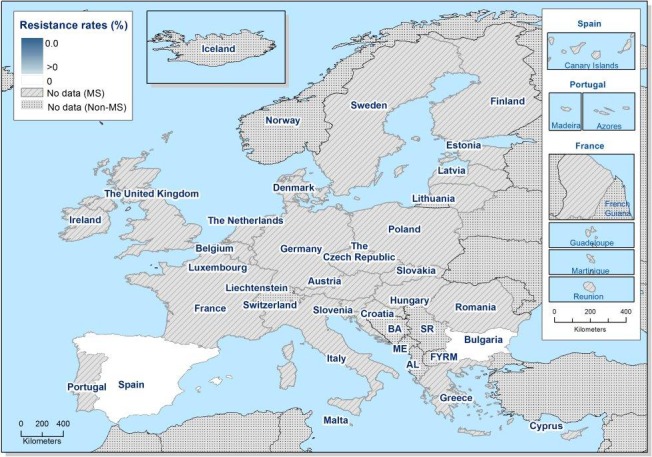
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin among Salmonella Meleagridis from carcases of calves of less than 1 year of age, 2 EU MSs, 2017
Figure C.9.
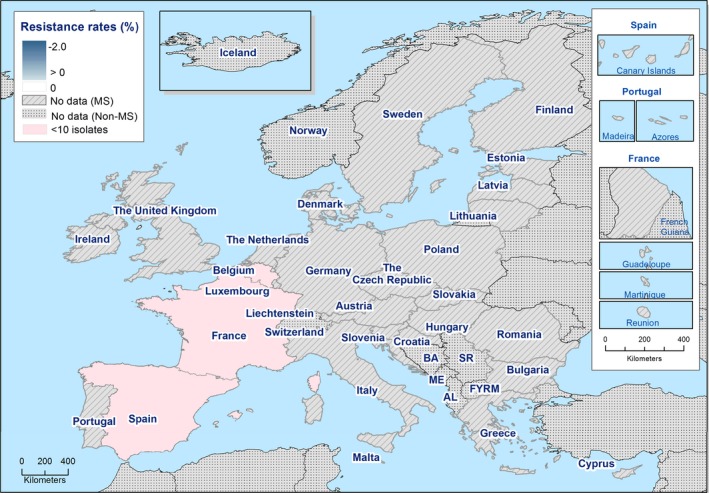
Spatial distribution of combined resistance to cefotaxime and ciprofloxacin among Salmonella Mbandaka from carcases of calves of less than 1 year of age, 3 EU MSs, 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Typhimurium, Mbandaka and Meleagridis from carcases of calves of less than 1 year of age
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among S. Typhimurium, S. Mbandaka and S. Meleagridis isolates recovered from calf carcases was not detected in any of the reporting MSs.
Appendix D – Antimicrobial resistance in Salmonella serovars recovered from the voluntary monitoring of caecal contents of cattle at slaughter
Distribution of Salmonella serovars in cattle
In 2017, serovar information was provided for all 176 Salmonella isolates recovered from cattle; the most common serovars detected (Table SERCATT) were S. Typhimurium (seven MSs and one non‐MS, 44.3%), monophasic S. Typhimurium (three MSs and one non‐MS, 14.8%) and the host‐adapted cattle serovar S. Dublin (five MSs, 13.6%) – see Figure D.1. Overall, MDR in S. Dublin was lower than that reported in S. Typhimurium, monophasic S. Typhimurium and Salmonella spp.
Figure D.1.
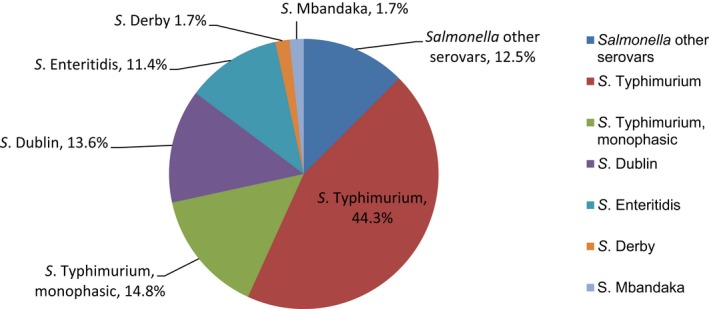
Breakdown of serovars among Salmonella isolates from cattle tested for susceptibility, 7 MSs and 1 non‐MS, 2017 (N = 176)
Resistance in S. Typhimurium from cattle
Resistance levels in S. Typhimurium from cattle
S. Typhimurium was the most frequently reported serovar recovered from cattle in 2017, accounting for 44.3% of Salmonella spp. serotyped (N = 176). Among a total of 78 isolates (seven MSs and one non‐MS, Table D.1), resistance to third‐generation cephalosporins, meropenem, azithromycin or colistin were not detected by any reporting country. Notably, in five reporting MSs, resistance levels were assessed on less than 10 isolates. Considering reporting MSs, resistance to sulfamethoxazole and tetracycline was extremely high (76.9% and 74.4%, respectively); Switzerland (N = 39) reported low and moderate levels of resistance to these compounds, respectively. Italy (N = 15), the Netherlands (N = 16) and Spain (N = 1) were the only MSs to report resistance to chloramphenicol and ampicillin, resulting in overall high and very high levels of resistance (46.2% and 56.4%, respectively). Switzerland reported low level resistance to chloramphenicol and ampicillin. Additionally, Italy and the Netherlands were the only countries to report resistance to gentamicin, resulting in an overall high level of resistance to this antimicrobial at 20.5%. Trimethoprim resistance was reported by three MSs, resulting in an overall high level of resistance (23.1%). Italy reported resistance to ciprofloxacin and nalidixic acid at equivalent very high levels (53.3%); as did Spain which reported extremely high levels of resistance to these antimicrobials (100%), although only one S. Typhimurium isolate was recovered by this country. Resistance to tigecycline was only reported by Italy. Considering all reporting MSs, overall resistance to (fluoro)quinolones was observed at high levels (23.1%), while tigecycline resistance was reported at a low level (2.6%) – see Table TYPHICATT.
Table D.1.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Typhimurium from cattle, using harmonised ECOFFs, 7 EU MSs and 1 non‐MS, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Croatiaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 100 | 0 |
| Estoniaa | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Finlanda | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Italy | 15 | 13.3 | 53.3 | 73.3 | 0 | 0 | 0 | 6.7 | 53.3 | 53.3 | 0 | 0 | 100 | 26.7 | 100 |
| Netherlands | 16 | 37.5 | 56.3 | 62.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 81.3 | 25 | 81.3 |
| Spaina | 1 | 0 | 100 | 100 | 0 | 0 | 0 | 0 | 100 | 100 | 0 | 0 | 100 | 0 | 100 |
| Swedena | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total (MSs 7) | 39 | 20.5 | 46.2 | 56.4 | 0 | 0 | 0 | 2.6 | 23.1 | 23.1 | 0 | 0 | 76.9 | 23.1 | 74.4 |
| Switzerland | 39 | 0 | 5.1 | 7.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7.7 | 0 | 17.9 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total of MSs data.
Spatial distribution of resistance in S. Typhimurium from cattle
Resistance to cefotaxime was not detected among isolates reported by seven MSs and one non‐MS (Figure D.2a). Spatial distributions of ciprofloxacin resistance in S. Typhimurium isolates recovered from cattle are shown in Figure D.2b. Fluoroquinolone resistance was reported by two MSs from southern Europe, with Italy and Spain registering very high and extremely high levels, respectively (53.3% and 100%). Notably, only one S. Typhimurium isolate was recovered from cattle by Spain.
Figure D.2.
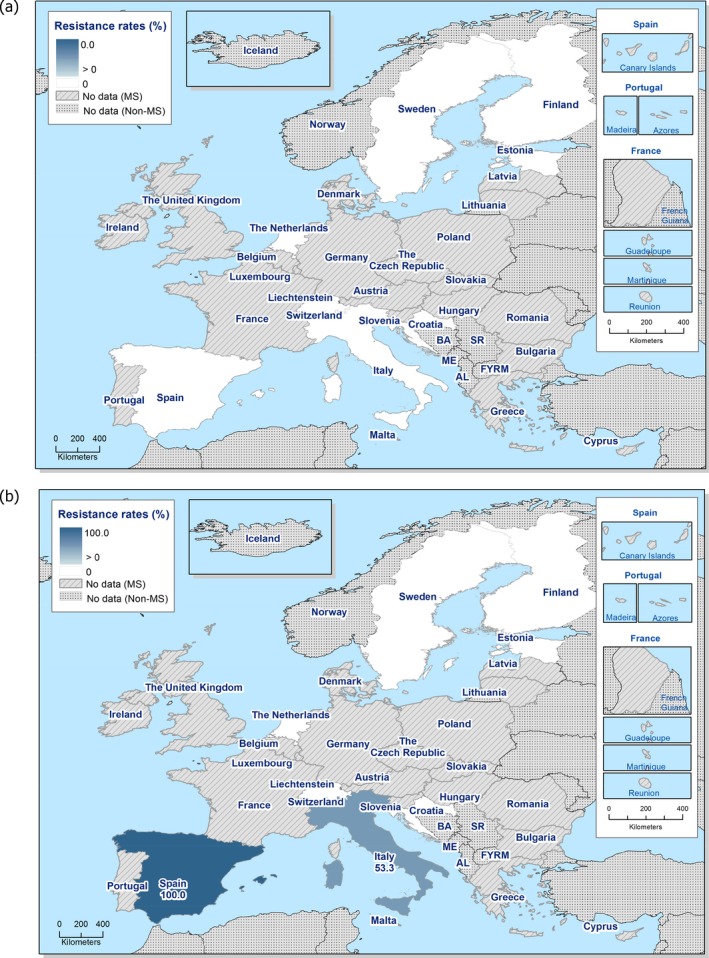
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella Typhimurium from cattle, 7 EU MSs and 1 non‐EU MS, 2017
Combined resistance to ciprofloxacin and cefotaxime in S. Typhimurium from cattle
‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime among S. Typhimurium isolates recovered from cattle was not detected by any reporting country.
Multidrug resistance and complete susceptibility in S. Typhimurium from cattle
Seven MSs and Switzerland reported data on 78 individual S. Typhimurium isolates, which were included in the MDR analysis (Figure D.3). Overall, 51.3% of S. Typhimurium isolates were susceptible to all 11 antimicrobials used in the analysis (complete susceptibility ranging from 0% to 100%), while 30.8% of S. Typhimurium isolates were multiresistant (MDR ranging from 0% to 73.3%) – see Table COMTYPHIBOV. Notably, where complete susceptibility was detected at 100%, less than five S. Typhimurium isolates were reported by MSs for assessment.
Figure D.3.
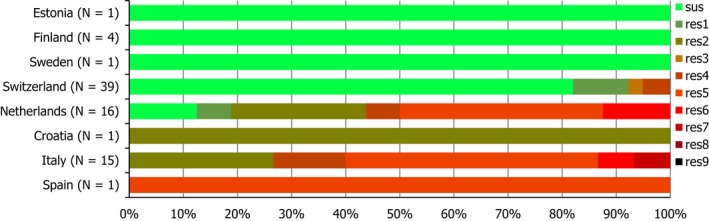
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Typhimurium from cattle, 7 EU MSs and 1 non‐EU MS, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; CZ: Czech Republic; UK: United Kingdom; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Resistance levels in monophasic S. Typhimurium from cattle
Resistance levels in monophasic S. Typhimurium from cattle
In 2017, monophasic S. Typhimurium was the second most commonly reported serovar recovered from cattle, accounting for 14.8% of Salmonella spp. serotyped (N = 176). Among a total of 26 isolates (three MSs and one non‐MS, Table D.2), resistance to third‐generation cephalosporins, meropenem, tigecycline, azithromycin or colistin was not detected by any reporting country. Notably in MSs, resistance levels were assessed on less than 10 isolates. Considering reporting MSs, resistance to ampicillin, sulfamethoxazole and tetracycline was extremely high (94.4%, 100% and 88.9%, respectively); Switzerland (non‐MS, N = 18) also reported extremely high levels of resistance to these compounds. Italy (N = 5) was the only country to report resistance to ciprofloxacin and nalidixic acid, where resistance was observed to both compounds at moderate levels (20%). Similarly, trimethoprim resistance was only reported by Italy, resulting in an overall moderate level (12.5%) of resistance considering reporting MSs. Gentamicin and chloramphenicol resistance was detected in 2/3 reporting MSs, resulting in an overall high level of resistance (25%) – see Table MOTYPHICAT.
Table D.2.
Occurrence of resistance (%) to selected antimicrobials in monophasic S. Typhimurium from cattle, using harmonised ECOFFs, 3 EU MSs and 1 non‐MS, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Italya | 5 | 20 | 20 | 80 | 0 | 0 | 0 | 0 | 20 | 20 | 0 | 0 | 80 | 20 | 100 |
| Netherlandsa | 2 | 50 | 50 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 |
| Spaina | 1 | 0 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 100 |
| Total (MSs 3) | 8 | 25 | 25 | 87.5 | 0 | 0 | 0 | 0 | 12.5 | 12.5 | 0 | 0 | 75 | 12.5 | 87.5 |
| Switzerland | 18 | 0 | 0 | 94.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 88.9 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance in monophasic S. Typhimurium from cattle
Spatial distributions of cefotaxime and ciprofloxacin resistance among monophasic S. Typhimurium isolates recovered from cattle are shown in Figure D.4. ‘Microbiological’ resistance to cefotaxime was not detected among monophasic S. Typhimurium isolates reported by three MSs and one non‐MS. Fluoroquinolone resistance was only reported by Italy (20%); however, less than 10 isolates were assessed. ‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime was therefore not detected in any reporting country.
Figure D.4.
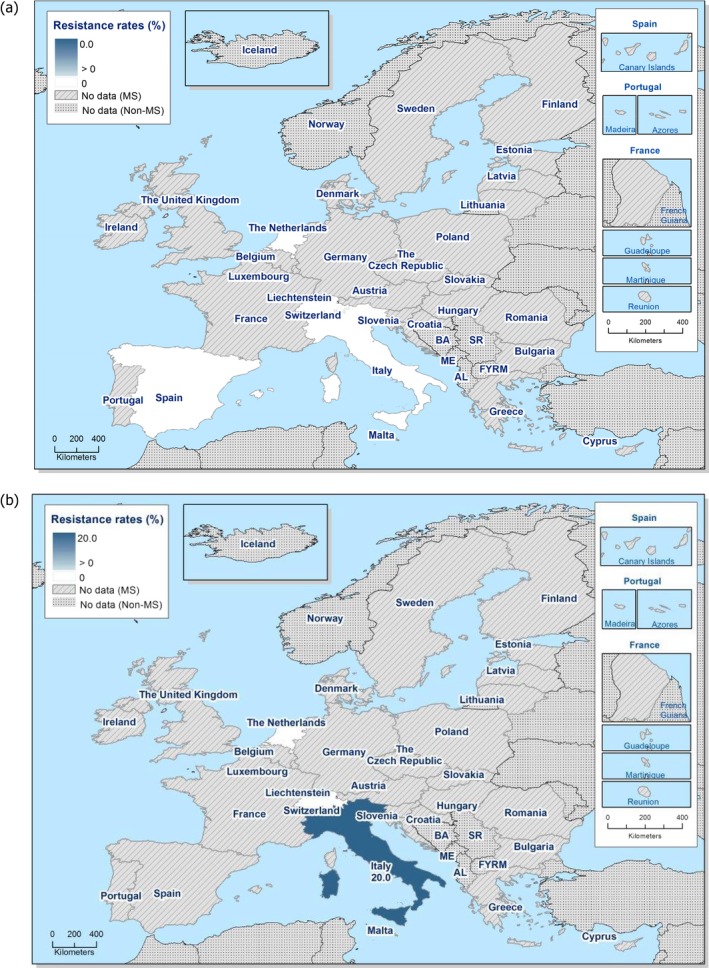
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among monophasic Salmonella Typhimurium from cattle, 3 EU MSs and one non‐MS, 2017
Multidrug resistance in monophasic S. Typhimurium from cattle
Three MSs and Switzerland reported data on 26 individual monophasic S. Typhimurium isolates, which were included in the MDR analysis (Figure D.5). In contrast to S. Typhimurium isolates recovered from cattle, a higher proportion of monophasic S. Typhimurium isolates were multiresistant; this figure was also higher than that reported for S. Dublin (16.7%) in cattle. Overall, most of the monophasic S. Typhimurium isolates were multiresistant (84.6%), at levels of 50% in the Netherlands, 80% in Italy, 88.9% in Switzerland and 100% in Spain. No isolates were susceptible to the full panel of antimicrobials – see Table COMMONTYBOV.
Figure D.5.
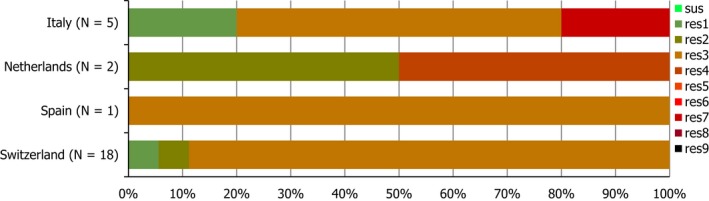
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in monophasic Salmonella Typhimurium from cattle, 3 EU MSs and 1 non‐MS in 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; CZ: Czech Republic; UK: United Kingdom; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to eleven antimicrobial classes of the common set for Salmonella.
Resistance in S. Dublin from cattle
Resistance levels in S. Dublin from cattle
S. Dublin was the third most frequently reported serovar recovered from cattle in 2017, accounting for 13.6% of Salmonella spp. serotyped (N = 176). Among a total of 24 isolates (five MSs, Table D.3), resistance to cefotaxime, ceftazidime, meropenem or tigecycline was not detected by any MS. Notably in most reporting countries, resistance levels were assessed on less than 10 isolates. Overall resistance to chloramphenicol, ampicillin, trimethoprim and tetracycline were observed at moderate levels, while sulfamethoxazole resistance was observed at a high level; the Netherlands (N = 18) were the only country to report resistance to these compounds. Similarly, the Netherlands were the only MS to report resistance to gentamicin, nalidixic acid and azithromycin, resulting in overall low levels of resistance to these antimicrobials. Resistance to ciprofloxacin was reported by three MSs, and overall resistance was observed at a moderate level. An overall very high level (66.7%) of resistance to colistin was reported, with the Netherlands (N = 18) detecting 14 colistin‐resistant S. Dublin isolates – see Table DUBCAT.
Table D.3.
Occurrence of resistance (%) to selected antimicrobials in Salmonella Dublin from cattle, using harmonised ECOFFs, 5 EU MSs, 2017
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estoniaa | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 | 0 | 0 | 0 |
| Italya | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 |
| Netherlands | 18 | 11.1 | 22.2 | 16.7 | 0 | 0 | 0 | 0 | 5.6 | 5.6 | 11.1 | 77.8 | 27.8 | 16.7 | 22.2 |
| Spaina | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Swedena | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Total (MSs 5) | 24 | 8.3 | 16.7 | 12.5 | 0 | 0 | 0 | 0 | 4.2 | 12.5 | 8.3 | 66.7 | 20.8 | 12.5 | 16.7 |
ECOFFs: epidemiological cut‐off values; N: number of isolates tested; MSs: Member States; GEN: gentamicin; CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: ceftazidime; MEM: meropenem; TGC: tigecycline; NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
The occurrence of resistance is assessed on less than 10 isolates and should only be considered as part of the total from all MSs data.
Spatial distribution of resistance in S. Dublin from cattle
‘Microbiological’ resistance to cefotaxime was not detected among S. Dublin isolates reported by five MSs (Figure D.6a). Spatial distributions of ciprofloxacin resistance among S. Dublin isolates from cattle are shown in Figure D.6b (and consider MSs reporting data on less than 10 isolates); resistance to this compound was reported at a low level in the Netherlands (5.6%) and at high levels in Italy (50%) and Estonia (50%). ‘Microbiological’ combined resistance to ciprofloxacin and cefotaxime was not detected by any reporting country.
Figure D.6.
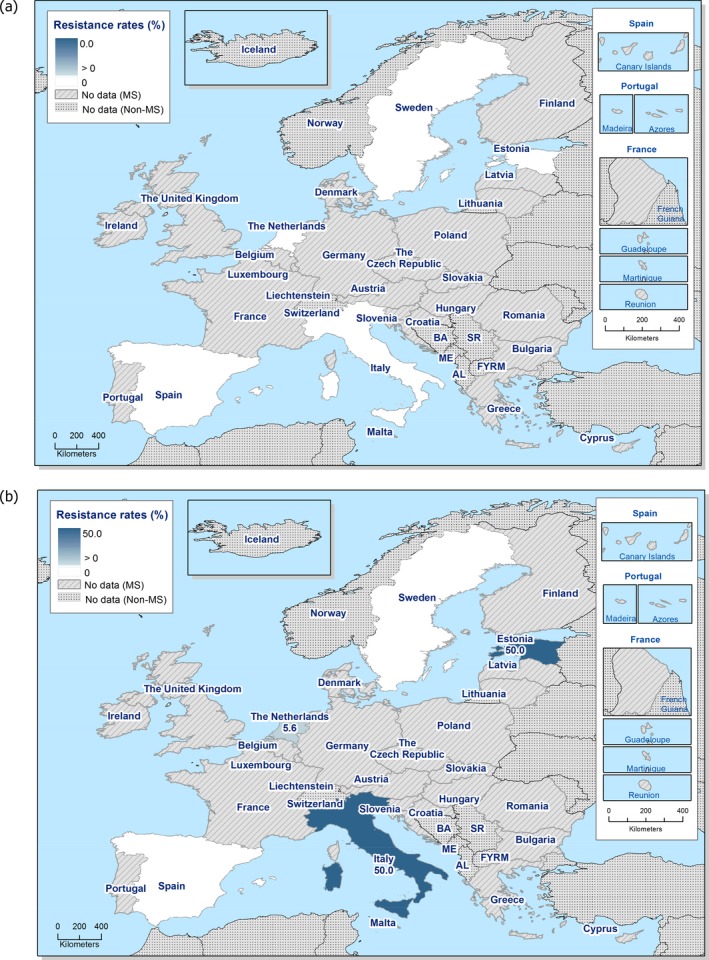
Spatial distribution of cefotaxime (a) and ciprofloxacin (b) resistance among Salmonella Dublin from cattle, 5 EU MSs, 2017
Multidrug resistance in S. Dublin from cattle
Five MSs reported data on 24 individual S. Dublin isolates, which were included in the MDR analysis (Figure D.7). In contrast to monophasic S. Typhimurium isolates recovered from cattle, a much lower proportion of S. Dublin were multiresistant; this figure was also lower than that reported in S. Typhimurium (30.8%). Overall, the majority of S. Dublin isolates were susceptible to the full panel of antimicrobials (66.7%), with susceptibility ranging from 50% to 100%, while only 16.7% of S. Dublin isolates were multiresistant (MDR ranging from 0% to 22.2%) ‐ see Table COMDUBLINBOV. Notably, where complete susceptibility was detected at 100%, only one S. Dublin isolate was reported by MSs for assessment.
Figure D.7.
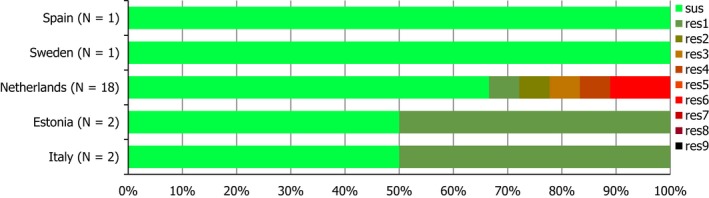
Frequency distribution of completely susceptible isolates and resistant isolates to one to nine antimicrobial classes in Salmonella Dublin from cattle, 5 EU MSs, 2017
- N: total number of isolates tested for susceptibility against the whole common antimicrobial set for Salmonella; UK: United Kingdom; sus: susceptible to all antimicrobial classes of the common set for Salmonella; res1–res9: resistance to one antimicrobial class/resistance to seven antimicrobial classes of the common set for Salmonella.
Appendix E – Additional data reported on antimicrobial resistance in indicator E. coli
In pigs and derived meat
For 2017, four MSs and one non‐MS also provided susceptibility data on indicator (commensal) E. coli from caecal content of fattening pigs for 2016 on a voluntary basis. Data were obtained according to the requirements laid down in Commission Implementing Decision 2013/652/EU. The ‘microbiological resistance’ to the harmonised set of antimicrobials (as opposed to clinical resistance) was interpreted using ECOFFs laid down in the Decision.17
Italy also provided on a voluntary basis data on E. coli from pig meat for 2017.
Antimicrobial resistance in indicator E. coli from pigs
Four MSs (Belgium, Croatia, Denmark, the Netherlands) and one non‐MS (Iceland) on a voluntary basis also reported data from fattening pigs for 2016 (Table E.2). For most antimicrobials, occurrence of resistance within a country was of the same magnitude in 2016 as in 2017. However, for several antimicrobials, Croatia reported lower resistance in 2017, notably for chloramphenicol, ampicillin, nalidixic acid, ciprofloxacin, sulfamethoxazole and tetracycline. Also, Denmark reported lower resistance to sulfamethoxazole in 2017 but higher ampicillin and tetracycline resistance. Iceland reported lower ampicillin, sulfamethoxazole and trimethoprim resistance in 2017 but higher nalidixic acid and ciprofloxacin resistance. Belgium in 2017 reported higher resistance than in 2016 for several antimicrobials, notably ampicillin, cefotaxime, ceftazidime, nalidixic acid and ciprofloxacin. No statistical evaluation of the data was made, and interpretation of numerical differences should be made with caution.
Table E.2.
Occurrence of resistance (%) to selected antimicrobials in indicator Escherichia coli from calves under 1 year of age, using harmonised ECOFFs, EU MSs, 2016
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 167 | 4.2 | 24.6 | 53.9 | 0 | 0 | 0 | 0 | 17.4 | 18.6 | 3 | 1.8 | 58.7 | 40.1 | 69.5 |
| Croatia | 95 | 1.1 | 10.5 | 22.1 | 0 | 0 | 0 | 0 | 6.3 | 8.4 | 0 | 0 | 32.6 | 9.5 | 29.5 |
| Denmark | 121 | 0 | 2.5 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 5.8 |
| Netherlands | 300 | 3.3 | 15 | 23.3 | 0.3 | 0.3 | 0 | 0 | 2.7 | 3.7 | 1.3 | 0 | 27 | 20 | 41.3 |
| Total (4 MSs) | 683 | 2.6 | 14.5 | 27.4 | 0.1 | 0.1 | 0 | 0 | 6.3 | 7.3 | 1.3 | 0.4 | 31.6 | 19.9 | 40.3 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin, CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; MEM: meropenem; TGC: tigecycline, NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
Antimicrobial resistance in indicator E. coli from pig meat
Resistance in E. coli from pig meat was reported on a voluntary basis by Italy. In all, data for 169 isolates were reported with occurrence of resistance to gentamicin 4.1%, chloramphenicol 26.0%, ampicillin 47.3%, cefotaxime 0.6%, ceftazidime 0.6%, meropenem 0%, tigecycline 0%, nalidixic acid 9.5%, ciprofloxacin 17.2%, azithromycin 0.6%, colistin 5.3%, sulfamethoxazole 43.8%, trimethoprim 37.9% and tetracycline 51.5%. In the data reported by Italy, occurrence of resistance was generally lower in isolates from meat of pigs than in isolates from caecal content of fattening pigs. Notably, resistance to chloramphenicol, ampicillin, sulfamethoxazole, trimethoprim and tetracycline in isolates from meat was 55–67% of the occurrence in isolates from caecal content. In contrast, resistance to colistin was more common in isolates from meat (5.3%) than in isolates from caecal content (0.6%). No statistical evaluation of the data was made, and interpretation of numerical differences should be made with caution.
In calves under 1 year of age and meat from bovine
For 2017, on a voluntary basis, four MSs also provided data on indicator E. coli from caecal content of calves under 1 year of age for 2016 (Table E.2). Data were obtained according to the requirements laid down in Commission Implementing Decision 2013/652/EU. And ‘microbiological resistance’ to the harmonised set of antimicrobials (as opposed to clinical resistance) was interpreted using ECOFFs laid down in the Decision.1
Italy also provided data on E. coli from meat of bovines for 2017 on a voluntary basis.
Antimicrobial resistance in indicator E. coli from Calves under one year
Four MSs (Belgium, Croatia, Denmark and the Netherlands) on a voluntary basis also reported data from calves under one year for 2016 (Table E.2). For most antimicrobials, occurrence of resistance within a country was of the same magnitude as in 2017. However, Croatia reported lower resistance for chloramphenicol and ampicillin in 2017 than in 2016. In contrast, Belgium reported higher resistance to ampicillin, ciprofloxacin and trimethoprim. Interestingly, in 2017, Belgium reported resistance to cefotaxime and ceftazidime, which was not detected in 2016. Moreover, the ratio of nalidixic acid resistance to and ciprofloxacin resistance was lower in and 2017 (0.52) than in 2016 (0.94). No statistical evaluation of the data was made, and interpretation of numerical differences should be made with caution.
Antimicrobial resistance in indicator E. coli from meat of bovines
Resistance in indicator E. coli from bovine meat was reported on a voluntary basis by Italy. In all, data for 160 isolates were reported with occurrence of resistance to gentamicin 8.8%, chloramphenicol 13.1%, ampicillin 35.6%, cefotaxime 0.6%, ceftazidime 2.5%, meropenem 0%, tigecycline 0%, nalidixic acid 13.8%, ciprofloxacin 20.0%, azithromycin 0.6%, colistin 3.1%, sulfamethoxazole 38.8%, trimethoprim 30.0% and tetracycline 40.0%. In the data reported by Italy, occurrence of resistance was generally lower in isolates from meat of bovines than in isolates from caecal content of calves under 1 year of age. Notably, occurrence of resistance to gentamicin, chloramphenicol, ampicillin, nalidixic acid, ciprofloxacin, sulfamethoxazole, trimethoprim and tetracycline in isolates from meat was about half of the occurrence in isolates from caecal content. No statistical evaluation of the data was made, and interpretation of numerical differences should be made with caution.
Table E.1.
Occurrence of resistance (%) to selected antimicrobials in indicator Escherichia coli from fattening pigs, using harmonised ECOFFs, four EU MSs and one non‐MS, 2016
| Country | N | GEN | CHL | AMP | CTX | CAZ | MEM | TGC | NAL | CIP | AZM | COL | SMX | TMP | TET |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | 169 | 0.6 | 24.9 | 45.6 | 0 | 0 | 0 | 0 | 1.8 | 4.7 | 0.6 | 0.6 | 50.3 | 45 | 47.3 |
| Croatia | 74 | 0 | 20.3 | 48.6 | 0 | 0 | 0 | 0 | 13.5 | 21.6 | 0 | 0 | 47.3 | 29.7 | 60.8 |
| Denmark | 145 | 2.1 | 4.8 | 31.7 | 0.7 | 0.7 | 0 | 0 | 1.4 | 1.4 | 2.1 | 0 | 42.1 | 29.7 | 33.8 |
| Netherlands | 299 | 0 | 12.7 | 23.1 | 0.3 | 0.3 | 0 | 0 | 0.7 | 0.7 | 0.3 | 0 | 34.4 | 31.8 | 42.8 |
| Total (4 MSs) | 687 | 0.6 | 14.8 | 33.2 | 0.3 | 0.3 | 0 | 0 | 2.5 | 4.1 | 0.7 | 0.1 | 41.3 | 34.4 | 44 |
| Iceland | 21 | 0 | 4.8 | 23.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 23.8 | 19 | 4.8 |
ECOFFs: epidemiological cut‐off values; MSs: Member States; N: number of isolates tested; GEN: gentamicin, CHL: chloramphenicol; AMP: ampicillin; CTX: cefotaxime; CAZ: Ceftazidime; MEM: meropenem; TGC: tigecycline, NAL: nalidixic acid; CIP: ciprofloxacin; AZM: azithromycin; COL: colistin; SMX: sulfamethoxazole; TMP: trimethoprim; TET: tetracycline.
Suggested citation: EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control) , 2019. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2017. EFSA Journal 2019;17(2):5598, 278 pp. 10.2903/j.efsa.2019.5598
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00752
Acknowledgements: EFSA and ECDC wish to thank the members of the Scientific Network for Zoonoses Monitoring Data (EFSA) and the Food‐ and Waterborne Diseases and Zoonoses Network (ECDC) who provided the data and reviewed the report and the members of the Scientific Network for Zoonoses Monitoring Data, for their endorsement of this scientific output. Also, the contribution of EFSA staff members: Pierre‐Alexandre Beloeil, Beatriz Guerra, Anca‐Violeta Stoicescu, Kenneth Mulligan, Krisztina Nagy, Gina Cioacata, and Daniel Thomas, the contributions of ECDC staff member: Therese Westrell, and the contributions of EFSA’s contractors: Björn Bengtsson, Isabelle Kempf, Oskar Nilsson and Catherine Tallentire for the support provided to this scientific output. Also to the EURL‐AR, specifically Inge Marianne Hansen, Jette Sejer Kjeldgaard, Anne Mette Seyfarth, Pimlapas Leekitcharoenphon, Valeria Bortolaia, and Rene S. Hendriksen, as well as the EFSA Tasking Grant Iolanda Mangone, for the support specially for the confirmatory testing.
Approved: 31 January 2019
This article was originally published on the EFSA website http://www.efsa.europa.eu on 26 February 2019 as part of EFSA's urgent publication procedures.
Notes
Reduced susceptibility according to ECOFFs to at least three of the nine antimicrobial classes tested.
Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, pp. 26–39.
Decision No. 1082/2013/EU of the European Parliament and of the Council of 22 October 2013 on serious cross‐border threats to health and repealing Decision No. 2119/98/EC. OJ L 293, 5.11.2013, pp. 1–15.
Commission Decision 2012/506/EU amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No. 2119/98/EC of the European Parliament and of the Council. OJ L 262, 27.9.2012, pp. 1–57.
Regulation (EC) No. 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the EFSA and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Regulation (EC) No. 851/2004 of the European Parliament and of the Council of 21 April 2004 establishing a European centre for disease prevention and control. OJ L 142, 30.4.2004, pp. 1–11.
Commission Implementing Decision 2013/652/EU of 12 November 2013 on the monitoring and reporting of antimicrobial resistance in zoonotic and commensal bacteria. OJ L 303, 14.11.2013, pp. 26–39.
The epidemiological cut‐off (ECOFF) values separate the naive, susceptible wild‐type bacterial populations from isolates that have developed reduced susceptibility to a given antimicrobial agent (Kahlmeter et al., 2003). The ECOFFs may differ from breakpoints used for clinical purposes, which are set out against a background of clinically relevant data, including therapeutic indication, clinical response data, dosing schedules, pharmacokinetics and pharmacodynamics. The use of harmonised methods and ECOFFs ensures the comparability of data over time at the country level and also facilitates the comparison of resistance between MSs.
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
‘Quantitative data’ derived from dilution methods consisted of the number of isolates having a specific MIC value (measured in mg/L) relative to the total number of isolates tested, for each antimicrobial agent and specific food/animal category.
The same sampling design was used to collect indicator E. coli isolates, whether dedicated to the routine monitoring of AMR or the specific monitoring of ESBL‐/AmpC‐/carbapenemase‐producing E. coli.
Available online: http://www.eurl-ar.eu
Giving the percentage of isolates ‘microbiologically’ resistant out of those tested.
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
SIR stands for susceptible, intermediate or resistant.
This pattern of MDR (resistance to ampicillin, sulfonamide and tetracycline) typically also includes resistance to streptomycin; however, as described in the Materials and methods section, data on this antimicrobial are no longer included in this report.
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
All tables on occurrence of resistance mentioned in this section can be uploaded in the EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
Of particular note is that ‘microbiological’ resistance to ciprofloxacin was addressed using ECOFF CIP > 0.034 in this report.
Austria performed this monitoring on a voluntary basis.
All tables on occurrence of resistance mentioned in this section can be uploaded from EFSA Zenodo link: http://doi.org/10.5281/zenodo.2562858
References
- Agersø Y, Torpdahl M, Zachariasen C, Seyfarth A, Hammerum AM and Møller Nielsen EM, 2012. Tentative colistin epidemiological cut‐off value for Salmonella spp. Foodborne Pathogens Disease, 9, 367–369. https://doi.org/10.1089/fpd. 2011.1015 [DOI] [PubMed] [Google Scholar]
- Angen Ø, Stegger M, Larsen J, Lilje B, Kaya H, Pedersen KS, Jakobsen A, Petersen A and Larsen AR, 2017. Report of mecC‐carrying MRSA in domestic swine. Journal of Antimicrobial Chemotherapy, 72, 60–63. 10.1093/jac/dkw389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous , 2018. Annual Report on Zoonoses in Denmark 2017, National Food Institute, Technical University of Denmark. Available from: https://www.food.dtu.dk/-/media/Institutter/Foedevareinstituttet/Publikationer/Pub-2018/Rapport-Annual-Report-on-Zoonoses-2017.ashx?la=da&hash=EDBE0AD05571F1B5BCC74B9D6505D921B0E6E4B5
- Aviv G, Tsyba K, Steck N, Salmon‐Divon M, Cornelius A, Rahav G, Grassl GA and Gal‐Mor O, 2014. A unique megaplasmid contributes to stress tolerance and pathogenicity of an emergent Salmonella enterica serovar Infantis strain. Environmental Microbiology, 16, 977–994. 10.1111/1462-2920.12351 [DOI] [PubMed] [Google Scholar]
- Bangerter PD, Sidler X, Perreten V and Overesch G, 2016. Longitudinal study on the colonisation and transmission of methicillin‐resistant Staphylococcus aureus in pig farms. Veterinary Microbiology, 183, 125–134. 10.1016/j.vetmic.2015.12.007 [DOI] [PubMed] [Google Scholar]
- van Belkum A, 1999. The role of short sequence repeats in epidemiologic typing. Current Opinion in Microbiology, 2, 306–311. [DOI] [PubMed] [Google Scholar]
- Bengtsson B, Persson L, Ekström K, Unnerstad HE, Uhlhorn H and Börjesson S, 2017. High occurrence of mecC‐MRSA in wild hedgehogs (Erinaceus europaeus) in Sweden. Veterinary Microbiology, 207, 103–107. [DOI] [PubMed] [Google Scholar]
- Bolinger HK, Zhang Q, Miller WG and Kathariou S, 2018. Lack of Evidence for erm(B) Infiltration Into Erythromycin‐Resistant Campylobacter coli and Campylobacter jejuni from Commercial Turkey Production in Eastern North Carolina: A Major Turkey‐Growing Region in the United States. Foodborne Pathogens and Disease, 15, 698–700. 10.1089/fpd.2018.2477 [DOI] [PubMed] [Google Scholar]
- Borowiak M, Szabo I, Baumann B, Junker E, Hammerl JA, Kaesbohrer A, Malorny B and Fischer J, 2017. VIM‐1‐producing Salmonella Infantis isolated from swine and minced pork meat in Germany. Journal of Antimicrobial Chemotherapy, 72, 2131–2133. 10.1093/jac/dkx101 [DOI] [PubMed] [Google Scholar]
- Boye K and Westh H, 2010. Variations in spa types found in consecutive MRSA isolates from the same patients. FEMS Microbiology Letters, 314, 101–105. 10.1111/j.1574-6968.2010.02151.x [DOI] [PubMed] [Google Scholar]
- Brouwer MSM, Rapallini M, Geurts Y, Harders F, Bossers A, Mevius DJ, Wit B and Veldman KT, 2018. Enterobacter cloacae complex isolated from shrimps from Vietnam encoding blaIMI‐1, resistant to carbapenems but not cephalosporins. Antimicrobial Agents and Chemotherapy, 62, e00398–18. 10.1128/AAC.00398-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos J, Cristino L, Peixe L and Antunes P, 2016. MCR‐1 in multidrug‐resistant and copper‐tolerant clinically relevant Salmonella 1,4[5],12:i:‐ and S. Rissen clones in Portugal, 2011 to 2015. Euro Surveillance, 21: 5 pp. 10.2807/1560-7917.ES.2016.21.26.30270 [DOI] [PubMed] [Google Scholar]
- Carfora V, Giacinti G, Sagrafoli D, Marri N, Giangolini G, Alba P, Feltrin F, Sorbara L, Amoruso R, Caprioli A, Amatiste S and Battisti A, 2016. Methicillin‐Resistant and Methicillin‐Susceptible Staphylococcus Aureus in Dairy Sheep and in‐Contact Humans: An Intra‐Farm Study, 99, 4251–4258. 10.3168/jds.2016-10912 [DOI] [PubMed] [Google Scholar]
- Carnevali C, Morganti M, Scaltriti E, Bolzoni L, Pongolini S and Casadei G, 2016. Occurrence of mcr‐1 in colistin‐resistant Salmonella enterica isolates recovered from humans and animals in Italy, 2012 to 2015. Antimicrobial Agents and Chemotherapy, 60, 7532–7534. 10.1128/AAC.01803-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CA‐SFM (Comité de l'antibiogramme de la Société Française de Microbiologie), 2017. Recommendations 2017. V.1.0. Mars 2017
- Cavaco LM, Hasman H, Xia S and Aarestrup FM, 2009. qnrD, a novel gene conferring transferable quinolone resistance in Salmonella enterica serovar Kentucky and Bovis morbificans strains of human origin. Antimicrobial Agents and Chemotherapy, 53, 603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco L, Hendriksen R, Agersø Y, Aaby Svendsen C, Nielsen H, Guerra B, Peran R and Hasman H, 2016. Selectiveenrichment of ESBL, AmpC and carbapenemase producingE. coliin meat and cecal samples ‐ additionalvalidation for poultry samples. 26th European Congress Clinical Microbiology and Infectious Diseases (ECCMID,2016). Poster P0643, 10 April 2016, Amsterdam, the Netherlands.
- Ceccarelli D, vanEssen‐Zandbergen A, Veldman KT, Tafro N, Haenen O and Mevius DJ, 2017. Chromosome‐basedblaOXA‐48‐like variants inShewanellaspecies isolates from food‐producing animals, fish, and the aquaticenvironment. Antimicrobial agents and Chemotherapy, 61, pii: e01013–16. 10.1128/aac.01013-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick SG, Prasad A, Smith WL, Mordechai E, Adelson ME and Gygax SE, 2013. Detection of epidemic USA300 community‐associated methicillin‐resistant Staphylococcus aureus strains by use of a single allele‐specific PCR assay targeting a novel polymorphism of Staphylococcus aureus pbp3. Journal of Clinical Microbiology, 51, 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Tagg KA, Joung YJ, Bennett C, Francois Watkins L, Eikmeier D and Folster JP, 2018. Report of erm(B)(+) Campylobacter jejuni in the United States. Antimicrobial Agents and Chemotherapy, 62, e02615–e02617. 10.1128/AAC.02615-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI and EUCAST (Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing), 2016. Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI‐EUCAST Polymyxin Breakpoints Working Group. 22 March 2016. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/Recommendations_ for_MIC_determination_of_colistin_March_2016.pdf
- Collignon PC, Conly JM, Andremont A, McEwen SA and Aidara‐Kane A and World Health Organization Advisory Group, Bogotá Meeting on Integrated Surveillance of Antimicrobial Resistance (WHO‐AGISAR) , 2016. World Health Organization ranking of antimicrobials according to their importance in human medicine: a critical step for developing risk management strategies to control antimicrobial resistance from food animal production. Clinical Infectious Diseases, 63, 1087–1093. 10.1093/cid/ciw475 [DOI] [PubMed] [Google Scholar]
- Crespo MD, Altermann E, Olson J, Miller WG, Chandrashekhar K and Kathariou S, 2016. Novel plasmid conferring kanamycin and tetracycline resistance in the turkey‐derived Campylobacter jejuni strain 11601MD. Plasmid, 86, 32–37. 10.1016/j.plasmid.2016.06.001 [DOI] [PubMed] [Google Scholar]
- Crombé F, Argudín MA, Vanderhaeghen W, Hermans K, Haesebrouck F and Butaye P, 2013. Transmission dynamics of methicillin‐resistant Staphylococcus aureus in pigs. Frontiers in Microbiology, 4, 57 10.3389/fmicb.2013.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuny C, Abdelbary M, Layer F, Werner G and Witte W, 2015. Prevalence of the immune evasion gene cluster in Staphylococcus aureus CC398. Veterinary Microbiology, 177, 219–223. 10.1016/j.vetmic.2015.02.031 [DOI] [PubMed] [Google Scholar]
- Dearlove BL, Cody AJ, Pascoe B, Meric G, Wilson DJ and Sheppard SK, 2016. Rapid host switching in generalist Campylobacter strains erodes the signal for tracing human infections. The ISME Journal, 10, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F, Shen J, Zhang M, Wu C, Zhang Q and Wang Y, 2015. Constitutive and Inducible Expression of the rRNA Methylase Gene erm(B) in Campylobacter. Antimicrobial Agents and Chemotherapy, 59, 6661–6664. 10.1128/AAC.01103-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisi AM, Lucarelli C, Owczarek S, Luzzi I and Villa L, 2009. Characterization of the plasmid‐borne quinolone resistance gene qnrB19 in Salmonella enterica serovar Typhimurium. Antimicrobial Agents and Chemotherapy, 53, 4019–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisi AM, Owczarek S, Benedetti I, Luzzi I and García‐Fernández A, 2016. Extended‐spectrum β‐lactamase‐producing Salmonella enterica serovar Infantis from humans in Italy. International Journal of Antimicrobial Agents, 48, 345–346. 10.1016/j.ijantimicag.2016.06.025 [DOI] [PubMed] [Google Scholar]
- ECDC (European Centre for Disease Prevention and Control), 2014. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates. Stockholm: ECDC; 2014. Available online: http://www.ecdc.europa.eu/en/publications/Publications/harmonised-monitoring-antimicrobial-resistance‐ human‐salmonella‐campylobacter‐isolates‐EU‐protocol.pdf
- ECDC (European Centre for Disease Prevention and Control), 2016. EU protocol for harmonised monitoring of antimicrobial resistance in human Salmonella and Campylobacter isolates – June 2016. Stockholm: ECDC; 2016. Available online: http://ecdc.europa.eu/en/publications/Publications/antimicrobial-resistance-Salmonella-Campylobacter-harmonised-monitoring.pdf
- ECDC (European Centre for Disease Prevention and Control), 2018. European Centre for Disease Prevention and Control. Surveillance of antimicrobial resistance in Europe – Annual report of the European Antimicrobial Resistance Surveillance Network (EARS‐Net) 2017 . Stockholm: ECDC; 2018.
- ECDC, EFSA, EMEA and SCENIHR (European Centre for Disease Prevention and Control, European Food Safety Authority, European Medicines Agency and European Commission's Scientific Committee on Emerging and Newly Identified Health Risks), 2009. Joint opinion on antimicrobial resistance (AMR) focused on zoonotic infections. EFSA Journal 2009;7(11):1372, 78 pp. 10.2903/j.efsa.2009.1372 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA Journal 2008;6(4):141, 44 pp. 10.2903/j.efsa.2008.141r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009a. Joint scientific report of ECDC, EFSA and EMEA on meticillin resistant Staphylococcus aureus (MRSA) in livestock, companion animals and foods. EFSA‐Q‐2009‐00612 (EFSA Scientific Report (2009) 301, 1–10) and EMEA/CVMP/SAGAM/62464/2009. EFSA Journal 2009;7(6):301r, 10 pp. 10.2903/j.efsa.2009.301r [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009b. Scientific opinion of the Panel on Biological Hazards on a request from the European Commission on Assessment of the public health significance of meticillin resistant Staphylococcus aureus (MRSA) in animals and foods. EFSA Journal 2009;7(3):993, 73 pp. 10.2903/j.efsa.2009.993 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2009c. Analysis of the baseline survey on the prevalence of methicillin‐resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008, Part A: MRSA prevalence estimates; on request from the European Commission. EFSA Journal 2009;7(11):1376, 82 pp. 10.2903/j.efsa.2009.1376 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012a. Technical specifications for the analysis and reporting of data on antimicrobial resistance (AMR) in the European Union Summary Report. EFSA Journal 2012;10(2):2587, 53 pp. 10.2903/j.efsa.2012.2587 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2012b. Technical specifications for the harmonised monitoring and reporting of antimicrobial resistance in methicillin‐resistant Staphylococcus aureus in food‐producing animals and foods. EFSA Journal 2012;10(10):2897, 56 pp. 10.2903/j.efsa.2012.2897 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) and ECDC (European Centre for Disease Prevention and Control), 2014. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2012. EFSA Journal 2014;12(3):3590, 336 pp., 10.2903/j.efsa.2014.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2011. Scientific Opinion on the public health risks of bacterial strains producing extended‐spectrum β‐lactamases and/or AmpC β‐lactamases in food and food‐producing animals. EFSA Journal 2011;9(8):2322, 95 pp. 10.2903/j.efsa.2011.2322. Available online: http://www.efsa.europa.eu/efsajournal [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2013. Scientific Opinion on Carbapenem resistance in food animal ecosystems. EFSA Journal 2013;11(12):3501, 70 pp. 10.2903/j.efsa.2013.3501 [DOI] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2016. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2014. EFSA Journal 2016;14(2):4380, 207 pp. 10.2903/j.efsa.2016.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017a. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2015. EFSA Journal 2017;15(2): 4694, 212 pp. 10.2903/j.efsa.2017.4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2017b. ECDC/EFSA/EMA second joint report on the integrated analysis of the consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food‐producing animals – Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. EFSA Journal 2017;15(7):4872, 135 pp. 10.2903/j.efsa.2017.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018a. The European Union summary report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2016. EFSA Journal 2018;16(2):5182, 270 pp. 10.2903/j.efsa.2018.5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control), 2018b. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA Journal 2018;16(12):5500, 262 pp. 10.2903/j.efsa.2018.5500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea AL, Gagetti P, Lamberghini R, Faccone D, Lucero C, Vindel A, Tosoroni D, Garnero A, Saka HA, Galas M, Bocco JL, Corso A and Sola C, 2014. New patterns of methicillin‐resistant Staphylococcus aureus (MRSA) clones, community‐associated MRSA genotypes behave like healthcare‐associated MRSA genotypes within hospitals, Argentina. International Journal of Medical Microbiology, 304, 1086–1099. https://pdfs.semanticscholar.org/313e/a7681483ab77327a6a9d3aab2a871e7b1880.pdf [DOI] [PubMed] [Google Scholar]
- EMA (European Medicines Agency), 2013. Use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2013/07/WC500146813.pdf
- EMA (European Medicines Agency), 2016. Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211080.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing), 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 1.0 December 2013. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing), 2014. Screening for fluoroquinolone resistance in Salmonella spp. with pefloxacin 5 lg. Tentative quality control criteria for users and disk manufacturers. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/QC/Tentative_QC_criteria_for_pefloxacin_5__g.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing), 2017a. Calibration of zone diameter breakpoints to MIC values ‐ Campylobacter jejuni and coli, Jan 2017. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_criteria/Validation_2017/Camplyobacter_v_2.2_January_2017.pdf
- EUCAST (European Committee for Antimicrobial Susceptibility Testing), 2017b. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. Version 2.01 July 2017. Available online:http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/ EUCAST_detection_of_resistance_mechanisms_170711.pdf
- EURL‐AR (European Union Reference Laboratory for Antimicrobial Resistance), 2009. Laboratory Protocol, Isolation of MRSA from dust samples. Technical University of Denmark.
- EURL‐AR (European Union Reference Laboratory for Antimicrobial Resistance), 2018. Laboratory Protocol, Isolation of methicillin‐resistant Staphylococcus aureus (MRSA) from food‐producing animals and farm environment. Technical University of Denmark.
- EVIRA (Finnish Food Safety Authority), onlineAvailable online: https://www.ruokavirasto.fi/en/farmers/animal-husbandry/animal-medication/moniresistentit-bakteerit/frequently-asked-questions-about-mrsa/ [Accessed: January 2019]
- Fabre A, Oleastro M, Nunes A, Santos A, Sifré E, Ducournau A, Bénéjat L, Buissonnière A, Floch P, Mégraud F, Dubois V and Lehours P, 2018. Whole‐genome sequence analysis of multidrug resistant Campylobacter spp. isolates: a focus on aminoglycoside resistance determinants. Journal of Clinical Microbiology, 56, e00390–18. 10.1128/JCM.00390-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falgenhauer L, Ghosh H, Guerra B, Yao Y, Fritzenwanker M, Fischer J, Helmuth R, Imirzalioglu C and Chakraborty T, 2017. Comparative genome analysis of IncHI2 VIM‐1 carbapenemase‐encoding plasmids of Escherichia coli and Salmonella enterica isolated from a livestock farm in Germany. Veterinary Microbiology, 200, 114–117. 10.1016/j.vetmic.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Fernández J, Guerra B and Rodicio MR, 2018. Resistance to carbapenems in non‐typhoidal Salmonella enterica serovars from humans, animals and food. Veterinary Science, 5 pii: E40 10.3390/vetsci5020040.Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Hille K, Ruddat I, Mellmann A, Köck R and Kreienbrock L, 2017a. Simultaneous occurrence of MRSA and ESBL‐producing Enterobacteriaceae on pig farms and in nasal and stool samples from farmers. Veterinary Microbiology, 200, 107–113. 10.1016/j.vetmic.2016.05.021 [DOI] [PubMed] [Google Scholar]
- Fischer J, San Rosé M, Roschanski N, Schmoger S, Baumann B, Irrgang A Friese A, Roesler U, Helmuth R and Guerra B, 2017b. Spread and persistence of VIM‐1 carbapenemase‐producing Enterobacteriaceae in three German swine farms in 2011 and 2012. Veterinary Microbiology, 200, 118–123. 10.1016/j.vetmic.2016.04.026 [DOI] [PubMed] [Google Scholar]
- Florez‐Cuadrado D, Ugarte‐Ruiz M, Quesada A, Palomo G, Dominguez L and Porrero MC, 2016. Description of an erm(B)‐carrying Campylobacter coli isolate in Europe. Journal of Antimicrobial Chemotherapy, 71, 841–843. 10.1093/jac/dkv383 [DOI] [PubMed] [Google Scholar]
- Florez‐Cuadrado D, Ugarte‐Ruiz M, Meric G, Quesada A, Porrero MC, Pascoe B, Saez‐Llorente JL, Orozco GL, Dominguez L and Sheppard SK, 2017. Genome comparison of erythromycin resistant Campylobacter from turkeys identifies hosts and pathways for horizontal spread of erm(B) genes. Frontiers in Microbiology, 8, 2240 10.3389/fmicb.2017.02240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frana TS, Beahm AR, Hanson BM, Kinyon JM, Layman LL, Karriker LA, Ramirez A and Smith TC, 2013. Isolation and characterization of methicillin‐resistant Staphylococcus aureus from pork farms and visiting veterinary students. PLoS ONE, 8, e53738 10.1371/journal.pone.0053738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco A, Hasman H, Iurescia M, Lorenzetti R, Stegger M, Pantosti A, Feltrin F, Ianzano A, Porrero MC, Liapi M and Battisti A, 2011. Molecular characterization of spa type t127, sequence type 1 methicillin‐resistant Staphylococcus aureus from pigs. Journal of Antimicrobial Chemotherapy, 66, 1231–1235. 10.1093/jac/dkr115 [DOI] [PubMed] [Google Scholar]
- Franco A, Leekitcharoenphon P, Feltrin F, Alba P, Cordaro G, Iurescia M, Tolli R, D'Incau M, Staffolani M, Di Giannatale E, Hendriksen RS and Battisti A, 2015. Emergence of a clonal lineage of multidrug‐resistant ESBL‐producing Salmonella Infantis transmitted from broilers and broiler meat to humans in Italy between 2011 and 2014. PLoS ONE, 10, e0144802 10.1371/journal.pone.0144802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FSA (Food Standards Agency), 2017. Risk Assessment on Meticillin‐Resistant Staphylococcus aureus (MRSA), with a focus on Livestock‐associated MRSA in the UK Food Chain. Available online: https://pdfs.semanticscholar.org/4fca/b9277652e3d8cc34a8150b31de865a838e67.pdf
- García‐Álvarez L, Holden MTG, Lindsay H, Webb CR, Brown DFJ, Curran MD, Walpole E, Brooks K, Pickard DJ, Teale C, Parkhill J, Bentley SD, Edwards GF, Girvan EK, Kearns AM, Pichon B, Hill RLR, Larsen AR, Skov RL, Peacock SJ, Maskell DJ and Holmes MA, 2011. Meticillin‐resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infectious Diseases, 11, 595–603. 10.1016/S1473-3099(11)70126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Fierro R, Montero I, Bances M, González‐Hevia MÁ and Rodicio MR, 2016. Antimicrobial drug resistance and molecular typing of Salmonella enterica serovar Rissen from different sources. Microbial Drug Resistance, 22, 211–217. [DOI] [PubMed] [Google Scholar]
- Gibbons CL, Mangen MJ, Plass D, Havelaar AH, Brooke RJ, Kramarz P, Peterson KL, Stuurman AL, Cassini A, Fevre EM and Kretzschmar ME and the Burden of Communicable diseases in Europe (BCoDE) consortium , 2014. Measuring underreporting and under‐ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health, 11, 147 10.1186/1471-2458-14-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MJ, Bos ME, Duim B, Urlings BA, Heres L, Wagenaar JA and Heederik DJ, 2012. Livestock‐associated MRSA ST398 carriage in pig slaughterhouse workers related to quantitative environmental exposure. Occupational and Environmental Medicine, 69, 472–478. 10.1136/oemed-2011-100069 [DOI] [PubMed] [Google Scholar]
- Giske CG, 2015. Contemporary resistance trends and mechanisms for the old antibiotics colistin, temocillin, fosfomycin, mecillinam and nitrofurantoin. Clinical Microbiology and Infection, 21, 899–905. [DOI] [PubMed] [Google Scholar]
- González‐Sanz R, Herrera‐León S, de la Fuente M, Arroyo M and Echeita MA, 2009. Emergence of extended‐spectrum β‐lactamases and AmpC‐type β‐lactamases in human Salmonella isolated in Spain from 2001 to 2005. Journal of Antimicrobial Chemotherapy, 64, 1181–1186. [DOI] [PubMed] [Google Scholar]
- Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E and Heederik D, 2010. Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PLoS ONE, 5, e10990 10.1371/journal.pone.0010990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøntvedt CA, Elstrøm P, Stegger M, Skov RL, Skytt Andersen P, Larssen KW, Urdahl AM, Angen Ø, Larsen J, Åmdal S, Løtvedt SM, Sunde M and Bjørnholt JV, 2016. Methicillin‐resistant Staphylococcus aureus CC398 in humans and pigs in Norway: A “One Health” perspective on introduction and transmission. Clinical Infectious Diseases, 63, 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardabassi L, O'Donoghue M, Moodley A, Ho J and Boost M, 2009. Novel lineage of methicillin‐resistant Staphylococcus aureus, Hong Kong. Emerging and Infectious Diseases, 15, 1998–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra B, Fischer J and Helmuth R, 2014. An emerging public health problem: acquired carbapenemase‐producing microorganisms are present in food producing animals, their environment, companion animals and wild birds. Veterinary Microbiology, 171, 290–297. [DOI] [PubMed] [Google Scholar]
- Guo B, Lin J, Reynolds DL and Zhang Q, 2010. Contribution of the multidrug efflux transporter CmeABC to antibiotic resistance in different Campylobacter species. Foodborne Pathogens and Disease, 7, 77–83. 10.1089/fpd.2009.0354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsma JA, Geenen PL, Ethelberg S, Hansdotter F, Jansen A, Korsgaard H, O'Brien SJ, Scavia G, Spitznagel H, Stefanoff P, Tam CC and Havelaar AH on behalf of a Med‐Vet‐Net Working Group , 2013. Community incidence of pathogen‐specific gastroenteritis: reconstructing the surveillance pyramid for seven pathogens in seven European Union member states. Epidemiology and Infection, 141, 1625–1639. 10.1017/S0950268812002166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison EM, Paterson GK, Holden MTG, Larsen J, Stegger M, Larsen AR, Petersen A, Skov RL, Christensen JM, Bak Zeuthen A, Heltberg O, Harris SR, Zadoks RN, Parkhill J, Peacock SJ and Holmes MA, 2013. Whole genome sequencing identifies zoonotic transmission of MRSA isolates with the novel mecA homologue mecC . EMBO Molecular Medicine, 5, 509–515. 10.1002/emmm.201202413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasman H, Agersø Y, Cavaco L, Aaby Svendsen C, San José M, Fisher J, Schmoger S, Jahn S, Guerra B and Peran R, 2015a. Validation of methods for enrichment of ESBL and AmpC producing E. coli in meat and cecal samples. European Congress Clinical Microbiology and Infectious Diseases (ECCMID, 2015) Poster P0995, 27 April 2015. Copenhagen, Denmark
- Hasman H, Agersø Y, Cavaco L, Aaby Svendsen C, Nielsen H, San José M, Fischer J, Schmoger S, Peran R and Guerra B, 2015b. Evaluation of methods for enrichment of carbapenemase‐producing E. coli in pork meat and cecal samples of porcine and bovine origin. 25th European Congress Clinical Microbiology and Infectious Diseases (ECCMID, 2015), Poster EV0266, 25 April 2015, Copenhagen, Denmark.
- Havelaar AH, Ivarsson S, Löfdahl M and Nauta MJ, 2013. Estimating the true incidence of campylobacteriosis and salmonellosis in the European Union, 2009. Epidemiology and Infection, 141, 293–302. 10.1017/S0950268812000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xu J, Wang J, Chen Q, Hua X, Fu Y and Yu Y, 2016. Decreased susceptibility to tigecycline mediated by a mutation in mlaA in Escherichia coli strains. Antimicrobial Agents and Chemotherapy, 60, 7530–7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo A, Johler S, Karvonen L, Julmi J, Fredriksson‐Ahomaa M and Stephan R, 2016. New dominant spa type t2741 in livestock‐associated MRSA (CC398‐MRSA‐V) in Finnish fattening pigs at slaughter. Antimicrobial Resistance and Infection Control, 5, 6 10.1186/s13756-016-0105-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper DC and Jacoby GA, 2015. Mechanisms of drug resistance: Quinolone resistance. Annals of the New York Academy of Sciences, 1354, 12–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins KL, Kirchner M, Guerra B, Granier SA, Lucarelli C, Porrero MC, Jakubczak A, Threlfall EJ and Mevius DJ, 2010. Multiresistant Salmonella enterica serovar 4,[5],12:i:‐ in Europe: a new pandemic strain? Eurosurveillance, 15: 19580 Available online: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19580 [PubMed] [Google Scholar]
- Irrgang A, Fischer J, Grobbel M, Schmoger S, Skladnikiewicz‐Ziemer T, Thomas K, Hensel A, Tenhagen B‐A and Kasbohrer A, 2017. Recurrent detection of VIM‐1‐producing Escherichia coli clone in German pig production. Journal of Antimicrobial Chemotherapy, 2 pp. 10.1093/jac/dkw47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseppi R, de Niederhäusern S, Bondi M, Messi P and Sabia C, 2018. Extended‐Spectrum β‐Lactamase, AmpC, and MBL‐Producing Gram‐Negative Bacteria on Fresh Vegetables and Ready‐to‐Eat Salads Sold in Local Markets. Microbial Drug Resistance, 24, 1156–1164. 10.1089/mdr.2017.0198 [DOI] [PubMed] [Google Scholar]
- Kahlmeter G, Brown DF, Goldstein FW, MacGowan AP, Mouton JW, Osterlund A, Rodloff A, Steinbakk M, Urbaskova P and Vatopoulos A, 2003. European harmonization of MIC breakpoints for antimicrobial susceptibility testing of bacteria. Journal of Antimicrobial Chemotherapy, 52, 145–148. 10.1093/jac/dkg312 [DOI] [PubMed] [Google Scholar]
- Kempf I, Jouy E and Chauvin C, 2016. Colistin use and colistin resistance in bacteria from animals. International Journal of Antimicrobial Agents, 48, 598–606. 10.1016/j.ijantimicag.2016.09.016 [DOI] [PubMed] [Google Scholar]
- Kieffer N, Aires‐de‐Sousa M, Nordmann P and Poirel L, 2017. High Rate of MCR‐1–producing Escherichia coli and Klebsiella pneumoniae among Pigs, Portugal. Emerging Infectious Diseases, 23, 2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinross P, Petersen A, Skov R, Van Hauwermeiren E, Pantosti A, Laurent F, Voss A, Kluytmans J, Struelens MJ, Heuer O and Monnet DL and the European human LA‐MRSA study group , 2017. Livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA) among human MRSA isolates, European Union/European Economic Area countries, 2013. Eurosurveillance, 22: 16–00696. 10.2807/1560-7917.ES.2017.22.44.16-00696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köck R, Kreienbrock L, van Duijkeren E and Schwarz S, 2017. Antimicrobial resistance at the interface of human and veterinary medicine. Veterinary Microbiology, 200, 1–5. 10.1016/j.vetmic.2016.11.013 [DOI] [PubMed] [Google Scholar]
- Köck R, Daniels‐Haardt I, Becker K, Mellmann A, Friedrich AW, Mevius D, Schwarz S and Jurke A, 2018. Carbapenem‐resistant Enterobacteriaceae in wildlife, food‐producing, and companion animals: a systematic review. Clinical Microbiology and Infection, 24, 1241–1250. 10.1016/j.cmi.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Krupa P, Bystroń J, Podkowik M, Empel J, Mroczkowska A and Bania J, 2015. Population structure and oxacillin resistance of Staphylococcus aureus from pigs and pork meat in south‐west of Poland. BioMed Research International, 2015, 141475 10.1155/2015/141475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang D, Zhang j, Xu X, Shi W, Chen S, Yang X, Su X, Shi X and Meng J, 2018. Emerging high‐level ciprofloxacin resistance and molecular basis of resistance in Salmonella enterica from humans, food and animals. International Journal of Food Microbiology, 280, 1–9. [DOI] [PubMed] [Google Scholar]
- Larsen J, Stegger M, Andersen PS, Petersen A, Larsen AR, Westh H, Agersø Y, Fetsch A, Kraushaar B, Käsbohrer A, Feβler AT, Schwarz S, Cuny C, Witte W, Butaye P, Denis O, Haenni M, Madec J, Jouy E, Laurent F, Battisti A, Franco A, Alba P, Mammina C, Pantosti A, Monaco M, Wagenaar JA, de Boer E, van Duijkeren E, Heck M, Domínguez L, Torres C, Zarazaga M, Price LB and Skov RL, 2016. Evidence for human adaptation and foodborne transmission of livestock‐associated methicillin‐resistant Staphylococcus aureus . Clinical Infectious Diseases, 63, 1349–1352. 10.1093/cid/ciw532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen J, Sunde M, Islam MZ, Urdahl AM, Barstad AS, Larsen AR, Grøntvedt CA and Angen Ø, 2017. Evaluation of a widely used culture‐based method for detection of livestock‐associated meticillin‐resistant Staphylococcus aureus (MRSA), Denmark and Norway, 2014 to 2016. Euro Surveillance, 22: 30573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hello S, Bekhit A, Granier SA, Barua H, Beutlich J, Zając M, Münch S, Sintchenko V, Bouchrif B, Fashae K, Pinsard JL, Sontag L, Fabre L, Garnier M, Guibert V, Howard P, Hendriksen RS, Christensen JP, Biswas PK, Cloeckaert A, Rabsch W, Wasyl D, Doublet B and Weill FX, 2013. The global establishment of a highly‐fluoroquinolone resistant Salmonella enterica serotype Kentucky ST198 strain. Frontiers in Microbiology, 4, 395 10.3389/fmicb.2013.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levings RS, Lightfoot D, Partridge SR, Hall RM and Djordjevic SP, 2005. The genomic island SGI1, containing the multiple antibiotic resistance region of Salmonella enterica serovar Typhimurium DT104 or variants of it, is widely distributed in other S. enterica serovars. Journal of Bacteriology, 187, 4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ma L, Li Y, Jia H, Wei J, Shao D, Liu K, Shi Y, Qiu Y and Ma Z, 2017. Antimicrobial Resistance of Campylobacter Species Isolated from Broilers in Live Bird Markets in Shanghai, China. Foodborne Pathogens and Disease, 14, 96–102. 10.1089/fpd.2016.2186 [DOI] [PubMed] [Google Scholar]
- Liu Y‐Y, Wang T, Walsh TR, Yi L‐X, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu L‐F, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu J‐H and Shen J, 2015. Emergence of plasmid‐mediated colistin resistance mechanism MCR‐1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases, 16, 161–168. Available online: 10.1016/s1473-3099(15)00424-7 [DOI] [PubMed] [Google Scholar]
- Liebana E, Gibbs M, Clouting C, Barker L, Clifton‐Hadley FA, Pleydell E, Abdalhamid B, Hanson ND, Martin L, Poppe C and Davies RH, 2004. Characterisation of beta‐lactamases responsible for resistance to extended‐spectrum cephalosporins in Escherichia coli and Salmonella enterica strains from food‐producing animals in the United Kingdom. Microbial Drug Resistance, 10, 1–9. [DOI] [PubMed] [Google Scholar]
- Loncaric I, Künzel F, Licka T, Simhofer H, Spergser J and Rosengarten R, 2014. Identification and characterization of methicillin‐resistant Staphylococcus aureus (MRSA) from Austrian companion animals and horses. Veterinary Microbiology, 168, 381–387. 10.1016/j.vetmic.2013.11.022 [DOI] [PubMed] [Google Scholar]
- Luangtongkum T, Jeon B, Han J, Plummer P, Logue CM and Zhang Q, 2009. Antibiotic resistance in Campylobacter: emergence, transmission and persistence. Future Microbiology, 4, 189–200. 10.2217/17460913.4.2.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luini M, Cremonesi P, Magro G, Bianchini V, Minozzi G, Castiglioni B and Piccinini R, 2015. Methicillin‐resistant Staphylococcus aureus (MRSA) is associated with low within‐herd prevalence of intra‐mammary infections in dairy cows: Genotyping of isolates. Veterinary Microbiology, 178, 270–274. 10.1016/j.vetmic.2015.05.010 [DOI] [PubMed] [Google Scholar]
- Luo N, Pereira S, Sahin O, Lin J, Huang S, Michel L and Zhang Q, 2005. Enhanced in vivo fitness of fluoroquinolone‐resistant Campylobacter jejuni in the absence of antibiotic selection pressure. Proceedings of the National Academy of Sciences of the United States of America, 102, 541–546. 10.1073/pnas.0408966102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macori G, Giuseppina G, Bellio A, Gallina S, Bianchi DM, Sagrafoli D, Marri N, Giangolini G, Amatiste S and Decastelli L, 2017. Molecular epidemiology of Staphylococcus aureus in the ovine dairy chain and in farm‐related humans. Toxins, 9, E161 11 pp. 10.3390/toxins9050161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec JY, Haenni M, Nordmann P and Poirel L, 2017. Extended‐spectrum b‐lactamase/AmpC‐ and carbapenemase‐producing Enterobacteriaceae in animals: a threat for humans? Clinical Microbiology and Infection, 23, 826–833. 10.1016/j.cmi.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson‐Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT and Monnet DL, 2012. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18, 268–281. [DOI] [PubMed] [Google Scholar]
- MARAN , 2016. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2015. Published June 2016. Available online: https://www.wur.nl/upload_mm/0/b/c/433ca2d5-c97f-4aa1-ad34-a45ad522df95_92416_008804_NethmapMaran2016+TG2.pdf
- MARAN , 2017. Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2016. Available online: https://www.wur.nl/upload_mm/6/9/5/4f37c335-224c-4595-82e4-be6182c0a5e1_74ce6009-b112-428d-aeb7-99b95063aab6_Maran%20report%202017.pdf
- Mathers AJ, Peirano G and Pitout JDD, 2015. The role of epidemic resistance plasmids and international high‐risk clones in the spread of multidrug‐resistant Enterobacteriaceae. Clinical Microbiology Reviews, 28, 565–591. 10.1128/CMR.00116-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschek E, Westrell T and Kahlmether G, 2015. Establishment of zone diameter ECOFFs for Salmonella spp. – a joint EUCAST and ECDC project. Poster session presented at: 25th European Congress of Clinical Microbiology and Infectious Diseases, 25–28 April 2015, Copenhagen, Denmark.
- Matuschek E, Åhman J, Webster C and Kahlmeter G, 2017. Evaluation of five commercial MIC methods for colistin antimicrobial susceptibility testing for Gram‐negative bacteria. Poster session presented at: 27th European Congress of Clinical Microbiology and Infectious Diseases, 22–25 April 2017, Vienna, Austria. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Warnings/Matuschek_colistin_ECCMID_2017.pdf
- McCrackin MA, Helke KL, Galloway AM, Poole AZ, Salgado CD and Marriott BP, 2016. Effect of Antimicrobial Use in Agricultural Animals on Drug‐resistant Foodborne Campylobacteriosis in Humans: A Systematic Literature Review. Critical Reviews in Food Science and Nutrition, 56, 2115–2132. 10.1080/10408398.2015.1119798 [DOI] [PubMed] [Google Scholar]
- Mollenkopf DF, Stull JW, Mathys DA, Bowman AS, Feicht SM, Grooters SV, Daniels JB and Wittum TE, 2017. Carbapenemase‐Producing Enterobacteriaceae Recovered from the Environment of a Swine Farrow‐to‐Finish Operation in the United States. Antimicrob Agents Chemother. pii: e01298‐16. 10.1128/AAC.01298-16. Print 2017 Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monecke S, Coombs G, Shore AC, Coleman DC, Akpaka P, Borg M, Chow H, Ip M, Jatzwauk L, Jonas D, Kadlec K, Kearns A, Laurent F, O'Brien FG, Pearson J, Ruppelt A, Schwarz S, Scicluna E, Slickers P, Tan H‐L, Weber S and Ehricht R, 2011. A field guide to pandemic, epidemic and sporadic clones of methicillin‐resistant Staphylococcus aureus . PLoS ONE, 6(4), e17936 10.1371/journal.pone.0017936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossong J, Mughini‐Gras L, Penny C, Devaux A, Olinger C, Losch S, Cauchie HM, van pelt W and Ragiumbeau C, 2016. Human campylobacteriosis in Luxembourg, 2010‐2013: a case‐control study combined with multilocus sequence typing for source attribution and risk factor analysis. Scientific Reports, 6, 20939; 10.1038/srep20939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nohra A, Grinberg A, Midwinter AC, Marshall JC, Collins‐Emerson JM and French NP, 2016. Molecular epidemiology of Campylobacter coli strains isolated from different sources in New Zealand between 2005 and 2014. Applied and Environmental Microbiology, 82, 4363–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi T, Aoki K, Ishii Y, Usui M, Tamura Y, Kawanishi M, Ohnishi K and Tateda K, 2017. Molecular epidemiological analysis of human‐ and chicken‐derived isolates of Campylobacter jejuni in Japan using next‐generation sequencing. Journal of Infection and Chemotherapy, 23, 165–172. 10.1016/j.jiac.2016.11.011 [DOI] [PubMed] [Google Scholar]
- Ohno H, Wachino J, Saito R, Jin W, Yamada K, Kimura K and Arakawa Y, 2016. A highly macrolide‐resistant Campylobacter jejuni strain with rare A2074T mutations in 23S rRNA genes. Antimicrobial Agents and Chemotherapy, 60, 2580–2581. 10.1128/AAC.02822-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaitan AO, Morand S and Rolain J‐M, 2014. Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Frontiers in Microbiology, 5, 643, 18 pp. 10.3389/fmicb.2014.00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi A, Caruso M, Normanno G, Latorre L, Sottili R, Miccolupo A, Fraccalvieri R and Santagada G, 2016. Prevalence, antimicrobial susceptibility and molecular typing of methicillin‐resistant Staphylococcus aureus (MRSA) in bulk tank milk from southern Italy. Food Microbiology, 58, 36–42. 10.1016/j.fm.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Paterson GK, Harrison EM and Holmes MA, 2014. The emergence of mecC methicillin‐resistant Staphylococcus aureus . Trends in Microbiology, 22, 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin‐Guyomard A, Bruneau M, Houee P, Deleurme K, Legrandois P, Poirier C, Soumet C and Sanders P, 2016. Prevalence of mcr‐1 in commensal Escherichia coli from French livestock, 2007 to 2014. EuroSurveillance, 21, 10.2807/1560-7917.es.2016.21.6.30135 [DOI] [PubMed] [Google Scholar]
- Petersen A, Stegger M, Heltberg O, Christensen J, Zeuthen A, Knudsen LK, Urth T, Sorum M, Schouls L, Larsen J, Skov R and Larsen AR, 2013. Epidemiology of methicillin‐resistant Staphylococcus aureus carrying the novel mecC gene in Denmark corroborates a zoonotic reservoir with transmission to humans. Clinical Microbiology and Infection, 19, E16–E22. 10.1111/1469-0691.12036 [DOI] [PubMed] [Google Scholar]
- Pletz MW, Wollny A, Dobermann UH, Rödel J, Neubauer S, Stein C, Brandt C, Hartung A, Mellmann A, Trommer S, Edel B, Patchev V, Makarewicz O and Maschmann J, 2018. A nosocomial foodborne outbreak of a VIM carbapenemase‐expressing Citrobacter freundii . Clinical Infectious Disease, 67, 58–64. 10.1093/cid/ciy034 [DOI] [PubMed] [Google Scholar]
- Pornsukarom S, Patchanee P, Erdman M, Cray PF, Wittum T, Lee J and Gebreyes WA, 2015. Comparative phenotypic and genotypic analyses of Salmonella Rissen that originated from food animals in Thailand and United States. Zoonoses and Public Health, 62, 151–158. [DOI] [PubMed] [Google Scholar]
- Qi W, Ender M, O'Brien F, Imhof A, Ruef C, McCallum N and Berger‐Bächi B, 2005. Molecular epidemiology of methicillin‐resistant Staphylococcus aureus in Zürich, Switzerland (2003): Prevalence of type IV SCCmec and a new SCCmec element associated with isolates from intravenous drug users. Journal of Clinical Microbiology, 43, 5164–5170. 10.1128/JCM.43.10.5164-5170.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Wang Y, Zhang Q, Chen X, Shen Z, Deng F, Wu C and Shen J, 2012. Identification of a novel genomic island conferring resistance to multiple aminoglycoside antibiotics in Campylobacter coli . Antimicrobial Agents and Chemotherapy, 56, 5332–5339. 10.1128/AAC.00809-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Wang Y, Zhang Q, Zhang M, Deng F, Shen Z, Wu C, Wang S, Zhang J and Shen J, 2014. Report of ribosomal RNA methylase gene erm(B) in multidrug‐resistant Campylobacter coli . Journal of Antimicrobial Chemotherapy, 69, 964–968. 10.1093/jac/dkt492 [DOI] [PubMed] [Google Scholar]
- Rogers BA, Sidjabat HE and Paterson DL, 2011. Escherichia coli O25b‐ST131: a pandemic, multiresistant, community‐associated strain. Journal of Antimicrobial Chemotherapy, 66, 1–14. [DOI] [PubMed] [Google Scholar]
- Roschanski N, Friese A, von Salviati‐Claudius C, Hering J, Kaesbohrer A, Kreienbrock L and Roesler U, 2017. Prevalence of carbapenemase producing Enterobacteriaceae isolated from German pig‐fattening farms during the years 2011‐2013. Veterinary Microbiology, 200, 124–129. 10.1016/j.vetmic.2015.11.030 [DOI] [PubMed] [Google Scholar]
- Rosner BM, Schielke A, Didelot X, Kops F, Breidenbach J, Willrich N, Gölz G, Alter T, Stingl K, Josenhans C, Suerbaum S and Stark K, 2017. A combined case‐control and molecular source attribution study of human Campylobacter infections in Germany, 2011‐2014. Scientific Reports, 7, 5139; 10.1038/s41598-017-05227-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- San José M, Hasman H, Fischer J, Agersø Y, Schmoger S, Jahn S, Thomas K, Guiral E, Helmuth R and Guerra Roman B, 2014. Evaluation of methods for detection of VIM‐1‐carbapenemase‐producing Enterobacteriaceae in bovine minced meat. 24th European Congress Clinical Microbiology and Infectious Diseases (ECCMID, 2014). Poster eP334. 10 May 2014. Barcelona, Spain.
- Schwarz S and Johnson AP, 2016. Transferable resistance to colistin: a new but old threat. Journal of Antimicrobial Chemotherapy, 71, 2066–2070. 10.1093/jac/dkw274 [DOI] [PubMed] [Google Scholar]
- Shore AC, Deasy EC, Slickers P, Brennan G, O'Connell B, Monecke S, Ehricht R and Coleman DC, 2011. Detection of staphylococcal cassette chromosome mec Type XI carrying highly divergent mecA, mecI, mecR1, blaZ, and ccr genes in human clinical isolates of clonal complex 130 methicillin‐resistant Staphylococcus aureus . Antimicrobial Agents and Chemotherapy, 55, 3765–3773. 10.1128/AAC.00187-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov R, Matuschek E, Sjolund‐Karlsson M, Ähman J, Petersen A, Stegger M, Torpdahl M and Kahlmeter G, 2015. Development of a pefloxacin disk diffusion method for detection of fluoroquinolone‐resistant Salmonella enterica . Journal of Clinical Microbiology, 53, 3411–3417. 10.1128/JCM.01287-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella S, Soncini G, Ziino G, Panebianco A, Pedonese F, Nuvoloni R, Di Giannatale E, Colavita G, Alberghini L and Giaccone V, 2016. Prevalence and quantification of thermophilic Campylobacter spp. in Italian retail poultry meat: analysis of influencing factors. Food Microbiology, 62, 232–238. 10.1016/j.fm.2016.10.028 [DOI] [PubMed] [Google Scholar]
- Stolle I, Prenger‐Berninghoff E, Stamm I, Scheufen S, Hassdenteufel E, Guenther S, Bethe A, Pfeifer Y and Ewers C, 2013. Emergence of OXA‐48 carbapenemase‐producing Escherichia coli and Klebsiella pneumoniae in dogs. Journal of Antimicrobial Chemotherapy, 68, 2802–2808. [DOI] [PubMed] [Google Scholar]
- SWEDRES , 2011. A report on Swedish antibiotic utilisation and resistance in human medicine. Available online: https://www.folkhalsomyndigheten.se/contentassets/822c49b59bc44d58b18f64c913b6ce59/swedres-svarm-2011.pdf
- Swedres‐Svarm , 2017. Consumption of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala ISSN1650‐6332. Available online: http://www.sva.se/globalassets/redesign2011/pdf/om_sva/publikationer/swedres_svarm2017.pdf
- Tang Y, Dai L, Sahin O, Wu Z, Liu M and Zhang Q, 2017. Emergence of a plasmid‐borne multidrug resistance gene cfr(C) in foodborne pathogen Campylobacter. Journal of Antimicrobial Chemotheraphy, 72, 1581–1588. 10.1093/jac/dkx023 [DOI] [PubMed] [Google Scholar]
- Tate H, Folster JP, Hsu C‐H, Chen J, Hoffmann M, Li C, Morales C, Tyson GH, Mukherjee S, Brown AC, Green A, Wilson W, Dessai U, Abbott J, Joseph L, Haro J, Ayers S, McDermott PF and Zhao S, 2017. Comparative analysis of extended‐spectrum‐β‐lactamase CTX‐M‐65‐producing Salmonella enterica serovar Infantis isolates from humans, food animals, and retail chickens in the United States. Antimicrobial Agents and Chemotherapy, 61(7), e00488–17. 10.1128/aac.00488-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover FC and Goering RV, 2009. Methicillin‐resistant Staphylococcus aureus strain USA300: origin and epidemiology. Journal of Antimicrobial Chemotherapy, 64, 441–446. 10.1093/jac/dkp241 [DOI] [PubMed] [Google Scholar]
- Torralbo A, Borge C, García‐Bocanegra I, Méric G, Perea A and Carbonero A, 2015. Higher resistance of Campylobacter coli compared to Campylobacter jejuni at chicken slaughterhouse. Comparative Immunology, Microbiology and Infectious Diseases, 39, 47–52. 10.1016/j.cimid.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Urdahl AM, Bergsjø B, Norström M and Grøntvedt CA, 2015. The surveillance programme for methicillin resistant Staphylococcus aureus in pigs in Norway 2015. Surveillance programmes for terrestrial and aquatic animals in Norway. Annual report 2015. Oslo: Norwegian Veterinary Institute 2016.
- Urdahl AM, Norström M, Bergsjø B and Grøntvedt CA, 2016. The surveillance programme for methicillin resistant Staphylococcus aureus in pigs in Norway 2016. Surveillance programmes for terrestrial and aquatic animals in Norway. Annual report 2016. Oslo: Norwegian Veterinary Institute 2017.
- Urdahl AM, Norström M, Bergsjø B and Grøntvedt CA, 2017The surveillance programme for methicillin resistant Staphylococcus aureus in pigs in Norway 2017. Surveillance programmes for terrestrial and aquatic animals in Norway. Annual report 2017. Oslo: Norwegian Veterinary Institute 2018.
- Wang Y, Zhang M, Deng F, Shen Z, Wu C, Zhang J, Zhang Q and Shen J, 2014. Emergence of multidrug‐resistant Campylobacter species isolates with a horizontally acquired rRNA methylase. Antimicrobial Agents and Chemotherapy, 58, 5405–5412. 10.1128/AAC.03039-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang R, Li J, Wu Z, Yin W, Schwarz S, Tyrrell JM, Zheng Y, Wang S, Shen Z, Liu Z, Liu J, Lei L, Li M,Zhang Q, Zhang Q, Wu C, Zhang Q, Wu Y and Walsh T, 2017. Comprehensive resistome analysis reveals the prevalenceof NDM and MCR‐1 in Chinese poultry production. Nature Microbiology, 2, art. 16260. 10.1038/nmicrobiol.2016.260 [DOI] [PubMed] [Google Scholar]
- Wei B and Kang M, 2018. Molecular Basis of Macrolide Resistance in Campylobacter Strains Isolated from Poultry in South Korea. BioMed Research International, 2018, 4526576, 9 pp. 10.1155/2018/4526576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse CA, Young S, Li C, Hsu CH, Martin G and Zhao S, 2018. Use of whole‐genome sequencing for Campylobacter surveillance from NARMS retail poultry in the United States in 2015. Food Microbiology, 73, 122–128. 10.1016/j.fm.2018.01.018 [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization ‐ Advisory Group on Integrated Surveillance of Antimicrobial Resistance), 2017. Critically important antimicrobials for human medicine, 5th Revision 2016. 41 pp. Available online: http://www.who.int/foodsafety/publications/antimicrobials-fifth/en/
- Wieczorek K, Kania I and Osek J, 2013. Prevalence and antimicrobial resistance of Campylobacter spp. isolated from poultry carcasses in Poland. Journal of Food Protection, 76, 1451–1455. 10.4315/0362-028X.JFP-13-035 [DOI] [PubMed] [Google Scholar]
- Woodford N, Wareham DW, Guerra B and Teale C, 2013. Carbapenemase‐producing Enterobacteriaceae and non‐ Enterobacteriaceae from animals and the environment: an emerging public health risk of our own making? Journal of Antimicrobial Chemotherapy, 69, 287–291. [DOI] [PubMed] [Google Scholar]
- Yang W, Zhang M, Zhou J, Pang L, Wang G and Hou F, 2017. The Molecular Mechanisms of Ciprofloxacin Resistance in Clinical Campylobacter jejuni and Their Genotyping Characteristics in Beijing, China. Foodborne Pathogens and Diseases, 14, 386–392. 10.1089/fpd.2016.2223 [DOI] [PubMed] [Google Scholar]
- Yao H, Shen Z, Wang Y, Deng F, Liu D, Naren G, Dai L, Su C, Wang B, Wang S, Wu C, Yu EW, Zhang Q and Shen J. 2016. Emergence of a potent multidrug efflux pump variant that enhances Campylobacter resistance to multiple antibiotics. MBio, 7, e01543–16. 10.1128/mBio.01543-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Liu D, Wang Y, Zhang Q and Shen Z, 2017. High Prevalence and Predominance of the aph(2″)‐If Gene Conferring Aminoglycoside Resistance in Campylobacter. Antimicrobial Agents and Chemotherapy, 61(5), e00112–e00117. 10.1128/AAC.00112-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Song L, Liang H, Gu Y, Zhang C, Liu X, Zhang LJ and Zhang M, 2016. Molecular subtyping and erythromycin resistance of Campylobacter in China. Journal of Applied Microbiology, 121, 287–293. 10.1111/jam.13135 [DOI] [PubMed] [Google Scholar]
- Zhao S, Mukherjee S, Chen Y, Li C, Young S, Warren M, Abbott J, Friedman S, Kabera C, Karlsson M and McDermott PF, 2015. Novel gentamicin resistance genes in Campylobacter isolated from humans and retail meats in the USA. Journal of Antimicrobial Chemotheraphy, 70, 1314–1321. 10.1093/jac/dkv001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong Z, 2012. Discovery of bla OXA‐199, a chromosome‐based bla OXA‐48‐Like variant, in Shewanella xiamenensis . PLoS ONE, 7, e48280. [DOI] [PMC free article] [PubMed] [Google Scholar]


