| Code/trivial namea | IUPAC name/SMILES notation/InChiKeyb | Structural formulab |
|---|---|---|
|
Thiacloprid YRC 2894 |
{(Z)‐3‐[(6‐chloro‐3‐pyridyl)methyl]thiazolidin‐2‐ylidene}cyanamide N#C/N=C1SCCN\1CC2=CC=C(Cl)N=C2 HOKKPVIRMVDYPB‐UVTDQMKNSA‐N |
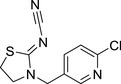
|
|
M01 4‐hydroxy‐YRC 2894 |
(Z)‐N‐(3‐((6‐chloropyridin‐3‐yl)methyl)‐4‐ ydroxythiazolidin‐2‐ylidene)cyanamide ClC1=NC=C(CN2C(O)CS/C2=N\C#N)C=C1 XXFNYZIMZUWOAV‐UVTDQMKNSA‐N |
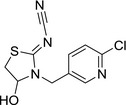
|
|
M02 thiacloprid‐amide Amide‐YRC2894 |
(Z)‐1‐(3‐((6‐chloropyridin‐3‐yl)methyl)thiazolidin‐2‐ylidene)urea O=C(N)/N=C1SCCN\1CC2=CC=C(Cl)N=C2 LEZHOZPJYAQQNU‐UVTDQMKNSA‐N |
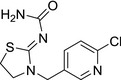
|
|
M03 6‐chloronicotinic acid IC‐0 6‐CNA |
6‐chloronicotinic acid ClC1=NC=C(C(O)=O)C=C1 UAWMVMPAYRWUFX‐UHFFFAOYSA‐N |
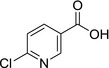
|
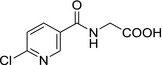
| M07 |
(6‐chloronicotinoyl)glycine ClC1=NC=C(C=C1)C(NCC(O)=O)=O VGSLNHSCEKVAIM‐UHFFFAOYSA‐N |
|
|
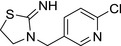
M29 thiacloprid‐des‐cyano |
3‐((6‐chloropyridin‐3‐yl)methyl)thiazolidin‐2‐imine N=C1SCCN1CC2=CC=C(Cl)N=C2 WJLMZDWUGGHQEH‐UHFFFAOYSA‐N |
|
|
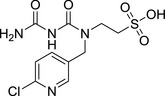
M30 thiacloprid‐sulfonic acid YRC2894 sodium Sulfonate |
2‐(3‐carbamoyl‐1‐((6‐chloropyridin‐3‐yl)methyl)ureido)ethane‐1‐sulfonic acid ClC1=CC=C(C=N1)CN(C(NC(N)=O)=O)CCS(=O)(O)=O UCZRQNICFJGZAI‐UHFFFAOYSA‐N |
|
|
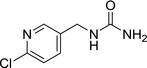
M31 6‐chloropicolyl urea |
1‐((6‐chloropyridin‐3‐yl)methyl)urea O=C(N)NCC1=CC=C(Cl)N=C1 GDAKHACCQXCCDP‐UHFFFAOYSA‐N |
|
|
M34 thiacloprid‐sulfonic acid amide |
2‐(1‐((6‐chloropyridin‐3‐yl)methyl)ureido)ethane‐1‐sulfonic acid ClC1=CC=C(CN(CCS(=O)(O)=O)C(N)=O)C=N1 NTWIWWZFSUKDGZ‐UHFFFAOYSA‐N |
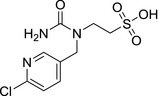
|
|
M36 6‐CPA (free) |
(6‐chloropyridin‐3‐yl)methanol OCC1=CN=C(Cl)C=C1 GOXYBEXWMJZLJB‐UHFFFAOYSA‐N |
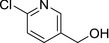
|
|
M37 4‐hydroxy YRC2894 Amide |
(Z)‐1‐(3‐(4‐chlorobenzyl)‐4‐hydroxythiazolidin‐2‐ylidene)urea NC(/N=C1SCC(N\1CC2=CC=C(C=C2)Cl)O)=O RSPMUINZMOFWAG‐KAMYIIQDSA‐N |
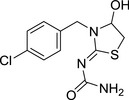
|
|
M38 YRC 2894 olefin |
(Z)‐N‐(3‐((6‐chloropyridin‐3‐yl)methyl)thiazol‐2(3H)‐ylidene)cyanamide ClC1=CC=C(C=N1)CN2C=CS/C2=N\C#N GSPNWTCHDVQYCA‐UVTDQMKNSA‐N |
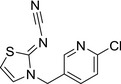
|
|
M46 thiacloprid‐thiadiazine Z5 |
4‐((6‐chloropyridin‐3‐yl)methyl)‐1,2,4‐thiadiazinan‐3‐one 1,1‐dioxide O=C(N(CC1=CC=C(Cl)N=C1)CC2)NS2(=O)=O VGRVYZQGUVEBCR‐UHFFFAOYSA‐N |
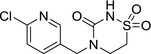
|
| M49 |
1‐((6‐chloropyridin‐3‐yl)methyl)‐2‐hydroxy‐5‐oxopyrrolidine‐2‐carboxylic acid OC(C1(CCC(N1CC2=CN=C(Cl)C=C2)=O)O)=O VMMDPMFMLQDEDM‐UHFFFAOYSA‐N |
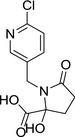
|
IUPAC: International Union of Pure and Applied Chemistry; SMILES: simplified molecular‐input line‐entry system; InChiKey: International Chemical Identifier Key.
The metabolite name in bold is the name used in the conclusion.
ChemBioDraw v.13.0.2.3021.
