Table A.1.
Specification summary of the substances in the flavouring group evaluation 201 revision 2 (JECFA, 2003; EFFA, 2018)
| FL‐no JECFA‐no | EU register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravitye | Comments |
|---|---|---|---|---|---|---|---|---|
|
02.174 1617 |
2‐Methylbut‐2‐en‐1‐ol |
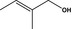
|
– 10258 4675‐87‐0 |
Liquid C5H10O 86.13 |
– Freely soluble |
137 – – 95% |
1.439–1.445 0.863–0.869 |
|
|
05.033 1216 |
2‐Ethylhept‐2‐enal |

|
2438 120 10031‐88‐6 |
Liquid C9H16O 140.23 |
Insoluble Soluble |
55–60 (5 hPa) – NMR 97% |
1.460–1.466 0.891–0.898 |
|
|
05.090 1209 |
2‐Methylpent‐2‐enal |
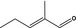
|
3194 2129 623‐36‐9 |
Liquid C6H10O 98.15 |
Insoluble Soluble |
137 – IR MS 95% |
1.445–1.453 0.855–0.865 |
Mainly E‐isomer (> 90%). Secondary components (E) and (Z)‐2‐methyl‐2‐pentenoic acid (< 0.5% in fresh samples, up to 1% in samples after storage) |
|
05.095 1201 |
2‐Methylcrotonaldehyde |
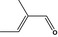
|
3407 2281 497‐03‐0 |
Liquid C5H8O 84.12 |
Slightly soluble Soluble |
117–118 – IR NMR 95% |
1.445–1.450 0.868–0.873 (20°) |
Mainly E‐isomer (> 95%). Secondary components: acetic acid (up to 0.1%), tiglic acid (up to 0.5%) and paraldehyde (up to 1%) (i.e. 2,4,6‐trimethyl‐1,3,5‐trioxane, which is a condensation product – cyclic ether) |
|
05.105 1214 |
2‐Butylbut‐2‐enal |
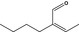
|
3392 10324 25409‐08‐9 |
Liquid C8H14O 126.20 |
Insoluble Soluble |
50 (18 hPa) – NMR 97% |
1.447–1.453 1.449–1.459 (20°) |
|
|
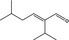
05.107 1215 |
2‐Isopropyl‐5‐methylhex‐2‐enal |
|
3406 10361 35158‐25‐9 |
Liquid C10H18O 154.25 |
Insoluble Soluble |
189 – NMR 95% |
1.448–1.454 0.840–0.846 |
|
|
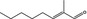
05.126 1217 |
2‐Methyloct‐2‐enal |
|
3711 10363 49576‐57‐0 |
Liquid C9H16O 140.23 |
Insoluble Soluble |
70–75 (10 hPa) – IR 96% |
1.449–1.459 0.872–0.882 |
|
| 05.130 | alpha‐Sinensal |

|
3141 10380 17909‐77‐2 |
– – 218.34 |
Substance not in the Register | |||
|
05.178 1227 |
beta‐Sinensal |

|
3141 10381 60066‐88‐8 |
Liquid C15H22O 218.34 |
Insoluble Soluble |
180 (1 hPa) – NMR 99% |
1.504–1.513 0.917–0.923 |
Substance not supported by industry |
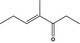
| 07.261 | 4‐Methyl‐3‐hepten‐5‐one |
|
22319‐31‐9 |
Liquid C8H14O 126.20 |
Insoluble Freely soluble |
179 – MS 96.12% |
1.442–1.462 0.851–0.871 |
Substance from FGE.204 |
|
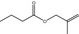
09.177 1207 |
2‐Methylallyl butyrate |
|
2678 572 7149‐29‐3 |
Liquid C8H14O2 142.20 |
Insoluble Soluble |
168 – NMR97% |
1.422–1.428 0.873–0.883 |
Substance not supported by industry |
|
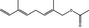
09.931 1226 |
2,6‐Dimethyl‐2,5,7‐octatriene‐1‐ol acetate |
|
3886 – 999999‐91‐4 |
Liquid C12H18O2 194.28 |
Insoluble Soluble |
70 (3 hPa) – IR NMR MS 96% |
1.490–1.500 0.937–0.947 |
Substance already evaluated in FGE.207 |
|
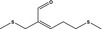
12.065 471 |
2,8‐Dithianon‐4‐en‐4‐carboxaldehyde |
|
3483 11904 59902‐01‐1 |
Liquid C8H14OS2 190.32 |
Slightly soluble |
104–105 (13 hPa) – IR NMR 98% |
1.557–1.567 1.105–1.107 |
Substance from FGE.225 |
|
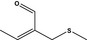
12.079 470 |
2‐(Methylthiomethyl)but‐2‐enal |
|
3601 11549 40878‐72‐6 |
Liquid C6H10OS 130.21 |
Insoluble |
77 (7 hPa) – 99% |
1.5228–1.5328 0.982–0.987 |
Substance from FGE.225 |
FL‐no: FLAVIS number; JECFA: Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: Identity; NMR: nuclear magnetic resonance; IR: infrared; MS: mass spectrometry.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa, if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
