Abstract
The EFSA Panel on Contaminants in the Food Chain (CONTAM) established a tolerable daily intake (TDI) for fumonisin B1 (FB 1) of 1.0 μg/kg body weight (bw) per day based on increased incidence of megalocytic hepatocytes found in a chronic study with mice. The CONTAM Panel considered the limited data available on toxicity and mode of action and structural similarities of FB 2–6 and found it appropriate to include FB 2, FB 3 and FB 4 in a group TDI with FB 1. Modified forms of FBs are phase I and phase II metabolites formed in fungi, infested plants or farm animals. Modified forms also arise from food or feed processing, and include covalent adducts with matrix constituents. Non‐covalently bound forms are not considered as modified forms. Modified forms of FBs identified are hydrolysed FB 1–4 (HFB 1–4), partially hydrolysed FB 1–2 (pHFB 1–2), N‐(carboxymethyl)‐FB 1–3 (NCM‐FB 1–3), N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB 1 (NDF‐FB 1), O‐fatty acyl FB 1, N‐fatty acyl FB 1 and N‐palmitoyl‐HFB 1. HFB 1, pHFB 1, NCM‐FB 1 and NDF‐FB 1 show a similar toxicological profile but are less potent than FB 1. Although in vitro data shows that N‐fatty acyl FBs are more toxic in vitro than FB 1, no in vivo data were available for N‐fatty acyl FBs and O‐fatty acyl FBs. The CONTAM Panel concluded that it was not appropriate to include modified FBs in the group TDI for FB 1–4. The uncertainty associated with the present assessment is high, but could be reduced provided more data are made available on occurrence, toxicokinetics and toxicity of FB 2–6 and modified forms of FB 1–4.
Keywords: fumonisins, modified forms, group health‐based guidance values
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1148/full
Summary
Following a request from the European Commission, the EFSA Panel on Contaminants in the Food Chain (CONTAM) assessed whether it is appropriate and feasible to set a group health‐based guidance value (group HBGV) for fumonisins B1 and B2 (FB1 and FB2) and their modified forms related to their presence in food and feed, and to consider, whether it would be appropriate to use the parent compound as a marker for toxicity.
In the context of this opinion, modified mycotoxins comprise all forms that differ in their chemical structure from the parent toxin. These include phase I and II metabolites formed in fungi or infested plants used for food and feed production, or food and feed products of animal origin. It does not include metabolites formed in humans, even if these may be similar. Moreover, modified forms include products of food and feed processing, and covalent adducts with matrix constituents. In contrast, non‐covalent binding to the matrix is not considered as a modification of the mycotoxin as it does not change the chemical structure of the toxin. Such forms are considered as ‘hidden’ forms.
Previous risk assessments on fumonisins and on modified mycotoxins have been used as a starting point for the present assessment. In addition, a systematic literature search has been carried out to obtain up‐to‐date and comprehensive information on fumonisins and its modified forms. In this opinion, the general principles for risk assessment were followed. Before assessing whether other fumonisins can be included in a group HBGV for FB1 and FB2 and also if modified forms can be included in such a group HBGV, the CONTAM Panel decided to review new relevant data on fumonisins and its modified forms since the year 2000 and to evaluate whether the Scientific Committee for Food (SCF) tolerable daily intake (TDI) for FB1, FB2 and FB3 alone or in combination needed to be revised, and, in addition, if there was a need also to set an acute reference dose (ARfD) for FBs and their modified forms.
Fumonisins are mycotoxins produced predominantly by Fusarium verticillioides and Fusarium proliferatum. They are long‐chain aminopolyols with two tricarballylic acid side chains. The most relevant compounds are the B‐type fumonisins FB1–FB4 which differ in the number and position of hydroxy‐groups in the backbone. Of relevance are also modified FBs, predominantly the hydrolysed FBs (HFBs) and partially hydrolysed FBs (pHFBs) which are formed upon alkaline hydrolysis as well as FB sugar conjugates which have been detected in food samples. Plant and fungal metabolites such as N‐ and O‐fatty acyl FBs are also described, however, only traces have been detected in food samples so far. Besides HFBs, pHFBs, N‐fatty acyl fumonisins with acyl‐chain lengths ranging from C16:0 to C24:1 are the only known FB in vivo metabolites. Their formation is catalysed by ceramide synthases (CerS), key enzymes in sphingolipid metabolism which are inhibited by FBs. N‐fatty acyl fumonisins are much more cytotoxic in vitro as compared to FBs.
Analytical methods for FB1–4 and for modified forms of FB1 are well established and are mainly based on mass spectrometry. However, the strong physical interaction of fumonisins with food matrix, may significantly affect the analytical performance. Therefore, indirect methods, usually based on alkaline hydrolysis of the matrix, have been proposed. Only FB1–3 are available on the market as calibrant solutions, while FB4 can be purchased as purified powder. Except for HFB1, analytical standards for modified forms are not commercially available.
The occurrence of FB1–3 is well documented in maize and products thereof, whereas little information is available for occurrence of FB4. Occurrence of HFB1–3 has been reported following food processing (e.g. nixtamalisation). Very few data are available on other modified FBs such as O‐fatty acyl and N‐fatty acyl FBs and it can be assumed that these modified FBs occur at low concentrations compared to their parent compounds. No information was identified on the transfer of modified forms of fumonisins to food and feed of animal origin.
FBs are poorly absorbed (< 4% of an oral dose) from the gastrointestinal tract and absorbed FBs and their metabolites are rapidly excreted, mainly in the bile of experimental animals, resulting in low plasma, tissue and urinary concentrations. Metabolism comprises the hydrolysis of the ester groups of the parent FBs and the formation of N‐fatty acyl FBs. Metabolic activity is low in mammalian tissues and hydrolytic metabolism involves the colonic microbiome. Few studies have been identified on the toxicokinetics of modified FBs. There is preliminary evidence for the partial release of FB1 from N‐(1‐deoxy‐d‐fructos‐1‐yl)‐fumonisin B1 (NDF‐FB1) in rats after oral ingestion.
The key event in the toxic mode of action of FBs is inhibition of CerS. FBs and in particular HFBs are structural analogues of sphingoid bases and they competitively inhibit CerS, causing disruption of sphingolipid metabolism and pathological changes seen after FBs exposure. Modified FBs may cause inhibition of CerS, but apparently with variable potencies, which could not be established precisely based on the studies available.
Although FBs are poorly absorbed, unchanged FBs excreted into urine have been used as a biomarker of exposure in humans. In animal studies changes in sphinganine (Sa) and sphingosine (So) and the Sa/So ratio can be determined in urine following FB exposure. A dose related increase in the sphinganine 1‐phosphate (Sa 1‐P)/sphingosine 1‐phosphate (So 1‐P) ratio in blood spots which correlated with urinary FB1 levels has been reported in human studies. This result is consistent with fumonisin inhibition of CerS in humans.
Toxicity studies deal mainly with effects of FB1, but FB2–4 are considered as having similar toxicological profiles and potencies. FB1 is considered not to be acutely toxic. In repeated dose studies with rodents, FB1 causes liver and kidney toxicity. Apoptosis, necrosis, proliferation, regeneration and hyperplasia of the bile duct are early signs of liver toxicity. Early signs of kidney toxicity were increases in free sphingoid bases, apoptosis and cell regeneration in the renal tubules of the outer medulla. Upon chronic exposure liver and kidney tumours are observed. FB1 is not mutagenic in bacteria and does not cause unscheduled DNA synthesis in mammalian cells, but is clastogenic via an indirect mechanism (induction of oxidative stress). FB1 caused embryotoxicity in mice, rats and rabbits, but only at doses where maternal toxicity was observed. In Syrian hamsters, such effects were observed in the absence of maternal toxicity. There are indications that FB1 causes neural tube defects (NTD) in sensitive mice strains but, overall, the evidence is inconclusive. In in vitro studies FB1–4 were approximately equipotent inhibitors of CerS and cause cytotoxicity in several mammalian cell types in vitro.
As compared to FB1, only limited in vivo data on modified FBs are available. HFB1 is less toxic than FB1 but shows a similar toxicological profile. Also pHFB1, N‐(carboxymethyl)‐fumonisin B1 (NCM‐FB1) and NDF‐FB1 are less toxic than FB1 showing a similar toxicological profile, however, the data base is even more limited than that for HFB1. No in vivo toxicity data were available for N‐fatty acyl FBs and O‐fatty acyl FBs. In brine shrimp, N‐palmitoyl‐HFB1 is more toxic than HFB1 and has about the same toxicity as FB1 suggesting that acylation could potentially increase toxicity in shrimp. Overall, the available data on modified forms suggest a similar toxicological profile as their parent compounds but the data are too limited and inconsistent to assess their relative potencies in quantitative terms.
There are only limited data available on the in vitro toxicity of modified fumonisins. For HFB1–2 in vitro toxic potencies relative to FB1 vary between 0.01 and 0.9. Notably, HFB1 is taken up by cells more rapidly and completely than FB1. For pHFB1–2, there were no data available for assessing the toxicity relative to their parent compounds. In one single study, NCM‐FB1 had a relative potency of 0.02 as compared with FB1. There is no information available on in vitro toxicity of O‐fatty acyl FBs. N‐fatty acyl FB1 and N‐fatty acyl HFB1–2 are up to 10 times more toxic in vitro than FB1. Notably, these compounds are more rapidly and to a greater extent taken up by cells than FB1 and also HFB1. Overall, the available in vitro data on modified FBs do not allow extrapolations to the human in vivo situation.
Several clinical effects have been discussed in humans (such as oesophageal cancer, liver cancer, NTD or growth impairment), but so far none of these have been causally related to fumonisin exposure.
Data from humans indicate that inhibition of CerS (changes in Sa 1‐P and the Sa 1‐P/So 1‐P ratio as measure in blood) may occur above a total FB1–3 exposure resulting in 0.5–1 ng FB1/mL in urine, corresponding to a total intake of FBs of about 1.7 μg FBs/kg body weight (bw) per day.
A dose–response analysis was conducted using data from a chronic feeding study in mice in which the incidence of liver lesions and an increase in Sa levels were observed at low doses. Because of a likely non‐genotoxic mechanism of tumourigenicity, the CONTAM Panel considered it appropriate to conduct dose–response analyses of liver effects. Increased incidence of megalocytic hepatocytes in the liver was established as the critical effect and a BMDL10 of 0.1 mg FB1/kg bw per day was derived. The CONTAM Panel used the BMDL10 of 0.1 mg/kg bw per day and an uncertainty factor (UF) of 100 for intra and interspecies variability to derive a TDI of 1.0 μg FB1/kg bw per day.
Based on structural similarity, and the limited data available indicating similar toxic profile and toxic potencies in the same order of magnitude, the CONTAM Panel decided that FB2, FB3 and FB4 should be included in a group TDI with FB1. It should be noted that the in vivo toxicology database for FB2–4 is very limited. Because of the currently insufficient data modified forms of FB1–4 could not be included in this group TDI. The CONTAM Panel noted that based on the available evidence it can be assumed that modified forms of FB1–4 exert lower toxicity than their parent compounds. However, this could not be quantified.
Standards and calibrants for FB2–6 and for modified forms of FBs are needed for analytical and toxicological purposes as well as more information on occurrence of FB2–6 and of modified FBs in order to prioritise toxicity testing. More information on the in vivo toxicokinetics for modified forms of FBs and also for FB2–6 is needed together with in vivo toxicity data on FB2–6 and of any modified FBs using pure compounds and in particular on the toxicity of hydrolysed FBs using pure compounds to assess if toxicity mitigation measures (e.g. nixtamalisation) are effective.
1. Introduction
1.1. Background Terms of Reference as provided by the requestor
Following a request from the European Commission, the risks to human and animal health related to modified forms of the Fusarium toxins zearalenone, nivalenol, T‐2 and HT‐2 toxins and fumonisins were evaluated in the scientific opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed1, adopted by the EFSA Panel on Contaminants in the Food Chain (CONTAM) on 25 November 2014.
The CONTAM Panel considered it appropriate to assess human exposure to modified forms of the various toxins in addition to the parent compounds, because many modified forms are hydrolysed into the parent compounds or released from the matrix during digestion. In the absence of specific toxicity data, toxicity equal to the parent compounds was assumed for modified mycotoxins. Risk characterization was done by comparing exposure scenarios with reference doses of the parent compounds.
The regulatory follow‐up to this scientific opinion was discussed at the Expert Committee “Agricultural contaminants” on 15 January 2015. The Standing Committee on Plants, Animals, Food and Feed has been informed thereof at its meeting on 11 February 20152.
Before taking regulatory measures as regards the modified mycotoxins, it was agreed to request EFSA to assess whether it is appropriate and feasible to set a group health based guidance value for the parent compound and its modified forms and to consider, if relevant, the appropriateness to use the parent compound as a marker for presence and toxicity of the parent compound and its modified forms.
1.2. Terms of Reference as provided by the requestor
In accordance with Art. 29 (1) (a) of Regulation (EC) No 178/2002, the Commission asks EFSA for scientific opinions to assess whether it is appropriate and feasible to set a group health based guidance value for the parent compound and its modified forms for zearalenone, fumonisins, nivalenol and T‐2 and HT‐2 toxin and to consider, if relevant, the appropriateness to use the parent compound as a marker for presence and toxicity of the parent compound and its modified forms for these mycotoxins.
The four requested scientific opinions are:
assessment whether it is appropriate and feasible to set a group health based guidance value for zearalenone and its modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed, and to consider, if relevant, the appropriateness to use the parent compound as a marker for presence and toxicity of zearalenone and its modified forms.
assessment whether it is appropriate and feasible to set a group health based guidance value for fumonisin B1 and B2 and their modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compounds as a marker for presence and toxicity of fumonisin B1 and B2 and their modified forms.
assessment whether it is appropriate and feasible to set a group health based guidance value for nivalenol and its modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compound as a marker for presence and toxicity of nivalenol and its modified forms.
assessment whether it is appropriate and feasible to set a group health based guidance value for T‐2 and HT‐2 toxin and their modified forms identified in the CONTAM opinion on the risks for human health related to the presence of modified forms of certain mycotoxins in food and feed and to consider, if relevant, the appropriateness to use the parent compound as a marker for presence and toxicity of T‐2 and HT‐2 toxin and their modified forms.
1.3. Introduction to mycotoxins and their modified forms
Mycotoxins are secondary metabolites of filamentous fungi. They are usually low molecular weight compounds and serve no function in the intermediary metabolism of the fungus, but provide advantages with respect to its competition for nutrients and habitat. Consequently, many mycotoxins are toxic for bacteria and other microorganisms. As mycotoxins are also toxic for humans and animals, their presence in food and feed may pose a potential health risk.
Numerous mycotoxins have been characterised to date. These toxic fungal secondary metabolites, also called parent mycotoxins, may occur as free compounds in infested food and feed items, but may also be converted into products with altered physicochemical, chemical and biological properties in fungi, or in plants and animals used for food and feed production, and during food and feed processing and storage. It is increasingly realised that such ‘modified’ forms of the parent ‘free’ mycotoxins occur in food and feed and should be taken into account for risk assessment, because they may contribute to the toxicity of the parent toxins.
The chemical structure of the ‘modified’ mycotoxin is different from that of the parent toxin. This is consistent with the recent proposal of a comprehensive definition of modified and other forms of mycotoxins by Rychlik et al. (2014). There are several possibilities to convert free parent mycotoxins into ‘modified’ forms:
Biotransformation in the fungus, infested plant and mammalian organism. This includes phase I metabolism through oxidation, reduction or hydrolysis of the parent toxin, as well as phase II metabolism involving conjugation with endogenous molecules. Phase II metabolites formed in the plant through conjugation with polar low molecular weight molecules such as glucose or sulfate have also been called ‘masked’ mycotoxins because they were difficult to detect by routine analysis. However, after intake with the food or feed such conjugates may be hydrolysed in the digestive tract, thereby releasing the parent free toxin which may add to the total exposure. Therefore, phase II metabolism in plants or fungi is of paramount importance for the risk assessment of mycotoxins.
-
Alteration of the chemical structure of the free parent mycotoxin by non‐enzymatic reactions, in particular:
2a) Processing of food and feed by thermal and/or chemical treatment, for example, degradation reactions during roasting, frying and extruding, and hydrolytic reactions during acidic or alkaline treatment (i.e. nixtamalisation).
2b) Covalent binding to food and feed matrix, for example to matrix components such as proteins and starch. From a chemical perspective, such covalent binding products can be considered to arise from a conjugation reaction, e.g. of a carboxylic acid group of the mycotoxin with an amino or hydroxy group of matrix components such as starch or proteins to form an amino or hydroxy group to form an amide or ester bond, respectively.
Products of non‐covalent binding of the parent mycotoxin to food or feed matrix constituents are not regarded as modified mycotoxins in this opinion3, because there is no change of the chemical structure involved. Such non‐covalent interactions, commonly named physical entrapments, may be mediated by hydrogen‐ or ionic bonding and any other kind of non‐covalent binding and appear to be of particular importance for fumonisins as such physical entrapment can seriously affect the analytical determination of parent fumonisins in food and feed, leading in some cases to underestimation of their content (see Section 5.3). Due to their difficult analysis, matrix‐associated mycotoxins have also be named ‘hidden’ or ‘bound’, although these designations appear not to differentiate between covalent and non‐covalent binding and are therefore not used in the present opinion.
In recent years many newly discovered modified mycotoxins have been described. Nonetheless, there are many knowledge gaps about modified mycotoxins. Currently, the terms ‘modified’, ‘masked’, ‘hidden’ and ‘bound’ are not used consistently and unambiguously in the scientific literature. Moreover, other terms sometimes lead to confusion, because they have a different meaning in chemical and biological disciplines: For example, conjugates are mainly considered as phase II metabolites in toxicology, but more broadly as the products of any reaction between two functional groups in chemistry.
In conclusion, in the context of risk assessment of mycotoxins in food and feed, modified mycotoxins comprise all forms that differ in their chemical structure from the parent toxin. These include phase I and II metabolites formed in fungi or infested plants used for food and feed production, or food and feed products of animal origin. Moreover, modified forms include products of food and feed processing, and covalent adducts with matrix constituents. In contrast, non‐covalent binding to the matrix is not considered as a modification of the mycotoxin as it does not change the chemical structure of the toxin but rather as an analytical issue leading to poor recoveries.
The modified forms of fumonisins which are regarded as relevant for this opinion are described in detail in Section 4.3 and their analysis in Section 5.3.
1.4. Legislation
Article 2 of Council Regulation (EEC) No 315/934 stipulates that food containing a contaminant in an amount unacceptable for public health shall not be placed on the market, that contaminant levels should be kept as low as can reasonably be achieved and that, if necessary, the EC may establish maximum levels for specific contaminants. These maximum levels (MLs) are laid down in the Annex of Commission Regulation (EC) No 1881/20065 and may include MLs for the same contaminants in different foods, analytical detection limits and reference to the sampling and analysis methods to be used. MLs for the sum of fumonisins B1 and B2 are listed for unprocessed maize and maize‐based foods ranging from 200 to 2,000 μg/kg. Fumonisins B3 and B4 and modified forms of FBs are not considered in the legislation.
1.5. Interpretation of Terms of Reference
The CONTAM Panel took the assumption that the previous risk assessment of FB1–3 by the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (FAO/WHO, 2012) is comprehensively covering all relevant aspects of FB1–3 and therefore used it together with the recent opinion on modified mycotoxins (EFSA CONTAM Panel, 2014) as a starting point for the present assessment.
The CONTAM Panel noted that, next to FB1 and FB2, mentioned in the Terms of Reference (ToR), also FB3 and FB4 are among the more common forms of fumonisins, and therefore decided to also consider these in the assessment as well. For FB5 and FB6, two other fumonisins of the B‐type, very little is known about their occurrence, and for FB5, the structure is not yet fully elucidated. Other groups of fumonisins are the A, C and P series, usually representing less than 5% of total fumonisins. These were not further considered as they were not part of the Terms of Reference (see Sections 4 and 6).
The CONTAM Panel reviewed the new relevant data on FB1–4 (i.e. published after 2011) to evaluate whether the group tolerable daily intake (TDI) established for FB1–3 by the Scientific Committee for Food (SCF 2003) needs to be revised and whether or not FB4 should be included in the group TDI. In addition, for the modified forms of FB1–4 identified to date the methods currently available for their analysis were reviewed.
In line with the previous EFSA opinion on modified mycotoxins (EFSA CONTAM Panel, 2014), modified forms of fumonisins arising from both plant and fungal metabolism, formed as a consequence of food processing and transfer from feed to animal tissues used as food were considered for possible inclusion in the group health‐based guidance values (HBGVs).
Moreover, for the evaluation of a group HBGV for fumonisins and their modified forms, the CONTAM Panel has decided to include only chemically characterised compounds, for which it could be possible to derive a relative potency factor compared to parent compounds. Therefore, only covalent bound forms of fumonisins or other chemically characterised modified forms such as hydrolysed fumonisins are considered for possible inclusion in a HBGV.
2. Data and methodologies
2.1. Methodology for data collection and study appraisal
A pilot search in Web of Science6 in December 2015 for publications that could potentially be relevant for the present assessment was carried out. From this, it became clear that due to the sheer amount of publications, a review of abstracts and identification of potentially relevant publications could not be done with the resources available at EFSA and the and the EFSA Working Group (WG), and the given deadline for the present mandate. Therefore, a call for a literature search and review was launched in March 2016 within the Framework Contract No OC/EFSA/AMU/2014/01 Lot 2 Chemical/toxicological – FWC 6 with the aim to identify and collect relevant literature related to fumonisins and their modified forms to support preparatory work for the present opinion and that on animal health risk assessment. A final project report has been delivered in November 2016 and was published together with the present opinion (NFI‐DTU, 2018). Briefly, nine search strings were designed to identify potentially relevant studies and after removal of duplicates and applying inclusion/exclusion criteria (as described in NFI‐DTU, 2018) potentially relevant references were identified. The year of publication of the SCF opinion on fumonisins that was considered as a starting point for the present assessment) and consequently papers published in the period from 1/1/2000 until 21/7/2016 were considered. The first number in the brackets give, per scientific area, the total number of hits obtained, the second the publications identified as potentially relevant: Chemistry and analysis (4,456/532), Toxicokinetics (2,262/114), Mode of Action (1,649/273), In vivo Toxicity (3,555/87), In vitro toxicity (1,632/138), Observations in humans (2,424/38), Adverse effects in farm and companion animals (5,087/270), Occurrence in food (3,284/709) and Occurrence in feed and animal exposure (3,283/270). The report contains as an annex all abstracts screened together with an evaluation of their relevance and the key points of the individual publications.
The abstracts proposed as potentially relevant in the report were then screened by the WG members and by applying expert judgement used in the assessment if relevant. The last comprehensive risk assessment of fumonisins publicly available at the time of drafting this opinion was that of JECFA (FAO/WHO, 2012). The technical report from a more recent JECFA evaluation (FAO/WHO, 2017) was also available to the Panel, however this did not contain the details of the evaluation presented as an Addendum that was still in press at that time. It was assumed that all relevant information on chemistry, analysis, occurrence, in vitro and in vivo toxicity, biomonitoring and epidemiology of fumonisins had been considered therein and therefore for these fields only studies published after 2011 have been considered in addition to those already referenced in the JECFA assessment. Key studies on in vivo toxicity presented by JECFA have been re‐evaluated and presented again in the present assessment. After careful review, the CONTAM Panel concluded that modified forms of fumonisins had not been considered in depth in the last JECFA assessment or in other previous risk assessments available. Therefore, in vitro and in vivo studies on modified forms available have been considered for the present opinion without any restriction to a time period.
Since a series of previous assessments were available (IARC, 1993, 2002, EHC, 2000; SCF, 2000, 2003; FAO/WHO 2001, 2012, 2017, EFSA, 2005; EFSA CONTAM Panel, 2014) these were also considered for the present assessment. Whenever necessary, original publications referenced in these assessments were retrieved.
In addition to the systematic search and the use of previous evaluations for retrieval of relevant literature, a ‘forward snowballing’ approach7 was applied by all WG members (see Jalali and Wohlin, 2012) in order to obtain any relevant information published until adoption of the opinion.
2.2. Methodology applied for hazard assessment
The CONTAM Panel applied the general principles of the risk assessment process for chemicals in food as described by WHO/IPCS (2009), which include hazard identification and characterisation, exposure assessment and risk characterisation. In addition to the principles described by WHO/IPCS (2009), any EFSA guidance relevant for the present assessment has been duly considered for the present assessment.
3. Previous assessments
In 2000, the SCF has published an opinion on FB1 (SCF, 2000). The Committee concluded that there was insufficient evidence that FB1 is genotoxic and that in short‐term, subchronic and chronic studies with mice and rats, liver and kidney were targets of FB1 toxicity. In short term studies with pigs adverse effects on lung and in horses equine leukoencephalomalacia (ELEM), secondary to cardiovascular effects, was observed. Reproductive and developmental effects where either not observed or only at dose levels with pronounced maternal toxicity. In chronic studies, FB1 induced tumours in liver and kidney in rodents. The Committee noted that fumonisins interfere with the de novo synthesis of ceramide and more complex sphingolipids which is reflected in early changes in the sphinganine/sphingosine (Sa/So) ratio and which results in disturbance of cell growth, differentiation, morphology, permeability and increased apoptosis. The latter appears to play a major role in FB1 toxicity including tumour formation. Considering the mode of action (MoA) and the lack of adequate evidence on genotoxicity the SCF found it justified to apply a threshold approach for risk assessment and set a TDI of 2 μg FB1/kg body weight (bw) based on an overall no observed adverse effect level (NOAEL) of 0.2 mg/kg bw per day for effects in liver and kidney in rodents (Voss et al., 1995; NTP, 1999) and by applying an uncertainty factor (UF) of 100.
In 2001, JECFA published a risk assessment on FB1–3 (FAO/WHO, 2001). The assessment was essentially based on FB1 data because for FB1 and FB2, which were considered having very similar toxicological profiles, only little information was available. Similarly to the previous evaluation of the SCF (2000), JECFA concluded that in repeated dose animal studies liver and kidney were the targets of FB1 toxicity. Early signs of toxicity in liver were apoptosis, necrosis, proliferation and regeneration and hyperplasia of the bile duct and elevated sphinganine (Delongchamp and Young, 2001; Kodell et al., 2001) while in kidney early signs were increases in free sphingoid bases, apoptosis and cell regeneration. In pigs, pulmonary oedema and hydrothorax and in horses, ELEM were observed upon oral application of FB1. In mice, rats and rabbits embryotoxicity occurred only at doses paralleled by maternal toxicity, whereas in one study with hamsters it was also observed in the absence of maternal toxicity. In chronic studies, kidney tumours were observed in male rats and liver tumours were observed in male rats and female mice. Neither FB1 nor other FBs have been shown to be clearly genotoxic. There was only limited evidence for a carcinogenic effect of fumonisins in humans. With regard to organ toxicity, JECFA noted that FB1 acts via interference with cellular lipid metabolism, secondary to ceramide synthase inhibition. A group provisional maximum tolerable daily intake (PMTDI) of 2 μg/kg bw for FB1–3 was allocated on the basis of a no observed effect level (NOEL) of 0.2 mg FB1/kg bw per day for renal toxicity observed in a subchronic and a chronic rat study (Voss et al., 1995; NTP, 1999) and by applying an UF of 100. It should be noted that elevated levels of Sa and the Sa/So ratio were observed in urine and kidney of male rats at the NOEL (NTP, 1999). The total dietary human exposure to FB1 was estimated to range from 0.2 μg/kg bw per day (European diet) to 2.4 μg/kg bw per day (African diet).
After publication of the JECFA assessment, the SCF was requested to evaluate if the TDI of 2 μg FB1/kg bw established in 2000 was applicable also for FB2–3. As these fumonisins are assumed to exert similar effects when tested in male BD IX rats at a dose of 1,000 mg/kg diet for 21 days (Gelderblom et al., 1993), the SCF concluded that the TDI for FB1 can be used as a group TDI for FB1–3 (SCF, 2003). However, a 28‐day dose–response feeding study in B6C3F(1) mice using approximately equimolar concentrations of purified FB1, FB2 or FB3 at concentrations of FB1 known to cause liver tumours, found no evidence of any effect by FB2 or FB3 but clear evidence of FB1 hepatotoxicity and disruption of sphingolipid metabolism (Howard et al., 2002).
In 2002, the International Agency for Research on Cancer (IARC) evaluated fumonisins considering additional data becoming available after their previous assessment from 1993 (IARC, 1993) and concluded that FB1 is possibly carcinogenic to humans (Group 2B) (IARC, 2002).
In 2005, EFSA published an opinion related to fumonisins as undesirable substances in animal feed (EFSA, 2005) in which NOAELs and lowest observed adverse effect levels (LOAELs) for different livestock species and farmed animals were established. Horses and pigs were identified as the most sensitive species (no NOAELs could be derived) and LOAELs of 0.2 mg/kg bw per day were derived for FB1 based on increased Sa/So ratio detected at that dose in serum of both species (Ross et al., 1991; Zomborszky‐Kovács et al., 2002).
In 2012, JECFA published an assessment of fumonisins in which all relevant studies available since their previous assessment (FAO/WHO, 2001) were reviewed. The previously proposed disruption of lipid metabolism as MoA of fumonisin toxicity was confirmed by additional studies. New studies also confirmed the previous conclusion that FB1 is not directly genotoxic and supported the notion that FB1‐mediated deoxyribonucleic acid (DNA) damage is a consequence of reactive oxygen species (ROS) formation. Several new studies potentially useful for deriving a TDI became available, confirming essentially the established toxicity profile and the target organs for FB1 toxicity. JECFA considered the incidences of megalocytic hepatocytes observed upon oral application of FB1 in male mice in two different strains in a new 6‐month study (Bondy et al., 2010; unpublished) as most appropriate to derive a BMDL10. Incidence data from the two strains were pooled and doses were rounded for the calculations. Dose–response modelling was carried out using the USEPA BMD software (BMDS version 2.1.2.). A pathology score of 1 was selected as endpoint to be modelled. For that reason log‐probit and multistage models were excluded from analysis. Of the other seven models, the lowest BMDL10 of 165 μg FB1/kg bw per day was obtained with the log‐logistic model. This BMDL value selected as reference point for derivation of a PMTDI. Using an uncertainty factor of 100 for intraspecies and interspecies variation, after rounding the Committee derived a PMTDI of 2 μg/kg body weight per day that should be applied also for FB2 and FB3. Based on national and international estimates, mean exposure estimates to FB1 for the general population ranges from 0.12 × 10−3 to 7.6 μg/kg bw per day whereas 95th percentile exposure estimates were as high as 33.3 μg/kg bw per day. In FAO/WHO (2012) dietary exposure estimates for average consumers, ranged from 0.087 × 10−3 to 14.4 μg/kg bw per day, whereas for consumers with high consumption, exposure estimates would be up to 44.8 μg/kg bw per day.
In 2014, EFSA issued an assessment on the increase of the risk for public health related to a possible temporary derogation from the ML of deoxynivalenol (DON), zearalenone (ZEN) and fumonisins for maize and maize products (EFSA, 2014). As this assessment was conducted in response to an urgent request it was not possible to carry out a full hazard characterisation. Therefore, EFSA used the group PMTDI of 2 μg/kg bw established by JECFA (FAO/WHO, 2012). Average chronic exposures to fumonisins (applying current MLs) in the children age groups ranged between 0.17 and 2.11 μg/kg bw (minimum lower bound (LB) and maximum upper bound (LB)) per day and was thus in the region of the group PMTDI of 2 μg/kg bw as established by JECFA (FAO/WHO, 2012). At the 95th percentile, corresponding numbers were 0.54 and 4.39 μg/kg bw per day. Chronic average exposures in adult age groups ranged between 0.03 and 1.19 μg/kg bw per day and at the 95th percentile between 0.08 and 2.30 μg/kg bw per day (minimum LB and maximum UB, respectively).
In the Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel, 2014), no specific information on the toxic effects of the modified forms of fumonisins could be identified. However, the chemistry and toxicokinetics of fumonisins as well as general considerations of biotransformation suggested that modified fumonisins may be cleaved in the gastrointestinal tract releasing fumonisins. Taking a pragmatic approach until more information became available, the CONTAM Panel assumed that modified forms of fumonisins have the same toxicological profile and potency as their parent compounds. Based on occurrence data available at that time (2014) it was then assumed that modified forms of fumonisins add 60% to the exposure to fumonisins. It should be noted that in the opinion of 2014, the term ‘modified fumonisins’ included both covalently and non‐covalently bound forms (hidden forms).
In 2017, JECFA published a report of further assessment on fumonisins (FAO/WHO, 2017) in which new studies becoming available since their last evaluation (FAO/WHO, 2012) were considered. Overall, the previous conclusions were reaffirmed and the group PMTDI of 2 μg/kg bw for FB1–3 was retained based on the data of Bondy et al. (2010, unpublished), which was used in the benchmark dose (BMD) modelling in the 2011 evaluation (FAO/WHO, 2012).
Inclusion of modified forms of fumonisins in a group TDI with fumonisins was not considered in any of the previous assessments presented above.
4. Chemistry
4.1. Chemical structure of fumonisins
The basic structural element of fumonisins is a C20 (or C19) long‐chain aminopolyol with two methyl groups as substituents (for FB1: 2S‐amino‐12S,16R‐dimethyl‐3S,5R,10R,14S,15R‐pentahydroxyeicosane). In addition, two propane‐1,2,3‐tricarboxylic acid (TCA, also named tricarballylic acid) side chains are esterified to hydroxy groups at positions C14 and C15 of the aminopolyol backbone. Based on different structural features, fumonisins are classified as A‐, B‐, C‐ and P‐series as shown in Figures 1 and 2. Fumonisins of the B‐type such as fumonisins B1 (FB1), B2 (FB2), B3 (FB3) and B4 (FB4) are the most abundant and were described by Gelderblom et al. (1988) and Cawood et al. (1991). FBs vary in the number and position of hydroxy‐substituents at position 5 and 10 of the backbone as shown in Figure 1. Besides FB1–4 other FBs, namely FB5–6, have been identified (Musser and Plattner, 1997; Mansson et al., 2010). FB5 has the same structure as FB1 with an additional OH group in an unknown position. FB6 is an isomer of FB1 with a hydroxy group at C4 instead of C10.
Figure 1.
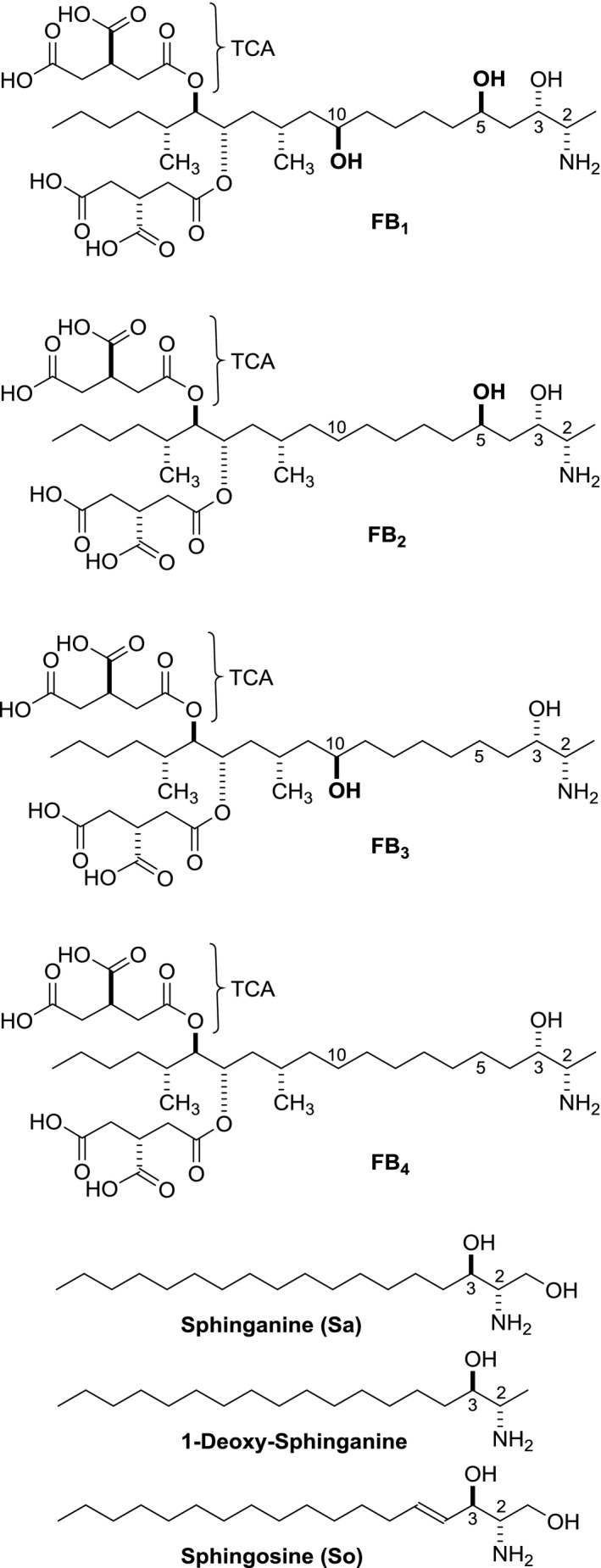
Structures of B‐series fumonisins (FB 1, FB 2, FB 3 and FB 4), sphinganine (Sa) and 1‐deoxy‐sphinganine and sphingosine (So)
Figure 2.
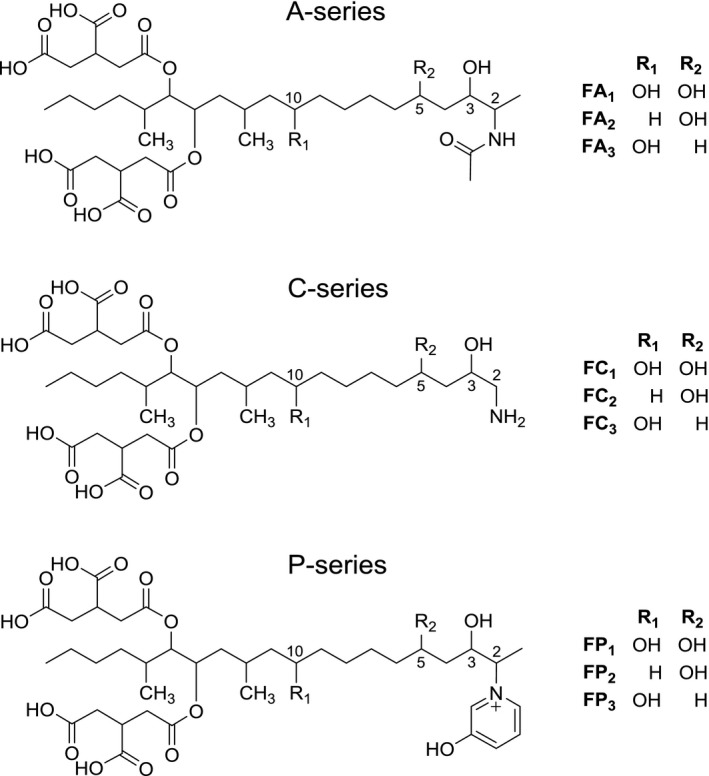
Structures of fumonisins of the A‐series (FA 1, FA 2, FA 3), C‐series (FC 1, FC 2, FC 3) and P‐series (FP 1, FP 2, FP 3)
All fumonisins are highly polar and water soluble compounds. Structurally, the fumonisin backbone resembles the sphingoid bases sphinganine (Sa) and sphingosine (So) especially with the amino and hydroxy functions in positions C2 and C3 (Figure 1). The sphingoid base most closely related structurally to fumonisin is 1‐deoxysphinganine (deoxySa), which can be found in mouse liver and kidney (Bondy et al., 2012).
FB1, (CAS No. 116355‐83‐0, C34H59NO15, molecular weight (MW) 721) contains 10 stereocenters (1,024 different possible stereoisomers) and intensive studies have determined the absolute configuration of the main isomer, as shown in Figure 1 (ApSimon, 2001; Hartl and Humpf, 2001). Other stereoisomers such as epi‐FB3 and epi‐FB4 with 2S,3R‐configuration as well as positional isomers such as iso‐FB1 (hydroxy group at C4 instead of C5) have been described (MacKenzie et al., 1998; Gelderblom et al., 2007; Bartók et al., 2010a).
The A‐type fumonisins (FAs) are characterised by an additional acetyl group at the amino function (Figure 2). Besides FA1–4 (Bezuidenhout et al., 1988; Musser and Plattner, 1997; Abbas et al., 2006) another A‐type fumonisin was identified as keto amide FAK1, which contains a keto function instead of the TCA side chain at C15 (Musser et al., 1995). Initially, it was suggested that the N‐acetylated fumonisins are possible artefacts of the isolation procedure that uses acetic acid, however, Musser and Plattner (1997) have shown that the A‐type fumonisins are also natural contaminants.
The C‐series fumonisins lack the 1‐methyl group resulting in a C19 long‐chain aminopolyol backbone (Figure 2). FC1 was described for the first time by Branham and Plattner (1993). The number and location of the hydroxy groups of C‐type fumonisins is based on the corresponding FBs.
The P‐series consisting of FP1, FP2 and FP3 have a characteristic N‐linked 3‐hydroxypyridinium moiety at C2 (Figure 2), and can occur at levels up to 30% of FB1 when grown on solid corn cultures (Musser et al., 1996). Further isomers of the P‐series have recently been identified in Fusarium verticillioides cultures (Bartók et al., 2014).
4.2. Biosynthesis
The fumonisin biosynthetic gene cluster has been identified by Proctor et al. (1999, 2003) in F. verticillioides and is summarised in a review of Huffman et al. (2010). FUM1 is encoding a polyketide synthetase (PKS) as the key enzyme that assembles the C3 to C20 part of the fumonisin backbone (see Figures 1 and 2) from one molecule of acetyl‐CoA, eight molecules of malonyl‐CoA and two molecules of S‐adenosyl methionine. The backbone is completed in the next step with the introduction of alanine by a 2‐oxoamine synthase (FUM8) (Seo et al., 2001) which confirmed earlier studies with labelled precursors (summarised in ApSimon, 2001). Further studies have shown that different orthologues of FUM8 have different specificity for alanine or glycine, which determine whether Fusarium produces B‐ or C‐type fumonisins (Proctor et al., 2008).
4.3. Modified fumonisins
Fumonisins are highly polar mycotoxins, carrying one amino and several hydroxy groups, two of which are esterified with TCA, leading to four free carboxyl groups in the TCA side chains (Figure 1).
These moieties can be hydrolysed as in the case of the TCA side chains or react with other molecules under thermal processing conditions commonly applied in food production, leading to modified forms of fumonisins.
Since the structure elucidation of FB1 in 1988, several modified forms and degradation/reaction products of fumonisins have been identified and are summarised in Figure 3. The first fumonisin degradation products described in the literature were the hydrolysed fumonisins HFBs (named also aminopentol or aminopolyol (APs) in some publications). They are formed under alkaline conditions by hydrolytic cleavage of the two tricarballylic acid side chains from the fumonisin backbone (reaction A, Figure 3) (Humpf and Voss, 2004). When the hydrolysis is not complete, partially hydrolysed fumonisins (pHFBs, Figure. 3) are formed by cleavage of only one of the two TCA side chains. As either one of the TCA‐side chains can be removed two forms of pHFBs exist which are named with ‘a’ or ‘b’ (Figure 3). Hydrolysed FB1 (HFB1) occurs mainly in nixtamalised corn products, but usually at lower concentrations than FB1 (Saunders et al., 2001). Nixtamalisation is a traditional alkaline cooking process of corn to produce masa and tortilla chips (Humpf and Voss, 2004). TCA, which is also liberated during alkaline hydrolysis, has also been evaluated in toxicity studies (see Section 10). Besides the formation during food and feed processing, HFB1 and pHFB1 have also been described as intestinal metabolites of FB1 in piglets (Fodor et al., 2007, 2008) and a non‐human primate (Shephard et al., 1994b).
Figure 3.
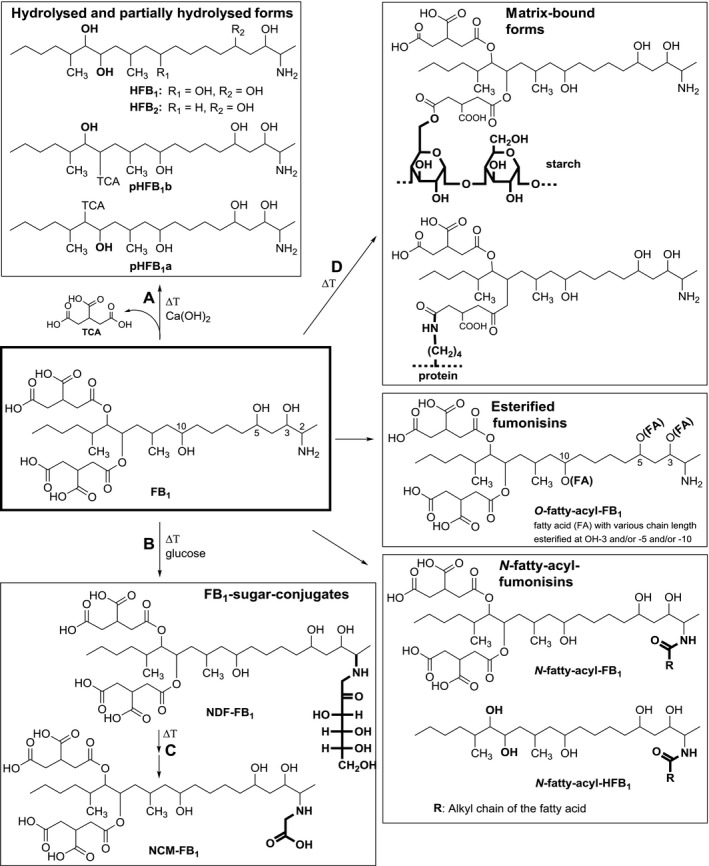
Modified forms of FB 1: hydrolysed fumonisin B1 (HFB 1), partially hydrolysed fumonisin B1 (pHFB 1a, pHFB 1b), N‐(carboxymethyl)‐fumonisin B1 (NCM‐FB 1), N‐(1‐deoxy‐d‐fructos‐1‐yl) fumonisin B1 (NDF‐FB 1), fatty acid (FA) esters of fumonisin B1 (O‐fatty‐acyl‐FB 1), N‐fatty‐acyl‐fumonisin B1 (N‐fatty‐acyl‐FB 1) and N‐fatty‐acyl‐hydrolysed fumonisin B1 (N‐acyl‐fatty‐HFB1)
Thermal reaction products of FB1, which are detectable in food samples, are N‐(carboxymethyl)‐fumonisin B1 (NCM‐FB1) and N‐(1‐deoxy‐d‐fructos‐1‐yl)‐fumonisin B1 (NDF‐FB1, Figure 3). Both compounds are formed during thermal food processing via a Maillard‐type reaction in the presence of reducing sugars. It was shown that the primary amino group of FB1 reacts with the carbonyl group of D‐glucose to yield a Schiff base which then undergoes Amadori rearrangement to form NDF‐FB1 (reaction B, Figure 3) and is further converted to NCM‐FB1 (reaction C, Figure 3) as stable end product (all basic reactions are summarised in Humpf and Voss, 2004). These reactions have been primarily shown for FB1 and HFB1 but all other fumonisins with a free primary amino group can react in the same way. Recently, NDF‐FB2 and NDF‐FB3 have been identified in corn samples (Matsuo et al., 2015). NCM‐FB1 has been detected in model experiments but also in processed food samples (Seefelder et al., 2001; Humpf and Voss, 2004; Meca et al., 2010). In the case of NDF‐FB1, the stability under gastrointestinal conditions has been evaluated. While NDF‐FB1 is already partially cleaved (about 41%) during simulated digestion, it remained rather stable towards human colon microflora (Cirlini et al., 2015). NDF‐FB1 was stable during drying and storage (Hahn et al., 2015).
Fumonisins can also covalently bind to macromolecules such as starch and proteins via their two reactive TCA side chains (see reaction D, Figure 3). These matrix‐bound forms of fumonisins were first described and partially characterised in model experiments with radiolabelled FB1 (Resch and Shier, 2000; Shier, 2000; Shier et al., 2000). Further studies characterised the covalent binding of FB1 via the TCA side chains to starch and protein model compounds by liquid chromatography–tandem mass spectrometry (LC–MS/MS) and in the case of starch also by NMR (Seefelder et al., 2003) (Figure 3). Such covalent binding has been described so far only for FB1, which is the most abundant fumonisin in crops. However, due to the chemical similarity of FB1 with other FBs, the formation of modified forms of FB2, FB3 and FB4, is very likely. Although these compounds have been isolated and characterised in model systems their direct determination in food as such is not possible, as the covalently bound fumonisins have to be first released by chemical hydrolysis. Therefore, these matrix‐bound forms of fumonisins can be determined indirectly by quantifying free FBs and HFBs before and after chemical hydrolysis or after digestion of the macromolecules (Dall'Asta et al., 2010) as described in Section 5
Besides covalently matrix‐bound forms of fumonisins as described above, the existence of non‐covalently bound (‘physically entrapped’) forms of fumonisins (see Section 1.3) has been postulated based on poor recovery rates from different food matrices in interlaboratory studies (Dall'Asta et al., 2009).
Furthermore it is expected that non‐covalent bound forms of fumonisins are released in the gastrointestinal tract, as starch and proteins are digested into their building blocks. Thus, the non‐covalently bound fumonisins are expected to preserve their full toxic potential; however, this has not been confirmed experimentally.
Other modified forms of fumonisins are fatty acid esters of FB1 (O‐fatty acyl FB1, in some publications also abbreviated as O‐acyl FB1 or esterified FB1 (EFB1)) and other fumonisins with variation in fatty acid chain length and position of esterification (3‐O‐, 5‐O‐ or 10‐O‐acyl‐fumonisins) (see Figure 3). These fumonisin esters are produced when F. verticillioides is grown on plant substrate such as maize and rice (Bartók et al., 2010b, 2013b; Falavigna et al., 2016). Besides O‐fatty acylfumonisins, the corresponding N‐fatty acyl fumonisins were also detectable in low amounts in Fusarium (Bartók et al., 2013b). N‐fatty acyl fumonisins and N‐fatty acyl hydrolysed fumonisins with fatty acid chain length ranging from C16:0 to C24:1 (specific fatty acids are indicated for example as C16:0‐HFB1 or N‐palmitoyl‐HFB1) are also described as in vitro and in vivo metabolites of fumonisins (Seiferlein et al., 2007; Harrer et al., 2013, 2015). Their formation is catalysed by ceramide synthases (CerS), a group of enzymes, which are responsible for the acylation of all free sphingoid bases including Sa, So and the corresponding 1‐deoxysphingoid bases (Zitomer et al., 2009). Six mammalian isoforms exist (CerS1–6), which differ in their tissue distribution as well as in their specificity towards the fatty acid chain length used for N‐acylation. Besides N‐fatty acyl FBs and hydrolysed/partially hydrolysed fumonisins as in vivo metabolites, no phase I or phase II metabolites are known.
Another compound described in the literature is the mono methylester of fumonisin FB1 (MME), which is an artefact formed during the isolation procedure of fumonisins when methanol is used as solvent (Cawood et al., 1991). The position of the methylester has not been specified. Nevertheless this compound has been used in some structure‐activity studies (see Gelderblom et al., 1991 and Section 10).
Table 1 provides an overview about the modified FBs described in the literature together with their synonyms as well as alternative names and abbreviations.
Table 1.
Modified forms of fumonisin Bs described in the literature, their abbreviations and synonyms (see Figures 1, 2, 3 for selected structures)
| Form of FBs | Abbreviations used in this document | Alternative names, abbreviations and explanations |
|---|---|---|
| Hydrolysed fumonisin B1–4 | HFB1–4 | Aminopentols/Aminopolyols (APs) |
| Partially hydrolysed fumonisin B1–2 | pHFB1–2a/pHFB1–2b | As either one of the TCA‐side chains can be removed two forms exist which are named with ‘a’ or ‘b’ (see Figure 3) |
| N‐(carboxymethyl) fumonisin B1 | NCM‐FB1 | – |
| N‐(1‐deoxy‐D‐fructos‐1‐yl)‐fumonisin B1–3 | NDF‐FB1–3 | – |
| O‐fatty acyl fumonisin B1 | O‐fatty acyl FB1 | Fatty acid esters of fumonisin B1, esterified fumonisin B1 (EFB1), O‐acyl‐FB1 |
| N‐fatty acyl fumonisin B1 | N‐fatty acyl FB1 | Fatty acid chain length ranging from C16:0 to C24:1. A specific fatty acid is named as C16:0‐HFB1 or N‐palmitoyl‐HFB1, N‐acyl‐FB1/HFB1 |
| N‐fatty acyl hydrolysed fumonisin B1–2 | N‐fatty acyl HFB1–2 | |
| N‐palmitoyl hydrolysed fumonisin B1 | N‐palmitoyl HFB1 | N‐fatty acyl‐HFB1 with palmitic acid as fatty acid, C16:0‐HFB1, PAP1 |
| N‐acetyl fumonisin B1 | FA1 | N‐acetylated FB1 |
| Mono methylester of fumonisin B1 | MME | Artefact formed during isolation and storage of fumonisins in methanol |
5. Analytical methods
5.1. Extraction and analysis of fumonisins
FB1–4 are soluble in water and polar solvents such as methanol and acetonitrile, owing to the presence of carboxyl moieties and hydroxy groups in FBs. They can be extracted from raw and processed materials with water/methanol or water/acetonitrile mixtures. Besides the composition of the extraction solvent, its temperature influences the effectiveness of the extraction (Lawrence et al., 2000). As for other mycotoxins, for sample clean‐up solid phase extraction (SPE) cartridges or immunoaffinity columns may be used (Hübner et al., 2012; Szekeres et al., 2013). However, antibodies for immunoaffinity columns are usually developed for FB1 and show a 100% cross‐reactivity for FB3, while a lower cross‐reactivity is reported for FB2 (40–60%). Very little information is available regarding affinity towards FB4.
Over the last decade, liquid chromatography/mass spectrometry (LC/MS) protocols have become the method of choice for analysis and have replaced LC‐fluorescence detector (LC‐FLD)‐based methods. However, the latter are still in use for routine testing. LC/MS techniques usually have a high sensitivity, reaching a limit of quantification (LOQ) for FB1 and FB2 in the range of 10–50 μg/kg.
Compared to other mycotoxins such as trichothecenes the inclusion of fumonisins in multi‐toxin methods is still difficult, due to differences in polarity and the increased matrix effect. The detection of fumonisins is hampered by relatively poor recovery (≤ 60%) and low accuracy in multianalyte methods. However, when fumonisins are analysed using a targeted method, covering only FB1–4, a better recovery can be obtained, usually in the range of > 90%.
5.2. Analytical issues related to non‐covalent binding to the matrix
FB1–4 may interact with matrix macroconstituents through non‐covalent binding, forming stable complexes. Such non‐covalent complexation can strongly affect the extractability of fumonisins from the matrix and pH, temperature and water proportion are crucial parameters for an effective recovery (Scott et al., 1999; Sewram et al., 2003).
Another crucial parameter for recovery is particle size, because a decrease in size results in an increased surface for extraction, thereby increasing extractability.
The non‐specific complexation of fumonisins can be disrupted by the use of sodium dodecyl sulfate as described by Kim et al. (2003). However, this approach may affect the chromatographic separation of analytes and instrumental performance.
A more general approach involves the alkaline hydrolysis of the matrix (i.e. by 2N aqueous KOH, see also Section 4.3).
5.3. Extraction and analysis of modified fumonisins
Methods for modified FBs differing clearly in their chemical structure from their parent FBs are commonly based on three different strategies: (i) direct analysis, (ii) alkaline hydrolysis and (iii) enzymatic digestion. According to the selected strategy, the resulting final analyte may be different, as summarised in Table 2. Comparison of results obtained by using different strategies may require extensive stoichiometric calculations.
Table 2.
Final analytes monitored for the detection of fumonisin B1–4 and their modified forms depending on the analytical approach
| Compound in sample | Direct analysis | Alkaline hydrolysis | Enzymatic digestion |
|---|---|---|---|
| FB1–4 | FB1–4 | HFB1–4 | FB1–4 |
| HFB1–4 or pHFB1–2 | HFB1–4 or pHFB1–2 | HFB1–4 | HFB1–4 or pHFB1–2 |
| Matrix‐bound FB1 (covalently bound) | Not directly detectable | HFB1 | FB1 + unknown products |
| N‐(carboxymethyl) FB1 | N‐(carboxymethyl) FB1 | N‐(carboxymethyl) HFB1 | N‐(carboxymethyl) FB1 |
| N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB1 | N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB1 | N‐(1‐deoxy‐d‐fructos‐1‐yl)‐HFB1 | N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB1 |
| O‐fatty acyl FB1 | O‐fatty acyl FB1 | HFB1 | Not tested |
| N‐fatty acyl FB1 | N‐fatty acyl FB1 | N‐fatty acyl HFB1 | Not tested |
| N‐acetylated FB1 | N‐acetylated FB1 | Not tested | Not tested |
FB1–4: fumonisin B1–4; HFB1–4: hydrolysed fumonisin B1–4; pHFB1–2: partially hydrolysed fumonisin B1–2.
5.3.1. Direct methods
Several different protocols for the direct determination of modified forms of fumonisins have been proposed in recent years. Most of the protocols are for the detection of HFB1–4 that may occur in processed maize products, such as masa flour.8 Extraction and analysis methods are very similar to those for the parent compound, and therefore FB1–4 and HFB1–4 are often determined within the same chromatographic run. Although in the past, many protocols were based on LC‐FLD with o‐phthaldialdehyde (OPA) derivatisation, more recent methods are mainly based on MS methods (De Girolamo et al., 2014). Partially hydrolysed FB1–2 (pHFB1–2) is less frequently measured, because of its lower stability, but the protocols in use are the same as those for FB1–4 and HFB1–4.
N‐alkyl conjugates of FB1, (i.e. NDF‐FB1 and NCM‐FB1) are extracted with the same methods as used for FB1, which are mainly based on the use of water/methanol or water/acetonitrile mixture (Castelo et al., 2001; Seefelder et al., 2001, 2003; Voss et al., 2001a,b).
Occurrence of fatty acid esters of FB1 has been reported in rice and maize (Bartók et al., 2010a; Falavigna et al., 2013). These less‐polar compounds are commonly extracted from the matrix using water: methanol (25/75, v/v), followed by analysis with LC–MS/MS. A similar LC‐ESI‐MS/MS based method for N‐acyl fatty acid FB1 has been proposed by Bartók et al. (2013a,b). The method was developed for fungal cultures of F. verticillioides and involves a SPE purification step before chromatographic analysis.
Following extraction, analysis of modified fumonisins is almost exclusively based on LC–MS/MS. The separation is obtained on a C18 column, using 0.1% aqueous formic acid or acetic acid and methanol/water or acetonitrile/water as mobile phase, under positive electrospray ionisation (ESI) as ionisation mode. Similar to parent compounds, determination of modified fumonisins is hampered by matrix effects. Therefore, the use of matrix‐matched calibration or of isotopic standards is required.
5.3.2. Indirect methods
It has been observed that performing alkaline hydrolysis of contaminated corn products often leads to higher amounts of released hydrolysed fumonisins than calculated by routine analytical methods. These additional amounts of FB1–4 may be due to the presence of both non‐covalently and covalently bound fumonisins and it is not possible to distinguish between the two forms.
Hydrolysis causes cleavage of the tricarballylic ester groups of FB1–4 releasing HFB1–4 that can be easily quantified by LC–MS. As sugar, starch, peptide or protein conjugates are also attached to the FB side chains through ester or amide bonds with the TCA side chain (see Figure 3), HFB1–4 can be released from these conjugates upon such treatment (Dall'Asta et al., 2009, 2010).
Originally, the analytical approach based on alkaline hydrolysis comprised of two steps: (i) extraction of ‘free’ fumonisins using water/methanol followed by LC–MS/MS determination of FB1–2; and (ii) alkaline treatment of the extracted sample followed by LC–MS/MS determination of HFB1–3 (Kim et al., 2003; Park et al., 2004).
Because this approach was time‐consuming and difficult in terms of sample handling, methods developed more recently are often based on a single step: after the alkaline hydrolysis of the sample, fumonisins are quantified as HFB1–4 by LC–MS/MS and the sum is referred as ‘total fumonisins’ (Dall'Asta et al., 2012) and recovery for HFB1–3 ranges from 92% to 98% with an LOQ of 70 μg/kg. Such indirect methods have been applied quantitatively only for FB1–3, and data on recovery of FB4 as HFB4 after hydrolysis have not been yet been reported.
Although indirect methods based on alkaline hydrolysis are often used for total FB determination, this approach is prone to bias because preformed HFBs are co‐determined with total FBs, especially when calculations are applied for free and bound FB1–3 (Dall'Asta et al., 2009; Bryla et al., 2014, 2016). Its main drawback is the lack of information obtained about the individual modified forms occurring in the samples, since all forms are detected as HFB1–3 and then the results are given as FB1–3 equivalents (this is also true for non‐covalently bound FB1–3 present in a given sample).
As an alternative approach, some authors proposed the application of a digestion protocol to completely degrade matrix macroconstituents (Dall'Asta et al., 2010).
Although it provides information on the pattern of modified forms occurring in the sample, this procedure is rarely applied as the time‐consuming digestion phase is not suitable for routine analysis.
5.4. Extraction and analysis of urinary exposure and effect biomarkers of fumonisins
Exposure to fumonisins can be assessed using urinary biomarkers. FB1–3 and HFB1 have been suggested as direct biomarkers of exposure by several authors (Shephard et al., 2007; Ediage et al., 2012; Torres et al., 2014; Heyndrickx et al., 2015). However, because of the poor urinary excretion of fumonisins and the consequent need for high sensitivity analytical procedures, the sample protocol requires an extensive clean‐up and concentration step, based on SPE C18 cartridge purification.
Fumonisin exposure may perturb sphingolipid metabolism and as a consequence changes in Sa and So or their ratio in urine may occur. The increase in urinary Sa and the Sa/So ratio in rats was primarily associated with dead cells sloughed into the urine (EHC, 2000). Although indicative of FB exposure, such changes are regarded as biomarkers of effect rather than exposure (Riley et al., 1994; Castegnaro et al., 1998; van der Westhuizen et al., 2011; Hahn et al., 2015). The protocol commonly used is based on a liquid‐liquid partition, using ethyl acetate or acetonitrile as organic phase, followed by LC–MS/MS analysis. To obtain an effective recovery of Sa and So from urine, strict control of the pH is crucial and often a hydrolysis step may be necessary. The use of sphingoid base analogues (i.e. phytosphingosine or d‐erythro‐C20‐dihydro‐So) as an internal standard is often reported to allow appropriate recovery correction.
6. Occurrence of fumonisin B1–4 and their modified forms
FB1–4 are mainly produced by Fusarium fujikuroi complex species, among these mainly F. verticillioides and F. proliferatum which colonie predominantly maize and sorghum. It was also shown that for F. verticillioides the pattern of FB1–4 production in maize and the relative amount of FB1 compared to FB2, FB3 and FB4, is related to climatic factors, such as water activity and temperature (Marin et al., 2010; Mylona et al., 2012).
FB2 and FB4, but not FB1 and FB3, are produced by Aspergillus sec. Nigri, mainly in vegetables and, to a lower extent, in cereals (Frisvad et al., 2007). However, data on the co‐occurrence of FB2 and FB4 produced by A. sec Nigri in grapes and raisins are still scarce (Logrieco et al., 2011; Knudsen et al., 2011; Susca et al., 2014; Qi et al., 2016).
While climatic conditions prior to harvest are the most important determinants for fumonisin production in the field, other important factors include maturity class of hybrids, nitrogen fertilisation, time of sowing and harvest and grain moisture (Battilani et al., 2008; Pietri and Bertuzzi, 2012).
6.1. Occurrence of fumonisin B1–4
Only FB1 and FB2 are currently considered in EU regulations on food and feed and occurrence data reported in the literature are mainly on these two compounds. Nevertheless, availability of MS‐based methods and appropriate analytical standards facilitated collection of information on the presence of FB3 in maize and products thereof over the last decade. Still, there are only very few studies reporting FB4 occurrence in grain.
A series of studies reported the occurrence of FB1 and FB2 in maize and products thereof in different European Countries (e.g. Candlish et al., 2000; D'Arco et al., 2009; Cano‐Sancho et al., 2012; Jaksic et al., 2012; Rubert et al., 2013; Christofidou et al., 2015). Although occurrence is widespread, concentration levels ranged only between 0.2 and 2 mg/kg, with generally higher levels in unprocessed material. Maize harvested in Italy in 2006–2008 showed mean FB concentrations (sum of FB1 and FB2) in the range of 4.8 to 10.9 mg/kg (Berardo et al., 2011).
Occurrence of FB1–4 in Triticum spp. (i.e. soft wheat and spelt) in association with F. proliferatum was reported (Castoria et al., 2005; Desjardins et al., 2007; Chehri et al., 2010; Cendoya et al., 2014). Several studies reported occurrence of fumonisins in spices and herbs, black tea, herbal infusions and maize‐based beer (Martins et al., 2001; Monbaliu et al., 2009, 2010; Bertuzzi et al., 2011).
Bakker et al. (2009) assessed the exposure of children to FB1–2 in the Netherlands, using a 24‐h diet recall and FB1 and FB2 were detected in about 28% and 7% of the samples, respectively. Estimated mean daily intake levels for FB1 and FB2 were 291 and 28 ng/kg bw per day, respectively.
FB2 is produced also by Aspergillus niger (Frisvad et al., 2007), which can infect grapes, wheat and maize (Logrieco et al., 2009, 2014; Nielsen et al., 2009; Mogensen et al., 2010; Chiotta et al., 2011). Although data on the occurrence of FB2 in raisins, must and wine are still scarce, it was shown that FB2 can co‐occur with ochratoxin A in grape‐based products (Logrieco et al., 2010; Abrunhosa et al., 2011). In raisins, FB2 co‐occurred with FB4 (Knudsen et al., 2011).
FB3 is often detected together with FB1 and FB2 in maize and products thereof, but its concentration usually does not exceed those of FB1 and FB2, and usually accounts for an additional 10–15% to FB1 levels (Hahn et al., 2015).
Occurrence data on FB4 in maize products are scarce. However, in a recent survey, FB4 was detected at concentrations above the limit of detection (LOD) in 28% of the analysed maize samples (n = 1,113), with a maximum concentration of 4.3 mg/kg accounting for up to 13% of the maximum concentration reported for FB1 (31.8 mg/kg) (Kovalsky et al., 2016). The same survey reported occurrence of FB3 in 40% of the analysed samples and at concentrations comparable to those of FB4.
6.2. Effect of processing on fumonisin B1–4
The effect of processing on FB1 distribution and occurrence in maize has been studied extensively. Due to structural similarities, results obtained for FB1 may likely be extrapolated to other FBs.
Fumonisins are heat‐stable, but when contaminated maize undergoes thermal processing, a reduction in FB content is often observed. Upon baking or canning, where temperatures are < 175°C, little or no loss of fumonisins is observed. Processes such as frying and extrusion cooking, where temperatures are > 175°C, result in greater losses (up to 90%) especially when reducing sugars are added. This is consistent with the formation of modified forms via Maillard‐type reaction (Bullerman et al., 2002). The choice of reducing sugar used for product formulation may affect FB reduction. Castelo et al. (2001) showed that concentrations of FB1 in maize grits decreased in the following order: addition of glucose > fructose > sucrose > no addition of sugars.
Extrusion cooking has been shown to decrease the content of FB1 in final products, which can be explained by Maillard‐type modification. Seefelder et al. (2001) demonstrated the formation of NCM‐FB1 upon extrusion cooking (160–180°C, 16–20% moisture content), at different amounts based on the sugar added (D‐glucose >> sucrose). However, the authors reported a total recovery of FB1, expressed as the sum of residual FB1 in the final product and formed NCM‐FB1, ranging between 10% and 40% of the initial contamination. Alkali‐treatment led to the further release of HFB1 (up to 15%), but not in sufficient amount to explain the mass unbalance. Therefore, the authors suggested the occurrence of matrix‐bound fumonisins (Seefelder et al., 2001).
Notably, NaCl, which is usually present in commercial products, may affect the reliability of fumonisin analysis when strong anion‐exchange (SAX) columns are used for the clean‐up step. The choice of proper analytical methodologies is thus crucial to effectively study the impact of processing on FB content.
Nixtamalisation, an alkaline treatment used for the production of masa flour, is known to cause FB reduction via TCA cleavage and formation of HFBs (Dombrink‐Kurtzman et al., 2000; Palencia et al., 2003; De La Campa et al., 2004; Voss et al., 2006; De Girolamo et al., 2011).
A significant reduction in fumonisin content was also reported in fermented maize (Mokoena et al., 2005; Chelule et al., 2010). Fermentation due to lactic acid bacteria is often used for staple food preparation in rural areas. However, the mechanism of reduction has not yet been elucidated.
Dry milling of maize revealed a heterogeneous distribution of fumonisins in the different parts of the grain, with higher levels in outer layers and lower levels in material from inner parts, such as corn meal and flaking grits (Castells et al., 2008; Aprodu and Banu, 2015). Levels are usually two to four times higher in germ and bran than in the whole corn. During milling, redistribution leads to a strong concentration in corn grits and middlings (Broggi et al., 2002). Similar results were obtained in the processing of precooked maize semolina (Generotti et al., 2015).
Becker‐Algeri et al. (2013) showed that thermal treatment is effective in reducing FB1 content in rice. In particular, cooking and dry heat treatment led to a reduction of 70–80%, while no significant reduction was obtained by autoclaving.
Bryla et al. (2014) studied the effect of baking on fumonisins content in gluten‐free products. Results indicate a significant reduction of about 30% in FB1–3 concentrations. However, after prior alkaline hydrolysis of the sample, further reduction of FBs was only 10%.
When FB1–3 content in maize‐based products (n = 88) was measured before and after alkaline hydrolysis, FB levels above the limit of quantification (LOQ) were found in 57% of all tested samples before hydrolysis (mean concentration: 390 μg/kg), whereas they were above the LOQ in 77% of the samples after alkaline hydrolysis (mean concentration: 574 μg/kg). The highest concentration was observed in maize snacks, and the lowest in maize‐based starch concentrate products. None of the tested products had FB1–3 concentrations above the LOQ before hydrolysis, whereas after alkaline hydrolysis, a mean FB1–3 concentration of 82 μg/kg was found. Overall, the differences were more pronounced in thermally processed products like corn flakes and snacks processed at higher temperatures than in maize flour, groats or raw popcorn grains (Bryla et al., 2016).
In a recent survey from Brazil (Oliveira et al., 2015), 72 maize samples were analysed using direct and indirect protocols. The ranges of concentrations of total fumonisins (expressed as HFB1–3) found were 1.5–3.8 times the concentration of free FB1–3, and in 25% of the samples, concentrations exceeded 5 mg/kg. A strong positive correlation was found between free and total fumonisins, in agreement with previous studies (Dall'Asta et al., 2012; Bryla et al., 2014, 2016).
6.3. Occurrence of modified fumonisin B1–4
Occurrence of modified FBs is reported from a number of studies, mainly aimed at investigating their formation and stability during maize processing. Most of these studies are based on model systems or originated from multi‐parameter experimental designs and only a few deal with occurrence in naturally contaminated samples.
6.3.1. Partially and totally hydrolysed fumonisin B1–4
Whereas in several studies occurrence and formation of HFB1–4 in alkali processed foods, was determined, little is known on the (co)‐occurrence of partially hydrolysed forms with their parent compounds, likely due to the lack of appropriate standards.
A recent study described the formation of HFB1–2 in naturally contaminated maize during the production of masa flour. FB1–2 and pHFB1–2 were found in raw maize, while no pHFBs or HFBs were detected. While concentrations of FB1 and FB2 ranged from 4.0 to 16.7 mg/kg and 1.2 to 3.7 mg/kg, respectively, levels of pHFB1 and pHFB2 were two orders of magnitude lower (i.e. ranging from 0.06 to 0.25 mg/kg and 0.05 to 0.26 mg/kg, respectively). During alkaline‐cooking processing, FB1–2 were converted to both pHFB1–2 and HFB1–2 and at the same time, pHFB1–2 were converted to HFB1–2. The authors reported that the total amount of FB1–2, pHFB1–2 and HFB1–2 measured after alkali‐cooking accounted for a total of 85–115% of the original amount (on a molar base) when maize was cooked without lime, 166–183% when maize was cooked with 1% lime and 153–165% when maize was cooked with 5% lime, suggesting that nixtamalisation releases matrix‐associated FB1–2 that are then converted to both pHFB1–2 and HFB1–2 (De Girolamo et al., 2016).
An exposure survey in Germany reported occurrence of HFB1–3 in thermally and/or alkali‐treated maize products, such as nibbles and extruder products, cereal grits and breakfast cereals including corn flakes. In corn flakes and cereal grits, HFB1–3 were more frequently found (62.4% vs 55.8%, respectively) and at higher concentration level than FB1–3 median concentration (13.0 μg/kg vs 10.0 μg/kg, respectively) (Zimmer et al., 2008).
6.3.2. N‐(carboxy methyl)‐fumonisin B1 and N‐(1‐deoxy‐d‐fructos‐1‐yl)‐fumonisin B1
While the formation of NCM‐FB1 and NDF‐FB1 has been extensively studied, their occurrence in food is rarely reported in the literature and limited to FB1 conjugates (Seefelder et al., 2001) measured the presence of NCM‐FB1, together along with FB1 and HFB1 in maize‐based retail products (n = 10) from the German market (Seefelder et al., 2001). All samples contained FB1 (22–194 μg/kg) and HFB1 (5–247 μg/kg and six out of ten samples also contained also NCM‐FB1 (10–76 μg/kg).
6.3.3. O‐fatty acyl fumonisin B1
Occurrence of O‐fatty acyl esters of FB1 (i.e. O‐linoleoyl‐FB1, O‐oleoyl‐FB1) was reported from highly contaminated raw maize. The mean concentration of FB1 (n = 3) was 321.7 mg/kg whereas mean concentration of the sum of O‐linoleoyl‐FB1 and O‐oleoyl FB1 was 2.1 mg/kg (i.e. 0.6% when compared with FB1) (Falavigna et al., 2013).
No information on the (co)occurrence of O‐acyl conjugates of FB2–4 has been identified by the CONTAM Panel.
6.3.4. N‐fatty acyl fumonisin B1
N‐fatty acyl FB1, i.e. N‐linoleoyl FB1, N‐oleoyl FB1, N‐stearyl FB1 and N‐palmitoyl FB1, have been analysed in retail alkali‐processed and fried maize foods (i.e. maize chips, taco shells, and tortilla chips). N‐acyl conjugates were found only in one out of 38 samples, at a total concentration of 65 μg/kg (Park et al., 2013).
Information on the occurrence of N‐acyl conjugates of FB2–4 has not been identified by the CONTAM Panel.
6.4. Transfer of fumonisins B1–4 and their modified forms
There is limited information about the transfer of fumonisins to food of animal origin. Gazzotti et al. (2009) reported the occurrence of FB1 in bovine milk in 8 out of 10 samples tested (mean concentration: 0.26 μg/kg). The same authors reported the occurrence of FB1 in five out of seven liver tissue samples from pigs fed for 7 weeks with naturally contaminated feed (two concentration levels in feed: 0.91 mg/kg for the first 3 weeks; 2.3 mg/kg for the next 4 weeks). The authors reported a mean concentration in liver of 28 μg/kg (range: 15.7–42.5 μg/kg), whereas HFB1 was found in 1 out of 7 samples and at a concentration of 17.3 μg/kg. Fodor et al. (2006) reported a mean accumulation of FB1 and FB2 in the liver (99.4 μg/kg and 1.4 μg/kg, respectively), kidney (30.6 μg/kg for FB1), and fat (2.6 μg/kg for FB2) in weaned barrows treated with 50 mg FB1, 20 mg FB2 and 5 mg FB3/animal per day for 22 days. A higher accumulation was reported by Meyer et al. (2003), with a mean FB1 concentration in pig liver of 231 μg/kg.
The CONTAM Panel did not identify information on the transfer of modified FBs.
7. Toxicokinetics of fumonisin Bs and their modified forms
Previous evaluations of fumonisins by the SCF (2000) and the JECFA (FAO/WHO, 2012) have concluded that FB1 is poorly absorbed after oral ingestion in farm animals (e.g. swine, cow, laying hen) and experimental animals (rat, mouse, monkey). The bioavailable amount (less than 4% of the dose) is rapidly distributed to all organs and eliminated by biliary excretion without biotransformation. Faecal excretion vastly predominates over urinary excretion. Small amounts of partly hydrolysed and fully hydrolysed FB1 were detected as metabolites in faeces and are believed to be generated by the colonic microbiome. Modified forms of fumonisins have not been addressed in depth in the previous evaluations (EHC, 2000; SCF, 2000, 2003; FAO/WHO, 2001, 2012).
In this opinion, the characteristic features of the toxicokinetics of fumonisins will be discussed in more detail, including more recent studies and modified forms.
The vast majority of the toxicokinetic studies on fumonisins (summarised by Shier, 2000; Voss et al., 2001a,b, 2007; Wang et al., 2016) have been conducted with FB1 or with a natural mixture of fumonisins obtained from fungal cultures, which contained predominantly FB1 and smaller amounts of FB2 and FB3. No studies have been identified on the toxicokinetics of FB3 and FB4, and only limited data have been identified on the modified forms HFB1, pHFB1 and NDF‐FB1 and no data on NCM‐FB1 although the latter compound is relevant as it was also detected in food samples (Seefelder et al., 2001). It is generally assumed that the toxicokinetics and metabolism of FB2, FB3 and FB4 are similar to that of FB1 due to the similarity of their chemical structures and polarities. However, there is some evidence that the toxicokinetics of FB2 and FB3 may be different than FB1 in that they may be less bioavailable and less accumulated in the liver and kidney as seen in some animal studies (Fodor et al., 2006; Riley et al., 2006) and less well excreted in urine as suggested in human studies (Riley et al., 2012; Torres et al., 2014). Modified forms resulting from hydrolysis or Maillard‐type reactions (see Section 4) have markedly different structures and polarities, and therefore their toxicokinetics may differ from that of FB1.
7.1. Absorption
7.1.1. Fumonisin Bs
Several studies in rats indicate that the gastrointestinal absorption of FB1 is very low (Norred et al., 1996; Voss et al., 2001a,b). For example, a bioavailability of 3.5% was determined for a single dose of 10 mg FB1/kg bw administered orally to male Wistar rats (Martinez‐Larranaga et al., 1999). Plasma levels of FB1 peaked at 1.0 h and declined thereafter with a half‐life of 3.1 h. The low but rapid intestinal absorption and short half‐life are consistent with an earlier study in bile‐duct cannulated male Wistar rats using 14C‐labelled FB1, whereas 67% of a single dose of 7.5 mg 14C‐FB1/kg bw were found in the 4‐h bile after i.p. administration, less than 0.2% were present in the bile after oral gavage of the same dose (Shephard et al., 1994a). This indicates low bioavailability resulting from low absorption and not from rapid biliary excretion. When a single dose of 7.5 mg 14C‐FB1/kg bw was injected i.p. into male BD IX rats, 32% of the radioactivity was recovered in the 24‐h urine, but only traces of urinary activity were found after oral gavage of the same dose (Shephard et al., 1992a,b). This finding again supports the low absorption after oral administration.
14C‐FB1 was also used to determine an oral bioavailability of only 3–6% in swine (dose 0.5 mg/kg bw, Prelusky et al., 1994) and 0.7% in laying hens (dose 2.0 mg/kg bw, Vudathala et al., 1994). No FB1 could be detected in plasma of cows after a single oral dose of 5 mg/kg bw of unlabelled FB1 (Prelusky et al., 1995). After administration of a single oral dose of 100 mg FB1/kg bw to turkey poults and ducks, plasma levels peaked at 3 h and at 1–2 h, and bioavailability was about 3% and 2% in turkey poults (Tardieu et al., 2008) and in ducks (Tardieu et al., 2009), respectively. In vervet monkeys receiving a single dose of 6.4 mg 14C‐FB1/kg bw by oral gavage, FB1 peaked in plasma within 2 h at a very low level (Shephard et al., 1995b).
Gastrointestinal absorption of FB2 has been studied in male BD IX rats (dose 7.5 mg FB2/kg bw, Shephard et al., 1995a) and vervet monkeys (7.5 mg FB2/kg bw, Shephard and Snijman, 1999), and has been found to be similar to that of FB1, i.e. very low.
Very limited data from humans consuming fumonisin‐contaminated maize diets suggest that the low gastrointestinal absorption of FB1 found in animal studies is also true in humans (Riley et al., 2012; and literature cited therein). This is based on the very low urinary excretion of FB1 consistently observed in all such reports (see Section 7.4.1). In vitro studies using differentiated Caco‐2 cells, (human epithelial colorectal adenocarcinoma cell line) which represent an established model for human intestinal absorption, confirm that FB1 is very poorly absorbed (De Angelis et al., 2005).
7.1.2. Modified fumonisin Bs
No in vivo studies on the gastrointestinal absorption of modified forms of FB1–4 in animals or humans have been identified. From a study on the urinary, biliary and faecal excretion of radiolabelled FB1, HFB1 and NDF‐FB1 in rats (Dantzer et al., 1999; see Section 7.4.2), it was concluded that HFB1 is about 2.5‐fold better absorbed than FB1 and the sugar conjugate NDF‐FB1. However, this finding was not supported by a more recent rat study (Hahn et al., 2015) using LC‐MS/MS and showing that the urinary excretion of HFB1 and NDF‐HBF1 is equally low as FB1 and marginal in comparison to faecal excretion (see Section 7.4.2).
In the Caco‐2 model for human intestinal absorption, HFB1 was found to cross the plasma membranes in both directions, although the passage from the apical (representing the intestinal lumen) to the basolateral (blood) side was lower than the reverse (De Angelis et al., 2005), suggesting that the rate of intestinal cell flux of HFB1 may exceed the rate of intestinal absorption. A somewhat similar situation was seen in cultured pig kidney renal epithelial cells (LLC‐PK1) where the rate of 14C‐FB1 accumulation in cells required greater than 8 h to reach equilibrium with the external concentration, indicative of passive accumulation, but the rate of efflux required only a few minutes suggesting an active process (Enongene et al., 2002).
7.2. Distribution
7.2.1. Fumonisin Bs
The general observation made in studies in numerous animal species was that FB1 is distributed to virtually all organs after absorption from the gastrointestinal tract, although liver, kidney and muscle appear to be preferred. As an example, radioactivity remaining in various organs of male vervet monkeys 24 h after a single oral dose of 6.4 mg 14C‐FB1/kg bw accounted for 0.6% of the dose in the liver, 0.14 in muscle, 0.03 in kidney, 0.02 in brain, and 0.01 or less in spleen, gonads, heart, lung, and red blood cells (Shephard et al., 1995a). In various rat strains (Sprague–Dawley, Wistar, BD IX) orally exposed to FB1, the toxin is found unchanged primarily in kidney and liver, and the differential sensitivity of these two organs appears to correlate with the tissue concentration (Riley and Voss, 2006 and references cited therein).
7.2.2. Modified fumonisin Bs
No studies on the distribution of modified forms have been identified by the CONTAM Panel.
7.3. Metabolism
7.3.1. Fumonisin Bs
The in vivo and in vitro metabolism of fumonisins has recently been reviewed by Wang et al. (2016). In summary, two metabolic pathways have been demonstrated for FB1 in mammals: (1) hydrolysis of the ester groups with the consecutive release of the two tricarballylic acid moieties, (2) fatty acylation of the amino group (Figure 4).
Figure 4.
Metabolic pathways of FB 1. For the N‐acyl (H)FBs the fatty acid acyl chain length ranges from C16:0 to C24:1
The hydrolytic pathway (1) gives rise to the isomeric pHFB1a and pHFB1b and eventually to HFB1, also termed aminopentol (AP or AP1). The two isomers of pHFB1 appear to be converted to each other in solution by intramolecular transesterification (migration of the tricarballylic acid between the hydroxy groups at C14 and C15). A mixture of the two pHFB1 isomers has been identified together with small amounts of HFB1 in the faeces of vervet monkeys receiving a single oral dose of 14C‐FB1 (8 mg/kg bw, Shephard et al., 1994b). pHFB1 and HFB1 were also found in the colon but were not detectable in the bile of vervet monkeys, even after i.v. administration of 14C‐FB1 (1.7 mg/kg bw, Shephard et al., 1995b). Likewise, the bile of rats after oral gavage of FB1 contained only unchanged FB1 and no evidence for any metabolic product (Shephard et al., 1994a). However, when rats were fed a diet containing FB1 for three weeks, small amounts of both pHFB1 isomers (together 2–6% of the total faecal metabolites) together with traces of HFB1 were detected in the faeces (Hahn et al., 2015). Therefore, it is likely that hydrolysis of FB1 does not occur in mammalian tissues, but is rather mediated by the colonic microbiome of some species (Shephard et al., 1995b; Fodor et al., 2008). In support of this notion are the observations that neither hydrolytic nor other metabolic products were detected upon incubation of FB1 with primary rat hepatocytes or with rat or bovine liver microsomes (Cawood et al., 1994; Spotti et al., 2001). Moreover, FB1 was not a substrate of rat hepatic triglyceride lipase or porcine pancreatic lipase (Cawood et al., 1994), On the other hand, FB1 was efficiently hydrolysed to pHFB1 and also to small amounts of HFB1 in anaerobic incubations with suspensions of pig caecal contents (Fodor et al., 2007). In contrast, no hydrolysis products of FB1 were found in anaerobic incubations with ruminal fluid (Caloni et al., 2000). There was also no indication of hydrolysis of FB1 by human intestinal bacteria, as its concentration did not decrease in the culture medium during a 72 h incubation period (Becker et al., 1997). Thus, bacterial hydrolysis of fumonisins may vary among species.
In contrast to the hydrolysis of fumonisins, the acylation of the amino group (pathway 2 in Figure 4) is clearly a mammalian metabolic reaction, probably mediated by CerS, the physiological role of which is the fatty acylation of free sphingoid bases (see MoA). Harrer et al. (2013) first demonstrated the formation of N‐fatty acyl FB1 in several mammalian cell lines, including cells overexpressing CerS. Acyl groups ranged from C16:0 (palmitoyl) to C24:1 (nervonoyl), and the extent of fatty acylation depends on the acyl group and the cell line. More recently, N‐fatty acyl FB1 metabolites were also identified in the kidney and liver of male Sprague–Dawley rats after i.p. administration of FB1 for five consecutive days (Harrer et al., 2015). While the metabolites in the kidney contained predominantly C16:0 acyl groups, C24 groups predominated in the liver. This tissue‐specific N‐fatty acylation is due to different isoforms of CerS expressed in kidney and liver (Harrer et al., 2015).
Deamination has thus far not been reported for fumonisins but has been reported for their hydrolysis products, which are considered modified forms (see below).
7.3.2. Modified fumonisin Bs
Limited information is available on the hydrolytic metabolism of pHFB1 and NDF‐FB1 from the study of Hahn et al. (2015), which is described in more detail in Section 7.4.2 Whereas the two isomers of pHFB1 are prone to further hydrolysis to HFB1, NDF‐FB1 appears to be hydrolysed to a significant extent to FB1 in the rat in vivo, possibly by the colonic microbiome.
Two pathways for the metabolism of the fully hydrolysed fumonisins HFB1 and HFB2 have been demonstrated, i.e. fatty acylation (Figure 3) and deamination.
Following the earlier report that CerS acylates HFB1 to N‐palmitoyl‐HFB1 ((C16:0‐HFB1, Humpf et al., 1998). Seiferlein et al. (2007) showed that HFB1 and HFB2 were converted to their respective N‐acylated metabolites by rat hepatic microsomes in the presence of the cosubstrates palmitoyl‐CoA or nervonoyl‐CoA. Moreover, the presence of N‐acyl HFB1 with acyl groups derived from fatty acids of various chain length (predominantly C24) was demonstrated in the liver of rats after i.p. dosing with HFB1 (Seiferlein et al., 2007).
Deamination, which represents the conversion of a free amino group to a carbonyl group, is a common reaction of aliphatic amines in mammalian cells. It has not been demonstrated as a pathway in the mammalian metabolism of fumonisins to date. However, it has been established in yeast and bacteria as the second step in the degradation of fumonisins, following the hydrolysis as a first step (Blackwell et al., 1999; Hartinger et al., 2011). As the free amino group is essential for the toxicity of fumonisins (see MoA), deamination is generally considered as an important detoxification reaction.
According to in vitro studies reported by Cirlini et al. (2015) the modified forms HFB1 and NDF‐FB1 appear to not to be stable in the human gastrointestinal tract. Although HFB1 was rather stable in an artificial system simulating human digestion in the small intestine, it was, however, partially metabolised to unknown compounds in an in vitro human colonic fermentation. Conversely, NDF‐FB1 was partially cleaved in the digestive model system, but was not affected by the human colon microflora.
7.4. Excretion
7.4.1. Fumonisins Bs
Numerous studies in experimental and farm animals have shown that the vast majority of orally ingested FB1 and FB2 is excreted unchanged with the faeces and only a minor proportion with the urine (Shier, 2000; Voss et al., 2001a,b, 2007; Wang et al., 2016). For example, only 0.5% of the total radioactivity of a single dose of 0.69 μmol 14C‐labelled FB1/kg bw, administered by oral gavage to male and female F344/N rats, was excreted in the urine within 84 h, whereas the faeces contained 90% after the same time (Dantzer et al., 1999). Most of the urinary and faecal excretion occurred during the first 12 and 48 h, respectively, and there was no gender difference. Biliary excretion in bile duct‐cannulated female Sprague–Dawley rats after the same dosing protocol amounted to 1.5% of the administered radioactivity within 4 h (Dantzer et al., 1999). Hence, the major fraction is not absorbed but is passed unchanged in the faeces.
Hahn et al. (2015) fed a diet equivalent to 13.9 μmol FB1/kg bw to male Sprague–Dawley rats and determined the pattern of parent toxin and hydrolytic metabolites in the 24‐h urine and faeces after 1, 2 and 3 weeks. Only traces of FB1 and no metabolites could be detected in rat urine by LC–MS. In contrast, considerable amounts of FB1 were observed in the faeces at all sampling points, together with small quantities of pHFB1a, pHFB1b and HFB1; the amount of FB1 was about 100‐fold higher in faeces than in urine, and the hydrolytic metabolites in faeces accounted for about 5% of the faecal FB1. This study confirms that faecal excretion predominates over urinary excretion, and is consistent with partial hydrolytic metabolism of FB1 in the rat digestive tract.
For humans, estimations of the urinary and faecal excretion of parent fumonisins are based on studies with volunteers eating food prepared from maize naturally contaminated with fumonisins. Very low concentrations of FB1 (usually below 1 ng/mL) were detected in urine in several studies (Gong et al., 2008; Xu et al., 2010; van der Westhuizen et al., 2011; Riley et al., 2012, 2015a; Robinson et al., 2012; Torres et al., 2014). In one study, it was estimated that urinary excretion accounted for 0.05–0.1% of the ingested amount of FB1 (van der Westhuizen et al., 2011). Although the contaminated maize, in addition to FB1 also contained FB2 and FB3, the latter two fumonisins were either not detected in urine (Riley et al., 2012) or present only at much lower concentrations compared to their levels relative to FB1 in maize (Riley et al., 2012; Torres et al., 2014). This suggests that FB2 and FB3 are less well absorbed or less excreted in the urine compared to FB1. Results from studies in pigs, rats and mice are also consistent with the hypothesis that FB2 is either absorbed or eliminated to a different extent than FB1. In rats (Riley and Voss, 2006) and pigs (Fodor et al., 2006), the amounts of accumulated FB2 relative to FB1 were less than what would be expected based on their relative amounts in the diets which contained both FB1 and FB2. The possible difference in how FB2 is absorbed or excreted is also consistent with the results of the study of Howard et al. (2002) where FB2 was without any effects in mice but FB1 was both hepatotoxic and disrupted sphingolipid metabolism in the liver.
After ingestion of fumonisin‐contaminated maize food by volunteers, faecal concentrations of FB1 in the range of several μg/g (about the same levels as in maize) have been reported by Chelule et al. (2001). Thus, like in other mammalian species, faecal excretion appears to markedly predominate over urinary excretion of parent fumonisins in humans.
7.4.2. Modified fumonisin Bs
Dantzer et al. (1999) also studied the urinary, faecal and biliary excretion of radioactivity in rats after oral administration of 14C‐labelled HFB1 and NDF‐FB1 (equimolar single doses of 0.69 μmol/kg bw). Within 84 h, 4.4% of the dosed radioactivity was recovered in urine for NDF‐FB1 but markedly higher amounts for HFB1, i.e. 17.3% in females and 12.8% in males. Faeces of both sexes contained 92% of the radioactivity after dosing NDF‐FB1 and 89% after dosing HFB1. Biliary excretion of radioactivity within 4 h of administering NDF‐FB1 and HFB1 was 0.8 and 1.7%, respectively. The excreted radioactivity was not analysed for metabolites.
Hahn et al. (2015) fed a diet containing 13.9 μmol/kg bw of unlabelled pHFB1 (mixture of a and b isomer) or HFB1 or NDF‐FB1 to male Sprague–Dawley rats and analysed the 24‐h urine and 24‐h faeces after 0, 1, 2 and 3 weeks by LC–MS for FB1, pHFB1, HFB1a, pHFB1b and NDF‐FB1. Only traces of some of the modified forms were detected in urine. In the faeces of rats dosed with the mixture of the pHFB1 isomers, pHFB1a and pHFB1b were the main forms excreted, together with smaller amounts of HFB1. Faeces of rats fed HFB1 contained large amounts of HFB1, indicating that the hydrolytic metabolism of FB1 is irreversible. However, in the faeces of rats dosed with NDF‐FB1, large amounts of NDF‐FB1 were detected together with significant amounts of FB1 (about 30% of the faecal metabolites), suggesting some hydrolysis of NDF‐FB1 with the release of FB1.
7.5. Summary remarks on toxicokinetics
Animal studies indicate that FB1 is poorly absorbed from the gastrointestinal tract (less than 4% of the dose), rapidly cleared from the blood (with half‐lives of less than 4 h) by the biliary route, and preferentially excreted with the faeces (usually more than 90% of the dose).
Small amounts of FB1 are found in liver and kidneys, with even smaller amounts in other organs. Metabolic pathways of FB1 in mammals comprise (1) hydrolysis of the ester groups leading to two isomers of pHFB1 and to HFB1 and (2) formation of N‐acyl fumonisins with long‐chain fatty acids. The biotransformation of FB1 is low in mammalian tissues and pathway hydrolysis of the TCA moieties appears to be restricted mostly to the lower gastrointestinal tract of some species, involving the colonic microbiome. The few data on the excretion of FB1 in humans eating fumonisin‐contaminated maize food suggest that the toxicokinetics of FB1 in humans is the same as in other mammalian species.
8. Mode of action
8.1. Fumonisin Bs
The MoA of fumonisins has been described in detail in previous evaluations (IARC, 1993, 2002; EHC, 2000; FAO/WHO, 2001, 2012) and most recently in FAO/WHO (2017). The key event is fumonisin inhibition of CerS. Inhibition of CerS results in the disruption of sphingolipid metabolism and, as a consequence, alterations in other lipid pathways. Fumonisins are regarded as structural analogues of free sphingoid bases (see Figure 1 in Section 4) and they competitively inhibit CerS, a group of key enzymes in the biosynthesis of ceramide and more complex sphingolipid (Wang et al., 1991).
Ceramide synthases catalyse on the one hand the acylation of Sa to form (dihydro)‐ceramide and more complex sphingolipids. On the other hand, CerS are also responsible for the reacylation of sphingosine derived from the turnover of more complex sphingolipids. The de novo sphingolipid biosynthesis and turnover pathways as well as the cellular consequences of FB‐disrupted metabolism are summarised in Figure 5.
Figure 5.
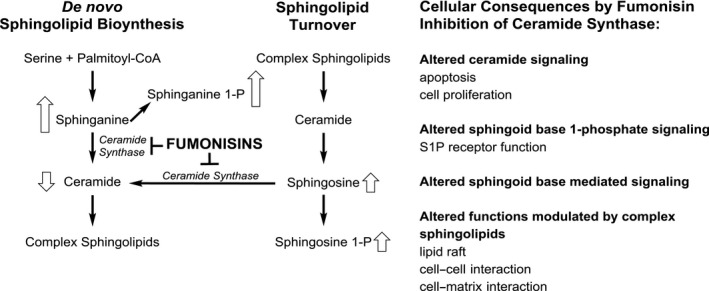
A simplified scheme of the de novo sphingolipid biosynthesis and turnover in mammalian cells indicating the inhibition of CerS by fumonisins and the cellular and biochemical consequences
- Open block arrows show the increase (arrow up) or decrease (arrow down) of respective metabolites. Note that Sa and Sa 1‐P increase more than So and So 1‐P leading to higher Sa/So and Sa 1‐P/So 1‐P ratios. The size of the open block arrows in the figure reflects relative quantitative response in tissues and cells.
Note that FB1 inhibition of CerS causes Sa and Sa 1‐P to increase more than So and So 1‐P leading to higher Sa/So ratios in the presence than in the absence of FB1 (see Figure 5). The sphingolipid pathway is rather complex and in the case of CerS six mammalian isoforms exist (CerS1–6) which differ in their tissue distribution as well as in their specificity of the fatty acid chain length used for N‐acylation (Tidhar and Futerman, 2013).
Knock‐out (KO) mice for CerS2 cannot synthesise very long acyl chain (C22–C24) ceramides. This phenomenon mimicking the FB1 MoA, results in elevated C16 ceramide and Sa levels. From 30 days of age, increased rates of hepatocyte apoptosis and proliferation were observed in the KO mice with nodules of regenerative hepatocellular hyperplasia progressing, at 10 months of age, to hepatomegaly and non‐invasive hepatocellular carcinoma (Pewzner‐Jung et al., 2010).
The inhibition of CerS leads to elevated levels of free sphingoid bases and sphingoid base 1‐phosphates, in particular Sa and Sa 1‐P and to a less extent also So and So 1‐P, in a dose dependent manner in blood and tissues and to a depletion of complex sphingolipids (summarised in Riley et al., 2015a and Riley et al., 2015b and FAO/WHO, 2012). Note that Sa and Sa 1‐P increase more than So and So 1‐P leading to higher Sa/So and Sa 1‐P/So 1‐P ratios (this is indicated by the size of the open block arrows in Figure 5). The increased levels of Sa and Sa 1‐P (also relative to corresponding sphingosines) are used as a biomarker for fumonisin exposure in animals as well as in humans (Riley et al., 2015b) (see Section 11). Recently, Masching et al. (2016) reported increased Sa/So ratios in piglets receiving low doses of 2 mg FB1+FB2 per kg feed, equivalent to 100 μg FB1+FB2/kg bw, for 42 days.
Besides inhibiting CerS, HFB1 and FB1 were shown to be substrates of CerS. They are converted in vitro and in vivo (Harrer et al., 2013, 2015) to N‐acyl fumonisins with various fatty acid chain length. N‐fatty acyl FB1 is more cytotoxic in vitro compared with FB1 (no in vivo data available). However, the role of these N‐fatty acyl fumonisins in the MoA is not clear yet. Note that these N‐fatty acyl fumonisins penetrate more readily into cells in vitro (Harrer et al., 2013)
While the role of N‐fatty acyl HFB1 in fumonisin toxicity in vivo is unknown, HFB1 has been shown repeatedly to be much less toxic compared to FB1 in feeding studies (Grenier et al., 2012; Voss et al., 2013; Masching et al., 2016). From these studies, it can be concluded that when HFB1 is fed to animals its possible metabolism to N‐fatty acyl HFB1 seems not to induce any toxic effects, although N‐fatty acyl HFB1 has been detected in vivo and is more cytotoxic in vitro. Likewise, in male Sprague–Dawley rats, the kidney is much more sensitive to FB1‐induced toxicity compared to liver and in the study by Harrer et al. (2015) the great majority (> 90%) of the total fumonisin in the kidney was unmetabolised FB1, whereas in the liver approximately half of the total fumonisin consisted of the N‐fatty acyl FB1 metabolites (Harrer et al., 2015). Clearly, revealing the role of N‐fatty acyl fumonisins should be a priority for future research.
Concerning structure–activity relationship it was shown in early studies that FB1–4 are inhibitors of CerS in rat liver slices at equimolar concentrations (Norred et al., 1997). Based on these data, it can be assumed that at the cellular level FBs have the same MoA, as the inhibition of CerS is the initial step in the down‐stream effects leading to fumonisin toxicity. However, toxicological outcomes are influenced by differences in absorption, distribution, metabolism, and excretion.
Sphingolipids are both highly bioactive compounds and important structural components in cell membranes. Ceramide, free sphingosine, and sphingoid base 1‐Ps are bioactive molecules in signal transduction pathways regulating cell growth and death. More complex sphingolipids play important roles in cellular physiology through direction of protein sorting, lipid raft function, mediation of cell‐to‐cell interactions and cell recognition (Bartke and Hannun, 2009). The disruption of sphingolipid metabolism is closely related at an early stage with fumonisin‐induced pathologies including tumour promotion, carcinogenicity and neural tube defects (NTDs) in sensitive animal strains. This was shown in many animal studies and more recently also confirmed with additional mechanistic details with CerS2 null mice (summarised in FAO/WHO, 2017). However, it is not known whether FB1 induced CerS inhibition is directly linked to any human disease.
Mitochondria were recently shown to be negatively affected by FB1 and effects in astrocytes were observed at concentrations of 0.5 μM and higher with indications that the complex I of the respiratory chain is the target of FB1 (Domijan and Abramov, 2011).
FB1 (28 μM) induced single strand breaks in DNA in human peripheral blood lymphocytes and this effect was related to oxidative stress (Domijan et al., 2015).
FB1 induced DNA hypomethylation and histone demethylation in HepG2 (human hepatoma cell line) cells which may be responsible for chromatin instability and represent an alternative MoA (Chuturgoon et al., 2014).
Similarly, histone modifications leading to the disruption of epigenetic events following FB1 exposure were observed in rat kidney epithelial cells (Sancak and Ozden, 2015).
FB1 (40 μM) induced an increase in nuclear Sa 1‐P and corresponding decrease in histone deacetylase activity and increased histone acetylation in mouse embryonic fibroblasts (MEF) suggesting a possible role of FB1 in epigenetic effects (Gardner et al., 2016).
8.2. Modified fumonisin Bs
Some of the modified forms of FB1–4 are also inhibitors of CerS. Although FB1–4 as well as FC4 on a molar basis have been identified as equipotent inhibitors of CerS in rat liver slices, the hydrolysed forms HFB1–3 were only 30–40% as potent as the parent compounds (Norred et al., 1997). N‐acetylated FB1 with a C2 chain (FA1, Figure 2 Section 4) did not inhibit CerS in this study; however, this seems to depend on the fatty acyl chain length as long‐chain derivatives such as N‐palmitoyl‐HFB1 inhibited CerS under in vitro conditions (Humpf et al., 1998). Interestingly FA1 does not inhibit CerS but is unstable and it can spontaneously rearrange to O‐acetylated forms. These rearrangement products are putative inhibitors of ceramide synthase (Norred et al., 2001). Although hydrolysed fumonisins HFB1–3 inhibited CerS in rat liver slices several animal experiments have shown that hydrolysed fumonisin HFB1 did not significantly elevate the Sa/So ratio, an early marker of CerS inhibition in vivo (reviewed in Voss et al., 2017a). In a recent animal study with rats, FB1 at a single dose of 10 mg/kg diet significantly increased the Sa/So ratio. In contrast, the modified fumonisins HFB1, pHFB1 as well as NDF‐FB1 did not raise the Sa/So ratio at a single dose equivalent to 10 mg FB1/kg diet (Hahn et al., 2015). In a dose–response feeding study in mice (Howard et al., 2002), NCM‐FB1 (approximately 0, 14, 70 and 140 μmol/kg diet) for 28 days had no effect on the Sa/So ratio, ceramide levels, serum analytes, organ weights, or hepatic structure, all of which were affected by FB1 (Howard et al., 2002) (for structures of modified forms of fumonisins see Figure 3).
In summary, the MoA of fumonisins is based on the inhibition of CerS, a group of key enzymes in the sphingolipid pathway. The disruption of the sphingolipid metabolism is linked at an early stage with fumonisin‐induced pathologies including porcine pulmonary oedema, ELEM, liver and kidney toxicity, tumour promotion, carcinogenicity and NTDs in animal studies.
9. Biomarkers
9.1. Biomarkers of exposure
Urinary FB1 itself is a biomarker of exposure. In several human studies, the fumonisin levels in food were correlated with urinary FB1 levels. The results show a clear correlation between fumonisin exposure and urinary FB1 levels (for a summary of the results until 2012 see FAO/WHO, 2012 and Turner et al., 2012).
Several human studies have successfully used urinary FB levels as a biomarker for human exposure to fumonisins (summarised by Van der Westhuizen et al., 2013 and Riley et al., 2015a). In a recent human study with more than 1,200 participants, urinary fumonisin levels were analysed in women from low‐ and high‐exposure communities in Guatemala and correlated with the total intake of FB1, FB2, and FB3 alone or in combination. Total FBs intake was estimated using the mean total FBs in maize at each sampling interval over a period of one year, each individual's reported tortilla consumption and each individual's body weight at each sampling time point. FB1‐levels in maize in high‐exposure communities were much higher (average: 3.69 μg/g) compared with low‐exposure communities (0.69 μg/g). The same trend was observed for the urinary FB1 levels, which were significantly higher in high‐exposure (average: 2.27 ng/mL) compared with low‐exposure communities (average: 0.26–0.38 ng/mL). The results clearly showed a correlation between urinary FB1 and estimated FB1 intake on an individual basis. Urinary FB1 levels above 0.1 ng/mL resulted in a dose dependent increase in the risk to exceed 2 μg/kg bw per day (i.e. the JECFA PMTDI) compared with women with no detectable urinary FB1. More than 50% of the participants exceeded 2 μg/kg bw per day when urinary FB1‐levels were above 0.5 ng/mL (Torres et al., 2014). The FB1 intake based on the average percentage of urinary FB1 excretion (0.5%, range 0.12–0.9%) determined experimentally in a recent kinetic study (Riley et al., 2012) was calculated. The predicted urinary FB1 concentration that coincided with 2 μg/kg bw per day was approximately 0.6 ng/mL, which fitted very well to the values obtained based on the individual maize consumption data (Torres et al., 2014).
Multimycotoxin biomarker studies have identified FB1 in human urine samples. However, these studies are difficult to compare as the LOD and LOQ are different and different units for concentration were used. Furthermore, it is not clear if spot urine, morning urine or 24 h urine samples have been used. Human urine samples from the following countries and cities have been analysed and the number of positive samples and mean urinary FB1 levels were reported: Belgium (Heyndrickx et al., 2015, 0; % positive samples), Bangkok (Warth et al., 2014, 0; % positive samples), Bangladesh (Gerding et al., 2015, 1% positive samples), Cameroon (Abia et al., 2013, 3% positive samples, mean urinary FB1: 0.33 ng/mg creatinine), Germany (Gerding et al., 2014, 2015, 0% positive samples), Haiti (Gerding et al., 2015, 3% positive samples, mean urinary FB1: 0.29 ng/mg creatinine), Italy (Solfrizzo et al., 2014, 56% positive samples, mean urinary FB1: 0.055 μg/L), Ivory Coast (Kouadio et al., 2014; men: 22.8% positive samples, mean urinary FB1: 0.5 μg/L; women: 32% positive samples, mean urinary FB1: 0.56 μg/L), Nigeria (Ezekiel et al., 2014, 13.3% positive samples, mean urinary FB1: 4.6 μg/L), Sweden (6% positive samples, mean urinary FB1: 0.004 μg/L) and South Africa (Shephard et al., 2013, single‐biomarker method: 87% positive samples, mean urinary FB1: 0.34 ± 0.46 ng/mg creatinine; multi‐biomarker method: 96% positive samples, mean urinary FB1: 1.52 ± 2.17 ng/mg creatinine).
In summary, fumonisin biomarkers are helpful to estimate human exposure and recent studies with large sample cohorts have shown a statistically significant correlation between fumonisin intake and urinary FB1 levels. Urinary fumonisin levels are indicative of recent exposure to fumonisins and allow the estimation of the individual chronic exposure especially in areas where maize is a main staple food.
9.2. Biomarkers of effect
Several animal studies have shown that the levels of free sphingoid bases and their 1‐phosphates increase in a dose‐dependent manner in tissues and blood when animals consume fumonisin‐contaminated feed material (as summarised in FAO/WHO, 2012; Riley et al., 2015a,b). Similar results were recently obtained in a large human study in Guatemala. In this study, urinary FB1 and Sa 1‐P as well as So 1‐P in blood samples were measured of 1539 women from high and low exposure communities (see Section 9.1). The results clearly show that high dietary FB1 intake is correlated with changes in Sa 1‐P and the Sa 1‐P/So 1‐P ratio in human blood in a manner consistent with FB1 inhibition of CerS (Riley et al., 2015a). It should be noted that the use of the Sa 1‐P and Sa 1‐P/So 1‐P ratio is not intended as a standalone biomarker of effect but is intended to be used in conjunction with urine samples collected so as to obtain an individual and time‐matched estimate of FB1 intake.
10. Toxicity
In this chapter, an overview and summary about the state of the art of the in vivo toxicity of fumonisins is presented that is essentially based on the assessment as presented by JECFA (FAO/WHO, 2012) which is the latest comprehensive assessment of fumonisin toxicity. Key studies discussed by JECFA are discussed again in detail below as are any relevant in vivo studies published after the JECFA assessment was issued.
10.1. Overview of fumonisin Bs toxicity as established in previous hazard assessments until 2012
The toxicity of fumonisins has been extensively reviewed by JECFA at three occasions, namely in 2001, 2011 and 2016 (FAO/WHO, 2001, 2012, 2017). However, only the technical report but not the detailed outcome of the last assessment (i.e. the Addendum) was available to the CONTAM Panel at the time of drafting the present opinion. The assessments published 2001 and 2012 were essentially based on FB1 data because the other FBs, FB2 and FB3, were considered to have very similar toxicological profiles. Similarly, in 2003 the SCF assessed FB1, FB2 and FB3, and included all three in a group TDI based on data on FB1 (SCF, 2003). This was based on the results of a comparative study on their relative cytotoxicity to primary rat hepatocytes, and their potential to induce hepatocyte nodules in an initiation/promotion model using male Fischer rats. All three fumonisins were able to induce hepatocyte nodules when fed at high dietary concentrations of 500 or 1,000 mg/kg feed over 21 days to the rats (Gelderblom et al., 1993). In addition, in a study with ponies FB2 and FB3 raised free sphinganine concentrations in liver and kidney of the animals although effects of FB3 were much less severe.
10.1.1. Acute toxicity
There were few studies available using FB1 and in none of these was lethality observed. In acute studies in rats, oral gavage doses up to 46.4 mg/kg bw have been tested. Other acute studies in rats showed that effects were similar to those occurring after repeated doses in longer term studies, i.e. kidney and liver toxicity. In pigs, early signs of pulmonary oedema occurred following a single oral dose of 5 mg/kg bw. Also the equine leukoencephalomalacia (ELEM) is considered a vascular effect. Vascular toxicity of FBs in humans cannot be excluded, but the only in vivo vascular effects reported in the literature are a chronic atherogenic effect associated with consumption by non‐human primates of diets containing fumonisins for extended periods of time (Fincham et al., 1992) but has not been reported. Overall, FB1 is considered not to be acutely toxic in humans.
10.1.2. Short term and long term toxicity
Following oral exposure to FB1, the toxic effects range from hepatotoxicity and renal toxicity in rodents, to species‐specific effects such as pulmonary oedema and hydrothorax in pigs, and ELEM in horses.
Early signs of FB1 liver toxicity in rodents were apoptosis, necrosis, proliferation and regeneration, and hyperplasia of the bile duct. Females exhibited hepatic effects at lower doses than males.
In chronic studies, liver tumours were observed in male rats (Gelderblom et al., 2001) and female mice (NTP, 1999). It is likely that the modulation of apoptotic and cell proliferative pathways accompanied by increased hepatocellular hypertrophy attributable to the disruption of sphingolipid, phospholipids and fatty acid metabolism plays a major role in the development of hepatocellular cancer in female B6C3F1 mice and male BD IX rats.
In a two‐year feeding study in male F344 rats fed diets containing pure FB1 (NTP, 1999), early signs of kidney toxicity in rats were increases in free sphingoid bases, apoptosis and cell regeneration in the renal tubules of the outer medulla. Kidney tumours were observed in male rats. Chronic nephropathy referred to as atypical tubule hyperplasia with increased renal tubule epithelial cell apoptosis, proliferation and increased incidence in renal tubule epithelial cell hyperplasia and hyperplastic lesions developing into adenomas, has been regarded as a precursor lesion for rat kidney carcinogenesis resulting in kidney cancer. The data from this study (NTP, 1999) and a 90‐day study (Voss et al., 1995) were used as the basis for setting the HBGVs at the 2001 meeting of JECFA (FAO/WHO, 2001).
In 2011, JECFA (FAO/WHO, 2012) used data on FB1 induced liver toxicity and adenoma formation in female wild type and p53+/− transgenic mice fed diets prepared with pure FB1 (Bondy et al., 2010) as a basis for derivation of a HBGV. The data were provided as an unpublished report of a study conducted by Health Canada. Megalocytic hepatocytes in male mice were considered as the most appropriate outcome for establishing a HBGV and a benchmark dose lower confidence limit 10% (BMDL10) of 165 μg/kg bw per day for FB1 was derived.
10.1.3. Reproductive and developmental toxicity
In mice, rats and rabbits, embryotoxicity occurred only at doses paralleled by maternal toxicity, whereas in one study with Syrian hamsters exposed to high doses of FB1 it was also observed in the absence of maternal toxicity. Results of studies using culture material from fumonisin‐producing F. verticillioides and FB1 indicated that they are not teratogenic in rodents and rabbits.
Because FB1 had been shown in cultured cells to disrupt the high affinity folate transporter located in sphingolipid enriched rafts in the cell membrane, there was a concern that exposure to FB1 in pregnancy, particularly in combination with folate deficiency, could be linked to an increased risk of NTDs (Marasas et al., 2004; Gelineau‐van Waes et al., 2005; Voss et al., 2011). This was further investigated in mouse models using either LM/Bc mice (a sensitive strain), or CD‐1 mice which are less sensitive (Voss et al., 2017b). FB1 induced NTDs when given by either i.p. injection at doses of about 20 mg/kg bw per day or by gavage at gestation days (GD) 7.5 and 8.5 at doses of about 20 mg/kg bw per day. Treatment (i.p.) with FB1 at 20 mg/kg bw per day caused reduced folate uptake in embryos and placenta and folate supplementation partially reversed the incidence of NTDs. NTDs were induced in one feeding study conducted in mice using cultured Fusarium material, but a follow‐up study that also included higher doses of FBs was unable to confirm these results.
10.1.4. Genotoxicity
FB1 was not mutagenic in bacterial assays. In mammalian cells in vitro, unscheduled DNA synthesis was not observed, but FB1 caused chromosomal breaks in rat hepatocytes in one study. 8‐hydroxy‐2′‐deoxyguanosine (8‐OH‐dG) adduct formation following lipid peroxidation was observed in an in vitro study in C6 glioma cells and MEF cells exposed to FB1 (Mobio et al., 2003). Theumer et al. (2010) found that FB1 induced DNA single strand breaks and micronuclei in vitro and in vivo. These effects were paralleled by in vitro and in vivo increases of malondialdehyde and catalase. Overall, the available data support the hypothesis of oxidative stress mediated genotoxicity of FB1.
10.2. In vivo toxicity studies with FBs published after 2011
For studies reporting only concentrations of the toxin in the feed, doses have been calculated to mg or μg/kg bw per day following the respective EFSA guidance (EFSA, 2012; EFSA FEEDAP Panel, 2012).
Studies with i.p. or i.v. administration have not been described in this section, except in cases when they were informative with regards to hazard characterisation by the oral route as they do not reflect oral bioavailability and in consequence oral toxicity of fumonisins.
10.2.1. Subacute toxicity studies
Mice
A group of 10 Swiss mice (5 males, 5 females) was administered an oral dose of 110 μg FB1/kg bw for 7 days (Kouadio et al., 2013). Treated female but not male mice had lower weights than control animals. While serum triglycerides and creatinine were enhanced in both sexes, cholesterol and protein content was only increased in males. Alanine transferase (ALT), aspartate amino transferase (AST), gamma glutamyl transferase (GGT) and creatine kinases were not affected by treatment. Based on their results, the authors suggest, that the NOAEL for FB1 is lower than 110 μg/kg bw per day.
Rats
Abdel Salam et al. (2012) fed groups of 15 male rats with diets to which FB1 containing Fusarium culture material was added. Groups were exposed to diets containing no FB1 for 8 weeks (control), 10 mg FB1/kg (equivalent to 1.2 mg/kg bw) for 8 weeks and 30 mg FB1/kg (equivalent to 3.6 mg/kg bw) for 1, 4 or 8 weeks. Relative body weight gain and relative lung weight was reduced in the high‐dose animals at four and eight weeks. FB1 also induced dose and time dependent increase of various gross and microscopic lung lesions such as pulmonary congestion, alveolar oedema, focal areas of interstitial oedema, areas of haemorrhage, proliferation of alveolar cells, with inflammatory cellular infiltration and alveolar septal oedema. At 8 weeks, scattered areas of atypia and endothelial cell damage, distortion of alveolar epithelium and increased alveolar macrophages with apoptotic changes were also observed. The CONTAM Panel noted that based on reporting of the results it is in many instances unclear which effects were seen already at the low dose.
In order to investigate the effect of fumonisins on the developing enteric nervous system Sousa et al. (2014) fed groups of 10 male Sprague–Dawley rats from day 21 to 63 of age with diets containing 0.159 mg/kg FB1 and no FB2 that served as negative control, and mixtures of 0.996 mg/kg fumonisins (0.73 mg FB1 + 0.267 mg FB2) and 2.819 mg/kg fumonisins (2.1 mg FB1 and 0.719 mg FB2). Fumonisins were obtained by adding Fusarium verticillioides culture material. The concentrations were equivalent to 0.12 and 0.39 mg FB1+FB2/kg bw, respectively. Five of the animals per group were killed on day 15 after treatment and 5 on day 42. The treatment did not affect body weight or serum ALT and AST activities or neuronal density in jejunum, whereas a reduction of the cellular area of immunoreactive neurons in jejunum was seen. Based on their results, the authors conclude that food containing fumonisins negatively affects myenteric neurons.
In order to investigate the effect of FB1 on kidney, Venancio et al. (2014) fed groups of 8 Wistar rats with diets containing either no FBs or 6 mg FB1/kg (obtained by addition of Fusarium verticillioides culture material) equivalent to 0.7 mg FB1/kg bw, for 42 days. FB1 did not affect feed intake, body weight and growth, creatinine levels in plasma, water intake, osmolarity and urinary excretion of sodium while increased urine volume and potassium excretion was observed which was paralleled by mild tubulointerstitial changes in the outer kidney cortex.
Hahn et al. (2015) fed groups of four male Sprague–Dawley rats diets containing purified FB1 at 10 mg FB1/kg equivalent to 1.2 mg FB1/kg bw for 21 days. Urinary Sa/So ratios were measured on days 0, 7, 14 and 21 and were increased from day 7 until the end of the study. Elevated Sa/So levels were also seen in kidneys. In this study, modified forms of FB1 were also investigated. The results from these investigations are therefore presented in detail in Section 10.3.
Hahn et al. (2015) fed groups of four male Sprague–Dawley rats diets containing purified FB1 at 10 mg FB1/kg equivalent to 1.2 mg FB1/kg bw for 21 days. Urinary Sa/So ratios were measured on days 0, 7, 14 and 21 and were increased from day 7 until the end of the study. Elevated Sa/So levels were also seen in kidneys. In this study, modified forms of FB1 were also investigated. The results from these investigations are therefore presented in detail in Section 10.3.
Abdellatef and Khalil (2016) gave 0, 50, 100 and 200 mg FB1/kg diet (equivalent to 0, 6, 12 and 24 mg/kg bw per day, respectively) to groups of six or seven male Sprague–Dawley rats for 4 weeks. The source of the FB1 was a strain of Fusarium moniliforme culture material. Fumonisin‐treated animals showed DNA fragmentation and decreases in glutathione (GSH) content, superoxide dismutase (SOD) activity, and total antioxidant capacity (TAC) in liver and kidney most pronounced at the highest dose. Lactic acid bacterial co‐treatments had a protective effect. Because effects were observed at the lowest dose tested the lowest observed adverse effect level (LOAEL) is 6 mg/kg bw per day.
Pigs
Grenier et al. (2012) gavaged groups of six piglets with F. verticillioides culture material extracts at 2.8 μmol FB1/kg bw per day (equal to 2.0 mg FB1/kg bw per day) for 14 days. FB1 induced increases in serum albumin, total protein, cholesterol, triglycerides, fibrinogen and GGT, changes in cytokine expression in liver, nuclear vacuolisation of hepatocytes and megalocytosis, cytokines expression in the gastrointestinal tract and lesions in the intestine. The Sa/So ratios were 8‐ to 10‐fold and 28‐fold higher in plasma and liver, respectively than in the control group. In this study also modified forms of FB1 were investigated. The results from these investigations are therefore presented in detail in Section 10.4.
Bracarense et al. (2012) fed piglets with diets prepared from F. verticillioides culture material containing 5.9 mg FBs/kg feed (4.1 mg FB1 and 1.8 mg FB2/kg), estimated by the authors to correspond to 260 μg FBs/kg bw per day for 5 weeks. Effects observed were atrophy and fusion of villi, decrease of villi height, cell proliferation in the jejunum, reduced number of goblet cells and lymphocytes. Tumour necrosis factor (TNF)‐a, interleukin (IL)‐1b, interferon (IFN)‐g, IL‐6 and IL‐10 were upregulated in the ileum or the jejunum and expression of the adherent junction protein E‐cadherin and the tight junction protein occludin in the intestine was seen.
A group of 12 specific pathogen‐free (SPF) piglets were fed with a diet prepared with maize naturally contaminated with FB1 and FB2 for 9 weeks (Burel et al., 2013). The final concentration in the contaminated feed was 11.8 mg FBs/kg (8.6 mg/kg FB1 and 3.2 mg/kg FB2). This concentration is equivalent to a dose of 0.6 mg FB1+FB2/kg bw per day (0.43 mg FB1 and 0.17 mg FB2/kg bw per day). In treated animals, an increase in Sa/So ratio compared to control animals was seen.
In the study of Loiseau et al. (2015), six male piglets were gavaged once a day with culture material extracts containing 1.5 mg FB1/kg bw (obtained from maize inoculated with F. verticillioides) for 9 days. In treated animals, Sa/So ratios were increased in both lung and liver. While total ceramide content in lung decreased by half, total sphingomyelin content doubled over the control group while in the liver total ceramide increased 3.5‐fold over the control group and total sphingomyelins content was reduced by 50%, overall showing that changes in ceramide content are counterbalanced by changes in sphingomyelin.
10.2.2. Subchronic toxicity studies
Mice
Alizadeh et al. (2015) fed a group of 15 female mice (strain not reported) with diets containing 150 mg FB1/kg (equivalent to 30 mg/kg bw per day) for 16 weeks. The source and purity of the FB1 was not specified. Compared to control animals (n = 14) parietal cell number was reduced together with gastric body glandular cell height and atrophy in gastric mucosa in treated animals. The authors attributed the effects to the increased apoptosis and the suppressed mitotic activity that was observed in the respective tissue.
Pigs
Gbore (2013) gave diets containing 0.2 (control), 5.0, 10.0 and 15.0 mg FB1/kg (equivalent to approximately 0.01, 0.25, 0.5 and 0.75 mg FB1/kg bw per day) to groups of six piglets for 6 months. The diets were prepared using F. verticillioides culture material. Total protein concentrations in the cerebellum, hypothalamus and the medulla oblongata and serum protein were significantly increased at the two highest doses in these groups. Many of the effects reported were not dose‐dependent. The authors concluded that FB1 in feed at concentrations higher than 5 mg/kg diet interferes with protein metabolism.
10.2.3. Long term toxicity studies
Bondy et al. (2012) gave (via the diet) 0, 0.39, 3.87 and 12.2 mg FB1/kg bw to groups of 10 male wild‐type p53 mice (WT p53+/+) and 0, 0.37, 3.88 and 12.6 mg FB1/kg bw, respectively, to groups of 10 male transgenic p53 mice (TG, p53+/−) for 26 weeks. The latter strain has a high sensitivity towards genotoxic carcinogens. While FB1 had no effect on body weight in WT mice, it was decreased in the TG mice receiving the highest dose, from week 22 onwards. Although FB1 had no significant effects on liver weight in either strain, kidney weight was elevated in TG mice treated with the highest dose. Spleen weights were elevated in both strains at the highest dose while thymus weights remained unaffected. Neutrophils and B lymphocytes (CD3‐19+ cells) were increased in high‐dose TG mice as compared to controls. Total plasma immunoglobulin A (IgA) and IgM increased in both strains at the highest dose while no change was seen in IgG levels. Likewise was plasma ALT activity increased in both strains at the highest dose. Liver nodes were observed with both strains at the highest dose. In liver, incidence of necrosis, multinucleated cells, focal inflammation and megalocytic hepatocytes increased from the lowest to the highest dose in both strains. Megalocytic hepatocytes were observed at the lowest dose, increased with dose and were widespread at the high dose in both strains, where they occurred in aggregates or nodules that often contained extremely enlarged hepatocytes with hyperchromatic chromatin and in which eosinophilic and vacuolated cytoplasm, cell necrosis and apoptosis were frequently observed. Between these cells, there were small vacuolated hepatocytes, oval cells and Kupffer cells.
A single liver adenoma was seen in a WT mouse in the lowest dose group and in the high‐dose group two animals with cholangiomas and three with adenomas were seen. With TG mice, neoplasms were only observed at highest dose (two mice had two adenomas each and further two mice had one and in one mouse a single cholangiocarcinoma was detected). No treatment related lesions were observed in either strain in kidney, oesophagus, stomach, ileum, Peyer's patch, mesenteric lymph nodes, spleen, thymus, heart and lung. In liver, sphingosine levels were not affected by treatment while sphinganine levels were increased at the mid‐ and high dose in WT mice and at high dose in TG mice. DeoxySa levels were increased with mid‐ and high dose in both WT and TG mice. No changes were seen in So 1‐P while Sa 1‐P was increased at high dose in both strains. In kidney, So, Sa, deoxySa and So 1‐P levels were enhanced in both strains at mid‐ and high dose while Sa 1 P levels were only elevated at the high dose. Overall, the authors concluded that because the TG mice (that carry a mutation in a tumour suppressor gene and is more sensitive to genotoxic carcinogens) were not more sensitive with regard to induction of tumours than the WT mice, FB1 acts via a non‐genotoxic MoA. The authors calculated BMDs combining both strains for the incidences of hepatic apoptosis, megalocytic hepatocytes and hepatic Sa concentrations. The resulting BMDL10 as calculated by the authors ranged from 0.16–0.46 mg/kg bw per day for apoptosis and 0.15–1.11 mg/kg bw per day for megalocytic hepatocytes.
The CONTAM Panel notes that the JECFA used preliminary report of this study (Bondy et al., 2010) for their previous evaluation (FAO/WHO, 2012). As noted in the technical report from the most recent JECFA evaluation (FAO/WHO, 2017), the final study published in 2012 (Bondy et al., 2012) differs slightly in the incidence of lesions and pathology scores for megalocytic hepatocytes and for apoptosis due to the addition of four mice (one in the control group, one in the low‐dose group and two in the high‐dose group) and the fact that for one mouse in the mid‐dose group the pathology score was adjusted from zero to one. JECFA concluded that these slight differences would not change the overall previous toxicological assessment and retained the PMTDI derived from Bondy et al. (2010).
10.2.4. Genotoxicity studies
BALB/c mice received i.p. single dose or repeated injections of pure FB1 (0.1, 1.0 and 10 mg/kg bw). Controls and positive controls were injected with single doses of saline and mitomycin C, respectively. FB1 did not cause an increase in the frequencies of micronucleated erythrocytes in the BALB/c mice neither in single nor in multiple dose studies (Karuna and Rao, 2013).
10.2.5. Developmental studies
Pellanda et al. (2012) fed groups of Wistar rat dams diets containing 0.9 mg/kg folate, 0.04 mg/kg vitamin B12 and 2,100 mg/kg choline either without (control, n = 13) or with addition of pure FB1 at 4 μg/kg bw per day (n = 2) and to methyl‐deficient diets (MDD, 0.01 mg/kg folate, 0 mg/kg vitamin B and 0.06 mg/kg choline) either without (n = 15) or with addition of 4 μg FB1/kg bw per day (n = 3), for 1 month before mating. Dams were sacrificed on GD 20 and gravid uteri were removed. For analysis 2 of 13 (control), 3 of 15 (MDD), 2 FB1 and 3 MDD/FB1 animals were used. The number of fetuses derived from each group was 23 (control), 39 (MDD), 25 (FB1) and 25 (FB1/MDD). For analyses 8 fetuses per group were randomly selected for further analyses. A significant decrease in body weight was seen in fetuses of the MDD and MDD/FB1 groups. Decreased liver folate concentrations were seen in fetuses of the MDD and MDD/FB1 group but not in the FB1 group. In dams the combination of MDD and addition of FB1 aggravated the pericentrolobular steatosis seen in the MDD group. A decrease in folate receptor messenger RNA as compared to controls was seen in the FB1 group, reinforcing, according to the authors, the hypothesis that FB1 alters folate transport via interference with sphingolipid metabolism. Overall, based on their results, the authors concluded that low doses of FB1 interact with MDD. The CONTAM Panel noted that reporting of results in this study lacks clarity in several instances.
Groups of 50 female LM/Bc mice were fed either control or folate‐deficient diets for 5 weeks. On E7 and E8, 15 dams per group were then given i.p. injections of 0, 2.5, or 10 mg FB1/kg bw. Fetuses were examined on E16. In the offspring of the control fed diet animals, 3 and 10 litters were affected in the low‐ and high‐dose groups, respectively, whereas in the folate‐deficient groups, only 4 of a total 11 litters were affected at the high dose. In a second trial following a similar study design, the earlier findings were corroborated as fewer litters were affected by NTD at the high dose in folate‐deficient animals. Overall, the authors concluded that folate deficiency does not exacerbate FB1 induced NTD in LM/Bc mice (Voss et al., 2014).
Pregnant LM/Bc mouse dams were orally gavaged with pure FB1 for three consecutive days on embryonic day (ED) 6.5, 7.5 and 8.5. The doses were 0 (n = 4), 5 (n = 2), 10 (n = 2), 15 (n = 3), 25 (n = 3), and 50 (n = 2) mg/kg bw per day (Riley et al., 2015a). The frequency of exencephaly in the LM/Bc fetuses increased in a dose‐dependent manner. No NTDs were observed in the control‐treated (0/4) or the 5 mg/kg bw per day dosed groups (0/2). Exencephalic fetuses were detected in the litters from the three groups dosed orally with ≥ 10 mg/kg bw per day (8/11 litters).
10.2.6. Other studies
In order to investigate combined effects of FB1 and aflatoxin B1, Qian et al. (2016) fed groups of 13 rats normal diets for 56 days (control), diets containing FB1 (35 days normal diet followed by 21 days of 250 mg FB1/kg diet, equivalent to 30 mg/kg bw), or aflatoxin B1 (14 days of 150 μg aflatoxin B1/kg diet, equivalent to 18 μg/kg bw, followed by 42 days of normal diet), or FB1 and aflatoxin B1 (14 days of 18 μg aflatoxin B1/kg bw, followed by normal diet for 21 days and then by 21 days of 30 mg FB1/kg bw). A group given a single injection of 200 mg diethylnitrosamine (DEN)/kg bw followed by a normal diet for 14 days and then followed by a diet containing 200 mg/kg 2‐acetylaminofluorene (2‐AAF, equivalent to 24 mg/kg bw) for 21 days serving as a positive control. A series of serum parameters (e.g. total protein, AST, creatinine, ALP, cholesterol) were altered upon treatment. In liver, glutathione S‐transferase P+ (GSTP+) foci, not detected in the control and FB1 group, were induced with aflatoxin B1. This induction was even more pronounced in animals receiving both toxins. For numbers of foci per area a more than additive effect was seen with animals receiving both toxins sequentially.
10.2.7. Summary remarks on in vivo toxicity of FBs
The relevant toxicity studies with FBs published after the last comprehensive risk assessment available (FAO/WHO, 2012), are described/summarised in the present chapter. The results of these new studies confirm and further corroborate the hazard identification and characterisation of FBs described in previous assessments (SCF, 2000; FAO/WHO, 2001, 2012; EFSA, 2005; EFSA CONTAM Panel, 2014). Also available to the current Panel was the Technical Report of the 83rd JECFA meeting (FAO/WHO, 2017). The Fumonisin Addendum prepared at the 83rd JECFA meeting was not published at the time of drafting of this opinion. The CONTAM Panel reviewed both the toxicity studies already described and evaluated in previous assessments and the newly available studies (which are described in detail in the present chapter) and concluded that the study from Bondy et al. (2012) is the most appropriate investigation, based on endpoints investigated, study design, results and reporting to be used for derivation of a HBGV for FB1. The CONTAM Panel concluded further that the potentially most appropriate endpoints to be considered for calculation of a BMDL for FB1 were incidence of hepatic adenoma, focal hepatic inflammation, liver Sa concentration, incidence of multinucleated hepatocytes, hepatic single cell necrosis and megalocytic (karyocytomegalic) hepatocytes.
10.3. In vivo toxicity of modified FBs
In the previous section on in vivo toxicity of FBs, with two exceptions, only in vivo oral studies published after 2011 are presented in detail. For the present chapter, no time limit was applied for studies to be evaluated and presented in detail and all relevant information was considered, since in the recent JECFA evaluations on fumonisins (FAO/WHO, 2012, 2017) HBGVs were only established for FBs but not for their modified forms. In addition, studies with routes of administration other than oral were considered for this section as they are potentially of value for deriving relative potencies of modified forms based on comparisons with their respective parent compounds. In Table 3, in vivo toxicity studies on modified FBs or comparative in vivo studies with FBs and their modified forms in which pure compounds were applied are summarised. In Table 4, these in vivo toxicity data are listed compound by compound.
Table 3.
Summary of in vivo toxicity studies on modified fumonisin Bs or comparative in vivo studies with fumonisin Bs and their modified forms in which pure compounds were applied
| Study design | Results | Reference |
|---|---|---|
| Groups of 3–5 male Fischer rats given diets of 60 mg/kg bw of FB1, FB2, FB3, HFB2, TCA, MME or 120 mg/kg bw of FA1, HFB1 for 21 days (then sacrifice of part of the animals); then 2 weeks control diet; then 20 mg/kg bw per day 2‐AAF for 3 days; then 14 days control diet followed by sacrifice of remaining animals |
After 21 days (before 2‐AAF treatment): FB1, FB2, FB3, MME: bw loss FA1, HFB1, HFB2, TCA: no effect 14 days after AAF injection: FB1, FB2, FB3, MME: ↑hepatocyte nodules FA1, HFB1, HFB2, TCA: no effect |
Gelderblom et al. (1993) |
|
Brine shrimp exposed for 48 h to different concentrations of FB1, FB2, HFB1, HFB2, N‐palmitoyl‐HFB1 (C16:0‐HFB1), NCM‐FB1 |
Mean LC50 (in μM): FB1: 2.74; FB2: 4.78; HFB1: 17.92; HFB2: 11.83, N‐palmitoyl‐HFB1: 3.55; NCM‐FB1: 285.34 |
Hartl and Humpf (2000) |
| Groups of 8 female B6C3F1 mice given diets containing 0, 2.8, 14 and 28 μmoles of each FB1, FB2, FB3, FP1, HFB1, NCM‐FB1 and FA1/kg bw for 28 days |
FB1: ↑serum cholesterol, ↑ALP bile acids Sa/So ratios and liver histopathology at the two higher doses. All other compounds: no effect |
Howard et al. (2002) |
| Groups of 30–31 pregnant rats (strain not reported) given diets of 0, 15, 30, 60, 120 mg/kg bw HFB1 from GD 3 to 16 | Maternal NOAEL set at 15 mg/kg bw per day based on ↓ in food consumption/bw gain at higher doses. No effect on maternal Sa/So ratios in liver, kidney brain and on reproduction/development at any dose. Fetal NOAELs set at highest dose. | Collins et al. (2006) |
| Inbred LM/Bc mice injected (i.p.) with 0, 2.5, 5.0, 10, 20 mg HFB1 or 10 mg FB1/kg bw on E7 and E8; Dams sacrificed on either E9 or E16 |
No effect on bw of dams with any compound. FB1: ↑ liver weights. ↓ fetal and placental weight. All litters affected by NTD. Marked changes in sphingolipid metabolism HFB1: No changes in dams no litters affected and only slight effects on sphingolipid metabolism at highest dose |
Voss et al. (2009) |
| Groups of six piglets (Pietrain/Duroc/Large‐white) gavaged with 0 or 2.8 μM FB1 or HFB1/kg bw for 14 days |
FB1: Increases in series of serum parameters and histopathology in liver and GI tract. Sa/So ratios 8–10‐ and 28‐fold above control in plasma/liver. HFB1: No effects besides slightly altered cytokine expression in GI tract. HFB1: Sa/So ratios unchanged in plasma, 2‐fold higher in liver |
Grenier et al. (2012) |
| Groups of 4 male Sprague–Dawley rats were fed diets of 1.2 mg FB1, 0.94 mg pHFB1, 0.7 mg HFB1 and 1.5 mg NDF‐FB1/kg bw per day for 21 days |
FB1: ↑ urinary Sa/So levels increased. Marked histopathological effects in kidney. No changes in liver. Besides mild effects in kidney with pHFB1 and HFB1 no changes with other compounds |
Hahn et al. (2015) |
FB1: fumonisin B1; FB2: fumonisin B2; FB1: fumonisin B3; HFB1: hydrolysed FB1; HFB2: hydrolysed FB2; TCA: tricarballylic acid; MME: mono methylester of fumonisin B1 (artefact during isolation and storage of fumonisins in methanol); FA1: N‐acetylated FB1; 2‐AAF: 2‐acetylaminofluorene; ↑: increase(d); ↓: decrease(d); GD: gestation day; NCM‐FB1: N‐(carboxymethyl) FB1; LC50: median lethal concentration; i.p.: intraperitoneal; E: embryonic day; ALP: alkaline phosphatase; NOAEL: no observed adverse effect level; NTD: Neurotubule defects; Sa/So: sphinganine/sphingosine; GI: gastrointestinal; NDF‐FB1: N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB1.
Table 4.
In vivo toxicity data of modified fumonisin Bs and their modified forms in which pure compounds were applied, listed compound wise
| Compound | Study design | Dose producing effect or highest dose used in experiment | Reference |
|---|---|---|---|
| HFB 1 | Male rats, 120 mg/kg bw for 21 days, then 2 weeks control then 20 mg/kg bw per day 2‐AAF for 3 days; then 14 days control diet |
> 120 mg/kg bw per day (no effect observed) |
Gelderblom et al. (1993) |
| Brine shrimp, exposed for 48 h | 17.92 μM (LC50) | Hartl and Humpf (2000) | |
| Female B6C3F1 mice, 0, 2.8, 14 and 28 μmoles per day for 28 days |
> 28 μM kg/bw per day (no effect on serum parameters Sa/So ratio, pathology) |
Howard et al. (2002) | |
| Pregnant rats, 0, 15, 30, 60, 120 mg/kg bw per day from GD 3 to 16 |
30 mg/kg bw per day (↓ in food consumption in dams > 120 mg/kg bw per day for offspring (no effect) |
Collins et al. (2006) | |
| Mice, 0, 2.5, 5.0, 10, 20 mg/kg bw per day |
20 mg/kg bw per day (slight effect on sphingolipid metabolism) |
Voss et al. (2009) | |
| Piglets, 2.8 μM /kg bw for 14 days |
2.8 μM/kg per day (slightly altered cytokine expression Sa/So ratios unchanged in plasma, 2 fold higher in liver) |
Grenier et al. (2012) | |
| Male rats, 0.7 mg/kg bw per day for 21 days |
0.7 mg /kg bw per day (mild effects in kidney) |
Hahn et al. (2015) | |
| HFB 2 | Male rats, 60 mg/kg bw for 21 days, then 2 weeks control diet, then 20 mg/kg bw per day of 2‐AAF for 3 days; then 14 days control diet |
> 60 mg/kg bw per day (no effect observed) |
Gelderblom et al. (1993) |
| Brine shrimp, exposed for 48 h | 11.83 μM (LC50) | Hartl and Humpf (2000) | |
|
N ‐palmitoyl‐HFB 1 (C16:0‐HFB 1 ) |
Brine shrimp, exposed for 48 h | 3.55 μM (LC50) | Hartl and Humpf (2000) |
| NCM‐FB 1 | Brine shrimp, exposed for 48 h | 285.34 μM (LC50) | Hartl and Humpf (2000) |
FB1: fumonisin B1; HFB1: hydrolysed FB1; 2‐AAF: 2‐acetylaminofluorene; ↑: increase(d); ↓: decrease(d); GD: gestation day; NCM‐FB1: N‐(carboxymethyl) FB1; LC50: median lethal concentration.
10.3.1. Subacute studies
Mice
In order to investigate comparatively the toxicity of FBs and modified forms of FB1, Howard et al. (2002) fed, diets containing approximately 0, 14, 70 and 140 μmol/kg diet (equivalent to 0, 2.8, 14 and 28 μmol/kg bw) of each of seven purified compounds: FB1, FB2, FB3, FP1, HFB1, NCM‐FB1 and N‐acetyl‐FB1 (described as FA1 in Figure 2) to groups of female B6C3F1 mice for 28 days. The control group comprised of 16 and the treated groups of 8 animals, respectively. None of the compounds affected bw or food consumption. Significant increases of serum cholesterol, ALP, and total bile acids were seen at doses of 14 and 28 μmol/kg bw FB1. None of the other compounds affected these parameters. Liver ceramide levels decreased significantly in the animals treated with 14 and 28 μmol/kg bw FB1 and increased Sa/So ratios were seen in all groups treated with FB1. These parameters were not affected by the other compounds. Histopathology was carried out in liver, brain, heart, kidney, thymus and mesenteric lymph nodes and changes (hepatocellular apoptosis, macrophage pigmentation, centrilobular hypertrophy and cytoplasmatic vacuolisation and Kupffer cell hyperplasia) were only observed in livers of animals treated with medium and high doses (14 and 28 μmol/kg bw) of FB1. At the highest dose also the relative liver weight was increased. Based on their results the authors concluded that FB2, FB3, FP1, HFB1, NCM‐FB1 and N‐acetyl‐FB1 tested in this study must be at least twofold less toxic than FB1.
Rats
Voss et al. (1996) fed groups of 10 Sprague–Dawley rats with diets containing 8 or 71 mg FB1/kg (equivalent to doses of 1 and 8.5 mg FB1/kg bw per day) or 58 mg/kg HFB1 (equivalent to 7 mg/kg bw per day, containing no detectable amounts of FB1) for 4 weeks, aiming at evaluating the influence of nixtamalisation of diets on FB toxicity. The diets were prepared using F. verticillioides culture material with and without nixtamalisation. A control group received a diet containing < 0.5 mg FB1/kg and < 0.002 mg/kg aflatoxin (not further specified). No changes in serum liver parameters were seen in controls and low‐dose FB1 group. ALT, AST and AP were increased in the HFB1 group while with the high FB1 group all serum liver parameters (ALT, AST, AP, GGT, cholesterol, triglycerides and bilirubin) were significantly increased. Relative kidney and liver weights were increased (compared to control) in the low FB1‐ and HFB1‐treated animals while in high FB1 animals only relative kidney weight was increased. The authors report minimal signs of hepatopathy in the low‐dose FB1 animals while clear signs of hepatopathy were observed in the high‐dose FB1 and HFB1 group, albeit to a much lesser extent in the latter (signs of hepatopathy were not further specified). Nephrotic lesions were observed in all treated groups without significant differences in their extent between the groups.
Voss et al. (1998) fed groups of 10 Sprague–Dawley rats with diets devoid of FB1 and HFB1, diets containing 11.1 μmol/kg FB1 (containing trace amounts of FB2), 98.5 μmol/kg of FB1 and 143 μmol/kg HFB1 (containing no FB1 and FB2) for 4 weeks. These concentrations are equivalent to doses of 0, 1.3 and 12 μmol FB1/kg bw per day and 17.2 μmol HFB1/kg bw per day. The diets were prepared using F. verticillioides culture material with and without nixtamalisation. Body weight, serum chemistry and liver and kidney effects did not differ between the FB1 and HFB1 diets (details not reported). Sa/So ratios in the control groups in kidney and liver were 0.19 and 0.49 respectively, increasing to 8.81 and 0.68 in the low‐dose (1.3 μmol FB1/kg bw per day) group (only levels in kidney differing significantly) increasing further in the high‐dose FB1 group (12 μmol/kg bw per day) to 15.0 and 4.85, respectively (significantly differing from control in both organs). Corresponding values in the HFB1 (17.2 μmol/kg bw per day) group were 11.0 and 1.51 in the kidney and liver, respectively (significantly differing from control group in both organs). The authors conclude that their results provide further evidence that inhibition of CerS may be a key event in toxicogenesis of fumonisins and related compounds noting that that their results need to be verified in studies using purified HFB1. The CONTAM Panel noted that based on the design of the study (in particular dosing) it was not possible to accurately estimate relative potency of HFB1 with regard to inhibition of CerS but the experiment suggests that HFB1 can disrupt sphingolipid metabolism in liver and kidney, albeit to a lesser extent than FB1.
In order to assess the impact of nixtamalisation on FB1 toxicity, Burns et al. (2008) fed uncontaminated maize, nixtamalised uncontaminated maize, contaminated maize or nixtamalised contaminated maize to seven groups of eight male Sprague–Dawley rats for 1 week (3 rats per group) or 3 weeks (5 rats per group). The uncontaminated (UC) diet contained 0.2 and 0.18 μmoles FB1 and HFB1/kg, respectively, approximately equivalent to 0.024 μmoles FB1/kg bw per day and 0.022 μmoles HFB1/kg bw per day. The other groups received diets resulting in FB1/HFB1 doses equivalent to 0.004/0.026 (nixtamalised uncontaminated, NUC), 1.5/0.07 (contaminated, CM, positive control), 0.35/0.38 (nixtamalised contaminated, NCM), 0.0083/0.5 (nixtamalised mixture of CM and ground corn, NCMC), 0.2/0.16 (sham nixtamalised9 CM, SCM) and 0.19/0.11 μM/kg bw per day (sham nixtamalised mixture of CM and ground corn, SCMC) of FB1 and HFB1, respectively. The source of the FB was F. verticillioides culture material. No differences were found in the different treatment groups with regard to body weight and relative kidney or liver weights. Kidney lesions (apoptosis, effects on mitosis, tubule regeneration and necrosis) were observed in the CM group. The severity of these lesions was reduced in nixtamalised CM (NCM, SCM). Total Sa levels in the different groups after 3 weeks were as follows CM = NCM > SCM = SCMC > NCMC > NUC = UC. Overall, the authors concluded that the fate of FB1 (besides the obvious conversion to HFB1) after nixtamalisation remains to be fully elucidated but that the method obviously reduces FB1 toxicity. The CONTAM Panel noted that the materials containing the highest amounts of FB1 (CM, NCM) produced the most pronounced effects on Sa levels while those seen with the diet containing relatively high amounts of HFB1 (NCMC) was very similar to that of the control. The CONTAM Panel also notes that in the experiment Fusarium maize cultures were used and that it cannot be excluded that other fumonisins or other mycotoxins (not tested) were present in the diet. In addition, in all groups FB1 and HFB1 were present, albeit in strongly varying amounts. Thereby any effects cannot be conclusively attributed to any specific compound.
Hahn et al. (2015) fed groups of four male Sprague–Dawley rats diets containing 0 (control group) or 10 mg/kg of FB1 (purity 97.2%, containing 1.3% FB3, 0.6% pHFB1a, 0.9% pHFB1b), 7.8 mg/kg pHFB1 (containing 3 mg/kg pHFB1a (purity 73.2%, containing 26.8% pHFB1b) and 4.8 mg/kg pHFB1b (purity 93.0%, containing 7.0% pHFB1a)), 5.6 mg/kg HFB1 (no impurities) and 12.2 mg/kg NDF‐FB1 (containing 2.5% FB1) for 21 days. These concentrations are equivalent to doses of 1.2 mg FB1, 0.94 mg pHFB1, 0.7 mg HFB1 and 1.5 mg NDF‐FB1/kg bw per day. Urinary Sa/So ratios were measured on days 0, 7, 14 and 21 and were significantly increased in the FB1 group from day 7 until end of the study. No changes were observed in the other groups. Significantly elevated Sa/So levels were seen in kidneys in the FB1 group (not seen in the other groups). In none of the groups was body weight affected and only minimal histopathological effects were observed in liver (not specified further). Effects in kidney were mild with HFB1 and pHFB1 while these were significantly elevated with FB1 (nature of effects were not described). Urine and faeces were collected on days 0, 7, 14 and 21. In urine, only FB1 and NDF‐FB1 were recovered and in similar amounts at days 7–21 while no other modified forms were recovered. In faeces of the FB1 group, considerable amounts of FB1, pHFB1 and traces of HFB1 were recovered on days 7–21. In the NDF‐FB1 group, significant amounts of FB1 were recovered on days 7–21. Based on their results, the authors concluded that NDF‐B1 is partly cleaved in the intestine to FB1 but as it is excreted via faeces it is not of toxicological relevance which was confirmed by the unaltered Sa/So ratio in urine seen with this compound. They also concluded that, overall, the modified forms of FB1 investigated in this study are of much lower toxicological relevance than FB1.
Pigs
In order to compare toxicity of FB1 and HFB1, Grenier et al. (2012) gavaged groups of six piglets with 0 and 2.8 μM/kg bw per day of FB1 and HFB1 for 14 days. FB1 induced increases in serum albumin, total protein, cholesterol, triglycerides, fibrinogen and GGT, changes in cytokine expression in liver, nuclear vacuolization of hepatocytes and megalocytosis, cytokines expression in the gastrointestinal tract and lesions in the intestine. No differences compared to the control were seen with HFB1 except for slightly altered cytokine expression in the intestine (mesenteric lymph nodes). The Sa/So ratios in animals treated with FB1 were 8‐ to 10‐fold and 28‐fold higher in plasma and liver, respectively, compared to the control group while in the HFB1 animals Sa/So ratios were not affected in plasma and were twofold higher in liver as compared to controls. The authors concluded that their results further corroborate that HFB1 is much less toxic compared to FB1. They noted that the toxicity attributed to HFB1 in studies using nixtamalised material could be in fact mediated instead by residual pHFB1 or by matrix bound FB1.
10.3.2. Developmental studies
Mice
In order to compare the potential of HFB1 and FB1 to induce NTD and to alter sphingolipid biosynthesis Voss et al. (2009) applied purified FB1 and HFB1 i.p. 0, 2.5, 5.0, 10 and 20 mg HFB1/kg bw per day and 10 mg FB1/kg bw per day to groups of LM/Bc mice dams (n = 8 to 10 per group) on embryonic day (E)7 and 8. Half of the animals were killed on E9 the rest on E16. Implantation sites were counted on E9 and weighed on E16. Uteri and fetuses of dams killed on E16 were examined. Treatment had no effect on body weight or body weight gain of the dams. Except for increased relative liver weights in the FB1 group treatments had no effect on organ weights. While FB1 caused liver toxicity this was not the case in HFB1 dams. HFB1 had no effect on fetal and placental weights while these were decreased in the FB1 group. NTDs were not found in either the litters of control or the high‐dose (20 mg/kg bw per day) HFB1‐treated dams (8–10 litters per group), whereas, in the 10 mg/kg bw per day FB1‐treated dams, all 10 litters examined had at least one NTD affected fetus. While the highest dose of 20 mg/kg bw per day of HFB1 had only slight effects on sphingolipid metabolism these effects were marked in the FB1 dams. It is notable that on E9 Sa levels were about 55‐fold higher than those of the control while at E 16 these were about twofold the control. Based on their results, the authors concluded that hydrolysed fumonisins are less toxic than their parent compounds and not a significant risk factor for NTD.
Rats
Collins et al. (2006) orally gavaged groups of 30–31 pregnant rats from GD 3 to 16 with doses of 0, 15, 30, 60 or 120 mg/kg bw of purified HFB1 (designated as AP1 in the publication). HFB1 decreased feed consumption and weight gain of dams but did not affect reproductive indices or fetuses. HFB1 did not affect Sa/So ratios in maternal liver, kidney or brain. Based on the results, a maternal NOAEL of 15 mg/kg bw per day and a fetal NOAEL of 120 mg/kg per day were established. The authors note that in a previous investigation with FB1 in pregnant rats significant increases in Sa/So ratios in liver, serum and kidney have been observed at doses of 50 mg/kg bw per day.
10.3.3. Other studies
In order to test the hypothesis that FB1 and its modified form could be initiators of carcinogenesis, Gelderblom et al. (1993) fed groups of 3–5 male Fisher rats with diets containing 500 mg/kg of purified FB1, FB2, FB3, HFB2 (designated as AP2 in the paper), TCA and MME10 or 1,000 mg/kg of purified FA1 and HFB1 (designated as AP1 in the paper) for 21 days. These concentrations are equivalent to 60 mg/kg bw per day of FB1, FB2, FB3, HFB2 and TCA or 120 mg/kg bw per day of FA1 and HFB1 (a group of eight animals receiving none of the test compounds was used as a negative control). After 21 days consuming, the diets all animals received a control diet for 2 weeks, then were given 20 mg/kg bw of 2‐AAF for 3 days and then were partially hepatectomised the day after the last treatment with 2‐AAF. Animals were either sacrificed 21 days after the start of the study or 14 days after the first 2‐AAF treatment. After 21 days of diet, body weight loss was observed in FB1‐, FB2‐, FB3‐ and MME‐treated animals while no effects were seen in animals treated with HFB1, HFB2 and TCA. In the FA1 treatment group, body weight gains were positive but slightly and significantly lower than the control group. Fourteen days after the first 2‐AAF treatment, hepatocyte nodules were observed in all remaining FB1‐, FB2‐ FB3‐ or MME‐treated animals but in none of the animals receiving FA1, HFB1, HFB2 or TCA. The authors concluded from their results that while FB1, FB2, FB3 and MME exhibited cancer initiating potential in the liver following 2‐AAF treatment. FA1, HFB1, HFB2 and TCA did not exert such a potential under the conditions of their experiment.
In order to investigate influence of nixtamalisation and nutrients on FB1 toxicity, more specifically on its cancer promotor potential Hendrich et al. (1993) injected 8 groups of six 10‐day‐old male F344/N rats with 15 mg DEN/kg bw. The rats where then exposed for 4 weeks to 45 and 48 mg FB1/kg diet (equivalent to 5.4 and 5.8 mg FB1/kg bw per day) or to 7.6 and 10.7 mg HFB1 (equivalent to 0.9 and 1.3 mg HFB1/kg bw per day). Animals exposed to 1.3 mg HFB1/kg or 0.9 mg HFB1/kg bw per day presented similar effects on body weight, relative liver weight and plasma glutamate‐pyruvate transaminase (GPT) as animals exposed to 5.4 and 5.8 mg FB1/kg bw per day. While in animals given diets devoid of FB1 or HFB1 neither liver adenomas or cholangiomas were found, incidences were 83% and 33% and 100% and 50% in the 5.8 mg and 5.4 mg FB1/kg bw per day group and 15% and 33% and 67% and 17% and in the 1.3 mg and 0.9 mg HFB1/kg bw per day groups. The authors concluded that the major toxic product of FB1 upon nixtamalisation is HFB1 but that it cannot be excluded that other breakdown products/metabolites not analysed/detected play a role in the effects seen. The CONTAM Panel noted that in the experiment Fusarium maize cultures were used and that therefore it cannot be excluded that other fumonisins (not analysed) were present in the diet. The CONTAM Panel also notes that the diets given in this study varied in their nutrient composition which might hamper comparative toxicity evaluation and notes further that that severity of effects seen with different doses of FB1 and HFB1 are not clearly dose related suggesting a possible impact of differences in diet and, more likely, the presence of other toxic compounds. Notably, the diet with the second highest concentration of HFB1 contained also significant amounts of FB1.
In order to study the cancer promotor potential of FB1 and its modified forms, Liu et al. (2001) injected 80 ten‐day‐old female F344/N rats i.p. with 15 mg/kg bw of DEN. At 4 weeks of age, the animals were divided into groups of 20 animals, receiving diets without addition of fumonisins, a diet containing 25 mg/kg of a ‘FB1‐glucose adduct’ (equivalent to approximately 3 mg/kg bw per day), and diets containing 8 and 25 mg/kg purified FB1 (equivalent to approximately 1 and 3 mg/kg bw per day). At 9 weeks of age 4, at 12 weeks another 5 and at 20 weeks of age all of the remaining rats of each group were killed. Treatment did not have any effect on body weight and relative liver weight in any group at any time point. In comparison with the control or FB1‐glucose adduct‐treated animals, rats given FB1 had increased ALT activity at 9 and 20 weeks, increased endogenous hepatic prostaglandin E2 and lower plasma cholesterol at 20 weeks placental glutathione S‐transferase (PGST)‐positive and (GGT)‐positive altered hepatic foci (AHF) occurred in rats given the high dose of FB1 at 20 weeks. Sa/So ratios in the liver were increased only in the high‐dose FB1 group at weeks 12 and 20 (3.5 and 0.8 vs 0.9 and 0.15 in the control group). Based on their results, the authors concluded that modification of FB1 with glucose prevents hepatotoxicity and they note that alteration of the Sa/So ratio was not the most sensitive biomarker of FB1. The CONTAM Panel notes that this study suffers from a lack of analytical characterisation of the ‘glucose adduct’. Normally, reaction of FB1 with glucose would result in formation of NDF‐FB1 or NCM‐FB1. Since the material tested in this study is poorly defined in the publication, the relevance of the results is unclear.
Hartl and Humpf (2000) exposed brine shrimp to different concentrations of purified FB1, FB2, HFB1, HFB2, N‐palmitoyl‐HFB1 (C16:0‐HFB1) and NCM‐FB1. The purified compounds were dissolved in seawater and then diluted into brine shrimp solution or in the case of N‐palmitoyl‐HFB1, dissolved into ethanol–dodecane (98:2) and then dissolved in seawater and finally diluted into brine shrimp solution. Mean LC50 values calculated by Probit analysis (in μM) were 2.74 (FB1), 4.78 (FB2), 17.92 (HFB1), 11.83 (HFB2), 3.55 N‐palmitoyl‐HFB1 and 285.34 (NCM‐FB1). The authors noted that the toxicity of N‐palmitoyl‐HFB1 is in the range of parent FBs which is in accordance with previous findings in cultured cells as is the comparatively low toxicity of NCM‐FB1 found in their study.
10.3.4. Summary remarks on in vivo toxicity of modified FBs
In Table 3, an overview about the comparative toxicity studies with FBs and their modified forms where pure compounds have been used is presented.
In Table 4, the doses of modified FBs producing effects, or the highest doses used in the different experiments are presented for each of the modified forms tested in studies where pure compounds have been used.
In summary, several repeated dose studies are available where the toxicity of FBs and their modified forms have been investigated. While in principle these studies should facilitate comparison of their relative toxicity, this comparison is hampered in practice because of the design of these studies. Some of the limiting factors are the use of fermented culture material instead of purified compounds, use of different doses among studies, use of a single dose levels for parent compound and modified form, choice of dose levels at which either no effects are seen or the response saturated in treated animals and insufficient documentation of the effects.
The modified form most studied for comparison to FB1 was HFB1. For the most part, either very marginal or no effects were observed for HFB1 at doses that caused clear effects with FB1. These findings suggest that HFB1 is devoid of or exerts only marginal toxic potency, albeit having similar toxic effects. Upon reviewing the available evidence, the CONTAM Panel concludes that HFB1 has a similar toxic profile but is of lesser toxic potency than FB1, albeit that based on the data available the actual potency cannot be accurately quantified. The information on other modified forms (NCM‐FB1, pHFB1, NDFB1) obtained in the in vivo studies presented above also suggest a similar toxic profile and likewise lesser toxic potency but as it is even much more limited than the data on HFB1 it is not possible to accurately estimate the relative toxicity of these modified forms as compared to the parent compound.
10.4. In vitro toxicity of fumonisin Bs
Earlier evaluations dealt only with the toxicity of FB1 (SCF, 2000) and FB1, FB2 and FB3 (SCF, 2003; FAO/WHO, 2012). Based on in vitro evidence the overall conclusion for FB1 was that there is ‘no adequate evidence that FB1 is genotoxic’ (SCF, 2000) and that it is ‘probably not genotoxic’ (FAO/WHO, 2012).
The SCF also considered the results of a comparative study of the fumonisins B1, B2 and B3 with respect to their relative cytotoxicity to primary rat hepatocytes (Gelderblom et al., 1993).
Furthermore, almost equal cytotoxicity was found for the FB1 and FB2 when tested in seven different rat hepatoma cell lines and in one dog kidney cell line (Shier et al., 1991).The SCF also noted that in primary rat hepatocytes, FB2 was as effective as FB1 in inhibiting the de novo biosynthesis of sphingolipids (Wang et al., 1991; Norred et al., 1992).
FB1, FB2, FB3 and FB4, tested at 0.05, 0.5 and 5 μM, exhibited approximately equipotent inhibition of CerS (Norred et al., 1997). Almost equal cytotoxicity was found for FB1 and FB2 when tested in a total of seven different rat hepatoma cell lines and in one dog kidney cell line (Shier et al., 1991). Overall, FB1 is moderately cytotoxic when tested in rat hepatocytes, rat hepatoma cells, pig and dog kidney cells and chicken macrophages (Eriksen and Alexander, 1998; Ribeiro et al., 2010).
FB1 has been considered as immunotoxic at concentrations of 10 μM as it reduced cell proliferation in mononuclear cells of pigs (Marin et al., 2007) and decreased IL‐4 and increased IFN‐γ synthesis in mononuclear cells of pigs and humans (Taranu et al., 2010).
Furthermore, the SCF considered it unlikely that FB1 causes developmental effects in humans even when considering embryotoxic effects observed in vitro and considering the worst–case scenario of complete transfer of FB1 through the human placenta (SCF, 2000). However, JECFA concluded that based on dose–response studies using mouse embryos (3–5 somite stage) that the no effect level for NTDs, was 1 μM FB1 (FAO/WHO, 2012).
10.4.1. In vitro toxicity of modified fumonisin Bs
For the present chapter, no time limit was applied for in vitro studies to be evaluated and presented in detail since in the recent JECFA evaluation on fumonisins in 2011 in vitro studies with modified FBs were not discussed (FAO/WHO, 2012). From the few in vitro studies available, toxic potencies (on a molar basis) of modified FBs relative to FB1 are summarised in Table 5. For the structures of the different modified FBs, see Figure 3 and for the abbreviations Table 1 of Section 4
Table 5.
In vitro potencies of modified fumonisin Bs relative to fumonisin B1
| Compound | Test system | Endpoint | Concentrations tested (incubation time) | Relative potency | Reference |
|---|---|---|---|---|---|
| HFB s and pHFB s | |||||
| HFB1 | Caco‐2 cells | Cell viability |
1.25, 2.5, 12.5, 25 μM (48 h) |
n.a. | Caloni et al.(2002) |
| HFB1 | HT29 cells | Cell viability |
1, 10, 50 μM (24 h) |
0.2 | Schmelz et al. (1998) |
| HFB1 | Turkey lymphocytes | Cell viability |
0.02–50 μMa (72 h) |
0.1 | Dombrink‐Kurtzman (2003) |
| HFB1 | Rat embryos | Neural tube development |
3, 10, 30, 100, 300 μM (45 h) |
0.01 | Flynn et al. (1997) |
| HFB1 | Precision‐cut rat liver slices | Sa concentrations, Sa/So ratio |
0.05, 0.5, 5 μM (2 h) |
0.2–0.7b | Norred et al. (1997) |
| HFB1 | Primary rat hepatocytes | LDH release |
125, 250, 500, 1,000 μM (48 h) |
0.2 | Gelderblom et al. (1993) |
| HFB2 | Primary rat hepatocytes | LDH release |
125, 250, 500, 1000 μM (48 h) |
0.2 | Gelderblom et al. (1993) |
| HFB2 | Precision‐cut rat liver slices | Sa concentrations, Sa/So ratio |
0.05, 0.5, 5 μM (48 h) |
0.2–0.9b | Norred et al. (1997) |
| HFB3 | Precision‐cut rat liver slices | Sa concentrations, Sa/So ratio |
0.05, 0.5, 5 μM (2 h) |
0.1–0.7b | Norred et al. (1997) |
| pHFB1 | Caco‐2 cells | Cell viability |
1.25, 2.5, 12.5, 25 μM (48 h) |
n.e. | Caloni et al. (2002) |
| pHFB2 | Caco‐2 cells | Cell viability |
1.25, 2.5, 12.5, 25 μM (48 h) |
n.e. | Caloni et al. (2002) |
| NCM‐FBs and NDF‐FBs | |||||
| NCM‐FB1 | Vero cells | Cell viability |
1.25, 2.5, 5, 25, 50, 100 μM (24 h) |
0.02 | Meca et al. (2010) |
| N‐fatty acyl‐FBs and N‐fatty acyl‐HFBs | |||||
| C16:0‐HFB1 and C24:1‐HFB1 | HT29 cells | Cell viability |
25 μM (24 h) |
n.a. | Seiferlein et al. (2007) |
| C16:0‐HFB2 and C24:1‐HFB2 | HT29 cells | Cell viability |
25 μM (24 h) |
n.a. | Seiferlein et al. (2007) |
| C16:0‐FB1, C18:0‐FB1 and C24:1‐FB1 | Hek, Hep3B, fibroblasts | Membrane integrity assay |
20 μM (8 h) |
10 | Harrer et al. (2013) |
| C16:0‐HFB1 | HT29 cells | Cell death |
1, 5, 50 μM (24 h) |
10 | Humpf et al. (1998) |
| C16:0‐HFB1 | 3T3 cells | Cell proliferation |
Concentrations tested not provided (72–120 h)d |
15c | Abou‐Karam et al. (2004) |
| C16:0‐HFB1 | KA31T cells | Cell proliferation |
Concentrations tested not provided (72–120 h)d |
8c | Abou‐Karam et al. (2004) |
| C16:0‐HFB1 | MDCK cells | Cell proliferation |
Concentrations tested not provided (72–120 h)d |
10c | Abou‐Karam et al. (2004) |
| C16:0‐HFB1 | H4TG cells | Cell proliferation |
Concentrations tested not provided (72–120 h)d |
5c | Abou‐Karam et al. (2004) |
n.a.: data not adequate for derivation of relative potency; n.e.: no effects on cell viability observed; HFB1: hydrolysed FB1; HFB2: hydrolysed FB2; HFB3: hydrolysed FB3; pHFB1: partially hydrolysed FB1; pHFB2: partially hydrolysed FB2; NCM‐FB1: N‐(carboxymethyl) FB1; NDF‐FB1: N‐(1‐deoxy‐d‐fructos‐1‐yl)‐FB1; Sa/So: sphinganine/sphingosine; Caco‐2: human epithelial colorectal adenocarcinoma cell line; HT29: human colonic cell line; Hek: human embryonic kidney cell line; Hep3B: human hepatoma cell line; 3T3: mouse fibroblast cell line; KA31T: mouse fibroblast cell line; MDCK: canine kidney epithelial cells; H4TG: rat hepatoma cells; LDH: lactate dehydrogenase.
Concentrations tested cannot be deduced from the provided figure;
Effects not concentration dependent;
Calculated from IC50 values;
Precise time not specified.
Hydrolysed fumonisin Bs
HFB1, HFB2 and HFB3 (tested at 0.05, 0.5 and 5 μM) were only 30–40% as potent as the parent compounds to inhibit CerS, measured as Sa concentration and as Sa/So ratio in precision cut liver slices in vitro (Norred et al., 1997). The potency relative to FB1 was not dose dependent but rather varied at the different concentrations tested (see Table 5).
HFB1 did not affect cell viability in differentiated Caco‐2 cells at the highest tested concentration of 25 μM suggesting a low toxicity of HFB1 for intestinal cells. No HFB1 was detected in the cells at a concentration of 2.5 μM while it could be detected intracellular when exposure concentrations were exceeding 12.5 μM (Caloni et al., 2002).
HFB1 was five times less potent (50 μM) than FB1 (10 μM) to reduce cell number of HT‐29 (human colorectal adenocarcinoma cell line) cells by 30% (Schmelz et al., 1998).
HFB1 was 10 times less potent than FB1 based on the IC50 values of 1 μM versus 10 μM to decrease cell proliferation in turkey peripheral blood lymphocytes (Dombrink‐Kurtzman, 2003).
HFB1 (100 μM) was 100 times less potent than FB1 to induce NTDs and inhibition of overall embryonic growth and development in cultured rat embryos (Flynn et al., 1997).
At high concentrations of 125 μM HFB1 and HFB2 were less toxic than FB1 and FB2 to primary rat hepatocytes with lactate dehydrogenase (LDH) leakage as the endpoint (Gelderblom et al., 1993).
Overall, based on the above reported studies, the potency relative to FB1 of HFB1, HFB2 and HFB3 ranged from 0.01 to 0.9 (see Table 5).
Notably, it has been shown that also HFB1 is taken up by cells more rapidly and completely than FB1 although not to the same extent as N‐fatty acyl conjugates (Harrer et al., 2013).
Partially hydrolysed fumonisin Bs
The partially hydrolysed metabolites pHFB1 and pHFB2 (a and b isomers not specified) did not induce any significantly decrease of cell viability in differentiated Caco‐2 cells at concentration of up to 32.5 μM. However, pHFB1 and pHFB2 were not detected within the cells at any concentration tested (< 32.5 μM) (Caloni et al., 2002). Therefore, no relative potency can be derived from this study (see Table 5).
N‐(carboxy methyl)‐fumonisin Bs and N‐(1‐deoxy‐d‐fructos‐1‐yl)‐fumonisin Bs
NCM‐FB1 (tested in the range 1–100 μM) was approximately 50 times less cytotoxic for Vero cells (monkey kidney cells) than FB1 (Meca et al., 2010). No studies with NDF‐FBs were identified.
O‐fatty acyl fumonisins
No in vitro investigations with O‐fatty acyl fumonisins were identified.
N‐fatty acyl fumonisins
N‐fatty acyl fumonisins with various fatty acid chain length (C16:0‐FB1, C18:0‐FB1, C24:1‐FB1) (20 μM) were cytotoxic to human embryonic kidney (Hek) and human hepatoma (Hep3B) cells, and human fibroblasts showing ca. 10 times higher relative potency than FB1 (Table 5). The N‐fatty acyl conjugates are much more rapidly accumulated and taken up in Hek cells than FB1 (Harrer et al., 2013).
The N‐acyl hydrolysed fumonisins acylation products C16:0‐HFB1, C24:1‐HFB1, C16:0‐HFB2 and C24:1‐HFB2 were cytotoxic to HT29 (human colonic cell line) cells at concentrations of 25 μM. C16‐HFB1 and C24:1‐HFB1 caused a 50% reduction in the number of viable cells following 24‐hour exposure while C16‐HFB2 and C24:1‐HFB2 caused only a 30% reduction of cell viability indicating lower toxicity. These results indicate that the N‐fatty acylated metabolites may be slightly more potent compared to FB1 and HFB1 (Seiferlein et al., 2007). N‐palmitoyl‐HFB1 (C16:0‐HFB1) significantly reduced the cell number of HT29, at concentrations of 1 μM and higher and was at least 10 times more potent than FB1 or HFB1 (Humpf et al., 1998).
N‐fatty acyl fumonisins (chain lengths from C2 to C16) of HFB1 showed higher cytotoxicity for the longer chain acylation products in two different mouse fibroblast cell lines (3T3 and KA31T), canine kidney epithelial (MDCK) cells and rat hepatoma (H4TG) cells when compared with HFB1 with IC50s ranging from 80 μM to 6.25 μM (Abou‐Karam et al., 2004).
N‐palmitoyl‐HFB1 (C16:0‐HFB1) did not induce apoptosis in human proximal tubule‐derived (IHKE) cells at concentrations of up to 25 μM (Seefelder et al., 2003).
10.4.2. Summary remarks on in vitro toxicity of modified forms
The various modified forms exhibit different toxicities in vitro compared with FB1.
N‐fatty acyl FB1, N‐fatty acyl HFB1 and N‐fatty acyl HFB2 show an in vitro toxicity of up to 10 times higher as compared with FB1. Notably, it has been shown that some N‐fatty acyl conjugates accumulated more rapidly and to a greater extent than FB1 in cells. The relevance of the increased cytotoxicity found with these compounds for the in vivo situation in humans is unclear.
For pHFB1 and pHFB2, relative in vitro potencies as compared with FB1 could not be established as no effects were observed.
For HFB1, relative in vitro potencies vary between 0.01 and 0.7 in the different studies. For HFB2, relative potencies vary between 0.1 and 0.9 and the respective factors for HFB3 range from 0.1 to 0.7 depending on which doses are compared. Notably, it has been shown that also HFB1 is taken up more rapidly and completely than FB1 although not to the same extent as N‐fatty acyl conjugates.
NCM‐FB1 has a relatively low relative potency of only 0.02 as compared to FB1.
The in vitro results are inconsistent, highly dependent on which doses are compared and their relevance for human hazard characterisation is unclear taking into account also the importance of toxicokinetics.
Hence, the available in vitro data on modified FBs do not allow extrapolations to the human in vivo situation and therefore no final conclusions can be drawn from these data.
11. Observations in humans
In the following text on human observations, information published prior to 2011 has been taken from previous evaluations by the SCF (SCF, 2000) and JECFA (FAO/WHO, 2001, 2012).
11.1. Cancer
11.1.1. Oesophageal cancer
In its opinion on FB1, the SCF (2000) noted that there were early epidemiological studies from South Africa and China that indicated that there might be an association between the intake of FB1 and increased incidence of oesophageal cancer (Rheeder et al., 1992; Chu and Li, 1994; IARC, 1993; van Jaskiewicz et al., 1987a; Scott et al., 1995; Marasas et al., 1979, 1981, 1988; Sydenham et al., 1990a,b; Zhen et al., 1984; Yoshizawa et al., 1994), whereas in other studies carried out in Italy such a correlation was not found (Logrieco et al., 1995; Pascale et al., 1995; EHC, 2000). The SCF concluded that the available studies, mostly of ecological design, were inconclusive. JECFA, in its 2000 evaluation of fumonisins, including a few additional studies, reached a similar conclusion (FAO/WHO, 2001). In 2011, JECFA (FAO/WHO, 2012) evaluated further ecological studies in which positive associations were found between fumonisin exposure and incidence of squamous epithelial oesophagus cancer in two studies in China, one in South Africa and one in Iran. A nested case–control study from China using changes in sphingolipids as exposure biomarkers did not find an association with the incidence of cancer in the oesophagus. Since 2011, one epidemiological study with ecological design in Iran investigated FB1 contamination in food and its relationship with oesophageal cancer in different geographical areas with either high or low oesophageal cancer‐risk. Exposure levels of FB1 were determined as frequencies of occurrences and contents in rice and maize. Frequencies of FB1 occurrence in rice samples obtained from the high and low‐risk areas were 75% and 21%, with a mean FB1 content of 43.8 μg/g and 8.9 μg/g, respectively. For maize samples, neither frequencies of FB1 contamination (57% and 47%) nor contents of FB1 (167 and 150 μg/g) were different between areas with high and low oesophageal cancer risk (Alizadeh et al., 2012).
In these studies, no dose–response relationship has been established. Except for the study using sphingolipid biomarkers as a proxy for fumonisin exposure that did not find an association with cancer, all the epidemiological studies conducted so far are ecological studies. In studies with such design, study unit is a population group rather than individuals, precluding taking into account individual factors, such as alcohol use, tobacco smoking, drinking of hot tea, opium use and poor hygiene, which may have an impact on disease outcome. Hence, studies determining exposure and outcome including covariates on an individual level are needed to determine whether or not dietary fumonisin exposure is causally related to oesophageal cancer.
11.1.2. Liver cancer
In 2000, JECFA (FAO/WHO, 2001) also evaluated studies investigating associations between exposure to fumonisins and liver cancer. In a study from China, FB in maize was investigated in regions with high and low rate of liver cancer, but there were no differences between the regions and most of the samples also contained aflatoxin B1 (Ueno et al., 1997). In studies from South Africa, no apparent association between the content of fumonisins in maize and liver cancer were found, whereas the rate correlated with aflatoxin B1 (Jaskiewicz et al., 1987b; Makaula et al., 1996). No additional studies were reported by JECFA in their 2011 evaluation (FAO/WHO, 2012).
In 2012, Persson et al. (2012) investigated the risk of hepatocellular carcinoma (HCC) from fumonisin exposure. The studies had a nested case‐control design and study subjects were included from two prospective cohorts in China, the Haimen City (a high‐risk area of liver cancer) cohort with 271 HCC cases and 280 controls, and the Linxian (a high‐risk area of gastric and oesophageal cancer) cohort with 72 HCC cases and 147 controls. FB1 in toenail samples was used as measure of exposure. In the Haimen City cohort, toenail FB1 levels in the cases (mean = 0.375 ng/mg) were not significantly different from that of the controls (mean = 0.143 ng/mg).Toenail concentrations were higher in the Linxian cohort, but no differences between the cases (mean = 1.96 ng/mg) and the controls (mean = 2.27 ng/mg) were observed. Neither the Haimen City nor the Linxian cohort showed any association between nail FB1 and HCC (odds ratio (OR): 1.10 confidence interval (CI): 0.64–1.89 and OR: 1.47, CI: 0.70–3.07, respectively). The analyses were adjusted for sex, age, residence area, alcohol drinking, and hepatitis B surface antigen. A meta‐analysis of both cohorts, in which study subjects were pooled, did not show any significant association between FB1 exposure and HCC (OR: 1.22, CI: 0.79–1.89) (Persson et al., 2012). The CONTAM Panel noted that toenails showed a low frequency of detectable FB1 in the study, that the validity of nail FB1 as a marker of exposure is not known and that dietary fumonisin exposure was not determined in this study.
11.1.3. Neural tube defects
In 2000, JECFA (FAO/WHO, 2001) also evaluated a possible role of fumonisins in NTD (FAO/WHO, 2001). Ecological studies in South Africa and China noted high incidences of NTD in areas with high exposures to fumonisins (Cornell et al., 1983; Ncayiyana, 1986; Sydenham et al., 1990a,b; Chu and Li, 1994; Venter et al., 1995; Moore et al., 1997). A high rate of NTD was also recorded in the lower Rio Grande valley in southern Texas, among the offspring of women who had conceived during 1990–1991 (Hendricks, 1999) and maize‐based foods obtained in that period also had a relatively high concentration of FBs (Sydenham et al., 1991). In 2011, JECFA (FAO/WHO, 2012) included an epidemiological study in Mexican American women living near the Texas–Mexico border (Missmer et al., 2006). This case–control study showed an association between the estimated fumonisin exposure during the first trimester of pregnancy and the incidence of NTDs in their babies. Fumonisin exposure was estimated using dietary intakes based on tortilla consumption and serum measurements of the Sa/So ratio. FB1 levels were detected in the study, whereas FB2 and FB3 levels were essentially non‐detect samples. The Sa/So ratio in serum as well as the estimated fumonisin exposure increased with the adjusted ORs for NTDs in the population in seven dose groups, except at the highest dose. The authors suggested that at the highest estimated fumonisin exposure, miscarriages might have occurred resulting OR for NTD. JECFA concluded that this study, combined with toxicological evidence (disturbance of sphingolipid metabolism and folate including induction of NTD in mice (Marasas et al., 2004; Gelineau‐van Waes et al., 2005) and earlier epidemiological studies, indicates that fumonisin exposure in pregnant women may be a contributing factor to increased NTD risk in their babies. No new studies after 2011 have been identified.
11.1.4. Childhood growth impairment (stunting)
Possible impairment of childhood growth by fumonisin exposure was reviewed by JECFA in 2011 (FAO/WHO, 2012). A study of 215 infants of half a year and older was conducted in Tanzania (Kimanya et al., 2010). Intakes of FB1 + FB2 + FB3 in maize flour ranged from 0.003 to 28.8 μg/kg bw per day. Height and weight were measured at 1 year of age. Infants (n = 26) with an estimated daily total fumonisin exposure exceeding 2 μg/kg bw (i.e. the JECFA PMTDI) were shorter (1.3 cm) and lighter (328 g) on average than the infants (n = 105) exposed to less than 2 μg/kg bw. Since 2011, two epidemiological studies conducted in Tanzania investigated the association between fumonisin‐aflatoxin co‐exposure and childhood growth, but no new studies on the impact of fumonisin exposure alone on childhood growth and have been published.
One study was conducted in 166 infants from Tanzania aged 6–14 months of age at inclusion (Shirima et al., 2015). At 6 and 12 months following recruitment length and weight were recorded and plasma aflatoxin‐albumin adducts and urinary FB1 were used as measures of exposure. Growth impairment, (stunting) was observed as the mean length for age z‐score (LAZ) and weight for age z‐score (WAZ) declined during this time period. There was a high prevalence of stunted children increasing from 44% to 56%, during the follow‐up. Urinary FB1 concentrations were 314 pg/mL at inclusion and at follow‐up they were 167 pg/mL (6 months) and 569 pg/mL (12 months), respectively. The association between urinary FB1 and childhood growth was analysed using multiple regression. In the analyses, the authors took account of and adjusted for breastfeeding and protein/energy intakes as well as maternal education, socioeconomic status and geographic location. LAZ and length velocity at 12 months from recruitment were negatively associated with the mean urinary FB1 at inclusion and follow‐up times. Urinary FB1 concentrations measured at inclusion were negatively associated with LAZ at both follow‐up time points suggesting that FB exposure could be a risk factor for growth impairment. Urinary FB1 levels were neither negatively associated with WAZ nor with weight‐for‐length z‐score (WLZ). AF‐alb was negatively associated with child growth, but this association was not statistical significance. Addressing the joint fumonisin‐aflatoxin effect in the statistical analyses gave results that could not be interpreted.
In another study from Tanzania (Magoha et al., 2016), 143 infants were followed up from birth at 1, 3 and 5 months of age when weight and length were recorded. Using the WHO Growth Standards, age related z‐scores were computed. As exclusive breastfeeding is rarely practiced in Tanzania a large fraction (80% and 97% at 3 and 5 months of age) receiving complementary food consisting mainly of maize flour. The intake of maize flour was estimated based on a 24‐h dietary recall and mycotoxins were determined in flour samples from the families. Of the flour samples (n = 67), 58% had detectable aflatoxins, 31% fumonisin and 22% both mycotoxins. The medians and ranges were 6 (0.33–69.47) μg /kg aflatoxin and 124 (48–1,224) μg/kg fumonisin. Independent of the mycotoxin contamination, a slightly higher weight and length gain from 3 to 5 months was found in exclusively breastfed infants (n = 23) in comparison with those also given complementary foods. The prevalence of underweight and stunting were 6% and 18% among those infants receiving maize‐containing complementary food. Among these infants those exposed to aflatoxin 3% were underweight and 15% stunted, and among those exposed to fumonisin alone, none were underweight and 5% stunted, and among those who were exposed both mycotoxins none were underweight and 7% stunted. No statistically significant associations between exposure to fumonisins or aflatoxins or both and underweight or stunting were found when these were examined using logistic regression (Magoha et al., 2016). The CONTAM Panel noted that in the statistical analyses it was apparently not controlled for multiple factors such as nutrient intake, frequent bacterial infections, socioeconomic status and mother's education and health that might have influenced the outcome. The authors also noted that in this study they did not take account of their previous findings, namely that breast milk samples from the same region were contaminated with aflatoxin M1 (100%) and FB1 (44%) and could serve as an additional source of exposure for children (Magoha et al., 2014a,b).
In a cross‐sectional study conducted in six villages in Cameroon with 220 children (Ediage et al., 2013), mycotoxins and their metabolites were detected in 160 of 220 (73%) urine samples. These included ochratoxin A, β‐zearalenol, aflatoxin M1, deoxynivalenol and FB1 (mean values: males 0.59 ng/mL, females, 0.01 ng/mL). No association was observed between the different malnutrition categories (stunted, wasting and underweight) and the mycotoxin concentrations detected in the urine of these children.
11.1.5. Human immunodeficiency virus (HIV)‐related mortality
In 2011, JECFA (FAO/WHO, 2012) assessed an epidemiological study on potential associations between fumonisin exposure and HIV‐related mortality, but as the HIV study did not include measurements of fumonisin levels in food or fumonisin exposure in humans, it was found insufficient to support an association between fumonisin exposure and HIV‐related mortality. No new studies after 2011 have been identified by the CONTAM Panel.
11.1.6. Acute mycotoxicosis
In 2000, JECFA (FAO/WHO, 2001) reported that in 1995 consumption of rain‐damaged, mouldy sorghum and maize by the inhabitants of 27 villages in the Deccan Plateau in southern India resulted in an episode of human mycotoxicosis characterised by gastrointestinal disease. The disease was characterised by abdominal pain, borborygmi11 and diarrhoea. Diarrhoea was reproduced in 1‐day‐old cockerels fed contaminated grain from the affected households. The dominant mycoflora in the sorghum were Aspergillus, Fusarium, and Alternaria spp. FB1 was the most common mycotoxin in both sorghum and maize samples, and a relatively high concentration of aflatoxin B1 was also detected in the maize.
11.1.7. Inhibition of ceramide synthases
Three surveys of fumonisin contamination in maize were conducted across Guatemala in order to select locations for two human studies to test the hypothesis that fumonisin intake will result in effects indicative of fumonisin inhibition of CerS (elevated Sa 1‐P and the Sa 1‐P/So 1‐P ratio) as seen in animal studies (Riley et al., 2015b). Communities were selected based on the surveys so as to maximise the likelihood of having populations enriched in either high or low fumonisin exposure individuals. One other goal of the studies was to estimate the FB1 intake in maize consumers in Guatemala using the urinary FB1 and to predict when individuals are at increased risk for exceeding the JECFA PMTDI of 2 μg/kg bw day (FAO/WHO, 2012).
FB1 intake was estimated using the urinary FB1 exposure biomarker and Sa 1‐P, So 1‐P and the Sa 1‐P/So 1‐P ratio (biomarkers of effect) were determined in blood spots collected on absorbent paper at the same time as urine collection. Maize samples were also collected from local markets in each community at the same time as urine and blood collection.
In the first study (Torres et al., 2014), blood spots and urine were collected every 3 months (March 2011–February 2012) from women living in low and high FB exposure communities (1,240 total recruits). The urinary FB1, Sa 1‐P/So 1‐P ratio, and Sa 1‐P/mL in blood spots were significantly higher in the high FB1 exposure community compared to the low FB1 exposure communities (Riley et al., 2015a). The results were confirmed in a follow‐up study (February to March 2013) involving 299 women living in three different low and high FB exposure communities (Riley et al., 2015a). In summary, high levels of FB1 intake are correlated with changes in Sa 1‐P and the Sa 1‐P/So 1‐P ratio in human blood in a manner consistent with FB1 inhibition of CerS (Riley et al., 2015a).
The results show that there was an apparent threshold below which the increase in the Sa 1‐P/So 1‐P ratio was not associated with a statistically significant increase in the urinary FB1 concentration relative to the group with the lowest Sa 1‐P/So 1‐P ratio. The urinary FB1 concentration at the breakpoint, in both studies, was estimated at 0.5–1.0 ng FB1/mL. For the Sa 1‐P/So 1‐P blood ratio, the first statistically significant increase occurred at the urinary FB1 window that was > 0.5 < 1.0 ng FB1/mL and for the Sa 1‐P concentration the window was > 1.0 < 5.0 ng FB1/mL.
In order to calculate an intake corresponding to 0.5 ng FB1/mL in urine, it was assumed that excretion is 0.5% of FB intake, that total urine output in the Guatemalan women is 1,000 mL, and the average weight was 60 kg. Based on these assumptions, 0.5 ng/mL urinary FB1 represents a total intake of 1.67 μg/kg bw per day (i.e. if 0.5 μg/L is 0.5% FBs daily intake, 100% is 100.2 μg/day, assuming 60 kg bw these are 1.67 μg/kg bw) (Riley et al., 2012, 2015a,b; Torres et al.,2014).
12. Dose–response analysis for fumonisin B1
12.1. Acute effects of fumonisin B1
Humans consuming mouldy sorghum and maize containing fumonisins have shown acute adverse effects such as gastrointestinal symptoms but there was no information on the dose or type of fumonisin and presence of other mycotoxins in the food consumed. Therefore, any effects cannot be clearly attributed to fumonisin alone and hence it is not possible based on these studies to decide on acute effects of FBs in humans.
There are few acute studies available using FB1 in experimental animals and in none of these was lethality observed. In acute studies in rats, oral gavage doses up to 46.4 mg/kg bw have been tested. Other acute studies in rats showed that effects were similar to those occurring after repeated doses in longer term studies, i.e. kidney and liver toxicity. In pigs, early signs of pulmonary oedema occurred following a single oral dose of 5 mg/kg bw. Also, ELEM in horses is considered a vascular effect. Although vascular toxicity of FBs in humans cannot be excluded, the only in vivo vascular effects reported in the literature are a chronic atherogenic effect associated with consumption by non‐human primates of diets containing fumonisins for extended periods of time (Fincham et al., 1992). Overall, FB1 is therefore considered not to be acutely toxic in humans.
12.2. Chronic effects of fumonisin B1
Reviewing the toxicological studies the CONTAM Panel came to the conclusion that study by Bondy and co‐workers (Bondy et al., 2012; for details on the study see Section 10.2.3) was the most appropriate for use in the dose–response evaluation. In this study, groups of 9–10 mice, were given daily doses of 0, 0.39, 3.87 and 12.2 mg FB1/kg bw (wild type p53+/+ mice (WT)) or 0, 0.37, 3.88 and 12.6 mg FB1/kg bw, (p53+/− transgenic mice (TG)) for 26 weeks. The endpoints considered of potential relevance for calculation of a BMD for FB1 were incidences and severity of various hepatic lesions which were adenoma, focal hepatic inflammation, multinucleated hepatocytes, hepatic single cell necrosis and megalocytic (also known as karyocytomegalic) hepatocytes and in addition liver Sa concentration, as seen in the chronic study of Bondy et al. (2012). The CONTAM Panel decided that induction of apoptosis and megalocytic hepatocytes and increases of Sa levels should be used for modelling and calculation of a BMD based on their relevance and sensitivity shown in the study. Although increased Sa levels were found in the study, the results did not allow calculation of a valid BMD when applying EFSA guidance (EFSA Scientific Committee, 2017).
The BMDs for induction of apoptosis and megalocytic hepatocytes in the present opinion were calculated considering both incidence and severity of the lesions observed in the study, following the procedure applied by Bondy et al. (2012) using the raw data for incidences and severity of the lesions provided by the author to EFSA. Briefly, severity of lesions was designated into six classes: 0 – not present; 1 – minimal; 2 – mild; 3 – moderate; 4 – marked; and 5 – severe. The cut‐off to consider a lesion as an incident was set to 1 for megalocytic hepatocytes and to 2 for apoptosis, i.e. lesions with a score of 1 or higher for megalocytic hepatocytes and with a score of 2 or higher for apoptosis, respectively, were considered as an incident in the calculations for a BMD (see Table Appendix A for details on incidences and severity scores).
The CONTAM Panel used a default benchmark response (BMR) of 10% (BMD10) for quantal data, combined the dose response results from both strains and used exact doses (as given by the authors), thus increasing the number of dose groups for the analysis. Consequently, covariates were not applied. This approach is justified as the responses did not differ between the WT strain and the TG strain which is more susceptible to genotoxic carcinogens. Using model averaging following EFSA guidance (EFSA Scientific Committee, 2017) the CONTAM Panel calculated the BMDL10–BMDU10 confidence interval as of 0.1–1.9 mg FB1/kg bw per day for incidence of megalocytic hepatocytes and as of 1.2–3.72 mg FB1/kg bw per day for incidence of apoptosis, respectively (for details on the BMD calculations, see Appendix B).
13. Establishment of health‐based guidance values
13.1. Acute reference dose (ARfD)/group ARfD
The CONTAM Panel noted that FBs have shown acute effects in certain species (e.g. horses) but concluded that the data available did not indicated a need for setting an acute HBGV for FBs or their modified forms.
13.2. Tolerable daily intake/group TDI
Following the guidance of EFSA (EFSA Scientific Committee, 2017) that recommends use of the lowest BMDL derived for a compound to set a HBGV, the CONTAM Panel decided to use the BMDL10 of 0.1 mg/kg bw per day derived for induction of megalocytic hepatocytes in mice for establishing a TDI for FB1. A UF of 100 for intra and interspecies variability was applied resulting in a TDI of 1.0 μg FB1/kg bw per day.
It is noted that data from humans indicate that biochemical effects, i.e. inhibition of CerS (changes in Sa 1‐P/So 1‐P ratio as measure in blood) may occur above a total FBs exposure resulting in 0.5–1.0 ng FB1/mL in urine, corresponding to an estimated total intake of FBs of about 1.7 μg FBs/kg bw per day (see also Section 11), which is in the region of the TDI established on the basis of the mouse study as explained above. This effect is a biochemical change and it is linked to adverse effects. However, in itself, it is not adverse and a quantitative relationship with adverse outcomes is not established. Therefore, the CONTAM Panel did not consider it appropriate to use this effect as basis for setting a TDI.
13.2.1. Inclusion of fumonisin Bs in a group tolerable daily intake (group TDI) with fumonisin B1
FB2–6 are structurally similar to FB1 and in precision‐cut rat liver slices in vitro FB2–4 exhibited inhibition of CerS approximately equipotent with that of FB1 (Norred et al., 1997). In primary hepatocytes, FB1, FB2 and FB3 showed approximately similar cytotoxicity in primary rat hepatocytes (Gelderblom et al., 1993). Moreover, almost equal cytotoxicity was found for FB1 and FB2 when tested in seven different rat hepatoma cell lines and in one dog kidney cell line (Shier et al., 1991). In primary rat hepatocytes, FB2 inhibited de novo biosynthesis of sphingolipids as effectively as FB1 (Wang et al., 1991; Norred et al., 1992).
In vivo, FB2–3, similar to FB1, were able to induce hepatic nodules when fed to rats (Gelderblom et al., 1993). Quite high doses were given, however, and all animals had nodules, which preclude comparison of potency between FB1 and FB2–3. In mice, receiving FB1–3, FB1, caused clear signs of liver toxicity and significantly increased liver Sa/So ratio and depressed liver ceramide, whereas FB2–3 did not (Howard et al., 2002).
When ponies were given maize material containing 75 mg/kg FB2 (containing also 3 mg/kg FB1 and less than 1 mg/kg FB3) or 75 mg FB3 (containing less than 1 mg/kg FB1 or FB2) the free Sa increased significantly in liver and in kidney although the increase was greater in the FB2 exposed ponies and there was no FB1‐treated ponies for concurrent comparison (Riley, 1997).
Based on the above data, the CONTAM Panel assuming dose addition of FBs, decided that FB2, FB3 and FB4 should be included in a group TDI with FB1.
Both FB5 and FB6 are of similar structure as FB1 and hence, based on structural similarity, both are likely to inhibit CerS and exhibit toxicity similar to that of the other FBs included in the group TDI. Due to a lack both of in vitro and in vivo data, the CONTAM Panel decided that FB5 and FB6 should not be included in the group TDI with FB1.
13.2.2. Inclusion of modified fumonisin Bs in a group tolerable daily intake (group TDI)
Because of the insufficient data modified forms of FB1–4 cannot be included in this group TDI. In the few in vivo studies available where pure compounds have been tested, HFB1 showed either very marginal or no effects in comparison to FB1.
Regarding HFB1, HFB2 and HFB3 the in vitro studies showed large variability in toxicity when compared with FB1, and relative potencies of hydrolysed forms ranged from 0.01 and 0.9 in the different studies, depending, among other factors, on which doses were compared (see Section 10.4). Although the CONTAM Panel recognised that some in vitro studies showed close to even enhanced toxicity, based on the overall in vivo evidence, the CONTAM Panel concluded that HFB1 acts via a similar MoA for toxicity (inhibition of CerS) but is of lower toxic potency than FB1. However, based on the data available the potencies cannot be quantified and therefore these modified forms should not be included in a group TDI with FB1–4.
For pHFB1, there is only one repeated‐single‐dose in vivo study showing only mild kidney effects while with FB1 at a similar dose level marked changes were seen. No relevant in vitro data on pHFBs are available. The CONTAM Panel concluded that these modified forms should not be included in a group TDI with FB1–4.
In vitro data with N‐fatty acyl FB1, N‐fatty acyl HFB1 and N‐fatty acyl HFB2 show a toxicity of up to 10 times higher as compared with FB1. Notably, it has been shown that some N‐fatty acyl conjugates are much more rapidly accumulated and to a greater extent taken up in cells in vitro in comparison to FB1. In an assay with brine shrimp, N‐palmitoyl‐HFB1 was equally toxic compared with FB1; however, the route of exposure was via the culture media and not via the food. The CONTAM Panel concluded that the database was insufficient for including N‐fatty acyl FBs in a group with FB1–4.
The information on in vivo and in vitro effects of other modified forms (NCM‐FB1, NDFB1) indicate a lower toxicity in comparison with FB1, but there are insufficient data to make conclusions on their toxicity and in consequence the CONTAM Panel concluded that NCM‐FB1 and NDFB1 should not be included in a group TDI with FB1–4.
14. Uncertainties
The CONTAM Panel identified several uncertainties in their evaluation of the appropriateness to set the group HBGVs for fumonisins and their modified forms.
The group TDI of 1.0 μg/kg bw day is based on a BMDL calculated for adverse effects of FB1. The database for setting a TDI for FB1 is relatively extensive. The inclusion of FB2, FB3 and FB4, however, is based on structural similarity, mechanistic considerations and limited toxicity data on these fumonisins, although there are indications that FB1 is the most active form. This constitutes a major uncertainty.
Due to a lack of appropriate toxicity data, the relative potency for any of the modified forms of FBs could not be quantified and therefore none of the modified forms where included in the group TDI. Despite the fact that relative potencies could not be numerically quantified based on the limited toxicity data available, HFBs, pHFBs, NCM‐FBs and NDF‐FBs are likely to be less toxic than their parent compounds. For N‐fatty acyl FB1, there are in vitro data suggesting a higher toxic potency than the parent compound, however, the reason for this and significance in vivo is unknown. Non‐inclusion of any modified forms in the group TDI is therefore associated with additional uncertainty.
14.1. Summary of uncertainties
In Table 6, a summary of the uncertainty evaluation is presented, highlighting the main sources of uncertainty and indicating an estimate of whether the source of uncertainty leads to over/underestimation of the resulting risk.
Table 6.
Summary of the qualitative evaluation of the impact of uncertainties on the assessment
| Sources of uncertainty | Directiona |
|---|---|
| Inclusion of FB2, FB3 and FB4 in a group TDI with FB1 based on limited toxicity data | + |
| Non inclusion of modified FBs in group TDI | − |
FB: fumonisin B; TDI: tolerable daily intake.
+ = uncertainty with potential to cause overestimation of exposure/risk; − = uncertainty with potential to cause under‐estimation of exposure/risk, +/− = extent of potential over/underestimation might differ in direction.
The overall uncertainty associated with the inclusion of FB2–4 into a group TDI with FB1 is considered as high and it would rather overestimate than underestimate the risk. The non‐inclusion of modified forms in the group TDI introduces additional uncertainty.
15. Conclusions
15.1. Introduction
Fumonisins are mycotoxins produced predominantly by F. verticillioides and F. proliferatum. Chemically, fumonisins are long‐chain aminopolyols with two tricarballylic acid side chains. The most relevant compounds are the B‐type fumonisins FB1‐FB4 which differ in the number and position of hydroxy‐groups at the backbone. Besides the B‐type fumonisins, other fumonisins such as the A‐, C‐ and P‐type have been described. However, these compounds are produced in much lower levels and are for this reason not of significance. Of relevance are several modified forms of fumonisins, predominantly the HFBs and pHFBs which are formed upon alkaline hydrolysis as well as NCM‐FBs and NDF‐FBs which have been detected in food samples. Plant and fungal metabolites such as N‐ and O‐fatty acyl fumonisins are also described, however, only traces have been detected in food samples so far. Besides HFBs, N‐fatty acyl fumonisins with acyl‐chain length ranging from C16:0 to C24:1 are the only known FB in vivo metabolites. Their formation is catalysed by CerS, key enzymes in the biosynthesis of sphingolipids which is inhibited by FBs. In vitro, N‐fatty acyl fumonisins are more cytotoxic compared to FBs.
Analytical methods for FB1–4 are well established and are mainly based on MS. Modified forms of FB1 are commonly analysed under the same conditions as their parent compound. However, the strong physical interaction of FBs with the food matrix, that is well documented in the literature, may significantly affect the analytical performance in a matrix‐related way. In order to mitigate this obstacle, several indirect methods, usually based on alkaline hydrolysis of the matrix, have been proposed. Only FB1–3 are available on the market as calibrant solutions, while FB4 can be purchased as purified powder. Except for HFB1, analytical standards for modified forms are not commercially available.
15.2. Occurrence of fumonisins B1–4 and their modified forms
The occurrence of FB1–3 is well documented in maize and products thereof, whereas little information is available for occurrence of FB4 and even less for occurrence of FB5–6.
Hydrolysed forms of FB1–3 (HFB1–3) have been reported following food processing (e.g. nixtamalisation).
Very few data are available on other modified FBs such as O‐fatty acyl and N‐fatty acyl FBs and it can be assumed that these modified FBs occur at low concentrations compared to occurrence levels of their parent compounds.
No information was identified on the transfer of modified forms of fumonisins to food and feed of animal origin.
15.3. Toxicokinetics of fumonisins B1–4 and their modified forms
FBs are poorly absorbed (< 4% of an oral dose) from the gastrointestinal tract. The absorbed FBs and their metabolites are rapidly excreted mainly in the bile of experimental animals resulting in low plasma, tissue and urinary concentrations.
Metabolism comprises the stepwise hydrolysis of the ester groups of the parent FBs and the formation of N‐fatty acyl FBs. Metabolic activity is low in mammalian tissues and hydrolytic metabolism involves the colonic microbiome.
Few studies have been identified on the toxicokinetics of modified FBs. There is preliminary evidence for the partial release of FB1 from NDF‐FB1 in rats after oral ingestion.
15.4. Mode of action for toxicity of fumonisins B1–4
The key event in the toxic MoA of FBs is inhibition of CerS. FBs and in particular HFBs are structural analogues of sphingoid bases and they inhibit CerS, causing disruption of sphingolipid metabolism and pathological changes seen after FBs exposure. Several modified FBs may cause inhibition of CerS, but apparently with variable potencies not well described.
15.5. Biomarkers
Although FBs are poorly absorbed in the body, unchanged FBs excreted into urine have been used as a biomarker of exposure in humans. Changes in Sa and So or their ratio can be determined in urine (due to presence of sloughed cells) following FB exposure. A significant FB1 dose‐related increase in the Sa 1‐P/So 1‐P ratios in matched blood spots has been reported in human studies.
15.6. Toxicity of fumonisins B1–4
Toxicity assessments are mainly based on results with FB1, but FB2–4 are considered as having similar toxicological profile and potency as FB1.
FB1 is considered not to be acutely toxic in humans.
In repeated dose studies with rodents FB1 causes liver and kidney toxicity. Apoptosis, necrosis, proliferation, regeneration and hyperplasia of the bile duct are early signs of liver toxicity. Early signs of kidney toxicity were increases in free sphingoid bases, apoptosis and cell regeneration in the renal tubules of the outer medulla. Upon chronic exposure, liver and kidney tumours are observed.
FB1 caused embryotoxicity in mice, rats and rabbits, but only at doses where maternal toxicity is observed. In Syrian hamsters, such effects were observed in the absence of maternal toxicity. There are indications that FB1 causes neural tube defects in sensitive mice strains but, overall, the evidence is inconclusive.
FB1–4 were approximately equipotent inhibitors of CerS and cause cytotoxicity in several mammalian cell types in vitro.
FB1 is not mutagenic in bacteria and does not cause unscheduled DNA synthesis in mammalian cells, but is clastogenic via an indirect mechanism, possibly by induction of oxidative stress.
15.7. Toxicity of modified fumonisins B1–4
15.7.1. In vivo toxicity
HFB1 shows a similar toxicological profile similar to FB1, but is less potent.
pHFB1, NCM‐FB1, NDF‐FB1 show a similar toxicological profile but are less potent than FB1, however, the data base is even more limited than that for HFB1.
No in vivo data were available for N‐fatty acyl FBs and O‐fatty acyl FBs.
In brine shrimp, N‐palmitoyl‐HFB1 is more toxic than HFB1 and has about the same toxicity as FB1
Overall, the available data on modified forms suggest a similar toxicological profile as their parent compounds but the data are too limited and inconsistent to assess their relative potencies in quantitative terms.
15.7.2. In vitro toxicity of modified fumonisins B
For HFB1–3, in vitro toxic potencies relative to FB1 vary between 0.01 and 0.9 Notably, HFB1 is taken up by cells more rapidly and completely than FB1.
For pHFB1–2, there were no data available for assessing toxicity relative to their parent compounds.
In a single study, NCM‐FB1 had a relative potency of 0.02 as compared with FB1.
There is no information available on in vitro toxicity of O‐fatty acyl FBs
N‐fatty acyl FB1 and N‐fatty acyl HFB1–2 are up to 10 times more toxic in vitro than FB1. Notably, these compounds are taken up more rapidly and to a greater extent by cells than FB1 and HFB1.
The available in vitro data on modified FBs do not allow extrapolations to the human in vivo situation.
15.8. Observations in humans
Several clinical effects have been discussed in humans (such as oesophageal cancer, liver cancer, neural tube defects, growth impairment), but so far none of these have been causally related to fumonisin exposure.
Data from humans indicate that inhibition of CerS leads to changes in Sa 1‐P/So 1‐P ratio as measured in blood and may occur above a total FB1–3 exposure resulting in 0.5–1 ng FB1/mL in urine, corresponding to a total estimated intake of FBs of about 1.7 μg FBs/kg bw per day.
15.9. Chronic dose–response analysis
The dose–response analysis was based on a chronic study in mice with FB1 resulting in a series of liver lesions including hepatic adenoma. The CONTAM Panel considered it appropriate to conduct dose response analyses of liver effects and establish a TDI. Increased incidence of megalocytic hepatocytes in the liver was considered as the critical effect and a BMDL10 of 0.1 mg/kg of FB1 per day was derived.
15.10. Establishment of group health‐based guidance values
15.10.1. Health‐based guidance values for fumonisins B1–4
The CONTAM Panel used the BMDL10 of 0.1 mg/kg bw per day and a UF of 100 for intra and interspecies variability resulting in a TDI of 1.0 μg FB1/kg bw per day.
Based on structural similarity and the limited data available indicating similar MoA and similar toxic potencies, the CONTAM Panel decided that FB2, FB3 and FB4 should be included in a group TDI with FB1.
15.10.2. Health‐based guidance values for modified fumonisins B1–4
Because of the insufficient data, modified forms of FB1–4 cannot be included in this group TDI. The CONTAM Panel noted that based on the available evidence it can be assumed that modified forms of FB1–4 excrete lower toxicity than their parent compounds; however, their relative toxicity could not be quantified.
16. Recommendations
Standards and calibrants for FB2–6 and for modified forms of FBs are needed for analytical and toxicological purposes.
More information on occurrence of FB2–6 and of modified FBs are needed in order to prioritise toxicity testing.
More information on the toxicokinetics for modified forms of FBs and also for FB2–4 are needed.
More information is needed on toxicity of FB2–6 and of any modified FBs using pure compounds and in particular on the toxicity of hydrolysed FBs using pure compounds.
The effectiveness of mitigation methods to reduce FB1 toxicity needs to be examined further.
Documentation provided to EFSA
Data on liver pathology (incidences of lesions and severity scoring) used for Bondy et al. (2012) were kindly provided to EFSA by Genevieve Bondy (Bureau of Chemical Safety, Food Directorate, Health Products and Food Branch, Health Canada, Ottawa) on 18 December 2017.
Abbreviations
- 3T3
mouse fibroblast cell line
- Ac
acetyl
- 2‐AAF
2‐acetylaminofluorene
- AHF
altered hepatic foci
- ALP
alkaline phosphatase
- ALT
alanine amino transferase
- AP
aminopentol/aminopolyol
- ARfD
acute reference dose
- AST
aspartate amino transferase
- BMD
benchmark dose
- BMDL05
the 95th percentile benchmark dose lower confidence limit
- BMDL10
the 90th percentile benchmark dose lower confidence limit
- BMDU05
the 95th percentile benchmark dose upper confidence limit
- BMDU10
the 90th percentile benchmark dose upper confidence limit
- BMR
benchmark response
- bw
body weight
- Caco
human intestinal cell line
- CAS
Chemical Abstracts Service
- CerS
ceramide synthases
- CI
confidence interval
- CM
contaminated
- CONTAM Panel
EFSA Panel on Contaminants in the Food Chain
- DART‐MS
direct‐analysis‐in‐real‐time mass spectrometry
- deoxySa
deoxysphinganine
- DEN
diethylnitrosamine
- DON
deoxynivalenol
- E
embryonic day
- EHC
Environmental Health Criteria
- ELEM
equine leukoencephalomalacia
- ELISA
enzyme‐linked immunosorbent assay
- FAO
Food and Agriculture Organization of the United Nations
- FA
fumonisin A
- FB
fumonisin B
- FC
fumonisin C
- FP
fumonisin P
- FEEDAP Panel
EFSA Panel on Additives and Products or Substances used in Animal Feed
- GC
gas chromatography
- GD
gestation day
- GGT
gamma‐glutamyl transferase
- GPT
glutamate‐pyruvate transaminase
- GSH
glutathione
- GST
glutathione‐S‐transferase
- GSTP+
glutathione S‐transferase P+
- H4TG
rat hepatoma cell line
- HBGV
health‐based guidance value
- HCC
hepatocellular carcinoma
- Hek
human embryonic kidney
- HepG2
human hepatoma cell line
- Hep3B
human hepatoma cell line
- HFB
hydrolysed fumonisin B
- HIV
human immunodeficiency virus
- HT29
human colonic cell line
- IARC
International Agency for Research on Cancer
- IC50
half maximal inhibitory concentration
- IF
interferon
- IgA
immunoglobulin A
- IgM
immunoglobulin M
- IHKE
human proximal tubule‐derived (cells)
- KA31T
mouse fibroblast cell line
- IL
interleukin
- i.p.
intraperitoneal
- IPCS
International Programme on Chemical Safety
- i.v.
intravenous
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- LC
liquid chromatography/left‐censored
- LC‐ESI‐MS/MS
liquid chromatography‐electrospray ionisation‐tandem mass spectrometry
- LC‐FLD
liquid chromatography–fluorescence detection
- LC–MS/MS
liquid chromatography–tandem mass spectrometry
- LC50
median lethal concentration
- LDH
lactate dehydrogenase
- LAZ
length for age z‐score
- LLC‐PK1
cultured pig kidney renal epithelial cells
- LOAEL
lowest observed adverse effect level
- LOD
limit of detection
- LOQ
limit of quantification
- M
molar
- MDCK
canine kidney epithelial cells
- MDD
methyl‐deficient diet
- MEF
mouse embryonic fibroblast
- ML
maximum level
- MME
mono methylester of fumonisin FB1
- MoA
mode of action
- mRNA
messenger RNA
- MS
mass spectrometry, mass spectrum
- MS/MS
tandem mass spectrometry
- MW
molecular weight
- NCM
nixtamalised contaminated
- NCMC
nixtamalised mixture of CM and ground corn
- NCM‐FB
N‐(carboxymethyl) fumonisins B
- NDF‐FB
N‐(1‐deoxy‐d‐fructos‐1‐yl) fumonisins B
- NFI‐DTU
National Food Institute‐Danish Technical University
- NMR
nuclear magnetic resonance
- NOAEL
no observed adverse effect level
- NOEL
no observed effect level
- NTD
neural tube defects
- NTP
National Toxicology Program
- OPA
o‐phthaldialdehyde
- OR
odds ratio
- PGST
placental glutathione S‐transferase
- pHFB
partially hydrolysed fumonisin B
- PKS
polyketide synthetase
- PMTDI
provisional monthly tolerable daily intake
- RNA
ribonucleic acid
- ROS
reactive oxygen species
- RP
Reference point
- Sa
d‐erythro‐sphinganine (or short: sphinganine)
- Sa 1‐P
sphinganine 1‐phosphate
- SAX
strong anion‐exchange
- SCF
Scientific Committee on Food
- SCM
sham nixtamalised CM
- SCMC
sham nixtamalised mixture of CM and ground corn
- So
d‐erythro‐sphingosine (or short: sphingosine)
- So 1‐P
sphingosine 1‐phosphate
- SOD
superoxide dismutase
- SPE
solid‐phase extraction
- SPF
specific pathogen‐free
- TAC
total antioxidant capacity
- TCA
tricarballylic acid
- TDI
tolerable daily intake
- TG
transgenic
- TLC
thin‐layer chromatography, total leukocyte
- TOF
time of flight
- TOF‐MS
time of flight‐Mass spectrometry
- ToR
Terms of Reference
- TNF
tumour necrosis factor
- t‐RNA
transfer RNA
- UC
uncontaminated
- UDP
uridine 5'‐diphosphate
- UF
uncertainty factor
- UPLC (RP‐C18)
ultra pressure liquid chromatography–reverse phase C18 column
- UV
ultraviolet
- WAZ
weight for age z‐score
- WBC
white blood cell
- WHO
World Health Organization
- WLZ
weight‐for‐length z‐score
- WT
wild type
Appendix A – Raw data used for derivation of a benchmark dose for incidence of megalocytic hepatocytes and apoptosis upon oral exposure to fumonisin B1 in mice
1.
Table A.1 shows the data used for derivation of BMDs for megalocytic hepatocytes and hepatic apoptosis.
Table A.1.
Summary of data used for derivation of benchmark doses for megalocytic hepatocytes and hepatic apoptosis (Bondy et al., 2012)
| Doses in mg/kg bw per day | Individual scores megalocytic hepatocytes (cut off = 1) | Combined incidence megalocytic hepatocytes (WT and TG) | Individual scores Apoptosis (cut off = 2) | Combined incidence apoptosis (WT and TG) |
|---|---|---|---|---|
|
0 (WT) 0 (TG) |
0 0 0 0 0 0 0 0 0 (WT) 0 0 0 0 0 0 0 0 0 0 (TG) |
0/19 |
1 1 1 1 1 1 1 1 1 (WT) 1 1 1 1 1 1 1 1 1 1 (TG) |
0/19 |
|
0.39 (WT) 0.37 (TG) |
0 0 0 0 0 0 1 0 2 0 (WT) 0 0 1 0 0 0 0 0 1 0 (TG) |
4/20 |
1 1 1 1 1 1 1 1 1 1 (WT) 1 1 1 1 1 1 1 1 1 1 (TG) |
0/20 |
|
3.87 (WT) 3.88 (TG) |
1 0 2 1 0 0 1 0 1 (WT) 2 2 2 0 0 0 0 1 0 0 (TG) |
9/19 |
4 3 4 4 2 2 2 1 3 (WT) 4 3 4 3 3 5 1 2 4 3 (TG) |
17/19 |
|
12.2 (WT) 12.6 (TG) |
5 5 5 5 4 3 3 5 5 5 (WT) 5 5 5 5 5 5 5 0 5 5 (TG) |
19/20 |
4 4 4 5 5 5 3 4 5 5 (WT) 4 3 3 4 4 4 4 3 5 5 (TG) |
20/20 |
WT: WG strain; TG: TG strain.
Note: for explanation of pathology scoring see Section 10.2.7.
Appendix B – Benchmark dose analysis
B.1. Introduction
Benchmark dose (BMD) analyses of the incidences of liver apoptosis and megalocytic hepatocytes in male mice (Bondy et al., 2012) were carried out according to the EFSA guidance (EFSA Scientific Committee, 2017). The benchmark response (BMR) is the estimated risk corresponding with the BMD of interest. A default BMR of 10% for quantal data was applied. A 90% confidence interval around the BMD was estimated, the lower bound is reported by BMDL and the upper bound by BMDU. Results were obtained using the R‐package bmdModeling. Fitting benchmark dose models is based on the R‐package proast61.3. Averaging results from multiple fitted benchmark dose models is based on the methodology described by Wheeler and Bailer (2008). Model averaging was used for all tested endpoints. There were no deviations from the recommended defaults (EFSA Scientific Committee, 2017). The BMD is defined as the dose that corresponds with an extra risk of 10% compared with the background risk. Fitted models applied for the calculations were the default models given in the EFSA guidance and selection of the BMDL was carried out following the flow chart in the guidance (EFSA Scientific Committee, 2017).
B.2. Incidence of megalocytic hepatocytes
The combined incidence and severity of megalocytic hepatocytes in two strains (WT and TG) of male mice treated orally with FB1 for 26 weeks (Bondy et al., 2012) were used for derivation of a BMD. Severity of lesions was designated into six classes and were: 0 – not present, 1 – minimal; 2 –mild; 3 – moderate; 4 – marked; 5 – severe. The cut‐off to consider a lesion was set to 1 for the megalocytic hepatocytes, i.e. lesion severity with score of 1 or higher were considered as an incident (see Table A.1, Appendix A)
Table B.1.
Observations of incidences with a severity of 1 or more of megalocytic hepatocytes in male mice treated with fumonisin B1
| Substance | Dose (mg/kg bw per day) | Incidence | N | Cov |
|---|---|---|---|---|
| FB1 | 0.00 | 0 | 10 | TG |
| 0.37 | 2 | 10 | TG | |
| 3.88 | 4 | 10 | TG | |
| 12.60 | 9 | 10 | TG | |
| 0.00 | 0 | 9 | WT | |
| 0.39 | 2 | 10 | WT | |
| 3.87 | 5 | 9 | WT | |
| 12.20 | 10 | 10 | WT |
bw: body weight; N: number of animals; Cov: Covariant (mice of WT or TG strain).
Table B.2.
Results for incidences of megalocytic hepatocytes
| Model | Number of parameters | Log‐ likelihood | AIC | Accepted AIC | BMDL | BMDU | BMD | Converged |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −52.80 | 107.60 | NA | NA | NA | NA | |
| full | 7 | −26.17 | 66.34 | NA | NA | NA | NA | |
| two.stage | 3 | −28.95 | 63.90 | yes | 0.337 | 1.840 | 0.51 | yes |
| log.logist | 3 | −29.43 | 64.86 | no | NA | NA | NA | yes |
| Weibull | 3 | −28.42 | 62.84 | yes | 0.038 | 0.656 | 0.24 | yes |
| log.prob | 3 | −29.35 | 64.70 | no | NA | NA | NA | yes |
| gamma | 3 | −28.22 | 62.44 | yes | 0.018 | 0.607 | 0.19 | yes |
| logistic | 2 | −30.09 | 64.18 | yes | 1.090 | 2.200 | 1.50 | yes |
| probit | 2 | −30.18 | 64.36 | yes | 1.120 | 2.100 | 1.50 | yes |
| LVM: Expon. m3‐ | 3 | −28.39 | 62.78 | yes | 0.047 | 1.290 | 0.26 | yes |
| LVM: Hill m3‐ | 3 | −28.69 | 63.38 | yes | 0.0423 | 1.610 | 0.27 | yes |
AIC: Akaike information criterion; BMD: benchmark dose; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit; NA: not applicable.
Table B.3.
Model weights in using model averaging
| Estimated model weights | two.stage | log.logistic | Weibull | log.prob | Gamma | Logistic | Probit | EXP | HILL |
| 0.09 | 0.06 | 0.16 | 0.06 | 0.19 | 0.08 | 0.07 | 0.16 | 0.12 |
Given the 1,000 generated data sets, the BMDL is the 5th percentile of all parametric bootstrap BMD values and the BMDU is the 95th percentile. Estimated BMD is based on the averaged response model which is a weighted average of the accepted models' response values.
Table B.4 shows final BMD, BMDL and BMDU values resulting from the calculations.
Table B.4.
Calculated BMD, BMDL and BMDU values (mg/kg bw per day) for combined incidences of megalocytic hepatocytes in male WT and TG mice after 26 weeks of oral application of fumonisin B1 using model averaging
| BMD | BMDL | BMDU |
|---|---|---|
| 0.3 | 0.1 | 1.9 |
Figure B.1 shows the different bootstrap curves based on model averaging.
Figure B.1.
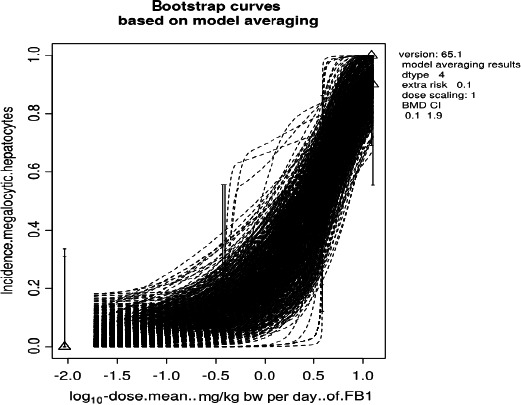
Averaged dose–response model for the incidence of megalocytic hepatocytes
B.3. Incidence of hepatic apoptosis
The combined incidence and severity of hepatic apoptosis in two strains of male mice (covariates WT and TG) treated orally with FB1 for 26 weeks (Bondy et al., 2012) were used for derivation of a BMD. Severity of lesions was designated into six classes: 0: not present, 1: minimal; 2: mild; 3: moderate; 4: marked; 5: severe. The cut‐off to consider a lesion was set to 2 for apoptotic lesions, i.e. lesions with score of 2 or higher were considered as an incident (see Table A.1, Appendix A)
Table B.5.
Observations of apoptotic lesions with a severity of 2 or more of apoptotic lesions in male mice treated with fumonisin B1
| Dose (mg/kg bw per day) | Incidence | N | Cov |
|---|---|---|---|
| 0.00 | 0 | 10 | TG |
| 0.37 | 0 | 10 | TG |
| 3.88 | 9 | 10 | TG |
| 12.60 | 10 | 10 | TG |
| 0.00 | 0 | 9 | WT |
| 0.39 | 0 | 10 | WT |
| 3.87 | 8 | 9 | WT |
| 12.20 | 10 | 10 | WT |
bw: body weight; N: number of animals; Cov: Covariant (mice of WT or TG strain).
Table B.7.
Results for incidences of apoptotic hepatocytes
| Model | Number of parameters | Log‐ likelihood | AIC | Accepted AIC | BMDL | BMDU | BMD | Converged |
|---|---|---|---|---|---|---|---|---|
| null | 1 | −53.96 | 109.92 | NA | NA | NA | NA | |
| full | 7 | −6.39 | 26.78 | NA | NA | NA | NA | |
| two.stage | 3 | −6.82 | 19.64 | no | NA | NA | NA | yes |
| log.logist | 3 | −6.39 | 18.78 | yes | 0.501 | 3.92 | 3.5 | yes |
| Weibull | 3 | −6.39 | 18.78 | yes | 0.511 | 3.92 | 3.3 | yes |
| log.prob | 3 | −6.39 | 18.78 | yes | 0.470 | 4.14 | 3.5 | yes |
| gamma | 3 | −6.39 | 18.78 | yes | 0.496 | 3.18 | 3.0 | yes |
| logistic | 2 | −6.39 | 16.78 | yes | 1.110 | 4.17 | 3.5 | yes |
| probit | 2 | −6.39 | 16.78 | yes | 0.972 | 4.12 | 3.5 | yes |
| LVM: Expon. m3‐ | 3 | −6.39 | 18.78 | yes | 0.491 | 3.68 | 3.4 | yes |
| LVM: Hill m3‐ | 3 | −6.39 | 18.78 | yes | 0.477 | 3.30 | 3.2 | yes |
AIC: Akaike information criterion; BMD: benchmark dose; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit; NA: Not applicable.
Table B.8.
Model weights in using model averaging
| Estimated model weights |
two. stage |
log. logistic |
Weibull |
log. prob |
gamma |
Log istic |
probit | EXP | HILL |
| 0.05 | 0.08 | 0.08 | 0.08 | 0.08 | 0.22 | 0.22 | 0.08 | 0.08 |
Given 1000 generated data sets, the BMDL is the 5th percentile of all parametric bootstrap BMD values and the BMDU is the 95th percentile. Estimated the BMD based on the averaged response model which is a weighted average of the accepted models' response values.
Table B.9.
Calculated BMD, BMDL and BMDU values (mg/kg bw per day) for combined incidences of apoptotic hepatocytes in male WT and TG mice after 26 weeks of oral application of fumonisin B1 using model averaging
| BMD | BMDL | BMDU |
|---|---|---|
| 3.26 | 1.2 | 3.72 |
BMD: benchmark dose; BMDL: benchmark dose lower confidence limit; BMDU: benchmark dose upper confidence limit.
Figure B.2.
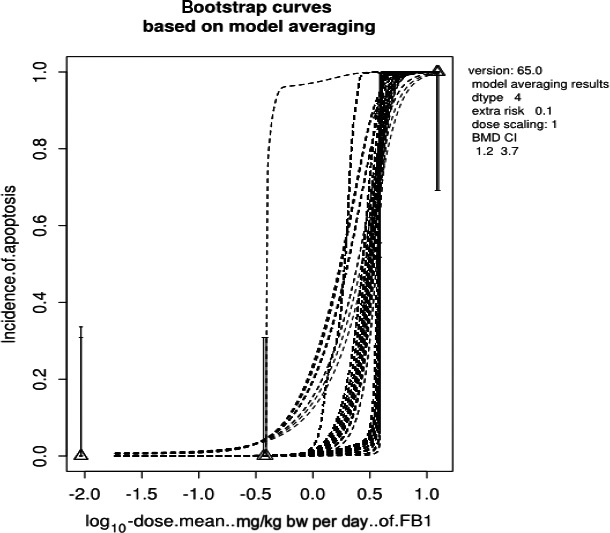
Averaged dose–response model for the incidence of apoptosis
Suggested citation: EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) , Knutsen H‐K, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, Cottrill B, Dinovi M, Edler L, Grasl‐Kraupp B, Hogstrand C, Hoogenboom LR, Nebbia CS, Petersen A, Rose M, Roudot A‐C, Schwerdtle T, Vleminckx C, Vollmer G, Wallace H, Dall'Asta C, Gutleb AC, Humpf H‐U, Galli C, Metzler M, Oswald IP, Parent‐Massin D, Binaglia M, Steinkellner H and Alexander J, 2018. Scientific opinion on the appropriateness to set a group health‐based guidance value for fumonisins and their modified forms. EFSA Journal 2018;16(2):5172, 75 pp. 10.2903/j.efsa.2018.5172
Requestor: European Commission
Question number: EFSA‐Q‐2015‐00227
Panel members: Jan Alexander, Lars Bårregard, Margherita Bignami, Beat Brüschweiler, Sandra Ceccatelli, Bruce Cottrill, Michael Dinovi, Lutz Edler, Bettina Grasl‐Kraupp, Christer Hogstrand, Laurentius (Ron) Hoogenboom, Helle Katrine Knutsen, Carlo Stefano Nebbia, Isabelle P Oswald, Annette Petersen, Martin Rose, Alain‐Claude Roudot, Tanja Schwerdtle, Christiane Vleminckx, Günter Vollmer and Heather Wallace.
Acknowledgements: The Panel wishes to thank the hearing expert Ronald T Riley for the support provided to this scientific output.
Adopted: 23 January 2018
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1148/full
Notes
EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. https://doi.org/10.2903/j.efsa.2014.3916 Available online: www.efsa.europa.eu/efsajournal
Summary report of the Standing Committee on Plants, Animals, Food and Feed, held in Brussels on 11 February 2015 (Section Toxicological Safety of the Food Chain), agenda item A.06. Report available at: http://ec.europa.eu/food/committees/regulatory/scfcah/toxic/docs/sum_20150211_en.pdf
It should be noted that in the previous opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed (EFSA CONTAM Panel 2014), the term modified mycotoxins included both covalently and non‐covalently bound forms for fumonisins in order not to underestimate exposure.
Council Regulation (EEC) No 315/93 of February 1993 laying down Community procedures for contaminants in food . OJ L 37, 13.2.1993, p. 1–5.
Regulation (EC) No 1881/2006 of the European Parliament and the Council of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. OJ L 364, 20.12.2006, p. 5–24.
Identifying articles that have been cited in articles found in a search.
Nixtamalised maize flour.
Processed in the same way as the nixtamalised material with the exception that the alkali was omitted from the cooking/steeping step.
Mono methylester is an artefact formed during the isolation procedure of fumonisins when methanol is used as a solvent.
Rumbling sounds caused by gas moving through the intestines, commonly referred to as stomach “growling”.
References
- Abbas HK, Cartwright RD, Xie W and Shier WT, 2006. Aflatoxin and fumonisin contamination of corn (maize, Zea mays) hybrids in Arkansas. Crop Protection, 25, 1–9. [Google Scholar]
- Abdel Salam G, Mehlab E and El‐Shishtawy M, 2012. Fumonisin lung toxicity: gross and microscopic changes are dose and time dependent. Journal of American Science, 8, 729–736. [Google Scholar]
- Abdellatef AA and Khalil AA, 2016. Ameliorated effects of Lactobacillus delbrueckii subsp. lactis DSM 20076 and Pediococcus acidilactici NNRL B‐5627 on Fumonisin B1‐induced hepatotoxicity and nephrotoxicity in rats. Asian Journal of Pharmaceutical Sciences, 11, 326–336. [Google Scholar]
- Abia WA, Warth B, Sulyok M, Krska R, Tchana AN, Njobeh PB, Dutton MF and Moundipa PF, 2013. Determination of multi‐mycotoxin occurrence in cereals, nuts and their products in Cameroon by liquid chromatography tandem mass spectrometry (LC‐MS/MS). Food Control, 31, 438–453. [Google Scholar]
- Abou‐Karam M, Abbas AK and Shier WT, 2004. N‐Fatty acylation of hydrolyzed Fumonisin B1, but not of intact Fumonisin B1, strongly enhances in vitro mammalian toxicity. Journal of Toxicology ‐ Toxin Reviews, 23, 123–151. [Google Scholar]
- Abrunhosa L, Calado T and Venancio A, 2011. Incidence of fumonisin B2 production by Aspergillus niger in portuguese wine regions. Journal of Agricultural and Food Chemistry, 59, 7514–7518. [DOI] [PubMed] [Google Scholar]
- Alizadeh AM, Rohandel G, Roudbarmohammadi S, Roudbary M, Sohanaki H, Ghiasian SA, Taherkhani A, Semnani S and Aghasi M, 2012. Fumonisin B1 contamination of cereals and risk of esophageal cancer in a high risk area in northeastern Iran. Asian Pacific Journal of Cancer Prevention, 13, 2625–2628. [DOI] [PubMed] [Google Scholar]
- Alizadeh AM, Mohammadghasemi F, Zendehdel K, Kamyabi‐Moghaddam Z, Tavassoli A, Amini‐Najafi F and Khosravi A, 2015. Apoptotic and proliferative activity of mouse gastric mucosa following oral administration of fumonisin B1. Iranian Journal of Basic Medical Sciences, 18, 8–13. [PMC free article] [PubMed] [Google Scholar]
- Aprodu I and Banu I, 2015. Co‐occurrence of fumonisins and T‐2 toxins in milling maize fractions under industrial conditions. CyTA‐Journal of Food, 13, 102–106. [Google Scholar]
- ApSimon JW, 2001. Structure, synthesis, and biosynthesis of Fumonisin B1 and related compounds. Environmental Health Perspectives, 109(Suppl. 2), 245–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker G, Sizoo E, Jekel A, Pereboom‐De Fauw DP, Schothorst R and Van Egmond H, 2009. Determination of mean daily intakes of aflatoxin B1, aflatoxin M1, ochratoxin a, trichothecenes and fumonisins in 24‐hour diets of children in the Netherlands. World Mycotoxin Journal, 2, 451–459. [Google Scholar]
- Bartke N and Hannun YA, 2009. Bioactive sphingolipids: metabolism and function. Journal of Lipid Research, 50(Suppl.), S91–S96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartók T, Tölgyesi L, Szekeres A, Varga M, Bartha R, Szécsi A, Bartók M and Mesterházy A, 2010a. Detection and characterization of twenty‐eight isomers of fumonisin B1 (FB1) mycotoxin in a solid rice culture infected with Fusarium verticillioides by reversed‐phase high‐performance liquid chromatography/electrospray ionization time‐of‐flight and ion trap mass spectrometry. Rapid Communications in Mass Spectrometry, 24, 35–42. [DOI] [PubMed] [Google Scholar]
- Bartók T, Tölgyesi L, Mesterházy Á, Bartók M and Szécsi Á, 2010b. Identification of the first fumonisin mycotoxins with three acyl groups by ESI‐ITMS and ESI‐TOFMS following RP‐HPLC separation: palmitoyl, linoleoyl and oleoyl EFB₁ fumonisin isomers from a solid culture of Fusarium verticillioides . Food Additives & Contaminants: Part A, 27, 1714–1723. [DOI] [PubMed] [Google Scholar]
- Bartók T, Tölgyesi L, Szécsi Á, Varga J, Bartók M, Mesterházy Á, Gyimes E and Véha A, 2013a. Identification of unknown isomers of fumonisin B5 mycotoxin in a Fusarium verticillioides culture by high‐performance liquid chromatography/electrospray ionization time‐of‐flight and ion trap mass spectrometry. Journal of Liquid Chromatography & Related Technologies, 36, 1549–1561. [Google Scholar]
- Bartók T, Szécsi Á, Juhász K, Bartók M and Mesterházy Á, 2013b. ESI‐MS and MS/MS identification of the first ceramide analogues of fumonisin B1 mycotoxin from a Fusarium verticillioides culture following RP‐HPLC separation. Food Additives & Contaminants: Part A, 30, 1651–1659. [DOI] [PubMed] [Google Scholar]
- Bartók T, Tölgyesi L, Szécsi Á, Mesterházy Á, Bartók M, Gyimes E and Véha A, 2014. Detection of previously unknown fumonisin P analogue mycotoxins in a Fusarium verticillioides culture by high‐performance liquid chromatography‐electrospray ionization time‐of‐flight and ion trap mass spectrometry. Journal of Chromatographic Science, 52, 508–513. [DOI] [PubMed] [Google Scholar]
- Battilani P, Pietri A, Barbano C, Scandolara A, Bertuzzi T and Marocco A, 2008. Logistic regression modeling of cropping systems to predict fumonisin contamination in maize. Journal of Agriculture and Food Chemistry, 56, 10433–10438. [DOI] [PubMed] [Google Scholar]
- Becker B, Bresch H, Schillinger U and Thiel PG, 1997. The effect of fumonisin B1 on the growth of bacteria. World Journal of Microbiology and Biotechnology, 13, 539–543. [Google Scholar]
- Becker‐Algeri TA, Heidtmann‐Bemvenuti R, Hackbart HCD and Badiale‐Furlong E, 2013. Thermal treatments and their effects on the fumonisin B1 level in rice. Food Control, 34, 488–493. [Google Scholar]
- Berardo N, Lanzanova C, Locatelli S, Lagana P, Verderio A and Motto M, 2011. Levels of total fumonisins in maize samples from Italy during 2006–2008. Food Additives and Contaminants: Part B, 4, 116–124. [DOI] [PubMed] [Google Scholar]
- Bertuzzi T, Rastelli S, Mulazzi A, Donadini G and Pietri A, 2011. Mycotoxin occurrence in beer produced in several European countries. Food Control, 22, 2059–2064. [Google Scholar]
- Bezuidenhout SC, Gelderblom WCA, Gorst‐Allman CP, Horak RM, Marasas WFO, Spiteller G and Vleggaar R, 1988. Structure elucidation of the fumonisins, mycotoxins from Fusarium moniliforme . Journal of the Chemical Society, Chemical Communications, 1988, 743–745. [Google Scholar]
- Blackwell BA, Gilliam JT, Savard ME, David Miller J and Duvick JP, 1999. Oxidative deamination of hydrolyzed fumonisin B(1) (AP(1)) by cultures of Exophiala spinifera . Natural Toxins, 7, 31–38. [DOI] [PubMed] [Google Scholar]
- Bondy GS, Mehta R, Caldwell D, Coady L, Armstrong C, Savard M, Miller JD, Chomyshyn E and Bronson R, 2010. Effects of long term exposure to fumonisin B1 on p53 +/− transgenic mice. Ottawa, Ontario, Canada, Health Canada, Health Products and Food Branch, Food Directorate, Bureau of Chemical Safety, Toxicology Research Division (unpublished).
- Bondy GS, Mehta R, Caldwell D, Coady L, Armstrong C, Savard M, Miller JD, Chomyshyn E, Bronson R, Zitomer NC and Riley RT, 2012. Effects of long term exposure to the mycotoxin fumonisin B1 in p53 heterozygous and p53 homozygous transgenic mice. Food and Chemical Toxicology, 50, 3604–3613. [DOI] [PubMed] [Google Scholar]
- Bracarense AP, Lucioli J, Grenier B, Drociunas Pacheco G, Moll WD, Schatzmayr G and Oswald IP, 2012. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. British Journal of Nutrition, 107, 1776–1786. [DOI] [PubMed] [Google Scholar]
- Branham BE and Plattner RD, 1993. Isolation and characterization of a new fumonisin from liquid cultures of Fusarium moniliforme . Journal of Natural Products, 56, 1630–1633. [Google Scholar]
- Broggi LE, Resnik SL, Pacin AM, Gonzalez HHL, Cano G and Taglieri D, 2002. Distribution of fumonisins in dry‐milled corn fractions in Argentina. Food Additives and Contaminants, 19, 465–469. [DOI] [PubMed] [Google Scholar]
- Bryla M, Roszko M, Szymczyk K, Jędrzejczak R, Słowik E and Obiedziński MW, 2014. The effect of baking on reduction of free and hidden fumonisins in gluten‐free bread. Journal of Agricultural and Food Chemistry, 09/2014, 10.1021/jf504077m [DOI] [PubMed] [Google Scholar]
- Bryla M, Roszko M, Szymczyk K, Jedrzejczak R and Obiedzinski MW, 2016. Fumonisins and their masked forms in maize products. Food Control, 59, 619–627. [Google Scholar]
- Bullerman LB, Ryu D and Jackson LS, 2002. Stability of fumonisins in food processing. Advances in Experimental Medicine and Biology, 2002, 195–204. [DOI] [PubMed] [Google Scholar]
- Burel C, Tanguy M, Guerre P, Boilletot E, Cariolet R, Queguiner M, Postollec G, Pinton P, Salvat G, Oswald IP and Fravalo P, 2013. Effect of low dose of fumonisins on pig health: Immune status, intestinal microbiota and sensitivity to Salmonella. Toxins, 5, 841–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns TD, Snook ME, Riley RT and Voss KA, 2008. Fumonisin concentrations and in vivo toxicity of nixtamalized Fusarium verticillioides culture material: Evidence for fumonisin–matrix interactions. Food and Chemical Toxicology, 46, 2841–2848. [DOI] [PubMed] [Google Scholar]
- Caloni F, Spotti M, Auerbach H, Op den Camp H, Fink‐Gremmels J and Pompa G, 2000. In vitro metabolism of Fumonisin B(1) by ruminal microflora. Veterinary Research Communications, 24, 379–387. [DOI] [PubMed] [Google Scholar]
- Caloni F, Spotti M, Pompa G, Zucco F, Stammati A and De Angelis I, 2002. Evaluation of fumonisin B1 and its metabolites absorption and toxicity on intestinal cells line Caco‐2. Toxicon, 40, 1181–1188. [DOI] [PubMed] [Google Scholar]
- Candlish A, Aidoo K, Smith J and Pearson S, 2000. A limited survey of aflatoxins and fumonisins in retail maize based products in the UK using immunoassay detection. Mycotoxin Research, 16, 2–8. [DOI] [PubMed] [Google Scholar]
- Cano‐Sancho G, Ramos AJ, Marin S and Sanchis V, 2012. Occurrence of fumonisins in Catalonia (Spain) and an exposure assessment of specific population groups. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 29, 799–808. [DOI] [PubMed] [Google Scholar]
- Castegnaro M, Garren L, Galendo D, Gelderblom WC, Chelule P, Dutton MF and Wild CP, 1998. Analytical method for the determination of sphinganine and sphingosine in serum as a potential biomarker for fumonisin exposure. Journal of Chromatography B: Biomedical Sciences and Applications, 720, 15–24. [DOI] [PubMed] [Google Scholar]
- Castells M, Marin S, Sanchis V and Ramos AJ, 2008. Distribution of fumonisins and aflatoxins in corn fractions during industrial cornflake processing. International Journal of Food Microbiology, 123, 81–87. [DOI] [PubMed] [Google Scholar]
- Castelo MM, Jackson LS, Hanna MA, Reynolds BH and Bullerman LB, 2001. Loss of Fumonisin B1 in extruded and baked corn‐based foods with sugars. Journal of Food Science, 66, 416–421. [Google Scholar]
- Castoria R, Lima G, Ferracane R and Ritieni A, 2005. Occurrence of mycotoxin in Farro samples from southern Italy. Journal of Food Protection, 68, 416–420. [DOI] [PubMed] [Google Scholar]
- Cawood ME, Gelderblom WCA, Vleggaar R, Behrend Y, Thiel PG and Marasas WFO, 1991. Isolation of the fumonisin mycotoxins: a quantitative approach. Journal of Agriculture and Food Chemistry, 39, 1958–1962. [Google Scholar]
- Cawood ME, Gelderblom WC, Alberts JF and Snyman SD, 1994. Interaction of 14C‐labelled fumonisin B mycotoxins with primary rat hepatocyte cultures. Food and Chemical Toxicology, 32, 627–632. [DOI] [PubMed] [Google Scholar]
- Cendoya E, Monge MP, Palacios SA, Chiacchiera SM, Torres AM, Farnochi MC and Ramirez ML, 2014. Fumonisin occurrence in naturally contaminated wheat grain harvested in Argentina. Food Control, 37, 56–61. [Google Scholar]
- Chehri K, Jahromi ST, Reddy KRN, Abbasi S and Salleh B, 2010. Occurrence of Fusarium spp. and fumonisins in stored wheat grains marketed in Iran. Toxins, 2, 2816–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelule PK, Gqaleni N, Dutton MF and Chuturgoon AA, 2001. Exposure of rural and urban populations in KwaZulu Natal, South Africa, to fumonisin B(1) in maize. Environmental Health Perspectives, 109, 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelule PK, Mbongwa HP, Carries S and Gqaleni N, 2010. Lactic acid fermentation improves the quality of amahewu, a traditional South African maize‐based porridge. Food Chemistry, 122, 656–661. [Google Scholar]
- Chiotta ML, Susca A, Stea G, Mule G, Perrone G, Logrieco A and Chulze SN, 2011. Phylogenetic characterization and ochratoxin A–fumonisin profile of black aspergillus isolated from grapes in Argentina. International Journal of Food Microbiology, 149, 171–176. [DOI] [PubMed] [Google Scholar]
- Christofidou M, Kafouris D, Christodoulou M, Stefani D, Christoforou E, Nafti G, Christou E, Aletrari M and Ioannou‐Kakouri E, 2015. Occurrence, surveillance, and control of mycotoxins in food in Cyprus for the years 2004‐2013. Food and Agricultural Immunology, 26, 880–895. [Google Scholar]
- Chu FS and Li GY, 1994. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from People's Republic of China in regions with high incidences of esophageal cancer. Applied Environmental Microbiology., 60, 847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuturgoon A, Phulukdaree A and Moodley D, 2014. Fumonisin B1 induces global DNA hypomethylation in HepG2 cells ‐ an alternative mechanism of action. Toxicology, 315, 65–69. [DOI] [PubMed] [Google Scholar]
- Cirlini M, Hahn I, Varga E, Dall'Asta M, Falavigna C, Calani L, Berthiller F, Del Rio D and Dall'Asta C, 2015. Hydrolysed fumonisin B1 and N‐(deoxy‐D‐fructos‐1‐yl)‐fumonisin B1: Stability and catabolic fate under simulated human gastrointestinal conditions. International Journal of Food Sciences and Nutrition, 66, 98–103. [DOI] [PubMed] [Google Scholar]
- Collins TF, Sprando RL, Black TN, Olejnik N, Eppley RM, Shackelford ME, Howard PC, Rorie JI and Bryant M, 2006. Ruggles DI 2006 Effects of aminopentol on in utero development in rats. Food and Chemical Toxicology, 44, 161–169. [DOI] [PubMed] [Google Scholar]
- Cornell J, Nelson MM and Beighton P, 1983. Neural tube defects in the Cape Town area, 1975–1980. South African Medical Journal, 64, 83–84. [PubMed] [Google Scholar]
- Dall'Asta C, Mangia M, Berthiller F, Molinelli A, Sulyok M, Schuhmacher R, Krska R, Galaverna G, Dossena A and Marchelli R, 2009. Difficulties in fumonisin determination: the issue of hidden fumonisins. Analytical and Bioanalytical Chemistry, 395, 1335–1345. [DOI] [PubMed] [Google Scholar]
- Dall'Asta C, Falavigna C, Galaverna G, Dossena A and Marchelli R, 2010. In vitro digestion assay for determination of hidden fumonisins in maize. Journal of Agriculture and Food Chemistry, 58, 12042–12047. [DOI] [PubMed] [Google Scholar]
- Dall'Asta C, Falavigna C, Galaverna G and Battilani P, 2012. Role of maize hybrids and their chemical composition in Fusarium infection and fumonisin production. Journal of Agricultural and Food Chemistry, 60, 3800–3808. [DOI] [PubMed] [Google Scholar]
- Dantzer WR, Hopper J, Mullin K, Hendrich S and Murphy PA, 1999. Excretion of 14C‐fumonisins B1, 14C‐hydrolyzed fumonisin B1 and 14C‐fumonisin B1‐fructose in rats. Journal of Agricultural and Food Chemistry, 47, 4291–4296. [DOI] [PubMed] [Google Scholar]
- D'Arco G, Fernandez‐Franzon M, Font G, Damiani P and Manes J, 2009. Survey of fumonisins B1, B2 and B3 in conventional and organic retail corn products in Spain and Italy and estimated dietary exposure. Food Additives & Contaminants Part B‐Surveillance, 2, 146–153. [DOI] [PubMed] [Google Scholar]
- De Angelis I, Frigge G, Raimondi F, Stammati A, Zucco F and Caloni F, 2005. Absorption of fumonisin B1 and aminopentol on an in vitro model of intestinal epithelium: the role of P‐glycoprotein. Toxicon, 45, 285–291. [DOI] [PubMed] [Google Scholar]
- De Girolamo A, Pascale M and Visconti A, 2011. Comparison of methods and optimisation of the analysis of fumonisins B1 and B2 in masa flour, an alkaline cooked corn product. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 28, 667–675. [DOI] [PubMed] [Google Scholar]
- De Girolamo A, Lattanzio VM, Schena R, Visconti A and Pascale M, 2014. Use of liquid chromatography‐high‐resolution mass spectrometry for isolation and characterization of hydrolyzed fumonisins and relevant analysis in maize‐based products. Journal of Mass Spectrometry, 49, 297–305. 10.1002/jms.3342 [DOI] [PubMed] [Google Scholar]
- De Girolamo A, Lattanzio VMT, Schena R, Visconti A and Pascale M, 2016. Effect of alkaline cooking of maize on the content of fumonisins B1 and B2 and their hydrolysed forms. Food Chemistry, 192, 1083–1089. [DOI] [PubMed] [Google Scholar]
- De La Campa R, Miller JD and Hendricks K, 2004. Fumonisin in tortillas produced in small‐scale facilities and effect of traditional masa production methods on this mycotoxin. Journal of Agriculture and Food Chemistry, 52, 4432–4437. [DOI] [PubMed] [Google Scholar]
- Delongchamp RR and Young JF, 2001. Tissue sphinganine as a biomarker of fumonisin‐induced apoptosis. Food Additives and Contaminants, 18, 255–261. [DOI] [PubMed] [Google Scholar]
- Desjardins AE, Busman M, Proctor RH and Stessman R, 2007. Wheat kernel black point and fumonisin contamination by Fusarium proliferatum . Food Additives & Contaminants, 24, 1131–1137. [DOI] [PubMed] [Google Scholar]
- Dombrink‐Kurtzman MA, 2003. Fumonisin and beauvericin induce apoptosis in turkey peripheral blood lymphocytes. Mycopathologia, 156, 357–364. [DOI] [PubMed] [Google Scholar]
- Dombrink‐Kurtzman MA, Dvorak TJ, Barron ME and Rooney LW, 2000. Effect of nixtamalization (alkaline cooking) on fumonisin‐contaminated corn for production of masa and tortillas. Journal of Agriculture and Food Chemistry, 48, 5781–5786. [DOI] [PubMed] [Google Scholar]
- Domijan AM and Abramov AY, 2011. Fumonisin B1 inhibits mitochondrial respiration and deregulates calcium homeostasis‐implication to mechanism of cell toxicity. International Journal of Biochemistry & Cell Biology, 43, 897–904. [DOI] [PubMed] [Google Scholar]
- Domijan A‐M, Gajski G, Jovanovic IN, Genic M and Garaj‐Vrhovac V, 2015. In vitro genotoxicity of mycotoxins ochratoxin A and fumonisin B1 could be prevented by sodium copper chlorophyllin – implication to their genotoxic mechanism. Food Chemistry, 170, 455–462. [DOI] [PubMed] [Google Scholar]
- Ediage EN, Diana Di Mavungu J, Song S, Van Peteghem C and De Saeger S, 2012. A direct assessement of mycotoxin biomarkers in human urine by liquid chromatography tandem mass spectrometry. Analytica Chimica Acta, 741, 58–69. [DOI] [PubMed] [Google Scholar]
- Ediage EN, Diana Di Mavungu J, Song S, Sioen I and De Saeger S, 2013. Multimycotoxin analysis in urines to assess infant exposure: a case study in Cameroon. Environment International, 57–58, 50–59. [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to fumonisins as undesirable substances in animal feed. EFSA Journal 2005;3(7):235, 32 pp. 10.2903/j.efsa.2005.235 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2014. Evaluation of the increase of risk for public health related to a possible temporary derogation from the maximum level of deoxynivalenol, zearalenone and fumonisins for maize and maize products. EFSA Journal 2014;12(5):3699, 61 pp. 10.2903/j.efsa.2014.3699 [DOI] [Google Scholar]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain), 2014. Scientific Opinion on the risks for human and animal health related to the presence of modified forms of certain mycotoxins in food and feed. EFSA Journal 2014;12(12):3916, 107 pp. 10.2903/j.efsa.2014.3916 [DOI] [Google Scholar]
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed), 2012. Guidance for the preparation of dossiers for sensory additives. EFSA Journal 2012;10(1):2534, 26 pp. 10.2903/j.efsa.2012.2534 [DOI] [Google Scholar]
- EFSA Scientific Committee , Hardy A, Benford D, Halldorsson T, Jeger MJ, Knutsen KH, More S, Mortensen A, Naegeli H, Noteborn H, Ockleford C, Ricci A, Rychen G, Silano V, Solecki R, Turck D, Aerts M, Bodin L, Davis A, Edler L, Gundert‐Remy U, Sand S, Slob W, Bottex B, Abrahantes JC, Marques DC, Kass G and Schlatter JR, 2017. Update: Guidance on the use of the benchmark dose approach in risk assessment. EFSA Journal 2017;15(1):4658, 41 pp. 10.2903/j.efsa.2017.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHC , 2000. Environmental Health Criteria 219: Fumonisin B1, International Programme on Chemical Safety (IPCS; UNEP, ILO and WHO). WHO, Geneva, 150 pp. [Google Scholar]
- Enongene EN, Sharma RP, Bhandari N, Meredith FI, Voss KA and Riley RT, 2002. Persistence and reversibility of the elevation in free sphingoid bases induced by fumonisin inhibition of ceramide synthase. Toxicological Sciences, 67, 173–181. [DOI] [PubMed] [Google Scholar]
- Eriksen GS and Alexander J (eds.), 1998. Fusarium toxins in cereals – a risk assessment. Nordic Council of Ministers; TemaNord 1998, 502, p. 7–27 and 45–58; Copenhagen.
- Ezekiel CN, Warth B, Ogara IM, Abia WA, Ezekiel VC, Atehnkeng J, Sulyok M, Turner PC, Tayo GO, Krska R and Bandyopadhyay R, 2014. Mycotoxin exposure in rural residents in northern Nigeria: a pilot study using multiurinary biomarkers. Environment International, 66, 138–145. [DOI] [PubMed] [Google Scholar]
- Falavigna C, Lazzaro I, Galaverna G, Battilani P and Dall'Asta C, 2013. Fatty acid esters of fumonisins: first evidence of their presence in maize. Food Additives and Contaminants. Part A, 30, 1606–1613. [DOI] [PubMed] [Google Scholar]
- Falavigna C, Lazzaro I, Galaverna G, Dall'Asta C and Battilani P, 2016. Oleoyl and linoleoyl esters of fumonisin B1 are differently produced by Fusarium verticillioides on maize and rice based media. International Journal of Food Microbiology, 217, 79–84. [DOI] [PubMed] [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2001. Safety evaluation of certain mycotoxins in food. WHO Food Additive Series 47 2001. Available online: http://www.inchem.org/documents/jecfa/jecmono/v47je01.htm
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2012. Safety evaluation of certain food additives and contaminants. Fumonisins. WHO Food Additives Series, 65, 325–794. [Google Scholar]
- FAO/WHO (Food and Agriculture Organization of the United Nations/World Health Organization), 2017. Evaluation of certain contaminants in food. Eighty third report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series 1002, 55‐74. Available online: http://apps.who.int/iris/bitstream/10665/254893/1/9789241210027-eng.pdf?ua=1
- Fincham JE, Marasas WFO, Taljaard JJF, Kriek NPJ, Badenhorst CJ, Gelderblom WCA, Seier JV, Smuts CM, Farber M, Weight MJ, Slazus W, Woodroof CW, Van Wyk MJ, Kruger M and Thiel PG, 1992. Atherogenic effects in a non‐human primate of Fusarium moniliforme cultures added to a carbohydrate diet. Atherosclerosis, 94, 13–25. [DOI] [PubMed] [Google Scholar]
- Flynn TJ, Stack ME, Troy AL and Chirtel SJ, 1997. Assessment of the Embryotoxic Potential of the Total Hydrolysis Product of Fumonisin B1 Using Cultured Organogenesis‐staged Rat Embryos. Food and Chemical Toxicology, 35, 1135–1141. [DOI] [PubMed] [Google Scholar]
- Fodor J, Meyer K, Riedlberger M, Bauer J, Horn P, Kovacs F and Kovacs M, 2006. Distribution and elimination of fumonisin analogues in weaned piglets after oral administration of Fusarium verticillioides fungal culture. Food Additives & Contaminants, 23, 492–501. [DOI] [PubMed] [Google Scholar]
- Fodor J, Meyer K, Gottschalk C, Mamet R, Kametler L, Bauer J, Horn P, Kovacs F and Kovacs M, 2007. In vitro microbial metabolism of fumonisin B1 . Food Additives & Contaminants, 24, 416–420. [DOI] [PubMed] [Google Scholar]
- Fodor J, Balogh K, Weber M, Mezes M, Kametler L, Posa R, Mamet R, Bauer J, Horn P and Kovacs F, 2008. Absorption, distribution and elimination of fumonisin B1 metabolites in weaned piglets. Food Additives & Contaminants, 25, 88–96. [DOI] [PubMed] [Google Scholar]
- Frisvad JC, Smedsgaard J, Samson RA, Larsen TO and Thrane U, 2007. Fumonisin B2 production by Aspergillus niger . Journal of Agriculture and Food Chemistry, 55, 9727–9732. [DOI] [PubMed] [Google Scholar]
- Gardner NM, Riley RT, Showker JL, Voss KA, Sachs AJ, Maddox JR and Gelineau‐van Waes JB, 2016. Elevated sphingoid base‐1‐phosphates and decreased histone deacetylase activity after fumonisin B1 treatment in mouse embryonic fibroblasts. Toxicology and Applied Pharmacology, 298, 58–65. [DOI] [PubMed] [Google Scholar]
- Gazzotti T, Lugoboni B, Zironi E, Barbarossa A, Serraino A and Pagliuca G, 2009. Determination of fumonisin B1 in bovine milk by LC–MS/MS. Food Control, 20, 1171–1174. [Google Scholar]
- Gbore FA, 2013. Protein profiles of serum, brain regions and hypophyses of pubertal boars fed diets containing fumonisins B1 . IFE Journal of Science, 15, 167–175. [Google Scholar]
- Gelderblom WCA, Jaskiewicz K, Marasas WFO, Thiel PG, Horak RM, Vleggaar R and Kriek NP, 1988. Fumonisins‐novel mycotoxins with cancer promoting activity produced by Fusarium moniliforme . Applied and Environment Microbiology, 54, 1806–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom WCA, Kriek NPJ, Marasas WFO and Thiel PG, 1991. Toxicity and carcinogenicity of the Fusarium moniliforme metabolite, fumonisin B1, in rats. Carcinogenesis, 12, 1247–1251. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Cawood ME, Snyman SD, Vleggaar R and Marasas WF, 1993. Structure‐activity relationships of fumonisins in short‐term carcinogenesis and cytotoxicity assays. Food and Chemical Toxicology, 31, 407–414. [DOI] [PubMed] [Google Scholar]
- Gelderblom WC, Abel S, Smuts CM, Marnewick J, Marasas WF, Lemmer ER and Ramljak D, 2001. Fumonisin‐induced hepatocarcinogenesis: mechanisms related to cancer initiation and promotion. Environmental Health Perspectives, 109(Suppl 2), 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderblom WCA, Sewram V, Shephard GS, Snijman PW, Tenza K, van der Westhuizen L and Vleggaar R, 2007. Structure and natural occurrence of stereoisomers of the fumonisin B series mycotoxins. Journal of Agriculture and Food Chemistry, 55, 4388–4394. [DOI] [PubMed] [Google Scholar]
- Gelineau‐van Waes J, Starr L, Maddox J, Aleman F, Voss KA, Wilberding J and Riley RT, 2005. Maternal fumonisin exposure and risk for neural tube defects: disruption of sphingolipid metabolism and folate transport in an in vivo mouse model. Birth Defects Research, 73, 487–497. [DOI] [PubMed] [Google Scholar]
- Generotti S, Cirlini M, Dall'Asta C and Suman M, 2015. Influence of the industrial process from caryopsis to cornmeal semolina on levels of fumonisins and their masked forms. Food Control, 48, 170–174. [Google Scholar]
- Gerding J, Cramer B and Humpf H‐U, 2014. Determination of mycotoxin exposure in Germany using an LC–MS/MS multibiomarker approach. Molecular Nutrition & Food Research, 58, 2358–2368. [DOI] [PubMed] [Google Scholar]
- Gerding J, Ali N, Schwartzbord J, Cramer B, Brown DL, Degen GH and Humpf H‐U, 2015. A comparative study of the human urinary mycotoxin excretion patterns in Bangladesh, Germany, and Haiti using a rapid and sensitive LC‐MS/MS approach. Mycotoxin Research, 31, 127–136. [DOI] [PubMed] [Google Scholar]
- Gong YY, Torres‐Sanchez L, Lopez‐Carrillo L, Peng JH, Sutcliffe AE, White KL, Humpf HU, Turner PC and Wild CP, 2008. Association between tortilla consumption and urinary fumonisin B1 levels in a Mexican population. Cancer Epidemiology and Biomarkers of Prevention, 17, 688–694. [DOI] [PubMed] [Google Scholar]
- Grenier B, Bracarense AP, Schwartz HE, Trumel C, Cossalter AM, Schatzmayr G, Kolf‐Clauw M, Moll WD and Oswald IP, 2012. The low intestinal and hepatic toxicity of hydrolyzed fumonisin B1 correlates with its inability to alter the metabolism of sphingolipids. Biochemical Pharmacology, 83, 1465–1473. [DOI] [PubMed] [Google Scholar]
- Hahn I, Nagl V, Schwartz‐Zimmermann HE, Varga E, Schwarz C, Slavik V, Reisinger N, Malachova A, Cirlini M, Generotti S, Dall'Asta C, Krska R, Moll WD and Berthiller F, 2015. Effects of orally administered fumonisin B1 (FB1), partially hydrolysed FB1, hydrolysed FB1 and N‐(1‐deoxy‐D‐fructos‐1‐yl) FB1 on the sphingolipid metabolism in rats. Food and Chemical Toxicology, 76, 11–18. [DOI] [PubMed] [Google Scholar]
- Harrer H, Laviad EL, Humpf H‐U and Futerman AH, 2013. Identification of N‐acyl‐fumonisin B1 as new cytotoxic metabolites of fumonisin mycotoxins. Molecular Nutrition & Food Research, 57, 516–522. [DOI] [PubMed] [Google Scholar]
- Harrer H, Humpf HU and Voss KA, 2015. In vivo formation of N‐acyl‐fumonisin B1. Mycotoxin Research, 31, 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartinger D, Schwartz H, Hametner C, Schatzmayr G, Haltrich D and Moll WD, 2011. Enzyme characteristics of aminotransferase FumI of Sphingopyxis sp. MTA144 for deamination of hydrolyzed fumonisin B₁. Applied Microbiology and Biotechnology, 91, 757–768. 10.1007/s00253-011-3248-9. Epub 2011 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl M and Humpf HU, 2000. Toxicity assessment of fumonisins using the brine shrimp (Artemia salina) bioassay. Food and Chemical Toxicology, 38, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Hartl M and Humpf H‐U, 2001. Combined synthetic/CD strategy for the stereochemical assignment of the tricarballylic acid side chains of fumonisin B1 . Journal of Organic Chemistry, 66, 3678–3681. [DOI] [PubMed] [Google Scholar]
- Hendrich S, Miller KA, Wilson TM and Murphy PA, 1993. Toxicity of fusarium proliferatum‐fermented nixtamalized corn‐based diets fed to rats: effect of nutritional status. Journal of Agricultural and Food Chemistry, 41, 1649–1654. [Google Scholar]
- Hendricks K, 1999. Fumonisins and neural tube defects in south Texas. Epidemiology, 10, 198–200. [DOI] [PubMed] [Google Scholar]
- Heyndrickx E, Sioen I, Huybrechts B, Callebaut A, De Henauw S and De Saeger S, 2015. Human biomonitoring of multiple mycotoxins in the Belgian population: Results of the BIOMYCO study. Environment International, 84, 82–89. [DOI] [PubMed] [Google Scholar]
- Howard Paul C, Couch LH, Patton RE, Eppley RM, Doerge DR, Churchwell MI, Marques MM and Okerberg CV, 2002. Comparison of the toxicity of several fumonisin derivatives in a 28‐day feeding study with female B6C3F1 mice. Toxicology and Applied Pharmacology, 185, 153–165. [DOI] [PubMed] [Google Scholar]
- Hübner F, Harrer H, Fraske A, Kneifel S and Humpf HU, 2012. Large scale purification of b‐type fumonisins using centrifugal partition chromatography (CPC). Mycotoxin Research, 28, 37–43. [DOI] [PubMed] [Google Scholar]
- Huffman J, Gerber R and Du L, 2010. Recent advancements in the biosynthetic mechanisms for polyketide‐derived mycotoxins. Biopolymers, 93, 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpf H‐U and Voss KA, 2004. Effects of thermal food processing on the chemical structure and toxicity of fumonisin mycotoxins. Molecular Nutrition & Food Research, 48, 255–269. [DOI] [PubMed] [Google Scholar]
- Humpf H‐U, Schmelz E‐M, Meredith FI, Vesper H, Vales TR, Wang E, Menaldino DS, Liotta DC and Merill AH Jr, 1998. Acylation of naturally occurring and synthetic 1‐deoxysphinganines by ceramide synthase. Formation of N‐palmitoyl‐aminopentol produces a toxic metabolite of hydrolyzed fumonisin, AP1, and a new category of ceramide synthase inhibitor. Journal of Biological Chemistry, 1998, 19060–19064. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 56, Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Press, Lyon. pp. 445–466. [Google Scholar]
- IARC (International Agency for Research on Cancer), 2002. Fumonisin B1. IARC (International Agency for Research on Cancer). Monographs on the Evaluation of Carcinogenic Risk to Humans, 82, 275–366 [Google Scholar]
- Jaksic S, Abramovic B, Jajic I, Balos MZ, Mihaljev Z, Despotovic V and Sojic D, 2012. Co‐occurrence of fumonisins and deoxynivalenol in wheat and maize harvested in Serbia. Bulletin of Environment Contamination and Toxicology, 89, 615–619. [DOI] [PubMed] [Google Scholar]
- Jalali S and Wohlin C, 2012. Systematic literature studies: Database searches vs. backward snowballing. 6th ACM‐IEEE International Symposium on Empirical Software Engineering and Measurement. Lund. Available online: http://www.diva-portal.org/smash/get/diva2:834640/FULLTEXT01.pdf
- van Jaskiewicz K, Rensberg SJ, Marasas WFO and Gelderblom WCA, 1987a. Carcinogenicity of Fusarium moniliforme culture material in rats. Journal of the National Cancer Institute, 78, 321–325. [PubMed] [Google Scholar]
- Jaskiewicz K, Marasas WFO and Van der Walt FE, 1987b. Oesophageal and other main cancer patterns in four districts of Transkei, 1981–1984. South African Medical Journal, 72, 27–30. [PubMed] [Google Scholar]
- Karuna R and Rao BS, 2013. Lack of micronuclei induction by fumonisin B1 mycotoxin in BALB/c mice. Mycotoxin Research, 29, 9–15. [DOI] [PubMed] [Google Scholar]
- Kim E‐K, Scott PM and Lau BP‐Y, 2003. Hidden fumonisins in corn flakes. Food Additives & Contaminants, 20, 161–169. [DOI] [PubMed] [Google Scholar]
- Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C and Kolsteren P, 2010. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Molecular Nutrition & Food Research, 54, 1659–1667. [DOI] [PubMed] [Google Scholar]
- Knudsen PB, Mogensen JM, Larsen TO and Nielsen KF, 2011. Occurrence of furnonisins B2 and B4 in retail raisins. Journal of Agricultural and Food Chemistry, 59, 772–776. [DOI] [PubMed] [Google Scholar]
- Kodell RL, Young JF, Delongchamp RR, Turturro A, Chen JJ, Gaylor DW, Howard PC and Zheng Q, 2001. A mechanistic approach to modelling the risk of liver tumours in mice exposed to fumonisin B1 in the diet. Food Additives & Contaminants, 18, 237–253. [DOI] [PubMed] [Google Scholar]
- Kouadio JH, Moukha S, Brou K and Gnakri D, 2013. Lipid metabolism disorders, lymphocytes cells death, and renal toxicity induced by very low levels of deoxynivalenol and fumonisin B1 alone or in combination following 7 days oral administration to mice. Toxicology International, 20, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouadio JH, Lattanzio VM, Ouattara D, Kouakou B and Visconti A, 2014. Assessment of mycotoxin exposure in Côte d'Ivoire (Ivory Coast) through multi‐biomarker analysis and possible correlation with food consumption patterns. Toxicology International, 21, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalsky P, Kos G, Nährer K, Schwab C, Jenkins T, Schatzmayr G, Sulyok M and Krska R, 2016. Co‐occurrence of regulated, masked and emerging mycotoxins and secondary metabolites in finished feed and maize‐an extensive survey. Toxins (Basel), 8, pii: E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence JF, Menard C, Yeung J and Ben Rejeb S, 2000. Evaluation of silica‐ and sepharose‐based immunoaffinity sorbents for sample cleanup in determination of fumonisins B1 and B2 in corn products. Journal of AOAC International, 83, 597–603. [PubMed] [Google Scholar]
- Liu H, Lu Y, Haynes JS, Cunnick JE, Murphy P and Hendrich S, 2001. Reaction of fumonisin with glucose prevents promotion of hepatocarcinogenesis in female F344/N rats while maintaining normal hepatic sphinganine/sphingosine ratios. Journal of Agriculture and Food Chemistry, 49, 4113–4121. [DOI] [PubMed] [Google Scholar]
- Logrieco A, Moretti A, Ritieni A, Bottalico A and Corda P, 1995. Occurrence and toxigenicity of Fusarium proliferatum from preharvest maize ear rot, and associated mycotoxin, Italy. Plant Disease, 79, 727–731. [Google Scholar]
- Logrieco A, Ferracane R, Haidukowsky M, Cozzi G, Visconti A and Ritieni A, 2009. Fumonisin B2 production by Aspergillus niger from grapes and natural occurrence in must. Food Additives and Contaminants, 26, 1495–1500. [DOI] [PubMed] [Google Scholar]
- Logrieco A, Ferracane R, Visconti A and Ritieni A, 2010. Natural occurrence of fumonisin B2 in red wine from Italy. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 27, 1136–1141. [DOI] [PubMed] [Google Scholar]
- Logrieco AF, Ferracane R, Cozzi G, Haidukowsky M, Susca A, Mule G and Ritieni A, 2011. Fumonisin B2 by Aspergillus niger in the grape‐wine chain: An additional potential mycotoxicological risk. Annals of Microbiology, 61, 1–3. [Google Scholar]
- Logrieco AF, Haidukowski M, Susca A, Mule G, Munkvold GP and Moretti A, 2014. Aspergillus section Nigri as contributor of fumonisin B2 contamination in maize. Food Additives and Contaminants Part A‐Chemistry Analysis Control Exposure & Risk Assessment, 31, 149–155. [DOI] [PubMed] [Google Scholar]
- Loiseau N, Polizzi A, Dupuy A, Therville N, Rakotonirainy M, Loy J, Viadere JL, Cossalter AM, Bailly JD, Puel O, Kolf‐Clauw M, Bertrand‐Michel J, Levade T, Guillou H and Oswald IP, 2015. New insights into the organ‐specific adverse effects of fumonisin B1: Comparison between lung and liver. Archives of Toxicology, 89, 1619–1629. [DOI] [PubMed] [Google Scholar]
- MacKenzie SE, Savard ME, Blackwell BA, Miller JD and ApSimon JW, 1998. Isolation of a new fumonisin from Fusarium moniliforme grown in liquid culture. Journal of Natural Products, 61, 367–369. [DOI] [PubMed] [Google Scholar]
- Magoha H, De Meulenaer B, Kimanya M, Hipolite D, Lachat C and Kolsteren P, 2014a. Fumonisin B1 contamination in breast milk and its exposure in infants under 6 months of age in Rombo, Northern Tanzania. Food and Chemical Toxicology, 74, 112–116. [DOI] [PubMed] [Google Scholar]
- Magoha H, Kimanya M, De Meulenaer B, Roberfroid D, Lachat C and Kosteren P, 2014b. Association between aflatoxin M1 exposure through breast milk and growth impairment in infants from Northern Tanzania. World Mycotoxin Journal, 7, 277–284. [Google Scholar]
- Magoha H, Kimanya M, De Meulenaer B, Roberfroid D, Lachat C and Kolsteren P, 2016. Risk of dietary exposure to aflatoxins and fumonisins in infants less than 6 months of age in Rombo, Northern Tanzania. Maternal & Child Nutrition, 12, 516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makaula NA, Marasas WFO, Venter FS, Badenhorst CJ, Bradshaw D and Swanevelder S, 1996. Oesophageal and other cancer patterns in four selected districts of Transkei, Southern Africa: 1985–1990. African Journal of Health Sciences, 3, 11–15. [PubMed] [Google Scholar]
- Mansson M, Klejnstrup ML, Phipps RK, Nielsen KF, Frisvad JC, Gotfredsen CH and Larsen TO, 2010. Isolation and NMR characterization of fumonisin B2 and a new fumonisin B6 from Aspergillus niger . Journal of Agriculture and Food Chemistry, 58, 949–953. [DOI] [PubMed] [Google Scholar]
- Marasas WF, Kriek NP, Wiggins VM, Steyn PS, Towers DK and Hastie TJ, 1979. Incidence, geographical distribution and toxigenicity of Fusarium species in South African corn. Phytopathology, 69, 1181–1185. [Google Scholar]
- Marasas WFO, Wehner FC, Van Rensburg SJ and Van Schalkwyk DJ, 1981. Mycoflora of corn produced in human oesophageal cancer areas in Transkei, Southern Africa. Phytopathology, 71, 792–796. [Google Scholar]
- Marasas WFO, Jaskiecz K, Venter FS and Van Schalkwyk DJ, 1988. Fusarium moniliforme contamination of maize in oesophageal cancer areas in Transkei. South Africa Medical Journal, 74, 110–114. [PubMed] [Google Scholar]
- Marasas WFO, Riley RT, Hendricks KA, Stevens VL, Sadler TW, Gelineau‐van Waes J, Missmer SA, Cabrera J, Torres O, Gelderblom WC, Allegood J, Martínez C, Maddox J, Miller JD, Starr L, Sullards MC, Roman AV, Voss KA, Wang E and Merrill AH Jr, 2004. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin‐contaminated maize. Journal of Nutrition, 134, 711–716. [DOI] [PubMed] [Google Scholar]
- Marin DE, Gouze ME, Taranu I and Oswald IP, 2007. Fumonisin B1 alters cell cycle progression and interleukin‐2 synthesis in swine peripheral blood mononuclear cells. Molecular Nutrition & Food Research, 51, 1406–1412. [DOI] [PubMed] [Google Scholar]
- Marín P, Magan N, Vázquez C and González‐Jaén MT, 2010. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum . FEMS Microbiology Ecology, 73, 303–311. 10.1111/j.1574-6941.2010.00894.x Epub 2010 Apr 23. [DOI] [PubMed] [Google Scholar]
- Martinez‐Larranaga MR, Anadon A, Diaz MJ, Fernandez‐Cruz ML, Martinez MA, Frejo MT, Martinez M, Fernandez R, Anton RM, Morales ME and Tafur M, 1999. Toxicokinetics and oral bioavailability of fumonisin B1. Veterinary and Human Toxicology, 41, 357–362. [PubMed] [Google Scholar]
- Martins ML, Martins HM and Bernardo F, 2001. Fumonisins B1 and B2 in black tea and medicinal plants. Journal of Food Protection, 64, 1268–1270. [DOI] [PubMed] [Google Scholar]
- Masching S, Naehrer K, Schwartz‐Zimmermann HE, Sarandan M, Schaumberger S, Dohnal I, Nagl V and Schatzmayr D, 2016. Gastrointestinal degradation of fumonisin B1 by carboxylesterase FumD prevents fumonisin induced alteration of sphingolipid metabolism in turkey and swine. Toxins, 8, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo Y, Takahara K, Sago Y, Kushiro M, Nagashima H and Nakagawa H, 2015. Detection of N‐(1‐deoxy‐D‐fructos‐1‐yl) Fumonisins B₂ and B₃ in corn by high‐resolution LC‐Orbitrap MS. Toxins, 7, 3700–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meca G, Fernández‐Franzón M, Ritieni A, Font G, Ruiz MJ and Mañes J, 2010. Formation of fumonisin B1‐glucose reaction product, in vitro cytotoxicity, and lipid peroxidation on kidney cells. Journal of Agriculture and Food Chemistry, 58, 1359–1365. [DOI] [PubMed] [Google Scholar]
- Meyer K, Mohr K, Bauer J, Horn P and Kovacs M, 2003. Residue formation of fumonisin B1 in porcine tissues. Food Additives & Contaminants, 20, 639–647. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Suarez L, Felkner M, Wang E, Merrill AH Jr, Rothman KJ and Hendricks KA, 2006. Exposure to fumonisins and the occurrence of neural tube defects along the Texas‐Mexico border. Environmental Health Perspectives, 114, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobio TA, Tavan E, Baudrimont I, Anane R, Carratu MR, Sanni A, Gbeassor MF, Shier TW, Narbonne JF and Creppy EE, 2003. Comparative study of the toxic effects of fumonisin B1 in rat C6 glioma cells and p53‐null mouse embryo fibroblasts. Toxicology, 183, 65–75. [DOI] [PubMed] [Google Scholar]
- Mogensen JM, Frisvad JC, Thrane U and Nielsen KF, 2010. Production of fumonisin B2 and B4 by Aspergillus niger on grapes and raisins. Journal of Agriculture and Food Chemistry, 58, 954–958. [DOI] [PubMed] [Google Scholar]
- Mokoena MP, Chelule PK and Gqaleni N, 2005. Reduction of fumonisin B1 and zearalenone by lactic acid bacteria in fermented maize meal. Journal of Food Protection, 68, 2095–2099. [DOI] [PubMed] [Google Scholar]
- Monbaliu S, Van Poucke C, Van Peteghem C, Van Poucke K, Heungens K and De Saeger S, 2009. Development of a multi‐mycotoxin liquid chromatography/tandem mass spectrometry method for sweet pepper analysis. Rapid Communications in Mass Spectrometry, 23, 3–11. [DOI] [PubMed] [Google Scholar]
- Monbaliu S, Wu A, Zhang D, Van Peteghem C and De Saeger S, 2010. Multimycotoxin UPLC‐MS/MS for tea, herbal infusions and the derived drinkable products. Journal of Agricultural and Food Chemistry, 58, 12664–12671. [DOI] [PubMed] [Google Scholar]
- Moore CA, Li S, Li Z, Hong S, Gu H, Berry RJ, Mulinare J and Erickson JD, 1997. Elevated rates of severe neural tube defects in a high‐prevalence area in northern China. American Journal of Medical Genetics, 73, 113–118. [PubMed] [Google Scholar]
- Musser SM and Plattner RD, 1997. Fumonisin composition in cultures of Fusarium moniliforme, Fusarium proliferatum, and Fusarium nygami . Journal of Agriculture and Food Chemistry, 45, 1169–1173. [Google Scholar]
- Musser SM, Eppley RM and Mazzola EP, 1995. Identification of an N‐acetyl keto derivative of fumonisin B1 in corn cultures of Fusarium proliferatum . Journal of Natural Products, 58, 1392–1397. [DOI] [PubMed] [Google Scholar]
- Musser SM, Gay ML and Mazzola EP, 1996. Identification of a new series of fumonisins containing 3‐hydroxypyridine. Journal of Natural Products, 59, 970–972. [DOI] [PubMed] [Google Scholar]
- Mylona K, Sulyok M and Magan N, 2012. Relationship between environmental factors, dry matter loss and mycotoxin levels in stored wheat and maize infected with Fusarium species. Food Additives and Contaminants, 29, 1118–1128. [DOI] [PubMed] [Google Scholar]
- Ncayiyana DJ, 1986. Neural tube defects among rural blacks in a Transkei district. South African Medical Journal, 69, 618–620. [PubMed] [Google Scholar]
- NFI‐DTU (National Food Institute‐Technical University of Denmark), 2018. Extensive literature search for studies related to fumonisins and their modified forms. EFSA supporting publication 2018:EN‐1148. 90 pp. 10.2903/sp.efsa.2018.en-1148 [DOI]
- Nielsen KF, Mogensen JM, Johansen M, Larsen TO and Frisvad JC, 2009. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Analytical and Bioanalytical Chemistry, 395, 1225–1242. [DOI] [PubMed] [Google Scholar]
- Norred WP, Wang E, Yoo H, Riley RT and Merril AH, 1992. In vitro toxicology of fumonisins and the mechanistic implications. Mycopathologia, 117, 73–78. [DOI] [PubMed] [Google Scholar]
- Norred WP, Voss KA, Riley RT and Plattner RD, 1996. Fumonisin toxicity and metabolism studies at the USDA. Fumonisin toxicity and metabolism. Advances in Experimental Medicine and Biology, 392, 225–236. [DOI] [PubMed] [Google Scholar]
- Norred WP, Plattner RD, Dombrink‐Kurtzman MA, Meredith FI and Riley RT, 1997. Mycotoxin‐induced elevation of free sphingoid bases in precision‐cut rat liver slices: specificity of the response and structure‐activity relationships. Toxicology and Applied Pharmacology, 147, 63–70. [DOI] [PubMed] [Google Scholar]
- Norred WP, Riley RT, Meredith FI, Poling SM and Plattner RD, 2001. Instability of N‐acetylated fumonisin B1 (FA1) and the impact on inhibition of ceramide synthase in rat liver slices. Food and Chemical Toxicology, 39, 1071–1078. [DOI] [PubMed] [Google Scholar]
- NTP (National Toxicology Program), 1999. Toxicology and Carcinogenesis Studies of Fumonisin B1 (CAS No 116355‐83‐0) in F344/N Rats and B6C3F, Mice (Feed Studies) (Draft NTP Technical Report TR 496; NIH Publication No 99‐3955. Final report approved 2001), Research Triangle Park, North Carolina.
- Oliveira MS, Diel ACL, Rauber RH, Fontoura FP, Mallmann A, Dilkin P and Mallmann CA, 2015. Free and hidden fumonisins in Brazilian raw maize samples. Food Control, 53, 217–221. [Google Scholar]
- Palencia E, Torres O, Hagler W, Meredith FI, Williams LD and Riley RT, 2003. Total fumonisins are reduced in tortillas using the traditional nixtamalization method of Mayan communities. Journal of Nutrition, 133, 3200–3203. [DOI] [PubMed] [Google Scholar]
- Park JW, Scott PM, Lau BP‐Y and Lewis DA, 2004. Analysis of heat processed corn foods for fumonisins and bound fumonisins. Food Additives & Contaminants, 21, 1168–1178. [DOI] [PubMed] [Google Scholar]
- Park JW, Scott PM and Lau BPY, 2013. Analysis of n‐fatty acyl fumonisins in alkali‐processed corn foods. Food Science and Biotechnology, 22, 147–152. [Google Scholar]
- Pascale M, Doko MB and Visconti A, 1995. Determination of fumonisins in polenta by high performance liquid chromatography. In: Atti 2 Congresso Nazionale di Chimica degli Alimenti, Giardini‐Naxos, Italy, 1067–1071 (Italian).
- Pellanda H, Forges T, Bressenot A, Chango A, Bronowicki JP, Guéant JL and Namour F, 2012. Fumonisin FB1 treatment acts synergistically with methyl donor deficiency during rat pregnancy to produce alterations of H3‐ and H4‐histone methylation patterns in fetuses. Molecular Nutrition & Food Research, 56, 976–985. [DOI] [PubMed] [Google Scholar]
- Persson EC, Sewram V, Evans AA, London WT, Volkwyn Y, Shen Y‐J, Van Zyl JA, Chen G, Lin W, Shephard GS, Taylor PR, Fan J‐H, Dawsey SM, Qiao Y‐L, McGlynn KA and Abnet CC, 2012. FB1 and risk of hepatocellular carcinoma in two Chinese cohorts. Food and Chemical Toxicology, 50, 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pewzner‐Jung Y, Park H, Laviad EL, Silva LC, Lahiri S, Stiban J, Erez‐Roman R, Brügger B, Sachsenheimer T, Wieland F, Prieto M, Merrill AH Jr and Futerman AH, 2010. A critical role for ceramide synthase 2 in liver homeostasis: I. alterations in lipid metabolic pathways. Journal of Biological Chemistry, 285, 10902–10910. 10.1074/jbc.m109.077594. Epub 2010 Jan 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri A and Bertuzzi T, 2012. Simple phosphate buffer extraction for the determination of fumonisins in masa, maize, and derived products. Food Analytical Methods, 5, 1088–1096. [Google Scholar]
- Prelusky DB, Savard ME and Trenholm HL, 1994. Pharmacokinetic fate of 14C‐labelled fumonisin B1 in swine. Natural Toxins, 2, 73–80. [DOI] [PubMed] [Google Scholar]
- Prelusky DB, Trenholm HL and Savard ME, 1995. Pilot study on the plasma pharmacokinetics of fumonisin B1 in cows following a single dose by oral gavage or intravenous administration. Natural Toxins, 3, 389–394. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Desjardins AE, Plattner RD and Hohn TM, 1999. A polyketide synthase gene required for biosynthesis of fumonisin mycotoxins in Gibberella fujikuroi mating population A. Fungal Genetics and Biology, 7, 100–112. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Brown DW, Plattner RD and Desjardins AE, 2003. Co‐expression of 15 contiguous genes delineates a fumonisin biosynthetic gene cluster in Gibberella moniliformis . Fungal Genetics and Biology, 38, 237–249. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Busman M, Seo JA, Lee YW and Plattner RD, 2008. A fumonisin biosynthetic gene cluster in Fusarium oxysporum strain O‐1890 and the genetic basis for B versus C fumonisin production. Fungal Genetics and Biology, 45, 1016–1026. [DOI] [PubMed] [Google Scholar]
- Qian G, Tang L, Lin S, Xue KS, Mitchell NJ, Su J, Gelderblom WC, Riley RT, Phillips TD and Wang JS, 2016. Sequential dietary exposure to aflatoxin B1 and fumonisin B1 in F344 rats increases liver preneoplastic changes indicative of a synergistic interaction. Food and Chemical Toxicology, 95, 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi TF, Renaud JB, McDowell T, Seifert KA, Yeung KK and Sumarah MW, 2016. Diversity of Mycotoxin‐Producing Black Aspergilli in Canadian Vineyards. Journal of Agricultural and Food Chemistry, 64, 1583–1589. 10.1021/acs.jafc.5b05584. Epub 2016 Feb 11 [DOI] [PubMed] [Google Scholar]
- Resch P and Shier WT, 2000. The fate of fumonisin during thermal food processing. Lebensmittelchemie, 54, 33. [Google Scholar]
- Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, Shephard GS and Van Schalkwyk DJ, 1992. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology, 82, 353–357. [Google Scholar]
- Ribeiro DH, Ferreira FL, da Silva VN, Aquino S and Correa B, 2010. Effects of aflatoxin B1 and fumonisin B1 on the viability and induction of apoptosis in rat primary hepatocytes. International Journal of Molecular Sciences, 11, 1944–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RT, Showker JL, Owens DL and Ross PF, 1997. Disruption of sphingolipid metabolism and induction of equine leukoencephalomalacia by Fusarium proliferatum culture material containing fumonisin B2 or B3 . Environmental Toxicology and Pharmacology, 3, 221–228. [DOI] [PubMed] [Google Scholar]
- Riley RT and Voss KA, 2006. Differential sensitivity of rat kidney and liver to fumonisin toxicity: Organ‐specific differences in toxin accumulation and sphingoid base metabolism. Toxicological Sciences, 92, 335–345. [DOI] [PubMed] [Google Scholar]
- Riley RT, Hinton DM, Chamberlain WJ, Bacon CW, Wang E, Merrill AH Jr and Voss KA, 1994. Dietary fumonisin B1 induces disruption of sphingolipid metabolism in Sprague‐Dawley rats: A new mechanism of nephrotoxicity. Journal of Nutrition, 124, 594–603. [DOI] [PubMed] [Google Scholar]
- Riley RT, Torres O, Showker JL, Zitomer NC, Matute J, Voss KA, Gelineau‐van Waes J, Maddox JR, Gregory SG and Ashley‐Koch AE, 2012. The kinetics of urinary fumonisin B1 excretion in humans consuming maize‐based diets. Molecular Nutrition and Food Research, 56, 1445–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RT, Torres O, Matute J, Gregory SG, Ashley‐Koch AE, Showker JL, Mitchell T, Voss KA, Maddox JR and Gelineau‐van Waes JB, 2015a. Evidence for fumonisin inhibition of ceramide synthase in humans consuming maize‐based foods and living in high exposure communities in Guatemala. Molecular Nutrition & Food Research, 59, 2209–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RT, Showker JL, Lee CM, Zipperer CE, Mitchell TR, Voss KA, Zitomer NC, Torres O, Matute J, Gregory SG, Ashley‐Koch AE, Maddox JR, Gardner N and Gelineau‐van Waes JB, 2015b. A blood spot method for detecting fumonisin‐induced changes in putative sphingolipid biomarkers in LM/Bc mice and humans. Food Additives and Contaminants Part A, 32, 934–949. [DOI] [PubMed] [Google Scholar]
- Robinson A, Johnson NM, Strey A, Taylor JF, Marroquin‐Cardona A, Mitchell NJ, Afriyie‐Gyawu E, Ankrah NA, Williams JH, Wang JS and Jolly PE, 2012. Calcium montmorillonite clay reduces urinary biomarkers of fumonisin B1 exposure in rats and humans. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 29, 809–818. Published online 2012 Feb 10. 10.1080/19440049.2011.651628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PF, Rice LG, Reagor JC, Osweiler GD, Wilson TM, Nelson HA, Owens DL, Plattner RD, Harlin KA and Richard JL, 1991. Fumonisin B1 concentrations in feeds from 45 confirmed equine leukoencephalomalacia cases. Journal of Veterinary Diagnostic Investigation, 3, 238–241. [DOI] [PubMed] [Google Scholar]
- Rubert J, Soler C, Marin R, James KJ and Manes J, 2013. Mass spectrometry strategies for mycotoxins analysis in european beers. Food Control, 30, 122–128. [Google Scholar]
- Rychlik M, Humpf HU, Marko D, Dänicke S, Mally A, Berthiller F, Klaffke H and Lorenz N, 2014. Proposal of a comprehensive definition of modified and other forms of mycotoxins including “masked” mycotoxins. Mycotoxin Research, 30, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak D and Ozden S, 2015. Global histone modifications in fumonisin B1 exposure in rat kidney epithelial cells. Toxicology in Vitro, 29, 1809–1815. [DOI] [PubMed] [Google Scholar]
- Saunders DS, Meredith FI and Voss KA, 2001. Control of fumonisin: effects of processing. Environmental Health Perspectives, 109(Suppl 2), 333–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCF (Scientific Committee on Food), 2000. Opinion on Fusarium Toxins. Part 3: Fumonisin B1 (FB1). SCF/CS/CNTM/MYC/ 24 FINAL, 1–33.
- SCF (Scientific Committee on Food), 2003. Updated opinion of the Scientific Committee on Food on Fumonisin B1, B2 and B3. SCF/CS/CNTM/MYC/28 Final, 1–4.
- Schmelz EM, Dombrink‐Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T and Merrill AH Jr, 1998. Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicology and Applied Pharmacology, 148, 252–260. [DOI] [PubMed] [Google Scholar]
- Scott PM, Kanhere SR, Lawrence GA, Daley EF and Farber JM, 1995. Fermentation of fumonisin of wort containing added ochratoxin A and fumonisin B1 and B2 . Food Additives and Contaminants, 12, 31–40. [DOI] [PubMed] [Google Scholar]
- Scott PM, Lawrence GA and Lombaert GA, 1999. Studies on extraction of fumonisins from rice, corn‐based foods and beans. Mycotoxin Research, 15, 50–60. 10.1007/BF02945215 [DOI] [PubMed] [Google Scholar]
- Seefelder W, Hartl M and Humpf H‐U, 2001. Determination of N‐(carboxymethyl)fumonisin B1 in corn products by liquid chromatography/electrospray ionization–mass spectrometry. Journal of Agriculture and Food Chemistry, 49, 2146–2151. [DOI] [PubMed] [Google Scholar]
- Seefelder W, Knecht A and Humpf H‐U, 2003. Bound fumonisin B1: Analysis of fumonisin‐B1 glyco and amino acid conjugates by liquid chromatography‐electrospray ionization‐tandem mass spectrometry. Journal of Agriculture and Food Chemistry, 51, 5567–5573. [DOI] [PubMed] [Google Scholar]
- Seiferlein M, Humpf H‐U, Voss KA, Sullards MC, Allegood JC, Wang E and Merrill AH Jr, 2007. Hydrolyzed fumonisins HFB1 and HFB2 are acylated in vitro and in vivo by ceramide synthase to form cytotoxic N‐acyl‐metabolites. Molecular Nutrition & Food Research, 51, 1120–1130. [DOI] [PubMed] [Google Scholar]
- Seo JA, Proctor RH and Plattner RD, 2001. Characterization of four clustered and coregulated genes associated with fumonisin biosynthesis in Fusarium verticillioides . Fungal Genetics and Biology, 34, 155–165. [DOI] [PubMed] [Google Scholar]
- Sewram V, Mshicileli N, Shephard GS and Marasas WFO, 2003. Fumonisin mycotoxins in human hair. Biomarkers, 8, 110–118. [DOI] [PubMed] [Google Scholar]
- Shephard GS and Snijman PW, 1999. Elimination and excretion of a single dose of the mycotoxin fumonisin B2 in a non‐human primate. Food and Chemical Toxicology, 37, 111–116. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG and Sydenham EW, 1992a. Initial studies on the toxicokinetics of fumonisin B1 in rats. Food and Chemical Toxicology, 30, 277–279. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG, Sydenham EW, Alberts JF and Gelderblom WC, 1992b. Fate of a single dose of the 14C‐labelled mycotoxin, fumonisin B1, in rats. Toxicology, 30, 768–770. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG, Sydenham EW and Alberts JF, 1994a. Biliary excretion of the mycotoxin fumonisin B1 in rats. Food and Chemical Toxicology, 32, 489–491. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG, Sydenham EW, Vleggaar R and Alberts JF, 1994b. Determination of the mycotoxin fumonisin B1 and identification of its partially hydrolysed metabolites in the faeces of non‐human primates. Food and Chemical Toxicology, 32, 23–29. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG, Sydenham EW and Snijman PW, 1995a. Toxicokinetics of the mycotoxin fumonisin B2 in rats. Food and Chemical Toxicology, 33, 591–595. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Thiel PG, Sydenham EW and Savard ME, 1995b. Fate of a single dose of 14C‐labelled fumonisin B1, in vervet monkeys. Natural Toxins, 3, 145–150. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Van Der Westhuizen L and Sewram V, 2007. Biomarkers of exposure to fumonisin mycotoxins: a review. Food Additives & Contaminants, 24, 1196–1201. [DOI] [PubMed] [Google Scholar]
- Shephard GS, Burger H, Gambacorta L, Gong YY, Krska R and Rheeder JP, 2013. Multiple mycotoxin exposure determined by urinary biomarkers in rural subsistence farmers in the former Transkei, South Africa. Food and Chemical Toxicology, 62, 217–225. [DOI] [PubMed] [Google Scholar]
- Shier WT, 2000. The fumonisin paradox: a review of research on oral bioavailability of fumonisin B1, a mycotoxin produced by Fusarium moniliforme . Journal of Toxicology: Toxin Reviews, 19, 161–187. [Google Scholar]
- Shier WT, Abbas HK and Mirocha CJ, 1991. Toxicity of the mycotoxins fumonisins B1 and B2 and Alternaria alternata f. sp. lycopersici toxin (AAL) in cultured mammalian cells. Mycopathologia, 116, 97–104. [DOI] [PubMed] [Google Scholar]
- Shier WT, Resch P, Badria FA and Abbas HK, 2000. Biological consequences of fumonisins. Bulletin of the Institute for Comprehensive Agricultural Sciences, Kinki University, 8, 67–74. [Google Scholar]
- Shirima CP, Kimanya ME, Routledge MN, Srey C, Kinabo JL, Humpf HU, Wild CP, Tu YK and Gong YY, 2015. A prospective study of growth and biomarkers of exposure to aflatoxin and fumonisin during early childhood in Tanzania. Environmental Health Perspectives, 23, 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solfrizzo M, Gambacorta L and Visconti A, 2014. Assessment of multimycotoxin exposure in Southern Italy by urinary multi‐biomarker determination. Toxins, 2, 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa FC, Schamber CR, Amorin SSS and Natali MRM, 2014. Effect of fumonisin‐containing diet on the myenteric plexus of the jejunum in rats. Autonomic Neuroscience‐Basic & Clinical, 185, 93–99. [DOI] [PubMed] [Google Scholar]
- Spotti M, Pompa G and Caloni F, 2001. Fumonisin B1 metabolism by bovine liver microsomes. Veterinary Research Communications, 25, 511–516. [DOI] [PubMed] [Google Scholar]
- Susca A, Moretti A, Stea G, Villani A, Haidukowski M, Logrieco A and Munkvold G, 2014. Comparison of species composition and fumonisin production in Aspergillus section Nigri populations in maize kernels from USA and Italy. International Journal of Food Microbiology, 188, 75–82. [DOI] [PubMed] [Google Scholar]
- Sydenham EW, Gelderblom WCA, Thiel PG and Marasas WFO, 1990a. Evidence for the natural occurrence of fumonisin B1, a mycotoxin produced by Fusarium moniliforme in corn. Journal of Agriculture and Food Chemistry, 38, 285–290. [Google Scholar]
- Sydenham EW, Thiel PG, Marasas WFO, Shephard GS, Van Schalkwyk DJ and Koch KR, 1990b. Natural occurrence of some Fusarium mycotoxins in corn from low and high oesophageal cancer prevalence areas of the Transkei, Southern Africa. Journal of Agriculture and Food Chemistry, 38, 1900–1903. [Google Scholar]
- Sydenham EW, Shephard GS, Thiel PG and Marasas WFO, 1991. Fumonisin contamination of commercial corn‐based human foodstuffs. Journal of Agriculture and Food Chemistry, 39, 2014–2018. [Google Scholar]
- Szekeres A, Lorantfy L, Bencsik O, Kecskemeti A, Szecsi A, Mesterhazy A and Vagvolgyi C, 2013. Rapid purification method for fumonisin B1 using centrifugal partition chromatography. Food Additives & Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 30, 147–155. [DOI] [PubMed] [Google Scholar]
- Taranu I, Marin DE, Burlacu R, Pinton P, Damian V and Oswald IP, 2010. Comparative aspects of in vitro proliferation of human and porcine lymphocytes exposed to mycotoxins. Archives of Animal Nutrition, 64, 383–393. [DOI] [PubMed] [Google Scholar]
- Tardieu D, Bailly JD, Skiba F, Grosjean F and Guerre P, 2008. Toxicokinetics of fumonisin B1 in turkey poults and tissue persistence after exposure to a diet containing the maximum European tolerance for fumonisins in avian feeds. Food and Chemical Toxicology, 46, 3213–3218. [DOI] [PubMed] [Google Scholar]
- Tardieu D, Bailly JD, Benlashehr I, Auby A, Jouglar JY and Guerre P, 2009. Tissue persistence of fumonisin B1 in ducks and after exposure to a diet containing the maximum European tolerance for fumonisins in avian feeds. Chemico‐Biological Interactions, 182, 239–244. [DOI] [PubMed] [Google Scholar]
- Theumer MG, Canepa MC, Lopez AG, Mary VS, Dambolena JS and Rubinstein HR, 2010. Subchronic mycotoxicoses in Wistar rats: assessment of the in vivo and in vitro genotoxicity induced by fumonisins and aflatoxin B1, and oxidative stress biomarkers status. Toxicology, 268, 104–110. [DOI] [PubMed] [Google Scholar]
- Tidhar R and Futerman AH, 2013. The complexity of sphingolipid biosynthesis in the endoplasmic reticulum. Biochimica et Biophysica Acta, 1833, 2511–2518. [DOI] [PubMed] [Google Scholar]
- Torres O, Matute J, Gelineau‐van Waes J, Maddox JR, Gregory SG, Ashley‐Koch AE, Showker JL, Zitomer NC, Voss KA and Riley RT, 2014. Urinary fumonisin B1 and estimated fumonisin intake in women from high‐ and low‐exposure communities in Guatemala. Molecular Nutrition & Food Research, 5, 973–983. [DOI] [PubMed] [Google Scholar]
- Turner PC, Flannery B, Isitt C, Ali M and Pestka J, 2012. The role of biomarkers in evaluating human health concerns from fungal contaminants in food. Nutrition Research Reviews, 25, 162–179. [DOI] [PubMed] [Google Scholar]
- Ueno Y, Iijima K, Wang S‐D, Sugiura Y, Sekijima M, Tanaka T, Chen C and Yu S‐Z, 1997. Fumonisins as a Possible Contributory Risk Factor for Primary Liver Cancer: A 3‐Year Study of Corn Harvested in Haimen, China, by HPLC and ELISA. Food and Chemical Toxicology, 35, 1143–1150. [DOI] [PubMed] [Google Scholar]
- van der Westhuizen L, Shephard GS, Burger HM, Rheeder JP, Gelderblom WCA, Wild CP and Gong YY, 2011. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiology Biomarkers and Prevention, 20, 483–489. [DOI] [PubMed] [Google Scholar]
- Van der Westhuizen L, Shephard GS, Gerderblom WCA, Torres O and Riley RT, 2013. Fumonisin biomarkers in maize eaters and implications for human disease. World Mycotoxin Journal, 6, 223–232. [Google Scholar]
- Venancio JC, Emerich SS, Branquinho NTD, Carlos de Sousa FC, Natali MRM and Baroni EA, 2014. Effect of administering a diet contamined with fumonisins on the kidneys of Wistar rats. Acta Scientiarum. Biological Sciences. Maringá, 36, 333–341. [Google Scholar]
- Venter PA, Christianson AL, Humato CM, Makhura MP and Gericke GS, 1995. Congenital anomalies in rural black South African neonates—A silent epidemic. South African Medical Journal, 85, 15–20. [PubMed] [Google Scholar]
- Voss KA, Chamberlain WJ, Bacon CW, Herbert RA, Walters DB and Norred WP, 1995. Subchronic feeding study of the mycotoxin fumonisin B1 in 32 B6C3F1 mice and Fischer 344 rats. Fundamental and Applied Toxicology, 24, 102–110. [DOI] [PubMed] [Google Scholar]
- Voss KA, Bacon CW, Meredith FI and Norred WP, 1996. Comparative subchronic toxicity studies of nixtamalized and water‐extracted Fusarium moniliforme culture material. Food and Chemical Toxicology, 34, 623–632. [DOI] [PubMed] [Google Scholar]
- Voss KA, Riley RT, Bacon CW, Meredith FI and Norred WP, 1998. Toxicity and sphinganine levels are correlated in rats fed fumonisin B1 (FB1) or hydrolyzed FB1 . Environmental Toxicology and Pharmacology, 5, 101–104. [DOI] [PubMed] [Google Scholar]
- Voss KA, Riley RT, Norred WP, Bacon CW, Meredith FI, Howard PC, Plattner RD, Collins TF, Hansen DK and Porter JK, 2001a. On overview of rodent toxicities: liver and kidney effects of fumonisins and Fusarium moniliforme . Environmental Health Perspectives, 109(Suppl. 2), 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss KA, Poling SM, Meredith FI, Bacon CW and Saunders DS, 2001b. Fate of fumonisins during the production of fried tortilla chips. Journal of Agricultural and Food Chemistry, 49, 3120–3126. [DOI] [PubMed] [Google Scholar]
- Voss KA, Norred WP, Meredith FI, Riley RT and Saunders DS, 2006. Fumonisin concentration and ceramide synthase inhibitory activity of corn, masa, and tortilla chips. Journal of Toxicology and Environmental Health‐Part A‐Current Issues, 69, 1387–1397. [DOI] [PubMed] [Google Scholar]
- Voss KA, Smith GW and Haschek WM, 2007. Fumonisins: Toxicokinetics, mechanism of action and toxicity. Animal Feed Science and Technology, 137, 299–325. [Google Scholar]
- Voss KA, Riley RT, Snook ME and vanGelineau‐Waes J , 2009. Reproductive and sphingolipid metabolic effects of fumonisin B1 and its alkaline hydrolysis product in LM/Bc mice: hydrolyzed fumonisin B1 did not cause neural tube defects. Toxicological Sciences, 112, 459–467. [DOI] [PubMed] [Google Scholar]
- Voss KA, Riley RT and Gelineau‐Van Waes J, 2011. Fumonisins. In: Gupta R (ed.). Reproductive and developmental toxicology. Elsevier, New York, NY. 725–737. [Google Scholar]
- Voss KA, Riley RT, Moore ND and Burns TD, 2013. Alkaline cooking (Nixtamalization) reduced the in vivo toxicity of fumonisin‐contaminated corn in a rat feeding bioassay. Food Additives and Contaminants, 30, 1415–1421. [DOI] [PubMed] [Google Scholar]
- Voss KA, Riley RT and Gelineau‐van Waes J, 2014. Fumonisin B1 induced neural tube defects were not increased in LM/Bc mice fed folate‐deficient diet. Molecular Nutrition & Food Research, 2014, 1190–1198. [DOI] [PubMed] [Google Scholar]
- Voss KA, Ryu D, Jackson L, Riley R and Gelineau‐van Waes J, 2017a. Reduction of fumonisin toxicity by extrusion and nixtamalization (alkaline cooking). Journal of Agriculture and Food Chemistry, 10.1021/acs.jafc.6b05761, in press. [DOI] [PubMed] [Google Scholar]
- Voss KA, Riley RT, Gardner NM and Gelineau‐van Waes J, 2017b. Fumonisins. In: Gupta R (ed.). Reproductive and Developmental Toxicology. Academic Press, New York. pp. 925–943. [Google Scholar]
- Vudathala DK, Prelusky DB, Ayroud M, Trenholm HL and Miller JD, 1994. Pharmacokinetic fate and pathological effects of 14C‐fumonisin B1 in laying hens. Natural Toxins, 2, 81–88. [DOI] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT and Merrill AH Jr, 1991. Inhibition of sphingolipid biosynthesis by fumonisins. Implications for diseases associated with Fusarium moniliforme . Journal of Biological Chemistry, 266, 14486–14490. [PubMed] [Google Scholar]
- Wang X, Wu QH, Wan D, Liu QY, Chen DM, Liu ZL, Martinez‐Larranaga MR, Martinez MA, Anadon A and Yuan ZH, 2016. Fumonisins: oxidative stress‐mediated toxicity and metabolism in vivo and in vitro . Archives of Toxicology, 90, 81–1001. [DOI] [PubMed] [Google Scholar]
- Warth B, Petchkongkaew A, Sulyok M and Krska R, 2014. Utilising an LC‐MS/MS‐based multi‐biomarker approach to assess mycotoxin exposure in the Bangkok metropolitan area and surrounding provinces. Food Additives and Contaminants. Part A, Chemistry, Analysis, Control, Exposure & Risk Assessment, 31, 2040–2046. [DOI] [PubMed] [Google Scholar]
- Wheeler MW and Bailer AJ, 2008. Model averaging software for dichotomous dose response risk estimation. Journal of Statistical Software, 26, 1–15.19777145 [Google Scholar]
- WHO/IPCS (World Health Organization/International Programme on Chemical Safety), 2009. Principles and Methods for the Risk Assessment of Chemicals in Food. A joint publication of the Food and Agriculture Organization of the United Nations and the World Health Organization. International Programme on Chemical Safety, Environmental Health Criteria 240. Available online: http://whqlibdoc.who.int/ehc/WHO_EHC_240_5_eng_Chapter2.pdf
- Xu L, Cai Q, Tang L, Wang S, Hu X, Su J, Sun G and Wang JS, 2010. Evaluation of fumonisin biomarkers in a cross‐sectional study with two high‐risk populations in China. Food Additives and Contaminants, 27, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Yoshizawa T, Yamashita A and Luo Y, 1994. Fumonisin occurrence in corn from, high‐ and low‐risk areas for human oesophageal cancer in China. Applied Environmental Microbiology, 60, 1626–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen YZ, 1984. The culture and isolation of fungi from cereals in five high and three low incidence counties of esophageal cancer in Henan Province. Journal Chinese Tumorigenesis (in Chinese). [PubMed] [Google Scholar]
- Zimmer I, Usleber E, Klaffke H, Weber R, Majerus P, Otteneder H, Gareis M, Dietrich R and Martlbauer E, 2008. Fumonisin intake of the German consumer. Mycotoxin Research, 24, 40–52. [DOI] [PubMed] [Google Scholar]
- Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier‐Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH Jr and Riley RT, 2009. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1‐deoxy‐sphinganine: A novel category of bioactive 1‐deoxy‐sphingoid bases and 1‐deoxy‐dihydroceramides biosynthesized by mammalian cell lines and animals. Journal of Biological Chemistry, 284, 4786–4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomborszky‐Kovács M, Vetési F, Horn P, Repa I and Kovács F, 2002. Effects of prolonged exposure to low dose fumonisin B1 in pigs. Journal of Veterinary Medicine Series B, 49, 197. [DOI] [PubMed] [Google Scholar]


