Table 2.
Overview of data submitted for subgroup 1.1.1 (IOFI, 2013)
| Test substance | Test | Test conditions | Reference |
|---|---|---|---|
Hex‐2(trans)‐enal [05.073] representative substance (purity: 98.2%)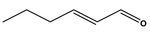
|
Bacterial reverse mutation assay | Salmonella Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 with and without metabolic activation up to 5,000 μg/plate | Sokolowski (2007a), Bhatia et al. (2010) |
| In vivo micronucleus assay |
Muta™mouse blood reticulocytes (days 1, 4 and 31) Treatment by oral gavage at doses of 120, 235 and 350 mg/kg bw per day for 28 days |
Beevers (2013) | |
| Induction of lacZ‐mutations in Muta™Mouse | Muta™Mouse treatment by oral gavage at doses of 120, 235 and 350 mg/kg bw per day for 28 days. Mutation frequencies (day 31) determined in the liver and the duodenum | Beevers (2013) | |
| In vivo micronucleus assay | Treatment by oral route at doses of 250, 500, 1,000 mg/kg bw per day. Sampling of bone marrow was done 24 and 48 h after treatment | Honarvar (2007a) | |
| In vivo rat liver unscheduled DNA synthesis (UDS) assay | Treatment by oral route at doses of 200 and 500 mg/kg bw per day. Liver was perfused at 16 and 3 h after dosing | Durward (2009) | |
2‐Dodecenal [05.037] not representative (purity: 99.4%)
|
Bacterial reverse mutation assay | S. Typhimurium strains TA98, TA100, TA102, TA1535 and TA1537 with and without metabolic activation up to 1,000 μg/plate | Sokolowski (2007b), Bhatia et al. (2010) |
| In vivo micronucleus assay | Treatment by oral route at doses of 500, 1,000 and 2,000 mg/kg bw per day. Sampling of bone marrow was done 24 and 48 h after treatment. 2,000 PCE scored at 24 h (3 doses) and 48 h (top dose) | Honarvar (2007b), Bhatia et al. (2010) | |
2‐Nonenal [05.171] not representative (purity: 96.2%)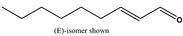
|
In vivo Micronucleus assay | Treatment by oral route at doses of 500, 1,000 and 2,000 mg/kg bw per day. Sampling of bone marrow was done 24 and 48 h after treatment. 2,000 PCE scored at 24 h (3 doses) and 48 h (top dose) | Honarvar (2008), Bhatia et al. (2010) |
bw: body weight; PCE: polychromatic erythrocytes.
