Table B.1.
Summary of Safety Evaluation of the JECFA Substances in the Present Group (JECFA, 1996, 1999, 2001, 2003, 2005, 2007, 2009)
| FL‐no JECFA‐no | EU register name | Structural formula | EU MSDIa US MSDI (μg/capita per day) | Classb Evaluation procedure pathc | JECFA outcome on the named compoundd ore | EFSA conclusion on the named compound |
|---|---|---|---|---|---|---|
|
02.020 1354 |
Hex‐2‐en‐1‐ol |

|
653.48 291 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.049 1184 |
Nona‐2,6‐dien‐1‐ol |

|
9.07 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.050 1793 |
Pent‐2‐en‐1‐ol |
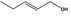
|
2.44 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.090 1365 |
Non‐2(trans)‐en‐1‐ol |
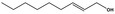
|
0.02 0.03 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.112 1369 |
Non‐2(cis)‐en‐1‐ol |
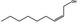
|
0.01 2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.137 1794 |
Dec‐2‐en‐1‐ol |
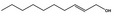
|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.156 1374 |
Hex‐2(cis)‐en‐1‐ol |
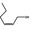
|
0.01 10 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 02.192 | Oct‐2‐en‐1‐ol |
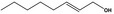
|
7.71 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
02.210 1384 |
Undec‐2‐en‐1‐ol |

|
0.01 1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 02.231 | trans‐2, cis‐6‐Nonadien‐1‐ol |
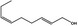
|
8.73 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.037 1350 |
2‐Dodecenal |
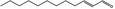
|
1.19 2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
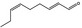
05.058 1186 |
Nona‐2(trans),6(cis)‐dienal |
|
15.78 24 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.060 1363 |
Oct‐2‐enal |

|
0.84 0.9 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.070 1360 |
2‐Heptenal |
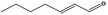
|
8.17 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.072 1362 |
trans‐2‐Nonenal |
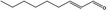
|
1.70 0.12 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.073 1353 |
Hex‐2(trans)‐enal |

|
2761 409 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.076 1349 |
Dec‐2‐enal |

|
12.94 6 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.078 1359 |
Tridec‐2‐enal |

|
0.97 0.7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.102 1364 |
Pent‐2‐enal |

|
0.37 0.1 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.109 1366 |
2‐Undecenal |

|
0.65 0.4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.111 1182 |
Octa‐2(trans),6(trans)‐dienal |
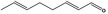
|
0.01 0.007 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.114 1208 |
4‐Methylpent‐2‐enal |

|
0.01 0.2 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.120 1197 |
Dodeca‐2,6‐dienal |

|
0.01 0.009 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.144 | Dodec‐2(trans)‐enal |
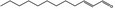
|
0.75 | No evaluation | Not evaluated by the JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.150 1360 |
Hept‐2(trans)‐enal |

|
16.27 30 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.171 1362 |
Non‐2‐enal |

|
9.89 0.4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.172 1187 |
Nona‐2(trans),6(trans)‐dienal |
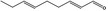
|
6.52 0.007 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
05.179 1803 |
Tetradec‐2‐enal |

|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.184 | Undec‐2(trans)‐enal |
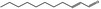
|
0.84 ND |
No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.189 | 2‐Hexenal |
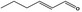
|
1.22 409 |
Class I No evaluation |
Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure. |
| 05.190 | trans‐2‐Octenal |
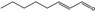
|
0.79 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.191 | trans‐2‐Decenal |

|
8.10 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 05.195 | trans‐2‐Tridecenal |
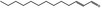
|
0.12 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
06.025 946 |
1,1‐Diethoxynona‐2,6‐diene |
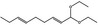
|
0.01 0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
06.031 1383 |
1,1‐Diethoxyhex‐2‐ene |
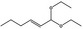
|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
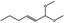
06.072 1728 |
1,1‐Dimethoxyhex‐2(trans)‐ene |
|
0.01 ND |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
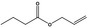
09.054 2 |
Allyl butyrate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
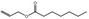
09.097 4 |
Allyl heptanoate |
|
12.92 28 |
Class II A3: Intake above threshold, A4: Endogenous |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
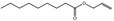
09.109 6 |
Allyl nonanoate |
|
0.01 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.119 5 |
Allyl octanoate |
|
0.01 1.3 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.146 9 |
Allyl undec‐10‐enoate |
|
0.01 < 0.01 |
Class II A3: Intake above threshold, A4: Endogenous |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.233 1 |
Allyl propionate |
|
0.57 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.244 3 |
Allyl hexanoate |
|
3583.8 820 |
Class II B3: Intake above threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
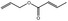
| 09.247 | Allyl crotonate |
|
0.04 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.276 1367 |
Oct‐2‐enyl acetate |

|
0.03 0.7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.277 1368 |
Oct‐2(trans)‐enyl butyrate |

|
0.15 0.7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.303 1799 |
Hept‐2‐enyl isovalerate |

|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
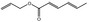
09.312 8 |
Allyl hexa‐2,4‐dienoate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.385 1798 |
Hept‐2‐enyl acetate |

|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.394 1355 |
Hex‐2(trans)‐enyl acetate |

|
272.73 56 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
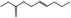
09.395 1378 |
Hex‐2(trans)‐enyl propionate |
|
0.08 4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.396 1375 |
Hex‐2‐enyl butyrate |
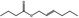
|
5.62 4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.397 1376 |
Hex‐2‐enyl formate |

|
0.01 7 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.398 1381 |
Hex‐2‐enyl hexanoate |

|
1.36 0.09 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.399 1377 |
Hex‐2‐enyl isovalerate |
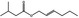
|
1.44 4 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
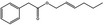
| 09.400 | Hex‐2‐enyl phenylacetate |
|
0.01 |
Class I No evaluation |
Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
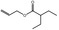
09.410 11 |
Allyl 2‐ethylbutyrate |
|
0.01 0.02 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
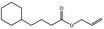
09.411 14 |
Allyl cyclohexanebutyrate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
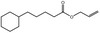
09.469 15 |
Allyl cyclohexanevalerate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
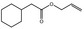
09.482 12 |
Allyl cyclohexaneacetate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
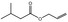
09.489 7 |
Allyl isovalerate |
|
0.06 0.19 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
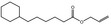
09.492 16 |
Allyl cyclohexanehexanoate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.493 10 |
Allyl 2‐methylcrotonate |
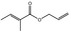
|
0.01 < 0.01 |
Class II B3: intake below threshold B4: adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.498 13 |
Allyl cyclohexanepropionate |
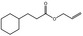
|
96.57 110 |
Class II B3: intake below threshold B4: adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
09.678 1795 |
Pent‐2‐enyl hexanoate |
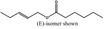
|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.701 18 |
Allyl phenoxyacetate |
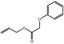
|
9.93 2.5 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
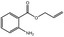
09.719 20 |
Allyl anthranilate |
|
0.01 0.09 |
Class III B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
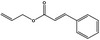
09.741 19 |
Allyl cinnamate |
|
0.01 0.28 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
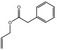
09.790 17 |
Allyl phenylacetate |
|
0.01 < 0.01 |
Class II B3: Intake below threshold, B4: Adequate NOAEL exists |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. Evaluated by JECFA before 2000 |
|
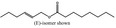
09.841 1796 |
2‐Hexenyl octanoate |
|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 09.866 | Allyl valerate |
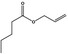
|
0.01 | No evaluation | Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
09.947 1188 |
(E,Z)‐2,6‐Nonadienyl acetate |
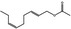
|
0.01 |
Class I A3: Intake below threshold |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
| 09.948 | (2E)‐2‐Nonenyl acetate |

|
0.01 |
Class I No evaluation |
Not evaluated by JECFA | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
|
13.004 21 |
Allyl 2‐furoate |
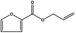
|
0.01 < 0.01 |
Class III B3: Intake below threshold, B4: No adequate NOAEL JECFA evaluated at step B5: intake below 1.5 μg/person per day |
d | Evaluated in FGE.200Rev1 as of no genotoxicity concern. The substance can be evaluated through the Procedure |
JECFA: The Joint FAO/WHO Expert Committee on Food Additives; FL‐no: FLAVIS number; MSDI: maximised survey‐derived daily intake; FGE: Flavouring Group Evaluation; NOAEL: no‐observed‐adverse‐effect‐level; ND: not determined.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg/capita per day. EU MSDI values calculated based on the most recent EFFA poundage information (kg/year) for the year 2015 (EFFA, 2018).
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
