Abstract
This update on the African swine fever (ASF) outbreaks in the EU demonstrated that out of all tested wild boar found dead, the proportion of positive samples peaked in winter and summer. For domestic pigs only, a summer peak was evident. Despite the existence of several plausible factors that could result in the observed seasonality, there is no evidence to prove causality. Wild boar density was the most influential risk factor for the occurrence of ASF in wild boar. In the vast majority of introductions in domestic pig holdings, direct contact with infected domestic pigs or wild boar was excluded as the route of introduction. The implementation of emergency measures in the wild boar management zones following a focal ASF introduction was evaluated. As a sole control strategy, intensive hunting around the buffer area might not always be sufficient to eradicate ASF. However, the probability of eradication success is increased after adding quick and safe carcass removal. A wider buffer area leads to a higher success probability; however it implies a larger intensive hunting area and the need for more animals to be hunted. If carcass removal and intensive hunting are effectively implemented, fencing is more useful for delineating zones, rather than adding substantially to control efficacy. However, segments of fencing will be particularly useful in those areas where carcass removal or intensive hunting is difficult to implement. It was not possible to demonstrate an effect of natural barriers on ASF spread. Human‐mediated translocation may override any effect of natural barriers. Recommendations for ASF control in four different epidemiological scenarios are presented.
Keywords: African swine fever, epidemiology, risk factor, seasonality, wild boar, domestic pigs, management, prevention
Short abstract
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1521/full
Summary
The European Commission requested the European Food Safety Authority (EFSA) to provide an updated epidemiological analysis based on the data collected from the Member States (MS), affected by African swine fever (ASF) Genotype II. Analysis of the data was only performed for the data provided by the Baltic States and Poland, but a narrative update of the situation was provided for nine affected MS.
ASF has been introduced into nine European Union (EU) MS, through two distinct spread processes: continuous wild boar‐mediated spread through wild boar populations and meta‐populations, for which the speed of propagation is notably slower than for some other infectious diseases in wild boar; and human‐mediated translocations leading to the establishment of new ASF clusters distant from areas of previous ASF occurrence. In affected areas within the established ASF range, there has been continued sporadic detection of cases despite very low wild boar densities. The focal introduction in the Czech Republic was the only occasion in which ASF spread in wild boar was apparently controlled. Elsewhere, ASF continues to expand into new areas.
In most affected countries, there have been many cases in wild boar and relatively few outbreaks in domestic pigs. In Romania, however, the opposite has been observed. The observed pattern in Romania should be interpreted with caution until the potential for under‐detection of ASF in wild boar populations can be excluded. This will require systematic surveillance activities in wild boar populations. Under‐detection of ASF in wild boar could also occur in other regions, and should be avoided through intense passive surveillance of wild boar.
Term of reference 1 (TOR1) requested to provide an insight into possible temporal patterns of ASF.
The temporal patterns in the proportions of tested samples that are positive are consistent with the different epidemiological situations in the countries. For example, in Lithuania, there is both spatial expansion of the ASF‐affected area and an increase in polymerase chain reaction (PCR)‐positive animals among wild boar found dead. By contrast, in Estonia, there is a reduction in the proportion of PCR‐positive results in the last reporting period among wild boar found dead, given that infection has been present throughout the whole country for several years.
Overall, among wild boar found dead, the proportions of animals that tested PCR positive were generally much higher than the proportion of animals testing enzyme‐linked immunosorbent assay (ELISA) antibody positive. The proportions of wild boar testing positive are much higher in animals found dead than in hunted animals. That confirms surveillance of dead wild boar as the most efficient method of ASF surveillance. Of particular importance, during wild boar surveillance is the finding of more than one dead wild boar during a singular mortality event.
Among hunted animals, both the proportion of wild boar testing PCR or ELISA positive remains low, i.e. below 5%, although there is some local variation.
The possible seasonality of the infection in wild boar was investigated, both visually and statistically.
Visual inspection revealed that there were apparent peaks in winter and summer in the proportion of wild boar found dead that were testing positive, while in domestic pigs only a summer peak was evident in the outbreaks. Among hunted wild boar, there appeared to be a slight decline in the proportion testing positive in spring (February–April). For the rest of the year, more or less the same proportions of positive samples over sampling effort were observed in hunted animals.
Statistical analysis demonstrated that the probability of ASF occurrence among hunted or found dead wild boar was not equally distributed across the different months and seasons of the year. Winter and summer peaks are observed in wild boar found dead.
Several driving forces could explain an increase in the proportions of positive samples that are tested either in winter or summer, related to the characteristics of the virus, the wild boar ecology, the pig farming husbandry, the involvement of arthropod vectors or human behaviour. As yet, however, there is a lack of evidence to support causal associations with any of those factors.
The speed of propagation of the ASF infection in the wild boar population was studied using a network analysis. The median speed of propagation of ASF infection in the Baltic States and Poland was estimated to be between 8 and 17 km/year. Similar estimates were obtained using other methods. Furthermore, from calculations based on the notifications to the Animal Disease Notification System (ADNS), there is a summer increase in the local spread velocity of the ASF infection moving through wild boar populations.
The ASF hot spot areas (areas with higher density of ASF notifications to ADNS) in the Baltic countries have moved in a south‐western direction over the 4 past years. In Latvia, these hot spot areas have reduced in size and are currently only present in the west of the country, whereas in Estonia they have disappeared entirely.
The study of possible sources of introduction of African Swine Fever Virus (ASFV) in pig holdings was based on information generated from epidemiological investigations in affected MS.
Domestic pig outbreaks were correlated in time and space with cases in wild boar, suggesting an association between the risk of introduction and the level of contamination of the environment.
The specific routes of ASF introduction into affected domestic pig farms in the EU could only be identified in very few of all outbreaks for which detailed investigations were conducted. In the vast majority of introductions, direct contact with infected domestic pigs or wild boar could be excluded as the likely route of introduction. Inadequate biosecurity is likely to have contributed to introduction of ASF into domestic farms via indirect contact through contaminated fomites or environment.
A systematic literature review on survival time of ASFV and the infectious period of ASFV in swine was carried out to update current knowledge on the possible duration in which different matrices or live swine could be a potential source of introduction of ASFV in domestic pig holdings. The virus has been demonstrated to survive for more than 2 years in frozen organs or for almost 2 years in chilled blood. Virus in faeces, urine or slurry will survive for a much shorter period (up to a week) in chilled or room temperature conditions. Therefore, anything that contains frozen and chilled blood or organs from infected pigs can be a very important source of introduction for prolonged periods of time (years), whereas fomites contaminated with faeces or urine, either chilled or at room temperature, will survive for about a week.
Term of reference 2 (TOR2) requested to identify risk factors involved in the occurrence, of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures. The risk factor analysis was updated for the occurrence of ASF in wild boar populations using both Bayesian hierarchical and general additive models, conducted on data provided by Estonia, as these were the most complete data with sufficient spatial and temporal resolution, allowing the analysis to be performed.
A risk factor analysis for the occurrence of ASF in domestic pig population was not carried out as there were relatively few outbreaks in Latvia and Estonia, the countries with the most detailed data sets. In the Bayesian hierarchical model, an increased density of domestic pigs and of wild boar and a decreased density of roads were associated with a significant increase in ASF occurrence in wild boar. Of these three risk factors, wild boar density was the most influential. The same results were obtained using a general additive model. There were insufficient data to finish the risk factor analysis for the occurrence of ASF in domestic pigs.
Term of reference 3 (TOR3) requested to review the control measures applied by the affected MS for controlling the spread of the disease in wild boar and for eradicating it. This assessment was based on a spatiotemporally explicit individual‐based model approach in structured geographic landscapes.
These conclusions pertain to emergency measures implemented in three management zones, the core areas, the buffer area and the intensive hunting area around a focal introduction of ASF in a wild boar population.
The model revealed that in the absence of carcass removal in the core area and assuming that carcass contact occurs immediately after death, the probability of success of the control measures can exceed 80% if the intensity of hunting in the intensive hunting area is much higher than during times of sustainable wild boar management. This could be possible in practice because the intensive hunting area is limited in size (3–12 wild boar home ranges). However, given the known limited efficacy of intensive regular hunting, intensive hunting around the buffer area as sole measure (e.g. without carcass removal or fence around the core area) might not be sufficient to control a focal introduction of ASF.
The model also showed that the hunting efficacy in the intensive hunting zone could be less (e.g. with a hunting efficacy similar to that achieved during sustainable wild boar management) when carcass removal is being implemented in the core area. With a carcass removal rate of 20%, the probability of success can exceed 80% with an intensive hunting area of limited size (width of 3–12 wild boar group home‐range diameters). The probability of success is further increased if carcass removal rates are doubled and/or carcasses are removed more quickly following death.
Following a focal introduction of ASF, the affected area is small and relatively limited numbers of wild boar are present that may subsequently become infected and die. In this situation, a high carcass removal rate in the core area can override carcass creation. Therefore, eradication might potentially be achieved without the need for extreme hunting efficacy in the intensive hunting area. Additionally, it was shown that the impact of hunting on the wild boar population should be maximised over as short a time period as possible (i.e. to obtain the same population reduction in less hunts).
When looking more closely at the different scenarios for the core and buffer zone, the model showed that a lengthy delay after the establishment of zoning and before culling in the core and buffer zones is detrimental with respect to maximising the probability of eradication success. This is contrary to expert opinion, in which it is recommended – to avoid perturbation of the population during the epidemic – that culling commences only once the epidemic peak has been reached.
The width of the buffer area will influence the probability of eradication success, with wider buffer areas leading to higher success rates. However, increases in the buffer area will also lead to a larger intensive hunting area, where intensive hunting efforts may be harder to achieve in practice, and more animals would need to be culled in the buffer area. It should be noted that the delineation of zones (including the core and buffer zones) is guided by the detection of carcasses, rather than the detection of actual infected animals. For this reason, eradication success may be imperfect even in the presence of wild boar‐proof fencing.
If carcass removal and intensive hunting are effectively implemented, fencing is more useful for delineating zones, rather than adding substantially to the control efforts. However, segments of fencing will be particularly useful in those areas where carcass removal or intensive hunting is difficult to implement.
Recent focal introduction and large‐scale area expansion should be considered different management problems in the context of ASF control. The success of the approach following focal introduction is closely linked to the eventual size of the outbreak, and cannot be observed in large‐scale affected areas, even with perfect measures. Every effort should be made to limit the size of these outbreaks following focal introduction, as there appears to be no point of return if this strategy fails.
Term of reference 4 (TOR4) sought a review and assessment of the robustness and effectiveness of the different types of geographical artificial or natural boundaries (e.g. roads, rivers) used for the determination/demarcation of the restricted areas. A predictive epidemiological model was used to assess if spread through the wild boar populations with barriers in the modelled landscape were more similar to the spread observed from the ADNS data, than spread without the barriers. Based on this comparison, it was not possible to demonstrate an effect of natural barriers on ASF spread. It appears that assumed human‐mediated translocations are particularly influential in overwhelming any positive effect of such barriers.
Term of reference 5 (TOR5) required to provide recommendations for measures for managing the wild boar populations in four separate geographical areas.
-
In disease‐free areas, far away from any ASF occurrence, long‐term actions should be taken to prepare for a possible future incursion of ASFV, considering the possibility of a human‐mediated trans‐location of ASFV.
Maintain control of borders, including the controls on the implementation on the ban on cross‐border trade of wild boar.
Establish and maintain systems of passive surveillance for early detection of ASF in wild boar.
Complete contingency planning, clearly outlining protocols, roles and responsibilities, etc., if there is an ASF incursion.
Increased understanding of local wild boar ecology.
Improve biosecurity and biosecurity awareness, both in domestic pig holdings, and at hunting grounds.
Collect discarded rubbish material on roads/in parks, etc., noting the potential for both urban and sylvatic wild boar.
Increase awareness and understanding among hunters and others who visit or work in the forest, of the importance of passive surveillance for early detection of ASF and efficient and biosecure hunting strategies.
Assess current approaches to hunting, seeking opportunities to improve hunting efficiency for wild boar population reduction.
Implement preventive measures to stabilise wild boar density. Take action on habitat carrying capacity including a ban on the feeding of wild boar and strategies to improve crop protection. Take action in order to substantially increase hunting pressure.
-
Disease‐free areas neighbouring infected or restricted areas at higher risk of getting the infection mainly via natural spread of the disease through scenario 1, with the following adjustments:
-
Preventive measures to stabilise wild boar density, focusing both on habitat carrying capacity and the hunting of wild boar, will be even more urgent. In non‐affected areas in close proximity to infected areas, hunting of wild boar should be conducted at the highest levels achievable in that area. Furthermore, it is recommended that hunting of wild boar is conducted throughout this area, including in protected areas (such as national parks). Collectively, these measures will be beneficial in reducing both:
-
–
the probability of ASF introduction through natural movement of wild boar;
-
–
the probability of establishment of ASF following introduction;
-
–
the efforts needed for potential emergency actions (such as carcass removal) if an ASF incursion were to occur.
-
–
There is a need for a planned, active and systematic approach to passive surveillance, to maximise the probability of early detection following introduction and the accuracy of subsequent efforts to delineate the geographic extent of the infected wild boar population.
-
-
Areas where the disease was recently introduced in wild boar.
-
Following focal ASF introductionFollowing initial focal ASF introduction, the infected area should be defined as outlined above, based on passive surveillance and if possible demarcated based on natural and artificial barriers:
Within the core and buffer areas, the wild boar populations should be kept undisturbed throughout the period of active ASF transmission (e.g. a complete hunting ban on all species should be imposed and a strategy of ensuring the needs of wild boar are met should be developed and implemented to limit animal movement). Carcass removal should be undertaken to limit infection in the environment, but under conditions of high biosecurity. Following the decline in the epidemic, as demonstrated through passive surveillance, active population management under strict biosecurity, including rapid population reduction (culling) and carcass removal, should be reconsidered.
Within the intensive hunting area, there should be a drastic and sustained reduction in the wild boar population. The modelling results highlight the interaction between multiple factors in the intensive hunting area (the area size, the intensity of the hunting effort, the concentration of the hunting effort) and the core and buffer areas (carcass removal, timing of carcass removal following death, timing of culling after initial detection). The intensity and concentration of hunting effort required in the intensive hunting area will be influenced by these other factors.
-
Geographic expansion of known ASF‐infected areas.In theory, the strategies recommended in response to focal ASF introduction are also suited to ASF introduction following the geographic expansion of known ASF‐infected areas. In practice, however, some modifications will be needed, as the latter will generally result in a much larger affected area. At these larger scales, culling can be more difficult to implement, fencing is likely to be impractical and broader societal and political issues need to be considered. Given this background, the following strategies are recommended:
Passive surveillance is particularly important, both for early detection and to delineate the geographic extent of the infected wild boar population.
Larger buffer areas can be considered, to account for expected wild boar movement.
Biosecurity and biosecurity awareness are particularly important, to minimise the risk of human‐mediated spread.
-
-
Areas where the disease has been present in the wild boar population for quite some time (more than 1 year).
There should be ongoing hunting of wild boar populations, both to slow infection and to monitor progress through active surveillance. The age profile of seropositive animals should be assessed.
There is an ongoing need for passive surveillance and carcass removal, to identify hot spot areas and limit ASF presence in carcasses/the environment.
There should be an ongoing feeding ban. Baiting should be kept to a minimum, and alternatives used where possible.
-
Further research is needed:
-
–
to clarify the pathways that facilitate ASF persistence in affected areas over a number of years;
-
–
to assist the interpretation of seropositivity in the context of ASF infection;
-
–
to define a pathway to ASF freedom following detection of the last known infected animal/carcass.
-
–
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
African swine fever (ASF) is an exotic disease occurring for the first time in certain eastern areas of the EU. The persistence of the disease in wild boar and the limited number of control measures available represents a challenge for the whole EU agricultural sector, in particular the pig farming industry.
From the beginning of 2014 to date, ASF Genotype II has been notified in the Czech Republic, Estonia, Latvia, Lithuania, Poland and Romania, causing very serious concerns. The disease has also been reported in Belarus, Moldova, Russia and Ukraine and creates a constant risk for all the Member States (MS) bordering with these third countries. There is knowledge, legislation, technical and financial tools in the EU to properly face ASF.
EU legislation primarily targets domestic pig and, when needed, lays down specific aspects related to wild boar. The main pieces of the EU legislation relevant for ASF are:
Council Directive 2002/60/EC1 of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and African swine fever: it mainly covers prevention and control measures to be applied where ASF is suspected or confirmed either in holdings or in wild boars to control and eradicate the disease.
Commission Implementing Decision 2014/709/EU2 of 9 October 2014 concerning animal health control measures relating to African swine fever in certain Member States and repealing Implementing Decision 2014/178/EU: it provides the animal health control measures relating to ASF in certain Member States by setting up a regionalisation mechanism in the EU. These measures involve mainly pigs, pig products and wild boar products. A map summarising the current regionalisation applied is available online.3
Council Directive No 82/894/EEC4 of 21 December 1982 on the notification of animal diseases within the Community which has the obligation for Member States to notify the Commission of the confirmation of any outbreak or infection of ASF in pigs or wild boar.
In addition, an ASF Strategy for the EU5 has been developed based on earlier scientific recommendations by EFSA. This strategy is constantly evolving based on new science available and on new experiences gained.
The Commission is in need of an updated epidemiological analysis based on the data collected from the MS affected by ASF Genotype II. This analysis should take into account the previous EFSA opinions and technical reports on ASF. The use of the EFSA Data Collection Framework is encouraged, given it promotes the harmonisation of data collection. Any data that are available from neighbouring non‐EU countries should be used as well.
Therefore, in the context of Article 31 of Regulation (EC) No. 178/2002, EFSA should provide the technical and scientific assistance to the Commission based on the following Terms of Reference (TOR):
Analyse the epidemiological data on ASF from MS and non‐EU countries affected by ASF Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar with a view to identifying patterns (ranges and speed) of transmission and also introduction of the virus in different types of domestic pig holdings.
Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures.
Review the control measures applied by the affected MS for controlling the spread of the disease in wild boar and for eradicating it. Assess their effectiveness and review scientific literature addressing these measures.
Review and assess the robustness and effectiveness of the different types of geographical artificial or natural boundaries used for the determination/demarcation of the restricted areas.
-
Based on the latest science and epidemiological data, review the measures for managing the wild boar populations in four separate geographical areas:
Disease‐free areas, far away from any ASF occurrence, which should take long‐term actions for preparing for a future possible incursion of the disease considering the human factor.
Disease‐free areas neighbouring infected or restricted areas at higher risk of getting the infection mainly via natural spread of the disease through wild boar.
Areas where the disease was recently introduced in wild boar.
Areas where the disease has been present in the wild boar population for quite some time (more than 1 year).
1.2. Interpretation of the Terms of Reference
TOR1: Analyse the epidemiological data on ASF from Member States and non‐EU countries affected by ASF Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar with a view to identifying patterns (ranges and speed) of transmission and also introduction of the virus in different types of domestic pig holdings.
As epidemiological data were provided by the affected MS of the EU, this report focuses on the ASF epidemiology in EU countries only. Analysis of the data was only performed for the data provided by the Baltic States and Poland, but a narrative update of the situation was provided for nine affected MS. To provide an insight into possible temporal patterns, timelines of ASF detections were provided and the possible seasonality of the infection in wild boar was investigated, both visually and statistically. A quantitative analysis using a network analysis and a predictive epidemiological model studied the speed of propagation of the ASF infection in the wild boar population. The study of possible sources of introduction of African Swine Fever Virus (ASFV) in pig holdings was based on information generated through the epidemiological investigations in the affected MS. However, as this information did not allow any quantitative analysis, it was summarised in a narrative section. A systematic literature review on survival time of ASFV and the infectious period of ASFV in swine was carried out to update the current knowledge on the possible duration in which different matrices or live swine could be a potential source of introduction of ASFV in domestic pig holdings
TOR2: Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthen biosecurity and other risk mitigation measures.
The risk factor analysis was updated for the occurrence of ASF in wild boar populations with both a Bayesian hierarchical model and a general additive model, carried out on data provided by Estonia, as these were the most complete data with sufficient spatial and temporal resolution, allowing the analysis to be performed.
A risk factor analysis for the occurrence of ASF in domestic pig population was not carried out as there were relatively few outbreaks in Latvia and Estonia, the countries with the most detailed data sets.
TOR3: Review the control measures applied by the affected Member States for controlling the spread of the disease in wild boar and for eradicating it. Assess their effectiveness and review the scientific literature addressing these measures.
A predictive epidemiological model was used to evaluate the control measures to stop the spread of ASF in wild boar in four different scenarios:
Disease‐free areas, far away from any ASF occurrence, which should take long‐term actions for preparing for a future possible incursion of the disease considering the human factor.
Disease‐free areas neighbouring infected or restricted areas at higher risk of getting the infection mainly via natural spread of the disease through wild boar.
Areas where the disease was recently introduced in wild boar.
Areas where the disease has been present in the wild boar population for quite some time (more than 1 year).
TOR4: Review and assess the robustness and effectiveness of the different types of geographical artificial or natural boundaries used for the determination/demarcation of the restricted areas.
A predictive epidemiological model was used to assess if spread through the wild boar populations including barriers in the modelled landscape were more similar to existing Animal Disease Notification System (ADNS) data, than spread without the barriers.
TOR5: Based on the latest science and epidemiological data, review the measures for managing the wild boar populations in four separate geographical areas.
Narrative section based on the above analysis, to provide recommendations for four scenarios.
2. Data and methodologies
2.1. Data
2.1.1. ASF notifications
Data on ASFV detections in wild boar and domestic pigs reported between 24 January 2014 and 8 October 2018 were extracted from the ADNS. The numbers of ASF outbreaks in domestic pigs and wild boar cases are presented in Table 1.
Table 1.
Number of African swine fever (ASF) outbreaks in domestic pigs and cases in wild boar notified to the Animal Disease Notification System from 24 January 2014 until 31 October 2018
| Country | Outbreaksa in domestic pigs | Casesb in wild boar | |
|---|---|---|---|
| Found dead | Hunted | ||
| Lithuania | 118 | 5,183 | 841 |
| Poland | 210 | 4,378 | 443 |
| Latvia | 63 | 2,376 | 2,236 |
| Estonia | 27 | 3,853 | |
| Czech Republic | 0 | 211 | 19 |
| Romania | 1,073 | 128 | 27 |
| Hungary | 0 | 46 | 2 |
| Bulgaria | 1 | 1 | 5 |
| Belgium | 0 | 128 | 4 |
An outbreak of ASF in domestic pigs refers to one or more cases detected in a pig holding.
A case of ASF in wild boar refers to any wild boar or wild boar carcass in which clinical symptoms or post‐mortem lesions attributed to ASF have been officially confirmed, or in which the presence of the disease has been officially confirmed as the result of a laboratory examination carried out according to the diagnostic manual.
2.1.2. Sample‐based data
The data on ASF tests from the Laboratory Information Management System (LIMS) of the national laboratories of the Baltic States and Poland have been collected in EFSA's Data Collection Framework (DCF) (EFSA, 2017). The data reported to the DCF by the different MS contained the information on samples tested for ASF between January 2014 and 31 August 2018.
Samples were tested for ASF using polymerase chain reaction (PCR) (testing for virus); and AB‐enzyme‐linked immunosorbent assay (ELISA), immunoblotting (IB) and immunoperoxidase (IPT) (tests for antibodies).
The data contain:
the date and location of samples (Local Administrative Level 1 and 2 or exact location: longitude and latitude)
the age and sex of animals
decomposition stage of the carcass
testing method
hunted/found dead wild boar.
2.1.3. Risk factor analysis
Risk factor analysis was carried out only for Estonia, as sufficient detailed data with the temporal and spatial resolution, allowing the risk factor analysis to be performed, were provided. For details about the data needs for the risk factor analysis, see the templates for the data provided in Annex A.
2.1.3.1. Domestic pig population data
Data on the domestic pig population and its distribution were provided by the Estonian Agricultural Registers and Information Board (ARIB) (online). Table 2 provides a summary of the type of data made available to EFSA for the assessment. The number of small pig farms (< 10 head) has been used as a potential risk factor as it was assumed that these small farms would often implement suboptimal biosecurity measures.
Table 2.
Data items provided by the relevant Member States on pig population and distribution
| MS | Data | Spatial resolution | YEARS | Temporal resolution |
|---|---|---|---|---|
| Estonia | Farms/holding | Longitude and latitude | 2014–2018 | Yearly |
| Number of pigs in the holding | ||||
| Latvia | Farms/holding | Longitude and latitude | 2014–2018 | 6 months |
| Number of pigs in the holding | ||||
| Lithuania | Number of pigs | Local administrative unit | 2014–2016 | Yearly |
| Number of holdings | LAU2 | |||
| Poland | Number of pigs and holdings | Municipalities | 2014–2017 | 6 months |
| Number of small (1–10 heads) holdings | ASF restricted area in 2014–2016 |
2.1.3.2. Wild boar population data
Data on the size of wild boar populations (based on estimates from the national hunters’ organisations of the population size in the springs of 2014–2018) were provided by the Estonian Environment Agency. The data were provided with sufficient detail per hunting ground, including the hunting efforts (i.e. dogs, baiting places, number of hunters) to carry out the risk factor analysis.
2.1.3.3. Available wild boar habitat and regional roads
A raster map of the quality of available habitats (QAHs), developed by CISA‐INIA (Spain), was used (Bosch et al., 2016; EFSA, 2017). The average QAH was calculated based on the raster inputs for each of the spatial regions considered using the zonal statistics tool of the ArcMap software (ESRI). The shape files of the roads were obtained from the website of the GIS‐LAB Project specialising in geographic information systems (GIS) (NEXTGIS, online). The total lengths of all types of roads were measured for each administrative unit and used as an indicator of human activity.
2.1.3.4. Demographic data and density of settlements
The 2015 data on the human population at district (LAU 2) level were extracted from the official website of the National Statistic Institution of Estonia (Statistics Estonia, online).
The locations of settlements were obtained from the website of the GIS‐LAB Project (GIS‐Lab, online) as shape files.
2.2. Methodologies
2.2.1. Descriptive epidemiology – TOR1
2.2.1.1. Update of the ASF situation in eastern Europe
A short narrative section was provided describing the ASF situation in the affected MS in the EU since the last report of EFSA, published in 2017 (EFSA, 2017), and a map was provided to visualise the spread of ASFV since the previous reporting period.
2.2.1.2. Timelines of proportions of positive samples tested with AB‐ELISA or PCR in wild boar hunted and found dead
The proportion of positive samples reported through the DCF (either tested by PCR or antibody‐ELISA (AB‐ELISA)) were calculated as the number of positive animals divided by the total number of tested animals (either hunted or found dead) per month, in the Baltic States and Poland. Local regression or local fitting smoothing (LOESS) (Cleveland et al., 1988) was used to estimate average profiles describing the global trends of the PCR‐ or ELISA‐positive samples. Confidence bands are also presented to show uncertainties in the estimation of the smoothing curves.
Two timelines were provided per country, the first showing the proportions in all the LAU 2 areas of the MS where animals were sampled (including also the ASF‐free LAU 2 areas), from introduction of the disease in the MS. The second graphs display only those proportions in the affected areas where at least one positive case has been found, from the first positive detection in that area onwards. Data were available on LAU 2 level from year 2016 onwards. The regions affected contributed to the estimation of proportion of positive only in the months after the first infection was found.
2.2.1.3. Seasonality of proportions of positive samples in wild boar hunted and found dead
To evaluate the seasonality of ASF occurrence, after its first detection in a region, the numbers of cases reported through the DCF were analysed. The data were arranged considering the sampled region, sampling date and test result (a sample was considered positive if it tested PCR positive). The starting time was considered as the date on which a positive sample was reported for the first time in the LAU 2 region. LOESS smoothing (Cleveland et al., 1988) was used to estimate average profiles describing the global trends. Confidence bands were also presented to show uncertainties in the estimation of the smoothing curves. A statistical assessment of the comparison of monthly incidence using a generalised linear mixed model and a Tukey pairwise comparison between each pair of months was performed.
2.2.1.4. Speed of propagation of ASF in wild boar population
Two methods were used to calculate the speed of propagation of infection in the wild boar population, namely the speed estimated with a network analysis and with a predictive epidemiological model (see Section 2.2.3 for detailed description for the predictive epidemiological model methodology).
Three networks for Latvia, Lithuania and Estonia were created using data up to May 2018. Two scenarios were created assuming:
a case can be caused by any of the previous cases in time and the network pairs are created based on the minimum distance and time elapsed between two cases;
a case can be caused by any of the previous cases in time, provided that at least 7 days have elapsed and network pairs are created based on minimum distance between two cases;
the outcomes of the different methods were compared.
2.2.1.5. Hot spot analysis
To describe the spatial distribution of the disease and potentially identify hot spots, the study area was partitioned into a regular grid of 14968 hexagons, each with a 5‐km edge. All wild boar cases reported to the ADNS between 2014 and 2015, 2015 and 2016, 2016 and 2017, and 2017 and 2018 were included in this analysis. Counts of wild boar cases were aggregated to the hexagon level and analysed. The outcome variable was the number of cases reported in the wild boar population in each hexagon during the study periods. Hot Spot Analysis was performed using the Hot Spot Analysis (Getis‐Ord Gi*) tool of ArcMap (Spatial Statistics toolboxes, ESRI). This tool identifies significant spatial clusters of high values (hot spots) and low values (cold spots) using the Getis‐Ord Gi* statistic (Spatial statistic tool of ArcGIS 10.2 for Desktop, ESRI Inc.).
The vicinity of the hot spots and speed of propagation was discussed in a narrative section and areas at risk were highlighted.
2.2.1.6. Wild boar–domestic pig interface
Sources of introduction of ASFV in domestic pig sector: field evidence
The main sources of ASFV introduction into domestic pig holdings were discussed in a narrative section, based on robust evidence collected during epidemiological investigations of outbreaks, as provided by the affected MS.
Human‐mediated spread
To evaluate the possible human‐mediated spread of ASF, the ADNS database of notifications was explored on extreme distances or velocities necessary to cover a distance. The most plausible previous notification was searched for each individual ADNS notifications based on the shortest distance or the slowest velocity of spread. Therefore, two measured values were assigned to the individual cases reported to the ADNS, i.e. first, the distance to the closest case report older than 7 days; and second, the report older than 7 days that required the minimum velocity to bridge the distance between the two cases. Then, the velocity and distance values were ranked. The resulting rank sum was noted for each case report (distance and velocity between two reported cases). Finally, the geographical maps of all recordings were coloured according to the percentile into which the values fell in the ordered distribution of: (i) distances; and (ii) rank‐sums. In particular, the upper 1% of values (i.e. 99–100th percentile) were marked on the map indicating extreme events that are very unlikely to be caused by wild boar movement‐related transmission of the infection.
Survival of virus on different matrices at different temperatures (as possible sources of introduction)
In 2014, the EFSA AHAW Panel ranked the ability of different matrices to contain and maintain infectious ASFV. This ranking was based on an extensive literature review followed by an expert elicitation (EFSA AHAW Panel, 2014). In 2018, a systematic literature review was carried out by an external procurement project for a Data Collection for Risk Assessments on Animal Health (DACRAH2), to identify published information on the duration of survival time of ASFV in different matrices. The detailed review protocol can be found in the Supplementary Material 1, and the data extracted from the papers in Supplementary Materials 2 and 3. A summary of this review was provided in this report.
2.2.2. Risk factor analysis – TOR2
A Besag, York and Mollié (BYM) model was fitted to identify risk factors for ASF occurrence in wild boar. Details about the model used can be found in EFSA (2017) and in the Zenodo repository (Varewyck et al., 2017).
The backward model‐building procedure was used. First, the model was fitted with all risk factors available. Using a backward elimination procedure, risk factors were reduced one by one, given their lack of significant contribution to model.
All risk factors considered were aggregated spatially on the basis of the shape file of the administrative units at LAU 2 level. Table 3 lists the risk factors considered. Only data from Estonia were provided with detailed enough spatial and temporal resolution to perform the analysis, and the data did not allow a risk factor analysis for determining the persistence of the disease, but only the probability of occurrence of an ASF case in wild boar.
Table 3.
Potential risk factors based on the available data used in the analysis
| Acronyms | Description | Explanation |
|---|---|---|
| Potential risk factors related to wild boar habitat | ||
| QAH | Quality of available habitat of wild boar (average) | Habitat quality could drive wild boar density |
| WBDNS | Wild boar density (estimated number/km2) | Wild boar density could have an effect on the occurrence of the disease |
| SNOWDEPTH | Average yearly snow depth | Climatic conditions could have an effect on the presence of the virus |
| TEMPERATURE_MIN | Average yearly minimum temperature | |
| Potential risk factors related to hunting activity and wild boar management | ||
| huntersDNS | Density of hunters/km2 | Describe hunting and managerial activities |
| dogDNS | Density of hunting dogs/km2 | |
| feedsDNS | Density of feeding/baiting places/km2 | |
| huntedDNS | Density of hunted wild boar/km2 | |
| Potential risk factors related to the pig farming system | ||
| PgFrmDNS | Density of pig farms (in total) | Pig density could have an effect on the occurrence of the disease (assuming circulation in domestic pigs) |
| PgDNS | Density of pigs (in total) | |
| PgFrmSDNS | Density of small pig farms (pig holding with up to 10 heads) | Small pig farms are assumed to have lower biosecurity measures in place, and lower reporting willingness? rate?, which could have an effect on the occurrence of the disease |
| PgSDNS | Density of pigs in small holdings (pig holding with up to 10 heads) | |
| Potential anthropogenic risk factors | ||
| StlmDNS | Human settlements density/km2 | A higher human activity in an area could have an effect on the occurrence of the disease |
| RdDNS | Total road length (km)/km2 of admin unit area | |
| HumPopDNS | Human population density (ind./km2) | |
2.2.3. Review wild boar depopulation/density reduction measures for controlling the spread of ASF – TOR3
2.2.3.1. Model frame work and documentation
This section is based on a spatiotemporally explicit individual‐based model approach in structured geographic landscapes. The model framework has been developed and applied in the context of multiple infections of wild boar, i.e. Classical swine fever (CSF), Foot and mouth disease (FMD), ASF. The model compiles: (i) an ecological component detailing processes and mechanisms related to the ecology, sociology and behaviour of wild boar in natural free‐roaming populations of the species Sus scrofa; (ii) an epidemiological component reflecting individual disease course characteristics and transmission pathways including direct contact transmission on different spatial scales and environmental transmission caused by ground contamination or contacts with carcasses of succumbed infected host animals; and (iii) a management component implementing surveillance and control scenarios in a spatiotemporal explicit manner. The model is stochastic in relation to all three components and parameterised using reported distributions from the literature including variability and uncertainty. The model is simulated on heterogeneous landscapes of several thousand square kilometres, including real geographies, e.g. CORINE Land Cover. Model population emerges from birth and dead probabilities depending on habitat quality maps on the level of individual social groups. The model is documented according to the ODD protocol (Overview, Design, Details following Grimm et al., 2006, 2010). The documentation is accessible via http://ecoepi.eu/ASFWB.
2.2.3.2. Model simulation
Relevant definitions
From a legal perspective, only the ‘infected area’ is defined. According to European legislation on ASF (Council Directive 2002/60/EC, Articles 15 and 16), as soon as ASF has been confirmed in wild boar the competent authority of a MS shall immediately study the epidemiological situation and define an infected area. When defining the infected area, the authority shall take into account:
the results of the epidemiological investigations carried out and the geographical distribution of the disease;
the wild boar population in the area;
the existence of major natural or artificial obstacles to movements of wild boar;
the size of the infected area is not regulated.
In this report, the legally defined term ‘infected area’, will be used in the regulatory context only e.g. when explaining historical measures. For the assessment of management options of wild boar populations, the terms as defined as below will be used (Figure 1).
Figure 1.

Different wild boar management zones considered in the model
The core zone refers to the smallest circle around all detected ASF‐positive carcasses at the moment of the start of the application of zoned measures.
The buffer zone is surrounding the core area and is meant to separate the core and the intensive hunting zone from each other to minimise disturbance of the former by the hunting activities in the latter.
The intensive hunting zone is surrounding the buffer zone and demarcates the area to which intensified hunting measures are applied for population reduction.
Depopulation measures inside the core and buffer zone are called ‘culling’ as non‐conventional depopulation methods may be used. Population reduction measures in the intensive hunting zone are called ‘hunting’ as regular ‘hunting’ tools are used.
Management scenarios in the model
Compared with previous modelling efforts evaluating the management options in wild boar populations adjacent to large‐scale ASF‐affected areas (EFSA AHAW Panel, 2015) the following scenarios relate to testing of a variety of assumptions in the context of a focal introduction of ASF into a wild boar population.
The model evaluates the direct implementation of the spatiotemporal regime proposed in the context of focal management using core, buffer and intensive hunting zone (Figure 2).
Figure 2.
The model realisation of the management scenarios of the focal approach
The inner circle (core zone) encircles the detected carcasses (visualised here only live infected animals). Moreover, the model assigns arbitrary number of additional ring‐like zones around the core zone to represent alternative spatial designs and temporal management activity plans. Left: at the moment of implementation of the zones. Right: after eradication of ASF using different culling and hunting scenarios in different zones.
Simulations vary systematically, as follows:
dimension of the management zones
hunting intensity either proportional or by fixed number of targets set per year
number of management campaigns per year
carcass removal intensity
issues related to the probability of carcass detection i.e. determining time until and percentage of carcass detections.
semi‐permeable fences.
The simulations analysis addresses the following output measures: time to eradication, hunting effort required and carcasses occurring.
Figure 3 shows an example of an arbitrary outcome of the model, applying a specific combination of management concepts in the model population. The individual parameters specify, for example, the width of the differently coloured zones, or the effort and timing of their population reduction measures are varied systematically. The following details summarise the standard parameters and, in parentheses, the variations if the respective parameter was subjected to variation during the assessment.
Figure 3.

Outcome of the zoning submodel shown by one arbitrary run applying random carcass detection during 4 weeks before delineation of the core zone
Red: core zone; yellow: buffer zone; blue: intensive hunting zone.
Core zone (red) encircles all detected ASF‐positive carcasses 4 weeks (pre‐set) after the first notification plus two wild boar home ranges. Core zone is delineated according to random carcass detection within 4 weeks, or perfect knowledge about carcasses (not the same as those removed).
Buffer zone (yellow) is around the core zone and adds another three home ranges (variation 1, 2, 3, 4, 5) in each direction.
Core + buffer zone are culled after waiting 26 weeks (4, 8, 13, 26, 39, 52 weeks) with 90% effort (varied 0–100%) in one 4‐week campaign.
Intensive hunting zone (blue) is around the buffer and adds another 0, 3, 6, 9, 12 home ranges around in each direction (e.g. width 0, 9, 18, 27, 36 km if home‐range dimension is assumed to be 3 km).
Hunting protocols are implemented via campaigns:
Campaigns have a duration over which a specified effort has to be delivered, i.e. 4 weeks.
Campaigns are scheduled two times per year (1, 2, 4, 13) and repeated for the horizon of the simulation.
Core + buffer zone are subjected to one culling campaign after 26 weeks (4, 8, 13, 26, 39, 52).
Hunting proportions of live animals (DepopProp): advice to hunt 75% (varied 0–100%) of the accessible animals per year. Effectively, animals are hunted per week with adequately adjusted partial probability x% considering the advice, the length of one campaign and the frequency.
Hunting numbers per wild boar group (DepopNumber): advice to hunt animals (0, 5, 10, 20, 40) per wild boar group per year, i.e. over the duration of a campaign. Effectively, the prescribed number of animals is hunted every week comprising the advice, the length of one campaign and the frequency.
Hunting disturbance: increases space use of infected animals by one home range (1, 2, 3, 4, 5 h) and lasts 4 weeks.
Carcass removal: was applied at 0% (10%, 20%, 40%, 80%) carcass removal rate.
Carcass infection: 2 weeks delay or 0 weeks delay.
Fence around core zone: has no effect on the system in the standard simulations. The fence was assumed to be 100% permeable, i.e. being a line delineating core zone. Increasing the effect of the fence (90%, 50%, 10%, 5%, 0% permeable) will limit movements/contacts of infectious animals and hence make all outcomes more optimistic while true fence efficiency is unknown.
2.2.4. Review natural/artificial borders – TOR4 for the determination/demarcation of the restricted areas
The conceptual understanding of the impact of natural barriers on the spread of ASF in wild boar populations (those transmissions caused directly between individual wild boar, either dead or alive, and without human interference) is still unclear. Therefore, modelling will provide a descriptive rather than an explanatory understanding of the effect of natural barriers. The simulations mimic the continental spread of ASF on the maps of the Baltic countries. Uncertainty of the approach is very large, as wild boar experts deny, for example the effect of large rivers, while anecdotal observations show certain slow‐down of continental spread at, for example particular segments of big rivers (Rossi et al. with CSF in wild boar at the river Rhine).
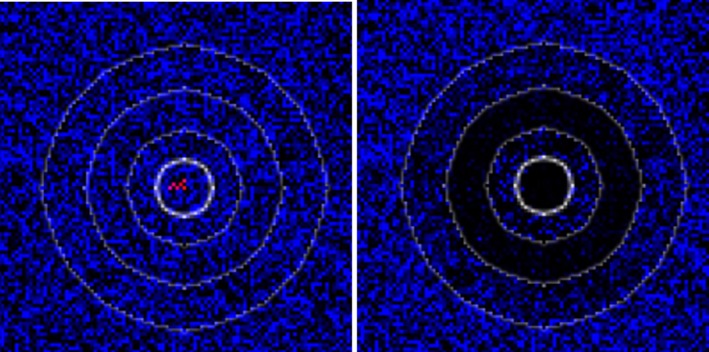
The model introduced in Section 2.2.3.1 (see http://ecoepi.eu/ASFWB or Lange et al., 2018 for complete documentation) is applied based on ADNS notification data (see Section 2.1.1). First, the local spread velocity was estimated and likely human‐mediated translocations were identified based on the time and distance between the last and all previous notifications. Re‐enforcing the likely human‐mediated translocations (n = 258), the continental spread is simulated on the habitat map of the region under study using the wild boar distribution map according to Pittiglio et al. (2018). An alternative map was produced, incorporating rivers and highways as suggested physical barriers to wild boar movement. For the latter, the continental spread of ASF infection was simulated with barriers preventing transmission events across the areas. Simulations assuming fully permeable barriers are compared with simulations with perfect barriers. The assessment will reveal whether considering barriers that prevent transmission would improve the similarity of simulated epidemics with the spatiotemporal structure of the ADNS data (Figure 4).
Figure 4.
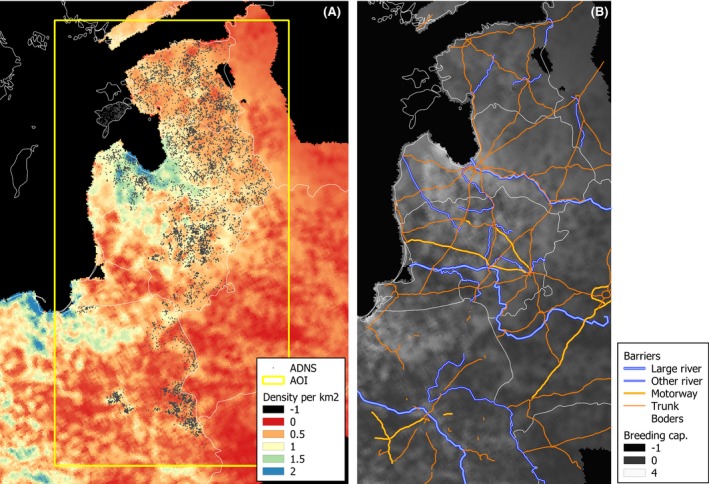
Habitat model of wild boar and physical barriers for the Baltic countries
Left: Carrying capacity values (expressed in density per km2) according to the distribution model proposed by Pittiglio et al. (2018). Right: The carrying capacity overlaid with barriers. Sources: see Lange et al., 2018.
2.2.5. Recommend measures for four scenarios − TOR5
This narrative section will be based on the outcomes of the epidemiological analysis, the risk factor analysis, the literature review of the wild boar management options model, and available field evidence from the affected MS.
3. Assessment
3.1. Descriptive epidemiology‐TOR1
3.1.1. Update of the ASF situation in eastern Europe
Figure 5 (left) shows the notifications to the ADNS, in the period since the first introduction into the EU (January 2014), up to the end of the previous report period (EFSA, 2017) and Figure 5 (right) shows the notification in the period covered by this report (from November 2017 until November 2018).
Figure 5.
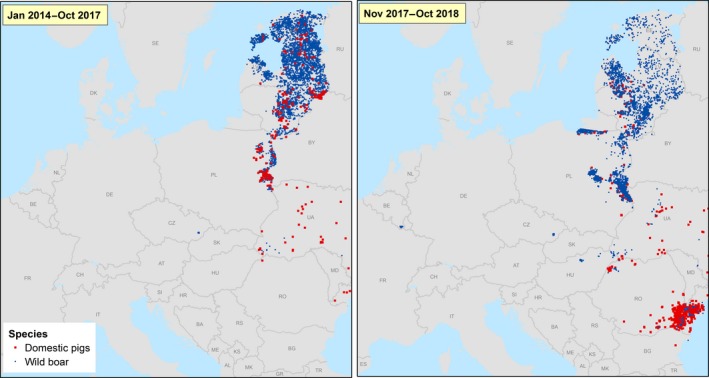
Notifications to the ADNS. Left: notifications from January 2014 to October 2017. Right: notifications from November 2017 to October 2018
ASF continued to spread slowly in the wild boar populations in the EU from the epidemic front in the Baltic MS and Poland and from the EU neighbouring countries Belarus and Ukraine in a west‐ and southwards direction.
At the same time, however, some long distance jumps of the disease have led to focal introductions of ASF in naïve wild boar populations in previously non‐affected areas/countries (e.g. in the Czech Republic, Hungary, and just recently in Belgium) or in both domestic pig and wild boar in new areas in Poland, Bulgaria and Romania, demonstrating the involvement of humans in the spread of the disease. Whereas the outbreak in the Czech Republic has apparently been controlled successfully, a fast spread of the disease was observed from Tulcea county into the surrounding counties in Romania and from the Warsaw area in Poland.
3.1.1.1. Lithuania
ASF was detected for the first time in eastern European Union countries in Lithuania, where it was officially reported on 24 January 2014. In 2014, Lithuania reported 76 cases of ASF in wild boar (54 found dead and 22 hunted) and 6 ASF outbreaks in domestic pigs. In 2015, 132 cases in wild boar (59 found dead and 73 hunted) and 13 outbreaks in domestic pigs were reported. Since February 2016, Lithuania has set up a compensation scheme for the notification of found dead wild boar and every person who notified a dead wild boar carcass was granted 30 euros. In the same year, ASF entered areas that were densely populated by wild boar and in 2016 Lithuania reported 478 ASF cases in wild boar (379 found dead and 99 hunted) and 19 outbreaks in domestic pigs. Some ASF disease ‘jumps’ have also been observed in 2017, when the disease was found 30–35 km from the previous cases. These jumps in disease spread were more likely to be caused by human activity, rather than by the natural movement of the infected wild boar.
In 2017, ASF spread slowly, but covered areas within commercial hunting grounds where the wild boar population was much higher (Figure 6). During the year 2017, Lithuania reported 2,456 ASF cases in wild boar (2,146 found dead and 310 hunted) and the increase in the number of the cases compared with 2016 was almost six times higher. In 2017, 30 outbreaks in domestic pigs were reported, 28 in non‐commercial pig farms (backyards) and two in commercial farms (one with 24,336 pigs and the second with 164 pigs). These outbreaks were most likely to have been associated with high infection pressure in the local environment. ASF cases in wild boar and outbreaks in domestic pigs are very interdependent; in the areas where the number of cases in wild boar drastically increased, during the summer season, the number of outbreaks in domestic pigs also increased and this was very likely to be due to the lack of biosecurity in the non‐commercial pig farms. Through direct or indirect contact with ASF‐affected wild boars or a contaminated environment, ASF virus entered the farms.
Figure 6.
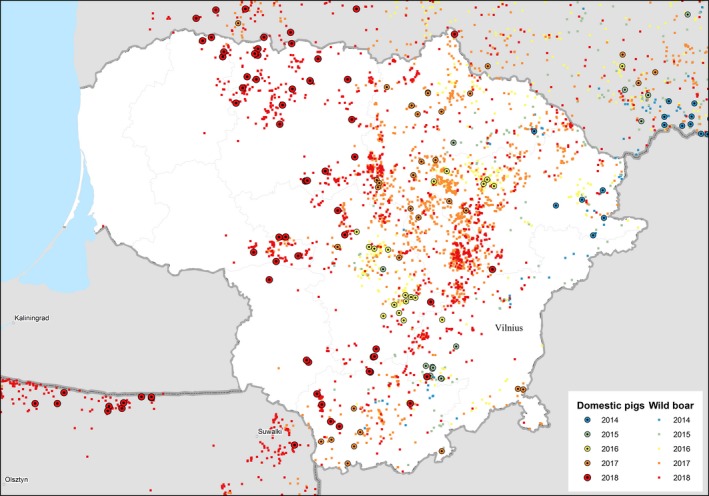
ASF outbreaks and cases in domestic pigs and wild boar, respectively, reported to the ADNS in Lithuania (2014–2018)
WB = wild boar; ADNS = Animal Disease Notification System.
ASF virus continues to spread and to enter new areas (Figure 6). Several cases were reported in three districts that bordered the neighbouring country, Latvia, apparently due to the continuous forest system, as ASF cases were found on both sides of the border.
In 2018, the number of cases in wild boar grew continuously, allowing ASF to occupy new areas of Lithuania and slowly move to the west, to the Baltic (see Figure 6).
In the period from January to 18 September 2018, 1,220 ADNS reports have been made on 2,749 cases in wild boar (2,461 found dead and 288 hunted).
A very clear seasonality was observed in the occurrence of ASF in domestic pigs in 2018. The first ASF outbreak was suspected on 2 June, and confirmed a few days later. From June to August 2018, 49 ASF outbreaks were confirmed in 43 non‐commercial and 6 commercial farms (in which 43, 204, 230, 934, 944 and 20,171 pigs were kept, respectively). All outbreaks in commercial farms occurred in the regions where numerous cases of ASF were confirmed in wild boar.
The virus has been introduced into these farms most likely because of improper biosecurity measures, through indirect contact with ASFV brought into the farms from the contaminated environment. The exact mechanism of introduction, however, remains unknown.
3.1.1.2. Poland
ASF emerged in Poland at the beginning of 2014 (Woźniakowski et al., 2016). Up to 21 August 2018, 2,812 cases in wild boars and 213 outbreaks in pigs have been confirmed. The current status of the ASF epidemiological situation is shown in Figure 7. The recent events of uncontrolled spread of ASF in the previously ASF‐free areas of Warsaw showed that there is a lack of awareness on disease epidemiology, which may lead to the spread of disease over distances longer than 160 km. So far, over 350 ASF cases have been confirmed only within the area surrounding Warsaw. ASF cases are currently located in the National Nature Park Kampinoski, where the wild boar population cannot be strictly controlled. In parallel, due to the emergence of ASF in the Kaliningrad area of the Russian Federation, new ASF cases were observed in areas bordering with it – the Bartoszyce, Braniewo, Węgorzewo, Kętrzyn, Gołdap and Elblag counties (Figure 7).
Figure 7.
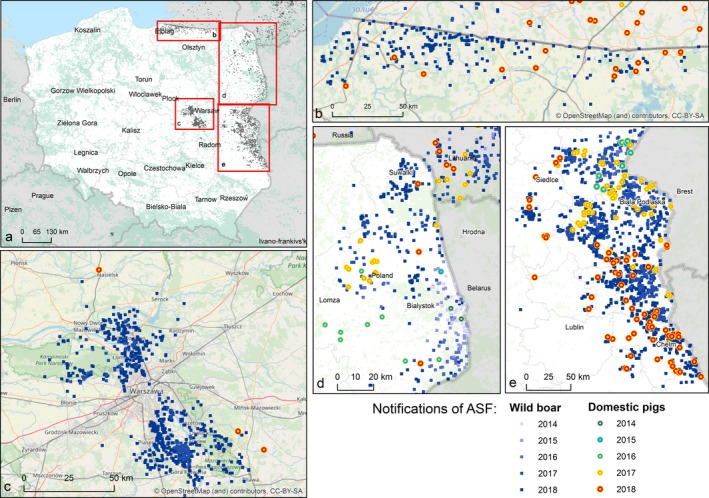
ASF outbreaks and cases in domestic pigs and wild boar, respectively, reported to the ADNS in Poland
In 2017, 81 outbreaks of ASF in domestic pigs were reported, while in 2018 the number of outbreaks increased to 109. At the same time, 1,980 ASF cases were found in wild boar across the country.
In 2018, a new cluster of ASF outbreaks has been identified in southern Poland in the Lubaczowski county. Because of the lack of previous reports on the occurrence of ASF cases within this area, the possible source of three outbreaks in pigs is related to human activity. However, it should be mentioned that this area is close to the Ukrainian border where ASF outbreaks and cases were previously reported. The statistical analysis of data indicates some relationships between the presence of ASFV in the wild boar population and the occurrence of the disease in domestic pigs. In 2018, ASF outbreaks were reported in four voivodeships in Poland namely: Podlaskie, Mazowieckie, Lubelskie and Warmińsko‐Mazurskie. Almost all (95%) of ASF outbreaks in pigs occurred in the counties (powiats) where ASF was also found in wild boars. However, some of the outbreaks that occurred in 2018 were distant from the natural reservoir for ASF, which may indicate that wild boar could be the main source, but not the only factor responsible for ASF spread in domestic pigs. The Polish experiences with ASF showed that eradication of ASFV in the country can be a long‐lasting procedure. Today, it can be stated that the primary objective of the multidirectional efforts of institutions involved in the ASF eradication programme should be focused on the prevention of disease spread in the swine population as well as on the limitation of ASF spread among wild boars. So far, none of these goals has been achieved.
3.1.1.3. Latvia
The first case of ASF in Latvia was confirmed on 25 June 2014 in a dead wild boar found by border guards on the border with Belarus. In the following weeks, several ASF cases were detected in the same region, the eastern part of Latvia. The first long distance ‘jump’ of the ASFV was observed in July 2014 when ASF was detected in dead wild boar in the northern part of Latvia, about 250 km from the initial cases. In August, other wild boar cases were found about 100 km from primary cases. These jumps in disease spread were most likely to have been caused by human activities, and were not related to the natural movement of infected wild boar. In total, Latvia reported 217 ASF cases in wild boar (178 found dead and 39 hunted) and 32 outbreaks in domestic pigs.
In 2015, disease spread slowly within the local wild boar subpopulation, making clusters around areas infected in 2014. In these areas, the wild boar population density was higher than the eastern part of Latvia (close to the Belarus border). As a result, in 2015 Latvia reported 1,048 ASF cases in wild boar (626 found dead and 422 hunted) and 10 outbreaks in domestic pigs.
In 2016, ASF cases in wild boar were detected already in half of the territory of Latvia. In summer 2016, ASF reached the central part of Latvia and later in autumn ‘jumped’ to the western part of Latvia where several wild boar cases were confirmed. This jump in disease spread was most likely to have been caused by human activity. In 2016, Latvia reported 1,146 ASF cases in wild boar (529 found dead and 617 hunted) and 3 outbreaks in domestic pigs. In 2017, the disease spread slowly within the local wild boar subpopulation around areas infected in 2016. Most ASF cases were found in the western part of Latvia (due to higher population density) moving the epidemiological front slowly south‐westwards. However, a small proportion of the total number of ASF cases was still found in areas where disease was introduced and the epidemiological front had passed. An increase in the number of wild boar with seropositive results was observed in 2017. In total, Latvia reported 1,431 ASF cases in wild boar (776 found dead and 655 hunted) and eight outbreaks in domestic pigs in 2017.
By 25 September 2018, almost 90% of the territory of Latvia was affected by ASF. The disease has been confirmed in 743 wild boars (264 found dead and 479 hunted), and 10 outbreaks in domestic pigs were reported. Most ASF cases in wild boar in 2018 were confirmed in hunted animals testing only as seropositive. Most ASF cases in wild boar are still located in the western part of Latvia with the minority of cases in areas affected during the previous years (Figure 8).
Figure 8.
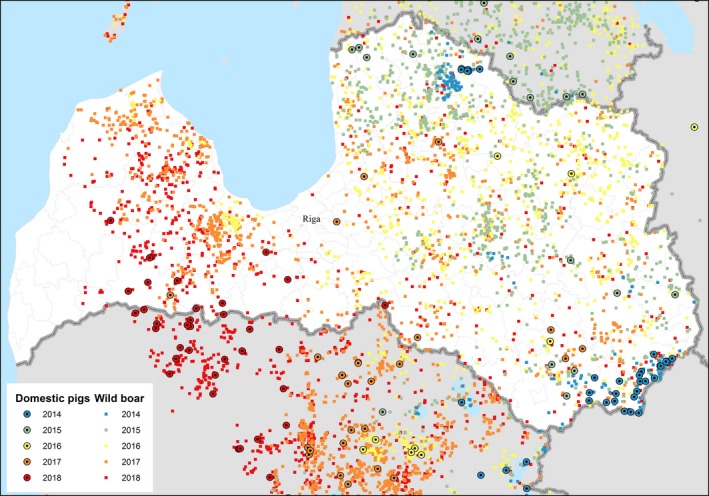
ASF outbreaks and cases in domestic pigs and wild boar, respectively, reported to the ADNS in Latvia
3.1.1.4. Estonia
ASF was first detected at the beginning of September 2014 in wild boar in the south of Estonia, close to the Latvian border. This can be considered a result of the expansion of the infected area in Latvia and natural spread of the virus through the wild boar population over the border to Estonia. However, after 2 weeks, a second focus of affected wild boar was detected in north‐eastern (N‐E) Estonia, 200 km from the focus detected first, indicating human‐mediated introduction. Further studies have revealed that the N‐E introduction was most likely to have been an independent event from the ‘southern’ introduction and had occurred earlier in time. The course of the epidemic in N‐E was distinct from the one in the south (Nurmoja et al., 2017b); the virus isolated from the N‐E focus has different biological characteristics: an attenuated phenotype (Nurmoja et al., 2017a) and, due to a deletion, it lacks a significant part at the 5’‐end of the genome, making it traceable by molecular tools (Zani et al., 2018). So far, the N‐E strain has not been detected anywhere else than in the limited area in N‐E Estonia (Ida‐Virumaa county). The virus was introduced to the N‐E most likely with infected pork or wild boar meat for own consumption carried by travellers who were arriving from affected countries.
In 2014 and in the first quarter of 2015, the virus slowly propagated in local wild boar populations (average expansion of the infected area was up to 3 km/month). No outbreaks among domestic pigs were detected. In spring 2015, the speed of spread of the infection among wild boar started to increase, reaching its peak in August (12 km/month). A second peak in the speed of expansion of the infected area was registered in November 2015 (Figure 9). In July 2015, first outbreaks among domestic pigs were detected, resulting in a total number of 18 reported outbreaks by September 2015. In 2016, a similar pattern of the disease spread among wild boar and the occurrence in domestic pigs was repeated. The occurrence of the disease in domestic pigs has been strictly seasonal in Estonia, coinciding with increased spread of the infection among wild boar (Figure 10). Last outbreaks occurred in summer 2017. The domestic pig outbreaks occurred, with few exceptions, in areas where the active spread of the virus among wild boar has been in progress, where the larger herds have been at higher risk of being infected and the route of transmission of the virus to the farms has been by indirect transmission, most likely through contaminated fomites, vehicles or clothing of farm workers, although transmission by mechanical vectors could not be excluded (Nurmoja et al., 2018).
Figure 9.
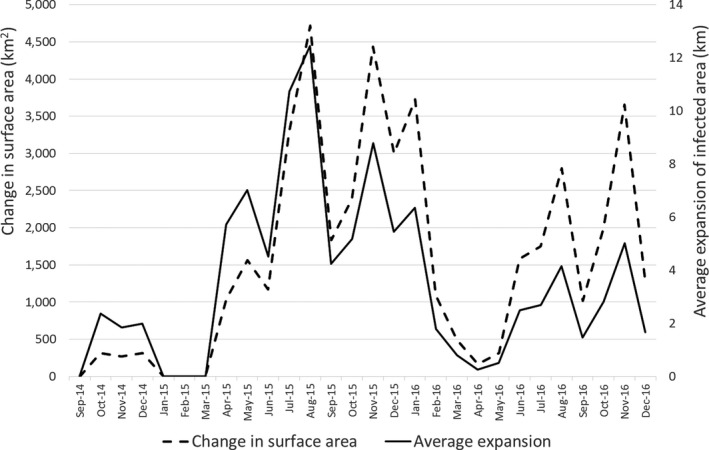
Expansion of the ASF‐infected area in Estonia from September 2014 until December 2016
Figure 10.
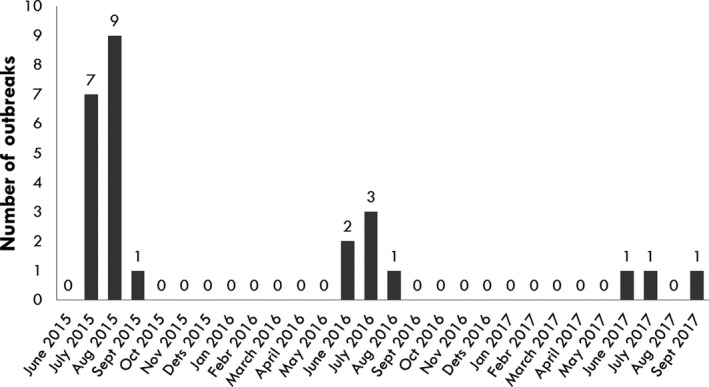
Occurrence of ASF in domestic pig herds in Estonia from June 2015 to September 2017
By the end of 2016, the whole mainland and the largest island of Estonia, Saaremaa, were considered as an ASF wild boar infected area. The number of detected ASF virus (PCR)‐positive wild boar increased steadily through the years from September 2014 to 2016 (77, 1,004 and 1,019, respectively) along with the expansion of the infected area. In 2017, the total number of detected PCR‐positive wild boars was 497. The vast majority (94%) of these animals was detected in the western part of the country, where the infection was in the epidemic phase. In 2018, by the end of August, 53 PCR‐positive wild boars were detected, out of these five were in the eastern part of the country. In summary (Figure 11), it is apparent that in areas that have experienced the ASF epidemic and where the density of wild boar has drastically decreased, the spread of the infection has decreased as well. This is reflected by lower numbers of detected cases, as well as in a lower prevalence of PCR‐positive animals among tested animals. Nevertheless, these areas still cannot be considered disease free as sporadic cases continue to emerge, often 6–8 months apart from previous detection in the same area (e.g. hunting grounds).
Figure 11.
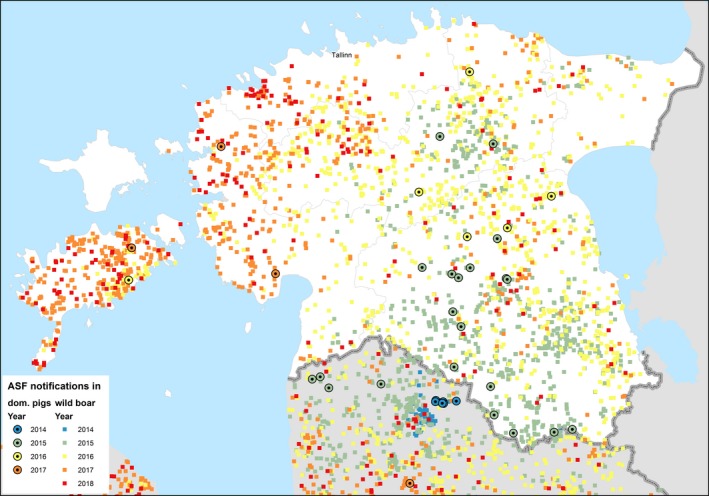
ASF outbreaks and cases in domestic pigs and wild boar, respectively, reported to the ADNS in Estonia
3.1.1.5. Czech Republic
The first case of ASF in the wild boar population was confirmed in the Czech Republic on 26 June 2017, in two wild boar found dead in the Zlín district. As of 3 April 2018, 200 cases of ASF have been registered among wild boars found dead in the Zlín district. With the exception of 10 cases found outside the fences, all the other cases have been recorded in the high‐risk area (inside the fences). Nearly 80% of the 245 found dead wild boars in the high‐risk area were positive. In total, 99 found dead wild boars have been tested from the infected area outside the fences. In total, 101 found dead wild boars have been tested from further districts in the Zlín region outside the infected area, all with negative results. In total, 648 wild boars were shot or trapped in the so‐called red zone up to 31 January 2018. Seventeen animals from this group were ASF positive; 1,874 wild boars were shot in the so‐called green zone in the same period. All tests from this group were negative.
Since 1 February 2018, the infected area has been reduced; 287 wild boars were hunted in the new infected area until the 3 April 2018. Only one positive case was recorded from this group. In total, 14,884 wild boars were hunted in the area (size 9,004 km2) of intensified hunting set up from 13 July 2017 until 3 April 2018; the highest number was found in the Uherské Hradiště (2,809) and Kroměříž (2,503) districts. No positive case of ASF has been recorded in this area.
The infected area was set up in the Zlín district immediately after the confirmation of the first ASF case, including a hunting ban. Later on, exception from the hunting ban has been allowed but only for approved hunters who had attended training on biosecurity rules during the hunting and transport of hunted animals, to ensure disease spread prevention. However, only individual hunting has been allowed after obtaining sufficient data on disease spread, this meant at first hunting only in the low‐risk area approximately a month after the first findings and, since September 2017, also in the high‐risk area. All wild boars hunted in the infected area have to be safely disposed of in rendering plants and tested for ASF; 2,809 wild boars were hunted in the infected area until 3 April. Eighteen wild boars from this group were ASF positive. To support hunting in the infected area in 2017, 3,000 CZK were paid to hunters for wild boar until 50 kg of weight and 4000 CZK over 50 kg of weight. In 2018, the payment increased to 4,000 CZK and 8,000 CZK. Further compensation is paid to hunters from the Ministry of Agriculture according to the Veterinary Act for wild boar that cannot be used as venison and have to be safely disposed of in rendering plants.
A so‐called buffer zone has been set up around the infected area within the area of intensified hunting. Hunting in this area was supported with 1,000 CZK in 2017 for each shot wild boar. In 2018, support increased to 2,000 CZK. All hunted boar have to be tested for ASF. The passive surveillance in the area of intensified hunting – finding of dead wild boar and its testing – has been generally accepted as one of the most significant steps among the approved measures. It is more important to collect carcasses than hunt. For this reason, 3,000 CZK are paid for each wild boar found dead.
From 16 October 2017, after 2 weeks with no positive finding in carcasses, at the end of epidemic phase, hunting of wild boar in the infected area by police snipers started. Hunted wild boars were in total 157, and 8 of these were positive for ASF. Snipers were trained for wild boar hunting and for biosecurity during hunting. Police snipers were employed in the high‐risk zone. They were split into eight teams of two men each, shooting wild boar at 3‐day interval. All shot wild boar were collected by the State Veterinary Administration (SVA), safely transported to the nearest road and then sampled at the rendering plant.
One year after the first case, ASF is still located in a very small territory and it is not spreading, thanks to a combination of the measures taken and continuously adjusted development of the disease situation (Figure 12). SVA closely collaborates with many subjects (regional government, municipalities, farmers, hunters’ organisations, etc.). The SVA issued several extraordinary veterinary measures to prevent spreading and enable disease eradication. Feeding of wild boar is forbidden in the whole territory of the Czech Republic with the exception of baiting for hunting. The installation of odour and electric fences, and the non‐harvesting of some fields in the high‐risk infected area could be taken as examples of other measures taken.
Figure 12.
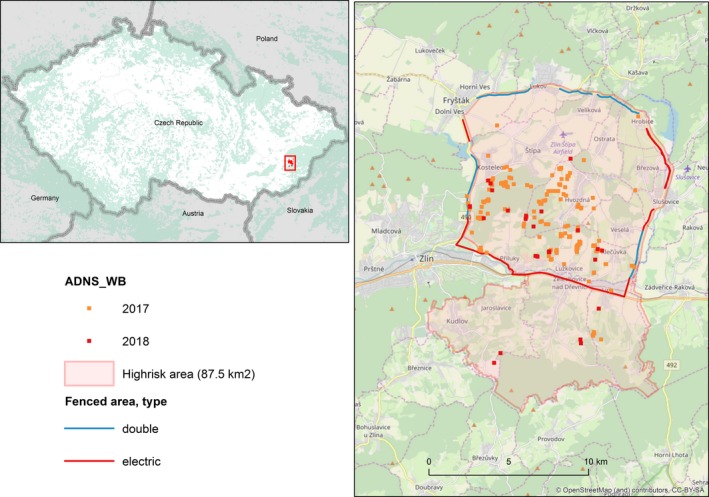
ASF cases in wild boar reported to the ADNS in the Czech Republic
At the same time, the SVA focused on the prevention of ASF introduction to the domestic pig population. Since July 2017, extraordinary official controls of holdings targeted on biosecurity have been carried out. To increase biosecurity and avoid contact between wild boar and domestic pigs is one of the main duties ordered in the infected area. Movement of pigs is allowed only after approval of the SVA. It is also forbidden to use straw and grass as feed, and to feed cereals from the last harvest from the infected area. It is ordered to keep pigs inside stables and to use special working clothing and shoes. Awareness on the necessity to follow strong biosecurity measures in pig holdings was repeatedly mentioned on various occasions (information campaign, leaflets, methodological instruction, webpage of SVA – http://www.svscr.cz). The ban on keeping pigs in non‐registered holdings (one pig for home slaughter – own consumption) has been endorsed in high‐risk areas. Municipalities in the whole Zlín region had to perform a census of all pig holdings until the end of January 2018. Duty to check all pig movements, a system of early detection, regular visits and checks by veterinary inspectors and a system of testing sick/dead animals in pig holdings also contributed to minimise the risk of ASF spread.
Finally, we can summarise the experience with eradication of a focal outbreak of ASF in wild boar. Passive surveillance, and the collection and testing of dead wild boar represent the key aspects of the early detection system and for effective measures. After confirmation of the disease, it is necessary to define the infected area based on passive surveillance. Because at the beginning, we missed information about infected areas and the exact wild boar population, the infected area is bigger and must be finalised according to passive surveillance. Stopping hunting and feeding during the epidemic phase of infection proved to be effective measures to prevent spreading of ASF. Hunting is applicable at the final stage of the epidemic phase after determining the infected area and assuring biosecurity during hunting biological safety during hunting. Intensive targeted hunting by trained people seems to be a very effective method for decreasing the population at the end of the epidemic phase. Active searching of carcasses in the infected area, after decreasing the population and crop harvesting, is very important because carcasses can be sources of infection for a long time. Very important is effective communication among all stakeholders. In the infected area and the high‐risk area, it is necessary to impose preventive measures in domestic pigs farms. Measures are focused mainly on improving biosecurity levels, especially in backyard farms.
3.1.1.6. Romania
Romania was free of ASF until July 2017, when two outbreaks were confirmed in domestic pigs in two backyards in Satu Mare county (Figure 13).
Figure 13.
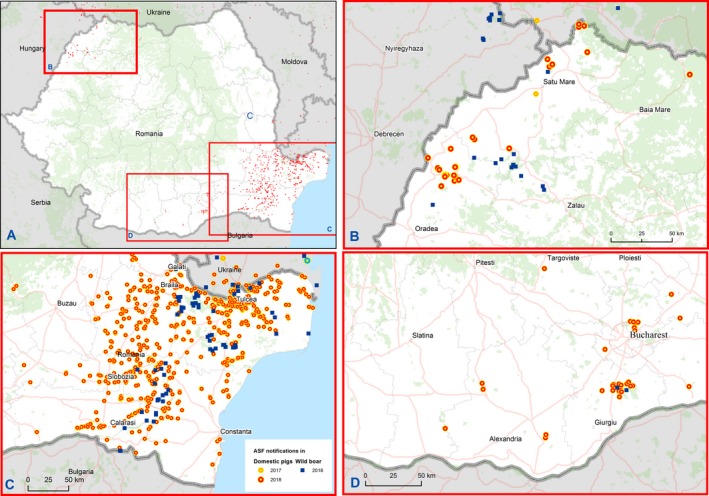
ASF outbreaks and cases in domestic pigs and wild boar, respectively, reported to the ADNS in Romania
In 2018, ASF had very different epidemiological changes in two geographic areas of Romania (Figure 13):
Northwest region: The first case of ASF in wild boars was confirmed on 29 May, in Satu Mare county. By 9 October, ASF had been confirmed in 15 wild boars (13 found dead and 2 hunted) and in 35 outbreaks in domestic pigs from backyards. Even if the disease was initially confirmed in the domestic pig population and subsequently in the wild boar population, at present, the virus in both populations is closely related.
Southeast region: On 10 June 2018, in Tulcea county, the first outbreak in domestic pigs was confirmed in a backyard holding. A few days later, the first wild boar case was confirmed (12 June 2018). Subsequently, the epidemiological situation experienced an aggressive change in this region of Romania, where, in a short time, a large number of outbreaks was confirmed. By 9 October, ASF has been confirmed in 72 wild boars (61 found dead and 11 hunted), in 943 outbreaks in backyards domestic pigs, in 15 outbreaks in commercial farms and in one slaughterhouse. The main epidemiological hypothesis of the ASFV introduction into the Danube Delta Biosphere Reservation is represented by the epidemic wave in wild boar from infected areas across the border, but human‐mediated translocation is still considered as the main risk factor that leads to the further spread of ASF, although a possible role of mechanical vectors for disease dynamics should not be ignored, and might need further investigation.
As described above, ASF has evolved into two geographical regions in Romania: the northwest and southeast area, with totally different dynamics in each area.
Change in the southeast area has been devastating, both through the rapidity of virus transmission and the number of affected farms. If there would be an explanation for the rapid dissemination of the virus in Tulcea county, the question remains what is the cause of the slower diffusion in the neighbouring counties?
The official veterinarians working in the field found, for example, in the county of Ialomita (the county at the border with Tulcea county) hypothesised, following the epidemiological investigations, that the existence of possible risk factors, such as haematopoietic insects, geographical positioning, weather conditions, could have accentuated the rapid dissemination of the virus.
As a result of epidemiological investigations in the counties affected by the ASF, it was observed that most locations where outbreaks appeared were near the water (especially near the Danube River).
At the same time, it could be noticed that the first outbreaks appeared in the areas located on the outskirts of the locality. In several localities, the outbreaks appeared for the first time in farmers’ backyards with two or three pigs, where the owners were elderly people who had no contact with the sylvatic environment, the field, the forest and even the neighbours. They did not buy food, used only products from their own household, nor did they handle swill in pig feed.
Another observed coincidence was that the spread of the virus increased in the period following the rains, during which time favourable conditions for insect resurgence were created.
These events led to the hypothesis of the existence of vectors transmitting the virus, which the literature does not mention, such as hematophagous insects (mosquitoes, flies). Alternatively virus transported on melons or in water was suggested to be the cause of spread of the disease (see the three commercial farms in Braila County).
All of these potential risk factors in the transmission and spreading of ASF require thorough investigation to be confirmed.
3.1.1.7. Hungary
Heves county
The first ASF case in Hungary was detected on 21 April 2018 in Heves county. On 19 April, a dead wild boar was found around the locality of Gyöngyös; a sample was taken and sent to the National Reference Laboratory (Veterinary Diagnostic Directorate of National Food Chain Safety Office, Budapest). The presence of ASF virus was confirmed on 21 April by PCR test. The virus isolation was positive on 27 April; sequencing of p72, p54 and B602L genes showed a 99–100% identity with the Georgia 2007 strain. Some bigger factories in the area hire a great number of workers from the Ukraine, residing in hostel‐like facilities in neighbouring villages. Therefore, ASF introduction was most likely to have been caused by human activities.
After the confirmation of the first case, a temporary infected area was set up as a direct Chief veterinary officer (CVO) order in the part of Heves county north from the M3 motorway (E71), in accordance with Council Directive 2002/60/EC.6 Furthermore, an exceptional controlled area within the infected area was also set up with further measures. Later, the temporary infected area was changed according to the risk analysis carried out after the first case and a 5‐km wide buffer zone around the exceptional controlled area was set up. On 21 September 2018, on the basis of the risk analysis carried out in August 2018, the infected area was extended to 16 hunting units in Nógrád County.
In Heves county, until 24 October 2018, 22 cases were confirmed in wild boar: 2 cases in April, 6 cases in May, 4 cases in June, 4 cases in July, 4 cases in August, 1 case in September and 3 cases in October. Of 22 cases, 21 were dead wild boars and 1 animal was shot due to clinical signs. The following map shows the geographical distribution of the cases (Figure 14).
Figure 14.
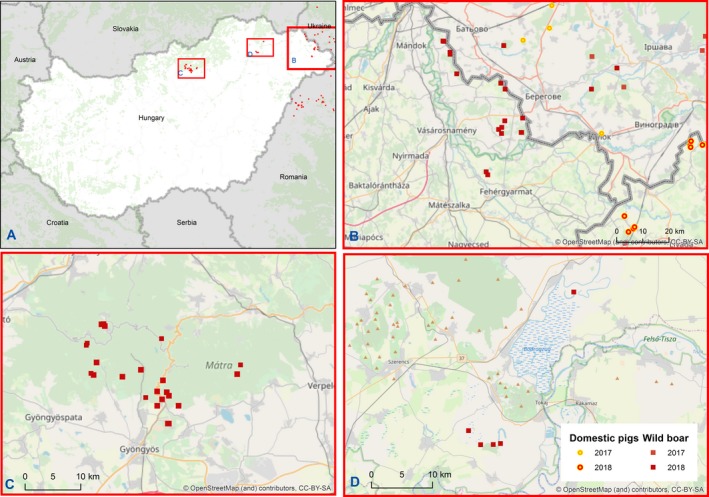
ASF cases in wild boar reported to the ADNS in Hungary (A); cases in Szabolcs‐Szatmár‐Bereg county (B); Heves county (C); and in Borsod‐Abaúj‐Zemplén county (D)
Szabolcs‐Szatmár‐Bereg county
In May 2018, ASF was detected in Szabolcs‐Szatmár‐Bereg county: on 14 May, a dead wild boar was found around the locality of Tiszakerecseny, around 1 km from the Ukrainian border. Samples were taken and sent to the National Reference Laboratory and ASF virus was confirmed on 16 May by PCR test. Virus isolation was positive on 24 May and the sequencing showed 100% identity with Heves county isolates. The most likely source of the infection is the natural spread from Ukraine, taking into consideration the distance in the infected neighbouring regions in the Ukraine and seasonal movements of wild boars.
The infected area has been set up according to the CVO order in the area, which was previously identified as the high risk area. An exceptionally controlled area within the infected area and a buffer zone around the exceptionally controlled area have been set up as well.
In Szabolcs‐Szatmár‐Bereg county, until 24 October 2018, 18 cases were confirmed in wild boars: 3 cases in May, 10 cases in June, 3 cases in July and 2 cases in August. All 18 cases were dead wild boars. The following map shows the geographical distribution of the cases (Figure 14).
Borsod‐Abaúj‐Zemplén county
At the beginning of October, ASF was detected in the third Hungarian county, the Borsod‐Abaúj‐Zemplén county. A healthy wild boar was shot (in the context of diagnostic shooting to decrease the wild boar population) around the locality of Tarcal in Borsod‐Abaúj‐Zemplén county, near to the border with Szabolcs‐Szatmár‐Bereg county, on the night of 27 September. Samples were taken, sent to the National Reference Laboratory and the ASF virus was confirmed on 2 October by PCR test. The epidemiological investigation is being carried out but, taking into consideration the distance from the cases found earlier, this case cannot be linked to those. At this stage of the epidemiological investigation, there is no meaningful information about the source of the infection. On 19 October one, and on 21 October two, ASF cases were confirmed by PCR in dead wild boars near to the first case.
The temporary infected area has been set up as a direct CVO order and an exceptional controlled area within the infected area (core area and a buffer zone) has also been set up with further measures. The following map (Figure 14) shows the localisation of this case and the areas.
3.1.1.8. Bulgaria
In July 2018, the construction of a fence along the land border with Romania was initiated (BFSA, 2018).
On 31 August 2018, the National Reference Laboratory (NRL) for ASF in Sofia confirmed the first positive result for ASF in domestic pigs in a backyard holding located in a village, Tutrakantsi, Provadiaya municipality, Varna region. The location of the affected farm is presented in Figure 15. In total, seven 1‐year‐old fattening pigs were reared in the affected holding and four of these were found dead. Samples were taken in the context of passive surveillance and sent to NRL in Sofia on 30 August 2018.
Figure 15.
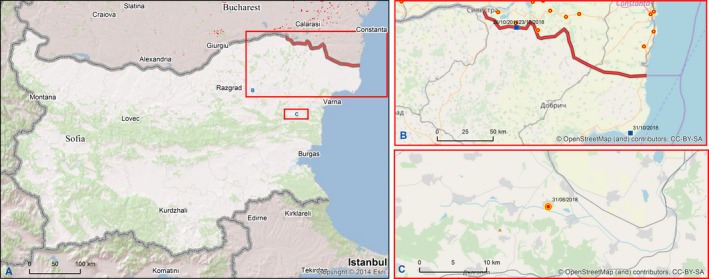
ASF outbreak domestic pigs reported to the ADNS in Bulgaria
The epidemiological investigation is inconclusive so far. Different scenarios were considered regarding the most likely source of infection. The farm was well fenced and the possibility for direct contact with wild boar is very low. The infected area is far from the main roads, approximately 30 km from Varna and 100 km from the border with Romania.
Control and eradication measures were implemented, with culling and disposal of all pigs reared in the village of Tutrakansi (in total 87 pigs in 30 holdings). Protection and surveillance zones were set up where sampling/testing of all pigs was carried out and the movement of domestic pigs was restricted in the Varna region.
Passive surveillance has been increased in both domestic pigs and wild boar in the whole territory of the country. For wild boar, a ban on trade into the territory of Bulgaria and individual hunting all over the year were enforced. Special focus was given on raising awareness in hunters, including numerous trainings to hunters on epidemiology, sampling and enhanced biosecurity measures in the context of ASF control.
On 23 October 2018, the NRL, for classical swine fever and ASF, in Sofia confirmed by PCR the first positive result for ASF in wild boar. The wild boar was found dead at the border with Romania, in Kaynardzha municipality, Silistra region, Bulgaria (Figure 15B). During the investigation of the place where the wild boar was found dead, a second wild boar with abnormal behaviour was shot a few metres away and later tested as positive for ASF by PCR.
3.1.1.9. Belgium
On Saturday, 8 September 2018, a huntress notified the discovery of three wild boar remains that were located close to each other in the Bois de Buzenol, a forest administratively attached to the municipality of Etalle in the south‐east of Belgium, about 12 and 17 km, respectively, from the borders of France and the Grand Duchy of Luxembourg. The said remains consisted of three vague aggregates of bones associated with a few fragments of desiccated skin. On Monday, 10 September, foresters of the Public Service of Wallonia who had been dispatched to the scene did not find the said remains, but soon fell on the foul smelling decaying body of an adult female wild boar. The forest officers decided to transport the carcass to the Faculty of Veterinary Medicine at the University of Liège for autopsy and sampling. After carrying the corpse a few hundred metres, they fortuitously stumbled upon a weak, staggering and ataxic young wild boar, which they euthanised.
On Tuesday, September 11, the autopsy of the young boar displayed lesions consistent with a diagnosis of ASF. On Wednesday, September 12, spleen/kidney samples of both animals were brought to the NRL and proved positive by quantitative polymerase chain reaction (qPCR) for the genome of the ASF virus. The two first cases were notified on Thursday, 13 September. Samples were sent to the European Union Reference Laboratory (EURL) in Spain for confirmation and gene typing. At the same time, a second team was dispatched to the Bois de Buzenol to search the remains of the three wild boars notified first. This time, the search was successful and the rinsing fluids of the medullary cavity of the long bones proved similarly positive, raising the number of infected animals found on the primordial spot to five. On 14 September, the EURL confirmed the ASF cases and identified the strain based on the sequence of the p72 protein as p72 genotype ll that had been circulating in eastern European countries since the first introduction in Georgia in 2007. Further subtyping throughout the analysis of three independent ASFV genome regions, clustered the Belgium wild boar viruses within the CVR‐I, IGR‐2 and MGF1 variants. From the data available at the EURL, these are the variants mostly circulating within the EU countries as well as described in Moldova, Ukraine, Belarus, and in certain areas of the Russia Federation.
Since then, a systematic search of the potentially infected zone of 63,000 ha defined by the Public Service of Wallonia and the Federal Agency for the Safety of the Food Chain and approved by the European Commission has been put in place. At the time of writing this document on 30 October 2018, 237 bodies of wild boar in different stages of decomposition have been detected over this area. The genome of the virus was detected in 155 boars, all of which came from a restricted geographical area extending over about 9,136 ha. The sweeping effort, to pick up as many wild boar remains as possible over the zone, is amplified every day to quickly circumscribe the geographical extension of the zone infected. Figure 16 reports cases in wild boar reported to ADNS from Belgium.
Figure 16.
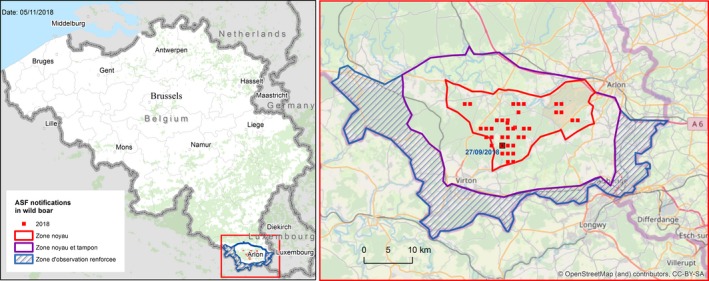
Sixteen ASF cases in wild boar reported to the ADNS in Belgium
3.1.1.10. Slovakia (not affected, but neighbouring‐affected areas)
Since the introduction of ASF in neighbouring countries of Slovakia (Ukraine, the Czech Republic and Hungary) an updated surveillance programme has been in place. In 2017, two buffer zones, one on the border with Ukraine and, from July 2017, also on the border with the Czech Republic were set up. A new ASF buffer zone on the border with Hungary was set up from May 2018 and monitoring of ASF in wild boars has been carried out as following:
investigation of all found dead wild boars on the whole territory;
investigation of all sick and suspicious wild boars on the whole territory;
investigation of all hunted wild boars in the ASF buffer zones.
A new hunting management strategy was introduced from wild boars (i.e. ban of feed, only baits, targeted hunting, disposal of carcasses, new benefits for hunters).
Additionally, greater emphasis was placed on biosecurity measures in all pig holdings.
Until 30 September 2018, 11,707 wild boars have been virologically investigated and 1,058 domestic pigs. No positive results in wild boars and domestic pigs have been detected.
3.1.2. Temporal patterns
3.1.2.1. Proportions of positive samples tested either by PCR or antibody‐ELISA in Baltic States and Poland since first detection
Since the introduction of ASF in the Baltic States and Poland, up to and including October 2018, the proportion of positive samples tested either by PCR or AB‐ELISA from hunted wild boar remained low, but there was a gradual increase in the proportion of positive samples from wild boar that were found dead (Figure 17).
Figure 17.
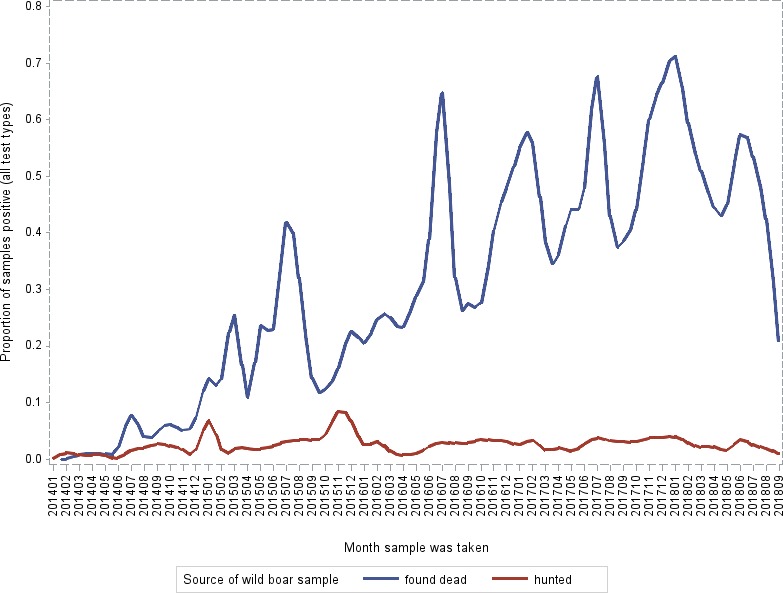
Proportion of positive sample over tested samples (PCR and AB‐ELISA) in hunted wild boar and wild boar found dead in the Baltic Countries and Poland since the first introduction
Figures 18, 20, 22 and 24 show the observed proportions of positive samples of hunted wild boar population tested by PCR and AB‐ELISA (dot‐dashed blue line) as well as LOESS‐smoothed data (blue and red lines) and confidence bands (blue and red regions), over all the samples tested in the LAU 2 areas (both affected and not affected areas) in Lithuania, Poland, Latvia and Estonia, respectively.
Figure 18.
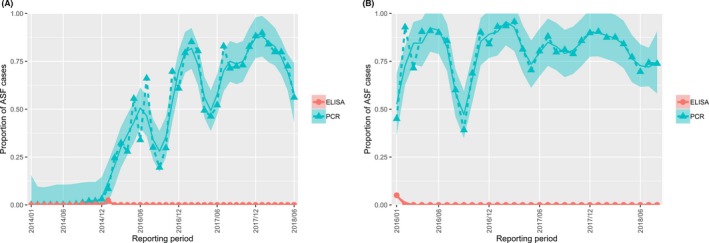
Proportion of ASF‐positive samples over tested samples from all wild boars found dead in the sampled areas of Lithuania (A) and from the affected areas only (B)
Figure 20.
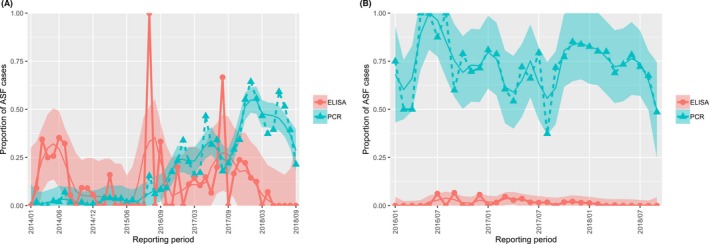
Proportion of ASF‐positive samples over tested samples from all wild boars found dead in the sampled areas of Poland (A) and from the affected areas only (B)
Figure 22.
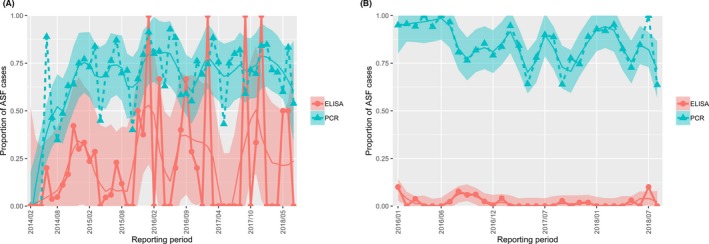
Proportion of ASF‐positive samples over tested samples from all wild boars found dead in the sampled areas of Latvia (A) and from the affected areas only (B)
Figure 24.
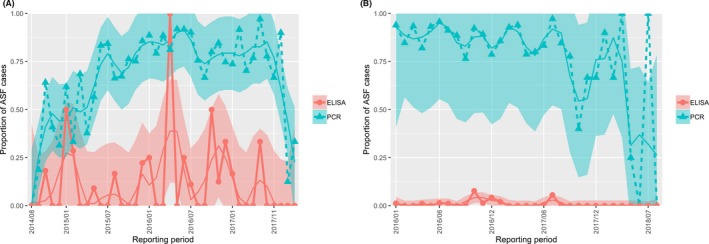
Proportion of ASF‐positive samples over tested samples from all wild boars found dead in the sampled areas of Estonia (A) and from the affected areas only (B)
Figures 19, 21, 23 and 25 show the same proportions, but this time only the affected LAU2 regions were included in the analysis, from the first positive detection onwards.
Figure 19.
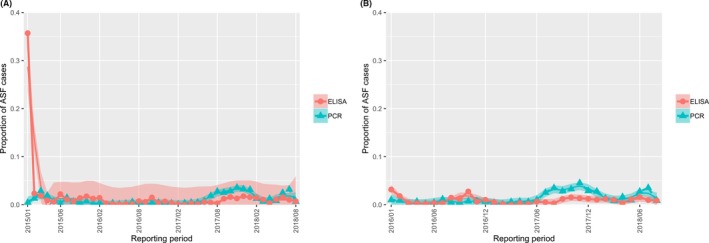
Proportion of ASF‐positive samples over tested samples from all hunted wild boar in the sampled areas of Lithuania (A) and from the affected areas only (B)
Figure 21.
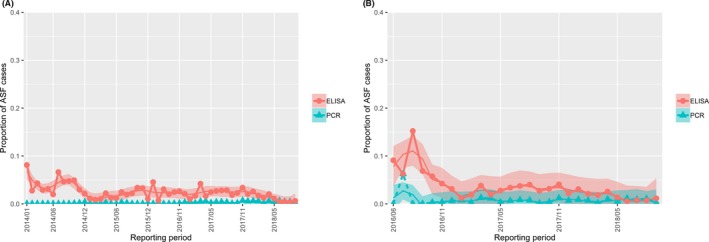
Proportion of ASF‐positive samples over tested samples from all hunted wild boars in the sampled areas of Poland (A) and from the affected areas only (B)
Figure 23.
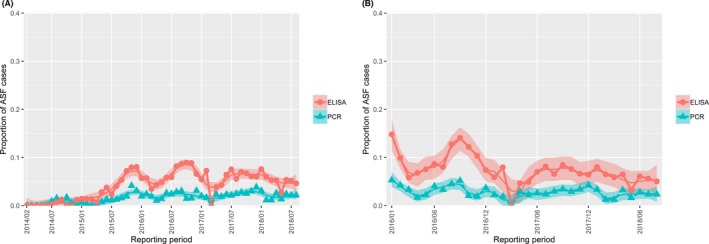
Proportion of ASF‐positive samples over tested samples from all hunted wild boars in the sampled areas of Latvia (A) and from the affected areas only (B)
Figure 25.
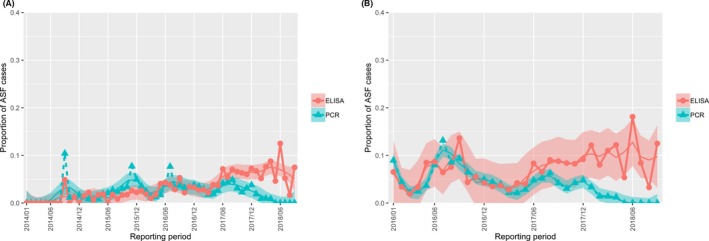
Proportion of ASF‐positive samples over tested samples from all hunted wild boars in the sampled areas of Estonia (A) and from the affected areas only (B)
The proportions of wild boar testing positive are always higher in animals found dead than in hunted animals.
Proportions of animals testing positive with PCR are generally much higher than proportions testing ELISA AB‐positive in animals found dead.
The increasing trends in proportions of positive testing animals in the animals that were found dead as observed in Figure 17 for all the Baltic countries and Poland pooled is only visible in Lithuania and Poland when looking at all the sampled areas, and only with the PCR testing. However, when looking at the same proportions in the affected areas only, this increasing trend was not visible. It appears also that, in the affected areas, the proportion of PCR‐positive tested wild boar is decreasing in the last half year, in animals found dead.
Conversely, it seems that in Estonia the proportions of seropositive hunted wild boar increased in the last reporting period.
Overall, in hunted animals, both the proportions of wild boar testing PCR and ELISA positive remained low, although some seasonal peaks are visible. Possible seasonality is studied in more detail in Section 3.1.2.2.
3.1.2.2. Seasonality: quantitative analysis supported by data
Figures 26 and 27 show the seasonal distribution of ADNS notifications from the Baltic Countries and Poland from 2014 to 2018.
Figure 26.
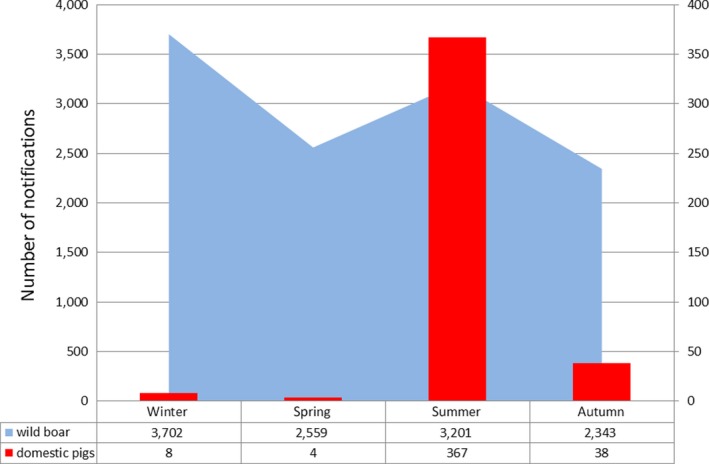
Seasonal distribution of ADNS notifications in wild boar and pigs from the Baltic countries and Poland from 2014–2018
Figure 27.
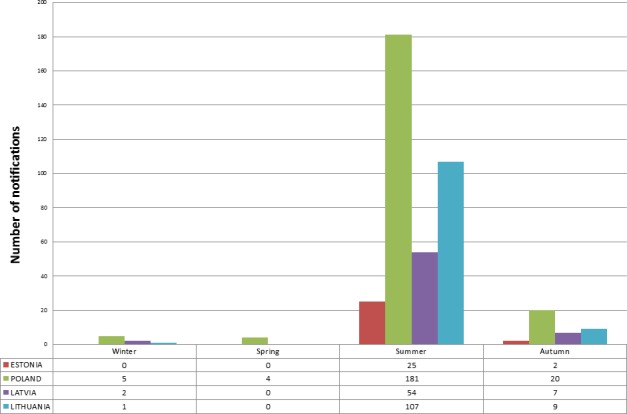
Seasonal distribution of ADNS notifications in pigs from the Baltic countries and Poland from 2014–2018
Seasonality of proportions of positive samples over the samples submitted for ASFV testing
Figures 28, 30, 32 and 34 show the seasonal patterns of the total numbers of samples from wild boar found dead submitted for PCR and ELISA testing and the proportions testing PCR and ELISA positive from Lithuania, Poland, Latvia and Estonia, respectively, since the first introduction of ASFV, until and including October 2018.
Figure 28.
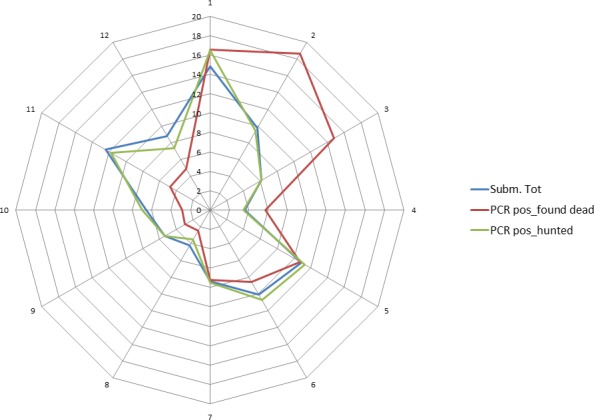
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive samples from wild boar found dead (red) and hunted wild boar (green) in Lithuania
Figure 30.
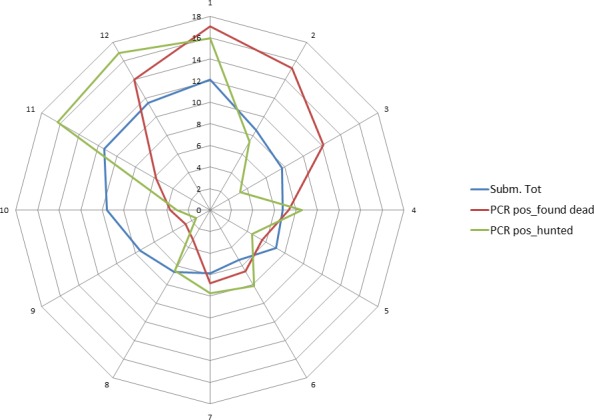
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive samples from wild boar found dead (red) and hunted wild boar (green) in Poland
Figure 32.
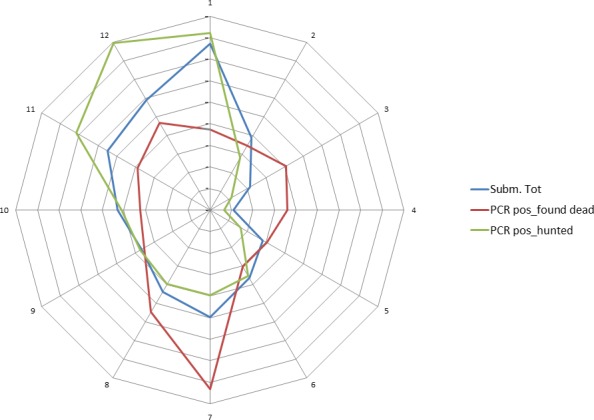
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive samples from wild boar found dead (red) and hunted wild boar (green) in Latvia
Figure 34.
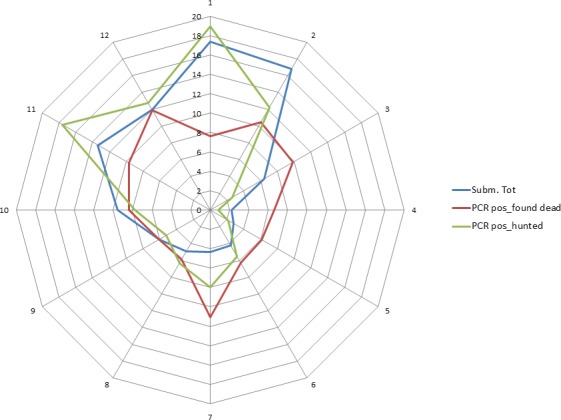
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive samples from wild boar found dead (red) and hunted wild boar (green) in Estonia
Figures 29, 31, 33 and 35 show the seasonal patterns of the total numbers of samples from hunted wild boar submitted for PCR and ELISA testing and the proportions testing PCR and ELISA positive from Lithuania, Poland, Latvia and Estonia.
Figure 29.
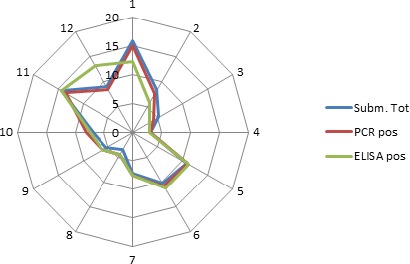
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive (red) and ELISA‐positive samples (green) from hunted wild boar in Lithuania
Figure 31.
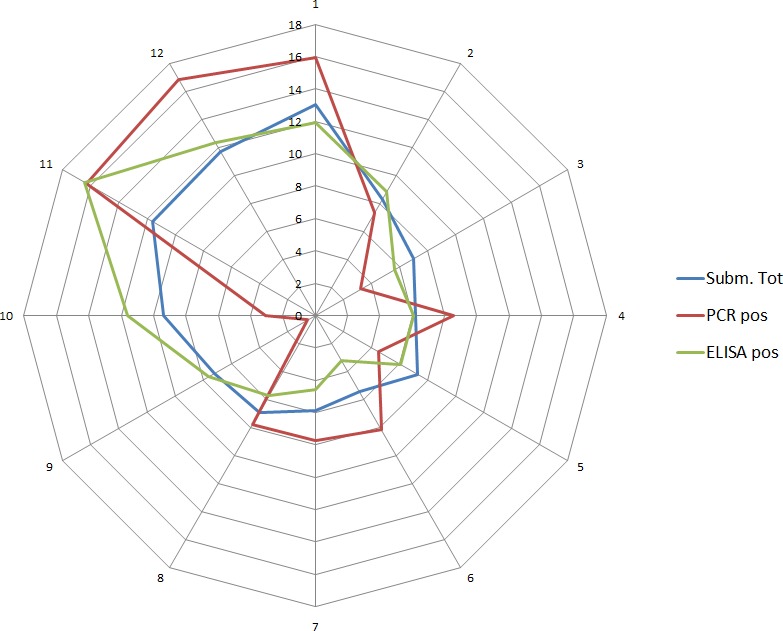
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive (red) and ELISA‐positive samples (green) from hunted wild boar in Poland
Figure 33.
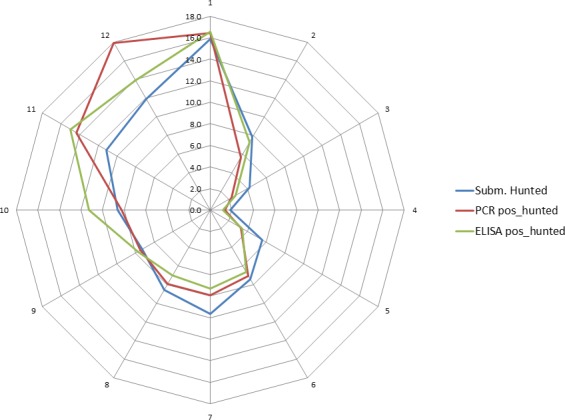
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive (red) and ELISA‐positive samples (green) from hunted wild boar in Latvia
Figure 35.
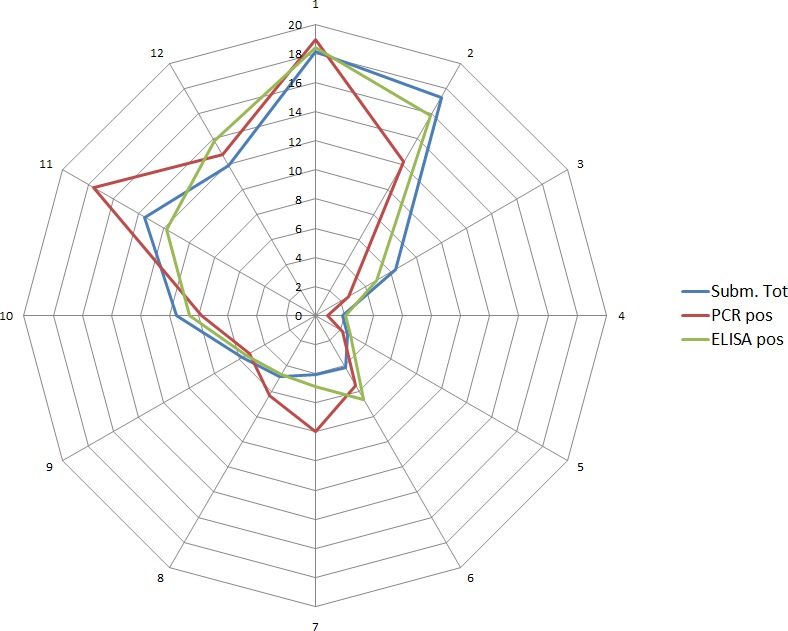
Total numbers of samples submitted for testing (blue) and proportions of PCR‐positive (red) and ELISA‐positive samples (green) from hunted wild boar in Estonia
For all these spider diagrams, the proportions of positive samples were calculated for samples taken in the whole country and not only in the infected regions.
It can be seen in Figure 28, for example that, on average, in the period 2014–2018, a peak was observed in February for the proportions of PCR‐positive samples from wild boar found dead in Lithuania, when about 18% of the proportions of positive PCR samples of the year in wild boar found dead were reported. A much smaller peak of the proportions of positive PCR samples in summer was observed. Similar patterns of the distribution of ELISA‐ and PCR‐positive samples can be observed in hunted wild boar (Figure 29).
The proportions of the numbers of positive samples tested for ASFV over the numbers of samples tested from wild boar which were found dead or hunted in the Baltic States and Poland are reported in Figures 36–39.
Figure 36.
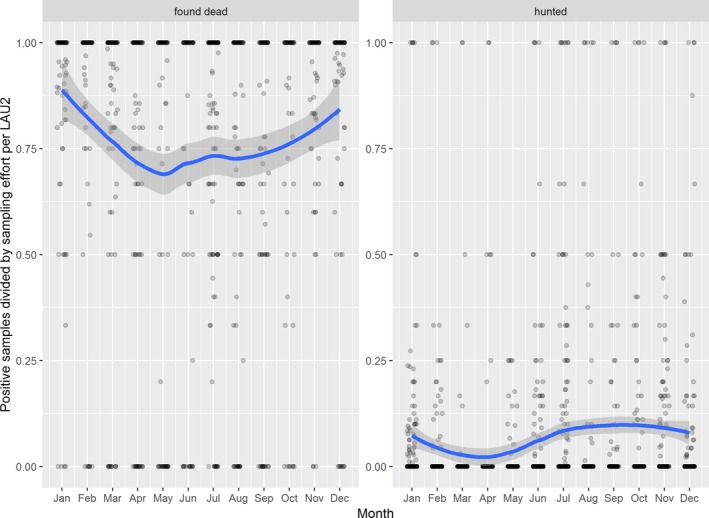
Lithuania (data available from 2016 to August 2018)
Figure 39.
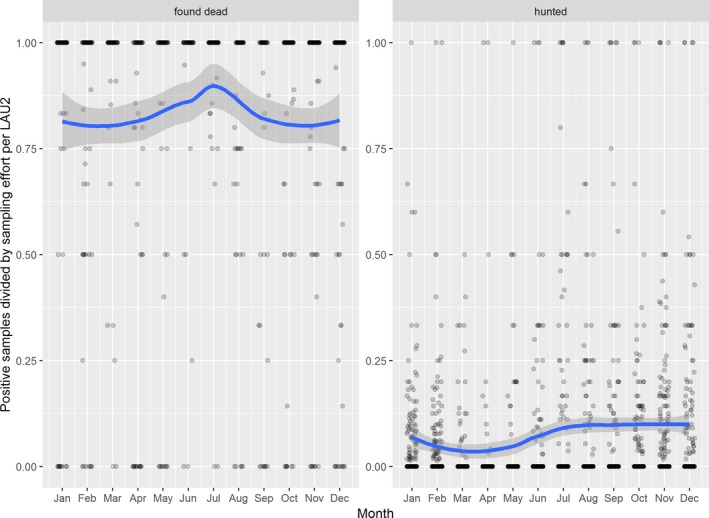
Estonia (data from available 2014 to August 2018)
There was an apparent peak in the summer for the proportion of wild boar that were found dead that were testing positive over the sampling effort (well visible in Poland, Latvia and Estonia, which is less visible for Lithuania).
Conversely, there seems to be also an apparent peak in winter of the proportions of samples that tested positive in the wild boar that were found dead in LT, PL, LV that was not observed in EE.
For the wild boar that are hunted there seems to be a slight decline in spring (February–April), but more or less the same proportion of positive samples over the sampling effort for the rest of the year is observed.
There could be several explanations for both the winter and summer peaks (see Section 3.1.2.3). Several driving forces could explain increased proportions of positive samples that are tested either in winter or summer, which could be related to the virus, to the wild boar ecology, to the pig farming husbandry, to the involvement of arthropod vectors or to human behaviour. The causality of none of those was proven statistically, but none of these can be excluded either.
Figure 37.
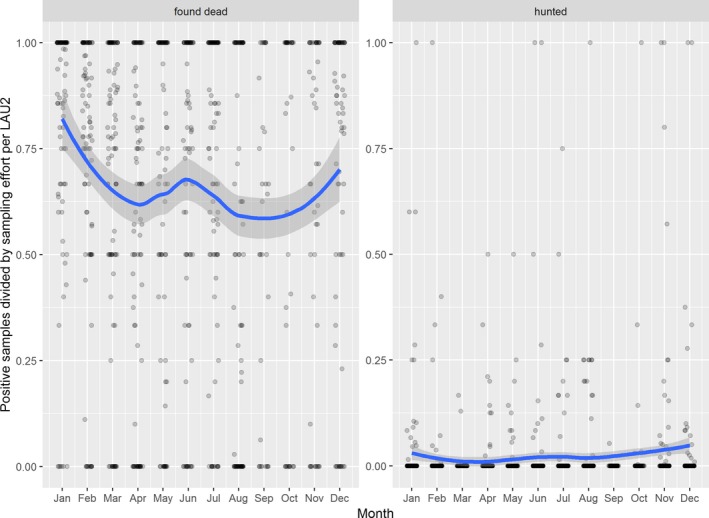
Poland (data available from 2014 to August 2018)
Figure 38.
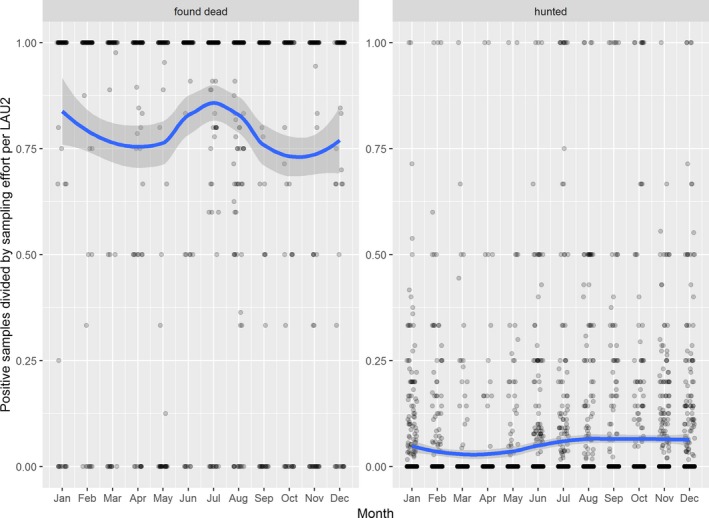
Latvia (data available from 2014 to August 2018)
Figures 40, 41, 42–43 show the results of the statistical assessment of the comparison of incidence using a generalised linear mixed model and a Tukey pairwise comparison between each pair of seasons. Winter is defined as the months of December, January and February, spring comprises March, April and May, summer are June, July and August, and the rest (September, October and November) are considered autumn. It can be noticed that several seasons per year have equal probabilities for the occurrence of ASF, which clearly differ from the other seasons.
Figure 40.
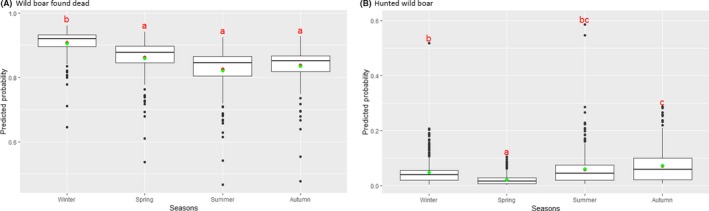
Comparison of seasonal ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Lithuania
Figure 41.
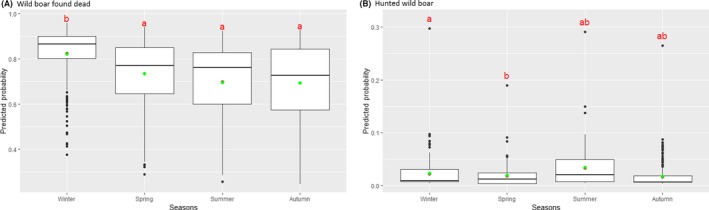
Comparison of seasonal ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Poland
Figure 42.
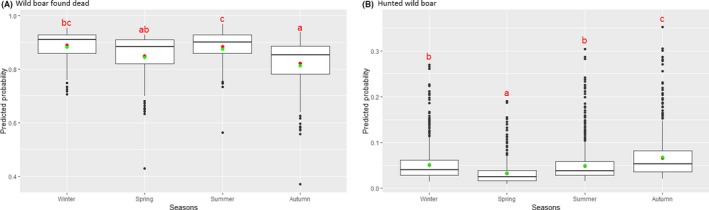
Comparison of seasonal ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Latvia
Figure 43.
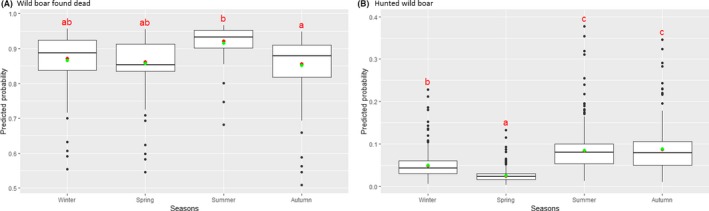
Comparison of seasonal ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Estonia
For example, it can be seen that the probability of occurrence of ASF in hunted wild boar in Lithuania and Poland in wild boar found dead in winter (resulting as ‘b’ in the pairwise comparison) is significantly different from the other seasons of the year (which all result as ‘a’ in the pairwise comparison).
It is very difficult, however, to draw a general conclusion from this assessment, as the patterns are different for the countries and the results will change according to the choice of how to group the months together as ‘seasons’ for the analysis. What could be concluded, however, is that the probability of ASF occurrence in wild boar found dead and hunted is not equally observed across the year with some periods showing a lower or higher probability than in other periods, which statistically confirms seasonality.
Seasonality of velocity of infection
In addition, the ADNS notification data reveal seasonality in local average velocity of the spread of the infection (in kilometres per year or km/a) (Figure 44). The local spread velocity increases in summer. Seasonality of local velocity may also trigger the demonstrated seasonality in case notifications as faster spread includes quicker attack to new local epidemics in naïve wild‐boar subpopulations.
Figure 44.
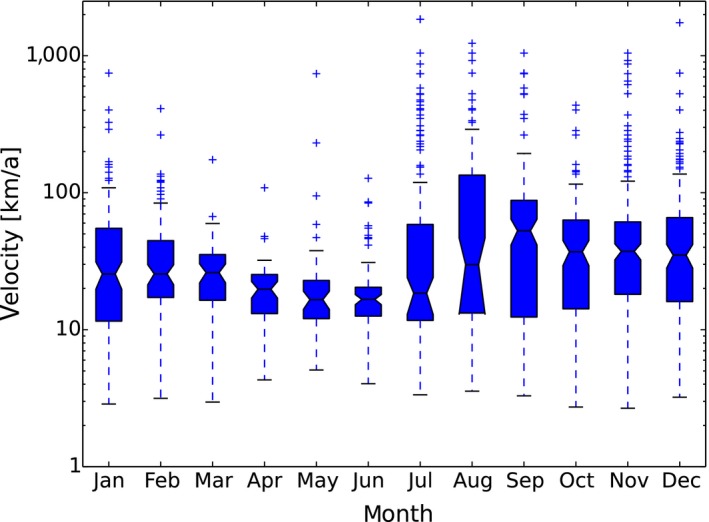
Seasonal variation of local expansive spread or local velocity (10 × 10 km) of infection measured by notifications (source: ADNS). Human‐mediated longer distance translocations are disentangled from the wild boar‐related spatial spread
3.1.2.3. Possible contributing factors to seasonality
Virus and environment
The duration of ASFV survival in different matrices increases with decreasing temperature (see Table 5 for examples of the duration of survival of ASFV at different temperatures). This results in a longer ‘infectious period’ for indirect transmission in winter both for wild boar (e.g. via contaminated environment, carcasses) and domestic pigs (e.g. via contaminated fomites, clothes, vehicles).
Pig farming activities
Depending on the type of pig farming system, there are different activities that can show seasonal patterns. For example, in indoor pig farming systems, the supply of freshly cut harvested litter or enriching materials will happen mainly during or shortly after the summer, when in the (party) outdoor farming systems, pigs are generally kept in sheds during the cold winter months, and roam outside during the warmer period of the year. It should be noted, however, that outdoor farming was prohibited in the Baltic States since the introduction on ASF. But also other farming activities, which are not strictly related to pig rearing, are seasonal. Harvesting of crops in the summer, for instance, or preparing the fields in autumn or spring could result in an increased traffic of vehicles in and out of pig farms, to and from potentially contaminated areas. In addition, pig feed composition may change according to the seasonal availability of ingredients.
Pig and pork trade patterns fluctuate over the year as well as the consumption patterns of pork meat and products, as reflected in the price fluctuations of pig produces (Eurostat, 2017). In backyard farms, slaughtering of pigs can be linked to traditional or religious events that occur in particular periods of the year, e.g. Christmas in winter, or Easter in spring.
Wild boar ecology
This section addresses the plausible ecological drivers of seasonality of ASF occurrence in wild boar. Seasonality in ASF notifications in wild boar, as opposed to occurrence, might be mediated by the timing of the hunting season and other sources of bias (see Figures 28–35). However, several aspects of wild boar ecology, including environmental factors, can plausibly lead to seasonality in ASF occurrence. These include the factors causing seasonal aggregation (for instance local food or water availability); factors facilitating close or aggressive interactions (for instance the mating season or interactions at feeding sites); juvenile dispersal; and seasonal (and geographical) variation in carcass availability and decomposition.
Factors causing spatial and temporal aggregation in wild boar include availability of food and water. Food availability is depending on the distribution and abundance of natural, cultivated and artificially provided resources, and will vary through time depending on mast cycles, crop type and management, and feeding and baiting activities. Whenever food is scarce or a local food resource becomes attractive, aggregation will take place and aggressive interactions are more likely to occur (Újváry et al., 2012; Figure 45). This could take place in seasons when supplementation takes place and natural food is scarce. In dry regions, water availability does also drive spatial aggregation (Vicente et al., 2007), mainly in the summer. Spatial aggregation of wild boar is as a known risk factor for other infections in wild boar (Acevedo et al., 2007; Vicente et al., 2007).
Figure 45.

Aggressive interactions among wild boar, a likely means of ASFV transmission, can take place at local attractive resources such as feeding sites, or during the mating season
Aggressive interactions, likely to facilitate ASFV transmission, are not only mediated by food or water resource distribution. Other drivers include the mating season, when sexual contacts take place and aggressive interactions among males may become more frequent, too. In most of Europe, the main wild boar mating season takes place from October to January (Rosell, 2012), roughly coinciding with the main hunting season. Although the farrowing season may peak in spring, births (and hence mating) can take place in any time of the year (Albrycht et al., 2016), particularly if supplementary feeding is provided.
Wild boar dispersal might increase ASFV transmission between social groups. Juvenile male wild boar will probably leave their maternal group during the mating season, generating a dispersal peak in autumn (Keuling et al., 2008). However, long‐range movements of older wild boar have also been recorded (Casas‐Díaz et al., 2013). Hence, long‐range movements may occur in any season. It should be kept in mind, though, that the infectious period is around 1–2 weeks and infected animals rapidly (in ~3 days) become immobile due to the disease (Table 6).
Finally, on wild boar carcass availability, hunting will cause the main portion of wild boar mortality, generating hunting remains (of field‐dressed wild boar) and non‐retrieved carcasses mostly in autumn–winter during the main hunting season. Little information is known on natural wild boar mortality, but summer peaks have been described in Mediterranean climates (Barasona et al., 2016), during droughts, while winter peaks due to starvation and severe cold are likely in northern climate (Melis et al., 2006; Pittiglio et al., 2018). Carcasses will last longer in the field in winter, when insect activity is low or lacking.
Concluding, all the above‐listed drivers could contribute to the seasonality in ASF occurrence in wild boar.
Arthropod vectors
For infectious diseases that are mainly transmitted through mechanical or biological arthropod vectors, a seasonal pattern corresponding to vector activity and abundance can be expected. As shown above (Section 3.1.2.2), the ongoing ASF epidemic has revealed such seasonal patterns with peaks during the summer months that is particularly evident for outbreaks in domestic pig herds. This seasonal pattern, in combination with occurrence of outbreaks also in high biosecurity settings, which are difficult to explain, has prompted concerns related to a potential role of arthropod vectors (mechanical and or biological) for ASF transmission at the wild boar–domestic pig interface.
To date, the only known biological arthropod vectors of ASFV are argasid ticks within the genus Ornithodoros, and O. erraticus and O. moubata are accepted as natural reservoirs for the virus with a well‐established role in the local persistence in nature in some settings (EFSA AHAW Panel, 2010). Ornithodoros ticks, however, are not believed to be present in the parts of EU currently affected by the epidemic, and so not to be involved in the epidemiology of the disease.
For potential mechanical arthropod vectors, stable flies, Stomoxys calcitrans, have been shown to be able to maintain and transmit the virus to pigs under experimental conditions (Mellor et al., 1987) and also to be able to infect pigs through the oral route (Olesen et al., 2018a,b) and other biting flies such as tabanids have also been discussed (Olesen et al., 2018a,b). Even though there is no scientific evidence on the capacity of any mechanical arthropod vectors to transmit ASFV under field conditions to date, a potential role in the epidemiology, and so in the seasonal pattern of the current epidemic, cannot be ignored. However, this needs further investigation.
Seasonality of human activities in wild boar habitat
Hunting‐related activities are generally seasonal. Depending on the country, the hunting season can be yearlong, or only during the winter months. Also the number of visitors, for recreational purposes will vary according the season, but is generally higher in the warmer periods of the year, or when the area is more accessible.
Harvesting of forest products, such as timber is also seasonal, and will depend on either the accessibility of the area (e.g. forests accessible for heavy machinery only in dry or cold season), and the ecological cycle of trees or other produces, such as fruits or mushrooms.
3.1.3. Spatiotemporal patterns
The speed with which ASFV is propagating through the wild boar population can vary and depends on several factors such as the season, landscape structure or the demographic characteristics of the population. There are several methods that can be used to calculate the speed of propagation, one more complex than the other, using less or more complicated calculation methods, taking into account less or more of those influencing factors and assumptions. The outcomes of two methods, namely a network analysis and a predictive epidemiological model, were compared in the two sections below.
Whatever method used, however, it is important to notice that the obtained values are within similar ranges. For instance, between 10 and 20 km/year estimated with the Network Analysis (assuming a network of paired cases with at least 7 days between the pairs, and the closest distance, and excluding extreme spread) and less than 50 km per year was estimated by the predictive epidemiological model.
These values also are in the same range as the speed of propagation of 14 km year, as reported for Estonia (Figure 8) or reported by 25 km/year, as reported by Podgórski and Śmietanka (2018).
Although a median value can be calculated by all the methods, there are also extreme outcomes for speed, for whatever method used because events such as the first introduction in a country or human‐mediated spread can cause a skewed distribution.
3.1.3.1. Speed of propagation estimated with network analysis
Figure 46 displays a network between the ASF cases in wild boar notified to the Animal Disease Notification System (ASDS) of Estonia, which can be used to calculate the speed of propagation of the infection. The particular network in Figure 46 was constructed assuming that subsequent cases in wild boar are caused by the one reported previously that is closest in distance and time.
Figure 46.
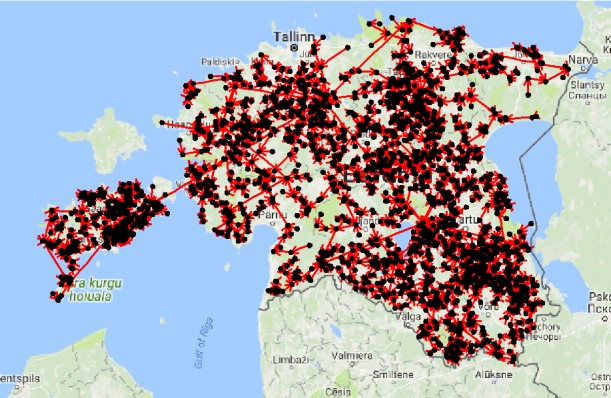
Network representation of ASF detections in Estonia, with outbreaks paired based on closest time and distance
Other scenarios were constructed for the network to evaluate the possible speed of propagation for each country (not displayed by figures), namely that at least 7 days had elapsed between the cases and the network pairs were also created based on minimum distance between the cases. The outcomes of the different methods are compared in Table 4.
Table 4.
Speed of propagation (km/year) of ASF infection in wild boar population assuming a different time–distance combination between the paired cases in Network Analysis
| Country | Distance | Time | Speed of propagation (km/year) | Mean (excluding extreme) | ||
|---|---|---|---|---|---|---|
| P‐25 | Median | P‐75 | ||||
| Estonia | Closest | Closest | 3.6 | 10.2 | 36.1 | 19.0 |
| Estonia | Closest | > 7 days | 3.3 | 9.0 | 27.0 | 13.9 |
| Latvia | Closest | Closest | 2.9 | 9.4 | 38.3 | 19.7 |
| Latvia | Closest | > 7 days | 2.8 | 8.2 | 28.7 | 14.4 |
| Lithuania | Closest | Closest | 4.4 | 16.3 | 62.9 | 33.5 |
| Lithuania | Closest | >7 days | 3.9 | 12 | 37.8 | 18.9 |
The 2.5, 25, 50, 75 and 97.5 percentiles of the speed of propagation are provided in Table 4, as well as the mean speed excluding the most extreme 10% of the speed (which could be considered as spread through human intervention). The median velocity of the infection in Latvia, Lithuania and Estonia as estimated with the network analysis was between 8 and 17 km/year. When looking at all the Baltic countries and Poland together, the speed of propagation of the infection through the wild boar populations was in a similar range, namely 11.7 km/year (results not shown in Table 4).
Hot spot analysis
Figure 47 visualises hot spots, or areas with higher densities of cases in wild boars, could be observed in the different years since the first incursion of ASF in eastern countries of the EU. Some of these hot spots have expanded dramatically in the first 2 years, whereas the centre of some hot spots moved gradually as the disease was spreading slowly through the wild boar population.
Figure 47.
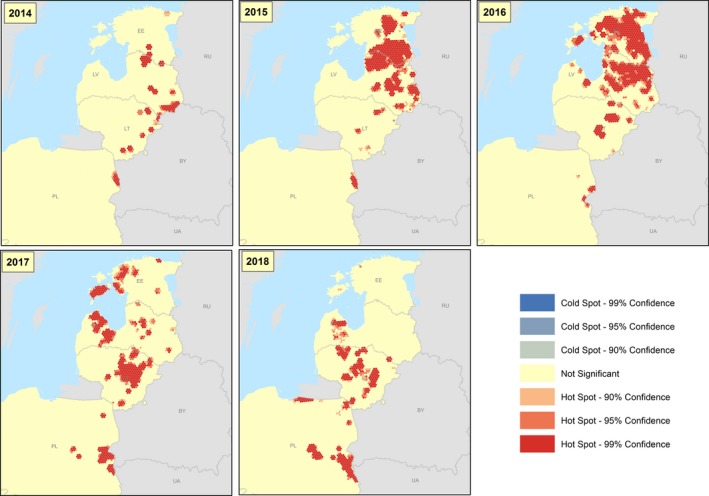
Statistically significant hot spots using the Getis‐Ord Gi* statistic (taking into account the number of cases)
Some of the hot spots origins could not be explained by wild boar‐mediated spread; as they were formed far from the infected areas in a short time and clearly indicated the involvement of humans in spread of the disease.
Overall, the hot spots in the Baltic countries have moved into a south‐western direction over the 4 past years. In Latvia, they have reduced again in size and are currently only present in the west of the country, whereas in Estonia they have disappeared entirely.
3.1.4. Wild boar‐domestic pig interface
3.1.4.1. Information from field investigations on possible sources of introduction of ASFV in domestic pig farms
Estonia
Narrative section:
The possible routes of introduction of the infection into pig farms in Estonia were set up by the epidemiology team (two or three people) conducting investigations at farms or analysing the data collected by the veterinary officials. The possible route was set up by exclusion starting from most dangerous contacts (risk factors) like animal movements, direct contact with wild boars, handling of wild boar carcasses/meat on farm, feeding of possibly contaminated food waste and moving further along various biosecurity bridges possibly leading to introduction via indirect transmission routs.
Based on collected information:
Introduction of infected domestic pigs: in none of 28 farms (including two secondary outbreak farms).
Direct contact with infected wild boar: two farms (one outdoor, one with outdoor access) of 28 farms.
Feeding of food waste containing infected pig/wild boar meet: one small‐holder (owner hunter) of 28 farms.
Handling wild boar carcasses on farm: 1 (see point c) of 28 farms.
However, in all farms one or more biosecurity breaches (sometimes very minor) were set up, which may have led to introduction via indirect transmission routes (see Annex B). In addition, transmission via mechanical arthropod vectors could not be excluded in most of the herds.
Latvia
Epidemiological investigations with the aim to identify the possible source of infection were performed in all ASF‐affected farms by official veterinarians (team of two or three people) representing the Food and Veterinary Service of Latvia. Information relevant for the analysis was collected during the interviews with animal owners of the affected farms as well as data from National Animal Identification and registration data base (register). Epidemiological investigations using a hypothesis‐based approach were conducted to find the possible source of infection.
The first ASF outbreak was confirmed in the eastern part of Latvia in June 2014. In total, 32 ASF outbreaks were confirmed in Latvia, the large majority of these in backyard pig farms. Most outbreaks (28) were primary outbreaks. Biosecurity shortcomings and feeding of potentially contaminated fresh grass or crops were considered to be the most serious factors possibly responsible for virus introduction into the holdings. However, swill feeding as a source could not be excluded. Basic biosecurity measures, change of footwear, outer clothing and disinfection as required by national legislation were not followed (Oļševskis et al., 2016).
During the following years (2015–2018), ASF was confirmed in 31 pig farm (27 backyard farms and four large commercial farms). All outbreaks were confirmed in areas where wild boar cases were reported before. All 27 backyard pig farms were defined as primary outbreaks without any links to previous ASF outbreaks. Due to the risk of ASFV presented in the environment by infected wild boar, in all backyard farms, the lack of the proper implementation of biosecurity procedures (indirect contact with ASF virus from the contaminated environment) was considered as most probable source of ASFV introduction.
For commercial pig farms, one out of four ASF‐affected farms was determined as a secondary outbreak, as it belonged to the same owner that had an ASF outbreak before. The source of the infection was the movement of pigs. For other three commercial farms, it was really difficult to find the exact source of infection. In all three ASF‐affected farms, some minor biosecurity shortcomings linked to deficiencies in the proper implementation of daily procedures as well as human‐mediated spread was assumed.
Considering the seasonality of outbreaks, the role of arthropod vectors in virus transmission cannot be excluded.
Lithuania
The first ASF outbreak in Lithuania was reported on 24 July 2014 in the commercial pig farm with 19,411 pigs. Despite the epidemiological investigation and involvement of the prosecution service in the investigation process on how and by which means the virus was introduced into the farm, the source of infection was not clarified. Another five outbreaks in non‐commercial pig holdings, lacking biosecurity, occurred within 1 month from the first detection. The most likely source was direct or indirect contact with the infected wild boar due to the close vicinity of the positive cases of ASF detected in the wild boar.
In 2015, a clear seasonal dynamic was observed and 12 out of 13 outbreaks occurred in the non‐commercial holdings during the summer time – in July and August. Out of all 13 outbreaks, only in 1 source of ASF virus could be identified, sick piglet's purchased from an infected holding, where 1 week later ASF was detected. All other 12 outbreaks were epidemiologically investigated, but the source of the ASF virus was not found.
In 2016, the number of outbreaks in domestic pigs increased probably due to an increase in the number of cases in the wild boar population and 19 outbreaks in the non‐commercial holdings were detected. The first outbreak was detected at the end of June and, during summer time, 17 outbreaks were detected. One outbreak was detected in September and one in November, in a slaughtered pig in the context of ASF passive surveillance.
In 2017, the real source of ASF virus and the way, how it is entering the holding, both non‐commercial and commercial, remain unclear. The ASF was detected in different types of farms – some of them have fully implemented national biosecurity requirements and some of them lacking some of the biosecurity items, but all outbreaks were located in the areas, where number of ASF cases were detected, which leading to the hypothesis, that ASF virus was introduced by the human means through direct or indirect contact with the wild boar and contaminated environment.
Poland
From the epizootic data collected by the veterinary service, the most common risk factor in ASF spread among pig holdings in Poland was suspected to be green forage, hay and straw.
Romania
In Romania, ASF had very different epidemiological changes in two geographic areas:
N‐W region. From epidemiological investigations performed by the veterinary officials, the possible routes of introduction of the infection into backyards, at the border with the Ukraine, are represented by the unlawful trade of meat and pork products and the epidemic wave in wild boars from infected areas. Even if the disease was initially confirmed in the domestic pig population and subsequently in the wild boar population, at present, the virus in both populations is closely related.
S‐E region. The main epidemiological hypothesis of the ASFV introduction into the Danube Delta Biosphere Reservation is represented by the epidemic wave in wild boar from infected areas across the border, but human‐mediated translocation is still considered as the main risk factor that leads to the further spread of ASF. The low level of biosecurity in backyards and the traditional sociocultural particularities of the pig raising system in Romania facilitated the introduction of ASF virus in so many backyards in a short period of time. The circulation of ASF virus between non‐professional holdings was mediated through pigs, meat products, people, vehicles, feed, water, etc. The high virological pressures of the environment, combined with possible breaches in biosecurity, lead to the introduction of ASF virus in commercial farms.
3.1.4.2. Human‐mediated spread
The ADNS data set was used and a combined plausibility network constructed. Every notification is assigned with the minimum distance to a previous notification at least 7 days apart from each other. For this minimum distance, the related velocity is calculated by dividing the distance value by the time between the two notifications. All ADNS notifications are then ordered according to this spread velocity index. Finally, the points are plotted on a map and coloured according to their position in the ranked list. Very exceptional notifications (0.1% percentile) are coloured pink, whereas red and blue was used for the 1% and 2% percentile. Due to their extreme position, these notifications are plausibly not related to wild boar‐to‐wild boar transmission but translocated with non‐wild boar movement velocity. Interestingly, the procedure identifies substantial numbers of confirmed anthropogenic translocations. Relaxing the percentile threshold may further support identification of human‐mediated translocations. This separation is important to disentangle wild boar‐ and human‐mediated events when for example validating wild boar spread models (Figure 48).
Figure 48.
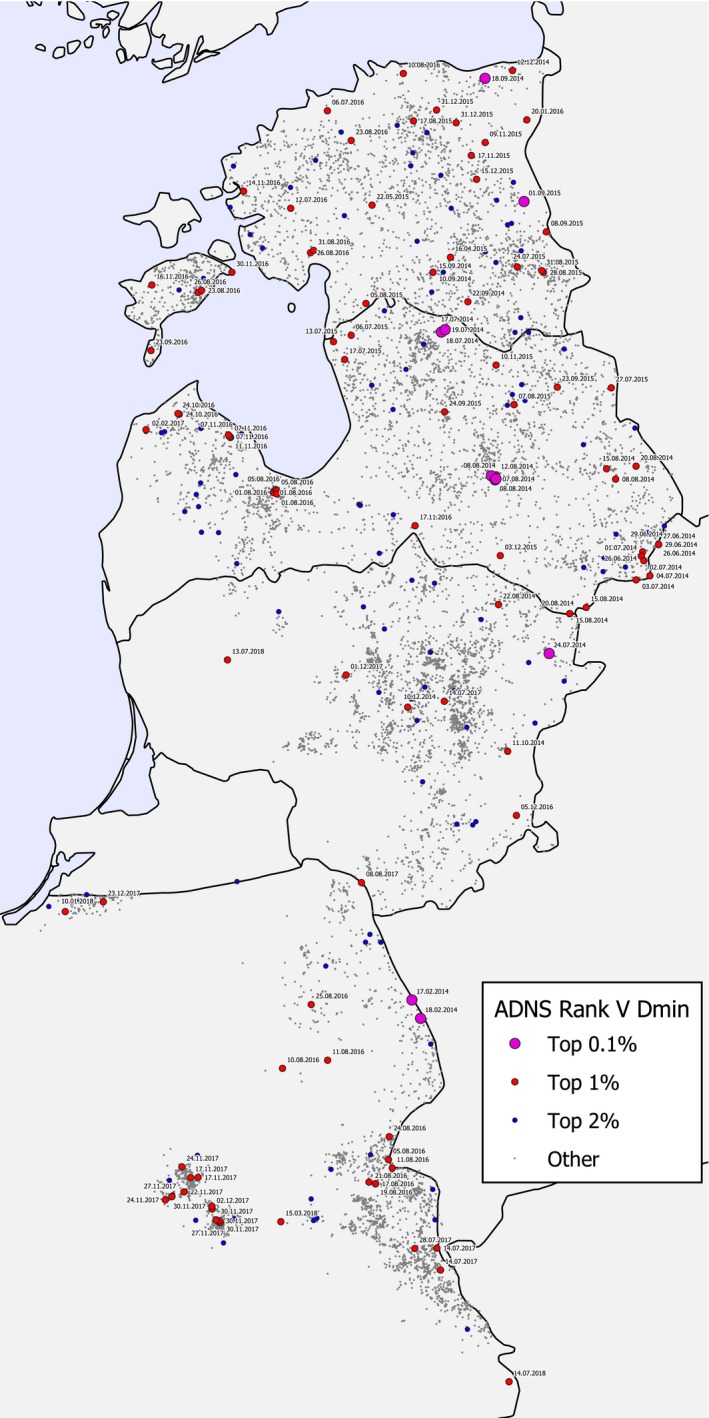
Human‐made translocations
3.1.4.3. Potential for the matrices to serve as a route of introduction of ASFV in pig holdings based on experimental data and expert elicitation
A summary of the outcomes of a systematic literature review (see Supporting Material) carried out to identify published information on the duration of survival time of ASFV in different matrices is provided in Table 5. Only results were shown where ASFV could be isolated, and where the documents were identifying the diagnostic method and temperatures at which the experiment was carried out. Review papers were excluded. The virus has been demonstrated to survive for more than 2 years in frozen organs or for almost 2 years in chilled blood. Virus in faeces, urine or slurry is surviving less long (up to about a week, under chilled or room temperature conditions).
Table 5.
Summary of maximum time to detect infectious ASFV (based on expert elicitation procedures)
| Matrix | Temperature range (°C) | Maximum number of days to detect infectious virus Med (min–max) | References |
|---|---|---|---|
| Unprocessed matrices | |||
| Aerosol | Room (15–25) | b | Donaldson and Ferris (1976) |
| Blood | Chilled (4–10) | 525 (525–525a) | Plowright and Parker (1967) |
| Collagen | Chilled (4–10) | 30 (30–30a) | Wieringa‐Jelsma et al. (2011) |
| Room (15–25) | 30 (30–30a) | Wieringa‐Jelsma et al. (2011) | |
| Faeces | Chilled (4–10) | 5 (5–5) | Davies et al. (2015) |
| Room (15–25) | 3 (1–5) | Davies et al. (2015) | |
| Organs (e.g. heart, kidneys, liver) | Frozen (−16 to 20) | 60 (60–735) | Sindryakova et al. (2016); Plowright and Parker (1967) |
| Room (15–25) | 16 (16–16) | Sindryakova et al. (2016) | |
| Skin‐fat | Frozen (−16 to 20) | 60 (60–60) | Sindryakova et al. (2016) |
| Chilled (4–10) | 0 (0–0) | Sindryakova et al. (2016) | |
| Slurry | Room (15–25) | 25a (25–25)c | Turner and Williams (1999) |
| Urine | Chilled (4–10) | 5 (5–5) | Davies et al. (2015) |
| Room (15–25) | 5 (1–5) | Davies et al. (2015) | |
| Processed matrices | |||
| Cooked cured meat (e.g. cooked ham) | Chilled (4–10) | 5 (2–5) | McKercher et al. (1978) |
| Precooked products (e.g. corned meat d ) | Frozen (−16 to 20) | 60 (60–60a) | Sindryakova et al. 92016) |
| Chilled (4–10) | 60 (60–60a) | Sindryakova et al. (2016) | |
| Room (15–25) | 16 (16–16) | Sindryakova et al. (2016) | |
| Pre‐cooked products (e.g. canned stew meat) | Frozen (−16 to 20) | 60 (60–60a) | Sindryakova et al. (2016) |
| Raw cured meat (e.g. raw ham) | Frozen (−16 to 20) | 183 (94–183) | McKercher et al. (1987) |
| Combination of tempe | 112 (56–112) | Mebus et al. (1993) | |
| No temperature specified | 140 (112–140) | Mebus et al. (1997) | |
| Raw (dry) fermented sausages (e.g. salami) | Chilled (4–10) | 30 (30–30) | McKercher et al. (1978) |
| Other matrices | |||
| Feed | Frozen (−16 to 20) | 60 (60–60) | Sindryakova et al. (2016) |
| Chilled (4–10) | 30 (30–30) | Sindryakova et al. (2016) | |
| Room (15–25) | 5 (5–5) | Sindryakova et al. (2016) | |
| Water | Frozen (−16 to 20) | 60 (60–60)a | Sindryakova et al. (2016) |
| Chilled (4–10) | 60 (60–60)a | Sindryakova et al. (2016) | |
| Room (15–25) | 60 (60–60)a | Sindryakova et al. (2016) | |
End of experiment.
Unclear method, time reported in range from 1 s to 5 min.
Time reported in hours.
Inoculated products.
Combination of different consequent temperature treatments, i.e. +60°C, −40°C and room temperature.
Anything that contains frozen and chilled blood or organs of infected pigs, therefore, can be a very significant source of introduction for a prolonged period of time (years), whereas on fomites contaminated with blood, urine, in chilled or room temperatures the virus will survive for about a week.
Table 6 summarises results of experimental infections and provides the maximum days post inoculation/infections for demonstrating the virus in live animals, or in organs post‐mortem. The incubation period is between 1 and 2 weeks, and the infectious period is also between 1 and 2 weeks (see Supporting Material for the review protocol and detailed outcomes).
Table 6.
Incubation and infectious period of suids infected with ASFV (genotype II when not specified)
| Matrix | Route of infection | Maximum days of incubation median (min–max) | Maximum number of days to detect infectious virus median (min–max) | References |
|---|---|---|---|---|
| Ante‐mortem | ||||
| Blood | Direct contact | 12 (5–16) | 13 (10–16) | Olesen et al. (2017); Wilkinson et al. (1981) (gen I) |
| Indirect contact | 14 (7–18) | 14 (7–18) | Olesen et al. (2017); Olesen et al. (2018a,b) | |
| Intramuscular | 7 (4–10) | 7 (5–10) | Carlson et al. (2016); (genotype NR); Karalyan et al. (2017); O'Donnell et al. (2016); Popescu et al. (2017); Sánchez‐Cordón et al. (2017) (gen I) | |
| Intranasal | 11 (11–20) | 11 (11–13) | Nurmoja et al. (2017a); Olesen et al. (2017); Post et al. (2017) (gen I) | |
| Oro‐nasal fluid | Intramuscular | 10 (10–10) | 10 (10–10) | Carlson et al. (2016) (genotype NR) |
| Post mortem | ||||
| Organs (e.g. heart, kidneys, liver) | Indirect contact | 7 (7–7) | 7 (7–7) | Olesen et al. (2018a,b) |
| Intranasal | 20 (20–20) | 13 (13–13) | Nurmoja et al. (2017a) | |
| Not reported | Intramuscular | 7 (7–7) | 7 (7–7) | Karalyan et al. (2016) |
| Not reported | 8 (5–9) | 8 (4–8) | O'Donnell et al. (2017) | |
Genotype NR: genotype not reported.
In the current mandate, the EFSA AHAW Panel was also asked to identify possible sources of introduction of ASF into domestic pig herds based on experiences and outbreak investigations from the affected countries in combination with available data on survival time in different matrices. However, in none, or only very few, of all outbreaks investigated, the specific routes of introduction could be identified. Rather, potential routes were selected based on exclusion during the investigations. This fact, in combination with the heterogeneity of the farms affected by location, farming system or biosecurity level, which will affect the risk of introduction through any specific route, would imply a very high level of uncertainty for identifying the exact sources of introduction.
Based on the experiences from affected countries, with the exception of Romania, which has a different disease pattern than the other affected MS, however, it is clear that the vast majority of introductions was caused by indirect contacts rather than direct contacts with infected domestic pigs or wild boar. It is also clear that, in most but not all cases, inadequate biosecurity contributed. Domestic pig outbreaks in the Baltic States and Poland occurred mainly in summer and correlated in time as well as in space with cases in wild boar, indicating an association between the risk of introduction and the level of contamination of the environment. The use of locally produced green forage, hay and straw in pig holdings has specifically been mentioned as a potential risk factor for ASF introduction. Although this would fit well with the seasonal pattern observed, any evidence is currently lacking. Also, transmission through mechanical arthropod vectors would fit well with the seasonal pattern, as stated above, but also the evidence for this is lacking.
3.2. Risk factor analysis – TOR2
3.2.1. Bayesian hierarchical model
A risk factor analysis on ASF in wild boars was performed using the Bayesian hierarchical model described in Section 2.2.2. First, several non‐significant (α = 0.05) risk factors that did not contribute to the model, were eliminated from the model, such as the risk factors related to wild boar habitat (i.e. average quality of available habitat of wild boar, average yearly snow depth, average yearly minimum temperature). Also, the not significant risk factors related to hunting activity and wild boar management (i.e. Density of hunters/km2, Density of hunting dogs/km2, Density of feeding/baiting places/km2, Density of hunted wild boar/km2) were removed from the model.
The Bayesian hierarchical model determined the density of total numbers of pigs in the area (PgDNS), the wild boar density (estimated number/km2) (WBDNS), the total road length per admin unit area (km)/km2 (RoadDENS) as significant risk factors (Table 7). The results indicate, for example, that the odds of observing an ASF‐positive wild boar increase by 2.21 for each unit increase in wild boar density (animals/km2). A similar interpretation can be made for the other parameters.
Table 7.
Parameter estimates and 95% confidence intervals, median and mode of the posterior distributions in the Bayesian hierarchical model
| Mean | Standard deviation | Lower 95% CI | Median | Upper 95% CI | Mode | Significant | |
|---|---|---|---|---|---|---|---|
| (Intercept) | −6.42 | 1.90 | −10.21 | −6.41 | −2.72 | −6.38 | NO |
| as.factor(timeId)2 | 3.24 | 0.42 | 2.45 | 3.23 | 4.12 | 3.20 | YES |
| as.factor(timeId)3 | 4.53 | 0.48 | 3.63 | 4.52 | 5.53 | 4.49 | NO |
| as.factor(timeId)4 | 3.07 | 0.48 | 2.17 | 3.06 | 4.06 | 3.03 | YES |
| as.factor(timeId)5 | 2.60 | 0.90 | 0.78 | 2.62 | 4.32 | 2.65 | NO |
| PgDNS | 0.02 | 0.01 | 0.01 | 0.02 | 0.03 | 0.02 | YES |
| WBDNS | 2.21 | 0.60 | 1.06 | 2.19 | 3.43 | 2.17 | YES |
| RoadDENS | −0.29 | 0.11 | −0.53 | −0.29 | −0.08 | −0.28 | YES |
The estimated temporal trends (Figure 49) demonstrate an increased probability of ASF occurrence in wild boar during the first 2 years (2015–2016) followed by a decline in 2017 and 2018 in Estonia.
Figure 49.
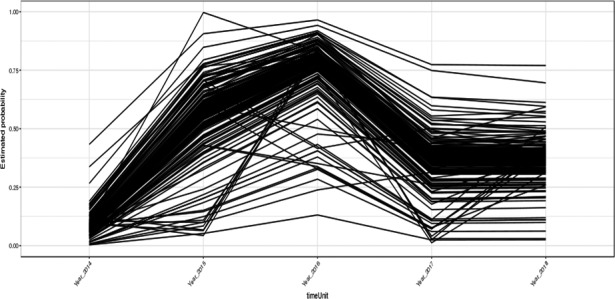
Temporal estimated probabilities for each LAU2 region showing the probability of observing African swine fever cases in Estonia for each year since the introduction
The spatial predictions (Figure 50) clearly indicate the same increase over time in the probability of ASF detections in Estonian wild boar for all LAU2 regions up to 2016 and a decrease for 2017 and 2018.
Figure 50.
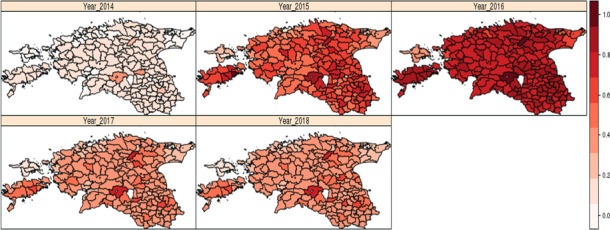
Spatial predictions for each LAU2 region in Estonia of probabilities of observing African swine fever for each year since the introduction
The same results and temporal trends confirming existence of significant relationships in time and space between presence of the virus and susceptible animal populations densities were observed after backward eliminations of non‐significant potential risk factors in the general additive model (GAM) statistical model.
The negative relationships between the detection of the virus and road density in a LAU2 region revealed from the two models could be related to the presence of more hunting grounds in relatively ‘remote’ places rather than in urban areas. The estimated temporal trends are similar and clearly indicate an increase over time in the probability of ASF detections in wild boar for all LAU2 regions in Estonia up to 2016, and a decrease for 2017–2018.
3.3. Review wild boar management measures for controlling the spread of ASF – TOR3
A spatiotemporally explicit individual‐based model approach in structured geographic landscapes was used to assess wild boar depopulation and density reduction measures for controlling the spread of ASF (see Section 2.2.3). Systematic variation of the parameter ‘bc’ which is the transmission probability of carcasses (transmission probability of carcasses includes the finding of carcasses, approaching it, and becoming infected). The parameter addresses the unknown chance of transmission related to a carcass succumbed to ASF infection to in‐contact animals per week. The parameter was tested and the results are sensitive to the assumption (see Lange et al., 2018).
3.3.1. General
The model outcome is expressed as the probability that a given strategy combination will fail to contain ASF following its focal introduction (i.e. 1 − probability of failure is the probability of success for a given strategy). The strategy combinations include both the intensity of measures and the size of the zones. Failure results in the breakout of the respective hunting zone dimension, i.e. drops in failure rate between radii values refer to successful termination of the simulated spread within the respective distance from the buffer zone.
In general, there remains considerable uncertainty about many aspects of ASF epidemiology in wild boar, including the carcass contact rate, the contact rate between groups, and the role of insects. For this reason, the modelling results are presented as a comparison between scenarios rather than evaluating absolute values. The model considers three different wild boar management zones: core buffer and intensive hunting zone (see Figure 1).
As technical illustration, not as strategy outcome, Figure 51 is presented to understand the variability of the output measure i.e. the survival curve of ASF summarised over 100 runs. The curve reveals the rate of failure as function of the distance to the outer edge of the buffer zone or by 1 minus y‐axis the success rate of the simulated strategy. In Figure 45, the ‘same’ curve was reproduced 30 times, always using 100 runs, 3,000 runs in total, with parameters unchanged. The ‘band’ filled by the 30 curves resembles the variability (i.e. credibility range). Any single line curve presented in the following should be read indicative of the thicker credibility range.
Figure 51.
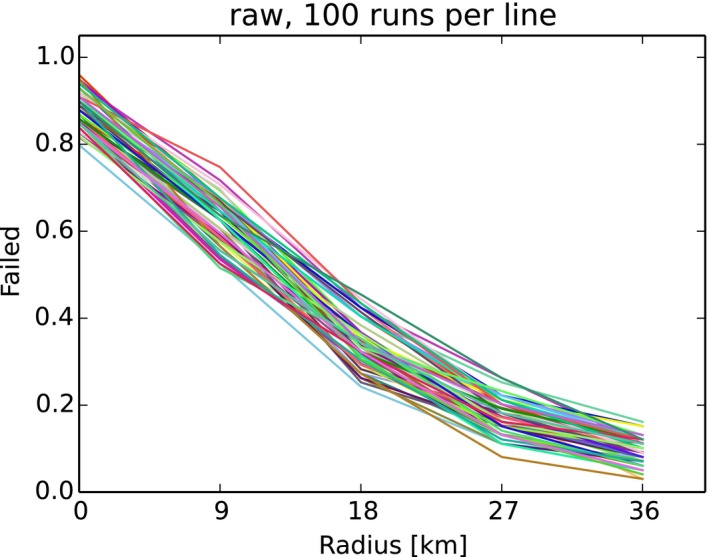
Example and variability of the model output. The probability is shown that the implemented measures fail to prevent ASF spread out of the intensive hunting area as function to its width. Thirty lines show variability by representing the same output measure produced of the standard approach, i.e. 100 simulation runs (total 3,000 simulation runs). Every run applies identical parameters
Figure 51 illustrates an example of a representation of the model output. The x‐axis reveals the distance to the edge of the buffer zone or the width of the intensive hunting area measured in wild boar group home‐range diameters, here scaled in kilometres, assuming 3 km as wild boar group home‐range diameter. The y‐axis reports the proportion of 100 repetitions that had ASF‐infected wild boar outside the buffer for increasing distance from the buffer edge as shown on the x‐axis. One minus y values at zero of the x‐axis shows the proportion of runs where the infection was successfully halted inside the core + buffer zones. For instance, from Figure 51 can be interpreted that for 10–20% runs the infection was terminated inside the core + buffer zones, as the y value (i.e. failure rate) at x value 0 is 80–90%. Furthermore, succeeding with a greater than 80% chance (less than 20% failure) was achieved in this particular simulation with an intensive hunting zone of minimum 27 km width (i.e. nine times the wild boar home‐range diameter of 3 km). The diagram is an arbitrary example and the outcome is not representative for all strategy options discussed in the following paragraphs.
3.3.2. Effect of alternative measures and effort levels in differently sized zones
3.3.2.1. No carcass removal, no fence encircling the core zone
Figure 52 shows the results of the model simulations of different hunting efficacy and different width of the intensive hunting zone without carcass removal and without fence around the core zone on the probability of failure of the measures (or the success, which is 1 – probability of failure).
Figure 52.
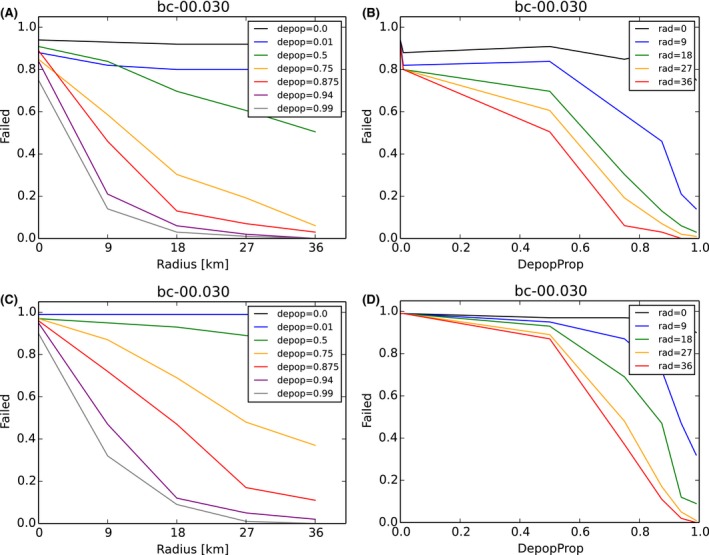
Simulation output on effect size of alternative hunting efforts at different width of the intensive hunting zone (without carcass removal and without fence around core zone) or percentage of wild boar population hunted in the intensive hunting zone
Top: carcass contacts only after 2 weeks (in line with Probst et al., 2017). Bottom: carcass contacts possible right after death. The same data are summarised as a function of hunting zone width (left) and annual depopulation efficacy (right). In the intensive hunting zone annual depopulation efficacy is set in per cent (coloured line graphs) and achieved every year with two campaigns. Each campaign was completed within 4 weeks. The protocol is repeated annually until the end of the simulations. In the core + buffer zones one campaign is performed after a half‐year waiting period, applying 90% reduction of animals still present.
Uncertainty discussion: Since the hypothesis on 2‐week contact delays with carcasses as reported by Probst et al. (2017) is no longer suggested by the authors, the issue was addressed by an alternative scenario, assuming contact since week of death. The outcome is extremely pessimistic. The hypothesis of delayed contact with carcasses will affect all following simulations: not only without, but also with carcass removal.
Interpretation: In the absence of both carcass removal and a fence encircling the core zone, eradication success will be difficult to achieve through depopulation efforts conducted within two campaigns per year in the intensive hunting area. This confirms earlier simulations for large affected areas, adjacent to the ASF epidemic front (EFSA, 2017). Substantial depopulation over maximised hunting zones would be required. In particular, if carcass contact was modelled to occur immediately after death, the efficacy of depopulation in the intensive hunting area (with a radius of the intensive hunting zone greater than six wild boar group home ranges (i.e. 18 km in the scaled diagrams)) would need to exceed 75% to reduce eradication failure below 20% (Figure 52, bottom left). Given the reported limitations of the efficacy of intensive regular hunting, intensive hunting focused solely in the intensive hunting zone might therefore be insufficient to control ASF following focal introduction.
3.3.2.2. Carcass removal, no fence encircling the core zone
Next, carcass removal was added to the simulated hunting measures (Figure 53).
Figure 53.
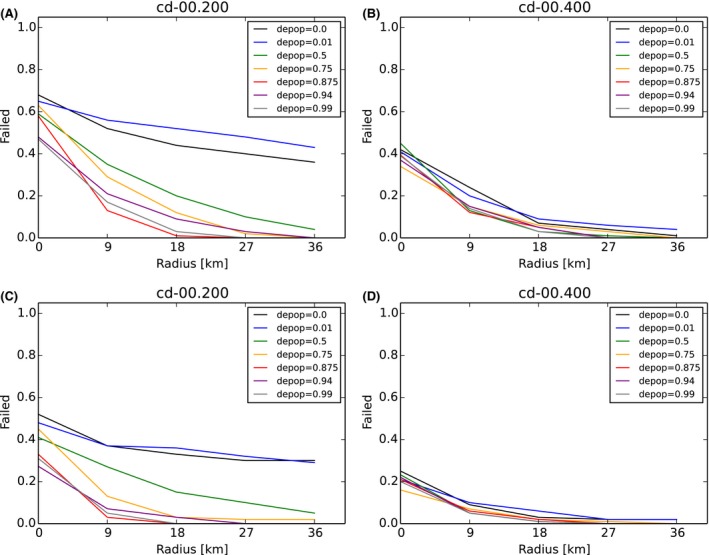
Simulation output on the effect of alternative carcass removal rate (carcass removal = cd, see diagram title) and width of as well as efficiency in the intensive hunting zone (x‐axis)
The core zone is delineated using either random carcass detections within 4 weeks (top two diagrams) or perfect knowledge on carcass distribution prior to zoning (bottom two diagrams; remember only knowledge of carcass was perfect not removal, latter as in top row). Independent of the annual depopulation efficacy in (coloured lines) or width of the intensive hunting zone (x‐axis), logically, only carcass removal rate in the core + buffer zone can determine the failure rate out of the core + buffer zone (equally coloured line graphs at x‐axis zero value compared between left and right at CD = 20% and CD = 40% carcasses removed). As before, increased hunting effort in the intensive hunting zone further improves success chance (coloured lines per diagram). The hunting target was achieved every year with two campaigns. Each campaign was completed within 4 weeks. The protocol is repeated annually until the end of the simulations. In the core + buffer zones one campaign is performed after a half‐year waiting period, applying 90% culling of animals still there.
Uncertainty discussion: Carcass removal is a significant measure in the model because the role of transmission was used according to the acknowledged expert understanding of ASF perpetuation in wild boar carcasses (EFSA AHAW Panel, 2015; EFSA, 2017). Beyond its possibility and plausibility, this mechanism was not biologically demonstrated, however. Nevertheless all control concept discussions depend on the plausibility of carcass‐mediated transmission, so the model does the same. Furthermore, indicative simulations have shown that only live‐to‐live transmission (likewise CSF in wild boar) would make it impossible to match simulated ASF spread velocity with spread dynamics as observed through ADNS data. Hence, if not carcasses than any other localised environmental reservoir might have a more significant role in transmission compared with live animal‐to‐live animal of different social groups.
Uncertainty discussion: In the model configuration and on such a small scale, carcass removal rate can reasonably override carcass creation in the intensive hunting zone. Beyond that threshold (between 20% and 40% carcasses safely removed, left vs right in the figure), increased hunting efforts in the intensive hunting zone are of marginal relevance.
Interpretation: Carcass removal and a high level of precision in the detection of carcasses to accurately delineate the core and buffer zones have the potential to substantially improve the model outcome. Perfect carcass detection compared with a 10% chance of carcass detection would have an equivalent effect as an extension in the width of the intensive hunting zone by three home ranges. Carcass removal rate can override carcass creation. Therefore, eradication could potentially be achieved without the need for intensive hunting efforts in the intensive hunting area. NB: Carcass contact delayed by 2 weeks.
3.3.3. Alternative timing of hunting activities over the year
Figure 54 displays the model outputs varying intervals of hunting campaigns in the intensive hunting zone over the year.
Figure 54.
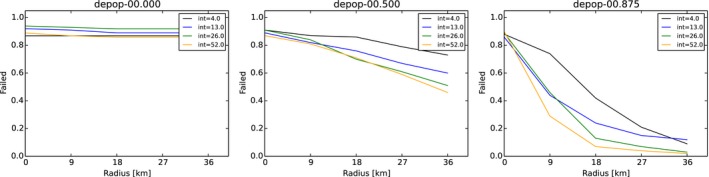
Simulation output on effect of alternative scheduling of hunting campaigns (i.e. varying the interval between hunting campaigns) while guaranteeing equal overall annual hunting efficacy (without carcass removal)
The depopulation efficacy over the year is 0%; 50%; 88% (see diagram title).The parameter interval (int) refers to the interval in weeks between the performance of the consecutive hunting campaigns in the intensive hunting zone; int = 52 means one 4‐week campaign per year with greatest efforts; int = 4 means 13 four‐week campaigns per year i.e. hunting every week with lesser efforts. Effort per campaign is adjusted to achieve in summary the annual efficacy of depopulation per cent. NB: The culling measures in the core + buffer zone did not alter over all simulations and were set at 90% as after 26 weeks waiting time
Interpretation: The impact of hunting on the wild boar population should be maximised over as short a time period as possible.
3.3.4. Changing the efforts assigned to the core + buffer zone
Figure 55 displays the model output for the probability of failure of the applied measures (or the probability of success, being 1‐probability of failure), for different waiting times between the start of all applied measures (also in the intensive hunting zone) and culling in the core + buffer zone. Three different efficacies of culling in the core + buffer zone (dpc) are shown from left to right: 0%, 75% and 99%.
Figure 55.
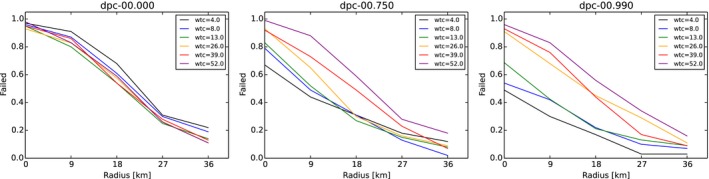
Culling efficacy in the core + buffer zone in one campaign for different waiting times between the start of all applied measures and the start of culling in the core + buffer zone
Failure rate is given for different waiting times (waiting times = wtc; coloured lines) between start of measures and culling in the core + buffer zone. Three scenarios for the culling efficacy (see dpc; diagram title) of 0% (left), 75% (middle) and 99 % right are shown.
3.3.4.1. Waiting time until culling in the core + buffer zone
Figure 55 displays the probability of failure of the measures when varying the efficacy of the culling in the core + buffer zone, and the waiting time from the start of all the measures and culling in the core + buffer zone.
Uncertainty discussion: Thinking of culling as a large‐scale disturbance effect may create substantial uncertainty to the result (see as partial solution in Lange et al., 2018).
Interpretation: A lengthy delay after the establishment of zoning and prior to culling in the core + buffer zone is detrimental with respect to maximising the probability of eradication success. This is contrary to expert opinion, where it is recommended – to avoid perturbation of the population during the epidemic – that culling commences only once the epidemic peak has been reached. Increasing culling efficiency in a single campaign in the core + buffer zone was only helpful for waiting times below 13 weeks, i.e. if applied before breakout from the core + buffer zone.
3.3.4.2. Changing the width of the buffer zone
Figure 56 displays the model outcomes for varying widths of the buffer zone and carcass removal rates.
Figure 56.
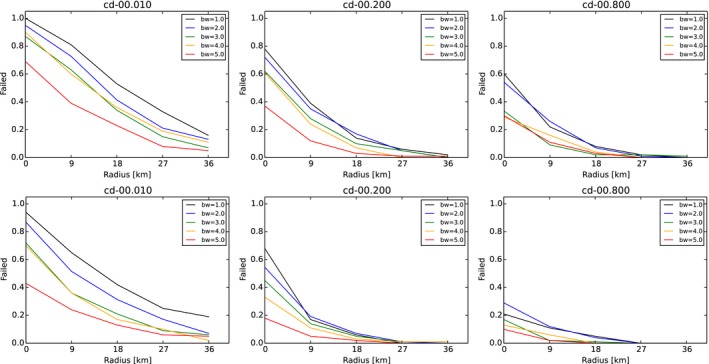
The effect of the width of the buffer zone on the final success assuming alternative carcass removal rates
The width of the buffer zone (bw) is represented by the coloured lines: black 1; blue 2; green 3; yellow 4; and red 5 times the wild boar group home range). The standard parameter used in scenarios above refers to buffer width (bw) = 3. Carcass detections and removal rate (cd; diagram title) is either 10% (left), 20% (middle); 80% right. Independently, the core zone is delineated based on random carcass detections during 4 weeks after ASF confirmation (top row) or perfect knowledge on carcass distribution (bottom row). The hunting efficiency in the intensive hunting zone is set to 75% distributed over two campaigns per year. Waiting time until culling of the core + buffer zone was 26 weeks and achieved by one campaign with 90% efficacy.
Uncertainty discussion: The numbers of hunted/culled animals as well the volume of carcasses removed are opposed. A twofold analysis would be useful.
Interpretation: The width of the buffer zone will influence the probability of eradication success, with wider buffer zones leading to higher success rates. However, increases in the width of the buffer zone will also lead to a larger intensive hunting zone, where intensive hunting efforts may be harder to achieve in practice.
3.3.4.3. Adding fences around the core zone
The simulated fences at the border of the core zone are built 1 week after zones are delineated using the carcass retrieval information, i.e. either a 10% chance during 4 weeks after first notification (standard) or experimentally using perfect knowledge about all carcasses in the landscape (note these will not be automatically removed!). Fences are assumed to be of varying permeability (90, 50, 10, 5, 0%). Here, 100% equals the above scenarios in which the fence is only a fictitious line in the landscape; 0% is assuming a wild boar‐proof wall. All results should be read having in mind that the core zone, as well the fence line, is set up around the detected carcasses and not based on infected animals (Figure 57).
Figure 57.
Example of simulation analysis showing true infection status of the wild boar groups at the moment of zoning (4 weeks following first notification) based on perfect knowledge about distribution of carcasses from animals that died from ASFV infection
The 100% wild boar‐proven fence was built around the core zone (white area) – while already newly infected animals have reached the buffer zone (light grey area). As a consequence, simulations assuming a 100% wild boar‐proven fence can also fail to stop the spread of ASF.
The following simulation outputs (Figure 58) reveal the effect of fences vs carcass removal vs depopulation efficacy (in one annual campaign). The first figure summarises the effect of the core + buffer measures as the off‐set of the left axis i.e. lower failure level at x‐axis zero represents increased contribution of the core + buffer zone; decline in the graphs reveals the effect of preventive hunting in the intensive hunting zone to stop possible breakouts from the buffer zone (failure rate at zero of x‐axis). The second figure reveals as a heat map the chance of certain wild boar groups being affected by ASF infection in the 100 repetitions per scenario.
Figure 58.
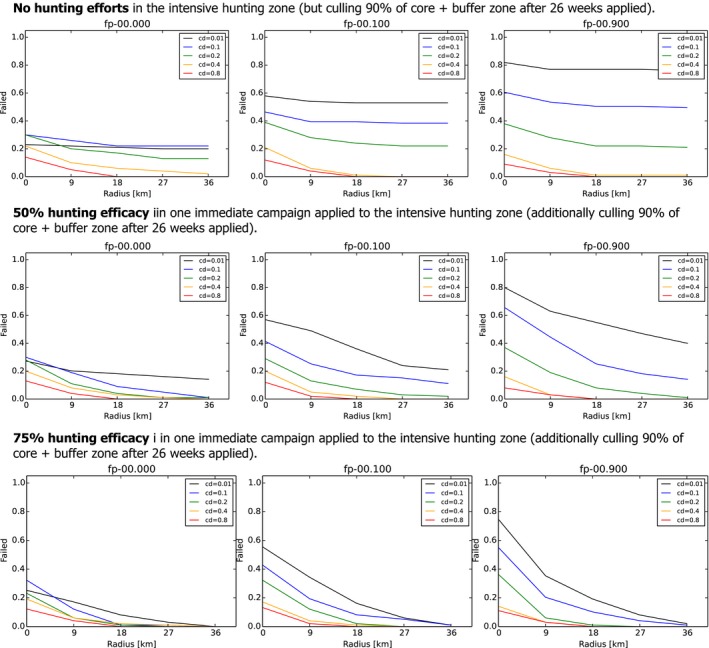
Simulation output as failure rate out of 100 repetitions of scenarios. Scenarios differ by different fence permeability, hunting efficacy, carcass detection rate and the width of the intensive hunting zone
Row‐wise: Different hunting efficacy (top 0%; 50%; 75% i.e. during the annual 4‐week campaign, with any animal having the chance to be hunted with the given percentage value);
Column‐wise: Different permeability of the fence (left 0% i.e. wild boar proof; middle 10%; right 90% nearly absent);
Colour of the lines: Different carcass detection rate (black 1%; blue 10%; green 20%; yellow 40%; red 80%);
x‐axis: width of the intensive hunting zone (0, 3, 6, 9, 12 times the wild boar group home ranges of 3 km); y‐axis: Proportion of runs failed to be halted, or 1‐success rate of the measure at a given hunting zone width.
Figure 59.
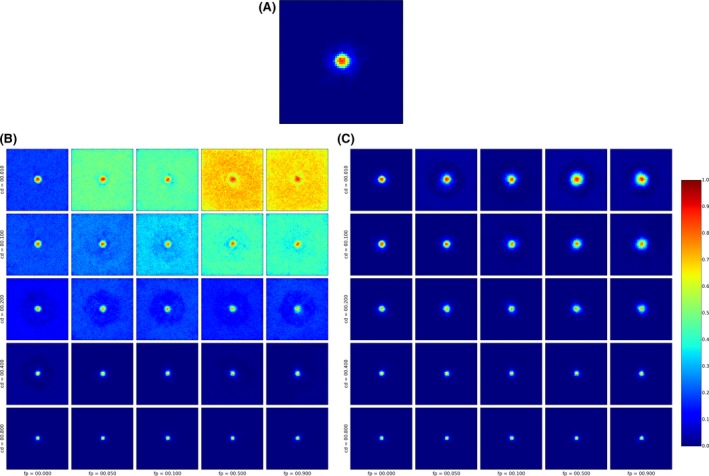
Spatial simulation output shown as a heat map of the proportion of 100 repetitions of alternative scenarios
Scenarios differed by applied control measures. (A) Enlargement of top‐left in (C) assuming the presence of a wild boar‐proof fence around the core zone, red area approximates the overlaid core zones of 100 runs, yellow, i.e. a much lower proportion, the buffer zones. The surrounding halos reveal the limited spread due to very high hunting efforts in the intensive hunting zone. (B) and (C) Scenario comparison for different levels of hunting i.e. 0% in (B) and 75% in (C). Both (B) and (C) cross‐tabulate column‐wise different permeability of the fence (left 0% wild boar proof; 5%; middle 10%; 50%; right 90% fence nearly absent) vs row‐wise different carcass detection rates (top 1%; 10%; middle 20%; 40%; bottom 80%). Zoning is based on perfect knowledge about infectious carcass distribution at the moment of zoning (results for 10% chance of carcass detection, see Lange et al., 2018).
Uncertainty consideration: It is important to be reminded that the fence analysis targets the interaction of the alternative control activities, here fencing, carcass removal and preventive hunting in the intensive hunting zone. Moreover, the permeability of fences, i.e. the probability that movement across the fence line or animal contacts at the fence are prevented, is not fully quantified in the field.
Interpretation: The delineation of zones (including the core and buffer zones) is guided by the detection of carcasses, rather than the detection of actually infected animals. For this reason, eradication success may be imperfect even in the presence of wild boar‐proof fencing. Moreover, the simulation model outputs have shown that fencing is only useful if other control measures, such as carcass removal and intensified hunting, fail in their efficient implementation. At intermediate levels of hunting, intensive carcass removal did compensate for weak fences. In the absence of preventive hunting efforts in the intensive hunting zone, carcass removal has to be sufficiently intensive to cope with potential breakouts from the buffer zone. If high levels of hunting in the intensive hunting zone are assumed (here 75%), this can compensate for both weak fences and less carcass removal. The risk situation in the simulation was homogeneous around the outbreak. One could speculate that segments of fencing will be particularly useful in those areas where carcass removal or intensive hunting is difficult to implement.
Summary observation: As discussed in the reports of EFSA (2017) and EFSA (2018), it is erroneous to limit ASF control measures solely to the buffer and core zones. In the absence of perfect fencing and super‐efficient carcass removal, the inclusion of intensified hunting and ‘preparatory’ carcass removal (i.e. hopefully all negative) is an important component of the overall control strategy. Independent of the size of the buffer zone, and because the core + buffer zones are subject to a hunting ban prior to final culling, ASF will spread into the buffer zone and may subsequently break out (i.e. into the intensive hunting zone) before final culling has been scheduled in the core zone. Intensive hunting in the intensive hunting zone (this being adjacent to the buffer zone) will assist in containing ASF spread in those situations where ASF has not been contained within the buffer area. Logically, an extension to the buffer is not helpful given than final culling of the core and the buffer is an important component of the overall strategy following focal introduction. Increases in the buffer area will also make final culling increasingly difficult, noting the quadratic relationship between buffer width and the number of animals that will need to be killed during final culling.
3.3.5. Applying the focal concept to non‐affected areas at higher risk of ASF introduction via natural spread mediated by wild boar
The previous section addressed simulations mimicking the situation in areas where the disease was recently introduced into wild boar populations, i.e. the focal approach. For comparison, the same strategy approach was applied in the context of non‐affected areas at higher risk of ASF introduction via natural spread mediated by wild boar (see EFSA 2017/2018 for detailed model investigations in this risk context).
The following results in Figure 60 extrapolate the outcome from the focal strategy, i.e. designed for a very limited and spatially delineated problem with most likely very few infected animals at the start of measures, if the approach is copied onto the larger landscape showing the ASF‐affected area to the right (treated as ‘core’) and a proposed management belt (treated as ‘buffer’) and up to a 12 home ranges‐wide (here 36 km) intensive hunting zone.
Figure 60.
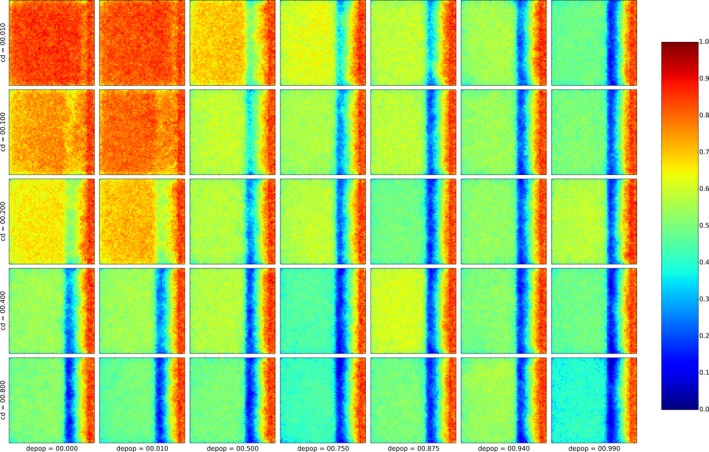
Spatial simulation output shown as a heat map of the proportion of 100 repetitions of alternative scenarios addressing the large‐scale control of ASF to stop wild boar‐mediated spread into areas at higher risk of ASF introduction by wild boar‐mediated spread
Scenarios differed by applied control measures. Column‐wise: different hunting efforts in the intensive hunting zone (left 0%; 1%; 50%; middle 75%; 87%; 94%, right 99%) vs row‐wise different carcass detection rates (top 1%; 10%; middle 20%; 40%; bottom 80%). The more reddish a pixel is coloured the higher the proportion of 100 repetitions for the scenario in which the respective wild boar group became affected.
As before, the following graph resembles the spatial outcome as heat map of 100 repetitions applying alternative hunting efficacy in the intensive hunting zone and carcass removal.
The focal approach does not involve appropriate aspects for the control items to provide sufficient countermeasures to stop ASF spread in an affected landscape. Even with perfect measures (99% hunting effort and 80% carcass removal from the intensive hunting zone) (blue colouration means usually nearly absent), this is not sufficient to prevent all breakthrough (area beyond the hunting zone affected at about 40% of all repetitions).
Interpretation: Recent focal introduction and large‐scale area expansion should be considered different management problems in the context of ASF control. The success of the approach following focal introduction is closely linked to the eventual size of the outbreak. Every effort should be made to limit the size of these outbreaks following focal introduction, as there appears to be no point of return if this strategy fails.
3.4. Review natural/artificial borders – TOR4 for the determination/demarcation of the restricted areas
The hypothesis was that simulated spatiotemporal spread on the continental scale could explain ADNS data better (i.e. as matched spatiotemporal occurrence of ASF‐positive samples) if physical barriers are included in the simulation landscape compared with simulation on a barrier‐free landscape. The results did not reveal the expected effect, although the barriers created structures on the heat map of infection probability (Figure 61).
Figure 61.
Probability to receive an ASF infection in wild boar dependent on physical barriers and likely human‐mediated translocations
Heat map of 100 repetitions. Higher value (reddish) shows greater probability. Left: Forced simulation assuming impermeable barriers (blocking walls); Middle: Forced simulation (see section 2.2.4) assuming fully permeable barriers; Right: As middle but without forcing from March 2015 onwards. White pixels represent forcing locations based on the excess values in local velocity.
Interpretation: Barriers lead to structure in the infection landscape, for example by partially isolating certain directions of spread. However, based on a comparison of model outputs and ADNS data, it was not possible to demonstrate an effect of natural barriers (e.g. roads, rivers) on ASF spread. Rather, it appears that likely human‐mediated translocations are particularly influential in overwhelming any potential effects of such barriers. To explain, any effect of barriers on ASF spread is quickly counteracted by longer‐distance transmission events, which are most likely to be associated with human‐mediated virus translocation.
Interpretation: The inclusion of likely human‐mediated translocations is mandatory to achieve useful matches with ADNS (left and middle panels in Figure 62). As a consequence, in those simulated epidemics considering perfect barriers, eventually any subarea is reached that was affected by the simulation without barriers in action. Hence, the overall similarity measure does not show a difference between the two simulation outcomes. It might be useful to consider that the barriers have an effect on a more narrow scale e.g. two sides of a river. However, for such level of detail, the ADNS data are of limited quality only. More importantly, although it might be possible to find segments of physical barriers where the particular ASF history in EU showed a kind of halting effect (e.g. in Latvia, E. Oļševskis pers. comm.), still it cannot be usefully extrapolated to such kind of barriers anywhere else. Speculating more, the inclusion of physical barriers, being a case‐by‐case outcome on effectiveness, might be trial and error in affecting the spatial spread of the infection, but – as with artificial fencing – allows sustainable demarcation of management zones in the landscape.
Figure 62.
Jaccard similarity map between ADNS and the final outcome of 100 repetitions considering combinations of physical barriers and likely human‐mediated translocations
Higher value (reddish) show greater similarity. Left: Forced simulation assuming impermeable barriers (blocking walls); Middle: Forced simulation assuming fully permeable barriers; Right: As middle but without forcing from March 2015 onwards. White pixels represent forcing locations based on the excess values in local velocity.
Uncertainty considerations: The investigations cannot provide absolute estimates of the effectiveness for halting or slowing continental spread of ASFV due to the great variation in permeability of natural barriers in real life by, for example, bridges over rivers, variation in width or occurrence of shallow segments. The approach is pragmatic and the difference in matching performance between the compared simulations is not mandatory for barriers having a certain effect on ASF spread, i.e. the effect of barriers can be so small that the stochastic nature of the problem will hide the barrier effect. Furthermore, the uncertainty of the practical implications is huge due to many processes acknowledged to alter continental spread of ASF in wild boar.
4. Conclusions
4.1. Descriptive epidemiology – TOR1
4.1.1. Update of ASF situation
-
ASF was introduced into nine EU MS, through two distinct spread processes:
-
–
Continuous wild boar‐mediated spread through wild boar populations and meta‐populations. The speed of propagation is notably slower than some other infectious diseases in wild boar.
-
–
Human‐mediated jumps leading to the establishment of new ASF clusters distant from areas of previous ASF occurrence.
-
–
Furthermore, in affected areas within the established ASF range, there has been continued sporadic detection of cases despite very low wild boar densities.
The focal introduction in the Czech Republic is the only occasion in which ASF spread in wild boar was apparently controlled. Elsewhere, ASF continues to expand into new areas.
In most affected countries, there have been many cases in wild boar and relatively few outbreaks in domestic pigs. In Romania, however, the opposite has been observed. The observed pattern in Romania should be interpreted with caution until the potential for under‐detection of ASF in wild boar populations can be excluded. This will require systematic surveillance activities in wild boar populations. Under‐detection of ASF in wild boar could also occur in other regions, and should be avoided through intense passive surveillance of wild boar.
4.1.2. Spatiotemporal patterns
Surveillance of dead wild boar is the most efficient method of ASF surveillance because the proportions of wild boar testing positive are much higher in animals found dead than in hunted animals. This is particularly important when more than one dead wild boar is found during a singular mortality event.
Among wild boar found dead, the proportions of animals that test PCR positive are generally much higher than the proportion of animals testing ELISA antibody positive.
The temporal patterns in the proportions of tested samples that are positive are consistent with the epidemiological situations in the countries. For example, in LT, there is both spatial expansion and an increase in PCR‐positive animals among wild boar found dead. By contrast, in Estonia, there is a reduction in the proportion of PCR‐positive results among wild boar found dead, given that infection has been present throughout the whole country.
Overall, among hunted animals, the proportion of wild boar testing PCR or ELISA positive remains low, i.e. below 5%, although there is some local variation.
Seasonality of ASF occurrence in wild boar:
There are apparent peaks in winter and summer in the proportion of wild boar found dead that are testing positive, while in domestic pigs only a summer peak is evident in the outbreaks.
For the wild boars that are hunted, there appears to be a slight decline in the proportion testing positive in spring (February–April). For the rest of the year, more or less the same proportion of positive samples over sampling effort is observed.
The probability of ASF occurrence among hunted or found dead wild boar is not equally dispersed across the year. Winter and summer peaks are observed in wild boar found dead.
Several driving forces could explain an increase in the proportions of positive samples that are tested either in winter or summer, such as the virus, the wild boar ecology, the pig farming husbandry, the involvement of vectors or human behaviour. As yet, however, there is a lack of evidence in support causal associations.
Speed of propagation of the ASF infection
The median speed of propagation of ASF infection in the Baltic States and Poland was estimated to be between 8 and 17 km per year based on a network analysis. Similar estimates of speed were obtained using other methods.
Based on ADNS notifications, there is a summer increase in the local spread velocity of ASF infection moving through wild boar populations.
Hot spots
Overall, the ASF hot spot areas in the Baltic countries have moved into a south‐western direction over the 4 past years. In Latvia, these hot spot areas have reduced in size and are currently only present in the west of the country, whereas in Estonia they have disappeared entirely.
Wild boar–domestic interface
The specific routes of ASF introduction into affected domestic pig farms in the EU could only be identified in very few of all outbreaks for which detailed investigations were conducted.
Based on the experiences from affected countries, in the vast majority of introductions direct contact with infected domestic pigs or wild boar could be excluded as the likely route of introduction.
Inadequate biosecurity is likely to have contributed to introduction of ASF into domestic farms via indirect contact through contaminated fomites or environment.
Domestic pig outbreaks were correlated in time and space with cases in wild boar, indicating an association between the risk of introduction and the level of contamination of the environment.
The virus has been demonstrated to survive for more than 2 years in frozen organs or for almost 2 years in chilled blood. Virus in faeces, urine or slurry will survive for a much shorter period (up to a week) in chilled or room temperature conditions. Therefore, anything that contains frozen and chilled blood or organic material from infected pigs can be a very significant source of introduction for prolonged periods of time (years), whereas fomites contaminated with blood or urine, either chilled or at room temperature, will survive for about a week.
Vectors were not mentioned as a possible risk factor, but were not considered.
4.2. Risk factor analysis – TOR2
In the Bayesian hierarchical model, an increased density of domestic pigs and of wild boar and a decreased density of roads were associated with a significant increase in ASF occurrence in wild boar. Of these three risk factors, wild boar density was the most influential. The same results were obtained using a general additive model.
There were insufficient data to finish the risk factor analysis for the occurrence of ASF in domestic pigs.
4.3. Review wild boar management measures for controlling the spread of ASF – TOR3
The following conclusions are based on the results of modelling.
There remains considerable uncertainty about many aspects of ASF epidemiology in wild boar, including the carcass contact rate, the contact rate between groups, and the role of insects. For this reason, the modelling results are presented as a comparison between scenarios rather than providing absolute values for management.
These conclusions pertain to emergency measures implemented after the focal introduction of ASF.
In the absence of carcass removal in the core area and assuming that carcass contact occurs immediately after death, the probability of success can exceed 80% if the intensity of hunting in the intensive hunting area is much higher than during times of sustainable wild boar management. This might be possible in practice because the intensive hunting area is limited (3–12 wild boar home ranges).
Given acknowledged limitations of intensive regular hunting, the sole application of intensive hunting zones around the buffer area may not be sufficient for focal emergency control measures.
Lesser hunting efforts are required in the intensive hunting area when carcass removal is being implemented in the core area. With a carcass removal rate of 20%, the probability of success can exceed 80% with an intensive hunting area of limited size (3–12 wild boar home‐range diameters width) and less intensive hunting efforts (similar to that achieved during sustainable wild boar management). The probability of success is further increased if carcass removal rates are doubled and/or carcasses are removed more quickly following death.
Following a focal introduction of ASF, the affected area is small and relatively limited numbers of wild boar are present that may subsequently become infected and die. In this situation, a high carcass removal rate in the core area can override carcass creation. Therefore, eradication could potentially be achieved without the need for intensive hunting efforts in the intensive hunting area.
The impact of hunting on the wild boar population should be maximised over as short a time period as possible.
A lengthy delay after the establishment of zoning and before culling in the core and buffer areas is detrimental with respect to maximising the probability of eradication success. This is contrary to expert opinion, in which it is recommended – to avoid perturbation of the population during the epidemic – that culling commence only once the epidemic peak has been reached.
The width of the buffer area will influence the probability of eradication success, with wider buffer areas leading to higher success rates. However, increases in the buffer area will also lead to a larger intensive hunting area, where intensive hunting efforts may be harder to achieve in practice, and more animals would need to be culled in the buffer area.
The delineation of zones (including the core and buffer zones) is guided by the detection of carcasses, rather than the detection of actual infected animals. For this reason, eradication success may be imperfect even in the presence of wild boar‐proof fencing.
If carcass removal and intensive hunting are effectively implemented, fencing is most useful in delineating zones, rather than adding substantially to the control efforts.
Segments of fencing will be particularly useful in those areas where carcass removal or intensive hunting is difficult to implement.
Recent focal introduction and large‐scale area expansion should be considered different management problems in the context of ASF control. The success of the approach following focal introduction is closely linked to the eventual size of the outbreak. Every effort should be made to limit the size of these outbreaks following focal introduction, as there appears to be no point of return if this strategy fails.
4.4. Review natural/artificial borders – TOR4 for the determination/demarcation of the restricted areas
Based on a comparison of model outputs and ADNS data, it was not possible to demonstrate an effect of natural barriers (e.g. roads, rivers) on ASF spread.
It appears that assumed human‐mediated translocations are particularly influential in overwhelming any positive effect of such barriers.
5. Recommendations (TOR5)
It is recommended that legal terminology in EU legislation (including the concept of an ‘infected area’) are separated from concepts that relate specifically to wild boar management zones, i.e. the core area, buffer zone and the intensive hunting zone.
5.1. Recommendations for non‐affected areas, far from any ASF occurrence, but at risk of human‐mediated ASF introduction
PREVENTION
5.1.1. Evidence in support
Evidence in support of the key role of passive surveillance for early detection in the current ASF epidemic was presented by EFSA in July 2018.
-
Biosecurity recommendations are underpinned by current understanding with respect to ASF epidemiology, including:
-
–
virus survival in carcasses/the environment (pork products, carcasses, contaminated fomites);
-
–
experiences during the current epidemic with evidence of human‐assisted movement of virus, on several occasions over long distances.
-
–
There is an observed year‐on‐year increase in the population density of wild boar in many parts of Europe (Massei et al., 2015).
There are several successful examples in which culling has substantially reduced the size of wild boar populations (up to 65% reduction compared with the initial total population) in defined local areas (e.g. Boadella et al., 2012; Quirós‐Fernández et al., 2017). However, this is not considered a sustainable long‐term option for population reduction over large areas because wild boar are extremely adaptable to different environmental circumstances and will respond to increased mortality through a compensatory larger reproductive success (Gethöffer et al., 2007; Hanson et al., 2009; Fonseca et al., 2010).
5.1.2. Recommendations for non‐affected areas, far from any ASF occurrence, but at risk of human‐mediated ASF introduction
Maintain control of borders, including the controls on the implementation on the ban on cross‐border trade of wild boar.
Establish and maintain systems of passive surveillance for early detection of ASF in wild boar.
Complete contingency planning, clearly outlining protocols, roles and responsibilities, etc. if there is an ASF incursion.
Increased understanding of local wild boar ecology.
Improved biosecurity and biosecurity awareness.
Improve biosecurity and biosecurity awareness, both in domestic pig holdings, and at hunting grounds.
Collect discarded rubbish material on roads/in parks, etc., noting the potential for both urban and sylvatic wild boar.
Increase awareness and understanding among hunters and others who visit or work in the forest, of the importance of passive surveillance for early detection of ASF and efficient and biosecure hunting strategies.
Increase the awareness of international travellers (i.e. tourists, foreign workers, transporters, etc.) coming from ASF‐infected countries about bringing meat with them and the eventual inappropriate disposal of such foods in areas accessible to wild boar, e.g. picnic sites, by the road, etc.
Assess current approaches to hunting, seeking opportunities to improve hunting efficiency for wild boar population reduction.
-
Implement preventive measures to stabilise wild boar density as this will be beneficial in reducing both the probability of establishment of ASF following introduction, and the efforts needed for potential emergency actions (such as carcass removal) if an ASF incursion were to occur. These measures should focus on:
-
–
Habitat carrying capacity. Key measures are needed to limit the carrying capacity of local habitats for wild boar including a complete ban on the feeding of wild boar and strategies to improve crop protection. Baiting must not become a substitute for feeding. It should be kept to a minimum, and alternatives used where possible.
-
–
Culling of wild boar. Hunting yields should be substantially increased to stabilise wild boar density and achieve sustainable management of these populations. Given the temporal trend of increasing wild boar population density that has been observed in Europe, these hunting efforts should include the harvest of animals of reproductive age (sows and boars), not excluding piglets. Hunting regulations and limitations should be made as flexible as possible to maximise opportunities for population reduction of wild boar. Once accurate estimates of wild boar density become available, it will be possible to refine the size of the hunting bag that will be required, consistent with sustainable population management in each region.
-
–
5.2. Non‐affected areas in close proximity to infected areas, or restricted areas at higher risk of ASF introduction primarily via natural spread mediated by wild boar
PREVENTION
5.2.1. Evidence in support
As above, in Section 5.1.1.
The July 2018 EFSA report provides details of methodology to assess the effectiveness of passive surveillance. As a rough guide, the number of carcasses detectable each year is approximately 1% of the total adult population (assuming 10% annual mortality and 10% of carcasses detectable). This provides a ‘baseline’ for passive surveillance (the number of carcasses that should be detected on an ongoing basis) in the absence of ASF.
5.2.2. Recommendations for non‐affected areas in close proximity to infected areas, or restricted areas at higher risk of ASF introduction primarily via natural spread mediated by wild boar
The recommendations are equivalent to those in Section 5.1.2, with the following adjustments:
-
Preventive measures to stabilise wild boar density, focusing both on habitat carrying capacity and the hunting of wild boar, will be even more urgent. In non‐affected areas in close proximity to infected areas, hunting of wild boar should be conducted at the highest levels achievable in that area. Furthermore, it is recommended that hunting of wild boar is conducted throughout this area, including in protected areas (such as national parks). Collectively, these measures will be beneficial in reducing both:
-
–
the probability of ASF introduction through natural movement of wild boar;
-
–
the probability of establishment of ASF following introduction;
-
–
the efforts needed for potential emergency actions (such as carcass removal) if an ASF incursion were to occur.
-
–
There is a need for a planned, active and systematic approach to passive surveillance, to maximise the probability of early detection following introduction and the accuracy of subsequent efforts to delineate the geographic extent of the infected wild boar population.
5.3. Areas where the disease was recently introduced into wild boar populations
EPIDEMIC
5.3.1. ASF presence following focal introduction (e.g. the Czech Republic)
5.3.1.1. Evidence in support
-
From the field. In large part, these recommendations are based on field experiences gained during the Czech outbreak, management of the Zlín outbreak is as yet the only example during the current epidemic in which ASF has been contained for an extended period of time. Approaches used during the Czech outbreak differed according to infection risk, as described in the July 2018 EFSA report, using terminology as per Section 2.2.3.2:
-
–
In the core and buffer areas: The core area is defined by a polygon that encompasses all dead positive wild boars, whereas the buffer area surrounds this with a width equivalent to wild boar home range over a year. In these areas, the approach to hunting evolved in line with the epidemic (hunting ban then sit‐and‐wait hunting then intensive hunting), there was active carcass removal with biosecurity, feeding was facilitated through unharvested crops, and there was no access to the public.
-
–
In the intensive hunting area (the infected area but outside the core and buffer areas): there was intensive hunting and carcass removal, passive surveillance and limited feeding.
-
–
The conclusions from modelling as presented previously.
5.3.1.2. Recommendations for areas with recent, focal introduction of ASF into wild boar populations
-
Following the initial focal ASF introduction, the infected area should be defined as outlined above, based on passive surveillance and if possible demarcated based on natural and artificial barriers:
-
–
Within the core and buffer areas, the wild boar populations should be kept undisturbed throughout the period of active ASF transmission (e.g. a complete hunting ban on all species should be imposed and a strategy of ensuring the needs of wild boar are met should be developed and implemented to limit animal movement). Carcass removal should be undertaken to limit infection in the environment, but under conditions of high biosecurity. Following the decline in the epidemic, as demonstrated through passive surveillance, active population management under strict biosecurity, including rapid population reduction (culling) and carcass removal, should be reconsidered.
-
–
Within the intensive hunting area, there should be a drastic and sustained reduction in the wild boar population. The modelling results highlight the interaction between multiple factors in the intensive hunting area (the area size, the intensity of the hunting effort, the concentration of the hunting effort) and the core and buffer areas (carcass removal, timing of carcass removal following death, timing of culling after initial detection). The intensity and concentration of hunting effort required in the intensive hunting area will be influenced by these other factors.
-
–
5.3.2. ASF presence in previously non‐infected areas as a consequence of geographic expansion of known infected areas (a moving front of infection)
5.3.2.1. Evidence in support
In the epidemic to date, geographic spread mainly presents in the form of a small‐scale epidemic. However, there have been multiple examples of human‐mediated spread.
The estimated speed of propagation in wild boar populations is approximately 10 km/year. In affected countries, there is evidence that local spread velocity increases in the summer.
With the exception of the Czech situation (which occurred as a result of focal introduction), no strategies have yet proved effective in the current epidemic in preventing the geographic expansion of known infected areas.
These recommendations are drawn from the experience of affected MS.
5.3.2.2. Recommendations following geographic expansion of the ASF‐affected areas
In theory, the strategies recommended in response to focal ASF introduction are also suited to ASF introduction following the geographic expansion of known ASF‐infected areas. In practice, however, some modifications will be needed, as the latter will generally result in a much larger affected area. At these larger scales, culling can be more difficult to implement, fencing is likely to be impractical and broader societal and political issues need to be considered.
Given this background, the following strategies are recommended:
Passive surveillance is particularly important, both for early detection and to delineate the geographic extent of the infected wild boar population.
Larger buffer areas can be considered, to account for expected wild boar movement.
Biosecurity and biosecurity awareness are particularly important, to minimise the risk of human‐mediated spread.
5.4. Areas where ASF is endemic (present in the wild boar population for more than 1 year)
ENDEMIC
5.4.1. Evidence in support
Based on experience to date, particularly in the Baltic States, infected wild boar have been detected in affected areas for some years after initial introduction, suggesting as yet poorly understood pathways to facilitate persistence of the virus.
Active and passive surveillance are both useful in an endemic situation:
As outlined previously, (EFSA, July 2018), the surveillance objectives will change during different phases following ASF introduction:
-
–
In infected populations with increasing prevalence, surveillance objectives include determining the extent of infected areas and identifying potentially useful interventions.
-
–
In infected areas once prevalence has reached a plateau, surveillance objectives include determining the extent of infected areas, identifying potentially useful interventions and monitoring the impact of interventions on the prevalence of infected animals.
-
–
In infected areas with decreasing prevalence, surveillance objectives include monitoring the effect of interventions on the prevalence of infected animals.
Active surveillance is generally the most suited approach for most of these surveillance objectives. However, passive surveillance is the most effective and efficient method of surveillance for early detection of ASF in wild boar (for example, into new areas or areas where ASF has not been detected in wild boar for some time; EFSA, July 2018).
The modelling results highlight the key role of carcass removal in limiting infection in affected wild boar populations.
5.4.2. Recommendations in areas of endemic ASF infection
There should be ongoing hunting of wild boar populations, both to slow infection and to monitor progress through active surveillance. The age profile of seropositive animals should be assessed.
There is an ongoing need for passive surveillance and carcass removal, to identify hot spot areas and limit ASF presence in carcasses/the environment.
There should be an ongoing feeding ban. Baiting should be kept to a minimum, and alternatives used where possible.
-
Further research is needed:
-
–
to clarify the pathways that facilitate ASF persistence in affected areas over a number of years;
-
–
to assist the interpretation of seropositivity in the context of ASF infection;
-
–
to define a pathway to ASF freedom following detection of the last known infected animal/carcass.
-
–
6. Other recommendations
There are significant gaps in knowledge about the epidemiology of ASF in Europe, including the carcass contact rate, the contact rate between groups, and potential role of vectors in ASF spread. Further research in each of these areas is recommended.
Abbreviations
- AB‐ELISA
antibody enzyme‐linked immunosorbent assay
- ADNS
Animal Disease Notification System
- ARIB
Estonian Agricultural Registers and Information Board
- ASDS
Animal Disease Notification System
- ASF
African swine fever
- ASFV
African Swine Fever Virus
- BYM
Besag, York and Mollié
- CSF
Classical swine fever
- CVO
Chief veterinary officer
- DACRAH
Data Collection for Risk Assessments on Animal Health
- DCF
Data Collection Framework
- ELISA
enzyme‐linked immunosorbent assay
- EURL
European Union Reference Laboratory
- FMD
Foot and mouth disease
- GAM
general additive model
- IB
immunoblotting
- IPT
immunoperoxidase
- GIS
geographic information systems
- LIMS
Laboratory Information Management System
- LOESS
local regression or local fitting smoothing
- MS
Member States
- N‐E
north‐eastern
- NRL
National Reference Laboratory
- PCR
polymerase chain reaction
- QAHs
quality of available habitats
- qPCR
quantitative polymerase chain reaction
- SVA
State Veterinary Administration
- TOR
Term of reference
Annex A – Templates for data collection for Epidemiological Analysis, including risk factor analysis
1.
Table A.1.
ASF DCF data model description (laboratory data)
| Element name | Controlled terminology | Description |
|---|---|---|
| localOrgId | – | Organisation reporting the data |
| progLegalRef | – | Reference to the legislation for the programme defined by programme code. Reference to the legislation on what to sample, how to evaluate the sample, etc. |
| sampStrategy (mandatory) | ST10A=Objective sampling, ST20A=Selective sampling, ST30A=Suspect sampling, ST40A=Convenient sampling, ST50A=Census, ST90A=Other, STXXA=Not specified | Typology of sampling strategy performed in the programme or project identified by programme code |
| progType | K028A=Survey ‐ national survey, K029A=Unspecified, K030A=Surveillance active, K031A=Surveillance passive, K023A=Monitoring –active, K024A=Monitoring – passive, K021A=Control and eradication programmes, K032A=Outbreak investigation | Indicate the type of programme for which the samples have been collected (National, EU programme, Total diet study, Control and eradication programme) |
| sampMethod | N001A=Individual/single, N002A=Pooled/batch, N003A=Animal, N004A=Flock, N005A=Holding, N006A=Herd, N007A=Slaughter batch, N008A=Unknown, N009A=According to Dir. 2002/63/EC, N010A=According to 97/747/EC | Reference to the method for sampling (e.g. EU legislation) |
| sampPoint (mandatory) | E101A=Farm, E180A=Hunting, E311A=Slaughterhouse, E012A=Zoo, E980A=Unknown, E310A=Meat processing plant, E350A=Animal feeds manufacturer, E191A=Natural habitat | Specify the type of location the sample was obtained from |
| progInfo | – | Additional info about programme |
| sampHoldingId | Unique code | Holding ID for multiple samples from domestic pigs from the same farm |
| animalID | Unique code | Unique identifier for the animal |
| sampId (mandatory) | Unique code | Unique identifier for the sample, this must be maintained when reporting all laboratory results linked to the sample |
| sampCountry (mandatory) | 2 digit country code | Country where the sample was taken for laboratory testing (ISO 3166‐1‐alpha‐2) |
| sampArea (mandatory) | NUTS 3 CODE | Area where the sample was collected (Nomenclature of territorial units for statistics – NUTS) |
| sampLAU1 | LAU1 CODE | Area at the first local administrative level where the sample was collected |
| sampLAU2 | LAU2 CODE | Area at the second local administrative level where the sample was collected at the lowest administrative unit available |
| longitude | – | Longitude of the representative sampling point in WGS84 decimal format |
| latitude | – | Latitude of the representative sampling point in WGS84 decimal format |
| sampY (mandatory) | 4 digit format | Year of sampling |
| sampM (mandatory) | 2 digit format | Month of sampling |
| sampD | 2 digit format | Day of sampling |
| sampInfo | – | Additional information on the sampling taken depending on specific requirements of the different data collection domains (e.g. day of arrival in the laboratory). |
| sampMatType (mandatory) | S000A=Animal sample, S019A=Food sample, S026A=Feed sample, S027A=Environmental sample, S030A=Unknown | Type of sample taken |
| sampMatCode | A056Y=Wild boar, A16AB=Wild boar‐domestic pig hybrids, A0C9X=Breeding pigs, A0C9Y=Fattening pigs, A0C9Z=Mixed pig herds, A0CAA=Breeding piglets, A0CAE=Fattening piglets | Type of animal tested |
| sampMatText | Hunted, clinical suspicion, found dead, alive, premovement testing, depopulation |
Additional info about how the sample was obtained ‘Clinical susp’ includes ‘euthanasia’ and ‘sick’ ‘Found dead’ includes ‘traffic accident’ Depopulation ‐ for wild boar, hunted in the framework of control measures |
| Decomposition (mandatory) | 1=Fresh, 2=Decomposed, 3=Bones | Degree of decomposition of carcasses |
| Age (mandatory) | Adult, Young, Unknown | ADULT=Greater 1 year, YOUNG=Up to 1 year, Unknown |
| Sex (mandatory) | M=Male, F=Female, U=Unknown | |
| sampMatInfo | Additional specific information and comments on the matrix sampled | |
| sampAnId | Identification code of sample analysed | |
| analysisY | Year when the analysis was completed | |
| analysisM | Month when the analysis was completed | |
| analysisD | Day when the analysis was completed | |
| anMatCode | A01XD=Animal liver, A01YG=Animal kidney, A01ZK=Animal other organs, A020P=Animal other slaughtering products, A0F1T=Animal blood, A021E=Animal bone marrow, A0CEY=Blood serum, A0F5E=Gelatine, A0CJN=Lymph nodes, A04MQ=Mixed organs, A01RG=Pig muscle, A16AA=Salivary glands, A06AK=Skin, A069Q=Spleen, A0EYE=Whole animal, A04CN=Wild boar carcase | Description of matrix analysed. It allows specifying the characteristics of the matrix analysed |
| anMatText | Description of the matrix analysed characteristics using free text | |
| labId | Identification code of the laboratory (National laboratory code if available). This code should be nationally unique and consistent through all data domain transmissions | |
| labCountry (mandatory) | 2 digit COUNTRY CODE | Country where the laboratory is located (ISO 3166‐1‐alpha‐2) |
| paramCode (mandatory) | RF‐00002657‐MCG = African swine fever virus | Encoding of the parameter/analyte according to the PARAM catalogue |
| paramText | Description of the parameter/analyte using free text. | |
| anMethCode | F086A=Polymerase chain reaction (PCR), F087A=Quantitative polymerase chain reaction (QPCR), F080A=Enzyme‐linked immunosorbent assay (ELISA), F151A=Immunoblotting (IB), F590A=Immunoperoxidase test (IPT), F089A=Genotyping, F563A=Virus isolation |
Encoding of the method or instrument used from the ANLYMD catalogue. PCR – virus, QPCR) – virus, Genotyping – virus, Virus isolation – virus, ELISA – antibodies, Immunoblotting (IB) – antibodies, Immunoperoxidase test (IPT) ‐ antibodies |
| anMethText | Additional description of the method or instrument using free text, particularly if ‘other’ was reported for ‘Analytical method code’ | |
| resId (mandatory) | Unique code | Unique identification of an analytical result |
| specificity | – | Analytical method specificity if available |
| sensitivity | – | Analytical method sensitivity if available |
| resUnit | – | Unit of measurement the result value when reporting quantitative values |
| resVal | The quantitative result of the analytical measure expressed in the unit specified in resUnit (e.g. CT or OD values) | |
| resQualValue (mandatory) | POS=Positive, NEG=Negative, EQU=Questionable | Qualitative result value: Positive or negative |
| resType | BIN=Qualitative Value (Binary) | Indicate the type of result, whether it could be quantified/determined or not |
| resInfo | – |
Free text to provide additional comments on laboratory result Additional specific information and comments on the result section depending on specific requirements of the different data collection domains. |
| ADNSId | Unique code | Code of the outbreak notified to the ADNS system |
| HGID | Unique code | Hunting ground ID |
Table A.2.
Domestic pig farms
| NUTS3 code | LAU1 code | LAU 2 code | FarmID | Type | Longitude | Latitude | Year | Month | Number of pigs | Number of sows |
|---|---|---|---|---|---|---|---|---|---|---|
| bg12321321 | A | 2001 | 01 | 100 | 10 | |||||
| if Long and Lat are not available | bg12321321 | A | 2001 | 02 | 200 | 10 | ||||
| … | ||||||||||
| … | ||||||||||
| bg12321322 | B | 2018 | 01 | 50 | 5 | |||||
| bg12321322 | B | 2018 | 06 | 70 | 5 | |||||
A. Non‐commercial farms (NCF): farms where pigs are kept only for fattening for own consumption and neither pigs nor any of their products leave the holding.
B. Commercial farms (CF): farms which sell pigs, send pigs to a slaughterhouse or move pig products off the holding.
C. Outdoor farms: pigs are kept temporarily or permanently outdoor.
Table A.3.
Domestic pig population if exact location (longitude and latitude) are not available
| NUTS 3 ID | LAU 1 ID | LAU 2 ID | Year | Num of pig holdings (tot) | Num of pigs (tot) | Number of pig holdings ≤ 10 heads (small pig holding) | Number of pigs in (small pig holdings) |
|---|---|---|---|---|---|---|---|
LAU: local administrative unit.
IDs in accordance with shape files of NUTS and LAU.
Table A.4.
Hunting activity and (including shape file of hunting grounds)
| Hunting Area ID | Hunting Management Unit (hunting ground or hunting district) ID | Name of Hunting Club Management Unit (hunting ground or hunting district) | Year | Number of wild boar estimated (spring census) | Number of active hunters per season | Number of hunting dogs in the area | Days of driven hunts | Number of hunted wild boar | Number of hunted females | Number of wild boar found dead | Number of wild boar feeding places in the area | Average amount of forage, t | Average number of piglets observed per sow |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| If monthly data are not available | |||||||||||||
Annex B – Results of epidemiological investigations in backyard outbreak farms in Estonia
1.
Table B.1.
Risk factors for introduction of ASF virus observed in backyard outbreak farms in Estonia in 2015–2017*
| Year/No. of the Outbreak | Total no. of pigs | Herd type | Outdoor access | Feeding grass | Contamination of feeda | Contamination of bedding | Production animals in barn | Pets in barn | Owner hunter | Berry picking |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015/1 | 1 | Fat | X | (Cats) | X | |||||
| 2015/7 | 3 | Fat | X | Chicken | Cat | |||||
| 2015/8 | 2 | Fat | (X)b | X | Cattle | (X) | X | |||
| 2015/13 | 5 | Fat | X | Cats | ||||||
| 2016/19 | 6 | Fat | X | X | X | X | Cattlec | (Cats) | ||
| 2016/20 | 7 | Fat | Cattle | Cat | ||||||
| 2016/21 | 3 | Fat | X | X | X | Cat | X | |||
| 2016/22 | 5 | FF | X | X | Cattle | Cat, Dogs |
Fat: fattener; FF: farrow to finish.
Possible secondary on‐site contamination of cereal feed at storage and/or preparation (milling, mixing).
Parentheses – can be suspected.
Cattle on the same territory as pigs had access.
* In all herds, feeding of kitchen waste could not be excluded. All herds had inadequate disinfection measures for vehicles and imperfect biosecurity procedures for people entering the premises.
Table B.2.
Risk factors for introduction of ASF virus observed in commercial outbreak farms in Estonia in 2015–2017
| Year/No. of the outbreak | Total no. of pigs | Herd type | Other animals in barn | Possible contamination of bedding | Possible contamination of feeda | Other vehicles on the territory | Missing fence | Inadequate disinfection of vehicles entering the territory | Inadequate disinfection of people or equipment at barn entrances | No separation in changing roomb | Miscellaneous |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015/2 | 186 | Bra | X | Car | X | NAd | X | NA | Owner is a hunter | ||
| 2015/3 | 355 | Fata | X | X | Car, Farm machine | X | NA | X | NA | ||
| 2015/4 | 32* | FF | Cat | X | NA | X | NA | ||||
| 2015/5 | 487 | FFe | Cattle, Cats | X | Farm machines | NA | X | X | Grass feeding | ||
| 2015/6 | 1186 | FF | X | Farm machines | X | X | X | ||||
| 2015/9 | 2149 | Fat | (X) | ||||||||
| 2015/10 | 6426 | Brb | Cats | X | Cars | X | (X) | X | |||
| 2015/11 | 3060 | FF | X | Farm machines | X | (X) | X | ||||
| 2015/12 | 1847 | FF | Dog | X | (X) | X | |||||
| 2015/14 | 2480 | Brb | Cars | X | (X) | X | |||||
| 2015/15 | 126 | FFe , f | X | X | NA | NA | Outdoor keeping | ||||
| 2015/16 | 3804 | Fat | X | X | X | ||||||
| 2015/18 | 104 | FFf | X | Farm machines | X | X | X | ||||
| 2016/23 | 4091 | FF | Cats | X | X | X | |||||
| 2016/24 | 2736 | Fat | X | NA | X | ||||||
| 2017/25 | 3415 | Fat | X | X | |||||||
| 2017/26 | 3232 | Fat | (Cats)c | X | X | ||||||
| 2017/27 | 6418 | FF | (X) |
Br: breeder/multiplier; Fat: fattener; FF: farrow to finish, a,b Denoting respective connected herds.
Possible secondary on‐site contamination of cereal feed at storage and/or preparation (milling, mixing).
No clear separation of clean and dirty zones in changing room for workers (NA – no dedicated changing room for workers).
Parentheses – can be suspected.
Not available.
Organic farm.
Crosses of domestic pigs and wild boar.
* At the time of the outbreak, four pigs was present. Thirty‐two pigs was the maximum number of pigs present at the farm during the 1 year period before the outbreak.
Annex C – Seasonality
1.
Figures C.1–C.4 show the results of the statistical assessment of the comparison of monthly incidence using a generalised linear mixed model and a Tukey pairwise comparison between each pair of months. It can be noticed that several months per year have equal probabilities for the occurrence of ASF, which clearly differ from the other months.
Figure C.1.
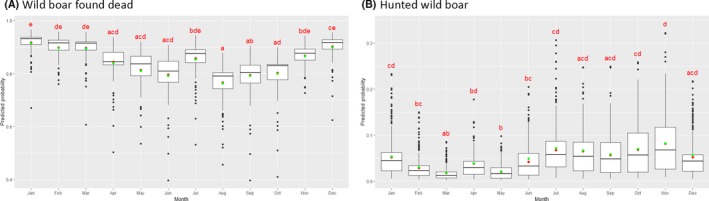
Comparison of monthly ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Lithuania
Figure C.4.
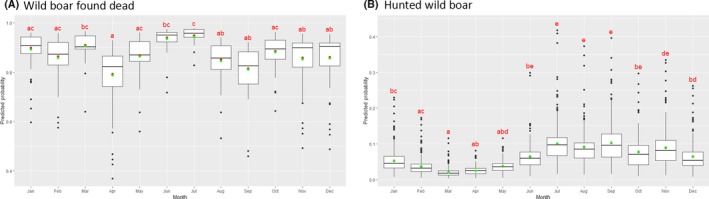
Comparison of monthly ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Estonia
For example, it can be seen that the probability of occurrence of ASF in hunted wild boar in Estonia in the summer months July, August and September is the same (resulting as ‘e’ in the pairwise comparison) and differs from all the other months.
It is very difficult, however, to draw a general conclusion from this assessment, as the patterns are different for the countries and the results will change according to the choice of how to group the months together as ‘seasons’ for the analysis. What could be concluded, however, is that the probability of ASF occurrence in wild boar found dead and hunted is not equally observed across the year, some it is equal in some periods, where a lower or higher probability is observed than in the other months of the year.
Figure C.2.
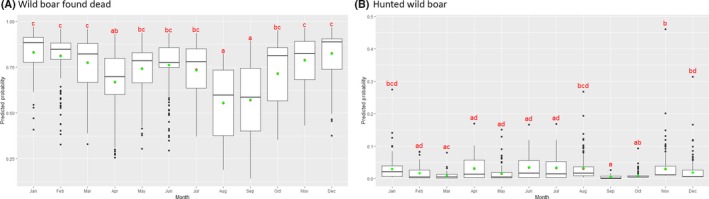
Comparison of monthly ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Poland
Figure C.3.
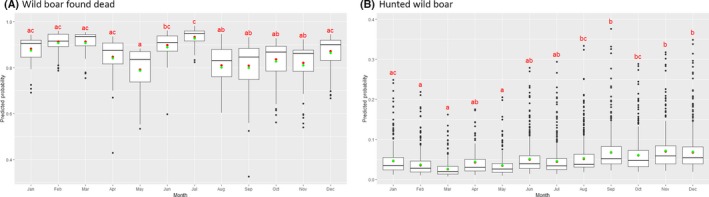
Comparison of monthly ASF incidence using a generalised linear mixed model and Tukey pairwise comparison for Latvia
Supporting information
SLR protocol
ASF survival data
Exp. Inf. data
Suggested citation: EFSA (European Food Safety Authority) , Boklund A, Cay B, Depner K, Földi Z, Guberti V, Masiulis M, Miteva A, More S, Olsevskis E, Šatrán P, Spiridon M, Stahl K, Thulke H‐H, Viltrop A, Wozniakowski G, Broglia A, Cortinas Abrahantes J, Dhollander S, Gogin A, Verdonck F, Amato L, Papanikolaou A and Gortázar C, 2018. Scientific report on the epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA Journal 2018;16(11):5494, 106 pp. 10.2903/j.efsa.2018.5494
Requestor: European Commission
Question number: EFSA‐Q‐2017–00471
Acknowledgements: EFSA wishes to thank all European competent institutions, Member State bodies and other organisations that provided data for this scientific output and specially the following experts for the support provided to this scientific output: Łukasz Bocian, Theodora Chesnoiu, Sándor Csanyi, Francesco Feliziani, Agniet≐ Grušauskien≐, Anna Hoffman, Kärt Jaarma, Marius Judickas, Jókay Katalin, Oliver Keuling, Melinda Kocsis, Daniela Korytarova, Katrin Lõhmus, Peep Männil, Maria Mihaita, Alexander Neil, Krystyna Pędrakowska, Claudia Pittiglio, Tomasz Podgorski, Mārtiņš Seržants, Carola Sautoer‐Louis, Jolanta Urbelionytė, Barbuli Velizar, Joaquin Vicente Banos, Richard Wallo and Ilgvars Zihmanis.
Approved: 8 November 2018
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2018.EN-1521/full
Notes
Council Directive 2002/60/EC of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and African swine fever https://publications.europa.eu/en/publication-detail/-/publication/8a4d70ec-c198-4eca-a412-284dd8a06179/language-en
References
- Acevedo P, Vicente J, Höfle U, Cassinello J, Ruiz‐Fons JF and Gortázar C, 2007. Estimation of European wild boar relative abundance and aggregation: a novel method in epidemiological risk assessment. Epidemiology and Infection, 135, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrycht M, Merta D, Bobek J and Ulejczyk S, 2016. The demographic pattern of wild boars (Sus scrofa) inhabiting fragmented forest in north‐eastern Poland. Baltic Forestry, 22, 251–258. [Google Scholar]
- Barasona JA, Acevedo P, Diez‐Delgado I, Queiros J, Carrasco‐García R, Gortázar C and Vicente J, 2016. Tuberculosis‐associated death among adult wild boars, Spain, 2009–2014. Emerging Infectious Diseases, 22, 2178–2180. 10.3201/eid2212.160677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- BFSA (Bulgarian Food Safety Agency), 2018. African swine fever control measures in Bulgaria. Presentation at the PAFF meeting, 12–13 July 2018, Brussels. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20180712_pres_asf_bul.pdf [Accessed: 31 October 2018].
- Boadella M, Vicente J, Ruiz‐Fons F, de la Fuente J and Gortázar C, 2012. Effects of culling Eurasian wild boar on the prevalence of Mycobacterium bovis and Aujeszky's disease virus. Preventive Veterinary Medicine, 107, 214–221. 10.1016/j.prevetmed.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Bosch J, Iglesias I, Muñoz MJ and Torre A, 2016. A cartographic tool for managing african swine fever in Eurasia: mapping wild boar distribution based on the quality of available habitats. Transboundary and Emerging Diseases, 64, 1720–1733. 10.1111/tbed.12559 [DOI] [PubMed] [Google Scholar]
- Carlson J, O'Donnell V, Alfano M, Salinas LV, Holinka LG, Krug PW, Gladue DP, Higgs S and Borca MV, 2016. Association of the host immune response with protection using a live attenuated African swine fever virus model. Viruses, 8, pii: E291. 10.3390/v8100291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas‐Díaz E, Closa‐Sebastià F, Peris A, Miño A, Torrentó J, Casanovas R, Marco I, Lavín S, Fernández‐Llario P and Serrano E, 2013. Recorded dispersal of wild boar (Sus scrofa) in northeast Spain: implications for disease‐monitoring programs. Wildlife Biology in Practice, 9, 13–26. 10.2461/wbp.2013.ibeun.3 [DOI] [Google Scholar]
- Cleveland WS, Devlin SJ and Grosse E, 1988. Regression by local fitting. Journal of Econometrics, 37, 87–114. [Google Scholar]
- Davies K, Goatley LC, Guinat C, Netherton CL, Gubbins S, Dixon LK and Reis AL, 2015. Survival of African swine fever virus in excretions from pigs experimentally infected with the Georgia 2007/1 isolate. Transboundary and Emerging Diseases, 64, 425–431. 10.1111/tbed.12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson AI and Ferris NP, 1976. The survival of some air‐borne animal viruses in relation to relative humidity. Veterinary Microbiology, 1, 413 10.1016/0378-1135(76), 90056-0 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2010. Scientific opinion on the role of tick vectors in the epidemiology of Crimean Congo hemorrhagic fever and African swine fever in Eurasia. EFSA Journal 2010;8(8):1703, 156 pp. 10.2903/j.efsa.2010.1703 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2014. Scientific Opinion on African swine fever. EFSA Journal 2014;12(4):3628, 77 pp. 10.2903/j.efsa.2014.3628 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare), 2015. Scientific opinion on African swine fever. EFSA Journal 2015;13(7):4163, 92 pp. 10.2903/j.efsa.2015.4163 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), Depner K, Gortazar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke H‐H, Viltrop A, Woźniakowski G, Cortiñas Abrahantes J, Gogin A, Verdonck F and Dhollander S, 2017. Epidemiological analyses on African swine fever in the Baltic States and Poland, (Update September 2016 – September 2017). Scientific report. EFSA Journal 2017;15(11):5068, 64 pp. 10.2903/j.efsa.2017.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), Depner K, Gortázar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke HH, Viltrop A, Woźniakowski G, Abrahantes JC, Gogin A, Verdonck F and Dhollander S, 2018. Epidemiological analyses of African swine fever in the Baltic States and Poland. EFSA Journal 2018;15(3):47321, 73 pp. 10.2903/j.efsa.2017.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estonian Agricultural Registers and Information Board (ARIB) , online. Homepage. Available online: http://www.pria.ee/en [Accessed: 15 October 2018].
- Fonseca C, da Silva AA, Alves J, Vingada J and Soares AMVM, 2010. Reproductive performance of wild boar females in Portugal. European Journal of Wildlife Research, 57, 363–371. 10.1007/s10344-010-0441-6 [DOI] [Google Scholar]
- Gethöffer F, Sodeikat G and Pohlmeyer K, 2007. Reproductive parameters of wild boar (Sus scrofa) in three different parts of Germany. European Journal of Wildlife Research, 53, 287–297. 10.1007/s10344-007-0097-z [DOI] [Google Scholar]
- GIS‐Lab , online. Info. Available online: http://gis-lab.info/index-eng.html [Accessed: 1 September 2017].
- Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss‐Custard J, Grand T, Heinz S, Huse G, Huth A, Jepsen JU, Jørgensen C, Mooij WM, Müller B, Pe'er G, Piou C, Railsback SF, Robbins AM, Robbins MM, Rossmanith E, Rüger N, Strand E, Souissi S, Stillman RA, Vabø R, Visser U and De Angelis DL, 2006. A standard protocol for describing individual‐based and agent‐based models. Ecology Modelling, 192, 115–126. [Google Scholar]
- Grimm V, Berger U, De Angelis DL, Polhill JG, Giske J and Railsback SF, 2010. The ODD protocol: a review and first update. Ecology Modelling, 221, 2760–2768. [Google Scholar]
- Hanson LB, Mitchell MS, Grand JB, Jolley DB, Sparklin BD and Ditchkoff SS, 2009. Effect of experimental manipulation on survival and recruitment of feral pigs. Wildlife Research, 36, 185–191. 10.1071/wr08077 [DOI] [Google Scholar]
- Karalyan Z, Zakaryan H, Arakelova E, Aivazyan V, Tatoyan M, Kotsinyan A, Izmailyan R and Karalova E, 2016. Evidence of hemolysis in pigs infected with highly virulent African swine fever virus. Veterinary World, 9, 1413–1419. 10.14202/vetworld.2016.1413-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalyan ZR, Ter‐Pogossyan ZR, Karalyan NY, Semerjyan ZB, Tatoyan MR, Karapetyan SA and Karalova EM, 2017. Hemophagocytic lymphohistiocytosis in acute African swine fever clinic. Veterinary Immunology and Immunopathology, 187, 64–68. 10.1016/j.vetimm.2017.03.008 [DOI] [PubMed] [Google Scholar]
- Keuling O, Stier N and Roth M, 2008. Annual and seasonal space use of different age classes of female wild boar Sus scrofa L. European Journal of Wildlife Research, 54, 403–412. [Google Scholar]
- Lange M, Guberti V and Thulke H‐H, 2018. Understanding ASF spread and emergency control concepts in wild boar populations using individual‐based modelling and spatio‐temporal surveillance data. EFSA supporting publication 2018:EN‐1521,46 pp. 10.2903/sp.efsa.2018.en-1521 [DOI]
- Massei G, Kindberg J, Licoppe A, Gačić D, Šprem N, Kamler J, Baubet E, Hohmann U, Monaco A, Ozoliņš J, Cellina S, Podgórski T, Fonseca C, Markov N, Pokorny B, Rosell C and Náhlik A, 2015. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Management Science, 71(4), 492–500. 10.1002/ps.3965 [DOI] [PubMed] [Google Scholar]
- McKercher PD, Hess WR and Hamdy F, 1978. Residual viruses in pork products. Applied and Environmental Microbiology, 35, 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKercher PD, Yedloutschnig RJ, Callis JJ, Murphy R, Panina GF, Civardi A, Bugnetti M, Foni E, Laddomada A, Scarano C and Scatozza F, 1987. Survival of Viruses in ‘Prosciutto di Parma’ (Parma Ham). Canadian Institute of Food Science and Technology, 20, 267–272. 10.1016/S0315-5463(87)71198-5 [DOI] [Google Scholar]
- Mebus CA, House C, Gonzalvo FR, Pineda JM, Tapiador J, Pire JJ, Bergada J, Yedloutschnig RJ, Sahu S, Becerra V and Sánchez‐vizcaíno JM, 1993. Survival of foot‐and‐mouth disease, African swine fever, and hog cholera viruses in Spanish serrano cured hams and Iberian cured hams, shoulders and loins. Food Microbiology, 10, 133–143. 10.1006/fmic.1993.1014 [DOI] [Google Scholar]
- Mebus C, Arias M, Pineda JM, Tapiador J, House C and Sánchez‐Vizcaíno JM, 1997. Survival of several porcine viruses in different Spanish dry‐cured meat products. Food Chemistry: Mediterranean Aspects of Meat Quality as Related to Muscle Biochemistry, 59, 555–559. 10.1016/S0308-8146(97)00006-X [DOI] [Google Scholar]
- Melis C, Szafrańska PA, Jędrzejewska B and Bartoń K, 2006. Biogeographical variation in the population density of wild boar (Sus scrofa) in western Eurasia. Journal of Biogeography, 33, 803–811. 10.1111/j.1365-2699.2006.01434.x [DOI] [Google Scholar]
- Mellor PS, Kitching RP and Wilkinson PJ, 1987. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Research in Veterinary Science, 43, 109–112. [PubMed] [Google Scholar]
- NEXTGIS , online. Data. Available online: https://data.nextgis.com/ru/ (Accessed: 01 October 2017).
- Nurmoja I, Petrov A, Breidenstein C, Zani L, Forth JH, Beer M, Kristian M, Viltrop A and Blome S, 2017a. Biological characterization of African swine fever virus genotype II strains from north‐eastern Estonia in European wild boar. Transboundary and Emerging Diseases, 64, 2034–2041. 10.1111/tbed.12614 [DOI] [PubMed] [Google Scholar]
- Nurmoja I, Schulz K, Staubach C, Sauter‐Louis C, Depner K, Conraths FJ and Viltrop A, 2017b. Development of African swine fever epidemic among wild boar in Estonia – two different areas in the epidemiological focus. Scientific Reports, 7, 12562 10.1038/s41598-017-12952-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurmoja I, Mõtus K, Kristian M, Niine T, Schulz K, Depner K and Viltrop A, 2018. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Preventive Veterinary Medicine, in press. 10.1016/j.prevetmed.2018.10.001 [DOI] [PubMed] [Google Scholar]
- O'Donnell VO, Holinka LG, Sanford B, Krug PW, Carlson J, Pacheco JM, Reese B, Risatti GR, Gladue DP and Borca MV, 2016. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Research, 221, 8 10.1016/j.virusres.2016.05.014 [DOI] [PubMed] [Google Scholar]
- O'Donnell VO, Risatti GR, Holinka LG, Krug PW, Carlson J, Velazquez‐Salinas L, Azzinaro PA, Gladue DP and Borca MV, 2017. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. Journal of Virology, 9, pii: e01760‐16. 10.1128/jvi.01760-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen AS, Lohse L, Boklund A, Halasa T, Gallardo C, Pejsak Z, Belsham GJ, Rasmussen TB and Botner A, 2017. Transmission of African swine fever virus from infected pigs by direct contact and aerosol routes. Vet Microbiol, 211, 92–102. [DOI] [PubMed] [Google Scholar]
- Olesen AS, Lohse L, Hansen MF, Boklund A, Halasa T, Belsham GJ, Rasmussen TB, Bøtner A and Bødker R, 2018a. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans). Transboundary and Emerging Diseases, 65, 1152–1157. [DOI] [PubMed] [Google Scholar]
- Olesen AS, Lohse L, Boklund A, Halasa T, Belsham GJ, Rasmussen TB and Bøtner A, 2018b. Short time window for transmissibility of African swine fever virus from a contaminated environment. Transboundary and Emerging Diseases, 65, 1024–1032. 10.1111/tbed.12837 [DOI] [PubMed] [Google Scholar]
- Oļševskis E, Guberti V, Seržants M, Westergaard J, Gallardo C, Rodze I and Depner K, 2016. African swine fever virus introduction into the EU in 2014: Experience of Latvia. Research in Veterinary Science, 105, 28–30. [DOI] [PubMed] [Google Scholar]
- Pittiglio C, Khomenko S and Beltran‐Alcrudo D, 2018. Wild boar mapping using population‐density statistics: from polygons to high resolution raster maps. PLoS ONE, 13(5), e0193295 10.1371/journal.pone.0193295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowright W and Parker J, 1967. The stability of African swine fever virus with particular reference to heat and pH inactivation. Archiv für die gesamte Virusforschung, 21, 383–402. 10.1007/BF01241738 [DOI] [PubMed] [Google Scholar]
- Podgórski T and Śmietanka K, 2018. Do wild boar movements drive the spread of African swine fever? Transboundary and Emerging Diseases, [Epub ahead of print] 10.1111/tbed.12910 [DOI] [PubMed] [Google Scholar]
- Popescu L, Gaudreault NN, Whitworth KM, Murgia MV, Nietfeld JC, Mileham A, Samuel M, Wells KD, Prather RS and Rowland RRR, 2017. Genetically edited pigs lacking CD163 show no resistance following infection with the African swine fever virus isolate, Georgia 2007/1. Virology, 501, 102–106. 10.1016/j.virol.2016.11.012 [DOI] [PubMed] [Google Scholar]
- Post J, Weesendorp E, Montoya M and Loeffen WL, 2017. Influence of age and dose of African swine fever virus infections on clinical outcome and blood parameters in pigs. Viral Immunology, 30, 58–69. 10.1089/vim.2016.0121 [DOI] [PubMed] [Google Scholar]
- Probst C, Globig A, Knoll B, Conraths FJ and Depner K, 2017. Behaviour of free ranging wild boar towards their dead fellows: potential implications for the transmission of African swine fever. Royal Society Open Science, 4, 170054 10.1098/rsos.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós‐Fernández F, Marcos J, Acevedo P and Gortázar C, 2017. Hunters serving the ecosystem: the contribution of recreational hunting to wild boar population control. European Journal of Wildlife Research, 63, 57 10.1007/s10344-017-1107-4 [DOI] [Google Scholar]
- Rosell C, 2012. Reproduction of wild boar in a cropland and coastal wetland area: implications for management. Animal Biodiversity and Conservation, 35(2), 209–217. [Google Scholar]
- Sánchez‐Cordón PJ, Chapman D, Jabbar T, Reis AL, Goatley L, Netherton CL, Taylor G, Montoya M and Dixon L, 2017. Different routes and doses influence protection in pigs immunised with the naturally attenuated African swine fever virus isolate OURT88/3. Antiviral Research, 138, 1–8. 10.1016/j.antiviral.2016.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sindryakova IP, Yu PM, Chichikin AY, Gazaev IK, Kudryashov DA and Tsybanov SZ, 2016. The influence of temperature on the Russian isolate of African swine fever virus in pork products and feed with extrapolation to natural conditions. Sel'skokhozyaistvennaya Biologiya, 51, 467–474. 10.15389/agrobiology.2016.4.467rus. [DOI] [Google Scholar]
- Statistics Estonia , online. Frontpage. National Statistic Institute Estonia. Available online: http://www.stat.ee/en [Accessed: 1 August 2017].
- Turner C and Williams SM, 1999. Laboratory‐scale inactivation of African swine fever virus and swine vesicular disease virus in pig slurry. Journal of Applied Microbiology, 87, 148–157. 10.1046/j.1365-2672.1999.00802.x [DOI] [PubMed] [Google Scholar]
- Újváry D, Horváth Z and Szemethy L, 2012. Effect of area decrease in a food competition situation in captive wild boars (Sus scrofa). Journal of Veterinary Behavior: Clinical Applications and Research, 7, 238–244. 10.1016/j.jveb.2011.06.003 [DOI] [Google Scholar]
- Varewyck M, Verbeke T and Cortinas Abrahantes J, 2017, September 12. Exploratory analysis for spatio‐temporal epidemiological data WEB app (spatial). Zenodo. 10.5281/zenodo.889551 [DOI]
- Vicente J, Höfle U, Garrido JM, Fernández‐de‐Mera IG, Acevedo P, Juste R, Barral M and Gortázar C, 2007. Risk factors associated with the prevalence of tuberculosis‐like lesions in fenced wild boar and red deer in south central Spain. Veterinary Research, 38, 451–464. [DOI] [PubMed] [Google Scholar]
- Wieringa‐Jelsma T, Wijnker JJ, Zijlstra‐Willems EM, Dekker A, Stockhofe‐Zurwieden N, Maas R and Wisselink HJ, 2011. Virus inactivation by salt (NaCl) and phosphate supplemented salt in a 3D collagen matrix model for natural sausage casings. International Journal of Food Microbiology, 148–157, 128 10.1016/j.ijfoodmicro.2011.05.010 [DOI] [PubMed] [Google Scholar]
- Wilkinson PJ, Wardley RC and Williams SM, 1981. African swine fever virus (Malta/78) in pigs. Journal of Comparative Pathology, 91, 277–284. 10.1016/0021-9975(81), 90033-5 [DOI] [PubMed] [Google Scholar]
- Woźniakowski G, Kozak E, Kowalczyk A, Łyjak M, Pomorska‐Mól M, Niemczuk K and Pejsak Z, 2016. Current status of African swine fever virus in a population of wild boar in eastern Poland (2014–2015). Archives of Virology, 161, 189–195. 10.1007/s00705-015-2650-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani L, Forth JH, Forth L, Nurmoja I, Leidenberger S, Henke J, Carlson J, Breidenstein C, Viltrop A, Höper D, Sauter‐Louis C, Beer M and Blome S, 2018. Deletion at the 5’‐end of Estonian ASFV strains associated with an attenuated phenotype. Scientific Reports, 8, 6510 10.1038/s41598-018-24740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SLR protocol
ASF survival data
Exp. Inf. data


