Abstract
The EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids was requested to consider evaluations of flavouring substances assessed since 2000 by the Joint FAO/WHO Expert Committee on Food Additives (JECFA), and to decide whether further evaluation is necessary, as laid down in Commission Regulation (EC) No 1565/2000. The present consideration concerns a group of four flavouring substances consisting of isopulegone and three other substances evaluated by JECFA at the 55th meeting. This revision is made due to additional toxicity data available for (1R,2S,5R)‐isopulegol [FL‐no: 02.067]. The substances were evaluated through a stepwise approach that integrates information on structure–activity relationships, intake from current uses, toxicological threshold of concern, and available data on metabolism and toxicity. p‐Mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is no longer supported by the flavour industry and was not evaluated. In agreement with JECFA, the Panel evaluated the candidate substances in this Flavouring Group Evaluation (FGE) via the B‐side of the Procedure. Based on a no observed adverse effect level (NOAEL) from a 90‐day oral toxicity study on [FL‐no: 02.067], adequate margins of safety for the three candidate substances could be calculated. Therefore, the Panel agrees with the JECFA conclusion, ‘No safety concern at estimated levels of intake as flavouring substances’ based on the maximised survey‐derived daily intake (MSDI) approach. Besides the safety assessment of these flavouring substances, the specifications for the materials of commerce have also been considered and found adequate. For the three substances evaluated in this FGE, use levels have become available and the modified theoretical added maximum daily intakes (mTAMDIs) were estimated. For [FL‐no: 02.067 and 07.067], the mTAMDI exceeds the toxicological threshold of concern for their structural classes and need more refined exposure assessment to finalise the evaluation.
Keywords: flavourings, isopulegone, isopulegol, isopulegyl acetate, pulegone, JECFA, FGE.57
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
The use of flavourings in food is regulated under Regulation (EC) No 1334/2008 of the European Parliament and Council of 16 December 20081 on flavourings and certain food ingredients with flavouring properties for use in and on foods. On the basis of Article 9(a) of this Regulation, an evaluation and approval are required for flavouring substances.
The Union list of flavourings and source materials was established by Commission Implementing Regulation (EC) No 872/20122. The list includes flavouring substances for which the scientific evaluation should be completed in accordance with Commission Regulation (EC) No 1565/20003.
On 29 January 2009, the EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) adopted an opinion on Flavouring Group Evaluation 57 (FGE.57): Consideration of two structurally related pulegone metabolites and one ester thereof evaluated by (JECFA) (55th meeting).4
In its opinion, the Panel stated that:
-
1
‐ It agreed with the application of the Procedure as performed by the JECFA for the three substances considered in this FGE until step B3. As no appropriate study could be identified to derive a no observed adverse effect level (NOAEL), the Panel concluded at step B4, contrary to JECFA, that for all three substances [FL‐no: 02.067, 07.067 and 09.219] additional toxicity data are required.
-
2
‐ For the three substances [FL‐no: 02.067, 07.067 and 09.219], evaluated through the Procedure, use levels are needed to calculate the modified theoretical added maximum daily intakes (mTAMDI) in order to identify those flavouring substances that need more refined exposure assessment and to finalise the evaluation.
-
3
‐ In order to determine whether the conclusion for the three JECFA evaluated substances can be applied to the materials of commerce, it is necessary to consider the available specifications. Specifications including purity and identity are available for one JECFA evaluated substance [FL‐no: 07.067]. Information on composition of mixture is incomplete for the other two substances [FL‐no: 02.067 and 09.219].
Thus, the Panel had reservations for all three substances. For two of the three substances, the composition of the mixture has to be specified [FL‐no: 02.067 and 09.219], and for all three substances [FL‐no: 02.067, 07.067 and 09.219], additional toxicity data are required.
Subsequently, the substances were included in the Union List with a Footnote 4.
On 20 December 2013 and on 29 January 2014, the applicant submitted additional relevant data for these 3 substances from FGE.57.
Terms of Reference as provided by the European Commission
The European Commission requests the European Food Safety Authority (EFSA) to evaluate this new information and, depending on the outcome, proceed to the full evaluation on this flavouring substance in accordance with Commission Regulation (EC) No 1565/2000.
1.2. Interpretation of the Terms of Reference
As additional genotoxicity data have been submitted, the European Commission requests EFSA to carry out a safety assessment on the flavouring substances [FL‐no: 02.067, 07.067 and 09.219] from FGE.57, in accordance with Commission Regulation (EC) No 1565/2000.
During the genotoxicity evaluation of p‐mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] in FGE.213Rev1 and FGE.213Rev2 (EFSA CEF Panel, 2014, 2015), the Panel noted that the chemical structure of the flavouring substance is more closely related to the structure of pulegone than to the structures used for the read‐across approach in FGE.213Rev1. In this case, the Panel decided to include also [FL‐no: 07.127] in the present revision of FGE.57Rev1.
p‐Mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is no longer supported by the flavour industry and will not be evaluated in the present revision.
2. Assessment
The approach used by EFSA for the safety evaluation of flavouring substances is referred to in Commission Regulation (EC) No 1565/2000, hereafter named the ‘EFSA Procedure’ (Appendix A). This Procedure is based on the Opinion of the Scientific Committee on Food (SCF, 1999), which has been derived from the evaluation procedure developed by Joint FAO/WHO Expert Committee on Food Additives (JECFA) (1995, 1996, 1997, 1999), hereafter named the ‘JECFA Procedure’. The Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (the Panel) compares the JECFA evaluation of structurally related substances with the result of a corresponding EFSA evaluation, focussing on specifications, intake estimations and toxicity data, especially genotoxicity data. The evaluations by EFSA will conclude whether the flavouring substances are of no safety concern at their estimated levels of intake, whether additional data are required or whether certain substances should not be evaluated through the EFSA Procedure.
The following issues are of special importance.
Intake
In its evaluation, the Panel as a default uses the maximised survey‐derived daily intake (MSDI) approach to estimate the per capita intakes of the flavouring substances in Europe.
In its evaluation, JECFA includes intake estimates based on the MSDI approach derived from both European and USA production figures. The highest of the two MSDI figures is used in the evaluation by JECFA. It is noted that in several cases, only the MSDI figures from the USA were available, meaning that certain flavouring substances have been evaluated by JECFA only on the basis of these figures. For Register substances for which this is the case, the Panel will need EU production figures in order to finalise the evaluation.
When the Panel examined the information provided by the European Flavour Industry on the use levels in various foods, it appeared obvious that the MSDI approach in a number of cases would grossly underestimate the intake by regular consumers of products flavoured at the use level reported by Industry, especially in those cases where the annual production values were reported to be small. In consequence, the Panel had reservations about the data on use and use levels provided and the intake estimates obtained by the MSDI approach. It is noted that JECFA, at its 65th meeting considered ‘how to improve the identification and assessment of flavouring agents, for which the MSDI estimates may be substantially lower than the dietary exposures that would be estimated from the anticipated average use levels in foods’ (JECFA, 2006).
In the absence of more accurate information that would enable the Panel to make a more realistic estimate of the intakes of the flavouring substances, the Panel has decided also to perform an estimate of the daily intakes per person using a mTAMDI approach based on the normal use levels reported by Industry.
As information on use levels for the flavouring substances has not been requested by JECFA or has not otherwise been provided to the Panel, it is not possible to estimate the daily intakes using the mTAMDI approach for the substances evaluated by JECFA. The Panel will need information on use levels in order to finalise the evaluation.
Threshold of 1.5 μg/person per day (step B5) used by JECFA
JECFA uses the threshold of concern of 1.5 microgram (μg)/person per day as part of the evaluation procedure:
‘The Committee noted that this value was based on a risk analysis of known carcinogens which involved several conservative assumptions. The use of this value was supported by additional information on developmental toxicity, neurotoxicity and immunotoxicity. In the judgement of the Committee, flavouring substances for which insufficient data are available for them to be evaluated using earlier steps in the Procedure, but for which the intake would not exceed 1.5 μg per person per day would not be expected to present a safety concern. The Committee recommended that the Procedure for the Safety Evaluation of Flavouring Agents used at the forty‐sixth meeting be amended to include the last step on the right‐hand side of the original procedure (‘Do the condition of use result in an intake greater than 1.5 μg per day?’) (JECFA, 1999).
In line with the Opinion expressed by the SCF (1999), the Panel does not make use of this threshold of 1.5 μg/person per day.
Genotoxicity
As reflected in the Opinion of the SCF (1999), the Panel has in its evaluation focussed on a possible genotoxic potential of the flavouring substances or of structurally related substances. Generally, substances for which the Panel has concluded that there is an indication of genotoxic potential in vitro will not be evaluated using the EFSA Procedure until further genotoxicity data are provided. Substances for which a genotoxic potential in vivo has been concluded will not be evaluated through the Procedure.
Specifications
Regarding specifications, the evaluation by the Panel could lead to a different opinion than that of JECFA, since the Panel requests information on e.g. isomerism.
Structural Relationship
In the consideration of the JECFA evaluated substances, the Panel will examine the structural relationship and metabolism features of the substances within the flavouring group and compare this with the corresponding FGE.
2.1. History of the evaluation of the substances in the Present FGE
In the scientific opinion on FGE.57 (EFSA, 2009), the Panel followed the JECFA approach for the evaluation of [FL‐no: 02.067, 07.067 and 09.219] and assessed the substances within the group of pulegone and menthofuran (JECFA, 2001a). However, none of the three substances is an α,β‐unsaturated compound like pulegone; moreover, there is no convincing evidence that pulegone and isopulegol are interconverted in vivo (or in vitro) (see Appendix C).
| FGE | Opinion adopted | Link | No. of substances |
|---|---|---|---|
| FGE.57 | 29 January 2009 | http://www.efsa.europa.eu/en/efsajournal/pub/1079.htm | 3 |
| FGE.57Rev1 | 31 January 2017 | http://www.efsa.europa.eu/en/efsajournal/pub/4727.htm | 4 |
For reasons detailed in Sections 2.2.1 and Appendix C, in the present revision of FGE.57, the Panel decided to deviate from JECFA and its previous evaluations and to no longer use pulegone as a supporting substance of (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219]. Nevertheless, the findings published on pulegone by the National Toxicology Program (NTP, 2011), the International Agency for Research on Cancer (IARC, 2014) and the European Medicines Agency (EMA, 2014) have been taken into consideration.
In 2011, NTP performed subchronic and chronic toxicity studies on pulegone (NTP, 2011), indicating that pulegone may cause bladder cancer in female rats. Based on the induced gene mutations observed when pulegone was tested in a bacterial reverse mutation assay, the authors linked the bladder tumours to the genotoxic potential of pulegone. Considering the above and also the reports by IARC (2014) and EMA (2014) on pulegone, the Panel concluded that the concern for genotoxicity of pulegone cannot be ruled out based on the lack of robust data.
In line with the NTP findings on pulegone and the fact that despite the absence of structural alert for isopulegone, reactive metabolites raising a genotoxicity concern might be formed from this substance via epoxidation, the Panel requested the applicant to test isopulegone [FL‐no: 07.067] in a bacterial reverse mutation assay and an in vitro micronucleus assay. Instead of isopulegone [FL‐no: 07.067], the flavour industry submitted data on the most available material, isopulegol [FL‐no: 02.067] (EFFA, 2016a). These data were acknowledged and considered by the Panel in the present revision of FGE.57 (FGE.57Rev1): a bacterial reverse mutation assay (Roy, 2015) and an in vitro micronucleus assay on (1R,2S,5R)‐isopulegol [FL‐no: 02.067] (Schulz, 2010).
p‐Mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is also going to be considered in the present revision of FGE.57. It is an α,β‐unsaturated alicyclic ketone evaluated in FGE.213Rev1, found to be structurally more closely related to pulegone than the supporting substances used to rule out the concern for genotoxicity in FGE.213Rev1 (EFSA CEF Panel, 2014, 2015). Because of its structural alert and structural similarity with pulegone, the Panel requested additional data on genotoxicity for the substance [FL‐no: 07.127]. Recent communication by the applicant, however, revealed that p‐mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is no longer supported by the flavour industry (EFFA, 2016b) and although included in the present revision, the substance will not be evaluated by the Panel.
In the scientific opinion on FGE.57, the Panel concluded that no NOAEL could be derived for (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219]. Accordingly, additional toxicity data were requested for the three substances.
In the present revision (FGE.57Rev1), a 14‐day range finding study (Mendes, 2012) and a 90‐day dietary study (Koetzner, 2013) have been provided for (1R,2S,5R)‐isopulegol [FL‐no: 02.067]. These studies, covering also the evaluation of the structurally related (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219], are evaluated by the Panel.
Information on the stereoisomeric composition of [FL‐no: 02.067, 07.067 and 09.219] and their use levels in food has also become available and included in the present output (EFFA, 2014, 2017).
2.2. Presentation of the substances in the JECFA Flavouring Group
2.2.1. Description
JECFA status
The JECFA Committee (JECFA, 2001a) has evaluated a group of six flavouring substances consisting of pulegone and five structurally related substances. The six substances are: pulegone, menthofuran, p‐mentha‐1,4‐(8)‐dien‐3‐one, (1R,2S,5R)‐isopulegol, (2R,5S)‐isopulegone and (1R,2S,5R)‐isopulegyl acetate (JECFA‐no: 753, 758, 757, 755, 754 and 756).
EFSA considerations
Pulegone (JECFA‐no: 753) and menthofuran (JECFA‐no: 758) are in Annex III of Regulation (EC) No 1334/2008 of the European Parliament and of the Council1 and accordingly cannot be used as chemically defined flavouring substances in the European Union (EU). p‐Mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is an α,β‐unsaturated ketone which has been considered together with other α,β ‐unsaturated ketones in FGE.213Rev1. The Panel decided to include [FL‐no: 07.127] in the present revision of this FGE because of its structural similarity with pulegone. However, meanwhile industry communicated that the substance is no longer supported (see Section 2.1). Therefore, [FL‐no: 07.127] will not be evaluated by the Panel. Thus, the current revision of FGE.57 deals with the evaluation of three flavouring substances [FL‐no: 02.067, 07.067 and 09.219].
Rational for rejecting pulegone as a supporting substance in this evaluation
Although it has been speculated that (2R,5S)‐isopulegone may be isomerised to pulegone (Gordon et al., 1987; Madyastha and Gaikwad, 1998), there is no convincing evidence for that since no experimental in vitro or in vivo data on metabolism could be identified to support the concept of interconversion of isopulegone to pulegone (for details see Appendix C).
Substances [FL‐no: 02.067, 07.067 and 09.219] are not α,β‐unsaturated compounds and therefore not sufficiently structurally related to pulegone for the latter to be a supporting substance. The additional data on genotoxicity submitted for isopulegol [FL‐no: 02.067] support this statement (see Section 4.3). Based on the above, the Panel decided that the findings on carcinogenicity for pulegone were not relevant for isopulegone. Consequently, pulegone is no longer considered as a supporting substance for the isopulegone‐related flavouring substances in FGE.57Rev1, contrary to what was done previously by JECFA (2001a) and the Panel (EFSA, 2009).
2.2.2. Isomers
Status
Substances with [FL‐no: 02.067, 07.067 and 09.219] evaluated by JECFA, have one or more chiral centres.
EFSA considerations
Adequate information on isomeric composition is available for all three substances (Table 1).
Table 1.
Specification summary of the substances in the FGE.57Rev1
| FL‐no JECFA‐no | EU Register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. indexd Spec. gravitye | EFSA comments |
|---|---|---|---|---|---|---|---|---|
|
02.067 755 |
(1R,2S,5R)‐Isopulegol |
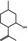
|
2962 2033 89‐79‐2 |
Liquid C10H18O 154.25 |
Slightly soluble Miscible |
218 IR 95% |
1.468–1.477 0.904–0.913 |
|
|
07.067 754 |
(2R,5S)‐Isopulegone |
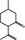
|
2964 2051 29606‐79‐9 |
Liquid C10H16O 152.24 |
Insoluble Miscible |
208 MS 95% |
1.465–1.473 0.925–0.932 |
|
|
07.127 757 |
p‐Mentha‐1,4(8)‐dien‐3‐one |
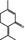
|
3560 11189 491‐09‐8 |
Liquid C10H14O 150.22 |
Insoluble Miscible |
233 MS 95% |
1.472–1.478 0.976–0.983 |
No longer supported by industry (EFFA, 2016b) |
|
09.219 756 |
(1R,2S,5R)‐Isopulegyl acetate |
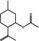
|
2965 2067 57576‐09‐7 |
Liquid C12H20O2 196.29 |
Insoluble Miscible |
232 IR 95% |
1.454–1.457 0.929–0.936 |
FL‐no: FLAVIS number; JECFA: the Joint FAO/WHO Expert Committee on Food Additives; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: identity; IR: infrared; MS: mass spectrometry.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1,013.25 hPa (1 atm), if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
2.2.3. Specifications
Status
Specifications for all JECFA substances evaluated in this FGE are reported in Table 1.
EFSA considerations
Specifications including complete purity criteria and identity are available for all substances (Table 1).
3. Intake estimation
3.1. Status
For all substances evaluated through the JECFA Procedure production volumes, based on which MSDI values can be calculated, are available for the EU, see Appendix E, Table E.1.
Table E.1.
Summary of the safety evaluation by JECFA (JECFA, 2001a)
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDI a US MSDI (μg/capita per day) | Class b Evaluation procedure path c | Outcome on the named compound [d or e] | EFSA conclusion on the named compound (Procedure steps, intake estimates, NOAEL, genotoxicity) | EFSA conclusion on the material of commerce |
|---|---|---|---|---|---|---|---|
|
02.067 755 |
1R,2S,5R‐Isopulegol |
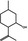
|
850 3,300 |
Class I B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
09.219 756 |
1R,2S,5R‐Isopulegyl acetate |
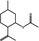
|
0.12 1.1 |
Class I B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
07.067 754 |
2R,5S‐Isopulegone |
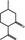
|
0.012 0.01 |
Class II B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
07.127 757 |
p‐Mentha‐1,4(8)‐dien‐3‐one |
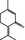
|
0.012 0.01 |
Class II B3: intake below threshold, B4: adequate NOAEL exists | No longer supported by the flavour industry (EFFA, 2016b) | No longer supported by the flavour industry (EFFA, 2016b) |
FL‐no: FLAVIS number; JECFA: Joint FAO/WHO Expert Committee on Food Additives; MSDI: maximised survey‐derived daily intake; NOAEL: no observed adverse effect level.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg per capita/day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
3.2. EFSA considerations
For all substances, industry has submitted production figures for the EU.
For (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219], the flavour industry submitted use levels for normal and maximum use (EFFA, 2017). Based on these normal use levels, the mTAMDI values were calculated. Isopulegyl acetate [FL‐no: 09.219] has mTAMDI intake estimate below the threshold of concern for its structural class. For (1R,2S,5R)‐isopulegol [FL‐no: 02.067] and (2R,5S)‐isopulegone [FL‐no: 07.067], the mTAMDI values are above the toxicological thresholds of concern for their structural classes of 1,800 and 540 μg/person per day, respectively. Therefore, for these two substances, more reliable exposure data are required in order to finalise their evaluation. On the basis of such additional data, [FL‐no: 02.067 and 07.067] should be reconsidered using the Procedure. Following this procedure, additional toxicological data might become necessary.
Use levels and mTAMDI values are presented in Appendix B, Tables B.1 and B.2.
Table B.1.
Normal and maximum use levels (mg/kg) for [FL‐no: 02.067, 07.067 and 09.129] in various food categories (EFFA, 2017)
| FL‐no | Food Categories | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal use levels (mg/kg) Maximum use levels (mg/kg) | |||||||||||||||||||
| 01.0 | 02.0 | 03.0 | 04.1 | 04.2 | 05.0 | 05.3 | 06.0 | 07.0 | 08.0 | 09.0 | 10.0 | 11.0 | 12.0 | 13.0 | 14.1 | 14.2 | 15.0 | 16.0 | |
| 02.067 | 12 | 10 | 50 | 5 | 14 | 5 | 5 | ||||||||||||
| 15 | 50 | 100 | 20 | 19 | 20 | 20 | |||||||||||||
| 09.129 | 5.1 | 6.1 | 3.1 | 6.5 | 1.4 | 1.4 | |||||||||||||
| 6.7 | 8.5 | 4.4 | 9.8 | 2.6 | 2.2 | ||||||||||||||
| 07.067 | 6.4 | 9.5 | 6.1 | 11 | 2.2 | 1.6 | |||||||||||||
| 11 | 12 | 7.0 | 15 | 3.0 | 2.6 | ||||||||||||||
Table B.2.
Estimated intakes based on the MSDI approach and the mTAMDI approach
| FL‐no | EU Register name | MSDI – EU (μg/capita per day) | mTAMDI (μg/person per day) | Structural class | Threshold of concern (μg/person per day) |
|---|---|---|---|---|---|
| 02.067 | 1R,2S,5R‐Isopulegol | 850 | 3,900 | Class I | 1,800 |
| 09.129 | 1R,2S,5R‐Isopulegyl acetate | 0.12 | 1,500 | Class I | 1,800 |
| 07.067 | 2R,5S‐Isopulegone | 0.012 | 2,500 | Class II | 540 |
MSDI: maximised survey‐derived daily intake; mTAMDI: modified theoretical added maximum daily intake.
4. Genotoxicity
4.1. Genotoxicity studies considered by JECFA (2001b)
No in vitro or in vivo genotoxicity studies were available on (1R,2S,5R)‐isopulegol [FL‐no: 02.067], 2R,5S‐isopulegone [FL‐no: 07.067] and 1R,2S,5R‐isopulegyl acetate [FL‐no: 09.219]. No conclusion was made by JECFA with respect to genotoxicity of (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219].
4.2. Genotoxicity studies on (1R,2S,5R)‐isopulegol [FL‐no: 02.067]
4.2.1. Bacterial reverse mutation assay
In order to investigate the potential of isopulegol (purity ≥ 99.4%) and/or its metabolites to induce gene mutations in bacteria, an Ames test was performed according to OECD Test Guideline 471 (OECD, 1997a) and following Good Laboratory Practice (GLP) in four strains of Salmonella typhimurium (TA98, TA100, TA1535 and TA1537) and Escherichia coli WP2uvrA, in the presence or absence of metabolic activation (S9‐mix) applying the standard plate incorporation method and the pre‐incubation test (Schulz, 2010). The first experiment (plate incorporation) was carried out using six different concentrations of isopulegol from 33 to 5,000 μg/plate. The second experiment was subsequently performed using the pre‐incubation test at concentrations of 10, 33, 100, 333, 1,000 and 2,500 with S. typhimurium strains, and 33, 100, 333, 1,000 and 5,000 μg/plate in E. coli. Appropriate positive control chemicals and dimethyl sulfoxide (DMSO) (as a vehicle control) were evaluated concurrently, and all test and control articles were evaluated in triplicate plates. All positive control chemicals induced significant increases in revertant colony numbers, confirming the sensitivity of the tests and the efficacy of the S9‐mix, while negative controls were within the historical control ranges. No precipitate was observed at any tested concentration in any tester strain with or without S9‐mix.
Toxicity, as evident by the absence or reduction in the mean number of revertant colonies and the absence or reduction in the background bacterial lawn, was observed in the plate incorporation test at 2,500 μg/plate and above in all tester strains in the presence and absence of S9‐mix; exception were TA1537 and WP2uvrA showing toxicity at 5,000 μg/plate. Applying the pre‐incubation assay, toxicity was observed at 1,000 μg/plate and above in S. typhimurium strains and 2,500 μg/plate and above in E. coli.
No increase in the mean number of revertant colonies was observed at any tested concentration in any tester strains with or without S9‐mix.
The Panel considered that isopulegol has no mutagenic activity under the conditions employed.
4.2.2. Micronucleus assay in vitro
Isopulegol (99.4% purity) was assayed for the induction of chromosome damage in mammalian cells by an in vitro micronucleus assay carried out according to OECD Test Guideline 487 (OECD, 2010) and following GLP. Human peripheral blood lymphocytes (HPBL) from healthy donors, stimulated with phytohaemagglutinin (PHA), were treated with isopulegol 48 h after culture initiation either for 4 h in the absence or presence of S9‐mix followed by 20 h recovery or for 24 h in the absence of S9‐mix (Roy, 2015). Cytochalasin B (final concentration of 6 μg/mL) was added to each culture after the 3 h treatment period, while in the 24 h treatment cultures were treated with the test article in the presence of cytochalasin B. Appropriate vehicle (DMSO) and positive controls were used (mitomycin C and vinblastine in the short and continuous treatment without S9‐mix, respectively; cyclophosphamide in the short treatment with S9‐mix). All positive control compounds induced a statistically significant increase in micronucleus frequency and the system was considered sensitive and valid. One thousand cells were scored in duplicate cultures (2,000 cells per concentration).
In a preliminary dose‐finding assay, performed at nine concentrations ranging from 0.154 to 1,540 μg/mL (10 mM), cytotoxicity was observed at the highest concentration in the short treatment with S9‐mix and 154 μg/mL and above after continuous treatment. Visible precipitate was observed in treatment medium at 1,540 μg/mL; at the end of the treatment period, all dose levels were soluble in treatment medium in all treatment conditions.
The concentration range suitable for the analysis of micronuclei in the main experiment was selected on the basis of these findings; however, due to toxicity pattern change, the experiment was repeated and the final concentrations tested in each experimental condition were as follows: (i) 250, 500, and 700 μg/mL for the short treatment with and without S9‐mix; (ii) 25, 50, and 100 μg/mL for the continuous treatment without S9‐mix. The levels of cytotoxicity (based upon cytokinesis‐blocked proliferation index (CBPI)) reached 58%, 57% and 50% at high concentrations in the three experimental conditions, respectively. In the repeat assay, the test substance was soluble in DMSO and in the treatment medium at all concentrations tested at the beginning and end of the treatment period. At the end of the short treatment with and without S9‐mix, haemolysis was observed at concentrations ≥ 900 μg/mL. No marked changes were observed with respect to osmolality and pH. No statistically significant increase in the frequency of micronuclei was observed after treatment with the test article at any concentration analysed.
The Panel considered that isopulegol did not induce micronuclei in cultured human peripheral blood lymphocytes under the conditions employed.
Therefore, the Panel concluded that, based on the bacterial gene mutation assay and the in vitro micronucleus assay, there is no concern with respect to genotoxicity of isopulegol [FL‐no: 02.067].
For a summary of in vitro genotoxicity data, see Appendix D, Table D.1.
Table D.1.
Genotoxicity Data on (1R,2S,5R)‐isopulegol from EFFA (EFFA, 2016a)
| Chemical name [FL‐no:] | Test system in vitro | Test object | Concentrations of substance and test conditions | Result | Reference | Comments |
|---|---|---|---|---|---|---|
|
(1R,2S,5R)‐isopulegol [02.067] |
Reverse mutation | S. typhimurium TA98, TA100, TA1535 and TA1537 | 0, 33, 100, 333, 1,000, 2,500 and 5,000 μg/plate | Negativea | Schulz (2010) | Test performed according to OECD Test Guideline 471 and GLP |
| 0, 10, 33, 100, 333, 1,000 and 2,500 μg/plate | Negativea , b | |||||
| Reverse mutation | E. coli WP2uvrA | 0, 33, 100, 333, 1,000, 2,500 and 5,000 μg/plate | Negativea | |||
| 0, 33, 100, 333, 1,000, 2,500 and 5,000 μg/plate | Negativea , b | |||||
| Micronucleus assay | Human peripheral blood lymphocytes | 250, 500 and 700 μg/mL | Negativea , c | Roy (2015) | Test performed according to OECD Test Guideline 487 and GLP | |
| 25, 50 and 100 μg/mL | Negatived |
FL‐no: FLAVIS number; OECD: Organisation for Economic Co‐operation and Development; GLP: Good Laboratory Practice.
With and without metabolic activation.
Assay modified with pre‐incubation.
4‐h treatment.
24‐h treatment, in the absence of S9‐mix.
4.3. EFSA considerations
Based on the results from the bacterial reverse mutation assays and the in vitro micronucleus assay, there is no concern for genotoxicity of (1R,2S,5R)‐isopulegol [FL‐no: 02.067] and the structurally related substances (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219].
5. Toxicity data on isopulegol
5.1. 14‐Day and 90‐day study on (1R,2S,5R)‐isopulegol [FL‐no: 02.067]
5.1.1. 14‐Day oral range‐finding toxicity study
A 14‐day range‐finding dietary study was performed with (1R,2S,5R)‐isopulegol [FL‐no: 02.067] (Mendes, 2012). The study was not in full compliance with GLP. Moreover, although it was stated to be performed according to OECD Test Guideline 407 (repeated dose 28‐day oral toxicity study in rodents), a full study according to OECD Test Guideline 407 would encompass a 28‐day exposure period and larger groups of animals. In addition, the number of parameters studied was far more limited than required for a full OECD Test Guideline 407 study (OECD, 2008).
CRL Sprague–Dawley CD® IGS rats (three per sex per group) were exposed to (1R,2S,5R)‐isopulegol (microencapsulated in acacia gum) via the feed at dietary levels of 2,400 (Group 2), 9,000 (Group 3) and 36,000 (Group 4) mg microencapsulated isopulegol‐containing acacia gum/kg feed. The control group received a feed containing 36,000 mg acacia gum/kg feed, (Group 1). The mean overall (days 0–14) daily intakes of (1R,2S,5R)‐isopulegol were 0, 230, 860 and 3,390 mg/kg body weight (bw) per day, in male rats and 0, 240, 860 and 3,150 mg/kg bw per day for the female animals. The study report is not explicit as the actual daily intake of isopulegol (neat) by the animals (on a mg/kg bw per day basis) was not provided.
The test substance was considered stable under the conditions of storage over the course of this study. Animals were observed daily for viability, signs of gross toxicity and behavioural changes, and on days 0, 7, and 14 for a battery of detailed observations. Body weights were recorded two times during the acclimatisation period including prior to test initiation (day 0), and on days 7, 10 and 14 prior to terminal sacrifice. Individual food consumption was also recorded to coincide with body weight measurements. No other parameters (e.g. urinalysis, haematology, histopathology, biochemistry) were studied.
There were no test substance‐related mortalities, clinical signs or macroscopic changes during this study.
In Group 4, female rats (36,000 mg/kg feed), (1R,2S,5R)‐isopulegol resulted in a statistically significant decrease in body weight gain from day 0–7 and for the overall study (days 0–14). Group 4 female rats also demonstrated a statistically significant reduction in food efficiency for the overall study (days 0–14). The decrease in Group 4 female rat body weight gain corresponded to reductions in food consumption and efficiency during the first half of the study. These reductions were partially made up in the latter half of the study, but overall do not adequately compensate for the initial loss. There were no statistical changes in body weight, body weight gain, food consumption or food efficiency associated with administration of (1R,2S,5R)‐isopulegol in male rats at all doses or in Groups 2 and 3 female rats.
5.1.2. 90‐Day oral toxicity study
A 90‐day dietary study was performed with (1R,2S,5R)‐isopulegol [FL‐no: 02.067] (Koetzner, 2013). The study was performed according to OECD Test Guideline 408 (OECD, 1998) and GLP.
CRL Sprague–Dawley CD® IGS rats (10 per sex per group) were exposed to (1R,2S,5R)‐isopulegol (microencapsulated in acacia gum) via the feed at dietary levels of approximately 3,000 mg (Group 3), 25,000 mg (Group 4) and 50,000 mg (Group 5) microencapsulated isopulegol‐containing acacia gum/kg feed; approx 20% of this material was isopulegol and 80% was acacia gum. Two control groups received a feed containing 0 or 50,000 mg acacia gum/kg feed (groups 1 and 2) without isopulegol. The test substance was considered stable under the conditions of storage over the course of this study. The mean overall daily intakes of microencapsulated isopulegol‐containing acacia gum were 0, 190, 1,750 and 3,500 mg/kg bw per day in male rats and 0, 190, 1,760 and 3,530 mg/kg per day for the female animals in groups 1 and 2 (controls), 3, 4 and 5, respectively.
There were no mortalities, clinical or ophthalmological changes attributable to (1R,2S,5R)‐isopulegol administration, and there were no consistent dose‐related or toxicologically relevant changes that could be attributed to exposure to isopulegol in clinical chemistry or urinalysis parameters. In males, there was a dose‐related decrease in eosinophils, which reached statistical significance in the highest dose group (down with 36%), compared with the vehicle control. Also, decreases in the other lymphocyte populations and in the total white blood cell counts were observed in the mid‐ and high‐dose group males. However, for these changes, statistical significance was not reached. There were no consistent dose‐related changes in other haematological parameters in males or in any of the haematological parameters in females.
Significant reductions in food consumption were observed in males administered the highest dose compared with basal and carrier control groups, and in all female groups administered (1R,2S,5R)‐isopulegol compared with the carrier control group. Significant decreases in male body weight and male and female body weight gain were considered the result of decreased food intake and related to the high dietary concentrations of (1R,2S,5R)‐isopulegol. There were no food consumption or food efficiency changes in carrier control group males and females compared with basal control groups for each sex. Since there were no consistent statistically significant changes in food efficiency in males and females, the body weight changes in males and females were considered to be related to the reduced feed intake and of no toxicological significance.
There were no clinical pathology or macroscopic findings attributed to the administration of (1R,2S,5R)‐isopulegol. Microscopic findings were observed in male rats administered (1R,2S,5R)‐isopulegol and included an increased incidence and severity of chronic progressive nephropathy and tubular hyaline droplets in Groups 4 and 5 male kidneys compared with the two control groups (1 and 2). These findings, along with granular casts in the renal tubules of males administered the highest dose of 3,500 mg/kg bw per day (1R,2S,5R)‐isopulegol, are consistent with alpha‐2u‐globulin nephropathy. There was no hyaline droplet accumulation or granular cast observation in the kidneys of the control group males or males administered the lowest concentration of (1R,2S,5R)‐isopulegol. There were no findings in the kidneys of basal control, carrier or (1R,2S,5R)‐isopulegol‐treated female rats, consistent with alpha‐2u‐globulin nephropathy. No other dose‐related histopathological findings were reported in either males or females.
Increased relative kidney weights in males administered the two highest dietary doses were considered the result of (1R,2S,5R)‐isopulegol administration, possibly related to the histological findings in the kidneys of male rats. Increases in relative male and female liver weights, at the two highest administered dietary doses, were without accompanying histological changes, and were considered toxicologically insignificant, also because in the clinical chemistry no indications of liver toxicity were observed.
No further evidence was provided that the renal changes in male rats were the result of (male rat specific) alpha‐2u‐globulin accumulation. Therefore, the Panel decided that these renal changes should be taken into account for the derivation of a NOAEL from this study, together with the decreased lymphocyte cell counts (observed in male rats at the two highest dose levels). Under the conditions of the study and based on the renal and haematological findings in male animals, the NOAEL for administration of (1R,2S,5R)‐isopulegol‐containing acacia gum in the diet was determined to be 190 mg/kg bw per day in male rats. Since the microencapsulated test material was analysed to contain 20% (1R,2S,5R)‐isopulegol (the rest was acacia gum), the equivalent NOAEL for (1R,2S,5R)‐isopulegol (neat) in the diet is calculated to be 38 mg/kg bw per day.
6. Application of the procedure
6.1. Application of the procedure to isopulegone and two structurally related flavouring substances by JECFA (2001a)
Step 1
According to JECFA, (1R,2S,5R)‐isopulegol [FL‐no: 02.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219] are allocated to structural class I and (2R,5S)‐isopulegone [FL‐no: 07.067] is allocated to structural class II (Cramer et al., 1978).
Step 2
At the estimated levels of intake, none of the three flavouring substances would be expected to saturate the available metabolic detoxication pathways, but they are not completely metabolised to innocuous products. JECFA evaluated these substances, together with pulegone via the B‐side of the Procedure.
Step B3
The daily per capita intakes of all the substances in this group are below the threshold for human intake for each class (class I, 1,800 μg; class II, 540 μg). Accordingly, evaluation of these substances proceeded to step B4.
Step B4
The lack of toxicity of pulegone at low levels of intake was demonstrated in a 90‐day study in rats fed peppermint oil that contained 1.1% pulegone. The no observed effect level (NOEL) of 40 mg/kg bw per day for nephropathy associated with hyaline droplets at a higher dose (Spindler and Madsen, 1992) corresponds to a NOEL of 0.44 mg/kg bw per day (26 mg/person per day) for pulegone. This NOEL is more than 10,000 times the intake of 0.033 μg/kg bw per day from use of pulegone as a flavouring agent. Since pulegone is metabolised to menthofuran [FL‐no: 13.035], data on pulegone can be used to evaluate the safety of menthofuran, although the latter was about three times more hepatotoxic after single doses (Gordon et al., 1982). (2R,5S)‐isopulegone [FL‐no: 07.067] was less hepatotoxic than pulegone after single doses. The NOEL of 0.44 mg/kg bw per day for pulegone in the 90‐day study is more than 1,000 times the daily intake of 0.4 μg/kg bw per day from use of menthofuran as a flavouring agent. (2R,5S)‐isopulegone [FL‐no: 07.067], (1R,2S,5R)‐isopulegol [FL‐no: 02.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219] are expected to be partly metabolised to menthofuran. Even if these compounds are assumed to be metabolised to menthofuran to the same extent as pulegone, however, the NOEL for pulegone is more than 10,000 times the daily intake from use of (2R,5S)‐isopulegone and (1R,2S,5R)‐isopulegyl acetate and is more than 1,000 times the daily intake from use of (1R,2S,5R)‐isopulegol as a flavouring agent.
In conclusion, JECFA evaluated all four substances as to be of no safety concern at the estimated level of intake as a flavouring substance based on the MSDI approach.
The evaluations of these flavouring substances by JECFA are summarised in Appendix E Table E.1.
6.2. EFSA considerations
The Panel agrees with JECFA (2001a) that the candidate flavouring substances in this FGE should be evaluated via the B‐side of the Procedure. However, the Panel decided that pulegone should not be considered as a supporting substance for the isopulegone‐related candidate substances in this FGE (see Section 2.2.1). A NOAEL of 38 mg/kg bw per day has become available for the candidate substance (1R,2S,5R)‐isopulegol which can be used to evaluate the three candidate substances in this FGE. Comparing the exposure estimates (MSDI) for (1R,2S,5R)‐isopulegol, (1R,2S,5R)‐isopulegyl acetate and (2R,5S)‐isopulegone [FL‐no: 02.067, 07.067 and 09.219] with the NOAEL of (1R,2S,5R)‐isopulegol, adequate margins of safety of 2.7 × 103, 1.9 × 107 and 1.9 × 108, respectively, can be calculated. Therefore, the Panel concluded that these three substances do not pose a safety concern at the current levels of intake as flavouring substances, based on the MSDI approach.
7. Conclusions
Following a request from the European Commission, the CEF Panel was asked to deliver a scientific opinion on the implications for human health of chemically defined flavouring substances used in or on foodstuffs in the Member States. In particular, the CEF Panel was requested to consider the JECFA evaluations of flavouring substances assessed since 2000, and to decide whether no further evaluation is necessary, as laid down in Commission Regulation (EC) No 1565/2000. These flavouring substances are listed in the Union List, which was adopted by Commission Regulation (EU) No 872/2012 and its consecutive amendments.
In FGE.57, EFSA considered a group of (2R,5S)‐isopulegone [FL‐no: 07.067] and three other substances, (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219] and p‐mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] evaluated by JECFA at its 55th meeting. Apart from [FL‐no: 02.067, 07.067, 07.127 and 09.219], two more substances were evaluated by JECFA in the group of pulegone and structurally related substances. These two substances, pulegone (JECFA‐no: 753) and menthofuran (JECFA‐no: 758), are in Annex III of Regulation (EC) No 1334/2008 of the European Parliament and of the Council1 and accordingly cannot be used as flavouring substances in the EU. p‐Mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127] is no longer supported by the flavour industry and has not been evaluated in this FGE. Therefore, the present revision of FGE.57, FGE.57Rev1, considers only three flavouring substances evaluated by JECFA.
Contrary to JECFA, the Panel decided not to use pulegone as a supporting substance for FGE.57Rev1.
On the basis of available data on (1R,2S,5R)‐isopulegol [FL‐no: 02.067], the Panel concluded that for (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219] there is no concern for genotoxicity and, therefore, they can be evaluated through the Procedure.
The Panel agrees with JECFA that these substances should be evaluated via the B‐side of the Procedure.
From a newly submitted 90‐day subchronic oral toxicity study, a NOAEL of 38 mg/kg bw per day can be derived for isopulegol. Based on this, for substances (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219], adequate margins of safety can be derived and these three substances would not pose a safety concern at the current levels of exposure, based on the MSDI approach.
In order to determine whether the conclusion for the three JECFA evaluated substances can be applied to the materials of commerce, it is necessary to consider the available specifications. Adequate specifications including complete purity criteria and identity became available for all the JECFA‐ substances evaluated in this FGE.
Thus, the Panel agrees with the JECFA conclusion on three substances [FL‐no: 02.067, 07.067, 09.219]: ‘No safety concern at estimated levels of intake as flavouring substances based on the MSDI approach’.
For (1R,2S,5R)‐isopulegol [FL‐no: 02.067], (2R,5S)‐isopulegone [FL‐no: 07.067] and (1R,2S,5R)‐isopulegyl acetate [FL‐no: 09.219], the flavour industry submitted use levels for normal and maximum use. Based on these normal use levels, the mTAMDI values were calculated. (1R,2S,5R)‐Isopulegyl acetate [FL‐no: 09.219] has the mTAMDI intake estimate below the threshold of concern for its structural class. For (1R,2S,5R)‐isopulegol [FL‐no: 02.067] and (2R,5S)‐isopulegone [FL‐no: 07.067], the mTAMDI values are above the toxicological thresholds of concern for their structural classes of 1,800 and 540 μg/person per day, respectively. Therefore, for these two substances more reliable data on use and use levels are required in order to finalise their evaluation. On the basis of such additional data, [FL‐no: 02.067 and 07.067] should be reconsidered using the Procedure. Following this procedure, additional toxicological data might become necessary.
Documentation provided to EFSA
EFFA (European Flavour Association), 2014. E‐mail from EFFA to FLAVIS Secretariat, Danish Food Institute, Technical University of Denmark, dated 2 June 2014. Information on substances [FL‐no: 02.067, 07.067, 07.127 and 09.219] in FGE.57Rev1. FLAVIS/8.241.
EFFA (European Flavour Association), 2016a. Updated Addendum of Additional Data Relevant to the Flavouring Group Evaluation of the Chemical Group 8 (Annex I of 1565/2000/EC) Consideration of Isopulegol evaluated by JECFA (46th meeting) as evaluated by EFSA in FGE.57 (2009). 02 September 2016. Unpublished report submitted by EFFA to EFSA.
EFFA (European Flavour Association), 2016b. Letter submitted from EFFA to EFSA in relation to the flavouring substance p‐mentha‐1,4(8)‐dien‐3‐one [FL‐no: 07.127]. 03 October 2016.
EFFA (European Flavour Association), 2017. Correspondence submitted from EFFA to EFSA in relation to the use levels of substances from FGE.57. 19 January 2017.
Koetzner L, 2013. Isopulegol (microencapsulated): a 90‐day dietary study in rats. Product Safety Labs. Study no. 35334. December 2, 2013. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Mendes O, 2012. Isopulegol: palatability/toxicity study: a 14‐day dietary study in rats. Product Safety Labs. Study no. 34780. September 28, 2012. Unpublished report submitted by EFFA to FLAVIS Secretariat.
Roy S, 2015. Isopulegol (CAS# 89‐79‐2): In vitro mammalian cell micronucleus assay in human peripheral blood lymphocytes (HPBL). Study number: AD90NJ.348.BTL. BioReliance Laboratories Ltd., Rockville, Maryland, USA. Unpublished report to the International Organization of the Flavor Industry, Brussels, Belgium.
Schulz M, 2010. L‐Isopulegol: Salmonella typhimurium/Escherichia coli reverse mutation assay (Standard Plate Test and Preincubation Test). Project No.: 40M0358/10M055. BASF SE, Ludwigshafen, Germany. Unpublished report provided by agreement to the International Organization of the Flavor Industry.
Abbreviations
- Bw
body weight
- CAS
Chemical Abstract Service
- CEF
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids
- CoE
Council of Europe
- DMSO
dimethyl sulfoxide
- EFFA
European Flavour Association
- EMA
European Medicines Agency
- FAO
Food and Agriculture Organization of the United Nations
- FEMA
Flavor and Extract Manufacturers Association
- FGE
Flavouring Group Evaluation
- FLAVIS (FL)
Flavour Information System (database)
- GC
gas chromatography
- GLP
Good Laboratory Practice
- HPBL
human peripheral blood lymphocytes
- HPLC
high‐performance liquid chromatography
- IARC
International Agency for Research on Cancer
- ID
Identity
- IOFI
International Organization of the Flavour Industry
- IR
infrared
- JECFA
Joint FAO/WHO Expert Committee on Food Additives
- MS
mass spectrometry
- MSDI
maximised survey‐derived daily intake
- mTAMDI
modified theoretical added maximum daily intake
- NOAEL
no observed adverse effect level
- NOEL
no observed effect level
- NTP
National Toxicology Program
- OECD
Organisation for Economic Co‐operation and Development
- PHA
phytohaemagglutinin
- SCF
Scientific Committee on Food
- WHO
World Health Organization
Appendix A – Procedure for the safety evaluation
1.
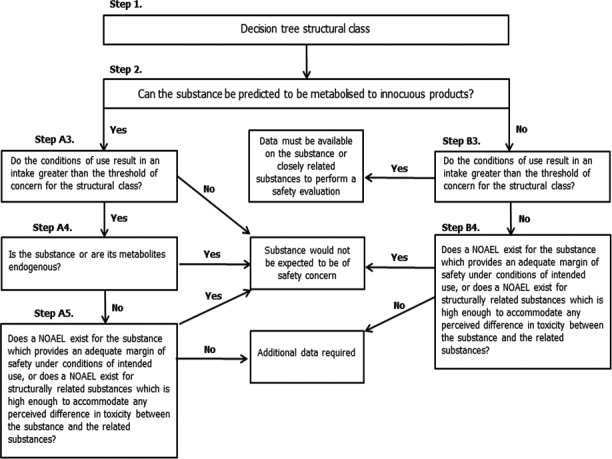
The approach for a safety evaluation of chemically defined flavouring substances as referred to in Commission Regulation (EC) No 1565/2000, named the ‘Procedure’, is shown in schematic form in Figure Figure A.1. The Procedure is based on the Opinion of the Scientific Committee on Food expressed on 2 December 1999 (SCF, 1999), which is derived from the evaluation Procedure developed by the Joint FAO/WHO Expert Committee on Food Additives at its 44th, 46th and 49th meetings (JECFA, 1995, 1996, 1997, 1999).
Figure Figure A.1.
Procedure for the safety evaluation of chemically defined flavouring substances
The Procedure is a stepwise approach that integrates information on intake from current uses, structure–activity relationships, metabolism and, when needed, toxicity. One of the key elements in the Procedure is the subdivision of flavourings into three structural classes (I, II and III) for which thresholds of concern (human exposure thresholds) have been specified. Exposures below these thresholds are not considered to present a safety concern.
Class I contains flavourings that have simple chemical structures and efficient modes of metabolism, which would suggest a low order of oral toxicity. Class II contains flavourings that have structural features that are less innocuous, but are not suggestive of toxicity. Class III comprises flavourings that have structural features that permit no strong initial presumption of safety, or may even suggest significant toxicity (Cramer et al., 1978). The thresholds of concern for these structural classes of 1,800, 540 or 90 μg/person per day, respectively, are derived from a large database containing data on subchronic and chronic animal studies (JECFA, 1996).
In step 1 of the Procedure, the flavourings are assigned to one of the structural classes. The further steps address the following questions:
Can the flavourings be predicted to be metabolised to innocuous products5 (step 2)?
Do their exposures exceed the threshold of concern for the structural class (steps A3 and B3)?
Are the flavourings or their metabolites endogenous6 (step A4)?
Does a NOAEL exist on the flavourings or on structurally related substances (steps A5 and B4)?
In addition to the data provided for the flavouring substances to be evaluated (candidate substances), toxicological background information available for compounds structurally related to the candidate substances is considered (supporting substances), in order to assure that these data are consistent with the results obtained after application of the Procedure.
The Procedure is not to be applied to flavourings with existing unresolved problems of toxicity. Therefore, the right is reserved to use alternative approaches if data on specific flavourings warranted such actions.
Appendix B – Exposure estimate
1.
Appendix C – Absorption, distribution, metabolism and elimination
1.
1.1.
1.1.1.
Interconversion of pulegone and isopulegone: the JECFA evidence
The Panel reassessed the earlier position of EFSA (taken over from a JECFA evaluation) that:
‘There are some indication that, to a small extent, isopulegone may be isomerised to pulegone (Gordon et al., 1987; McClanahan et al., 1988). Therefore, the Panel considered it relevant to include data on pulegone and the metabolically related menthofuran. Accordingly, in the consideration of the three substances in this FGE (isopulegol [FL‐no: 02.067], isopulegone [FL‐no: 07.067] and isopulegyl acetate [FL‐no: 09.219]) the Panel will take into account the SCF Opinion on pulegone and menthofuran (SCF, 2002), later revised by EFSA based on additional data (EFSA, 2005)’ (EFSA, 2009).
The above evaluations, based on a review by Speijers (2001) for the 55th JECFA meeting, deal with the safety evaluation of (iso)pulegone. They suggest that isopulegone and pulegone can be interconverted, in vivo, in animals.
In the Speijers review, however, no evidence is presented for conversion of isopulegone to pulegone. Indeed no such evidence could be retrieved from literature since publications on the metabolism of isopulegone were not found.
All evidence quoted to support the conversion of pulegone to isopulegone was based on the publication by Gordon et al. (1987). In this article, it is mentioned that ‘Isomerization of pulegone to isopulegone does occur during incubation of pulegone with mouse liver microsomes’. However, the authors do not give any evidence to support that statement, nor any reference to earlier findings either. In fact, no evidence from the literature could be retrieved to support this statement.
In a more recent review on pulegone metabolism (Gordon and Khojasteh, 2014), isopulegone is no longer included as a pulegone metabolite in the scheme of pulegone metabolism. Speijers (2001) refers to the publication by McClanahan et al. (1988), where it is presented the proposed mechanism of cytochrome P450‐catalysed formation of (deuterated) menthofuran‐d3 from pulegone‐d3. In this article, the potential conversion to isopulegone is not discussed.
In conclusion, no experimental data could be identified to support the concept of interconversion of isopulegone and pulegone.
Metabolism of isopulegone
No data on the metabolism of isopulegone could be retrieved from the literature. It can be assumed that cytochrome P450‐mediated oxidation may take place at various positions in the molecule, and that subsequent conjugation with sulfate or glucuronic acid occurs. However, no experimental data are available.
Metabolism of pulegone
Engel (2003) extensively studied the metabolism of pulegone in human volunteers. In this study, 35 mg of R‐(+)‐pulegone and 70 mg of S‐(–)‐pulegone were administered to three male and three female volunteers and urine was collected 24 h after oral dosing under carefully controlled conditions (Engel, 2003). The urine was treated with β‐glucuronidase/arylsulfatase to release conjugates, and extracted by diethyl ether into several fractions. It was compared to urine collected in a control period from the same volunteers. High‐performance liquid chromatography (HPLC) and high‐resolution gas chromatography–mass spectrometry (GC–MS) were used for the identification and the semiquantitative determination of the metabolites. The identification of all metabolites was confirmed by comparison with synthesised reference compounds. The major metabolites of S‐(–)‐pulegone are (conjugates of) 8‐hydroxymenthone (M1), 1‐hydroxymenthone (M2) and 10‐hydroxypulegone (M4). Minor metabolites are piperitone (M5) and 3‐p‐menthen‐8‐ol (M6). The author identified menthol (M3) as a pulegone metabolite. Interestingly, Engel (2003) found only traces of menthofuran in the urine. On eventual, other metabolites the author states: ‘there were traces of other compounds present only in test urines; however, due to their low amount it was impossible to identify them by interpreting the mass spectrometric data. Because their amount was much lower compared to the metabolites identified, they might also stem from impurities in pulegone and therefore might not be metabolites of the latter’. Therefore, Engel (2003), in this very thorough investigation, did not find isopulegone as a pulegone metabolite.
Chen et al. (2003) extensively investigated the disposition of R‐(+)‐pulegone in mice and rats and did not mention the presence of isopulegone.
Da Rocha et al. (2012) in a study on the action of R‐(+)‐pulegone on the urinary bladder evaluated the metabolism of pulegone, but did not mention isopulegone as metabolite in urine.
Thomassen et al. (1991) did not find isopulegone or isopulegol as biliary metabolites in rats orally administered with pulegone.
Madyastha and Gaikwad (1998) reported that they identified isopulegone in rat urine after dosing with S‐(–)‐pulegone. The amount of pulegone recovered was over 99%. To demonstrate the presence of isopulegone, the authors analysed the urine and identified the substance in ‘fractions containing small quantities of compounds’. The outcome of the GC–MS analysis revealed the presence of molecular ion peaks of six metabolites’ and isopulegone was one of them. The authors confirmed this analytical outcome by co‐injecting authentic standards along with the total fraction used for GC–MS analysis and observing the enhancement of the corresponding peaks. In any case and if indeed present, the quantity of isopulegone as a metabolite of pulegone was very minor. However, the Panel noted that in this publication it is not investigated the possibility that isopulegone identified in urine might be a small impurity of the initially administered pulegone preparation.
Conclusion
Based on the information presented above, the Panel concluded that there is no (convincing) evidence for interconversion of pulegone and isopulegone.
Appendix D – Genotoxicity
1.
Appendix E – Summary of the safety evaluation
1.
Suggested citation: EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids) , Silano V, Bolognesi C, Castle L, Cravedi J‐P, Engel K‐H, Fowler P, Franz R, Grob K, Gürtler R, Husøy T, Kärenlampi S, Milana MR, Penninks A, Tavares Poças MF, Smith A, Tlustos C, Wölfle D, Zorn H, Zugravu C‐A, Beckman Sundh U, Brimer L, Mulder G, Marcon F, Anastassiadou M, Carfí M and Mennes W, 2017. Scientific Opinion on Flavouring Group Evaluation 57, Revision 1 (FGE.57Rev1): consideration of isopulegone and three flavouring substances evaluated by JECFA (55th meeting). EFSA Journal 2017;15(3):4727, 24 pp. doi: 10.2903/j.efsa.2017.4727
Requestor: European Commission
Question numbers: EFSA‐Q‐2014‐00339, EFSA‐Q‐2014‐00340, EFSA‐Q‐2014‐00341 and EFSA‐Q‐2014‐00346
Panel members: Claudia Bolognesi, Laurence Castle, Jean‐Pierre Cravedi, Karl‐Heinz Engel, Paul Fowler, Roland Franz, Konrad Grob, Rainer Gürtler, Trine Husøy, Sirpa Kärenlampi, Wim Mennes, Maria Rosaria Milana, André Penninks, Maria de Fátima Tavares Poças, Vittorio Silano, Andrew Smith, Christina Tlustos, Detlef Wölfle, Holger Zorn and Corina‐Aurelia Zugravu.
Acknowledgements: The Panel wishes to thank the members of the Genotoxicity working group on flavourings: Mona‐Lise Binderup, Daniel Marzin and Pasquale Mosesso, the hearing experts Vibe Beltoft and Karin Nørby and EFSA staff Annamaria Rossi for the support provided to this scientific opinion.
Adopted: 31 January 2017
Notes
Regulation (EC) No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. OJ L 354, 31.12.2008, p. 34–50.
Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334/2008 of the European Parliament and of the Council and repealing Commission Regulation (EC) No 1565/2000 and Commission Decision 1999/217/EC. OJ L 267, 2.10.2012, p. 1–161.
Commission Regulation (EC) No 1565/2000 of 18 July 2000 laying down the measures necessary for the adoption of an evaluation programme in application of Regulation (EC) No 2232/96. OJ L 180, 19.7.2000, p. 8–16.
EFSA Journal (2009) ON‐1079, 1–17.
‘Innocuous metabolic products’: products that are known or readily predicted to be harmless to humans at the estimated intakes of the flavouring agent (JECFA, 1997).
‘Endogenous substances’: intermediary metabolites normally present in human tissues and fluids, whether free or conjugated; hormones and other substances with biochemical or physiological regulatory functions are not included (JECFA, 1997).
References
- Chen L‐J, Lebetkin EH and Burka LT, 2003. Metabolism of (R)‐(+)‐pulegone in F344 rats. Drug Metabolism and Disposition 29, 1567–1577. [PubMed] [Google Scholar]
- Cramer GM, Ford RA and Hall RL, 1978. Estimation of toxic hazard ‐ a decision tree approach. Food and Cosmetics Toxicology, 16, 255–276. [DOI] [PubMed] [Google Scholar]
- Da Rocha MS, Dodmane PR, Arnold LL, Pennington KL, Anwar MM, Adams BR, Taylor SV, Wermes C, Adams TB and Cohen SM, 2012. Mode of action of pulegone on the Urinary Bladder of F344 Rats. Toxicological Sciences 128, 1–8. doi: 10.1093/toxsci/kfs135 [DOI] [PubMed] [Google Scholar]
- EFSA , 2005. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food on a request from the Commission on pulegone and menthofuran in flavourings and other food ingredients with flavouring properties. EFSA Journal 2005;298:1–32 pp. doi: 10.2903/j.efsa.2008.298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA , 2009. Scientific Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in contact with food on a request from the Commission on Flavouring Group Evaluation 57: consideration of two structurally related pulegone metabolites and one ester thereof evaluated by JECFA (55th meeting). EFSA Journal 2009;ON‐1079:1–17. doi: 10.2903/j.efsa.2009.1079 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2014. Scientific Opinion on Flavouring Group Evaluation 213, Revision 1 (FGE.213Rev1): consideration of genotoxic potential for α,β‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.7 of FGE.19. EFSA Journal 2014;12(5):3661, 46 pp. doi: 10.2903/j.efsa.2014.3661 [DOI] [Google Scholar]
- EFSA CEF Panel (EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids), 2015. Scientific Opinion on Flavouring Group Evaluation 213, Revision 2 (FGE.213Rev2): consideration of genotoxic potential for α,β ‐unsaturated alicyclic ketones and precursors from chemical subgroup 2.7 of FGE.19. EFSA Journal 2015;13(9):4244, 49 pp. doi: 10.2903/j.efsa.2015.4244 [DOI] [Google Scholar]
- EMA (European Medicines Agency), 2014. Public statement on the use of herbal medicinal products containing pulegone and menthofuran. 24 November 2014 EMA/HMPC/138386/2005 Rev. 1. Committee on Herbal Medicinal Products (HMPC), 1–19.
- Engel W, 2003. In vivo studies on the metabolism of the monoterpene pulegone in humans using the metabolism of ingestion‐correlated amounts (MICA) approach: explanation for the toxicity differences between (S)‐(−)‐ and (R)‐(+)‐Pulegone. Journal of Agricultural and Food Chemstry, 51, 6589–6597. [DOI] [PubMed] [Google Scholar]
- Gordon P and Khojasteh SC, 2014. A decades‐long investigation of acute metabolism‐based hepatotoxicity by herbal constituents: a case study of pennyroyal oil. Drug Metabolism Reviews, 16, 1–9. [DOI] [PubMed] [Google Scholar]
- Gordon WP, Forte AJ, McMurtry RJ, Gal J and Nelson SD, 1982. Hepatotoxicity and pulmonary toxicity of pennyroyal oil and its constituent terpenes in the mouse. Toxicology and Applied Pharmacology, 65, 413–424. [DOI] [PubMed] [Google Scholar]
- Gordon WP, Huitric AC, Seth CL, McClanahan RH and Nelson SD, 1987. The metabolism of the abortifacient terpene, (R)‐(+)‐pulegone, to a proximate toxin, menthofuran. Drug Metabolism and Disposition, 15, 589–594. [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer), 2014. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 108.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1995. Evaluation of certain food additives and contaminants. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives. 14–23 February 1995. WHO Technical Report Series, no. 859. Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1996. Toxicological evaluation of certain food additives. Forty‐fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additives and contaminants. WHO Food Additives Series: 35. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1997. Evaluation of certain food additives and contaminants. Forty‐sixth report of the Joint FAO/WHO Expert Committee on Food Additives. Geneva, 6–15 February 1996. WHO Technical Report Series, no. 868. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 1999. Evaluation of certain food additives and contaminants. Forty‐ninth report of the Joint FAO/WHO Expert Committee on Food Additives. Rome, 17–26 June 1997. WHO Technical Report Series, no. 884. Geneva. [PubMed]
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001a. Evaluation of certain food addtives and contaminants. Fifty‐fifth report of the Joint FAO/WHO Expert Committee on Food Additives. WHO Technical Report Series, no. 901. Geneva, 6–15 June 2000.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2001b. Safety evaluation of certain food additives and contaminants. Fifty‐fifth Meeting of the Joint FAO/WHO Expert Committee on Food Additives, WHO Food Additives Series: 46. IPCS, WHO, Geneva.
- JECFA (Joint FAO/WHO Expert Committee on Food Additives), 2006. Sixty‐seventh Meeting. Rome, 20‐29 June 2006, Summary and Conclusions. Issued 7 July 2006.
- Madyastha KM and Gaikwad NW, 1998. Metabolic fate of S‐(–)‐pulegone in rat. Xenobiotica 28, 723–734. [DOI] [PubMed] [Google Scholar]
- McClanahan RH, Huitric AC, Pearson PG, Desper JC and Nelson SD, 1988. Evidence for a cytochrome P‐450 catalyzed allylic rearrangement with double bond topomerization. Journal of the American Chemical Society, 110, 1979–1981. [Google Scholar]
- NTP (National Toxicology Program), 2011. Toxicology and carcinogenesis. Studies of pulegone. (CAS NO. 89‐82‐7) in F344/N rats and B6C3F1 mice (gavage studies). National Toxicology Program, Research Triangle, NC, USA. TR‐563. NIH Publication No. 11‐5905. Available online: http://ntp.niehs.nih.gov/ntp/htdocs/LT_rpts/TR563.pdf [PubMed]
- OECD (Organisation for Economic Co/operation and Development), 1997a. Test No. 471: Bacterial Reverse Mutation Test. OECD Guideline for Testing of Chemicals. Section 4.
- OECD (Organisation for Economic Co/operation and Development), 1998. Test No. 408: Repeated Dose 90‐Day Oral Toxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co/operation and Development), 2008. Test No. 407: Repeated Dose 28‐day Oral Toxicity Study in Rodents. OECD Guidelines for the Testing of Chemicals, Section 4.
- OECD (Organisation for Economic Co/operation and Development), 2010. Test No. 487: In Vitro Mammalian Cell Micronucleus Test. OECD Guidelines for the Testing of Chemicals, Section 4.
- SCF (Scientific Committee for Food), 1999. Opinion on a programme for the evaluation of flavouring substances (expressed on 2 December 1999). Scientific Committee on Food. SCF/CS/FLAV/TASK/11 Final 6/12/1999. Annex I to the minutes of the 119th Plenary meeting. European Commission, Health & Consumer Protection Directorate‐General.
- SCF (Scientific Committee for Food), 2002. Opinion of the Scientific Committee on Food on pulegone and menthofuran (expressed on 2 July 2002). Scientific Committee on Food. SCF/CS/FLAV/FLAVOUR/3 ADD2 Final. 25 July, 2002. European Commission, Health & Consumer Protection Directorate‐General.
- Speijers GJA, 2001. WHO Food Additives Series 46: Pulegone and related substances. National Institute of Public Health and the Environment, Section on Public Health, Centre for Substances and Risk Assessment. Bilthoven, the Netherlands. Safety evaluation of certain food additives and contaminants. Fifty‐fifth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). WHO, Geneva 2001. Available online: http://www.inchem.org/documents/jecfa/jecmono/v46je10.htm
- Spindler P and Madsen C, 1992. Subchronic toxicity study of peppermint oil in rats. Toxicology Letters, 62, 215–220. [DOI] [PubMed] [Google Scholar]
- Thomassen D, Pearson PG, Slattery JT and Nelson SD, 1991. Partial characterization of biliary metabolites of pulegone by tandem mass spectrometry. Detection of glucuronide, glutathione, and glutathionyl glucuronide conjugates. Drug Metabolism and Distribution 19, 997–1003. [PubMed] [Google Scholar]


