Table E.1.
Summary of the safety evaluation by JECFA (JECFA, 2001a)
| FL‐no JECFA‐no | EU Register name | Structural formula | EU MSDI a US MSDI (μg/capita per day) | Class b Evaluation procedure path c | Outcome on the named compound [d or e] | EFSA conclusion on the named compound (Procedure steps, intake estimates, NOAEL, genotoxicity) | EFSA conclusion on the material of commerce |
|---|---|---|---|---|---|---|---|
|
02.067 755 |
1R,2S,5R‐Isopulegol |
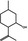
|
850 3,300 |
Class I B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
09.219 756 |
1R,2S,5R‐Isopulegyl acetate |
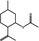
|
0.12 1.1 |
Class I B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
07.067 754 |
2R,5S‐Isopulegone |
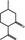
|
0.012 0.01 |
Class II B3: intake below threshold, B4: adequate NOAEL exists | d | No safety concern at the estimated level of intake based on the MSDI approach | No safety concern at the estimated level of intake based on the MSDI approach |
|
07.127 757 |
p‐Mentha‐1,4(8)‐dien‐3‐one |
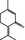
|
0.012 0.01 |
Class II B3: intake below threshold, B4: adequate NOAEL exists | No longer supported by the flavour industry (EFFA, 2016b) | No longer supported by the flavour industry (EFFA, 2016b) |
FL‐no: FLAVIS number; JECFA: Joint FAO/WHO Expert Committee on Food Additives; MSDI: maximised survey‐derived daily intake; NOAEL: no observed adverse effect level.
EU MSDI: Amount added to food as flavour in (kg/year) × 10E9/(0.1 × population in Europe (= 375 × 10E6) × 0.6 × 365) = μg per capita/day.
Thresholds of concern: Class I = 1,800 μg/person per day, Class II = 540 μg/person per day, Class III = 90 μg/person per day.
Procedure path A substances can be predicted to be metabolised to innocuous products. Procedure path B substances cannot.
No safety concern based on intake calculated by the MSDI approach of the named compound.
Data must be available on the substance or closely related substances to perform a safety evaluation.
