Abstract
Previous introductions of highly pathogenic avian influenza virus (HPAIV) to the EU were most likely via migratory wild birds. A mathematical model has been developed which indicated that virus amplification and spread may take place when wild bird populations of sufficient size within EU become infected. Low pathogenic avian influenza virus (LPAIV) may reach similar maximum prevalence levels in wild bird populations to HPAIV but the risk of LPAIV infection of a poultry holding was estimated to be lower than that of HPAIV. Only few non‐wild bird pathways were identified having a non‐negligible risk of AI introduction. The transmission rate between animals within a flock is assessed to be higher for HPAIV than LPAIV. In very few cases, it could be proven that HPAI outbreaks were caused by intrinsic mutation of LPAIV to HPAIV but current knowledge does not allow a prediction as to if, and when this could occur. In gallinaceous poultry, passive surveillance through notification of suspicious clinical signs/mortality was identified as the most effective method for early detection of HPAI outbreaks. For effective surveillance in anseriform poultry, passive surveillance through notification of suspicious clinical signs/mortality needs to be accompanied by serological surveillance and/or a virological surveillance programme of birds found dead (bucket sampling). Serosurveillance is unfit for early warning of LPAI outbreaks at the individual holding level but could be effective in tracing clusters of LPAIV‐infected holdings. In wild birds, passive surveillance is an appropriate method for HPAIV surveillance if the HPAIV infections are associated with mortality whereas active wild bird surveillance has a very low efficiency for detecting HPAIV. Experts estimated and emphasised the effect of implementing specific biosecurity measures on reducing the probability of AIV entering into a poultry holding. Human diligence is pivotal to select, implement and maintain specific, effective biosecurity measures.
Keywords: avian influenza, introduction, spread, mutagenesis, surveillance, biosecurity, zoning
Short abstract
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.5018/full
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1282/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1283/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1284/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1285/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1286/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1287/full
Summary
As a follow‐up to the highly pathogenic avian influenza (HPAI) H5N8 virus outbreaks in 2014/2015, the European Food Safety Authority (EFSA) has been requested by the European Commission to assess the risk of HPAI introduction into the European Union (EU) and into poultry holdings via wild birds and other possible entry routes, to assess the risk of low pathogenic avian influenza virus (LPAIV) introduction from the wild bird reservoir into poultry holdings and to assess the suitability of biosecurity, early detection and protection measures in poultry if there is HPAI occurrence in wild birds and the surveillance strategy. Additional questions were submitted to EFSA after the avian influenza (AI) outbreaks in France in 2015/2016, mainly to assess AI transmission characteristics and to analyse the mutation of LPAI to HPAI viruses as well as widening the questions on AI introduction and analysis of surveillance tools.
Mapping of HPAI and LPAI outbreaks
In the last decade, several clades of highly pathogenic avian influenza virus (HPAIV) H5 and members of the Eurasian lineage of HPAIV H7 have been detected in Europe. HPAIV H5 affected poultry and wild birds, whereas HPAIV H7 was only found in poultry. Distinct HPAI clades of goose/Guangdong‐like H5 subtype viruses are present in poultry populations in several subcontinental regions outside the EU, e.g. in South‐East and possibly Central Asia, China, Egypt and West Africa. None of the HPAI viruses recently detected in the EU revealed significant zoonotic potential, although highly zoonotic HPAIV are circulating in Asia (H7N9, H5N6 and H5N1) and in Egypt (H5N1) that may be introduced into Europe. The descendants of the original goose/Guangdong (gs/GD) HPAIV H5 clades 2.2.1.2, 2.3.2.1c and 2.3.4.4 were selected for detailed analysis of introduction via wild birds as these were considered the main virus types that could cause outbreaks in the EU in the near future.
H5 and H7 LPAI viruses are endemic in the European wild bird population. Potentially zoonotic LPAI viruses of subtype H9N2 (G1 lineage) are endemic in poultry in many parts of Asia, the Middle East and northern Africa. Although these viruses have not been detected so far in Europe, introduction is possible. LPAI viruses were analysed as one further group as in this scientific opinion they all have a similar impact on poultry.
Establishing a harmonised data collection system, integrating outbreak notification data, wild bird findings and epidemiological parameters, will aid in providing timely epidemiological analysis within the EU. It is also recommended that observations of global AI‐related epidemiology fare shared in due time to inform competent authorities and guide strategic preventive measures.
HPAI introduction via migratory and residential wild birds
Outbreaks of HPAI H5 in poultry in the EU since 2006 were initiated by primary incursions of infected migratory wild birds into Europe, but intrinsic generation from an LPAI precursor virus and secondary spread between poultry holdings has also been observed. Four potential different geographical routes for the entry of wild birds into the EU were defined here: the north‐eastern route (NE; EU border with Russia and Belarus), eastern route (E, EU border with Ukraine, Moldova, Black Sea, Turkey until the southern border of Turkey), southern route (S, EU border from the southern border of Turkey to the northern border of Portugal) and north‐western route (NW; EU border from north Portugal to north Russia). According to results obtained from the epizootic model generated in this opinion, the NE and E routes have been associated with a high risk of H5 HPAIV‐infected wild birds entering the EU. No H5 HPAIV incursion has been observed so far from the S and NW routes.
Upon introduction of HPAIV into a wild bird population within the EU, a critical number of wild birds is required before virus amplification and further wild bird‐associated geographical spread of the virus may take place. The lower the number of susceptible water birds entering daily, the later the prevalence starts to increase. Some scenarios are described in detail in the opinion to relative importance of different parameters, whereas extrapolation of the numbers to the real world is difficult given the model assumptions and high uncertainty around the data. An association was identified between the HPAIV occurrence in wild birds and the likelihood of infection of poultry holdings, which is supported by the association between detections in wild birds and poultry in the field. According to expert opinion, prevention of access of poultry to water bodies could result in a threefold reduction in HPAI entry probability. Combining this biosecurity measure with confining poultry to indoor housing was estimated to further reduce the HPAI entry probability twofold, and adding routine or high biosecurity would result in a further reduction of 4‐ and 44‐fold, respectively. The estimated effect of biosecurity measures was considered independently of the HPAI virus characteristics.
LPAI introduction via migratory and/or residential wild birds
For LPAIV, endemically infected wild bird populations play an important role as a source of primary incursions, but secondary spread by undiagnosed infected poultry flocks must be considered as well. According to results obtained from the epizootic model, LPAIV can reach similar maximum prevalence levels in wild bird populations when compared with HPAIV. At the same prevalence in the wild bird reservoir, the risk of LPAIV infection of a poultry holding was estimated to be lower than that of HPAIV. Experts considered the effect of implementing biosecurity on lowering the probability of LPAIV entry into a holding similar to that of HPAIV.
HPAI and LPAI introduction via non‐wild bird pathways
The risk of HPAIV and LPAIV introduction into the EU through non‐wild bird pathways is estimated to be lower compared with the wild bird pathway. The only non‐wild bird pathways that were considered to have a non‐negligible risk of HPAI introduction are intra‐EU movements and Third country trade of semen, intra‐EU movements of manure originating from holdings with Anseriformes species. For LPAI, the only non‐wild bird pathways that were considered to have a non‐negligible risk of introduction are intra‐EU movements of live poultry and day‐old chicks, intra‐EU movements of manure originating from holdings with any species. Introduction of AI into a poultry holding via feed and bedding was considered non‐negligible when accessible by wild birds during storage or any point during the transport route. Illegal introductions of HPAIV‐infected commodities (e.g. birds of prey, pet birds, unprocessed poultry meat) have been detected at the EU border. Risk assessments would benefit from studies analysing virus perseverance in semen and faeces/manure (unprocessed, storage, composting, effect of cleaning and disinfection procedures). It is also recommended that Member States (MSs) trading poultry semen report their national rules (based on OIE recommendations) and an estimate of the volumes traded so that a risk‐based approach can be developed to assess the risk of AI introduction and spread via semen.
HPAI and LPAI transmission and spread
The transmission rate between animals within a flock is assessed to be higher for HPAI viruses than LPAI viruses. In most cases, LPAIV remains restricted to a single farm, although horizontal spread has been observed in several occasions. Spread of HPAI viruses between farms is highly likely in the absence of control measures. It is recommended to perform epidemiological studies to obtain quantitative information on between‐flock transmission and to assess the effect of risk factors influencing between‐flock and between‐farm spread. Collection of standardised epidemiological data at the EU level from the holdings (e.g. location) and their houses (e.g. affected or not, number of susceptible birds, population structure) would be required on an ongoing basis.
Mutation from LPAI to HPAI
In very few cases, it could be proven that HPAI outbreaks were caused by intrinsic mutation of LPAIV to HPAIV and since 2005 the secondary spread of such HPAI viruses in the EU was limited except for one event, which has led to recurrent HPAIV outbreaks in a single region of France. No specific factors related to host species, environmental conditions or viral lineage were identified and likewise no molecular markers that would be useful predictors of increased risk of a specific LPAIV to mutate to an HPAI phenotype were identified. However, emergence of HPAI viruses from LPAI precursors in Europe has occurred more frequently for LPAI viruses of the H7 subtype than H5. Current knowledge does not allow a prediction as to if, and when, LPAI will mutate to HPAI. Standardising and connecting virological and epidemiological data collections across the EU and supporting research that applies a holistic approach to increase our ability to assess the role of specific viral, environmental and host factors on the pathogenicity evolution is recommended.
HPAI and LPAI surveillance and early detection
Introduction of HPAIV in gallinaceous poultry populations inevitably results in severe clinical disease and high mortality, whereas the clinical manifestation and mortality in anseriform poultry depends on the phenotypic characteristics of a HPAI virus. Passive surveillance through notification of suspicious clinical signs/mortality is the most effective method for early detection of HPAI outbreaks in gallinaceous poultry. For effective surveillance in anseriform poultry, passive surveillance through notification of suspicious clinical signs/mortality needs to be accompanied by active serological surveillance and/or a virological surveillance programme of birds found dead (bucket sampling). Pooling of such samples for polymerase chain reaction (PCR)‐directed diagnosis may be useful. Subclinically infected domestic Anseriformes have a higher likelihood of continued spreading of HPAIV when compared with clinically infected gallinaceous poultry, because it may go unnoticed. Therefore, MSs are recommended to focus their annual serological surveillance programme on Anseriformes and game bird populations.
Recognition and reporting of suspicion, sampling, testing and reporting of results is required to be done in a timely manner. Risk‐based surveillance is useful as it targets flocks where the likelihood of avian influenza virus (AIV) introduction is considered to be higher than average, although there is limited quantitative (EU‐wide) evidence to weigh the risk factors. Reporting of risk‐based surveillance approaches are not currently detailed enough to allow robust analysis and comparison of the results among MSs. There is currently a lack of data on non‐affected holdings and houses within the affected regions, which is required in order to establish the magnitudes of risk of infection for the various potential risk factors, such as location, holding‐ and flock sizes, biosecurity measures, etc. It is recommended to quantify the weighting of the risk factors used to design risk‐based surveillance and implement a detailed description of their use in national surveillance plans to facilitate analysis of the results and comparison of results among MSs.
The current serological surveillance programme is useful to detect major changes in regional LPAIV occurrence but results in the detection of active H5 or H7 infection only in a minority (around 20%) of follow‐ups conducted. The serological surveillance is unfit for early warning of LPAI outbreaks at the individual holding level but could be effective in tracing clusters of LPAIV‐infected holdings. Therefore, serosurveillance should aim at detecting clusters of LPAIV‐infected farms in order to identify those LPAI events with continuous between farm spread. Epidemiological follow‐up (tracing on/back) of serologically positive holdings should be carried out to determine if there is clustering of AIV‐infected holdings/flocks in space and/or time regardless of whether the seropositive birds are still at the holding or whether active virus infection has been detected. If the group (i.e. epidemiological unit such as shed or flock) of poultry that were sampled for serology are not available for PCR testing, then any other poultry (in particular seronegative birds) still remaining on that holding should be tested.
Passive surveillance is an appropriate method for HPAI surveillance in wild birds if the HPAIV infections are associated with mortality. Active wild bird surveillance efficiency is very low in detecting HPAI. When HPAIV has been detected in poultry within a given geographical area, active wild bird surveillance could play a role in detecting HPAIV infections in wild birds that are not associated with mortality as a possible source of virus introduction. A relative risk map of predicted H5 HPAIV occurrences in wild birds in Europe has been generated based on the wild bird events reported between 2005 and 2017, which could contribute to identification of priority locations in the EU where targeted active wild bird surveillance could be implemented during wild bird migration periods. Targeted active wild bird surveillance through virology tests (swabbing) combined with enhanced passive surveillance at a few priority regions in the EU may detect, if infection prevalence and sample sizes are sufficient, the presence of circulating AIV when these do not cause massive mortality among these birds. Serological analysis would be useful to increase our understanding of HPAIV dynamics in wild bird populations.
Biosecurity to prevent HPAI and LPAI entry and spread
Experts indicated that biosecurity measures play a key role in preventing AI spread from wild birds to poultry. Human diligence is pivotal to select, implement and maintain effective biosecurity measures. While certain general biosecurity principles universally apply to poultry holdings, unique features for each holding need to be considered for optimised protection.
According to expert opinion, the most feasible, sustainable and efficient measure to reduce the risk of AI entry in indoor poultry holdings is to prevent direct and indirect wild bird contact. Other measures with a high feasibility and sustainability are separation of waterfowl from other poultry species, provision of potable drinking water instead of surface water, the implementation of a hygiene lock for each poultry house, and biosecurity training of staff.
Outdoor poultry holdings bear an increased risk of AI incursions and the applicable biosecurity measures are more limited. According to expert opinion, restricting access of persons and providing biosecurity training are the most feasible, sustainable and efficient measures to reduce the risk of AI entry and spread in commercial holdings where poultry have access to outdoor areas.
For backyard holdings, experts assigned biosecurity training the highest overall rank to prevent AI entry and spread.
According to expert opinion, the highest ranked measures to prevent secondary spread of the virus are: to contain poultry and fomites (i.e. materials that were in contact with poultry) during transport, cleaning and disinfection of equipment, biosecurity training, cleaning and disinfection of transport vehicles, and the use of a hygiene lock for each poultry house. The risk of avian influenza virus (AIV) introduction and spread will remain high in production processes when: movement of animals, restricting access throughout the whole production cycle, and/or contact with wild birds is not reduced.
It is recommended at all times to restrict wild bird access to poultry holdings, avoiding the presence of open water bodies on the premises, feed should be provided indoors only, to implement hygiene locks, restrict access of people, and to limit contacts to other poultry holdings. Professional staff of poultry holdings should attend general biosecurity training but also receive holding‐specific biosecurity advice ideally from an expert (e.g. veterinarian) familiar with the particular holding. Also hobby keepers should receive information to achieve at least a minimal understanding of biosecurity to prevent entry and/or spread of AIV in their backyards and during markets and/or shows. Game bird hunting activities must be fully separated from rearing poultry and game birds should be tested for AI before release. Online biosecurity questionnaires could be used by farmers to check their current biosecurity level and subsequently to improve it based on the received feedback.
During high‐risk periods, it is recommended to prevent direct contact between wild birds and poultry through confinement, netting, or at least limitation of outdoor access area of domestic birds. Feed and water should be provided under a roof or a horizontal fabric. Biosecurity training and improved control of catching crews and other ‘mobile’ staff may be useful to limit indirect spread of HPAIV and LPAIV during large‐scale operations in commercialised poultry holdings.
Establishment of a control and monitoring area and risk zones
There is no scientific evidence to guide the sizes of a control and monitoring zone upon finding HPAIV in wild birds because it depends on the dynamics of the epizootic and the infected bird species. It is recommended that control and in particular monitoring areas are set up based on the ecological habitat and flight distance of the infected wild bird species. Setting up small‐sized restriction zones for the first cases at the beginning of a new wild bird epidemic may be instrumental in being able to implement increased surveillance activities in poultry in this zone. Informing poultry keepers on the detection of HPAIV in wild birds in the region will increase their awareness of the risk of virus introduction into their holding. In the progression of a wild bird epidemic, setting up wider zones, rather than a succession of small, restricted zones may be more appropriate. During an epidemic of HPAIV in wild birds, it is recommended that samples from new species and non‐previously reported areas be tested. Testing in reported areas can be restricted to check viral presence in relation to an ‘exit strategy’ from measures implemented. Sharing data and expert opinion at national and EU level on exit strategies would aid in terms of harmonised and structured approaches as well as interpretation of available data. Collaboration between authorities and stakeholders is crucial.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
1.1.1. EFSA‐Q‐2015‐00214 (April 2015)
The occurrence of highly pathogenic avian influenza (HPAI) outbreaks of the H5N8 subtype in Member States (MSs) triggered the immediate implementation of control measures according to Council Directive 2005/94/EC1. The Commission asked the European Food Safety Authority (EFSA) to issue a scientific report on the disease situation world‐wide and to assess possible virus entry routes into the European Union (EU) poultry holdings with a particular view to the role played by wild migratory birds.
Although there is knowledge about the direct or indirect migration routes from East Asia to Europe, several theories of HPAI H5N8 virus (and possibly other HPAI viruses) entry routes from East Asia into Europe involving infected migratory birds appear plausible. Transmission of HPAI H5N8 virus between different wild bird species at breeding and stopover places seems likely, but this theory needs further assessment. Also, the role of other virus entry routes such as through material contaminated by infected wild birds, human activities, movement of vehicles or equipment needs to be further examined for a more complete risk assessment on avian influenza virus (AIV) introduction into EU poultry holdings.
EU legislation on biosecurity and early detection measures to reduce the risk of HPAI H5N1 introduction into poultry holdings are laid down in Decision 2005/734/EC2, which sets out the criteria and risk factors to be considered by MSs when defining areas with an increased risk for avian influenza (AI) introduction into poultry holdings. The measures are intended to prevent contact between poultry and wild birds as well as separating domestic waterfowl from other poultry species. As the scope of those measures is limited to HPAI H5N1 it is necessary to assess the risk posed by other HPAI viruses and specifically HPAI H5N8 to verify if the provisions of Decision 2005/734/EC are suitable when facing further HPAI H5N8 outbreaks. In addition, Decision 2006/563/EC3 also provides for a comprehensive set of protection measures following HPAI H5N1 virus findings in wild birds. EFSA should assess if the measures in that Decision are properly addressing risks posed to poultry holdings when HPAI H5N8 and other HPAI viruses are detected in wild birds.
EU‐wide surveillance programmes for avian influenza in poultry and wild birds have been in place since 2003. Directive 2005/94/EC introduced a new legal basis for avian influenza surveillance which is first aimed at identifying the circulation of low pathogenic avian influenza (LPAI) viruses in different poultry species before they become widespread in the poultry population. It should contribute to the basis of a regularly updated risk assessment and to the current knowledge on the threats posed by wild birds in relation to any influenza virus of avian origin in birds. Following the HPAI H5N1 epidemic in 2006 and subsequent years, avian influenza surveillance was reviewed in the light of several EFSA Scientific Opinions, the work of the OIE‐FAO OFFLU initiative, the reports of the EU Reference Laboratory (EURL) for avian influenza and the input of the Task Force for Animal Disease Surveillance.
The revised guidelines for avian influenza surveillance laid down in Decision 2010/367/EU4 follow a risk‐based approach. The objectives shall provide for the most suitable surveillance strategy informing competent veterinary authorities on disease prevention and control purposes aimed at protecting poultry and other captive bird holdings from avian influenza infection. Following the current HPAI H5N8 outbreaks, it is deemed appropriate to assess if the EU strategy and guidelines for avian influenza surveillance are still suitable and sufficient, considering that active surveillance by laboratory testing of wild birds trapped or hunted is currently not foreseen in the EU‐approved surveillance programmes.
To this end, alternative surveillance designs based on active sampling of healthy wild birds to study the many different aspects of virus presence and characteristics should be considered within the context of risk management targeted to inform the risk manager in an efficient manner. Therefore, some principles of surveillance in wild birds and in poultry holdings need to be revised. In the light of the recent outbreaks, it is also necessary that EFSA studies certain aspects of the epidemiology of HPAI H5N8 virus which are related to biosecurity and confinement of poultry.
Control measures for LPAI outbreaks of the H5 and H7 subtypes were included in Directive 2005/94/EC as those avian influenza viruses have the potential to mutate to HPAI virus with possibly severe consequences for animal health and the poultry industry. The presence of LPAI viruses in the wild bird reservoir poses an ongoing risk for LPAI virus introduction into poultry holdings. A specific challenge for the management of biosecurity measures is to prevent contacts of wild birds with poultry constitutes holdings where poultry is kept in open air runs.
EFSA is therefore also requested to assess the risks of LPAI virus introduction into poultry holdings taking into account the conditions under which poultry is housed and the appropriate surveillance and biosecurity measures to be applied.
EFSA is requested to provide a scientific opinion in accordance with Article 29 of Regulation (EC) No 178/20025:
-
1
Assess the risks of introduction of HPAI H5N8 and possibly other HPAI viruses considering the possible entry routes into the EU.
-
2
Assess the risks posed by HPAI H5N8 and possibly other HPAI viruses for public and animal health and specifically with a view to assess the suitability of the provisions on:
biosecurity and early detection measures to reduce the risk of its introduction into poultry holdings laid down in Decision 2005/734/EC;
protection measures in poultry in case of its occurrence in wild birds laid down in Decision 2006/563/EC;
the surveillance strategy, in particular objectives and methodology, laid down in Decision 2010/367/EU.
-
3
Assess the current situation in the EU and elsewhere as regards the risk of a possible introduction of HPAI (H5N8) virus and possibly other HPAI viruses to EU poultry holdings.
-
4
Assess the continuous risk posed by LPAI (subtypes H5 and H7) for the introduction from the wild bird reservoir into poultry holdings taking into account risks for holdings where poultry is kept in open air runs and the suitability of surveillance and biosecurity measures aimed at protection of poultry against LPAI infection.
1.1.2. EFSA‐Q‐2016‐00348 (May 2016)
Highly pathogenic avian influenza is a highly contagious viral disease and causes high mortality in most bird and poultry species (except in many ducks and geese species). LPAI viruses mainly cause mild disease and may even remain undetected. Wild migratory birds are the natural reservoir for LPAI viruses. LPAI viruses of the H5 and H7 subtypes have the potential to mutate to HPAI viruses.
Until the adoption of Council Directive 2005/94/EC, EU control measures for avian influenza were only directed against HPAI.
Large HPAI epidemics world‐wide (e.g. USA/Pennsylvania 1983, Italy 1999/2000, the Netherlands 2003) that emerged by mutation from a circulating LPAI virus strain into its highly pathogenic form caused death and killing of more than 60 million poultry with devastating socioeconomic consequences. These experiences, supported by science including EFSA (2005) led to the introduction of control measures against LPAI viruses of the H5 and H7 subtypes into EU legislation.
Also, the World Organization for Animal Health (OIE) introduced in its Terrestrial Animal Health Code in addition to the existing recommendations for international trade for HPAI standards for LPAI and developed guidance on surveillance (OIE, 2010).
The EU control measures for LPAI and HPAI foresee the killing of all poultry on HPAI‐infected holdings. In case of LPAI infection, poultry may either be killed or be quarantined, further tested and may then go for slaughter under biosecure conditions. However, recently no MS has made use of the latter option.
LPAI‐infected poultry may not show clinical signs. It was therefore necessary to introduce compulsory EU‐wide active surveillance programmes to detect circulating LPAI virus and, in addition, circulating HPAI in domestic waterfowl as these species may not show disease even when infected with HPAI. The programmes are based on serosurveillance with virological follow‐up of positive results and are not aimed at early detection of infection. The surveillance programmes have been refined over the years defining the objectives and enabling targeting risk‐based strategies. Passive surveillance and early detection systems are complementing those active surveillance programmes. The variety of risk factors associated with different poultry species and production systems continues to make meaningful and affordable surveillance a challenge.
Surveillance for avian influenza has been carried out by MSs under cofinanced programmes since 2003, Directive 2005/94/EC with new control measures for HPAI and LPAI had to be implemented since mid‐2007. During these last 10 years, many MSs have made their own experiences with HPAI or LPAI outbreaks or have rehearsed the control measures in the framework of simulation exercises. Also, the entry of the HPAI H5N1 virus into Europe in 2005/2006, constituted an unprecedented event involving HPAI virus transmission mainly via wild migratory birds that became a prominent pathway for HPAI incursions prompting the adoption of a series of control measures.
The EU measures for the control of avian influenza have worked well so far, but the proportionality of some measures applied for HPAI and especially for LPAI remains a concern and should be based on risk assessment.
With respect to surveillance, the number of LPAI outbreaks in a country is considered to be primarily related to the monitoring intensity and quality of early warning procedures. Countries that have the most elaborate surveillance systems tend to detect LPAI incursions more frequently. This has also consequences for international trade. The OIE's LPAI free status in the Terrestrial Animal Health Code may not properly reflect the real LPAI status of a country considering the heterogeneity of LPAI surveillance systems implemented world‐wide ranging from almost non‐existing to the well‐structured active and passive surveillance programmes implemented in the EU. This status must also be seen against the background of the number of countries actually notifying LPAI to the OIE.
A question remains open as regards the extent intensive active and passive surveillance implemented in the EU has effectively led to preventing or reducing HPAI outbreaks by surveillance and control of LPAI outbreaks.
The new Animal Health Law Regulation (EU) 2016/4296 and its future delegated and implementing acts offer now the opportunity to review certain disease prevention and control measures.
Because of the above, and in accordance with Article 29 of Regulation (EC) 2002/178, the Commission asks EFSA for a scientific opinion and to specifically:
-
5
assess the different pathways, the most important routes and risk factors for avian influenza viruses ((a) HPAI and (b) LPAI) to enter poultry holdings in the EU including the threat posed by viruses circulating in wild birds;
-
6
assess the within‐flock, within‐farm and between‐farm transmission characteristics for both (a) HPAI and (b) LPAI viruses;
-
7
assess and, if possible, quantify the risk of mutation of a LPAI viruses to HPAI viruses and to identify the factors that influence the mutation frequency of avian influenza viruses in poultry flocks;
-
8
indicate which avian influenza surveillance tools are most suitable and which factors need to be taken into account for optimising an avian influenza surveillance programme.
1.2. Interpretation of the Terms of Reference
INTRODUCTION (Terms of Reference (TOR) 1, TOR 3, TOR 4 and TOR 5)
There is ample scientific evidence that HPAIV arises following adaptation and spontaneous mutation from LP precursor viruses, in particular during extended circulation and efficient replication in poultry. Metapopulations of wild birds, mainly of the Anseriformes and Charadriiformes orders, constitute the natural reservoir of LPAIVs, and virus transmission out of these reservoirs into poultry populations may start a sequence of events that culminates in the generation of AIV with an HP phenotype. Lateral spread of HPAI viruses in poultry may in turn lead to spill‐over transmission to wild bird species. Evidence has now accumulated indicating that HPAIV‐infected wild birds, depending on virus‐ and host species‐specific factors, may be responsible for long‐distance translocation of HPAIV leading to transmission to and outbreaks of HPAI in geographically distant poultry populations from outside the EU and among European countries (Lee et al., 2015; Verhagen et al., 2015).
Long‐distance geographical translocation of HPAIV by wild birds is envisaged to be associated with seasonal migration movements but may also be linked to shorter, regional movements of individuals within wild bird populations during the moulting period of some species (Reperant et al., 2010), or that are initiated by adverse weather conditions (cold spells, e.g. Ridgill and Fox, 1990).
The focus of the TOR 5 analysis is to present a global overview of HPAI and LPAI outbreaks during the previous 5 years, to underpin AI viruses that are included in a more detailed assessment of their risk of introduction during the next migration season(s) (TOR 1, TOR 2 and TOR 3). For HPAI, HPAI H7 viruses (including the recently emerged Chinese HPAIV H7N9) and three clades of Asian origin HPAI H5 viruses have been selected as they have already been reported within the EU (EU annual reports7 on active surveillance). On the LPAI side, viruses of subtypes H5 and H7 circulating in the EU were selected. LP viruses of subtype H7N9 of Chinese origin, and LPAI viruses of subtype H9N2, genogroup G1, are also taken into account. Although the latter two have not been reported in the EU so far, subtype H7N9 of Chinese origin bears substantial zoonotic potential and has recently mutated to a highly pathogenic phenotype that has spread among poultry in China and has already caused human cases of infection (Khan et al., 2015; Zhu et al., 2016, 2017). H9N2 genogroup G1 viruses, although they are not likely to mutate to HPAI, might well exert an economic impact on the European poultry industry if they were to be introduced (Pu et al., 2015). H9N2 viruses from other genogroups have been reported in Europe (Slomka et al., 2013; Lindh et al., 2014; Smietanka et al., 2014).
Different entry pathways are described via which infected wild birds can reach EU territory (HPAI, TOR 1 to TOR 5; LPAI, TOR 5) and the probability of introduction of the selected viruses during the next migration season is analysed by quantitative means when possible to estimate the probability of introduction. Furthermore, a map depicting the infection exposure likelihood in wild birds across the EU is provided (Si et al., 2010).
Assessing the risk of introduction into poultry holdings from wild bird reservoir is carried out quantitatively for those viruses for which data are available in the scientific literature. These will be used as a blueprint for comparison of viruses where data are insufficient to perform a quantitative assessment (HPAI, TOR 3 to TOR 5; LPAI, TOR 4 and TOR 5). In addition, a general model is generated to determine the influence of wild bird population density and composition on the probability of AIV incursion into a worst‐case poultry holding. Experts estimated the fold reduction of this probability when implementing four different levels of biosecurity. The model takes into account HPAIV amplification cycles in local wild bird populations after the introduction from sites outside the EU territory while LPAIV is considered to be endemic within the wild bird reservoirs of the EU territory, even though their subtypes may vary.
A qualitative assessment on the risk of AI introduction via non‐wild bird pathways is provided as well (HPAI, TOR 1 to TOR 3; LPAI, TOR 5), considering imported captive birds (for approved premises), pet birds, birds for competitions/exhibitions, imported live poultry (hatching eggs, day‐old and older birds) and imported poultry products (meat, eggs for consumption, semen, bedding, manure, feathers and down and poultry feed) as defined in previous EFSA Scientific Opinions (EFSA, 2006a,b, 2008). For manure, only movements, of unprocessed manure, within the EU are considered as the animal by‐product Regulation (EU) No 2011/1428 Article 25 prohibits imports from Third countries. The volume of traded/imported commodities is taken into account where data are available.
TRANSMISSION AND SPREAD (TOR 6)
Transmission of infection depends on characteristics of both the virus and the host. Combined they shape the likelihood of a bird becoming infected (dose–response curve) and the infectivity of a bird upon infection (excretion kinetics and total amount of virus shed by the bird). In concert with the contact network between birds, they determine the rate of transmission between individual birds. In addition, between‐flock transmission is determined by the number of birds infected in a flock (measure of the infectivity of a flock) and the contact network between flocks. Finally, at the level of farms, the total number of infected flocks on a farm is determining the infectivity of a farm and the contact network between farms links this to susceptible farms. To address TOR 6, it is assessed whether there are differences between HPAI and LPAI transmission dynamics in poultry.
Transmission parameters are identified for LPAIV and HPAIV between animals, between houses and between farms (different locations). Likely transmission parameters to be used are the transmission rate parameter (number of new infections per infectious bird/flock/farm per day), reproduction ratio (number of new infections per infectious bird/flock/farm during its entire infectious period) and the duration of infection. In addition, factors influencing transmission at the three levels will be identified. At the within‐flock (= between‐animal level), these are virus strain, poultry type (species/genotype, age, constitution, etc.) and possibly housing factors like population density/outdoor access, caging systems, etc. At the level of flocks (= between houses on a farm), these are (in addition to the factors influencing transmission between animals) flock size and information on the contact network between the houses (outdoor access or not) and biosecurity measures applied. At the level of farms (= between farms), these are (in addition to the factors influencing transmission between animals and flocks) farm size and information on the spatial relation and contact network between the farms and biosecurity measures applied.
This assessment does not include modelling the transmission across Europe because available models do not allow extrapolation beyond the local situation they have been developed for (Lycett et al., 2016).
In Section 3.5, a One Health perspective is briefly mentioned, since collection of data on AI spread among animals will also facilitate assessing human exposure risks to AI. Assessment on the zoonotic aspects of AI is beyond the mandate and will not be further analysed.
LPAI MUTATION TO HPAI (TOR 7)
Pathogenicity of AIV is measured by the intravenous pathogenicity index (IVPI) as laid down in Commission Decision 2006/437/EC9. HPAIVs can cause massive mortality, in particular in gallinaceous poultry. The amino acid sequence at the endoproteolytical cleavage site (CS) in the viral haemagglutinin protein (HA) can alternatively be used as a molecular marker of the HP phenotype for viruses of subtypes H5 and H7 (Commission Decision 2006/437/EC; Franҫa and Brown, 2014). All HP AIV found in the field so far were of the H5 or H7 subtype.
Published experimental data and outbreak analyses were screened and data were extracted (i) to describe the current understanding of the mechanisms that govern pathogenicity of AIV in poultry and (ii) to identify intrinsic and extrinsic factors which might influence LP‐to‐HP mutation rates.
SURVEILLANCE AND EARLY DETECTION (TOR 2, TOR 4 and TOR 8)
HPAI but not LPAI viruses can cause devastating disease and gross economic losses in most poultry species except domestic waterfowl. Therefore, HPAI detection in the EU historically was based on passive surveillance. In gallinaceous poultry, HPAI is mainly identified via passive surveillance as the virus causes overwhelming clinical disease and high mortality that is easily detectable (Franҫa and Brown, 2014). Conversely, HPAIV infections in Anseriformes may or may not lead to clinical signs (Kim et al., 2011; Lee et al., 2011), hence, passive surveillance may miss clinically mild or inapparent forms of infection and a more crucial role of active surveillance in domestic Anseriformes ensues. The surveillance components are described and the suitability of the current 10 HPAI serosurveillance in gallinaceous and Anseriformes poultry (Commission Decision 2010/367/EU) is analysed.
AI surveillance in both wild birds and poultry in the EU was broadened following outbreaks of HPAI which arose by mutating from LPAIV precursors in poultry (Italy 1999–2001, see; Netherlands 2003, see Stegeman et al., 2004; Monne et al., 2014) and following outbreaks caused by HPAIV H5N1 which was most likely introduced by wild birds (Starick et al., 2008) (Commission Decision 2010/367/EU). HPAI surveillance in wild birds was introduced, first in an active and passive manner but was later reduced to passive surveillance only as based on previous field experience which was, however, restricted to a single HPAIV H5 clade only (H5N1, clade 2.2). The suitability of the current HPAI surveillance system in wild birds (Commission Decision 2010/367/EU) is analysed (TOR 2 to TOR 8).
The ultimate objective of implementing LPAI surveillance in poultry is the detection of LPAIV of subtypes H5 and H7 before they mutate to HPAIV, as it was formulated in Commission Decision 2007/268/EC11. The current Decision 2010/367/EU describes LPAIV surveillance of subtypes H5 and H7 in gallinaceous birds (namely chickens, turkeys, guinea fowl, pheasants, partridges and quails) to support disease control. H9 surveillance is not included in the EU legislation nor OIE standards since it has not been reported to mutate into HPAIV and hence is not considered to induce HPAI‐like disease in poultry although tangible economic losses may ensue.12 The suitability of the current LPAI surveillance system to detect H5 and H7 LPAIV in poultry to prevent HPAI outbreaks is analysed (TOR 4 to TOR 8).
Recommendations to optimise detection of circulating AI viruses in the EU are provided, in particular regarding annual serosurveillance programs. The absence of detailed scientific data (at least at EU level) hampers the ability to weight the relative importance of risk factors and hence provide recommendations to harmonise risk‐based surveillance (RBS) approaches. Further development of risk‐based methodology could be possible after a period of detailed reporting by the MSs on the relative weights of risk factors currently used and the scientific evidence available at national level to underpin these weights. Establishing a RBS framework at EU level would help the analysis of RBS outcomes and their comparison among MSs. However, it is not expected that scientific evidence will become available to support a uniform AI surveillance approach for all MSs given the difference in AI history between MSs and the heterogeneity of poultry populations across the EU.
The early detection measures described in Commission Decision 2005/734/EC have been reviewed mainly based on the HPAI H5N8 outbreaks in the period October–November 2016 (EFSA, 2016a) taking into account the global processes of HPAI H5 evolution. In the present scientific opinion, recommendations are provided aimed at refining the existing early detection systems using voluntary surveillance components like poultry production parameter monitoring, bucket sampling and testing to exclude notifiable avian diseases.
BIOSECURITY (HPAI, TOR 2; LPAI, TOR 4)
The effects of biosecurity measures on mitigating the risk of HPAI/LPAI introduction into a poultry holding are pleiotropic in character and, so, difficult to quantify. Expert knowledge has been elicited (EKE) to identify and rank biosecurity measures applicable in commercial chicken holdings (EFSA, 2016a). In this opinion, an additional EKE has been performed to further analyse effects of biosecurity measures in indoor versus outdoor commercial and non‐commercial chicken production. The main objective was to identify and describe a set of biosecurity measures that can be applied in practice to reduce risks of AI entry and spread in high‐risk periods, for instance when an infected wild bird has been found in the area. Biosecurity should therefore aim at improving bioexclusion and biocontainment of poultry holdings. Biosecurity implementation in zoos and by poultry dealers have been discussed, as well as the effect of hunting and use of live decoys. Finally, a brief gap analysis is provided on data required to underpin future assessments on the effect of biosecurity measures in an evidence‐based way.
ESTABLISHMENT OF A CONTROL AND MONITORING AREA (TOR 2)
The provisions regarding the establishment of a control and monitoring area after HPAI detection in a wild bird (Commission Decision 2006/563/EC) have been analysed and described in the AI Statement (EFSA, 2016a). In this scientific opinion, the zoning around wild bird cases and poultry outbreaks are assessed based on the analysis of the AI outbreaks occurring in the EU between October 2016 and April 2017 (EFSA, 2017b). Recommendations are provided on how to perform a risk assessment on the actual usefulness of zoning measures, the timing and lifting of protection measures, aiming to provide science‐based advice to risk managers.
2. Data and methodologies
2.1. Approach to map HPAI and LPAI outbreaks (TOR 5)
The objective was to map the HPAI and LPAI outbreaks over the last 5 years with the aim of underpinning viruses selected for detailed analysis as they might cause outbreaks in the EU in the near future. Presenting findings in wild birds is included as scientific evidence when explaining why the opinion focuses on the AI introduction pathway via wild birds.
Data sources used were the Animal Disease Notification System (ADNS); EMPRES‐i database; OIE WAHID database; AI Consortium database; scientific literature, in particular observational studies; FAO Empres‐i situation update reports and ECDC rapid risk assessments, in particular for H7N9.
Viruses were selected to be assessed in TOR 1 and TOR 3 (might cause outbreaks in the EU in the near future): merging or dissecting separately certain virus groups and clades in the analysis such as HPAI viruses with distinct pathogenicity (e.g. H5N8 2.3.4.4), HPAIV with distinct geographic bindings (e.g. H5N1 2.2.1.2; G1‐like H9N2), and HPAIV that evolved from a common group of LPAI precursor viruses already endemic in the EU territory (e.g. all HPAIV of subtype H7 reported in the EU). The outcome is described in Section 3.1.
2.2. Approach to assess AI introduction into the EU by migratory wild birds (HPAI, TOR 1 and TOR 5; LPAI, TOR 5) and subsequently into a poultry holding (HPAI, TOR 3 and TOR 5; LPAI, TOR 5)
For HPAI, the objective was to assess the risk of virus introduction as the probability that at least one infected wild bird enters the EU and to provide a predictive risk map of HPAI occurrences in wild birds in Europe. Subsequently, the cumulative (seasonal) probability of poultry holdings without biosecurity becoming infected with HPAI was determined.
Four different risk pathways were defined, based on the entry routes of migratory wild birds crossing the EU border (Section 3.2.1). A mathematical (epizootic) model was generated, consisting of three components: entry assessment, AI dynamics in the wild bird reservoir, and exposure assessment with poultry holding incursions (see detailed description in Appendix C).
The parameters were informed based on: (i) data from experimental and observational studies collected by the Consortium ((Duncan et al., 2017a,b; data received as per procurement, Erasmus University Medical Centre, OC/EFSA/ALPHA2015/01, unpublished Gonzales; and data received as per procurement, coordinated by Linnaeus University SE) and Erasmus Medical Centre (NL), OC/EFSA/ALPHA 2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished) and the Working Group (WG) experts (see Appendix D), and (ii) EKE (semi‐formal and formal), performed using the Sheffield method. Parameters have been estimated by experts as probability distribution reflecting the uncertainty around the unknown true values (see Section C.6 in Appendix C). The model was used to assess entry of HPAIVs via the NE entry route and the outcomes are described in Section 3.2.2.
The probability of HPAIV entry via the E, S and NW routes was assessed in a comparative manner, considering the model outcome for HPAI clade 2.3.4.4. for the NE route as the benchmark. The probability terms used are defined in Table 1. The outcomes are described in Sections 3.2.3–3.2.6.
Table 1.
Definitions of probability terms used to describe the risk of HPAI introduction for a given clade and entry route in comparison to the benchmark (HPAI clade 2.3.4.4 entry via the NE route)
| Probability term | Subjective probability range |
|---|---|
| Extremely high/low | > 1,000‐fold the benchmark valuea |
| Much higher/lower | Between 10‐ and 1,000‐fold the benchmark valuea |
| Higher/lower | Between 3‐ and 10‐fold the benchmark valuea |
| Slightly higher/lower | Between 1.5‐ and 3‐fold the benchmark valuea |
| Similar | Up to 1.5‐fold the benchmark valuea |
The benchmark value is the model outcome on HPAI clade 2.3.4.4 entry into the EU via the NE route (see Section 3.2.2).
For LPAI, entry into the EU was not assessed as it is considered to be endemic. Only a description of new viruses that could enter the EU in the future is provided (see Section 3.3.1). The epizootic model was also used to simulate entry of LPAIV introduction into a new foraging area and the resulting probability of a poultry holding to become infected. The parameters were also informed on extracted data and EKE (see Section C.7 in Appendix C). The results are described in Section 3.3.2.
A sensitivity analysis was run for HPAI and LPAI to assess the importance of input variables on the model outcome (see Appendix E).
2.3. Approach to assess AI introduction by non‐wild bird pathways
The objective was to perform a brief qualitative assessment on the risk of AI introduction via non‐wild bird pathways in two scenarios: (i) Third country trade and (ii) intra‐EU movements. The focus of the current scientific opinion is on analysing scientific evidence suggesting a reduction in efficacy of HPAI/LPAI passive surveillance in: (i) wild bird and (ii) poultry populations due to altered pathogenesis and or epidemiology of the selected HPAI viruses. The exposure of such groups to the different trade pathways will therefore be part of the evidence assessment for surveillance.
Ten pathways and the corresponding commodities other than wild birds, by which there is potential to introduce AIV into a commercial poultry holding have been identified and qualitatively assessed for the two pathogenic groups of viruses (HPAI and LPAI), in two scenarios (third country trade and intra‐EU movements) with a description of the entry and exposure to poultry pathways, given the EU rules and requirements for trade (see Section 3.4). Illegal import is briefly described in the text but is not included in the qualitative assessment due to a lack of data.
Data were extracted from the UN ComTrade database (the United Nations International Trade Statistics Database) for various commodity codes related to trade in live poultry, captive birds, hatching eggs, day‐old chicks, birds as pets, products of poultry origin for human consumption, poultry by‐products, pharmaceuticals, germinal products, manure, bedding and poultry feed. The EUR‐LEX database for all relevant legislation related to the legal trade and intra‐EU movement of such commodities. An average of 3 years annual trade was used to give an average annual volume for either import into the EU or intra‐EU movements. Literature searches have been performed for the identified commodities and information was extracted on HPAI findings in different matrices, preservation of HPAIV in such matrices and clinical signs. The EU legislation and OIE documents have been used to identify checks and certificate requirements for consignments. Detailed information is provided in Appendix F.
For each scenario (commodity, HPAI or LPAI, Third country or intra‐EU trade), the WG identified the tests that can be used and assessed the probability of testing, the probability of virus detection, the probability of virus preservation during transport, the probability of poultry exposure to the commodity (see Table F.1, Appendix F) and the probability of AIV introduction into a commercial poultry holding (see Section 3.4). Each probability was assessed separately13 by discussion among the experts and reaching consensus. The used probability terms and corresponding subjective probability ranges are shown in Table 2.
Table F.1.
Clinical signs and/or cause of death of birds of prey reported in the scientific literature (2005–2015)
| Virus type, clade | Species | Infection | Clinical signs and/or cause of death | Reference |
|---|---|---|---|---|
| HPAI H5N1, 2.2 | Common buzzard, peregrine falcon | Natural | Encephalitis | Van den Brand et al. (2015) |
| HPAI H5N1, 2.3.2 | Common buzzard | Natural | No signs of infection | Reid et al. (2011) |
| HPAI H5N1, 2.3.2.1.c | Gyr falcons and hybrids of gyr and peregrine falcons | Natural | Signs of systemic disease | Naguib et al. (2015) |
| HPAI H5N1 | Vultures | Natural | Dyspnoea, neurological signs, asthenia, locomotion problems, diarrhoea, respiratory disorders, prostration, ruffled feathers | Ducatez et al. (2007) |
| HPAI H5N1, 2.2. | Gyr‐Saker (Falco rusticolus x Falco cherrug) hybrid falcons | Experimental | One falcon died on 3 dpi, three died on 4 dpi and one died on 5 dpi. Four had reduced food intake starting from the day of infection and three had a slightly bloody tracheal exudate detectable the day after exposure. One bird died with no clinical signs | Lierz et al. (2007) |
| HPAI H5N1, NS | Houbara bustards | Natural | Torticollis, paralysis of the leg and imbalance, swollen head, nasal discharge, greenish diarrhoea. Within 4 days, 38 out of 41 bustards died | Khan et al. (2009) |
| HPAI H5N1, NS | Falcons | Natural | Nervous signs and diarrhoea | |
| HPAI H5N1, NS | American kestrels | Experimental | Feather fluffing, rhythmic side to side head movements, ataxia, head held at an angle, loss of appetite, loss of balance and motor control, tremors | Hall et al. (2009) |
| HPAI H5N1, NS | Gyr‐Saker (F. rusticolus x F. cherrug) hybrid falcons | Experimental | The infected falcons died or were euthanised between 5 and 7 dpi after showing acute severe neurological signs | Bertran et al. (2012) |
| LPAI H7N2 | Gyr‐Saker (F. rusticolus x F. cherrug) hybrid falcons | Experimental | No clinical signs (although all seroconverted) | Bertran et al. (2012) |
dpi: days post‐infection; NS, not specified.
Table 2.
Probability terms and subjective probability ranges used to describe the probability of AI introduction via non‐wild bird pathways
| Probability term | Subjective probability range |
|---|---|
| Non‐negligible | From 10% up to 100% |
| Unlikely | From 2% up to 10% |
| Very unlikely | From 1% up to 2% |
| Extreme unlikely | Up to 1% |
| Negligible | Indistinguishable from 0 |
In terms of the levels of categorisation for the risk level, it was assumed that most risk managers will not distinguish between 10% and 100% likelihood; anything within this range will be managed. This reduces the levels of complexity in terms of assessing the risk, but it can lead to large boundaries of subjectivity where probability of occurrence of the ‘non‐negligible’ risk level is anything between 10% and 100%.
2.4. Approach to assess AI transmission and spread
The objective was to determine the transmission parameters (e.g. transmission rate parameter and reproduction ratio) for HPAI and LPAI transfer between animals, between houses and between sites and associated risk factors (virus, host, contact network). Also, the role of wild birds in AI transmission between houses and holdings is assessed.
Data have been extracted by the AI Consortium from the scientific literature (Duncan et al., 2017a,b). A descriptive analysis of the data is provided in Section 3.5 and tables with transmission characteristics are included in Appendix E.
2.5. Approach to assess mutation from LPAI to HPAI
The objective was to describe the current understanding of the mechanisms that govern pathogenicity of AIV in poultry and to identify factors that might influence LP‐to‐HP mutation rates with respect to virus, environmental and host species‐specific aspects. Data have been extracted by the AI Consortium from the scientific literature (Richard et al., 2017) and several databases have been used (GISAID, 2017; data collected by the Consortium as reported by Duncan et al., 2017a,b).
Data collected from experimental trials related to the LPAI/HPAI switch, from the genetic comparison between HPAI and LPAI precursors, as well as the genome sequences downloaded from public databases were used to evaluate the possible existence of molecular markers associated with the evolution of LPAIV to HPAIV.
Epidemiological information referring to documented and confirmed HPAI outbreaks since 1959 were analysed to evaluate the possible existence of environmental factors associated with the LPAIV evolution into HPAIV.
Information from the documented HPAI outbreaks as well as from the experimental trials on LPAI/HPAI switch were analysed to explore the possible existence of a host contribution (i.e. age of the animal, type of poultry, immune status) in the evolution of LPAIV to HPAIV.
Genetic sequences from GISAID were used to evaluate the overall degree of pathogenicity clustering among the HA genes. Sequences of the HA gene segment of HP and LP avian influenza viruses were retrieved from GISAID (online14). All the H5 and H7 sequences of avian origin for which at least 70% of the total length was available were downloaded. Based on phylogenetic clustering of avian influenza viruses (Krauss et al., 2007) and on computational needs, for each HA subtype, two distinct data sets, one including sequences from Eurasia, Africa and Oceania and one with the American sequences (H5 Eurasia–Africa–Oceania, H5 Americas, H7 Eurasia–Africa–Oceania and H7 Americas), were generated for a total of four distinct data sets (Table 3). As the HA genes of all the HPAI H5 viruses originating from A/goose/Guangdong/1/96 clustered all together, only 10 representative sequences belonging to this lineage were selected for the H5 data set.
Table 3.
Description of the data set used to assess mutation from LPAI to HPAI
| Data set | Segment | No. of sequences | Date |
|---|---|---|---|
| H5 Eurasia–Africa–Oceania | 4 | 464a | 1959–2016 |
| H5 America | 4 | 594 | 1966–2016 |
| H7 Eurasia–Africa–Oceania | 4 | 1,746 | 1902–2016 |
| H7 America | 4 | 894 | 1927–2016 |
No. of sequences after the partial removal of the HPAI A/goose/Guangdong/1/96 lineage.
Nucleotide sequence alignments were constructed for each data set using the online version of MAFFT v.7 program (MAFFT version 7, 2017, 15). Alignments were manually curated to remove low quality sequences and the non‐coding regions. To infer the evolutionary relationships for each gene segment, we employed the Bayesian method available in the MrBayes v.3.2.6 program (Ronquist and Huelsenbeck, 2003), incorporating a GTR model of nucleotide substitution with a gamma distribution of among‐site rate variation (with four rate categories, Γ4). All the trees were run for sufficient time to achieve an average standard deviation of split frequencies below 0.05. Posterior probabilities values were used to depict support for individual groupings. The consensus trees were visualised using FigTree v. 1.4.2 (Rambaut, 2007, 16) and the online tool iTOL (Letunic and Bork, 2016, 17).
Taxa in each tree were labelled according to:
Virus pathogenicity (LP and HP). All the HPAI viruses were identified based on: (i) the insertion of multiple basic amino acids at the cleavage site of the HA; (ii) the literature review or (iii) OIE reports.
Type of host (wild or domestic). Chickens, geese, turkeys, quails, guinea fowls, domestic ducks and ostriches were considered as domestic birds, while all the remaining species were categorised as wild birds.
Main genetic groups, defined by long branches and posterior probabilities higher than 90.
The outcomes are described in Section 3.6 whereas the figures and tables are provided in Appendix H.
2.6. Approach to assess AI surveillance
The poultry data from surveillance activities presented in the report are restricted to data that were collected between 2014 and 2016 according to the guidelines laid down in Commission Decision 2010/367/EU.
MS submitted data to the European Commission database in a standardised format, containing laboratory testing information and more detailed information on the positive holdings found for each poultry category. The data submitted by MS were extracted from the European Commission database and checked and analysed by the EURL. The standardised format for submission of data ensured that in the majority of MS the data were complete and could be analysed effectively.
Some MS sample holdings more than once within their approved surveillance programmes for the survey period. This was assumed to be the case where the reported number of holdings sampled for a poultry category exceeded the total number of holdings reported for that category.
The data from the 2016/2017 HPAI H5 epizootic was taken from ADNS which is uploaded by MS shortly after disease confirmation. These data are not subject to rigorous checks and some data entry errors may occur.
These data sets were visualised using ArcGIS 10.2.218 and qGIS 2.18.7.19
2.7. Approach to generate a risk map for HPAI H5 in wild birds
The methodology used to generate the risk map for HPAI H5 in wild birds is described in Si et al. (2010). Data on wild bird events are used from the EMPRES‐I database (HPAI H5, 2005–2017.5.31) and data provided by the AI consortium and MSs to EFSA (HPAI H5 2006–2017.5.19). Data were available on 1,841 outbreaks in wild birds. After removing duplicated records within 1 km distance, there were 1,271 locations left. Then, locations with Null data were removed, if they fall into Null data area in environmental layers, resulting in 1,127 locations left. Seventy per cent of the locations of H5 HPAI presence were used for model training (789), 30% for model validation (338). Five thousand locations of absence were created from the background, 1,000 training subsets were used in further calculation, each training subset contained 789 presence and 789 absence points. The final model was generated conducting univariate regression models, considering multicollinearity and autocorrelation, using stepwise regression to select important variable, and running multiple logistic regression models to determine the core variables and the final model (see Table 4). 338 independent validating samples were used to validate the risk map, and 89.65% of samples fell into a predictive high‐risk area.
Table 4.
Summary of the multiple logistic regression models for the occurrence of the highly pathogenic avian influenza (HPAI) H5 virus in wild birds in Europe. All values in the table are mean values obtained from 1,000 runs of the model
| Parameter | Description | Unit | Original data source | B | OR | 95% CIs | OR | p‐value |
|---|---|---|---|---|---|---|---|---|
| Intercept | Intercept of the model | No unit | NA | 2.801973 | 17.57614 | 5.643299 | 54.78154 | 1.93E‐05 |
| City | Distance to the nearest city | km | ESRI | −4.25E‐05 | 0.999958 | 0.999938 | 0.999977 | 0.000522 |
| GLWD | Distance to the nearest lake or wetland | km | ESRI | −2.04E‐05 | 0.99998 | 0.999971 | 0.999989 | 0.000195 |
| Metropolis | Distance to the nearest metropolis | km | ESRI | −5.91E‐06 | 0.999994 | 0.999992 | 0.999996 | 6.45E‐08 |
| prec_12 | Mean monthly precipitation in December | mm | WORLDCLIM | −0.02637 | 0.973983 | 0.963299 | 0.984785 | 5.49E‐05 |
| Ramsa | Distance to the nearest Ramsa site | km | Wetlands International | −9.68E‐06 | 0.99999 | 0.999987 | 0.999994 | 1.19E‐05 |
| roads | Distance to the nearest road | km | ESRI | −8.71E‐05 | 0.999913 | 0.999876 | 0.999949 | 3.70E‐05 |
| tmin_12 | Mean monthly minimum temperature in December | °C*10 | WORLDCLIM | 0.023218 | 1.023499 | 1.009271 | 1.037928 | 0.005659 |
| Slope | Slope gradient | ° | WORLDCLIM | −0.17368 | 0.840889 | 0.769036 | 0.919467 | 0.000754 |
| prec_07 | Mean monthly precipitation in July | mm | WORLDCLIM | 0.033028 | 1.033585 | 1.022292 | 1.045002 | 3.65E‐07 |
| Stmin_12 | Square term of mean monthly minimum temperature in December | No unit | WORLDCLIM | −0.00081 | 0.999187 | 0.998936 | 0.999438 | 1.32E‐08 |
| SNDVI01 | Square term of monthly NDVI (normalised difference vegetation index) in January | No unit | NASA | −7.06245 | 0.004311 | 3.38E‐05 | 0.561924 | 0.016615 |
| NDVI01 | Monthly NDVI in January | No unit | NASA | 3.363269 | 65.55596 | 1.255973 | 3446.199 | 0.165909 |
2.8. Approach to assess biosecurity in relation to AI
The biosecurity measures described in the AI Statement (EFSA, 2017a) served as a basis and were differentiated for commercial and non‐commercial (backyard) holdings with indoor housing only or with outdoor access. An EKE meeting was organised where two WG members and eight hearing experts discussed and edited the biosecurity measures to achieve a common understanding and to prevent overlap between the measures. The experts also ranked the measures for feasibility, sustainable implementation, effectiveness to prevent entry and effectiveness to contain the virus. For each parameter, the measures were ranked from highest (high rank) to lowest (low rank) importance. The experts were able to judge measures as equally important. Then, these were equally set to the middle rank of their positions:
Feasibility: proportion of the farmers willing to start implementing the given biosecurity measure.
Sustainable implementation: proportion of the farmers to maintain the given biosecurity measure continuously during 90 days of high risk.
Effectiveness to reduce entry: reduction in the amount of virus coming from the outside to the holding, able to reach poultry within the holding and cause infection by implementing the given biosecurity measure.
Effectiveness to contain the virus: reduction in the amount of virus that can be transferred between epidemiology units within (poultry houses) and outside of the affected holding by implementing the given biosecurity measure.
Average ranks were calculated from the individual rankings of the 10 experts (Figure J.1A, Appendix J). During the elicitation the deviation of the individual versus the average judgements (Figure J.1B, Appendix J), the variation of judgements on each statement and the conformance of each expert with the group were assessed and discussed. The final ranking was concluded by the group.
Figure J.1.
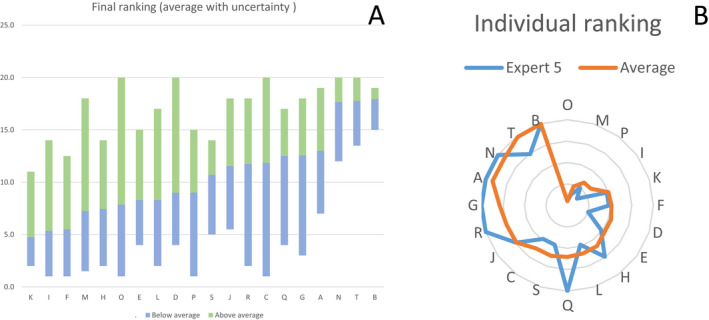
Example average ranking of biosecurity measures calculated from the individual rankings of the 10 experts (panel A) and example of deviation of the individual versus the average judgements (panel B)
The definitions of commercial and non‐commercial poultry holdings described in the EU legislation were used:
-
Commercial poultry holding means a holding where poultry is kept for commercial purposes.
-
1
–Non‐commercial holding/pet bird holding means a holding where poultry or other birds are kept by their owners: (a) for their own consumption of the poultry or other birds or their products or (b) as pets.
-
1
2.9. Uncertainty assessment
EFSA's Scientific Committee is developing a Guidance document (EFSA, 2016b) to offer a toolbox of methodologies – both quantitative and qualitative – for analysing scientific uncertainties in all its scientific assessments. Through the application of these tools, EFSA aims to give decision‐makers a clearer picture of the scientific uncertainties affecting each assessment.
The mandates on AI have been chosen as a case study for testing the applicability of the approach proposed in the draft Guidance on Uncertainty in EFSA scientific assessments. The experience gained with this specific risk assessment and the other case studies identified in each Unit in EFSA will be used to fine‐tune the Guidance document.
The draft Guidance identifies the following steps to be followed to analyse uncertainties in EFSA scientific assessments:
Identify sources of uncertainty.
Select which sources of uncertainty to assess individually.
Assess individual sources of uncertainty.
Quantify combined uncertainty (from individual sources).
Describe unquantified uncertainties.
Investigate influence/sensitivity.
Decide whether to refine the uncertainty analysis.
Document and report the uncertainty analysis.
The draft Guidance on uncertainty in scientific assessment was pilot‐tested on the entry of HPAI and LPAI via wild birds. The latter subquestions were deemed an adequate example to evaluate applicability and appropriateness of both the overall approach proposed in the Guidance and the methodologies suggested for performing the various steps of the U analysis.
For the introduction of HPAI via non‐wild bird pathways, bounded probabilities instead of probability distributions were used to describe the uncertain estimate of the probability of introduction into the EU since the evidence available was scarce and not very accurate.
For the other TORs, the approach for uncertainty analysis consisted in the identification and listing of the potential sources of uncertainty.
Steps recommended in the Uncertainty Guidance were followed with some adaptations since all uncertainties affecting a single parameter in the AI epizootic model were assessed jointly (for instance all limitations in the knowledge related to the HPAI prevalence at the EU border) and then combined with the overall uncertainty affecting each of the other parameters. Therefore, an uncertainty distribution was set up around each parameter instead of an individual source of uncertainty as suggested in steps 3 and 4.
The uncertainty analysis included two main types of uncertainty:
limitations in the evidence used to support the estimate of the assessment parameters;
assumptions made in the assessment and the structure of the assessment itself (including model structure if any).
Effect of the assumptions/structure of the assessment as source of uncertainty was assessed only for TOR 1 and TOR 3 addressed with the mathematical models, once the results of the quantification of the first type of uncertainty were available.
The approach taken to analyse the second type of uncertainty varies depending on the level of realism of the assumption, the expected effect on the outcome and the feasibility of quantifying it.
The effects of assumptions were not further analysed when belonging to the following categories: having a limited effect, representing the worst‐case scenario or representing the most realistic scenario.
The assumptions expected to introduce a high uncertainty on the results, have been investigated further either via scenario analyses (running model with different formulations of the assumptions) or via semi‐formal EKE (see Appendix C).
2.9.1.
Risk of AI introduction via wild birds
An AI epizootic model (described in Appendix C) has been generated to simulate HPAI entry into the EU and HPAI/LPAI into poultry holdings via wild birds.
The following steps were followed to analyse the uncertainty:
An a priori sensitivity analysis was performed aimed at prioritising components (parameters and variables) of each model whose uncertainty is expected to be collectively more influential on the uncertainty of the outcome. To this scope, for each parameter and variable, the sources of uncertainty collectively affecting each of them (uncertainty on inputs) were expressed in the form of a range (minimal assessment step).
-
Based on the prioritisation exercise performed in the first step, for each component of the model, the uncertainty assessment (quantifying collectively the effect of various sources of uncertainty on each component) was expressed as probability distribution derived using a:
semi‐formal EKE conducted among WG members for inputs with overall uncertainty expected to have limited influence on the model outcome;
formal EKE conducted among WG members and hearing experts for inputs expected to have high influence on the outcome uncertainty.
Uncertainty distributions around each component of the models were combined using Monte Carlo techniques ((EFSA, 2016a – Guidance Document Uncertainty EFSA, 2016b)).
The uncertainty sources related to the structure/assumptions of each model were investigated once the uncertainties related to the model inputs were quantitatively combined. The sources of uncertainty were listed and the expected direction and magnitude of the effect on the uncertainty of each model outcome were assessed with a semi‐formal EKE among WG members if sufficient evidence/knowledge was available to carry out this assessment. Otherwise the sources of uncertainty were simply listed. Scenario analyses were performed for some assumptions to assess the effect on the outcome of different formulations.
For HPAI entry via E, S or NW routes, the following steps were followed to estimate uncertainties:
The model outcome of the HPAI clade 2.3.4.4 entry was used as a benchmark.
Uncertainties identified in the extrapolation of the outcome of the reference scenario to other scenarios (other routes of wild bird exposure and clades) have been listed.
Whenever possible, the identified sources of uncertainty have been assessed collectively via a semi‐formal EKE process carried with WG members and an adjusted probability distribution has been derived as output for each route/clade.
To address the issue of separating uncertainty from variability in the inputs, the model inputs were defined as variability distribution centiles or location parameters (e.g. median, mean) or reference was made to the occurrence of a variable at a specific point in time (e.g. day of the migration season 2016–2017).
For each scenario and model, a summary table describes sources of uncertainty broadly classified, steps taken to assess uncertainty, components of the model considered in each step, method used to analyse uncertainty with reference to the Uncertainty Guidance.
Risk of AI introduction via non‐wild bird pathways
Evidence was collected on the following features: import volume and probabilities of testing, virus detection, virus preservation during transport and poultry exposure to the commodity. They were expressed in terms of ranges as available data were poor in quantity and quality. Uncertainties pertaining to the limitations in the evidence were used to derive probabilities of introduction via these pathways using a semi‐formal elicitation performed among WG members.
3. Assessment
3.1. Mapping of HPAI and LPAI outbreaks during the last 5 years (TOR 5)
The scientific opinion focuses on the introduction pathways of HPAI viruses into Europe and the assessments of the different TORs aimed to provide science‐based advice to risk managers on how to be prepared for future HPAI and LPAI (H5 and H7) outbreaks in Europe. This section of the opinion gives an overview of the HPAI and LPAI outbreaks in Europe and the other continents during the last 5 years, with the purpose of identifying HPAI and LPAI virus types that could cause outbreaks in the EU in the next years. These virus types are then assessed in more detail in the following chapters of the opinion.
3.1.1. HPAI detections in Europe and other continents
So far, only HPAI viruses of subtype H5 have been introduced into Europe from abroad while viruses of subtype H7 causing outbreaks of HPAI were intrinsically generated within Europe from European low pathogenic precursor viruses (see Appendix A for overview since 2010). The focus in the HPAI introduction assessment (Section 3.2) will therefore be on HPAIV H5 clades that are descendants of the original goose/Guangdong (gs/GD) H5N1 HPAI virus, which arose in 1996 in South China. Gs/GD representatives that were found circulating in Europe in the last decade were restricted to clades 2.2.1.x (HPAIV H5N1) 2.3.2.1c (HPAIV H5N1), and 2.3.4.4 (includes HPAIV subtypes H5N8, H5N5 and H5N6). HP H5N2 viruses of clade 2.3.4.4 caused widespread outbreaks in the United States in 2014/2015 but have not yet been found in Europe. Some recent papers (Kim et al., 2014; Pulit‐Penaloza et al., 2015; Richard et al., 2015) point out differences in virulence of new H5N8 and H5N2 2.3.4.4a strains as compared with previously circulating clades, in particular 2.2 descendants.
Apart from the clades/lineages that were selected for detailed incursion analysis here (2.2.1.2, 2.3.2.1c and 2.3.4.4), there are further clades of gs/GD HPAIV subtype H5(N1) which circulate endemically in SE Asian countries but have not escaped to other continents. Currently, these include clades 1.X (southern Vietnam), 2.1.3 (Indonesia), 2.3.2.1a (India, Bangladesh), 2.3.4.2 (Myanmar) and 7.X (China) (FAO/EMPRES‐i). These viruses remained confined to a geographic region within South‐east and South Asia. Likewise, HPAI viruses of subtype H5N6 belonging to clades 2.3.4.4c and d, which have been detected first in China in 2014 continued to spread in poultry in China, Laos, Cambodia, South Korea, Vietnam and Japan but not beyond (Heine et al., 2015). Some members of the 2.3.4.4c clade have shown zoonotic potential and a limited number of human cases (currently 19 in China) have been confirmed (Yang et al., 2017). It should be noted that the HPAI H5N6 virus of clade 2.3.4.4b detected 2017 in Greece is not related to the former zoonotic lineage! So, there seem to exist clades of gs/GD viruses that are highly mobile in geographical terms while other clades remain largely sessile even over more than a decade. The molecular or epidemiological basis for this behaviour remains unclear. So, in the future, other viruses of the gs/GD lineage may acquire geographic mobility for reasons that are not known or foreseeable at present. In addition, further evolution of these viruses in their epicentres is ongoing and will surely lead to the emergence of new, but unpredictable clades and phenotypes.
To build a risk analysis on incursions of such viruses into the EU, extrapolations into future viral developments are not regarded a firm base. Therefore, the range of gs/GD HPAIV H5 clades to be analysed here was restricted to 2.2.1.2, 2.3.2.1c and 2.3.4.4 for the following reasons:
Viruses of these clades, or their predecessors (2.2.1), have already been detected in the EU. So, they have proven geographic mobility potential.
The listed clades also had an out‐of‐Asia origin and are still in circulation in SE Asia (2.3.2.1, H5N1; 2.3.4.4, H5N8, H5N5, H5N6).
2.2‐like viruses also originated from an Asian reservoir and caused the first European outbreaks of HPAI viruses of the gs/GD lineage in 2005/2006. Nowadays, they seem to be extant in Egypt only. Descendants of clade 2.2.X therefore have shifted from a highly mobile behaviour, which brought them to Europe and Africa in 2005/2006, to a rather stationary but not static existence in Egypt and a few neighbouring countries such as Libya, Israel and Palestinian Auton. Territories (Soliman et al., 2012; El‐Zoghby et al., 2013; Sheta et al., 2014; Abdelwhab et al., 2016). Nevertheless, these viruses were included as they may be remobilised from Egypt which is situated at a crossing point of several avian migratory flyways. In addition, these viruses also bear substantial zoonotic potential, which has, so far, not been associated with representatives of above‐mentioned 2.3.4.4 lineage detected in Europe, and only rarely for viruses of the 2.3.2.1 clade (Figure 1).
Figure 1.
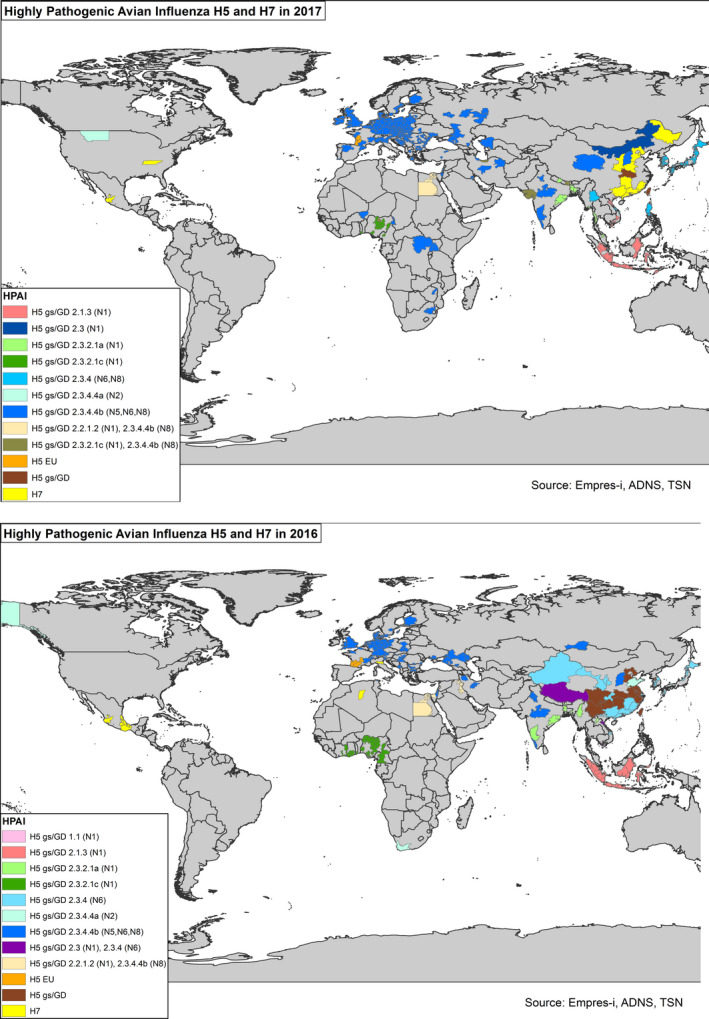
World‐wide distribution of influenza HPAI H5 and H7 subtypes and clades in domestic birds, wild birds and humans (1 January 2016–31 August 2017)
Further in the opinion, emphasis is put on distinctive phenotypic characteristics of the different clades and subtypes as these are important to consider when reviewing the legislation. Using clades based on the phylogenetic relationship of the HA gene to classify H5 viruses may not be perfect when assessing pathogenicity and transmissibility, but it is considered to be more appropriate than comparing subtypes. An internationally accepted and harmonised clade designation system currently in use for Asian origin H5 HPAI viruses has not yet been developed for H7 viruses, or viruses of other subtypes, although several proposals have been made, mainly from regional perspectives (Kang et al., 2014; Van der Auwera et al., 2014). This may, in part, be due to a stricter geographical restriction of outbreaks of subtype H7 HPAI: Although limited transboundary spread of these viruses has been observed in the past (e.g. during the 2003 outbreak of H7N7 HPAI in the Netherlands transmissions to Belgium and Germany occurred), no transcontinental avian‐associated spread as seen repeatedly with the gs/GD‐like H5 viruses has ever been reported for H7 HPAIV. Probably for the same reason, data on the comparative pathogenicity of the different H7 HPAIV subtypes and lineages are scarce (Post et al., 2013; Kalthoff et al., 2014a; Shichinohe et al., 2016). It is, therefore, conceivable to treat in this assessment all H7 HPAI viruses as a uniform group.
Cases of gs/GD‐like HPAIV H5 infection during the last 5 years in Europe have always been associated with infected wild birds. In most episodes, poultry holdings were affected as well although usually after the detection of infected wild birds (2005/2006; 2016/2017). An exception are outbreaks in 2014/2015 when the first poultry outbreak was detected slightly earlier than the first wild bird case. Nevertheless, detection of gs/GD‐like HPAIV H5 in both poultry and wild birds in Europe was always closely associated, both geographically and temporally, indicating a tight epidemiological link (Munster et al., 2005; Steensels et al., 2006; Starick et al., 2008; Artois et al., 2009; Brochet et al., 2009; Globig et al., 2009; Reid et al., 2011; Saito et al., 2015; Bi et al., 2016; Claes et al., 2016; Muzyka et al., 2016). Thoroughly conducted epidemiological tracing back studies of the index cases of each gs/GD‐related poultry outbreak failed to generate evidence that might have indicated an infection source within European poultry populations. Also, no epidemiological links were evident between the index cases of HPAIV H5N8 (2.3.4.4a) in poultry holdings in Germany, the Netherlands, the United Kingdom and Italy which had all occurred in a narrow timeframe in 2014 (Bouwstra et al., 2015; Conraths et al., 2016; Nunez et al., 2016). Instead, wild bird surveillance data and phylogenetic analyses of the viruses detected in wild birds and in poultry corroborated scientifically sound evidence that identified migratory wild birds as a more likely source of transboundary virus spread; this circumstantial evidence has been massively supported by recent analyses of the origin of the North American outbreaks of HPAIV H5N8 and its American reassortant, H5N2: migrating wild birds picked up the infection in Far East Asia during spring 2014 and set up a transmission chain across Beringia into Alaska and northern Canada. During autumn migration the same year, the virus was translocated further south into California and other southern US States (Adlhoch et al., 2014; Dalby and Iqbal, 2015; Ip et al., 2015; Lee et al., 2015; Verhagen et al., 2015). Therefore, the conclusion may be drawn that transboundary spread by infected migratory wild birds constitutes the highest likelihood of an introduction of Asian origin H5 HPAI viruses. Both South Korea in September 2014 and Japan in November that year reported findings of H5N8 in Tundra swans (Cygnus columbianus). These birds migrate to the Arctic and subarctic tundra for winter breeding grounds while the subspecies, the Bewick swan (C. c. bewicki) also visits breeding grounds in the tundra and coastal areas of Siberia, wintering in Northern Europe. The Whistling swan (C. c. columbianus) breeds in coastal plains of Alaska and Canada then moving to wintering grounds in north‐west USA. Although these are separate subspecies, their common breeding grounds may overlap, which could potentially lead to transmission of the H5N8 virus across three continents within one single breeding season (JNCC, 2001). There is limited evidence for migratory swans being implicated in the large geographic expansion of avian influenza viruses, and it should be noted that comingling with other migratory duck species may also be involved in a complex pattern of virus transmission (Newman et al., 2009).
Based on the results of passive surveillance and phylogenetic investigations, infected wild birds have also been implicated in the westward spread of clade 2.3.2.1c viruses from Asia. Between 2014 and 2016, the virus showed an apparently expanding range of geographical dispersal following confirmation of infections in wild birds and poultry in African, Middle Eastern and European countries (Naguib et al., 2015; Tassoni et al., 2016). Nevertheless, human‐related factors, including falconry may have been involved as well (Naguib et al., 2015). The 2.3.2.1c clade continues to spread in poultry across West and Central Africa, with Cameroon and Togo becoming the latest African countries to detect the disease in May and August 2016, respectively. The lack of a structured surveillance system in these territories and the limited number of genetic and epidemiological data available make it difficult to pin‐point how these viruses may have spread across the African regions.
Autochthonal generation of HPAI from LPAI precursor viruses in poultry populations must of course also be considered as a possible introductory pathway. The recently on‐going outbreaks of HPAIV H5 in south‐west France are caused by at least three reassortants (H5N1, H5N2 and H5N9) which all have their common origin in a single (unknown) H5 LPAI virus of European ancestry (Anonymous, 2016). In 2016/2017 the already complex situation further exacerbated with the introduction of HPAI H5N8 2.3.4.4b viruses into the same region. LPAIV of European lineages of subtype H5 are maintained in European metapopulations of aquatic wild birds; the HA of these viruses is distinctly different from those of the Asian gs/GD‐like ones (Olsen et al., 2006; Munster et al., 2007). The epidemiological data of the French epizootic suggest spill‐over infection of an LPAIV H5 precursor virus into waterfowl population in south‐west France, continuing unnoticed circulation of this virus over prolonged periods, de novo generation of an HPAI phenotype, further secondary spread of this phenotype between duck holdings and reassortment with at least two further LPAI viruses with disparate NA subtypes (Briand et al., 2017).
The same mechanism has also been at the basis of all European outbreaks of H7 HPAIV. There is no indication of transboundary spread of these viruses from outside Europe, neither by migratory wild birds nor by transmission chains within the poultry industries. None of the HPAIV of subtype H7 that arose and circulated for some time outside Europe (e.g. H7N7 in Mexico, H7N3 in Canada, H7N7 in Pakistan) found its way into European wild bird or poultry populations.
Apart from migratory wild birds, of course, further potential ways of introduction of HPAIV into Europe exist. These include import of infected game and captive birds or poultry and contaminated avian products, feed and other fomites. HPAI H5N1 viruses, for example, have been detected in smuggled wild birds from Asia (Van Borm et al., 2005; OIE WAHID, 2013). In addition, contaminated clothing and shoes of airline passengers from endemically infected regions must be considered as a possible source of introduction although no cases linked to this route were identified so far. Spread within or between EU MSs may also be related to co‐owned premises and heavy trading activities in different MSs (Hungary/UK, 2007). In addition, slaughtering and marketing of healthy ducks asymptomatically infected by HPAIV H5N1 had caused clinically overt HPAI outbreaks in backyard chicken holdings in Germany after offal from frozen duck carcasses had illegally been fed to scavenging chickens (Harder et al., 2009).
3.1.2. LPAI detections in Europe and other continents
There is longstanding and ample scientific evidence that metapopulations of wild birds, especially species in the orders Anseriformes and Charadriiformes, constitute a natural reservoir of all known subtypes of avian‐associated influenza A viruses which are maintained in low pathogenic forms (Yoon et al., 2014; Lang et al., 2016).
There are seasonal fluctuations in the overall prevalence of AIV in aquatic wild bird populations associated with population turnover rates and migration. For as yet unknown reasons, the relative prevalence of certain subtypes in these populations shows gross variations from year to year (Wille et al., 2013; Latorre‐Margalef et al., 2014). It is without doubt that transmission of H5 and H7 viruses from such reservoirs is at the basis of the index cases of notifiable LPAIV outbreaks in poultry. Domestic waterfowl appears to be fully permissive for wild bird‐derived H5 and H7 LPAIV; infection is usually productive but clinical signs are not induced although this can be modulated by the presence of other (opportunistic) co‐infections (Jourdain et al., 2010). Chickens, in contrast, seem not to be fully permissive for these viruses and cycles of adaptation may be required to achieve fully productive replication which, likewise, does not induce clinical signs that are easily perceived (Pillai et al., 2010a,b). So, a chain of transmission (and adaptation) events from wild birds to domestic waterfowl to gallinaceous poultry has been proposed to explain the occurrence of LPAIV H5 or H7 viruses in domestic chickens and turkeys; turkeys as well as quails seem to have a very high susceptibility to LPAIV of various origins and have been proposed as intermediate or bridging hosts between Anseriformes and gallinaceous species (Wan and Perez, 2006; Pillai et al., 2010a; Thontiravong et al., 2012; Pantin‐Jackwood et al., 2014). Due to the lack of conspicuous clinical signs, these infections can only be diagnosed by means of serological and/or virological laboratory tools. So, detection of such infections in Europe is directly related to the intensity of specific surveillance measures. This may explain, at least in part, the highly variable frequency of notifiable LPAI in different European countries over the last decade: the Netherlands and Italy have reported the highest frequency of LPAI H5 or H7 infections but also run the most intensive H5‐ and H7‐specific surveillance programmes (see annual reports on AI surveillance of the EU‐CRL (APHA, 2015)). The structure of the poultry production systems may also influence the frequency of notifiable LPAI infections by modulating the likelihood of direct and indirect contacts between LPAIV‐infected aquatic wild birds, their environment and domestic waterfowl. Countries with high contingents of free‐range waterfowl production are likely to experience (and detect) higher infection rates as is evident, e.g. for outdoor duck grazing holdings in western France or foie gras production in Bulgaria (Cappelle et al., 2014; APHA, 2015; Marinova‐Petkova et al., 2016).
The vast majority of LPAIV H5 or H7 outbreaks in Europe has affected only single holdings and only few cases with further secondary spread among poultry have been reported: an LPAIV H5N3 outbreak reported from Germany in late 2008 finally affected 29 turkey holdings (Mughini‐Gras et al., 2014); transmission was brought to a stop only in early 2009 following culling of affected flocks and the implementation of restocking restrictions. Following the identification of an outbreak caused by an H5N2 virus in an industrial turkey flock in northern Italy in late August 2012, strengthened monitoring activities resulted in the detection of seven additional LP H5N2 outbreaks in both industrial and rural poultry sectors of three distinct Italian regions, with the last outbreak notified in October 2012 (SCoFCAH, 2012). Prolonged unnoticed spread and circulation of LPAIV H5 among waterfowl in south‐western France, probably since 2013, had finally culminated in the generation of HPAI H5 strains in 2015 that did not induce overt clinical symptoms in ducks but caused fatal infections in chickens (Anonymous, 2016).
With respect to zoonotic propensities of low pathogenic H5 and H7 AIV, few data are available. These few studies point towards higher zoonotic risks for LPAIV H7 due to increased affinity of some isolates to mammalian/human‐like receptors. However, only single and sporadic cases of human LPAIV H7 infection have been reported until 2013. In that year, a new LPAIV H7N9 emerged in China as a reassortant of H7 and LPAI H9N2‐G1‐like viruses. These viruses proved to be highly zoonotic and continue to cause, in annual cycles, clusters of human cases that were confined to China with few cases in travellers from China to other countries. A high case‐fatality ratio was noticed although the virus was apathogenic in poultry including chickens. This has severely hampered efforts to unravel and drain an avian reservoir of these viruses. Human cases continue to occur until to date. Exposure to presumably infected poultry is the main route of infection while no sustained human‐to‐human transmission chains have been observed (Su et al., 2017). So far, these viruses remained largely confined to China and detection in feral20 and wild birds was on sporadic occasions only. Meanwhile the emergence of a highly pathogenic phenotype of Chinese H7N9 has been detected, and these viruses have already caused three human cases of infection (Kang et al., 2017). It is difficult to assess risks of incursions into Europe of these viruses through migrating wild birds due to the absence of prevalence data in avian reservoir host populations.
The same H9N2 G1‐like viruses which donated genome segments to the highly zoonotic Chinese H7N9 reassortants also expressed discrete zoonotic propensities themselves which have led, so far, to a limited number (n = 32, world‐wide) of mild clinical human cases only. Mutations in the HA receptor binding region of these viruses conferred a higher affinity to human‐like sialic acid receptors. Infections with LPAI H9N2 G1‐like viruses are extremely widespread in poultry in most Asian, Middle East and North African countries where they cause substantial economic damage. Detection in wild bird populations of G1‐like viruses has also been repeatedly reported. LPAI H9N2 viruses are also prevalent in European wild bird populations but these viruses are only distantly related to the G1 lineage and do not bear mutations that induce receptor binding shifting. G1‐like viruses have not been detected in poultry in Europe. An introduction to Europe of G1‐like H9N2 either via migrating wild birds along routes described for the HPAIV H5N1 of clade 2.3.2.1c or via poultry or poultry products does not seem unlikely.
3.2. HPAI introduction via migratory and residential wild birds (TOR 1)
3.2.1. Risk pathways
Clades
Based on the mapping of HPAI outbreaks during the last 5 years (see Section 3.1.1), an assessment was performed to determine the probability of H5 HPAIV introduction into the EU and subsequently into a poultry holding via wild birds. HPAIVs from the clades 2.3.2.1c, 2.2.1.x and 2.3.4.4 are differentiated as these viruses have different characteristics that may affect their risk of introduction.
Entry routes
For the sake of the current risk analysis, wild bird species present in the EU have been divided in two main groups of animals: water birds (ducks, geese, swans, grebes, etc.) and non‐water birds (raptors, song birds, waders, gulls, terns, storks, cranes, etc.) according to their general behaviour, habitat preference and known susceptibility to AIV. Although waders are water birds, they are in this opinion included in the non‐water birds group because their behaviour is different from that of Anatidae, in particular towards contact rates to poultry farm premises (Caron et al., 2017). More information is provided in Tables B.1 and B.2 (Appendix B). Within each group, subgroups of birds referring to the average common bird behaviour (e.g. habitat preferred) have been identified.
Table B.2.
List of species according to the sub‐group, the order and family they belong to in the non‐water birds group
| Sub‐category | Order | Family |
|---|---|---|
| Raptors | Falconiformes | Falconidae |
| Accipitriformes | Accipitridae, Pandionidae | |
| Strigiformes | Stigidae, Tytonidae | |
| Song birds | Passeriformes | Aegithalidae, Alaudidae, Bombycillidae, Certhiidae, Cinclidae, Corvidae, Emberizidae, Estrildidae, Fringillidae, Hirundinidae, Laniidae, Motacillidae, Muscicapidae, Oriolidae, Paridae, Ploceidae, Prunellidae, Remizidae, Sittidae, Sturnidae, Sylviidae, Troglodytidae, etc. |
| Columbiformes | Columbidea | |
| Waders | Charadriiformes | Charadiidea, Scolopaciea, Recurvirostridae, etc. |
| Gulls–Terns–Storks–Cranes | Charadriiformes | Laridae |
| Ciconiiformes | Ciconiidae | |
| Gruiformes | Gruidae |
Given the flyways of migratory water birds (see for instance Scott and Rose (1996) and data received as per procurement, coordinated by Linnaeus University (SE) and Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished (the Consortium report Waldenström et al., 2017)), four entry routes where migratory wild birds would cross the EU border were considered (Figure 2):
the NE route, via the EU border with Russia and Belarus, where migratory birds generally enter the EU during the autumn migration (and to a limited extent during cold spells in winter) along the East Atlantic flyway. HPAIV expected to be introduced via this area belong mainly to the 2.3.4.4 clade (Adlhoch et al., 2014; Bouwstra et al., 2015; Harder et al., 2015; Marchenko et al., 2015; Saito et al., 2015; Verhagen et al., 2015). A particular emphasis was given to this route, as it was considered to be the main migration corridor for European migratory birds breeding in Russia;
the eastern route (E), via the EU border with Ukraine, Moldova, Black Sea, Turkey until the southern border of Turkey. Here too, migratory birds generally enter the EU during autumn migration, although large‐scale movements from countries further to the east may also occur during cold spells. HPAIV so far detected in birds from this route mainly belong to the 2.3.2.1c clade (Marinova‐Petkova et al., 2012; Alkhamis et al., 2013), often originating from Central Asia (Bi et al., 2015, 2016; Naguib et al., 2015; Marchenko et al., 2016; Tosh et al., 2016; Ghafouri et al., 2017);
the southern route (S), via the EU border from the southern border of Turkey to the northern border of Portugal. Entry via this route is, as opposed to the above, mostly occurring during spring, as birds migrate back from their African wintering grounds. Virus clades considered to enter via the southern route belong to clades 2.2.1.2 (which is endemic to Egypt) and 2.3.2.1c (which is prevalent in sub‐Saharan West African countries, that also comprise major water bird winter quarters) (Arafa et al., 2015; Kammon et al., 2015; Monne et al., 2015); following the occurrence of viruses of clade 2.3.4.4b since winter 2016 in various African countries, these viruses must be included here as well;
the NW route, used by wild birds migrating from or across Greenland to the EU, entering the EU between the northern border of Portugal and the northern border of Russia. Only a very small proportion of migrating water birds actually enters the British Isles during autumn–winter migration following this route. HPAIV present in North America could enter the EU via this route (Saito et al., 2015; Bevins et al., 2016; Claes et al., 2016; Kaplan et al., 2016; Krauss et al., 2016; Lee et al., 2016).
Figure 2.
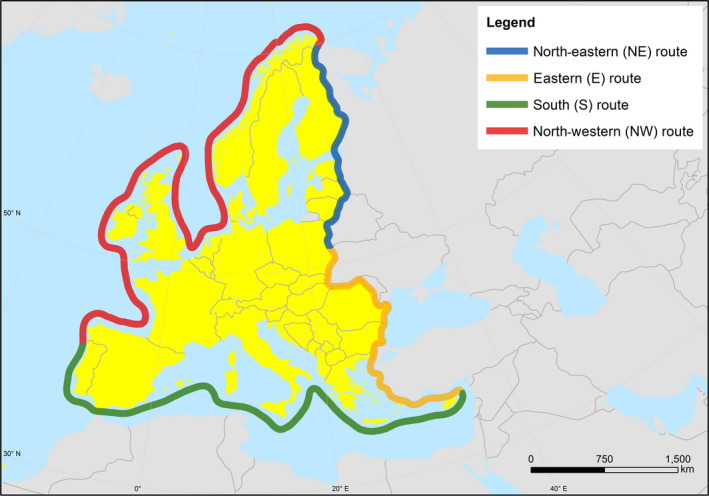
Map of the borders where migratory wild birds enter the EU via the four entry routes considered in this opinion
For each entry route and clade, three models were set up describing, respectively, the:
probability of HPAIV entry into the EU;
development of HPAIV prevalence in the wild bird reservoir on EU territory;
probability of HPAIV entry into a poultry holding without biosecurity measures on EU territory.
Figure 3 depicts the essential structures of the set‐up, which is shortly described in the paragraphs that follow.
Figure 3.
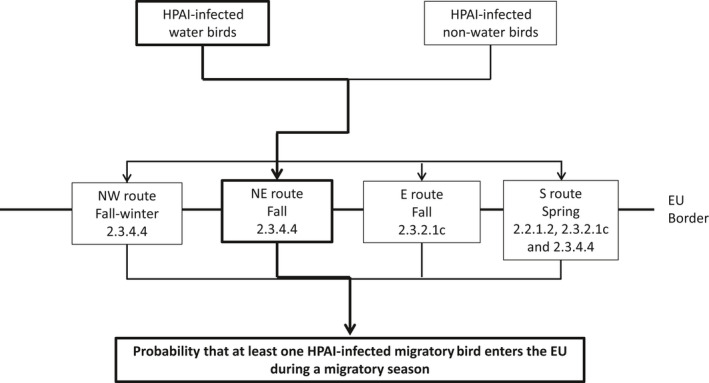
Proposed entry pathways of HPAI viruses belonging to clades 2.3.2.1c, 2.2.1.2 and 2.3.4.4 into the EU via migratory wild birds
- Bold lines, quantitative assessment; thin lines, qualitative assessment.
The sections below describe the probability of HPAI introduction into the EU and subsequently into a poultry holding without biosecurity, for the different wild bird entry routes and H5 HPAI viral clades.
3.2.2. NE route – clade 2.3.4.4
HPAIV clade 2.3.4.4 entry into the EU via the NE route
A mathematical model (see detailed description in Appendix C) was developed to quantitatively estimate the risk under the scenario where water birds infected with HPAIV clade 2.3.4.4 would enter the EU via the NE route during autumn migration. In essence, the probability of HPAI entry is a function of the number of migratory birds entering the EU in a migration season and the HPAI prevalence in this population. Based on the observation that detections of HPAI cases in wild non‐water birds are rare (e.g. EU report on AI surveillance, 2014; APHA, 2015)), in the model only migratory water birds were considered as a source of HPAI introduction in the model.21 The total number of migratory water birds entering the EU during a migration season was estimated by experts as an uncertainty distribution (mean 36,660,070 with standard deviation 11,140,680). The duration of the autumn migration period was estimated based on published data as 125 days (see Section D.1.1, Appendix D). Experts estimated the proportion of HPAIV clade 2.3.4.4 infected migratory water birds at the moment they cross the EU border (see Section D.1.2). All the parameter values used in the model are provided in Table C.6 of Appendix C.
Table C.6.
List of variables used in the HPAI clade 2.3.4.4 simulation model with description, type of EKE performed, type of distribution fitted and relative parameter estimates
| Parameter | Description | Source | Uncertainty distribution shape | U distr par1 | U distr par2 | U distr par3 | U distr par4 | More info | |
|---|---|---|---|---|---|---|---|---|---|
| MSLength | Length (in days) of the fall–winter migration season for wild birds entering the EU through the north‐east border | Semi‐formal EKE | Constant | 125 | NA | NA | NA | Section D.1.1 | |
| π MWB | Number of HPAI clade 2.3.4.4 infected water birds at the moment they cross the EU border out of 106 | Formal EKE | Weibull | Scale = 0.48 | Shape = 237.75 | NA | NA | Section D.1.2 | |
| Prob(contact NWB,NWB ) | Probability that a non‐water bird comes in contact with HPAI clade 2.3.4.4 infectious excretions of non‐water birds | Semi‐formal EKE | Beta | α = 0.72 | β = 69.84 | NA | NA | Section D.1.4 | |
| Prob(contact NWB,WB ) | Probability that a water bird comes in contact with HPAI clade 2.3.4.4 infectious excretions non‐water birds | Semi‐formal EKE | Beta | α = 0.55 | β = 74.55 | NA | NA | Section D.1.4 | |
| Prob(contact WB,NWB ) | Probability that a non‐water bird comes in contact with HPAI clade 2.3.4.4 infectious excretions non‐water birds | Semi‐formal EKE | Beta | α = 0.56 | β = 45.26 | NA | NA | Section D.1.4 | |
| Prob(contact WB,WB ) | Probability that a water bird comes in contact with HPAI clade 2.3.4.4 infectious excretions water birds | Semi‐formal EKE | Beta | α = 0.56 | β = 35.64 | NA | NA | Section D.1.4 | |
| 1/r NWB | Reciprocal of the duration in days of the HPAI clade 2.3.4.4 shedding period in non‐water birds | Semi‐formal EKE | Tnorm | Mean = 4.14 | SD = 4.3 | Lower bound = −0.15 | Upper bound = 17.28 | Section D.1.5 | |
| 1/r WB | Reciprocal of the duration in days of the HPAI clade 2.3.4.4 shedding period in water birds | Semi‐formal EKE | Weibull | Scale = 1.87 | Shape = 5.96 | NA | NA | Section D.1.5 | |
| md NWB | Mortality rate in non‐water birds due to HPAI clade 2.3.4.4 disease | Literature | Constant | 0.75 | NA | NA | NA | Section D.1.7 | |
| md WB | Mortality rate in water birds due to HPAI clade 2.3.4.4 disease | Literature | Constant | 0.07 | NA | NA | NA | Section D.1.7 | |
| Preservation | AIV preservation in the environment | Literature | Constant | 13 | Section D.1.9 | ||||
| Prob(Inf NWB | contact) | Probability that a susceptible non‐water bird becomes infected with HPAI clade 2.3.4.4 given a contact with excretions containing infectious virus in a forage area | Semi‐formal EKE | Beta | α = 0.78 | β = 1596.73 | NA | NA | Section D.1.10 | |
| Prob(Inf WB | contact) | Probability that a susceptible water bird becomes infected with HPAI clade 2.3.4.4 given a contact with excretions containing infectious virus in a forage area | Semi‐formal EKE | Beta | α = 0.74 | β = 235.89 | NA | NA | Section D.1.10 | |
|
|
Number of non‐water birds landing into a holding | Formal | lnorm | Mean = 6.75 | SD = 1.05 | NA | NA | Section D.1.12 | |
|
|
Number of water birds landing into a holding | Formal | Weibull | Scale = 0.46 | Shape = 111.36 | NA | NA | Section D.1.12 | |
| β b0 | Regression coefficient for the probability for a worst‐case holding to get the HPAI clade 2.3.4.4 infection given the presence of HPAI‐infected wild bird | Formal | beta | α = 0.38 | β = 561.41 | NA | NA | Section D1.13 | |
| TMWB | Total number of Migratory WB entering EU during migration season | Selected scenarios | Tnorm | Mean = 53403.94 | SD = 70370.43 | Lower bound = 1,6826.33 | Upper bound = 5,5632.39 | Section C.5.2 Model scenarios |
NA, not applicable.
The model outcome suggests, with a probability of 1 (median of the uncertainty distribution), that at least one migratory water bird infected with HPAIV clade 2.3.4.4 enters the EU via the NE route (see Figure 1, Appendix E). This assessment has been performed based on data that were available on HPAIV clade 2.3.4.4 until March 2016. The HPAI H5N8 detections in migratory water birds during the 2016–2017 winter are in line with what was predicted by the model.
HPAIV clade 2.3.4.4 prevalence in the wild bird reservoir
Once in the EU, infected migratory water birds could transfer HPAIV to the wild bird reservoir (Figure 4). All migratory non‐water birds are assumed not yet being infected (susceptible) when they enter the EU since only very low numbers of non‐water birds found positive for HPAI clade 2.3.4.4 in 2014. All residential wild birds are assumed to be susceptible to HPAIV infection at the start of the migration season, reflecting what is seen as a worst‐case scenario. Virus transfer among wild birds depends on their densities in the area where infected migratory water birds land. No data are available on the density of migratory and residential wild birds covering the whole EU territory. Wetland densities are sometimes used as a proxy for wild bird densities ((e.g. Empres Watch, September 2016, Volume 36 FAO EMPRES watch, 2016)), but expert elicitation made clear that this does not reflect the real situation because wild bird densities are also dependent on other characteristics such as food availability and surrounding vegetation (EFSA, 2016a). Also, inner coastal areas have seasonally high densities of wild migrating water birds, e.g. between the Danish islands. Therefore, a virtual contact area was considered in the model where the virus is transferred via infected excretions (including faeces, respiratory excretions, etc.), hence leading to virus amplification in the wild bird reservoir (Figure 4). Four scenarios were considered with respect to the proportion of migratory and resident wild birds and the proportion of water and non‐water birds out of the total population of wild birds in the virtual area (Table 5).
Figure 4.
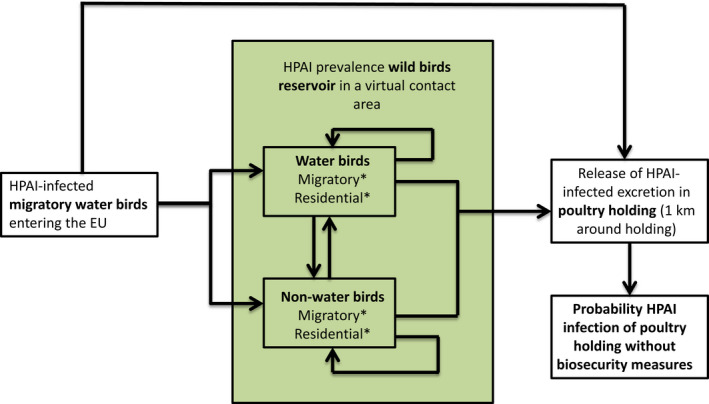
Proposed entry pathways of HPAI viruses from migratory water birds into a poultry holding
- The white fields represent components of the model based on real field situations, whereas the green fields represent components based on theoretical scenarios.
- *Four scenarios are considered with respect to proportion of migratory/resident wild birds and proportion of water/non‐water birds (see Table 5).
Table 5.
Four scenarios are considered with respect to proportion of migratory and resident wild birds and proportion of water and non‐water birds out of the total population
| Scenario | % migratory vs % resident wild birds | % water birds vs % non‐water birds | Representing a virtual contact area |
|---|---|---|---|
| 1 | 90% vs 10% | 90% vs 10% | Close to migration corridors and in a region with high presence of water bodies |
| 2 | 90% vs 10% | 10% vs 90% | Close to migration corridors and in a region with low presence of water bodies |
| 3 | 10% vs 90% | 90% vs 10% | Far from migration corridors and in a region with high presence of water bodies |
| 4 | 10% vs 90% | 10% vs 90% | Far from migration corridors and in a region with a low presence of water bodies |
The contact of a wild bird with an infected excretion could occur in wetlands or while foraging in surrounding terrestrial habitats. A random pattern is assumed for the release of infected excretions and for the contact of wild birds with excretions because wild birds move randomly and can defecate frequently. Water birds were considered more likely to get infected through other water bird excretions than by non‐water bird excretions because they live in the same ecological niches (Kear, 2005). The quantity of excretions is not considered as its effect on the probability of contact with excretions was assumed to be negligible compared with that of sharing the same habitat preferences with other (potentially infected) individuals. Conversely, the number of infected excretions is assumed to be directly proportional to the number of birds shedding the virus. The model also assumes that wild birds can become infected via contact with virus persisting for 13 days22 in excretions present in the environment. Once infected, birds shed the virus, subsequently they either die or become resistant for the rest of the simulated season. Based on available evidence, the model considered that once a wild bird becomes infected, it can transmit the virus to another susceptible wild bird from the next day onwards. More details on the model simulating AIV dynamics in the wild bird population can be found in Section C.3 of Appendix C.
The model outcomes suggest that entry of HPAIV‐infected water birds into the EU only leads to an epidemic in the wild bird reservoir when the number of susceptible wild water birds is above a critical number.23
When the number of water birds is high (scenarios 1 and 3), this will result in an increase of virus prevalence within the wild bird reservoir after the entry of infected wild water birds but only for total population sizes of 100,000 or 10,000. In scenario 1, due to the high number of migrating water birds arriving daily, prevalence of infected water birds increases a few days after entry. When 648 susceptible water birds enter daily (i.e. when the total population size at the end of the migratory season reaches 100,000 wild birds), the prevalence increases after 2 days and reaches a plateau around day 25 of the migration season (median 0.002, 95th percentile24 0.029 at the end of the migration season i.e. day 125) (see Figure 5: scenario 1, population capacity 100,000 birds). The lower the number of susceptible water birds entering daily, the later the prevalence starts to increase: when the number of susceptible water birds entering daily is reduced to 65,25 the virus prevalence in the wild bird population will decrease until day 13 when the critical number of wild birds is reached. Then, the virus prevalence increases gradually in the wild bird reservoir until the end of the migration season (median 0.000, 95th percentile 0.020 at day 125) (see Figure 5: scenario 1, population capacity 10,000 birds). In scenario 3, due to the high number of resident water birds, virus prevalence immediately increases within the wild bird reservoir after the entry of infected wild water birds (95th percentile 0.020 at day 125) (see Figure 5: scenario 3, population capacity 100,000 birds). Having sufficient susceptible resident wild birds but lowering the number of infected wild water birds entering, results in lower maximum prevalence levels in the wild bird reservoir (95th percentile 0.004 at day 125) (see Figure 5: scenario 3, population capacity 10,000 birds). It was not possible26 to use the model generated for this opinion to simulate whether and how long the virus could be perpetuated in the wild bird population. During the 2016–2017 outbreaks, when a high incidence of infection was detected in wild bird populations and significant contamination of the environment occurred, virus‐positive wild birds have been reported even 10 months after the first events (EFSA, ECDC, EURL, 2017a, 2017b).
Figure 5.
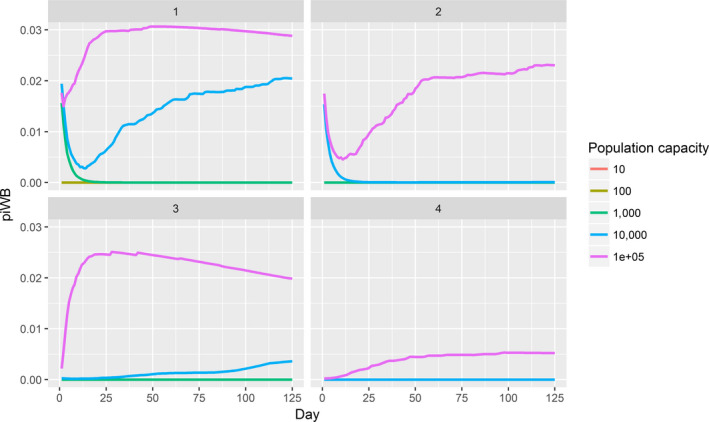
Modelled HPAI clade 2.3.4.4 worst‐case prevalence (95th percentile) in wild water birds (piWB) after entry of infected wild water birds at day 1 of the migration season. Different population sizes (10–100,000) and scenarios 1–4 are presented. Scenario 1: 90% water birds, 90% migratory birds; scenario 2: 10% water birds, 90% migratory birds; scenario 3: 90% water birds, 10% migratory birds; scenario 4: 10% water birds, 10% migratory birds
Reducing the number of water birds in the wild population (scenarios 2 and 4), will lead to an epizootic in the residential wild bird population only when considerable numbers of susceptible water birds enter (see Figure 5, scenario 2)27 or considerable numbers of susceptible water birds are resident (see Figure 5, scenario 4).28
Simulating entry of infected wild water birds in the middle of the migration season (day 60) suggests immediate onset of an epizootic in the wild bird reservoir as the critical number of susceptible water birds is already available (see Figure E.2, Appendix E). The HPAI prevalence in wild water birds at the end of the migration season is similar to the level obtained when infected wild birds arrive already in the beginning of the migration season (comparison Figure 5 with Figure E.2).
Figure E.2.
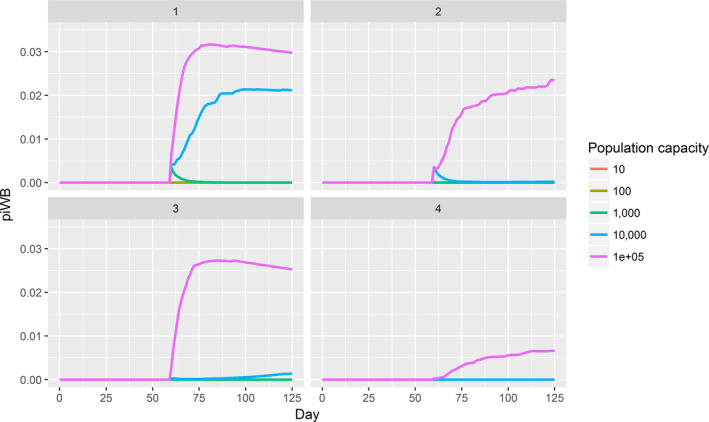
Modelled HPAI clade 2.3.4.4 prevalence (95th percentile) in wild water birds (piWB) after entry of infected wild water birds at day 60 of the migration season. Different population capacities (10–100,000) and scenarios (1–4, see definition in Table 5 in Section 3.2.2) are presented
It has to be noted that very low HPAI clade 2.3.4.4 prevalence levels in wild water birds are obtained in all simulated scenarios.
Probability of HPAIV clade 2.3.4.4 entry into poultry holdings without biosecurity measures
HPAI‐infected migratory water birds entering the EU and/or HPAI‐infected birds from the wild bird reservoir around a poultry holding could release infectious excretions in and/or around the poultry houses of a holding (Figure 4). Any mechanism of virus transfer from an infectious excretion in and/or around the poultry houses to the animals is considered because our understanding of these processes is not sufficient to further differentiate these. A study performed in the Netherlands showed a significant lower relative risk of LPAIV introduction into poultry holdings when the distance to wild waterfowl areas and medium size waterways was greater than 250 m (with slight further reduction in risk with increasing distances) (Bouwstra et al., 2017). In the model, therefore, the environment within an area with a radius of 1 km around a poultry holding (= 3.14 km2 surface) is considered crucial to spark an initial poultry infection following passive (i.e. via fomites) transfer of virus. The release of infected excretions within the area around the poultry holding is defined as a function of the number of wild birds landing and their HPAI prevalence (see details in Section C.4 of Appendix C). Finally, the probability of a poultry holding to become infected is dependent on the level of biosecurity measures implemented (extrapolation of data on the relation between LPAI infection and biosecurity; e.g. Parker et al., 2012). To assess the worst‐case scenario, a poultry holding without biosecurity measures implemented is considered, having outdoor housing (the place where poultry is kept is surrounded by fences but there is no horizontal protection to prevent access of wild birds), the presence of domestic waterfowl and of gallinaceous poultry, and presence of a water body on the premises accessible to both domestic waterfowl and wild birds. The effect of implementing biosecurity measures on the probability of a poultry holding becoming infected with AIV is described in section further below.
The evolution of the daily probability of a holding without biosecurity to become HPAI clade 2.3.4.4 infected during the migratory season (see Figure E.4, Appendix E) follows the kinetics of virus prevalence in wild water birds (Figure 5). HPAI clade 2.3.4.4 prevalence in wild non‐water birds (see Figure E.3, Appendix E) is more than 10‐fold lower compared with prevalence in water birds. A sensitivity analysis confirmed that mainly prevalence of migratory infected water birds (pi_MWB) drives the probability of a holding to become infected (see Figure E.5, Appendix E). This reflects the original assumption that only water birds can bring the virus into the EU.
Figure E.4.
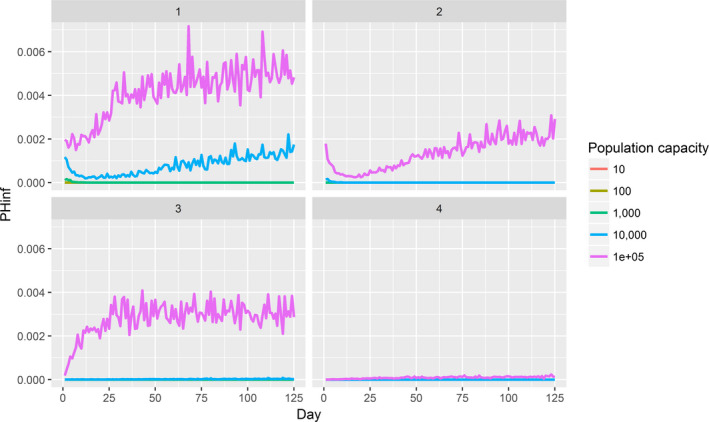
Daily probability (95th percentile) of a poultry holding without biosecurity to become infected with HPAI clade 2.3.4.4 after entry of infected wild birds at day 1 of the migration season. Different population sizes (10–100,000) and scenarios (1–4, see definition in Table 1) are presented
Figure E.3.
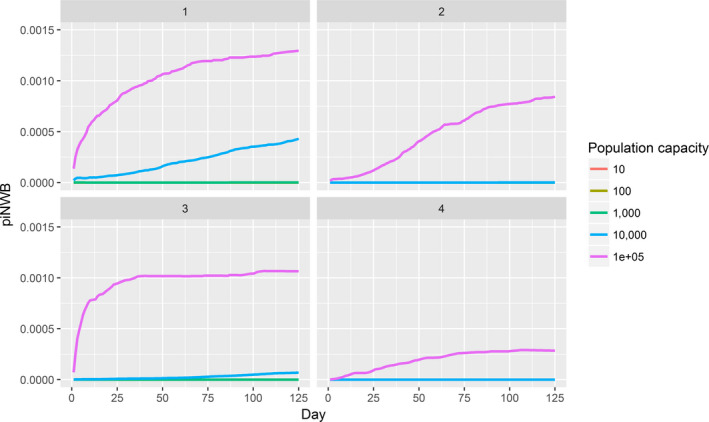
Modelled HPAI clade 2.3.4.4 prevalence (95th percentile) in wild non‐water birds after entry of infected wild water birds at day 1 of the migration season. Different population capacities (10–100,000) and scenarios (1–4, see definition in Table 5 in Section 3.2.2) are presented
Figure E.5.
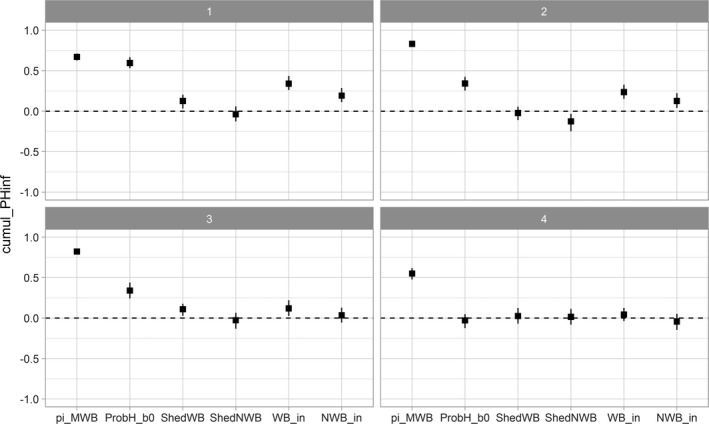
Sensitivity analysis on the seasonal probability that a poultry holding without biosecurity becomes infected after entry of infected wild birds at day 1 of the migration season, for the four scenarios (as defined in Table 5 in Section 3.2.2). A wild bird population capacity of 100,000 birds is considered
- pi_MWB, prevalence of migratory infected water birds; ProbH_b0, probability that a poultry holding is infected due to the presence of infected wild birds; ShedWB, shedding period for water birds; ShedNWB, shedding period for non‐water birds; WG_in, number of water birds present in the holding premise; NWB_in, number of non‐water birds present in the holding premise.
The model suggests that a poultry holding without biosecurity could become infected29 with HPAIV clade 2.3.4.4 when the wild bird reservoir consists of more than 1,000 birds (consisting of 90% migratory birds, 90% water birds; scenario 1). The median seasonal probability of poultry holdings without biosecurity becoming HPAIV clade 2.3.4.4 infected is 0 and 2 (94 and 407, 95th percentile) per 1,000 holdings when considering wild bird populations of 10,000 and 100,000 birds, respectively (Figure 6). The daily and seasonal probability of a poultry holding without biosecurity to become infected are lower in scenarios 2–4 compared with scenario 1 (see Table E.1, Appendix E). Furthermore, it should be noted that the risk period can be longer than a migration season of 125 days as considered here in the model. In the HPAI H5N8 2016/2017 outbreak, infected wild birds were found during more than 10 months (EFSA, ECDC, EURL, 2017).
Figure 6.
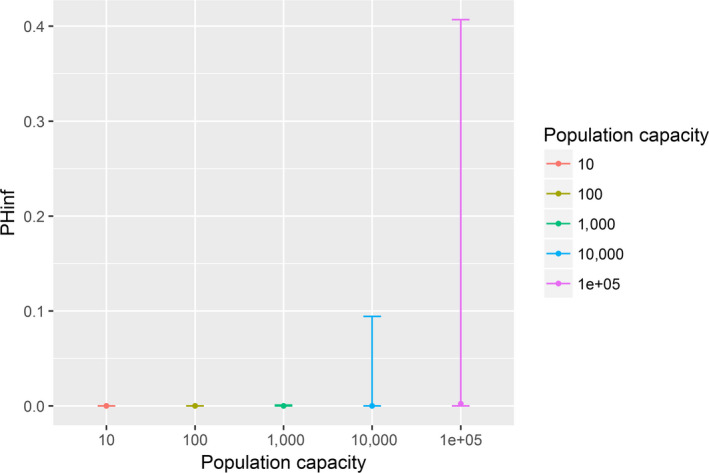
Seasonal probability (median (lower bar) – 95th percentile (upper bar)) of a poultry holding without biosecurity becoming infected with HPAIV clade 2.3.4.4 via wild birds over the entire migratory season, when this holding is located in an area where 10–105 wild birds are present (consisting of 90% migratory birds, 90% water birds; scenario 1)
Table E.1.
Daily and seasonal probability that a poultry holding without biosecurity could become infected after entry of infected wild birds at day 1 of the migration season. Different population sizes (ranging from 100,000 to 100) and scenarios (1–4, see definition in Table 5 in Section 3.2.2) are presented
| Scenario | Population capacity | Daily probability at day 125 | Seasonal probability | ||
|---|---|---|---|---|---|
| Median | 95th percentile | Median | 95th percentile | ||
| 1 | 100,000 | 0.0000347 | 0.004827 | 0.00227 | 0.406861 |
| 10,000 | 0 | 0.001743 | 0 | 0.094249 | |
| 1,000 | 0 | 0.00000145 | 0 | 0.000827 | |
| 100 | 0 | 0 | 0 | 0 | |
| 2 | 100,000 | 0 | 0.002918 | 0 | 0.156423 |
| 10,000 | 0 | 0.00000485 | 0 | 0.000778 | |
| 1,000 | 0 | 0 | 0 | 0 | |
| 3 | 100,000 | 0 | 0.002867 | 0 | 0.298166 |
| 10,000 | 0 | 0.0000422 | 0 | 0.002877 | |
| 1,000 | 0 | 0 | 0 | 0 | |
| 4 | 100,000 | 0 | 0.000128 | 0 | 0.011515 |
| 10,000 | 0 | 0 | 0 | 0 | |
It can be concluded that the HPAIV prevalence in water birds as well as the size and composition of the wild bird reservoir are determining the probability of a holding to become infected. It should be noted that comparing the scenarios gives useful insights whereas the specific numerical outcomes of the model should be considered with caution given all the assumptions of the model.
Reducing probability of HPAIV clade 2.3.4.4 entry into poultry holdings by implementing biosecurity measures
The text above describes the probability of poultry holdings becoming infected by HPAIV clade 2.3.4.4 when no biosecurity measures are implemented on the premises. An EKE was performed to estimate the fold reduction of HPAIV entry when implementing measures to attain increasing levels of biosecurity. Very few quantitative data on the effect of biosecurity measure related to prevention of AIV incursions are published (Ssematimba et al., 2013; Millman et al., 2017). More systematic research has been addressed to bacterial infections such as Salmonella or Campylobacter (e.g. Newell et al., 2011) but due to the distinct features of AIV and bacteria these data were deemed not convertible. The experts took the median probability value of a worst‐case poultry holding (no biosecurity) as the benchmark value (two holdings infected out of 1,000) and estimated the fold reduction of the holding probability to become infected when stepwise implementing biosecurity measures and considering exposure to 100 wild birds. The outcome of the expert elicitation suggests that preventing access of poultry to waterbodies on the premises could result in a threefold reduction in HPAIV entry probability. Combining this measure with confining poultry to indoor housing was estimated to further reduce the HPAI entry probability twofold. If in addition, routine (daily average practised) biosecurity30 or high biosecurity31 (as practised in nucleus or breeding herds) would be applied, the estimated fold reduction of HPAI entry was around 4 and 44, respectively.
The estimated effect of biosecurity measures was considered to be independent of the virus characteristics. Therefore, the results are relevant for HPAI and LPAI viruses. Based on these values, the implementation and maintenance of carefully selected and adapted biosecurity measures are key in the protection of poultry holdings from AIV incursions (Figure 7).
Figure 7.
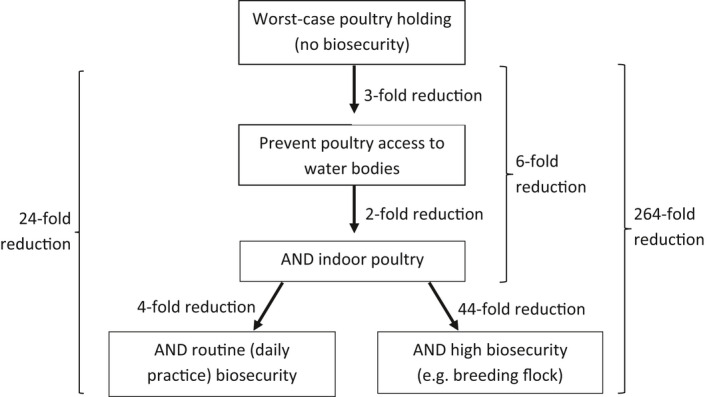
Based on median value of probability that holdings will become infected with HPAI clade 2.3.4.4 given the exposure to 100 infected wild bird, fold reductions were calculated for increasing level of biosecurity
3.2.3. NE route – clades 2.2.1.2 and 2.3.2.1c
The mathematical model was also used to assess entry of HPAI clades 2.2.1.2 and 2.3.2.1c, changing the values of parameters that were considered to be different from HPAI clade 2.3.4.4 (see Table C.7, Appendix C).
Table C.7.
List of variables used in the HPAI clades 2.3.2.1c and 2.2.1.2 simulation model (which are different compared to the HPAI clade 2.3.4.4 variables, see Table C.6) with description, type of EKE performed, type of distribution fitted and relative parameter estimates
| Parameter | Description | Source | Uncertainty distribution shape | U distr par1 | U distr par2 | U distr par3 | U distr par4 | More info |
|---|---|---|---|---|---|---|---|---|
| π MWB | Number of HPAI clade 2.3.2.1c infected HPAIV water birds at the moment they cross the EU border out of 106 | Semi‐formal EKE | Weibull | 0.53 | 191.45 | NA | NA | Table D.1 |
| Number of HPAI clade 2.2.1.2 infected HPAIV water birds at the moment they cross the EU border out of 106 | Semi‐formal EKE | Weibull | 0.62 | 14.67 | NA | NA | Table D.1 | |
| 1/r NWB | Reciprocal of the duration in days of the HPAI clades 2.3.2.1c and 2.2.1.2 shedding period in non‐water birds | Semi‐formal EKE | tnorm | −17.02 | 10.22 | −0.1 | 10.37 | Table D.1 |
| 1/r WB | Reciprocal of the duration in days of the HPAI clades 2.3.2.1c and 2.2.1.2 shedding period in water birds | Semi‐formal EKE | Weibull | 1.45 | 4.93 | NA | NA | Table D.1 |
| md NWB | Mortality rate in non‐water birds due to HPAI clade 2.3.2.1c disease | Literature | Constant | 1 | NA | NA | NA | Table D.2 |
| Mortality rate in non‐water birds due to HPAI clade 2.2.1.2 disease | Literature | Constant | 0.79 | NA | NA | NA | Table D.2 | |
| md WB | Mortality rate in water birds due to HPAI clade 2.3.2.1c disease | Literature | Constant | 0.63 | NA | NA | NA | Table D.2 |
| Mortality rate in non‐water birds due to HPAI clade 2.2.1.2 disease | Literature | Constant | 0.79 | NA | NA | NA | Table D.2 |
Entry into the EU
Experts estimated that the number of HPAI clade 2.3.2.1c infected wild water birds at the moment they cross the EU border is very similar to that of HPAI clade 2.3.4.4 infected water birds (median of 100 and 114 out of 1,000,000 birds, respectively), whereas this number is estimated to be much lower for HPAI clade 2.2.1.2 infected water birds (median of 8 out of 1,000,000 birds).
For clade 2.3.2.1c, the model outcome suggests similar probability that at least one infected migratory water bird enters the EU via the NE route as for HPAIV clade 2.3.4.4. For clade 2.2.1.2, the probability that at least one infected migratory water bird enters the EU via the NE route is similar as for HPAIV clade 2.3.4.4 (median probability 100%) but the number of infected water birds entering is more than 10‐fold lower.32
Entry into holding
Most parameters related to HPAI amplification in the wild bird reservoir are the same for the three clades, whereas available scientific evidence suggests differences on the prevalence of infected wild birds and shedding period. The sensitivity analysis of the model indicated that the prevalence value influences the seasonal probability of holdings becoming infected, whereas the duration of the shedding is less influential (Figure E.5, Appendix E). The differences in prevalence between the three clades for birds entering via the NE route are described in the paragraph above.
HPAIV H5N1 persistence in seawater has been noted as an additional possible route of further spread. Prolonged retention of viral infectivity was explicitly noticed for brackish seawater with lower salinity such as present in some parts of the Baltic Sea (Domanska‐Blicharz et al., 2010; Willeberg et al., 2010; Perez et al., 2011; Alkhamis et al., 2012). So dispersion of AIV, e.g. from avian faecal droppings, in shallow seawater may contribute to viral spread, at least locally. In contrast, tidal currents may cause huge dilution effects that counteract regional and even local spread.
Outcomes of the model suggest that the relative ranking of the seasonal probability of holdings becoming infected with one of the three clades during a migratory season is similar to their prevalence.
For HPAIV clade 2.3.2.1c, the median seasonal probability of poultry holdings without biosecurity becoming infected is between six‐ and three‐fold smaller33 than for HPAIV clade 2.3.4.4 for scenario 1. The seasonal probability of a poultry holding without biosecurity to become infected is lower in scenarios 2, 3 and 4 compared with scenario 1.
For HPAIV clade 2.2.1.2, the median seasonal probability of poultry holdings without biosecurity becoming infected is 10‐fold smaller34 than for HPAIV clade 2.3.4.4 for scenario 1. The seasonal probability of a poultry holding without biosecurity to become infected is negligible35 in scenarios 2, 3 and 4.
3.2.4. E route – clades 2.3.4.4, 2.2.1.2 and 2.3.2.1c
The assessment of the probability that HPAI enters the EU by migratory wild birds entering the EU via the E, S or NW route has been carried out by analysing the differences with respect to HPAI clade 2.3.4.4 entry via the NE route (which is used as a benchmark, see Figure E.1). The outcome is the identification of a possible fold change using the terminology defined in Section 2.2.
Entry into the EU
The eastern route comprises a sector reaching from Ukrainian/Moldavian borders with the EU across the Black Sea until the southern border of Turkey. Migrating wild birds or birds driven by cold spells out of Central Asia are expected to enter the EU territory via this route.
Using the above‐described benchmark of clade 2.3.4.4 entry via the NE entry route (see Section 3.2.2), potential sources of uncertainties related to the extrapolation of the benchmark probability of entry from NE to other entry routes are described with respect to the different viral clades of HPAIV H5. In principle, water birds entering the EU via either the NE or E routes are originating from the same breeding grounds scattered across Arctic and Palaearctic Siberia. These populations are believed to be at heightened risk of direct or indirect exposure to HPAIV of subtype H5 of Asian origin during migration. Although this has not been specifically studied, there is no reason to believe that autumn migration length and dates via the E route would be different that from the NE route. Introduction of HPAIV H5 via the E routes is, however, not necessarily restricted to the (autumn) migration season but might as well be triggered by sudden cold spells. Rapid regional relocation in an east‐to‐west direction of local water bird populations to escape cold spells that renders surface waters inaccessible for feeding have been shown to be associated with outbreaks of HPAIV H5N1 clade 2.2 in 2005–2006 in the EU (Ottaviani et al., 2010; Reperant et al., 2010). Cold spells in Europe often occur later in the winter (e.g. in January or February) but these can also occur earlier (for instance in December). It should be taken into account that the estimated population size of water birds in the Black Sea area is larger than the NE one in several species.36 For example, the estimation is 500,000 common teal (Anas crecca) in north‐west Europe compared with 1,000,000 individuals in the population comprising western Siberia, NE Europe the Black Sea and Mediterranean areas (Wetlands International, 2017b, 37). Similar figures for common pochard (Aythya ferina) are 250,000 and a range from 570,000 to 630,000, respectively.
Besides the number of migratory wild birds entering the EU via the E route, the probability of entry of HPAIV into the EU is also dependent on the virus prevalence in the migratory wild bird population.
For clade 2.3.4.4, there are a few (two as of 12 January 2017) reports of infected wild birds at the EU border with Ukraine and Moldova, although it should be noted that the surveillance intensity is low in this area. It is assumed that the prevalence of infected migrating wild birds would be similar compared with the NE route but has a higher uncertainty. So far, there is no evidence that HPAI clade 2.3.4.4 has been introduced into the EU via the E route in 2014 (Lycett et al., 2016). The introduction pathways for the 2016 outbreaks of HPAI 2.3.4.4 are still under investigation. Taken together, it can be concluded that the probability of HPAIV clade 2.3.4.4 entering the EU via the E route is slightly lower (between 1.5‐ and 3‐fold) compared with entry of such viruses via the NE route.
For HPAIV clade 2.2.1.2, there are no recent reports of infected wild birds close to the EU border with Ukraine, Moldova and Turkey, although it should be noted that the surveillance intensity is low in this area. In fact, 2.2.1.2 viruses have remained endemic in Egypt with few incursions into Israel and Libya; elsewhere viral descendants of the former clade 2.2 are no longer extant. In conclusion, it is expected that the probability of HPAIV clade 2.2.1.2 entering the EU via the E route is much lower (between 10‐ and 1000‐fold) compared with HPAIV 2.3.4.4 entry via the NE route.
For HPAIV clade 2.3.2.1c, there are various reports of infected wild birds (and poultry) in Central Asia (see Figure 1 in Section 3.1.1). Entrance of such viruses into the EU via the E route likely occurred for instance in March 2010 (Bulgaria and Romania) (Reid et al., 2011; Marinova‐Petkova et al., 2012). These could have been the result of classical migration or deviated migration due to a cold spell. It is hence concluded that the probability of HPAI clade 2.3.2.1c entering the EU via the E route is likely to be similar compared with HPAI 2.3.4.4 entry via the NE route.
3.2.5. S route – clades 2.3.4.4, 2.2.1.2 and 2.3.2.1c
Entry into the EU
The southern route concerns all wild birds with migrations originating south of Turkey, i.e. the Middle East and Africa.
Entry via this route is considered to occur during spring migration as birds fly back from wintering grounds in Africa. For a given species, the spring migratory season is often contracted compared with the more diffuse autumn migration (see for instance Guillemain et al., 2006 for common teal).
Comparison of the number of migratory birds entering the EU via the S route in comparison to the NE route is not easy. However, it is considered that the birds wintering in Africa are mostly breeding north‐east of the EU (mainly in the Russian Federation; Scott and Rose, 1996), so that birds entering in spring via the S route would be a fraction of those that entered via the NE route the previous autumn. Given that water birds have high mortality rates during autumn and winter, especially in juveniles (Guillemain et al., 2010, 2013), it was therefore considered that the number of birds entering the EU via the S route during spring would be approximately 50–75% of these entering the EU via the NE route.
An introduction of HPAIV into the EU via the S route would probably be restricted to the spring migration period since this is the only period when wild birds enter from the south. There is a generally higher prevalence of AIV infection in wild water birds in sub‐Saharan Africa gradually increased from the autumn to the end of the winter, as the migrants reached these wintering grounds, to reach the highest values from January to March when spring migration commences (Gaidet et al., 2012).
Besides the number of migratory wild birds entering the EU via the S route, the probability of entry into the EU is also dependent on the virus prevalence in the migratory wild bird population.
For HPAIV clade 2.3.4.4, the first virus detections in wild birds in Africa and the Middle East have been reported in 2016–2017 (e.g. Egypt, Israel, Tunisia, Uganda). Additionally, these viruses were detected in free‐ranging poultry in Nigeria and Cameroon. However, surveillance is limited in wild birds in northern Africa and the Middle East. Taken together, the probability of HPAIV clade 2.3.4.4 entering the EU via the S route is much lower compared with HPAIV 2.3.4.4 entry via the NE route.
For HPAIV clade 2.2.1.2, there are detections reported in wild birds in Egypt as well as in poultry in Egypt and its directly neighbouring countries such as Libya, Palestinian Territories (see Figure 1 in Section 3.1.1). The rare detections of clade 2.2.1.2 virus in wild birds might be due to inefficient spill‐over from poultry to migratory wild birds. This could be due to a lack of contact, although there are some important wintering grounds and likely migratory stopovers in the area, e.g. along the Nile Valley in Egypt (e.g. Scott and Rose, 1996). In conclusion, it is estimated that the probability of HPAIV clade 2.2.1.2 entering the EU via the S route is lower (between 3‐ and 10‐fold38) compared with HPAIV 2.3.4.4 entry via the NE route.
For HPAIV clade 2.3.2.1c, poultry outbreaks have been reported in multiple sub‐Saharan West African countries (see Section 3.1.1 and Figure 1). Moreover, the virus is present in several countries of the Middle Eastern region such as Lebanon, Iraq and Iran. So far, 2.3.2.1c viruses have not been transmitted from sub‐Saharan Africa into Egypt or other northern African countries. Therefore, the probability of HPAIV clade 2.3.2.1c entering the EU via the S route is considered lower (between 1.5‐fold and 3‐fold) compared with HPAIV 2.3.4.4 entry via the NE route.
3.2.6. NW route – clades 2.3.4.4, 2.2.1.2 and 2.3.2.1c
Entry into the EU
There are data suggesting migration of Anatidae and Charadriiformes species from Palaearctic NE America via Greenland and Iceland to the United Kingdom, Ireland and other NW EU areas (Scott and Rose, 1996; Guillemain and Elmberg, 2014).
The length of the migration season is assumed to be similar for the NW and NE route, although there are no data to underpin this hypothesis.
Within the metapopulation of all AIV subtypes, two geographically restricted clusters can be distinguished genetically: an American and a Eurasian one. This observation points towards prolonged and efficient separation of the reservoir replication pools of these groups. In fact, wild bird migration between the American continent and Asia or Europe is limited and poses an active barrier to intermingling of the respective virus groups. Nevertheless, ornithological data accumulate which indicate occasional transcontinental bird migration, in particular via the Bering Strait (Peters et al., 2012). Accordingly, interhemispheric reassortants of AIV have been detected repeatedly (Dugan et al., 2008; Kishida et al., 2008; Fries et al., 2013). These also include HPAIV of clade 2.3.4.4 that were introduced from Asia to North America in autumn 2014 by migrating wild birds, although most likely through the Bering Strait (Lycett et al., 2016). Reassortment with indigenous North American LPAI viruses yielded HPAIV H5N1 and H5N2 viruses, the latter of which became widespread in poultry in the USA in 2015 (Bevins et al., 2016; Claes et al., 2016; Lee et al., 2016). A natural translocation of ‘Americanised’ HPAIV of clade 2.3.4.4 would depend on complex transmission routes which involve relay‐like virus transmission between wild bird populations resident in America and breeding in Palaearctic NE America to geese and wader populations that breed in the same area but migrate via Greenland and Iceland into Western Europe in autumn. Virus transfer via this complex route is assumed to be a rare event.
Besides the number of migratory wild birds entering the EU via the NW route, the probability of entry of HPAIV into the EU is also dependent on the virus prevalence in the migratory wild bird population. HPAIV of clade 2.3.4.4 has been leaking into North America since autumn 2014 across the Bering Strait along the Pacific flyway in a southerly direction (Lee et al., 2015). In late winter of 2015, reversed virus dispersal in a northerly direction along the Rocky Mountain and Central Mississippi flyways was recorded (Saito et al., 2015; Bevins et al., 2016). Substantial numbers of poultry holdings along these flyways were found to be infected, although a large proportion of poultry cases were apparently due to secondary virus spread within the poultry industry (USDA APHIS VS, 2015). Detection ceased in summer of 2015 and a solitary redetection of HPAIV 2.3.4.4 was reported in September 2016 in a mallard from Alaska (OIE, 2016b). Taken together, the probability of HPAIV clade 2.3.4.4 entering the EU by wild birds via the NW route is much lower (between 10‐ and 1,000‐fold) compared with HPAIV 2.3.4.4 entry via the NE route. Viruses of clades 2.2.1.2 and 2.3.2.1c have never been reported in North America (Figure 1, Section 3.1.1), hence the probability that they enter the EU via the NW route is extremely low (more than 1,000‐fold lower) compared with HPAIV clade 2.3.4.4 entry via the NE route.
3.2.7. Overview of seasonal probability of holdings without biosecurity becoming infected for all routes and clades
The relative ranking of the seasonal probability of holdings without biosecurity becoming infected with one of the three clades during a migratory season is similar to the relative ranking of the probability that these viruses enter the EU by wild birds via a given migration route since both probabilities are proportional to the number of infected wild birds entering the EU.
An overview of the seasonal probability of holdings without biosecurity is provided in Table 6. The outcomes for the NE route are obtained via modelling (see Sections 3.2.2 and 3.2.3). For the E, S and NW routes, the probability is estimated using HPAIV clade 2.3.4.4 (NE route) as the benchmark and applying an uncertainty factor. The scientific evidence underpinning these estimates is provided in Sections 3.2.4–3.2.6. This assessment has been performed in January 2017 and should be updated when new epidemiological information becomes available.
Table 6.
Seasonal probability of poultry holdings without biosecurity becoming infected with HPAIV, following entry of infected migratory water birds via the NE, E, S or NW route
| Clade | NE routea | E routec | S routed | NW routee |
|---|---|---|---|---|
| 2.3.4.4 | Benchmarka | Slightly lower | Much lower | Much lower |
| 2.2.1.2 | Much lowerb | Much lower | Lower | Extremely low |
| 2.3.2.1c | Similarb | Similar | Lower | Extremely low |
Similar, up to 1.5‐fold higher or lower than the benchmark value; slightly lower, between 1.5‐ and 3‐fold lower than the benchmark value; Lower, between 3‐ and 10‐fold lower than the benchmark value; much lower, between 10‐ and 1,000‐fold lower than the benchmark value; extremely low, more than 1,000‐fold lower than the benchmark value.
Based on model outcome, described in Section 3.2.2, and used as benchmark.
Based on model outcome, described in Section 3.2.3.
Based on expert judgement, described in Section 3.2.4.
Based on expert judgement, described in Section 3.2.5.
Based on expert judgement, described in Section 3.2.6.
3.3. LPAI introduction via migratory and/or residential wild birds
3.3.1. LPAI introduction into the EU by migratory wild birds (TOR 5)
There is a continuum of LPAI subtype H5 and H7 viruses which is maintained in the Eurasian metapopulation of aquatic wild birds in an endemic manner (Munster et al., 2007). These viruses are regularly detected at fluctuating prevalences in wild birds in many European countries. Peak prevalences are usually seen during autumn migration.
Zoonotic properties for these viruses have, so far, only been described for several H7 LPAIV lineages which have caused mild ophthalmological or respiratory symptoms in people (Abdelwhab et al., 2014). Compared with viruses of the highly zoonotic LPAIV H7N9 lineage of Chinese origin, European origin H7 viruses appear to have grossly reduced zoonotic propensities (Kalthoff et al., 2014a). Representatives of the highly zoonotic Chinese H7N9 LPAIV lineages have to date not been detected in wild birds outside China. The reservoirs of these viruses in China have not definitively been unravelled, so it remains extremely difficult to assess risks of a translocation by wild birds to outside China (Wang et al., 2013, 2014; Jones et al., 2014; Zhao et al., 2014; Lam et al., 2015; Chen et al., 2016).
In addition to LPAIV of subtype H5 and H7, the H9N2 viruses must also be considered with respect to zoonotic risks. Asian origin H9N2 viruses of the G1 lineages have been shown to sporadically infect humans and other mammals including swine (Butt et al., 2010; Yu et al., 2011). Mutations in the HA of these H9N2 viruses have been associated with a shift of the receptor binding affinity towards alpha‐2,6 sialic acid residues which explains at least in part their increased zoonotic propensity (Wan et al., 2008; Imai and Kawaoka, 2012; Srinivasan et al., 2013; Li et al., 2014). In addition, G1‐like H9N2 viruses are frequently implicated as donators of internal gene segments in reassortments with H7 and H5 subtype viruses (Pu et al., 2015). The Chinese H7N9 zoonotic virus lineage carries six internal segments derived from genotype S of G1‐like H9N2 viruses (Gu et al., 2014). During the last decade, G1‐like H9N2 viruses have established endemic status in poultry populations in many Asian countries as well as in the Middle East and North African regions. Infection of poultry in these regions is extremely wide spread and frequent. The prevalence of G1‐like H9N2 infections in Eurasian wild birds in Far East and Central Asia is not known. The vast majority of circulating viral load of these viruses is associated with poultry, and wild birds are considered not to play an important role in their epidemiology.
In wild birds in Europe, viruses of this G1‐like lineage have not been detected although H9N2 infection is endemic within these populations as well; H9N2 viruses circulating in European wild bird populations constitute a separate phylogenetic lineage that is also distinguished from G1‐like viruses by their apparent lack of zoonotic potential (Lindh et al., 2014; Muzyka et al., 2016). Although present endemically in European wild bird populations, sporadic outbreaks of non‐G1 H9N2 infections in poultry are reported only occasionally from several European countries. An exception occurred in turkey populations in Germany, where the non‐G1‐like H9N2 infections circulated over several years and also caused infections in Poland through transboundary transport of infected pullets (Smietanka et al., 2014). Once established in poultry populations, non‐G1 H9N2 viruses can cause substantial economic losses due to significant morbidity of to 20% especially in young turkeys. These viruses may pose a challenge to control since H9N2 infections are not in the list of notifiable or reportable diseases in poultry and, hence, all measures of control remain at the disposal of the stockman.
Given that LPAIVs are endemic in the EU wild bird population, its introduction into the EU was not simulated with the current model. As described in Section D.1.3 (Appendix D), LPAI viruses of subtypes H5 and H7 are detected annually with seasonal variations in wild bird metapopulations in Europe. According to the EURL reports of the wild bird monitoring programmes of MSs, the data assembled by the Consortium of this working group and published data (Section D.1.3, Appendix D), the highest annual occurrences in Anseriformes wild birds are detected during autumn, but a scattered presence, especially of subtype H7 viruses, has also been described in spring.
3.3.2. LPAI introduction into a poultry holding from the wild bird reservoir (TOR 4 and TOR 5)
For the simulations of LPAIV entry into a poultry holding via wild birds, scenarios are selected to analyse the effect of H5, H7 and H9 LPAIV prevalence in the wild bird population (since this parameter had a large effect on the outcome of the HPAI model) and the effect of protective immunity (since this is an important biological factor in infectivity). LPAIV and HPAIV may infect and be transmitted by the same wild bird species, hence the same classification of wild birds into water birds and non‐water birds is used in both models (Tables B.1 and B.2, Appendix B). Experimental infections with LPAIV have shown that a transient, low‐level antibody response can be generated, which may be sufficient to provide partial protection against reinfection with viruses of the same subtype; it is less likely that the induced humoral response confers protection against heterologous reinfections (Fouchier and Munster, 2009). Yet, frequent reinfections of mallards with viruses of the same subtype have also been reported (Globig et al., 2013; Wille et al., 2014).
The LPAIV prevalence in Anseriformes wild bird populations is highly dependent on the time of the year and the geographical location (see Section D.1.3, Appendix D). Data from virus detection, isolation and characterisation were used from areas where LPAIV prevalence in wild birds was assessed over several years (Latorre‐Margalef et al., 2014; data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL) OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished (Consortium report Waldenström et al., 2017) to determine three values reflecting the most likely prevalence in many MSs (0.002), the most likely prevalence in northern MSs (0.02) and an extreme prevalence in northern MSs (0.06). These three prevalence values are considered to reflect low, moderate and high exposure scenarios that have been observed within the EU (Table 5).
The model was first used for a complete naïve wild bird population. The effect of immunity on the LPAI dynamics in the wild bird population and subsequently on the probability of LPAIV introduction into a poultry holding was assessed by running an additional simulation with prevalence 0.02 and with half of the wild bird population being immune.
For the LPAI model, the two highest wild bird population capacities (10,000 and 100,000 wild birds) are used as in the HPAI model, facilitating comparison of the model outcomes. As for the population composition, this was defined as 90% entering (10% resident) wild birds and 90% water birds (10% non‐water birds) since it was the highest risk scenario for HPAI. In fact, these conditions reflect the autumn period. The different scenarios analysed with the model are presented in Table 7. All parameters used in the LPAI model are provided in Table C.8 (Appendix C).
Table 7.
Scenarios analysed using the LPAIV model
| Prevalence | Scenario | Population size | Proportion entering wild birdsa | Proportion water birdsb | Proportion naïve birdsc |
|---|---|---|---|---|---|
| 0.06 (high exposure) | 1 | 10,000, 100,000 | 0.9 | 0.9 | 1.0 |
| 0.02 (moderate exposure) | 2 | 10,000, 100,000 | 0.9 | 0.9 | 1.0 |
| 3 | 10.000, 100,000 | 0.9 | 0.9 | 0.5 | |
| 0.002 (low exposure) | 4 | 10,000, 100,000 | 0.9 | 0.9 | 1.0 |
Proportion of wild birds entering the local area in relation to the (non‐entering) wild birds already present in this area.
Proportion of water birds (as opposed to non‐water birds) in the wild bird population.
Proportion of wild birds without protective immune response.
When the prevalence of infected entering water birds is high (0.06, see scenario 1 in Figure 8), an immediate onset of the epidemic and a rapid increase in the prevalence of infected water birds is observed during the first 10 days. When an average of 39 susceptible water birds enter daily, the prevalence immediately increases and reaches a maximum around day 10 (median 0.013, 95th percentile 0.017) and then decreases to 0.003 (95th percentile 0.007) at the end of the considered period (i.e. day 125) (see Figure 8: scenario 1, population capacity 100,000 birds). Reducing the prevalence of infected water birds entering the new foraging area (0.02 in scenario 2 and 0.002 in scenario 4), will lead to an epidemic in the residential wild bird population only when considerable numbers of (infected) water birds are present (which is the case in scenario 2 but barely the case in scenario 4 – because prevalence is low). For scenario 2, and with a population capacity 100,000, a maximum prevalence level of 0.004 (95th percentile 0.007) is reached in water birds at day 13, followed by a decrease of prevalence to 0.001 (95th percentile 0.004) at day 125. For scenario 4, LPAIV will only be maintained at a low level when a large wild bird population is present (100,000 birds).
Figure 8.
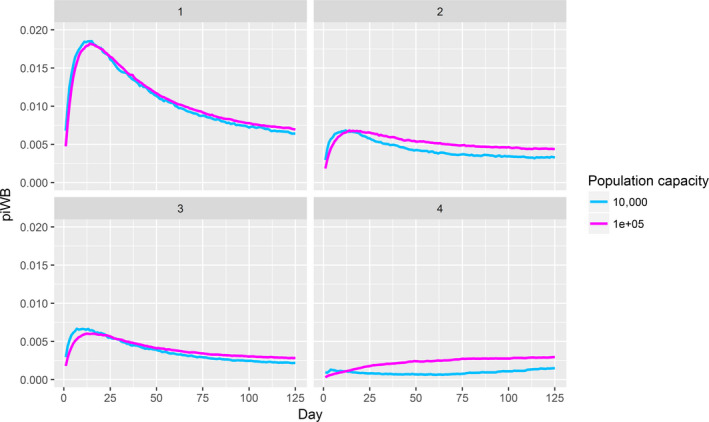
Modelled LPAI 95th percentile prevalence in wild water birds (piWB) after entry of infected wild water birds at day 1 of the migration season. Different population sizes (10,000 and 100,000) and scenarios 1–4 are presented. Scenario 1: 0.06 prevalence in infected entering water birds; scenario 2: 0.02 prevalence in infected entering water birds; scenario 3: 0.02 prevalence in entering infected water birds and 50% immune population; scenario 4: 0.002 prevalence in infected entering water birds
Simulating 50% of the wild bird population being immune (scenario 3) when the prevalence of entering infected water birds is 0.02 (same as in scenario 2), this leads to a similar prevalence pattern in time as when the total population was considered naïve. This can be explained by: (1) if 50% of the population is immune and R0 > 2, major outbreaks are still possible (Anderson and May, 1991) and (2) initially the population is only small, but it increases during the migratory season.
When compared with HPAI (see Figure 5 in Section 3.2.2), it can be concluded that LPAIV can reach similar maximum prevalence levels. However, sometimes after an initial peak, LPAIV levels are maintained at a lower level than HPAI in large populations of wild birds (100,000 birds). The model outcomes suggest that immunity in half of the birds (as occurs during autumn) does not really influence the LPAI spread in a wild bird population. The explanations here are the same as mentioned for HPAI above.
Probability of LPAI entry into poultry holdings without biosecurity measures
The evolution of the daily probability of a holding without biosecurity becoming LPAI infected during a period of 125 days (see Figure E.9, Appendix E) follows the kinetics of virus prevalence in wild water birds (Figure 8). LPAI prevalence in wild non‐water birds (see Figure E.8, Appendix E) is more than 10‐fold lower compared with prevalence in water birds.
Figure E.9.
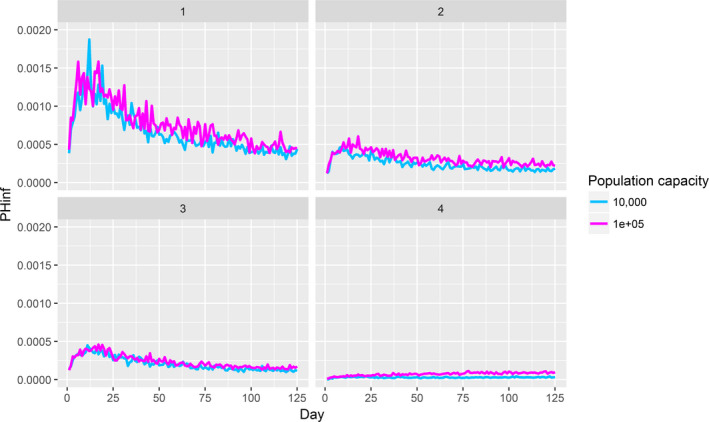
Daily probability (95th percentile) of a poultry holding without biosecurity to become infected with LPAI after entry of infected wild birds. Different population sizes (10–100,000) and scenarios (1–4, see definition in Table 7 in Section 3.3.2) are presented
The model suggests that a poultry holding without biosecurity could become infected with LPAI when the wild bird reservoir consists of 90% migratory birds and 90% water birds (scenario 1). The median probability of poultry holdings without biosecurity becoming LPAI infected over the season (125 days) is 9 and 12 (796 and 863, 95th percentile) per 10,000 holdings when considering wild bird populations of 10,000 and 100,000 birds, respectively (Figure 9). The daily and seasonal probability of a poultry holding without biosecurity to become infected are lower in scenarios 2, 3 and 4 when compared with scenario 1 (see Table E.2, Appendix E). The relative effect of implementing biosecurity on lowering the probability of LPAIV entry into a holding is similar to that which has been described for HPAIV (see end of Section 3.2.2).
Figure 9.
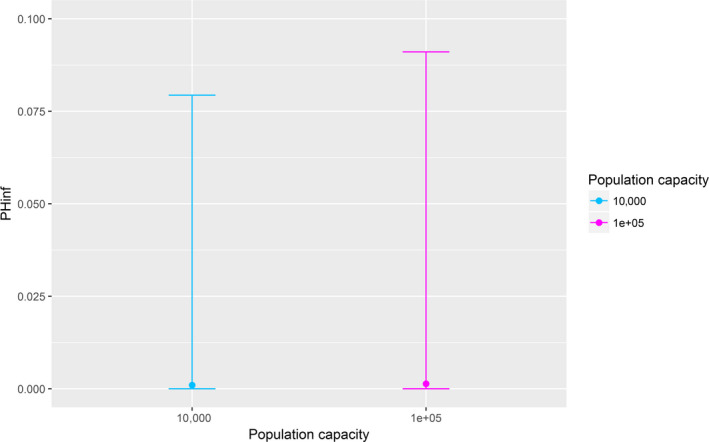
Seasonal probability (median (square) – 95th percentile (upper bar)) of a poultry holding without biosecurity becoming infected with LPAI via wild birds over the entire considered period (125 days), when this holding is located in an area where 10,000–105 naïve wild birds are present (consisting of 90% entering birds, 90% water birds; scenario 1)
Table E.2.
Daily and seasonal probability that a poultry holding without biosecurity could become infected after entry of LPAI‐infected wild birds. Different population sizes (ranging from 100,000 to 10,000) and scenarios (1–4, see definition in Table 7 in Section 3.3.2) are presented
| Scenario | Population capacity | Daily probability at day 125 | Seasonal probability | ||
|---|---|---|---|---|---|
| Median | 95th percentile | Median | 95th percentile | ||
| 1 | 100,000 | 7.56E‐06 | 0.000358 | 0.001286 | 0.086275 |
| 10,000 | 5.45E‐06 | 0.000361 | 0.000975 | 0.07956 | |
| 2 | 100,000 | 3.80E‐06 | 0.000202 | 0.000563 | 0.033405 |
| 10,000 | 2.78E‐06 | 0.000175 | 0.000385 | 0.028812 | |
| 3 | 100,000 | 2.78E‐06 | 0.000143 | 0.000464 | 0.031332 |
| 10,000 | 1.61E‐06 | 0.000109 | 0.000256 | 0.022919 | |
| 4 | 100,000 | 1.62E‐06 | 0.000104 | 0.000155 | 0.009643 |
| 10,000 | 3.32E‐07 | 2.55E‐05 | 2.63E‐05 | 0.003133 | |
It can be concluded that the LPAIV prevalence in water birds entering a forage area determine the probability of a holding becoming infected. In comparison to HPAI, the seasonal probability is fourfold lower for the 100,000 population capacity and similar for the 10,000 population capacity. This is in line with the observed lower probability of LPAI than HPAI outbreaks in poultry holdings.
It should be noted that comparing the scenarios gives useful insights, whereas the specific numerical outcomes of the model should be considered with caution given all the assumptions utilised in model.
3.4. HPAI and LPAI introduction via non‐wild bird pathways (TOR 1, TOR 3 and TOR 5)
A qualitative risk assessment has been performed for 10 pathways regarding HPAI and LPAI introduction (other than wild birds) into poultry holdings of MS in two scenarios: (i) third country trade and (ii) intra‐EU movements (see Section 2.3). The following terms were used to describe the probability of HPAI and/or LPAI introduction into a commercial poultry holding via a given commodity: negligible (indistinguishable from 0), extreme unlikely (up to 1%), very unlikely (from 1% up to 2%), unlikely (from 2% up to 10%) and non‐negligible (from 10% up to 100%) (see Section 2.3).
Several steps are required to delineate risks along the trade pathways. The country of origin for third country trade should be considered. For live birds, hatching eggs and day‐old chicks, there is a limited list of approved countries, under Commission Regulation (EC) No 798/200839 Part 1 of Annex I. For certain countries, only regions are approved. If a disease event occurs, the Annex is adapted to regionalise for disease‐free areas. All such decisions are made through the Standing Committee on Plants, Animals, Food and Feed (PAFF) meetings and are agreed with MSs. For other products, such as fresh or frozen meat, again the country list is limited, whereas for treated or processed products the list of approved countries is much broader as these commodities are considered a lower risk.
For imports from third countries, the commodity in many circumstances will be certified, which can include AI surveillance programmes for poultry and testing requirements and quarantine for captive birds (Regulation (EU) No 139/201340). Animal Health Certificates for imports from third countries into the EU have requirements for the country of origin, the disease‐free status of the premises or region, a veterinary check prior to travel and checks on arrival to the EU. For live birds which are moving with the owners into the Community, and therefore classified as pets, there are requirements for one of several options involving either 30‐day isolation and negative tests for H5N1 before movement or quarantine on arrival. Veterinary checks are required (Commission Decision 2007/25/EC41). All live animals must enter through a live animal border inspection post (BIP) and all products through a product BIP where additional checks on documents, identity and physical checks take place. At this stage, additional testing may be required if there are issues with the checks. Official veterinarians will be responsible for these checks. Once the commodity has entered the EU, it may move to another MS; for products, these are now in free circulation and no further checks are made unless there are food safety issues. At the place of destination, further checks may be made on live animals if there are concerns raised about the country or region of origin after the consignment was dispatched.
For intra‐EU movements, the health certificate requirements are less stringent as the overall disease status of the MSs is considered to be high and the onus is on the consignor to ‘never knowingly consign a commodity which could present a risk for disease spread’. Nevertheless, a veterinary inspection is still required before movement. The MS of destination may consider (with a risk assessment) the need for further checks at the destination, if there are concerns about undisclosed disease when the consignment was certified. The rationale behind this procedure is that there is transparency on the MSs’ disease statuses; harmonised disease control measures are implemented if there is an outbreak that restrict movements of commodities from the infected holdings and from holdings in established protection and surveillance zones.
For intra‐EU movements of pet birds, no veterinary checks or owner self‐certification are required. The owner of ‘birds other than poultry’ excluding pet birds must self‐certify the birds are free of disease (Articles 4 and 7 of Council Directive 92/65/EEC42), whereas, due to the risk of psittacosis, a commercial document signed by the competent veterinary authority is necessary for movements of psittacines only. Certain products, such as poultry semen, are not covered by harmonised certificates, but instead national rules may be used.
Specific pathogen‐free (SPF) eggs are not taken into account here, as they are considered, by their very nature, to be pathogen‐free and to enter specified approved bodies, such as laboratories or research establishments.
The main outcomes of the qualitative risk assessment are described in the sections below and Table 8, whereas the detailed assessment and the available scientific evidence is provided in Appendix F (see overview Table F.6).
Table 8.
Probability of HPAI and LPAI introduction into a commercial poultry holding via non‐wild bird pathways, separating third country trade and intra‐EU movements (for brevity, some categories have been grouped; relevant explanation of the probability scores is reported in Appendix F and summarised in Sections 3.4.1–3.4.10)
| Pathway | Third country trade | Intra‐EU movements | ||
|---|---|---|---|---|
| HPAI | LPAI | HPAI | LPAI | |
| Live birds, including birds as pets, bird of prey, pigeons or others | Extreme unlikely | Very unlikely | ||
| Live poultry (poultry for breeding or production (> 72 h old); day‐old chicks and hatching eggs) | Extreme unlikely (live poultry and day‐old chicks); very unlikely (hatching eggs) | Unlikely | Unlikely (hatching eggs); Non‐negligible (live poultry and day‐old chicks) | |
| Meat and eggs for human consumption | Very unlikely | Not relevant (meat); Very unlikely (eggs) | Very unlikely | Not relevant (meat); Very unlikely (eggs) |
| Semen | Non‐negligible | Not relevant | Non‐negligible | Not relevant |
| Feathers, skin and down | Extreme unlikely | Not relevant | Very unlikely | Not relevant |
| Feed | Extreme unlikely (non‐negligiblea) | |||
| Bedding | Extreme unlikely (non‐negligiblea) | |||
| Manure | Not relevant | Non‐negligible | ||
| Pharmaceuticals | Extreme unlikely | |||
| Other animal by‐products | Extreme unlikely | Not relevant | Extreme unlikely | Not relevant |
When the commodity is stored in way that wild birds can have access to it.
3.4.1. Live birds
This covers the captive birds such as Passeriformes, birds of prey, Psittacines, peacocks, swans, wild ducks, wild geese, snipe, woodcocks, grouse, birds as pets, racing pigeons (not for consumption) and zoo birds (i.e. originating in an approved body, institute or centre).
The overall risk of introduction of infection into a commercial poultry farm is considered extreme unlikely for third country trade because birds must be tested for AI and must enter quarantine.
For intra‐EU movements, the risk level is higher than for third country imports and was assessed as very unlikely which accounts for the lack of pre‐movement testing and quarantine, the increased volume of trade, but recognising that poultry will generally not be exposed.
For birds traded between approved bodies, institutes or centres, under Council Directive 92/65/EEC, there are requirements on the centres to have in place surveillance programmes for listed diseases, which include avian influenza. Therefore, the movement between approved premises are an even lower risk. For birds from registered holdings, there are no requirements for surveillance programmes, but there must be no reports of disease at the holding for the 30 days prior to consigning the birds.
A large proportion of the trade volume will be racing pigeons which are not raised for human consumption. Currently, available evidence suggests that pigeons appear to be less susceptible to infection than many other species of birds and are ineffective propagators and disseminators of virus (Kaleta et al., 2007; Werner et al., 2007; EFSA, 2008; Abolnik, 2014). Exceptions occur as cases of natural infections have been reported from countries with endemic HPAIV H5N1 status (Mansour et al., 2014) and in the 2016/2017 epizootic a few wild and farmed pigeons have tested positive; however, these may be considered spill‐over infections in areas where there are high levels of environmental contamination. The role of wild pigeons in onward transmission of avian influenza is not considered important in the overall epidemiology of disease, according to historical information gathered over the last 10 years or so (see Section F.4, Appendix F). Globally, pigeons are rarely found to be infected during either epizootics or in endemic areas where H5 and H7 viruses circulate, hence they do not play an important role in the spread of AIV. Nevertheless, experimental infection of pigeons with H5 and H7 viruses is possible (see Table F.2, Appendix F). Council Directive 92/65/EEC allows the movement of captive birds including racing pigeons, to another MS provided they originate in a holding free of AI for the previous 30 days and show no signs of disease on the day of travel. An owner declaration to such an effect is required. Once birds are released for racing, they will generally return to their home loft quickly and usually will not stray on to poultry farms and only land en route to drink.
Table F.2.
Results from experimentally infected pigeons
| Al‐Attar et al. ( 2008 ) |
|
Virus subtype and sample collection: H9N2, 60 pigeons were captured from Nineveh province around Mosul city (Iraq) in 2007 Virus amplification: 81.5% and 50% of birds were positive in ELISA and HI tests, respectively Virus shedding: not analysed |
| Brown et al. ( 2009 ) |
|
Virus subtype and sample collection: HPAI H5N1, experimental infection of 20 wild‐caught adult rock pigeons (Columba livia) Virus amplification: A high virus concentration (106.1 EID50/0.1 mL) was required to produce infection (3/5) or death (2/5) Virus shedding: When infection did occur, the duration of viral shedding was brief and viral titres were low (below detectable limit of the tissue culture titration assay (101.87 TCID50/mL) |
| Chang et al. ( 2014 ) |
|
Virus subtype and sample collection: not subtype specific, H5 AI seroprevalence analysis (HI tests) in live wild birds in Yunnan Province (China) Virus amplification: 2/2 samples from Columbiformes were seronegative Virus shedding: not analysed |
| Hayashi et al. ( 2011 ) |
|
Virus subtype and sample collection: HPAI H5N1, experimental infection (106 EID50) of groups of 8 pigeons with two HPAI H5N1 viruses Virus amplification: Replication of both viruses has been detected, a survival experiment was not conclusive since also control birds died Virus shedding: Shedding of both viral strains was observed 5 days post‐infection (3/3 or 1/3 birds with mean titres 102.8 and 102.7 EID50/mL) |
| Kalthoff et al. ( 2014a , b ) |
|
Virus subtype and sample collection: LPAI H7N9 and H7N7, experimental infection (106 TCID50) of racing pigeons Virus amplification: no clinical signs, antibodies detected in ELISA against NP and H7 (8/8), VN negative (8/8) and HI was negative (6/8) or weakly positive (2/8, titre 2 or 4) Virus shedding: very low RNA levels were detected in oropharyngeal and cloacal swabs (Cq values variation 26.2–34.2) |
| Klopfleisch et al. ( 2006 ) |
|
Virus subtype and sample collection: HPAI H5N1, experimental infection (108 EID50) of 14 4‐month‐old racing pigeons (Columbia livia f. domestica) Virus amplification: 3 birds died after disease associated with neurotropism whereas the remaining 9 pigeons showed neither clinical signs nor gross or histological lesions although H5 seroconversion (titres ranging from 1:32 to 1:64 in HI test) indicated that they had been infected Virus shedding: five SPF White Leghorn chickens were added to the aviary with the pigeons 48 h postinoculation and none of these seroconverted at 19 days postinoculation (whereas inoculation of chickens with the virus strain confirmed their susceptibility to the virus) |
| Pantin‐Jackwood et al. ( 2014 ) |
|
Virus subtype and sample collection: LPAI H7N9, experimental infection (106 EID50) of 6‐ to 12‐month‐old rock pigeons (Columbia livia domestica) Virus amplification: infection without clinical signs Virus shedding: very low viral shedding (101.7–2.5 EID50/mL mean virus titre) at 2 and 4 days postinoculation (5–7/11 and 1/8 birds, respectively), pigeons did not transmit the virus to direct contact pigeons |
| Shriner et al. ( 2016 ) |
|
Virus subtype and sample collection: not subtype specific, H5 AI seroprevalence analysis (ELISA and HI tests) in live wild birds captured around poultry farms during an acute HPAI H5N8 outbreak Virus amplification: 3/38 rock pigeons were suspected positive in ELISA but were below the detection level of the HI test (< 1:8 titre) Virus shedding: not analysed |
3.4.2. Live poultry
This regards breeding, production birds, hatching eggs and day‐old chicks of all poultry types (gallinaceous poultry, Anseriformes, ratites, gamebirds). For brevity, not all additional conditions, such as surveillance programmes and any premovement testing are specifically mentioned in the following text and the reader is recommended to view the appropriate legislation. However, the requirements have been taken account of in the accompanying risk tables in Appendix F.
According to the EU legislation (Directive 2009/158/EC43), these commodities (or their parent flocks) should come from approved establishments before moving: a residency period of 12 weeks in an approved establishment (certified according to Regulation (EC) No 798/2008) is required before being imported from third countries, the residency period is of 6 weeks in the case of intra‐EU movements. Approved breeding establishments are under official control and are required to have in place early detection, notification, heightened biosecurity and to take part in active serosurveillance programmes. The legislation also requires that breeding establishments are neither directly under disease control restrictions for notifiable disease nor in a region under such restrictions.
For third country imports, day‐old chicks and live poultry must be kept separately from other poultry present on arrival. They are then either observed for 6 weeks and, if disease is suspected, subjected to clinical examination and testing, or they are kept for 3 weeks and undergo testing. For hatching eggs, these must be hatched separately, or if this is not possible, the whole batch must then be considered as one epidemiological unit for which control measures must be applied. (Annex VIII of Regulation (EC) No 798/2008). These post‐movement requirements do not apply after intra‐EU movement to another MS.
For third countries trade of these commodities, the overall risk is considered very unlikely for hatching eggs and extremely unlikely for live birds. The difference in the risk level is due to very high numbers of hatching eggs in comparison to the numbers of live poultry and therefore takes account of the aggregated risk score, as opposed to the risk level for a single consignment. However, as the eggs will be in a designated hatchery the risk of exposure to other poultry should be mitigated.
For intra‐EU movements, however, there are varying risk levels, which reflects the high volume of trade, possible mixing of batches and lack of pre‐movement testing requirements. LPAIV incursion is of more concern because with the HPAI strains the required veterinary inspection should detect any clinical signs. Therefore, for the movement of day‐old chicks or live poultry, from a region where LPAIV is suspected to be present, the probability of AIV infection transfer was considered non‐negligible, but unlikely for HPAI. Regular testing of the (parent) flock should be considered in such regions. Hatching eggs are less of a concern for LPAI because they will not be infected (unlikely), and they should be disinfected in accordance with Article 8(1) of Directive 2009/158/EC and in line with Chapter 6.4 of the OIE code, such that the eggs are cleaned and sanitised as soon as possible after collection with an approved sanitising agent and all crates and packing materials should be new or sanitised. Nevertheless, fomite contamination (egg shells, crates, etc.) cannot be entirely discounted, through recontamination or incorrect sanitation procedures.
3.4.3. Meat and eggs for human consumption
This section refers to fresh, frozen, non‐processed meat and table eggs for human consumption. The limited list of approved third countries, the certificate requirements and the swill feeding ban in the EU limits the probability of incursion of HPAIV through these pathways for exposure of commercial poultry and these are considered very unlikely probability pathways.
For intra‐EU movements, given the veterinary inspection processes in slaughter houses and the likely clinical signs in parent flocks (which may at least include reduced egg production observed in Anseriformes for HPAI) as well as the swill feeding ban, again the probability of HPAI incursion is considered very unlikely, but it should be noted that there are very high volumes of these commodities and therefore reducing the exposure of commercial poultry is the most important rate limiting step.
For LPAI, as birds are rarely viraemic, meat or table eggs would not contain high levels of virus and therefore these pathways are not considered relevant for meat, while for table eggs it is the fomite risk which leads to a probability of very unlikely. Such commodities are also considered a negligible risk for public health, as food safety rules should ensure no sick animal enters the food chain and provided products are cooked according to food hygiene rules.
3.4.4. Semen
Semen for the artificial insemination of turkeys is commonly used in the EU. There is scientific literature on the infection of turkeys after being artificially inseminated and this was considered an important route for transmission of pandemic influenza A (H1N1) into turkeys (Pantin‐Jackwood et al., 2010). There are no harmonised rules for this commodity, and therefore, there are no trade statistics. For bilateral international trade, MSs should apply the requirements of the OIE code – that the donor stags are disease‐free and no disease has been reported in the past 21 days. However, the lack of data on the volumes used and the lack of trade rules means that the risk of this pathway for HPAI cannot be rejected, hence the risk might be considered non‐negligible, given the level of uncertainty. It is important to emphasise that the probability of ‘non‐negligible’ is between 10% and 100%, hence the uncertainty.
This pathway is considered not relevant for the introduction of LPAI, as there is no viraemia associated with infection.
3.4.5. Feathers, skin and down
According to Commission Regulation (EU) No 142/2011, the importation into and the transit through the EU of untreated feathers and parts of feathers and down is prohibited. Treatments comprise physical and/or chemical processes ensuring complete viral inactivation. For imports of treated feathers, parts of feathers and down, no health certificate is required. For LPAI, these commodities may be contaminated rather than infected, therefore these pathways are not considered relevant. There is an anomaly with the trade code. According to the Vet Checks Decision (2007/275/EC) EX 0505 is described as ‘Skins and other parts of birds, with their feathers or down, feathers and parts of feathers (whether or not with trimmed edges) and down, not further worked than cleaned, disinfected or treated for preservation; powder and waste of feathers or parts of feathers’. Therefore, it is considered that feathers and parts of feathers and down have been treated by ‘another method’ but not undergone a full steam treatment. This is why, once imported, these products have to go to an approved establishment in the EU for further treatment. Therefore they may appear in the trade database as ‘raw’ as they have not been fully processed or if ‘other’ is a viable option under the Combined Nomenclature (CN) codes (Council Regulation (EEC) No 2658/8744). The risk of HPAIV introduction into commercial poultry holdings via treated skin, feathers and down is therefore considered extreme unlikely for imports from third countries, whereas when transported between MSs, when treatment is not required, it is considered very unlikely taking into account the high volume.
3.4.6. Feed containing poultry products and other feed such as processed grain
Poultry products used as feed should not be fed to poultry themselves, except for poultry tallow. In the EU, there is an intraspecies recycling ban on feeding of processed animal protein (PAP), such as poultry blood meal. It is allowed to feed other treated blood products that are not PAP and also poultry tallow back to poultry. Poultry blood products can be fed to pigs/fish but not ruminants. Poultry PAP cannot be used for other livestock feed except for aquaculture. World‐wide, poultry by‐products (offal), as an offshoot of broiler production, may be processed for animal feed but the process itself reduces the pH to prevent bacterial and viral development. Whole poultry meal and processed poultry meal may all be used as feed and therefore these products could be diverted to poultry feed by mistake. In general, this is considered extremely unlikely to be a suitable pathway for incursion of AI into commercial poultry. Feed specifically for poultry, if commercially produced, is processed as part of pelleting manufacture, and again, it is extremely unlikely this would be a suitable pathway for disease incursion. However, the product itself could be exposed to virus‐infected wild birds or virus‐contaminated environment while being transported or on arrival when stored, therefore in these circumstances this pathway is considered non‐negligible. However, it is considered as part of the general wild bird indirect exposure pathways, which highlights the importance of biosecurity around storing feed away from possible contamination. In summary, therefore, the probability of AIV survival in feed is extreme unlikely but feed might become contaminated during storage, hence the risk level is given in parentheses in Table 8.
3.4.7. Bedding
Hay and straw must only enter the EU from third countries with a commercial document; however, there are no consignments notified in the UN ComTrade database from third countries. For intra‐EU movements, large volumes of such a commodity are moved in the EU. In general, the risk of introduction of AIV via this pathways is considered extreme unlikely, however, similarly to the feed pathway, if contamination occurs at time of harvest, during transport or storage, through contact with infected wild birds or contaminated vermin and because of the intended exposure to poultry, this pathway is scored as non‐negligible, with some uncertainty based on the time since harvest and the transport conditions which may affect viral perseverance. Contamination of imported bedding with wild bird faeces cannot currently be eliminated as a potential pathway and this possible risk score is given in parentheses in the relevant section of Table 8.
3.4.8. Manure
In an outbreak situation, manure cannot be removed from poultry holdings and spread in the protection and surveillance zone except for transport for treatment and storage following under control of competent authority. These prohibitions also apply in zones that reach into a neighbouring MS. According to Commission Regulation (EU) No 142/2011, the importation into and the transit through the EU of unprocessed manure is prohibited; therefore, this pathway is not relevant in the case of Third Countries trade. However, within the EU, the competent authorities of two MSs which share a common border may authorise the dispatch of manure between farms located in border regions of those two MSs subject to appropriate conditions for the control of any possible risks to public or animal health, such as obligations for the operators concerned to keep appropriate records, which are laid down in a bilateral agreement. In outbreak situations, the information in these records should be used for tracing the infection. The product itself consists of a mixture of bird manure, poultry feed, sawdust and bedding material that accumulates at the bottom of or on manure conveyor belts in poultry sheds and therefore can potentially include dead poultry. If not treated or pelleted, it can contain high levels of virus if taken from an infected poultry farm, and should always be stacked to reduce viral load (Directive 2005/94/EC on AI control), in accordance with disease control measures. Therefore, for manure originating in holdings with HPAI‐infected galliforme species it would be expected that the disease would have been confirmed and all manure treated. For manure originating from holdings with HPAI in Anseriformes species or LPAI in any poultry species, it is possible that disease would not be detected, and therefore, this is considered a non‐negligible pathway taking also into account reduced virus perseverance in manure. In terms of exposure directly to commercial poultry, it cannot be discounted as a potential pathway if the manure is spread near a poultry farm or if there is access to wild waterfowl.
3.4.9. Pharmaceuticals
Vaccines for notifiable avian influenza are rarely used in the EU, mostly because of the trade implications for products from vaccinated poultry, but also because of the costs associated with application of vaccines to individual birds and surveillance costs. They must comply with Directive 2001/82/EC and Regulation (EC) No 726/2004 (Article 52 of AI Directive). Vaccination requires explicit approval of vaccination plan by the European Commission on applications by MSs. All registered vaccines must be notified to the European Commission and each MS must submit plans for approval indicating vaccine to be used and keep records of birds vaccinated (captive birds in zoos and collections or in special circumstances, some poultry). Only one vaccine for avian influenza is approved for use in the EU: the Nobilis Influenza H5N2 for use in chickens. It is an inactivated vaccine manufactured in the EU. Nevertheless, other live vaccines for use in poultry (e.g. against Newcastle Disease virus) are often produced in eggs (SPF eggs) and contamination could occur, but is thought improbable given the controls in place with Good Manufacturing Practices with pharmaceutical companies and veterinary medicine authorities. Therefore, this is considered an extreme unlikely pathway, but all authorised vaccines are assessed for quality, safety and efficacy. As part of the quality assessment, the applicant has to provide information on any potential extraneous agents and there is a monograph which outlines which agents to test for and ensure that there is no risk. Also, if material(s) of animal origin is used during production of the vaccine then information has to be provided and a transmissible spongiform encephalopathy (TSE) risk assessment performed. If they are live vaccines that have been attenuated in some way, the potential to revert to virulence is assessed based on the safety data provided. Any concerns on safety such that a product could revert to a virulent state would be noted and taken into account when determining the benefit/risk of the product, and whether an authorisation is given. The final product tests in place should give assurance that there is no contamination, but in case it does, there are steps in place to manage the risk from such events. The tests that are performed on authorised live/inactivated vaccines reassures that there is no risk of extraneous agents, and these tests include one for influenza A.
Finished medicinal products, such as vaccines, are not covered by veterinary legislation for import and therefore there are no import data available. Intermediate products derived from Category 3 material and intended for technical uses in medical devices, in vitro diagnostics, laboratory reagents and cosmetics are included (See Commission Decision 2007/275/EC) but these products are not considered a risk of introducing avian influenza to poultry as they are destined for laboratories.
3.4.10. Other animal by‐products (ABPs)
There are many other products of poultry origin (such as processed meat, processed parts of birds including feathers, skin, feet, casings, blood), but they were discounted as not being a significant pathway (and scored as extremely unlikely for HPAI and not relevant for LPAI) as they were either for treatment, processing (e.g. for pet food) or for human consumption. ABP rules (Regulations (EC) No 1069/200945 and (EC) No 142/2011) about the disposal of categories 1 and 2 waste means high‐risk material will be rendered. Category 3 ABPs are also considered a very low risk category for AIV transmission. Unprocessed pet food such as dog chews made of poultry meat or frozen day‐old chicks are frequently traded and imported, with health certification. Both represent an extremely unlikely pathway for the introduction of HPAIV (and not relevant for LPAIV) into commercial poultry.
3.4.11. Illegal introduction
For any of the commodities mentioned, illegal introduction is theoretically possible but very difficult to quantify. Illegal consignments of captive birds or hatching eggs from captive birds are picked up at the EU border on occasion, but the number which are not identified are ipso facto, unknown (EFSA, 2008) and there have been three recorded occasions of such birds testing positive for H5N1 HPAI. Birds of prey have been implicated in a case of illegal introduction into the EU at Brussels airport in 2005 (Van Borm et al., 2005). In the UK in 2006, an African Grey parrot was tested positive for H5N1 HPAI while in quarantine. In 2013, a mixed consignment of psittacines and Passeriformes were illegally introduced into Austria and stopped at Vienna Airport. Of the nearly 100 birds of this consignment, 60 were alive and of the dead birds, four tested positive for H5N1 HPAI. Poultry meat (fresh or frozen) and table eggs are frequently intercepted in passenger luggage (information from UK Border Force seizures). However, if even those may be expected to be infected with a higher likelihood than their parallel legal commodities, the volumes involved are far lower.
3.5. HPAI and LPAI transmission and spread (TOR 6)
3.5.1. Transmission between birds in a poultry population
The transmission parameters of LPAI infections and HPAI infections are shown in Tables G.1 and G.2 (Appendix G). In chicken, the estimates of the basic reproduction ratios, R0, (the average number of secondary infections caused by one infectious bird) for LPAI and HPAI are similar, ranging from 0.8 to 9.1 and 1.6 to 10.7, respectively.46 However, due to high mortality, the average infectious period of HPAI is considerably shorter than that of LPAI, resulting in much quicker transmission as indicated by infection rate parameters, (β, the average number of secondary cases caused by one infectious individual per day) that is 0.76–4.5 for HPAI and 0.1–0.91 for LPAI. One should bear in mind that the variation of these parameters among LPAI and HPAI viruses is large, hampering a universal conclusion. However, van der Goot et al. (2003) showed that for an H5N2 subtype including a specific HPAI isolate and its LPAI precursor, both R0 and β were lower for LPAI than for HPAI (Van der Goot et al., 2003). So overall, it can be concluded HPAI viruses are transmitted much quicker in chicken populations than LPAI viruses. Although virus strain is a likely determinant of transmissibility, differences between studies and the wide confidence intervals of the estimates of transmission parameters do not allow definitive conclusions. Moreover, successful adaptation to the host species studied likely influences transmission.
Table G.1.
Parameters of LPAI transmission between birds in a poultry population (data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, De Koeijer et al., 2017). The mean values are presented with the lower (L) and upper (U) limits. IP, infectious period (days)
| Study | Serotype | Species | Beta per day (L–U) | IP (L–U) | R0 (L–U) | Design | Virus origin |
|---|---|---|---|---|---|---|---|
| Gonzales (2011) | H7N1 | Chicken | 0.49 (0.3–0.75) | 7.7 (6.7–8.7) | 3.8 (1.3–6.3) | Transmission | Poultry |
| Claes et al. (2013) | H7N1 | Chicken | 0.38 (0.11–0.44) | 6.1 (0.45–11.75) | 2.32 (0.12–4.52) | Transmission | Poultry |
| Gonzales et al. (2012a) | H7N7 | Chicken | 0.1 (0.04–0.18) | 7.1 (6.5–7.8) | 0.8 (0.4–1.8) | Transmission | Poultry |
| Gonzales et al. (2012b) | H7N3 | Chicken | 0.91 (0.45–1.62) | 10.03 (8.5–11.56) | 9.1 (3.6–19.5) | Transmission | Poultry |
| Gonzales et al. (2012b) | H7N3 | Chicken | 0.72 (0.68–0.77) | 7.69 (5.88–11.11) | 5.6 (4.3–7.7) | Field | Poultry |
| Gonzales et al. (2012b) | H7N3 | Chicken | 0.5 (0.45–0.55) | 9.09 (6.25–20) | 4.7 (3–8.6) | Field | Poultry |
| Lee et al. (2012) | H7N8a | Chicken | 0.78 (0.3–1.7) | ND | ND | Challenge | Wild bird |
| Lee et al. (2012) | H7N8a | Chicken | 0.26 (0.07–0.7) | ND | ND | Challenge | Wild bird |
| van der Goot et al. (2003) | H5N2 | Chicken | 0.24 (0.12–0.45) | 4.25 (2.57–5.93) | 1.17 (0.47–2.39) | Transmission | Poultry |
| Claes et al. (2013) | H5N2 | Chicken | 0.37 (0.14–0.61) | 5.5 (2.36–8.64) | 2.04 (0.79–3.28) | Transmission | Poultry |
| Yee et al. (2009) | H6N2a | Chicken | 2.46 (1.2–4.56) | ND | ND | Transmission | Poultry |
| Youn et al. (2012) | H9N2a | Chicken | 1.57 (0.9–2.74) | ND | ND | Challenge | Poultry |
| Bonfante et al. (2014) | H10N1b | Chicken | 0.43 (0.21–0.79) | 7.4 (6.1–8.8) | 3.2 (1.4–5.1) | Challenge | Wild bird |
| Li et al. (2010) | H11N9b | Chicken | 1.42 (0.75–2.45) | ND | ND | Transmission | Poultry |
| Claes et al. (2015) | H5N2 | Chicken | ND | ND | 2.11 (0.85–6.15) | Transmission | Poultry |
| Pillai et al. (2010a)b | H5N2 | Chicken | ND | ND | 1.1 (0.5–3.8) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Chicken | ND | ND | 0.36 (0.1–1.9) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Chicken | ND | ND | 0.22 (0.005–1.2) | Challenge | Turkey |
| Pillai et al. (2010a) | H5N3 | Chicken | ND | ND | 0.63 (0.22–3.08) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N2 | Chicken | ND | ND | 0 (0–0.62) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N2 | Chicken | ND | ND | 0 (0–0.62) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N5 | Chicken | ND | ND | 0 (0–0.66) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N1 | Chicken | ND | ND | 0.44 (0.01–2.28) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N7 | Chicken | ND | ND | 0 (0–0.75) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N9 | Chicken | ND | ND | 0 (0–0.75) | Challenge | Wild bird |
| Saenz et al. (2012) | H7N1 | Turkey | 2.01 (1.6–2.5) | 7.65 (7–8.3) | 15.3 (11.8–19.7) | Transmission | Poultry |
| Mondal et al. (2013) | H7N1b | Turkey | 0.08 (0.004–0.345) | ND | 0.56 (0.03–44.18) | Challenge | Wild bird |
| Mondal et al. (2013) | H6N8b | Turkey | 0.2 (0.03–0.62) | ND | 0.88 (0.03–44.18) | Challenge | Wild bird |
| Comin et al. (2011) | H7N1 | Turkey | ND | 8.1 (6.4–10.5) | 5.5 (3.36–18.33) | Challenge | Poultry |
| Pillai et al. (2010a)b | H5N2 | Turkey | ND | ND | 1.3 (0.05–2.12) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N2 | Turkey | ND | ND | Inf (1–inf) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Turkey | ND | ND | 2.1 (0.5–2.6) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N3 | Turkey | ND | ND | 0.9 (0.05–1.9) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N3 | Turkey | ND | ND | 1.83 (0.56–5.1) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N5 | Turkey | ND | ND | 0 (0–1.04) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N1 | Turkey | ND | ND | 1.6 (0.11–2.2) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N8 | Turkey | ND | ND | 1.45 (0.07–4.45) | Challenge | Wild bird |
| Niqueux et al. (2014) | H5N1 | Duck (M) | 1.84 (1.09–3.11) | 8.1 (4.9–13.4) | 14.9 (7.2–30.8) | Transmission | Duck |
| Niqueux et al. (2014) | H5N1 | Duck (M) | ND | 6.5 (3.9–10.7) | ND | Transmission | Poultry |
| Niqueux et al. (2014) | H5N2 | Duck (M) | 2.41 (1.41–4.13) | 5.1 (3.1–8.5) | 15.6 (7.4–32.7) | Transmission | Wild bird |
| Niqueux et al. (2014) | H5N3 | Duck (M) | 1.07 (0.64–1.78) | ND | 5.5 (2.7–11.3) | Transmission | Poultry |
| Li et al. (2010) | H11N9b | Duck (P) | 0.31 (0.13–0.6) | ND | ND | Transmission | Poultry |
| Pillai et al. (2010a) | H5N3 | Duck (P) | ND | ND | 2.55 (0.33–4.8) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N2 | Duck (P) | ND | ND | 0.7 (0.2–1.8) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Duck (P) | ND | ND | 0.7 (0.2–1.8) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Duck (P) | ND | ND | 2.7 (0.84–7.28) | Challenge | Poultry |
| Pillai et al. (2010a) | H5N2 | Duck (P) | ND | ND | 1.3 (0.06–2.37) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N5 | Duck (P) | ND | ND | 1.2 (0.04–1.7) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N1 | Duck (P) | ND | ND | 1.6 (0.12–2.3) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N7 | Duck (P) | ND | ND | 1.3 (0.05–1.95) | Challenge | Wild bird |
| Pillai et al. (2010a) | H5N9 | Duck (P) | ND | ND | 1.24 (0.04–1.71) | Challenge | Wild bird |
These were challenge experiments where contacts were introduced. Parameters values were estimated from manuscript data.
For all beta calculations 1 day latent period was assumed (the methodology used is described in the respective papers).
Table G.2.
Parameters of HPAI transmission between birds in a poultry population (data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, De Koeijer et al., 2017). The mean values are presented with the lower (L) and upper (U) limits. IP, infectious period (days)
| Study | Serotype | Species | Beta per day (L–U) | IP (L–U) | R0 (L–U) | Design |
|---|---|---|---|---|---|---|
| Bouma et al. (2009) | H5N1 | Chicken | 0.76 (0.42–1.2) | 2.1 (1.8–2.3) | 1.6 (0.9–2.5) | Transmission |
| Spekreijse et al. (2011a) | H5N1 | Chicken | 1.14 (0.7–1.72) | 1.6 (0.9–2.2) | 2.1 (0–5.2) | Transmission |
| Spekreijse et al. (2011a) | H5N1 | Chicken | ND | 1.7 (0–4.5) | 4.8 (0–7.5) | Transmission |
| Spekreijse et al. (2011b) | H5N1 | Chicken | 1.43 (0.27–7.56) | ND | ND | Transmission |
| Spekreijse et al. (2013) | H5N1 | Chicken | 1.45 (0.38–5.57) | ND | ND | Transmission |
| Spekreijse et al. (2013) | H5N1 | Chicken | 1.71 (0.38–5.57) | ND | ND | Transmission |
| Tiensin et al. (2007) | H5N1 | Chicken | 1.43 (1.2–1.71) | ND | 2.86 (2.41–3.41) | Field |
| van der Goot et al. (2003) | H5N2 | Chicken | 0.78 (0.42–1.47) | 6.8 (4.91–8.69) | 5.6 (2.9–8.4) | Transmission |
| Bos et al. (2010) | H7N1 | Chicken | 1.19 (0.93–1.52) | ND | ND | Field |
| Bos et al. (2009) | H7N7 | Chicken | 4.5 (2.68–7.57) | ND | ND | Field |
| van der Groot et al. (2005) | H7N7 | Chicken | 1.7 (0.8–3.9) | 6.3 (3.9–8.7) | 10.7 (5–17.7) | Transmission |
| Saenz et al. (2012) | H7N1 | Turkey | 2.04 (1.5–2.7) | 1.47 (1.3–1.7) | 3.01 (2.2–4) | Transmission |
| Bos et al. (2010) | H7N1 | Turkey | 1.43 (1.17–1.74) | ND | ND | Field |
| Bos et al. (2008) | H7N7 | Turkey | 1.26 (0.99–1.59) | 6.2 (5–8) | 7.8 (6–10.16) | Transmission |
| van der Groot et al. (2012) | H5N1 | Duck (P) | 4.7 (2.3–9.4) | 4.3 (3.8–4.8) | 20 (12.17–35.57) | Transmission |
| Wibawa et al. (2014) | H5N1 | Duck (P) | 1.6 (0.5–3.6) | 13.4 (12.3–14.5) | 21.5 (19.7–23.3) | Transmission |
| Kang et al. (2015) | H5N8 | Duck (P) | 1.22 (0.36–5.21) | ND | 1.22 (0.36–5.21) | Challenge |
| van der Groot et al. (2007) | H7N7 | Pheasants | 2.8 (1.4–5.5) | 12.2 (7.7–16.7) | 34 (17.18–64.77) | Transmission |
| van der Groot et al. (2007) | H7N7 | Teals | ND | 10.4 (7.6–13.2) | ND | Transmission |
For turkeys, less information is available than for chicken, however, the trend appears like that of chicken. Also, for ducks, the information is limited, although it points towards higher transmission parameters of LPAI viruses in ducks than in chickens. Additionally, the tables show high values of both R0 and β for HPAI H5N1 in ducks, whereas the value for R0 for H5N8 virus in ducks reported is quite moderate, but this reflects only a single publication. Although differences between isolates have been observed, results from Pillai et al. (2010a,b) indicate that a poultry derived isolate transmits better between chickens than a wild bird‐derived isolate (Pillai et al., 2010a). Wild bird isolates transmit better among turkeys and ducks than among chickens. The latter reflects the higher susceptibility of turkey compared with chickens to LPAI and probably the species jump is less of a hurdle from wild birds to domestic ducks than from wild birds to chicken.
3.5.2. Transmission between flocks within a farm
No information is available in the published scientific literature on transmission parameters of avian influenza viruses between flocks within a farm. In the Netherlands, during the 2014 H5N8 outbreaks, only a single poultry house was found affected in each of the five affected farms, although up to five poultry houses were present on the individual farms (Stegeman et al., 2015). Nevertheless, this only suggests that early warning was effective and the farms were depopulated before the virus was transferred to other poultry houses on the premises. Similar observations have been made in the turkey holding in Germany which had been affected first during the 2014/2015 epizootic (Conraths et al., 2016), while in the UK, on the infected duck holding, there were six sheds across two sites and birds tested positive in three sheds on one site, with only mild clinical signs reported; therefore, delaying the early detection and resulting in spread between the three sheds (APHA, 2014). The other site had been previously depopulated and the carcasses were recalled as a precautionary measure. Regarding LPAI, there is no information available.
3.5.3. Spread between farms
Interpreting AI spread between farms is difficult because transmission parameters have only been quantified during large epidemics, which creates bias in the reported data (Table G.3, Appendix G). Information suggests similar reproduction numbers for LPAI epidemics than for HPAI epidemics and a picture reflecting a lower infection rate parameter in the first compared with the latter. However, to enable a valid estimate of the overall spreading potential of HPAI and LPAI also the minor outbreaks, those outbreaks affecting only one or a few holdings, should be considered. This is hampered by quick depopulation of HPAI outbreaks (resulting from early warning because of the clear clinical manifestation of the disease) and limited surveillance for LPAI (many outbreaks will be missed). In addition, stamping out was applied during the HPAI outbreaks, whereas this was not applied during documented LPAI epidemics before 2006. Consequently, transmission potential of LPAI will be overestimated based on the published information, whereas it will be underestimated for HPAI. Kernel estimations in the Netherlands, however, suggest a much higher potential for HPAI to spread than for LPAI. Moreover, in a poultry‐dense region in the Netherlands most between herd spread was attributed to proximity to an infected holding and approximately 20% of the outbreaks could be attributed to wind‐borne spread (Ssematimba et al. 2013; Ypma et al., 2013). In addition, professional contacts by visitors, feed trucks, egg collection trucks and vehicles from the rendering company proved a significant risk for transmission.
Table G.3.
Parameters of HPAI and LPAI transmission between farms (data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, De Koeijer et al., 2017). The mean values are presented with the lower (L) and upper (U) limits
| Study | Virus | Pathogenicity | Country | Beta per day (L–U) | R0 (L–U) |
|---|---|---|---|---|---|
| Stegeman et al. (2004) | H7N7 | HPAI | Netherlands | 0.47 (0.3–0.7) | 6.5 (3.1–9.9) |
| Stegeman et al. (2004) | H7N7 | HPAI | Netherlands | 0.39 (0.2–0.9) | 3.1 |
| Garske et al. (2007) | H7N7 | HPAI | Netherlands | ND | 1.1 (0.9–1.5) |
| Garske et al. (2007) | H7N7 | HPAI | Netherlands | ND | 1.9 (1–3) |
| Mannelli et al. (2007) | H7N1 | HPAI | Italy | 0.15 (0.09–0.25) | 1.8 |
| Mannelli et al. (2007) | H7N1 | HPAI | Italy | 0.13 (0.1–0.18) | 1.5 |
| Garske et al. (2007) | H7N1 | HPAI | Italy | ND | 1.9 (1.2–2.7) |
| Garske et al. (2007) | H7N3 | HPAI | Canada | ND | 2.4 (1.4–3.6) |
| Busani et al. (2007) | H7N3 | LPAI | Italy | ND | 3 (2.3–3.9) |
| Mulatti et al. (2010) | H7N1 | LPAI | Italy | 0.4 | 2.15 |
| Mulatti et al. (2010) | H7N3 | LPAI | Italy | 0.4 | 2.06 |
3.5.4. One health perspective
Human infection with AIV is mainly due to close contact with infected birds, their infectious excretions or carcasses. Although most of the human cases identified were due to zoonotic transmission of HPAI viruses from infected birds, the term highly pathogenic is only referring to the pathogenicity of the virus in poultry and not related to disease severity in humans. In Europe, people with the highest exposure are often professionals involved in identification, analysis, culling and destroying of infected animals as well as cleaning and disinfection of contaminated houses and equipment. For instance, during the H7N7 outbreak in the Netherlands (2003), the virus was detected from 89 patients: 86 humans who handled affected poultry and in three of their family members (Fouchier et al., 2004). However, also wild bird ringers and other occupational groups with contact to infected live or dead (wild) birds might be at increased risk of infection with avian influenza viruses. Data on AI outbreaks, in particular on poultry holdings, need to be shared between‐animal and human health risk assessors since these data are required to assess the risk of transmission between holdings, the exposure of humans to AIV and the possibility of virus to jump species and infect humans (De Nardi et al., 2014). This information is also needed for risk managers to develop public health recommendations on personal protection measures, monitoring and follow up, antiviral pre‐ or post‐exposure prophylaxis administration or vaccination of directly exposed persons. The occurrence of avian influenza outbreaks needs also to be communicated with local practitioners and hospitals to raise awareness for the initiation of testing and ruling out of avian influenza infection of exposed people with respiratory symptoms or conjunctivitis.
Conversely, people returning from avian influenza affected countries in Asia should avoid entering poultry holdings when having respiratory symptoms or, in general, within a 10‐ to 14‐day period after returning, the maximum estimated incubation period of avian influenza.
3.6. Mutation from LPAI to HPAI (TOR 7)
The HA protein is a major determinant of pathogenicity for subtypes H5 and H7 in avian hosts (Bottcher‐Friebertshauser et al., 2014). Differences in the number of basic amino acids in the endoproteolytic CS of this protein distinguish low from highly pathogenic phenotypes. The presence of multiple basic amino acids in the CS is strongly correlated with an increase of virulence of avian influenza viruses of the H5 and H7 subtypes in gallinaceous hosts.
3.6.1. Current understanding of the mechanism
Endoproteolytic processing of the HA is pivotal to render progeny virions infective. As AIV do not encode a protease of their own, they depend on utilising host proteases. The CS of HPAI viruses of subtypes H5 and H7 is accessible and cleavable by subtilisin‐like proteases (Steinhauer, 1999; Kido et al., 2012). The amino acid consensus motif of subtilisin‐accessible CSs is –R–X–R/K–R–G, where ‘X’ stands for any amino acid except proline (P). In contrast, the CS of LPAI viruses is not accessible or cleavable by subtilisin‐like proteases. Instead, only trypsin‐like proteases have been shown to process this site, which harbours the common motif –X–X–R–G. Trypsin‐like proteases are located in the respiratory and gut‐associated epithelia of the avian host. This confines productive replication of LPAIV to these tissues and restricts replication locally. In contrast, subtilisin‐like proteases are expressed by virtually all cells of the avian host. Therefore, HPAIV replication is enabled in a systemic manner giving rise to massive tissue damage provoking severe clinical symptoms that ultimately may lead to the death of the infected host (Franҫa and Brown, 2014).
It has repeatedly been shown in vitro, and there is also evidence in vivo, that HPAI phenotypes can arise de novo from LPAIV precursor viruses by mutations in the HA CS sequence (Munster et al., 2005). In the simplest cases, two nucleotide substitutions were sufficient to induce non‐synonymous mutations that generated a subtilisin‐like from a trypsin‐like CS motif. More often, insertional mutations of untemplated adenosine and guanosine nucleotides at this site had caused the change in the CS motif. It is believed that a slippage mechanism of the viral polymerase complex is at the basis of this process as it increases the frequency of nucleotide insertion and then the acquisition of additional codons to generate the polybasic CS. The presence of consecutive adenine residues and a stem‐loop structure, in the viral RNA region encoding the HA CS motif of influenza viruses of the H5 subtype, have been experimentally demonstrated to play a crucial role in inducing the viral polymerase slippage and then the acquisition of the multiple insertions required to create a polybasic CS (Nao et al., 2017). Non‐homologous recombination resulting in the insertion of a foreign nucleotide sequence into the HA CS has been described as an additional, third mechanism for generating HPAI phenotypes. In these (rarer) cases, all of them restricted to American viruses of the H7 subtype, small fragments of the viral matrix gene or the viral nucleoprotein gene or the host 28S RNA were translocated by unknown mechanisms into the CS site (Suarez et al., 2004; Pasick et al., 2005; Maurer‐Stroh et al., 2013).
However, there are viral strains in which the HA CS amino acid sequence and the pheno/pathotype did not match in the predicted way (Londt et al., 2007). Discordant results between the molecular classification, derived by sequencing of the HA CS, and virulence for experimentally infected chickens have been observed in few AIVs of the H5 and H7 subtype. Two good examples for the H5 subtype are the A/chicken/PA/1/83 (H5N2) and the A/chicken/Texas/298313/04 (H5N2) viruses. Although these viruses contained multiple basic amino acids at the HA cleavage site that would be consistent with the classification as highly pathogenic AIVs, they were not virulent for experimentally inoculated chickens. Conversely, a Chilean H7N3 HPAIV which arose by intersegmental recombination displayed basic amino acid residues only at positions ‐1, ‐4 and ‐6 (PKTCSPLSRCRETR‐G) (Suarez et al., 2004) but clinically classified as HPAI.
In addition, there is experimental evidence demonstrating that the insertion of a multiple basic HA cleavage site may not be sufficient to transform all LPAIV into HPAIV, not even in H5 and H7 backgrounds (e.g. Stech et al., 2009). This indicates that virulence is influenced by further viral determinants either in the HA (Abdelwhab and Abdel‐Moneim, 2015) or beyond (Stech et al., 2015). However, the mechanisms driving the different mutations in the field and the reasons why HA CS changes are restricted to the H5 and H7 subtypes remain obscure. Experimentally, the presence of a polybasic HA cleavage site, even in non‐H5/H7 HA can support a highly pathogenic phenotype in the appropriate viral background (Veits et al., 2012). Therefore, the restriction of natural HPAIVs to certain viruses of the subtypes H5 and H7 subtypes is likely to be a result of their unique predisposition to acquire a polybasic HA cleavage site (Nao et al., 2017).
3.6.2. Factors that influence LPAI to HPAI mutation
Emergence of HPAI viruses in the field appears to be a rare event. Between January 2006 and June 2016 in Europe, only nine primary HPAI outbreaks caused by viruses that had likely arisen de novo from LPAI precursors, were reported against a number of 274 primary and secondary outbreaks of LPAI of the H5 and H7 subtypes that had been officially reported in the same period. Based on the data collected from 43 HPAI outbreaks identified over 56 years from a variety of host species at a global level and on the results of the molecular and phylogenetic characterisation of LP/HP viruses of the H5 and H7 subtypes, a qualitative assessment was performed to determine whether viral, environmental and host species‐specific aspects might influence LP‐to‐HP mutation rates. The main outcomes are described below and the tables along with result of phylogenetic analyses are available in Appendix H. The risk of transition from LPAIV to HPAIV is difficult to assess as it depends on a large variety of interconnected elements which comprise an unknown predisposing ‘optimal’ viral gene constellation and a combination of host‐ and environmental‐specific factors.
Intrinsic factors
By intrinsic factors, we refer to all the genotypic and phenotypic attributes of a virus strain that may determine whether it has the potential to evolve from LPAI precursor to HPAI type. The identification of the intrinsic factors associated with the potential of a LPAI virus to evolve into a HPAI genotype/phenotype is challenging, as it is presently restricted by the current scientific knowledge of which exact molecular determinants are necessary for the evolution of HPAI from LPAI precursors and by the limited awareness of the steps by which the genetic changes for HPAI emergence occur. The most relevant intrinsic risk factor known to influence the ability of a virus to switch from a LPAI precursor to a HPAI phenotype is its HA subtype, as to date only the H5 and H7 subtypes seem to be prone to the incorporation of multiple basic amino acids. Of the 43 primary HPAI outbreaks that occurred world‐wide between 1959 and 2017, 15 were caused by the H5 subtype and 28 by the H7 subtype combined with different neuraminidase subtypes (see Table 1 provided by the Consortium: Richard et al., 2017). More specifically, the HP H5 viruses were in combination with the N2 (n = 8 outbreaks), N1 (n = 4 outbreaks), N3 (n = 1 outbreak), N8 (n = 1 outbreak) and N9 (n = 1 outbreak) subtypes. Most of the HP H7 outbreaks were combined with the N7 (n = 13 outbreaks) and N3 (n = 9 outbreaks) while combination with N1, N8, N9 and N2 subtypes seemed to be related to more unique epidemic events. Although a different NA combination frequency has been identified, the existence of a certain degree of association between the geographic distribution of the outbreaks and the NA subtype (e.g. HPAI H7N7 in Europe versus H7N3 in the Americas), the lack of comprehensive information of the NA subtype of the LPAI H5 and H7 viruses cocirculating in the regions affected by the HP outbreaks and the scarcity of experimental evidence of the role of the HA/NA balance in the evolution of the AI pathogenicity prevent the identification of the NA subtype as a potential risk factor.
The LPAI precursor virus was known and available for analysis for 10 out of 41 (10/41) HP primary outbreaks described in poultry. Comparison of the genetic changes between HPAI viruses and LPAI precursors was carried out to evaluate whether it is possible to identify specific molecular traits that can increase the risk of transition from LPAI to HPAI genotype/phenotype. As well, the results of the in vitro and in vivo experiments carried out to assess the molecular changes and phenotypic markers associated with a switch from low to highly pathogenicity in avian influenza viruses were analysed with the same purpose (Tables 1, 2 and 3; by the Consortium: Richard et al., 2017). The main conclusion that can be reached following these analyses is that except for the multibasic amino acids in the CS, genetic changes for the evolution of HP viruses from LP precursor vary in type and number (from 9 to 68 substitutions/whole genome) according to the virus pair. Aside from changes at the cleavage site of HA, mutations mainly occurred in the HA in positions adjacent or apart from the CS, including the receptor binding site, and in the polymerase genes. M1, M2 and NEP genes turned out to be the components with the lowest number of substitutions, so suggesting a possible marginal role in the pathogenicity evolution of the AI pairs compared here. In the few cases in which the LPAI precursor was known, the duration of the known circulation time of the precursor LPAI varied and ranged from 10 days to 24 months, meaning that the LP/HP switch is an unpredictable evolutionary process.
As the HA gene is the major determinant of pathogenicity in AI viruses, the last potential intrinsic risk factor investigated was the phylogenetic relatedness of HA sequences of HP and LP viruses. 3,705 sequences of the HA gene segment of HP and LP avian influenza viruses of the H5 and H7 subtypes were retrieved from GISAID (GISAID, 2017). Based on global phylogenetic clustering of avian influenza viruses (Krauss et al., 2007), the downloaded sequences were divided into four distinct data sets, namely H5 Eurasia–Africa–Oceania, H5 Americas, H7 Eurasia–Africa–Oceania and H7 Americas which were then phylogenetically analysed to evaluate whether pathogenicity classes would correlate with phylogenetic clustering among the AI HA genes. Phylogenetic analyses indicated that the HA genes of HPAI H5 and H7 viruses identified over 114 years (1902–2017) from a variety of host species in Eurasia, Africa, Australia, and North and South America do not share the same common ancestors but fall within distinct genetic clusters with LPAI viruses identified from both domestic and wild birds (Figures H.1, H.2, H.3–H.4, Appendix H). This suggests that the inference of phylogenetic relationships between LPAI and HPAI cannot be used to predict whether a virus has the potential to evolve from a LPAI precursor to a HPAI type (Figures H.1, H.2, H.3–H.4), as it seems that HPAI viruses can emerge from genetic clusters with a distinct evolutionary history. Three monophyletic groups of LPAI viruses (defined by long branches and posterior probabilities higher than 90) that so far never evolved into a highly pathogenic phenotype were recognised in both the H5 and H7 phylogenetic trees: groups E5‐C1, A5‐C, and A7‐C1 (Figures H.1, H.2, H.3–H.4, Tables H.1, H.2, H.3–H.4). In particular, group E5‐C1 contains 165 H5 viruses, collected from 1993 to 2011 in Europe and Asia, shows only three spill overs in poultry according to the genetic sequences available (Figure H.1, Table H.1); group A5‐C consists of 338 H5 viruses, which circulated between 2000 and 2014 in the Americas and caused only few poultry infections (8 cases) (Figure H.2, Table H.2); group A7‐C1 includes 310 H7 subtype American viruses, which spread in poultry and wild birds in North America for 12 years (1994–2006), mainly in the State of New York (73%) (Figure H.4, Table H.4). Based on the genetic data available, two out of the four clusters (groups E5‐C1, A5‐C), entirely composed of LPAI viruses, have been mostly identified in wild birds (96–97%) and have been only sporadically reported in poultry species. Differently the group A7‐C1, despite its extensive circulation in poultry species (68%), never switched to the HPAI form. Interestingly, the existence of an additional monophyletic group (E7‐B2) in the H7 Eurasia–Africa–Oceania phylogeny which had been circulating for 8 years in poultry, wild birds and humans without showing the evolution to an HP genotype needs to be highlighted. This group includes 867 low pathogenic avian viruses which between 1999 and 2017 had only circulated in Asia (Figure H.3, Table H.3), as well, this group also contains the zoonotic LPAI H7N9 viruses which have been circulating in China since 2013 (Su et al., 2017). In February 2017, the emergence of HPAI H7N9 viruses was observed within this cluster, which once again demonstrates that it is not possible to predict the risk of pathogenicity evolution from the phylogenetic clustering. Therefore, more scientific evidence and comprehensive genetic data from LP and HP H5 and H7 outbreaks are needed to ascertain the risk of evolution towards an HP type for these specific LPAI clusters.
Figure H.1.
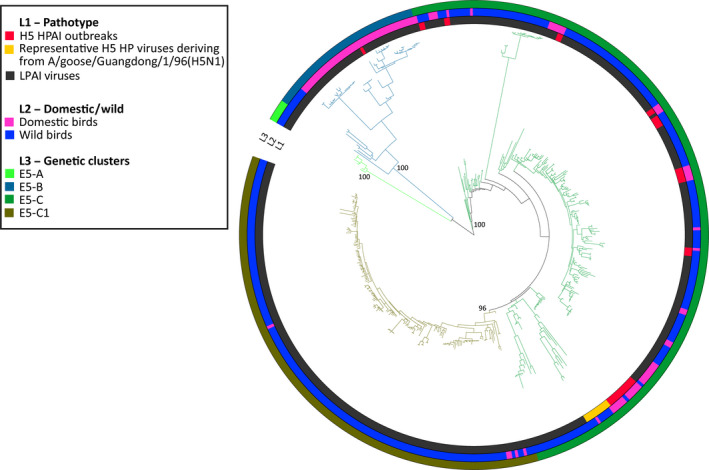
Phylogenetic tree constructed by Bayesian analysis of the H5 haemagglutinin gene segment of avian influenza viruses A collected in Europe, Asia, Africa and Oceania. Posterior probability values (expressed as a percentage) of the main clusters identified are indicated above the nodes (iTOL, 2016)
Figure H.2.
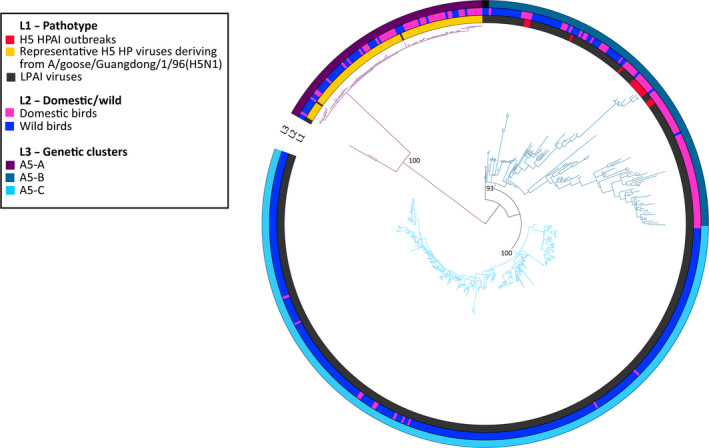
Phylogenetic tree constructed by Bayesian analysis of the H5 haemagglutinin gene segment of avian influenza viruses A collected in the Americas. Posterior probability values (expressed as a percentage) of the main clusters identified are indicated above the nodes (iTOL, 2016)
Figure H.3.
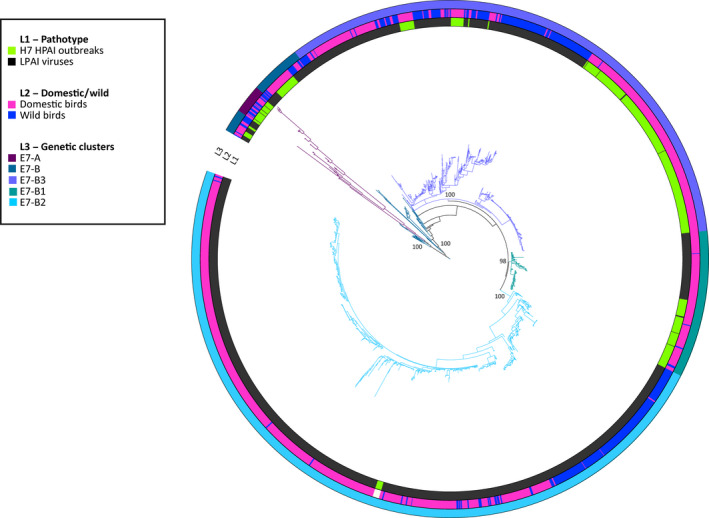
Phylogenetic tree constructed by Bayesian analysis of the H7 haemagglutinin gene segment of avian influenza viruses A collected in Europe, Asia, Africa and Oceania. Human and environmental samples are identified in L1 by a white band. Posterior probability values (expressed as a percentage) of the main clusters identified are indicated above the nodes (iTOL, 2016)
Figure H.4.
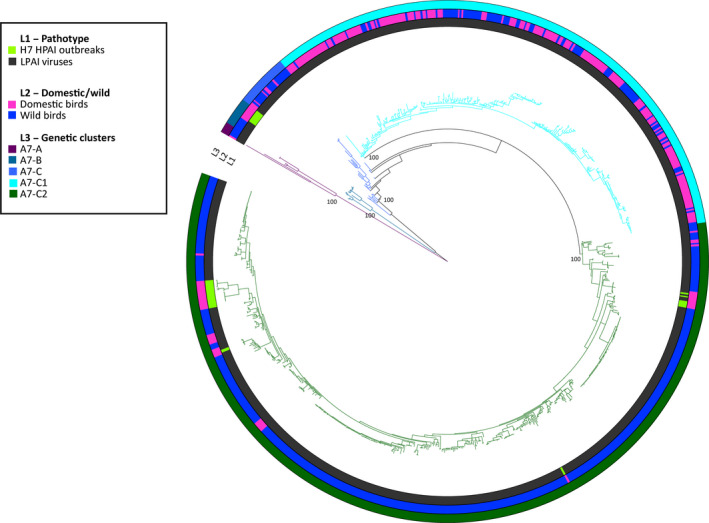
Phylogenetic tree constructed by Bayesian analysis of the H7 haemagglutinin gene segment of avian influenza viruses A collected in the Americas. Posterior probability values (expressed as a percentage) of the main clusters identified are indicated above the nodes (iTOL, 2016)
Table H.1.
Description of the genetic clusters identified in the H5 phylogeny of the H5 viruses collected in Europe, Asia, Africa and Oceania
| Colour | Group | No. of viruses | Location | Date | Spill over in poultry (based on sequence metadata) | |||
|---|---|---|---|---|---|---|---|---|
| E5‐C | 239 | Europe, Asia, Africa | 1959–2016 | 17 in Europe (6 evolved into HP) | ||||
| 3 in South Africa (2 evolved into HP) | ||||||||
| 3 China (1 evolved into HP) | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Italy | 1997–1998 | H5N2 | Domestic (poultry) | |||||
| England | 1991 | H5N1 | Domestic (poultry) | |||||
| Ireland | 1983 | H5N8 | Domestic (poultry) | |||||
| France | 2015 | H5N1 | Domestic (ck) | |||||
| France | 2015 | H5N2 | Duck | |||||
| France | 2015 | H5N9 | Duck | |||||
| South Africa | 2004–2006 | H5N2 | Domestic (ostrich) | |||||
| South Africa | 2011 | H5N2 | Domestic (ostrich) | |||||
| Goose‐Guangdong‐96 | 1996–to date | H5 | Domestic/wild birds | |||||
| South Africa | 1959–1961 | H5N3 | Wild (tern) | |||||
| Nigeria | 2006–2007 | H5N2 | Wild (spur‐winged goose) | |||||
| Notes | ||||||||
| Most widespread group | ||||||||
| Highest number of spill over in domestic poultry (23) | ||||||||
| Highest number of HP cases (11) | ||||||||
| Unique group with HP in wild birds | ||||||||
| Origin of the H5N1 goose‐Guangdong | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E5‐C1 | 165 | Europe, Asia | 1993–2011 | 1 in Italy | ||||
| 1 in France | ||||||||
| 1 in China | ||||||||
| Notes | ||||||||
| This group circulates mainly in wild birds (160/165 cases) | ||||||||
| It has never evolved into HP | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E5‐B | 52 | China, Taiwan, Japan, | 1984–2015 | 1 in Taiwan (1 evolved into HP) | ||||
| New Zealand, Antarctica | 1 in Japan | |||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Taiwan | 2014 | H5N2 | Domestic (poultry) | |||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E5‐A | 7 | Vietnam, Korea, Japan | 2004–2011 | |||||
| Notes | ||||||||
| This group contains 7 viruses | ||||||||
| It circulates only in wild birds/ducks | ||||||||
| It has never evolved into HP | ||||||||
Table H.2.
Description of the genetic clusters identified in the H5 phylogeny of the H5 viruses collected in the Americas
| Colour | Group | No. of viruses | Location | Date | ||||
|---|---|---|---|---|---|---|---|---|
| A5‐A | 101 | North America | 2014–2016 | |||||
| Notes | ||||||||
| This group includes only North American viruses collected between 2014 and 2016, deriving from the H5N1 A/goose/Guangdong/1/96 | ||||||||
| This group circulated both in domestic and wild birds | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry * | |||
| A5‐B | 155 | America | 1966–2004 | 13 in North America (4 evolved into HP) | ||||
| 1 in Central America (1 evolved into HP) | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Ontario | 1966 | H5N9 | Domestic (tk) | |||||
| Pennsylvania | 1983–1986 | H5N2 | Domestic (ck) | |||||
| Texas | 2004 | H5N2 | Domestic (ck) | |||||
| Texas | 1993 | H5N2 | Wild (emu) | |||||
| Mexico (Puebla) | 1994 | H5N2 | Domestic (ck) | |||||
| Notes | ||||||||
| This is the only group where LPAI viruses evolved into HPAI | ||||||||
| It can be divided into three subgroups. The 1st and the 2nd subgroups include North American viruses | ||||||||
| The 3rd subgroup includes Central and South American viruses, from Mexico (mainly) and Chile (1) | ||||||||
| This group circulated both in domestic and wild birds | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| A5‐C | 338 | America | 2000–2014 | 8 in North America | ||||
| Notes | ||||||||
| This group circulated mainly in wild birds (326/338 cases) | ||||||||
| It never evolved into HP | ||||||||
* Number of spill over should be considered as indicative as it is based on sequences available in public databases and on genetic clustering.
Table H.3.
Description of the genetic clusters identified in the H7 phylogeny of the H7 viruses collected in Europe, Asia, Africa and Oceania
| E7‐A | 32 | Europe, Asia, Africa, Oceania | 1902–2007 | 5 in Europe (5 evolved into HP) | ||||
| 5 in Australia (5 evolved into HP) | ||||||||
| 1 in Asia (1 evolved into HP) | ||||||||
| 1 in Africa (1 evolved into HP) | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Italy | 1902 | H7N7 | Domestic (ck) | |||||
| Netherland | 1927 | H7N7 | Domestic (ck) | |||||
| UK | 1933 | H7N7 | Domestic (ck) | |||||
| Germany | 1933–1934 | H7N1 | Domestic (ck) | |||||
| UK | 1963 | H7N3 | Domestic (turkey) | |||||
| Victoria | 1976 | H7N7 | Domestic(poultry) | |||||
| Victoria | 1985 | H7N7 | Domestic(ck)/wild(starling) | |||||
| Victoria | 1992 | H7N3 | Domestic (ck) | |||||
| Queensland | 1994–1995 | H7N3 | Domestic (ck) | |||||
| New South Wales | 1997 | H7N4 | Domestic (ck) | |||||
| Taiwan | 1993 | H7N7 | Duck | |||||
| Egypt | 1945 | H7N1 | Domestic(poultry) | |||||
| Notes | ||||||||
| Most viruses of this group are very old | ||||||||
| 12 HP outbreaks | ||||||||
| All the Australian viruses belong to group 1 | ||||||||
| The most recent viruses in this group (2005–2007) are from Australia | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E7‐B | 84 | Asia, Europa, Africa | 1972–2009 | 5 in Asia (2 evolved into HP) | ||||
| 6 in Europa (1 evolved into HP) | ||||||||
| 2 in Africa | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Pakistan | 1995–2004 | H7N3 | Domestic (ck) | |||||
| Pakistan | 2001 | H7N3 | Domestic (ck) | |||||
| Germany | 1979 | H7N7 | Domestic (ck/goose) | |||||
| Notes | ||||||||
| This group gave origin to groups 2.1, 2.2, 2.3 | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E7‐B1 | 166 | Italy | 1999–2001 | 1 in Italy | ||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Italy | 1999–2000 | H7N1 | Domestic (poultry) | |||||
| Notes | ||||||||
| This group includes only Italian viruses collected during the 1999–2001 epidemic | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E7‐B2 | 867 | Asia | 1999–2015 | 5 in Asia (1 evolved into HP) | ||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| China | 2016–2017 | H7N9 | Human/environment | |||||
| Notes | ||||||||
| This group circulated only in Asia | ||||||||
| LPAI H7N9 viruses circulating in China, responsible of several human cases, belong to this group | ||||||||
| H7N9 strain evolved into HP after several years of circulation in Asia. This HP strain caused human infection in China in 2016–2017 | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| E7‐B3 | 604 | Europa, Africa, Asia | 2000–2016 | 25 in Europe (5 evolved into HP) | ||||
| 1 in Asia | ||||||||
| 1 in Africa | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Netherland | 2003 | H7N7 | Domestic (ck) | |||||
| UK | 2008 | H7N7 | Domestic (ck) | |||||
| Italy | 2013 | H7N7 | Domestic (ck) | |||||
| UK | 2015 | H7N7 | Domestic (ck) | |||||
| Italy | 2016 | H7N7 | Domestic (ck) | |||||
| Notes | ||||||||
| This sub‐group is the most widespread within group 2 | ||||||||
| It is responsible of several recent HP epidemics in Europe | ||||||||
| All the HP viruses of this group belong to H7N7 subtype and circulated mainly in chicken (260 chicken/279 HP cases) | ||||||||
* Number of spill over should be considered as indicative as it is based on sequences available in public databases and on genetic clustering.
Table H.4.
Description of the genetic clusters identified in the H7 phylogeny of the H7 viruses collected in the Americas
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry * | |||
| A7‐A | 6 | North America | 1927–1994 | 1 in Texas (Victoria) | ||||
| Notes | ||||||||
| Small group (6 viruses) constituted only by old viruses (1927–1994) | ||||||||
| It has never evolved into HP | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry * | |||
| A7‐B | 16 | South America (Chile, Bolivia) | 2001–2014 | 1 in Chile (1 evolved into HP) | ||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Chile | 2002 | H7N3 | Domestic (ck) | |||||
| Notes | ||||||||
| This group circulated only in South America, mainly in Chile (15 viruses) | ||||||||
| The viruses of this group belong to H7N3 or H7N6 subtype | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| A7‐C | 30 | America | 1971–1994 | 4 in USA | ||||
| Notes | ||||||||
| This group gives origin to subgroups C1 and C2 | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| A7‐C1 | 310 | North America | 1994–2006 | Many in USA | ||||
| Notes | ||||||||
| This group circulated mainly in domestic birds (212/310 cases) | ||||||||
| It has never evolved into HP | ||||||||
| This group circulated only in North America, mainly in NY (226/310 cases) | ||||||||
| Colour | Group | N. of viruses | Location | Date | Spill over in poultry | |||
| A7‐C2 | 533 | America | 1993–2015 | 8 North America (3 evolved into HP) | ||||
| 2 Central America (2 evolved into HP) | ||||||||
| HPAI outbreaks | ||||||||
| Country | Date | Subtype | Host | |||||
| Canada (British Columbia) | 2004–2005 | H7N3 | Domestic (ck) | |||||
| Canada (SK) | 2007 | H7N3 | Domestic (ck) | |||||
| Indiana | 2016 | H7N8 | Domestic (tk) | |||||
| Mexico (Jalisco) | 2012 | H7N3 | Domestic (ck) | |||||
| Mexico (Jalisco‐Puebla) | 2015 | H7N3 | Domestic (ck) | |||||
| Notes | ||||||||
| This group circulated mainly in wild birds (481/533 cases) | ||||||||
* Number of spill over should be considered as indicative as it is based on sequences available in public databases and on genetic clustering.
Extrinsic factors
The extrinsic factors include host species‐specific and environmental aspects. This assessment has identified a significant gap in knowledge of the contribution of environmental and host‐related items in the emergence of HPAI viruses. So far, no transition of LPAIV to HPAIV has been clearly attributed to any specific aspect, such as host species, host immune status, holding type, poultry density and holding management. The role of these factors and their interaction is unclear and it seems that even minute permutations can lead to chaotic and unpredictable effects on virus pathogenicity evolution.
First, the role of host‐specific aspects was investigated. Information from 42 HPAI outbreaks revealed that chickens and turkeys were the species in which initial detection of HPAI was most frequently observed (81%). It is not clear whether the dominance of gallinaceous poultry in primary cases is due to the fact that gallinaceous poultry regularly produce massive clinical signs while waterfowl more often develop a delayed and milder course of disease; this would render HPAIV cases in gallinaceous poultry more prone to detection by syndromic surveillance. In South Africa, H5N2 HPAI outbreaks involving only farmed ostriches were also reported. During one of these (H5N2 in 2006) a LPAI virus was isolated from ostriches but only after the initial detection of HPAI. As well, in 1996 in a geese commercial holding in the Chinese province of Guangdong two different H5N1 pathotypes were recognised, suggesting that even this species might have been involved in the pathogenicity evolution of an avirulent virus (Abdelwhab et al., 2013). Multiple other domestic bird species other than the three described have been affected in secondary outbreaks: duck, guinea fowl, Chukar partridge, emu, pigeon, pheasant and quail. Viruses of the H5 or H7 subtype isolated from wild birds are almost invariably LPAI in poultry. With the exception of a large die‐off of terns in South Africa which had occurred in 1961 (Becker, 1966) and from which A/tern/South Africa/61 (H5N3) was isolated and of a molecular‐based identification of an HPAI H5N2 virus in healthy wild birds in Nigeria in 2007 (Gaidet et al., 2008), HPAI virus isolations from wild birds have been associated to contacts with infected poultry, usually as a result of surveillance of birds trapped or found dead in infected poultry holdings. More specifically, the species in which the switch from LPAI to HPAI had occurred was known only in 10 out 42 outbreaks, and chickens turned out to be the most widely affected group (no of outbreaks 8) followed by turkeys (no of outbreaks 2).
For the age of the animals, LPAI to HPAI switch or primary outbreaks were detected in breeders, broilers, laying hens, in meat and breeder turkeys. Although it has been shown that younger animals are the most susceptible to HPAI infections, which could give a selective advantage to HPAI over LPAI, there is no information from the field suggesting that a LPAI/HPAI switch occurs preferentially in younger animals. During one outbreak (Canada H7N3 2004), LPAI virus was isolated from older flocks (52 weeks of age) 10 days before HPAI was isolated from younger flocks (24 weeks of age) (Pasick et al., 2007). On the other hand, all the five European H7 HPAI outbreaks which have occurred since 2009 have involved layer hens; in four out of five cases this poultry category has been identified as the one where transition of LPAI viruses to HPAI phenotypes occurred.
Initial HPAI outbreaks were recorded in industrial, free‐range and backyard holdings. There was therefore no correlation between the size of the holding or the poultry density and the emergence of HPAI viruses. However, an in‐depth analysis of the 10 outbreaks for which the species involved in the LPAI to HPAI switch was known revealed that all the holdings except for one (South Africa H5N2 in ostriches) were characterised by a high poultry density in the affected holding. In addition, there was a strong positive correlation between the number of subsequent outbreaks and the poultry industry density in the affected geographic area (i.e. Italy in 1999–2000; Capua and Marangon, 2000), the Netherlands in 2003 (de Jong et al., 2009)). Therefore, poultry holding density should be considered a major contributing factor for the appearance of HPAI epidemics with a large effect.
The involvement of wild birds in these outbreaks was often unknown. During the H7N7 outbreak which hit the UK in 1979, wild birds were observed to be feeding on holdings due to the harsh winter conditions (Alexander and Spackman, 1981). In the H7N7 outbreak in 1985 in Australia, free‐living starlings also were affected, likely as a result of a spill‐over of the virus from poultry (Barr et al., 1986). In the H5N2 outbreak in South Africa, wild birds were observed to accumulate at ostrich holdings around watering troughs and feeders (Abolnik et al., 2009). Epidemiological data and the characteristics of the virus detected during the Italian HP H7N7 avian influenza epidemic in 2013 showed that the introduction in the first affected layer holding was due to contacts between free‐range hens and wild waterfowl (Bonfanti et al., 2014). In the H5N1 outbreaks which had started in 1996 and soon spread world‐wide, the long‐distance spread of the virus from Asia westwards after a spill‐over of the virus from poultry (Keawcharoen et al., 2008) was ascribed to wild birds. A similar mechanism was found to occur in the H5N8 outbreaks in 2014 and 2016 (Lycett et al., 2016).
As free‐range and outdoors holdings are known to be more exposed to the risk of AIV introduction through wild birds and considering that any introduction of H5 or H7 LPAI viruses could, according to the presently available data, result in a pathogenicity transition, these types of poultry holdings could be considered at a higher risk of being involved in HPAI transition events. As a matter of fact, in four out of the five H7 HPAI outbreaks which have occurred in Europe since 2009, the poultry holdings initially involved had outdoor access.
Mechanisms by which HPAI variants that emerged from LPAI precursors successfully compete with, gain ground and finally prevail in populations infected with the LPAIV precursor remain elusive. Faster transmission of HPAIV as indicated by increased infection rate parameters, (β, the average number of secondary cases caused by one infectious individual per day) may play a role (see Section 3.5.1).
3.7. HPAI and LPAI surveillance
3.7.1. Surveillance components
The surveillance system for AI in the EU consists of three mandatory components: (1) passive poultry surveillance, (2) active poultry serosurveillance and (3) passive wild bird surveillance. In addition, three voluntary components can be implemented: (1) testing to exclude (TTE)47 notifiable avian disease, (2) poultry production parameter monitoring and (3) active wild bird surveillance. A description of the surveillance system is provided in Section I.1 (Appendix I). The text below describes the results of the analysis of the current serosurveillance in gallinaceous and anseriforme poultry, and HPAI surveillance in wild birds.
3.7.2. Current HPAI serosurveillance in gallinaceous and Anseriformes poultry
3.7.2.1. Gallinaceous poultry
In gallinaceous birds, the value of serosurveillance to detect HPAI is extremely low. The very high mortality associated with HPAI in chickens and turkeys is quickly noticed by the poultry keeper and will almost always result in quick detection by passive surveillance (notification). In addition, most birds die before developing detectable antibodies due to the high case fatality rate. The acute nature of the disease also limits the utility of production parameters, such as drop in feed consumption, water consumption or egg production, for syndromic surveillance. A steep rise of mortality is by far the most prominent sign that is obvious to farmers and veterinarians. Compulsory notification in situations when more than 2% of the birds on a farm die within two days has been implemented previously (Commission Decision 2005/734/EC). However, in large farms with multiple poultry houses, this threshold is likely too high (e.g. on a 100.000 chicken farm the threshold would be 2,000 dead birds) and a legislative threshold might prevent poultry keepers from reporting before the threshold is reached. Establishing the threshold per house may be more effective, although compliance is more difficult to control. The effectiveness of TTE has not been properly evaluated, however, it is considered useful in areas at risk of HPAI incursion. It should not be used when there is HPAI suspicion, or when HPAI has already been introduced into the area. In the latter case, TTE could lead to a delay in diagnosis as the laboratories will test samples from suspected holdings with higher priority.
3.7.2.2. Anseriforme poultry
Duck and geese holdings are present across most of the EU although the density varies a lot (Figure I.1, Appendix I). HPAI incursion into dense areas of anseriforme poultry production is a threat as these species are related to the migratory waterfowl. This genetic similarity may mean that any HPAIV adapted to these migratory waterfowl is likely to be efficient in its transmission to anseriforme poultry. In addition, this adaptation may also lead to infection with a limited clinical response hindering detection through passive surveillance. If disease remains undetected, it may spread to contiguous holdings via direct movement of live birds, indirect spread through personnel, machinery or equipment or via other fomites. This may be accentuated by farming practices that cause significant movement of poultry populations as with duck gavage feeding, where mixing of infected flocks with other susceptible poultry populations might occur.
Figure I.1.
Duck and geese production by region reported by MSs to EFSA (autumn 2016) (taken from EFSA, ECDC, EURL, 2017a, 2017b)
These farming practices are likely to have played an important role in the French H5 episode in 2015/2016 which showed significant undetected spread of HPAI in duck populations in the south‐west of the country. Initial detection of HPAI was in a chicken farm where it was associated with significant mortality before other outbreaks were detected in ducks, geese and guinea fowl. The level of spread (over 80 premises infected with HPAI) found after further investigations suggested that the original infection was unlikely a recent exposure. In the 2016/2017 H5 (clade 2.3.4.4b) HPAI epizootic, this same population was again infected alongside similar populations in Hungary and Bulgaria using similar farming practices.
The 2016/2017 H5 HPAI (clade 2.3.4.4b) epizootic was associated with clear clinical signs and high mortality in most affected holdings, in contrast to the 2014/2015 H5 HPAI (clade 2.3.4.4a) and the French 2015/2016 H5 HPAI epizootics. As a result, there were high numbers of detections made by passive surveillance in anseriforme and game bird holdings during the 2016/2017 HPAI epizootic (Figure 10; see also EFSA scientific report 2017b).
Figure 10.
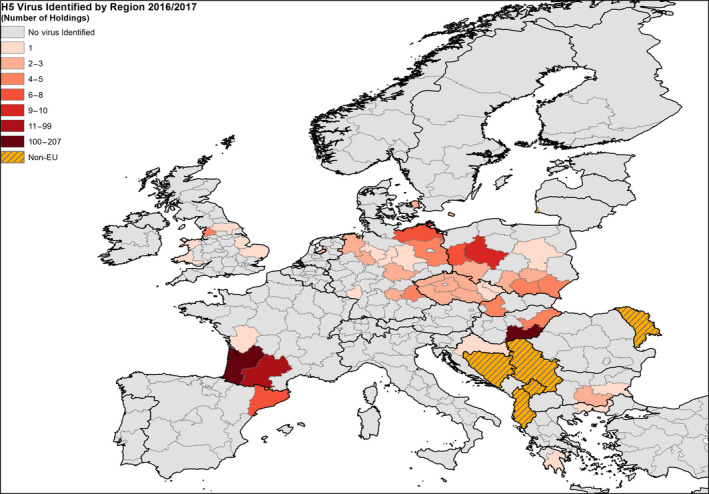
HPAI virus detected in Anseriformes and game birds during the 2016/2017 H5 HPAI (clade 2.3.4.4b) epizootic
Serological Surveillance
MSs carry out serological surveillance on a proportion of their poultry population according to EU legislation and the surveillance programme that they submit to the European Commission. Serosurveillance does not only detect prior exposure to HPAI viruses, but also to H5 and H7 LPAI viruses. The submitted programme can be risk‐based based on poultry demographics, proximity to waterbodies, production type/biosecurity level, trade, timing of sampling and reactive sampling (see Section I.1.4, Appendix I). Sampling carried out on ducks and geese between 2014 and 2016 can be found in Figure 11, while sampling carried out on game birds (both gallinaceous and anseriforme) over the same time period can be found in Figure 12.
Figure 11.
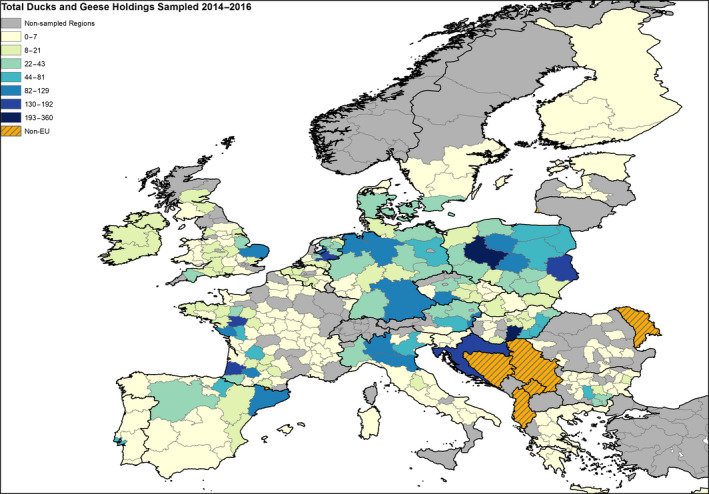
Active serological surveillance in duck and geese holdings samples between 2014 and 2016
Figure 12.
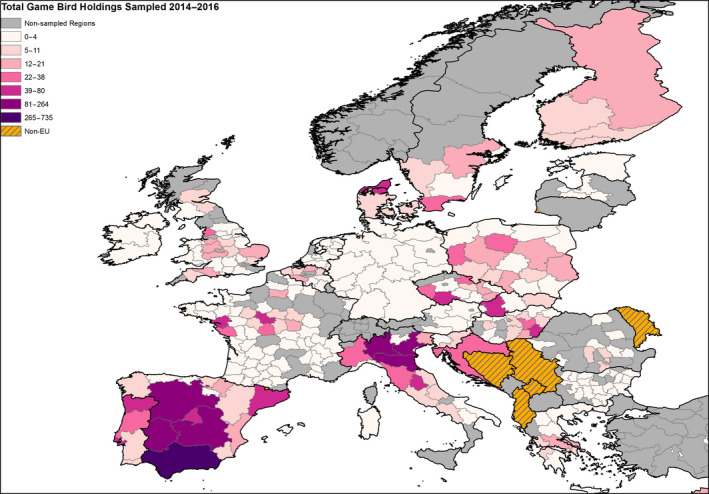
Active serological surveillance in game bird holdings samples between 2014 and 2016
The majority of sampling carried out is representative of the underlying demographics. Risk‐based surveillance is useful as it targets flocks where the risk of AI introduction is considered to be most likely, although its application varies with regards to their application of the risk factors and how they are weighted. The scientific evidence about individual risk factors is limited regarding what factors are important and is mostly not quantitative in nature. Quantitative information is mostly restricted to specific situations in single MS, which is often not available or applicable to other MSs and the EU level. Therefore, availability of specific advice as to the quantitative weighting of risk factors in particular countries is very limited. The lack of quantitative weighting information also hampers the comparison of surveillance results between MSs. Reporting quantitative information on the weighting factors used by MSs in relation to the available scientific evidence is needed to better understand how risk‐based surveillance is implemented and will subsequently help to improve the risk‐based approach and comparison between MSs.
Serological Surveillance Positives
Results of the active annual serological surveillance programme from 2014 to 2016 show that anseriforme poultry have more often been found AI seropositive (HI test) than gallinaceous poultry, with farmed anseriforme game birds particularly affected. See Table 9 for a complete breakdown of serological survey results across the EU.
Table 9.
Active serological surveillance of AI (number of holdings) in the EU by poultry category (data reported to EURL)
| Category species | Category type | 2014 | 2015 | 2016 | Total | Proportion positive (2014–2016) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sampled | Positive | Sampled | Positive | Sampled | Positive | Sampled | Positive | |||
| Fattening ducks | A | 619 | 14 | 971 | 26 | 999 | 22 | 2,589 | 62 | 2.4% |
| Fattening geese | A | 333 | 2 | 498 | 7 | 459 | 8 | 1,290 | 17 | 1.3% |
| Duck breeders | A | 252 | 7 | 330 | 6 | 632 | 74 | 1,214 | 87 | 7.2% |
| Farmed game birds (waterfowl) | A | 234 | 0 | 342 | 11 | 196 | 69 | 772 | 80 | 10.4% |
| Geese breeders | A | 208 | 10 | 296 | 23 | 265 | 19 | 769 | 52 | 6.8% |
| Farmed game birds (gallinaceous) | A* | 992 | 0 | 930 | 14 | 896 | 15 | 2,818 | 29 | 1.0% |
| Chicken breeders | G | 3,145 | 2 | 5,702 | 6 | 2,661 | 7 | 11,508 | 15 | 0.1% |
| Laying hens | G | 3,888 | 3 | 3,747 | 8 | 3,480 | 7 | 11,115 | 18 | 0.2% |
| Backyard flocks | G | 2,619 | 0 | 2,341 | 5 | 2,493 | 2 | 7,453 | 7 | 0.1% |
| Fattening turkeys | G | 2,254 | 0 | 2,443 | 16 | 2,144 | 12 | 6,841 | 28 | 0.4% |
| Free‐range laying hens | G | 1,782 | 5 | 2,884 | 10 | 1,812 | 13 | 6,478 | 28 | 0.4% |
| Broilers (at heightened risk) | G | 1,045 | 0 | 1,238 | 1 | 1,193 | 0 | 3,476 | 1 | 0.0% |
| Turkey breeders | G | 181 | 0 | 215 | 4 | 232 | 1 | 628 | 5 | 0.8% |
| Free‐range laying hens | G | 0 | 0 | 0 | 0 | 219 | 5 | 219 | 5 | 2.3% |
| Others | O | 1,278 | 3 | 1,343 | 19 | 942 | 12 | 3,563 | 34 | 1.0% |
| Ratites | O | 141 | 1 | 195 | 2 | 138 | 4 | 474 | 7 | 1.5% |
Category type: (A: Anseriforme poultry, G: Gallinaceous poultry, O: Others).
A*: While this category explicitly states gallinaceous poultry, their method of husbandry and their close contact with wild birds may attenuate clinical signs and are therefore categorised as anseriforme poultry.
Nb. Sampling is not uniform across all EU MSs, MSs can choose to use either representative or risk‐based sampling under EU legislation.
Samples were tested using the hemagglutination‐inhibition (HI) assay in accordance with the Diagnostic Manual for avian influenza, which lays down the procedures for confirmation and differential diagnosis of avian influenza (Commission Decision 2006/437/EC).
The distribution of game bird holdings, which can include both anseriforme and gallinaceous poultry, across the EU can be found in Figure I.2 (Appendix). Gallinaceous game birds may not always show high mortality and present clinical signs typical for HPAI in gallinaceous poultry (e.g. chickens and turkeys). The reason is that they are generally kept in close contact with wild birds and consequent exposure to LPAIV present in these populations. The resulting immune response may, in some cases, attenuate clinical disease manifestation of HPAI in the holding.
Figure I.2.
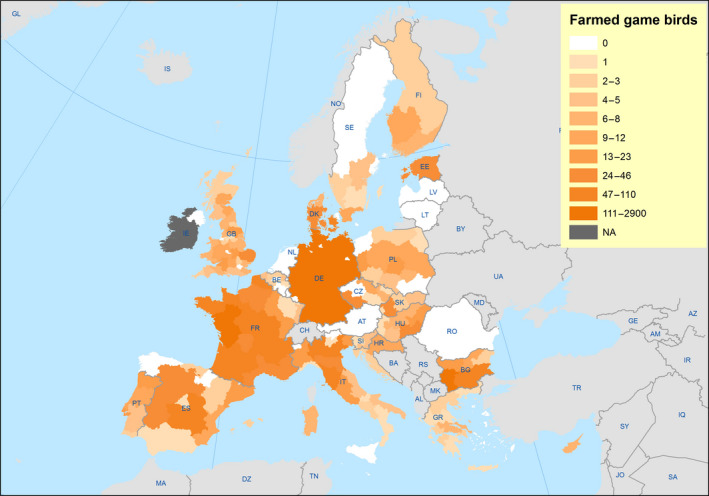
Game bird production by region reported by MSs to EFSA (autumn 2016) (taken from EFSA, ECDC, EURL, 2017a, 2017b)
From the above, it can be concluded that for Anseriformes, notification based on passive surveillance is less effective than for gallinaceous birds, and that serosurveillance in Anseriformes could be an effective complementary method to passive notifiable disease surveillance to detect HPAI, provided adequate follow up of positive findings. In the absence of such follow‐up, HPAIV circulation among Anseriformes can be prolonged as was shown in France 2015/2016 (Annex A). In addition to serosurveillance, regularly testing of anseriform birds that died during the production period (bucket sampling) for the presence of virus might prove useful to detect HPAI. Pooling of such samples for polymerase chain reaction (PCR)‐directed diagnosis may be useful. Although, HPAI may not induce clear clinical signs in anseriform birds, in combination with other infections present on the farm it might result in dead birds.
3.7.3. Analysis of the current HPAI surveillance in wild birds
Passive wild bird surveillance includes dead birds being reported to the veterinary authorities that are subsequently tested for HPAI virus. The intensity of reporting (e.g. numbers of birds found dead) differs between MSs or even between regions in a MS.48 The effectiveness of passive surveillance highly depends on the mortality associated with HPAI in wild birds, which has differed considerably between the outbreaks in Europe. During the HPAI H5N1 epizootic in 2005/2006, passive surveillance clearly demonstrated virus spread in the wild bird population as considerable numbers of dead birds were found H5N1 infected (Bragstad et al., 2007). The 2014/2015 HPAI H5 (clade 2.3.4.4a) epizootic presented itself, however, without noticeable mortality in wild birds. The 2016/2017 H5N8 (clade 2.3.4.4b) epizootic was associated with large numbers of birds found dead in many different regions and including many different species. Based on the wild bird HPAI findings during these epizootics, a predictive risk map was created using a logistic regression model analysing the relationship between reported wild bird events and environmental factors (Figure 13; see Section 2.7).
Figure 13.
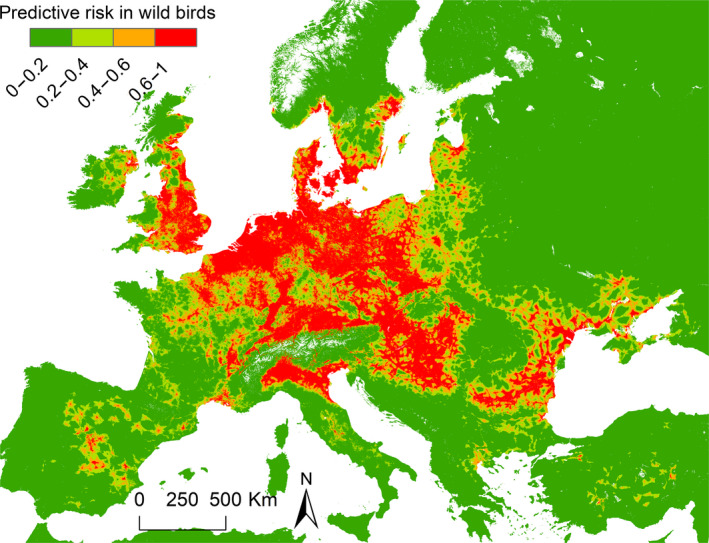
Relative risk map of predicted highly pathogenic avian influenza (HPAI) H5 occurrences in wild birds in Europe based on wild bird events reported in Europe between 2005 and 2017, and using the methodology as described in Si et al., 2010 (see Section 2.7)
In total, here have been 2,961 birds reported to be positive (on a total of 158,361 tested via passive surveillance) with HPAI between 2006 and April 2017 (Table 10), including 2,099 (70.9%) during the H5 H5Nx HPAI (clade 2.3.4.4b) epizootic in 2016–April 2017. From 2006 to April 2017, HPAIV was detected in 1.9% of the birds submitted to the NRLs as part of passive surveillance for avian influenza in the EU.
Table 10.
Results of active wild bird surveillance January 2006–April 2017
| Country | Total sampled | Negative | HPAI (% of sampled) | ALL LPAI (% of sampled) | H5 LPAI | H7 LPAI | Notifiable LPAI (% of sampled) | Non‐notifiable LPAI | Unknown pathotype (% of sampled) |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 5,598 | 5,396 | 2 (0.04) | 45 (0.80) | 34 | 10 | 44 (0.79) | 1 | 138 (2.47) |
| Belgium | 32,891 | 31,473 | 0 (0.00) | 378 (1.15) | 20 | 13 | 33 (0.10) | 345 | 897 (2.73) |
| Bulgaria | 538 | 533 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 5 (0.93) |
| Cyprus | 606 | 602 | 0 (0.00) | 4 (0.66) | 0 | 0 | 0 (0.00) | 4 | 0 (0.00) |
| Czech Republic | 2,156 | 1,977 | 0 (0.00) | 179 (8.30) | 16 | 0 | 16 (0.74) | 163 | 0 (0.00) |
| Denmark | 16,032 | 15,475 | 1 (0.01) | 468 (2.92) | 81 | 18 | 99 (0.62) | 369 | 75 (0.47) |
| Estonia | 505 | 503 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 2 (0.40) |
| Finland | 1,428 | 1,343 | 0 (0.00) | 75 (5.15) | 1 | 1 | 2 (0.18) | 73 | 10 (0.69) |
| France | 10,715 | 9,369 | 0 (0.00) | 428 (3.99) | 100 | 18 | 118 (1.10) | 310 | 514 (4.80) |
| Germany | 83,610 | 82,267 | 43 (0.05) | 168 (0.20) | 95 | 6 | 101 (1.12) | 67 | 749 (0.90) |
| Greece | 1,782 | 1,778 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 4 (0.22) |
| Hungary | 11,766 | 11,715 | 0 (0.00) | 51 (0.43) | 19 | 0 | 19 (0.16) | 32 | 0 (0.00) |
| Ireland | 1,146 | 1,125 | 0 (0.00) | 4 (0.35) | 2 | 0 | 2 (0.17) | 2 | 12 (1.05) |
| Italy | 19,862 | 19,418 | 0 (0.00) | 107 (0.54) | 14 | 11 | 25 (0.13) | 82 | 204 (1.03) |
| Latvia | 2,797 | 2,645 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 133 (4.76) |
| Lithuania | 2,497 | 2,301 | 0 (0.00) | 108 (4.33) | 0 | 2 | 2 (0.08) | 106 | 88 (3.52) |
| Luxembourg | 832 | 832 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Malta | 8 | 8 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Netherlands | 57,431 | 53,353 | 0 (0.00) | 2,778 (4.84) | 57 | 21 | 78 (0.14) | 2,700 | 1,229 (2.14) |
| Poland | 9,330 | 9,239 | 34 (0.36) | 22 (0.24) | 4 | 0 | 4 (0.04) | 18 | 35 (0.38) |
| Portugal | 4,262 | 4,238 | 0 (0.00) | 24 (0.56) | 13 | 3 | 16 (0.38) | 8 | 0 (0.00) |
| Romania | 4,297 | 4,297 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Slovakia | 557 | 551 | 2 (0.36) | 4 (0.72) | 0 | 2 | 2 (0.36) | 2 | 0 (0.00) |
| Slovenia | 1,405 | 1,351 | 0 (0.00) | 7 (0.05) | 1 | 0 | 1 (0.07) | 6 | 47 (3.35) |
| Spain | 30,239 | 30,153 | 0 (0.00) | 24 (0.08) | 4 | 9 | 13 (0.04) | 11 | 29 (0.10) |
| Sweden | 12,332 | 11,848 | 8 (0.06) | 270 (2.19) | 136 | 7 | 143 (1.16) | 127 | 206 (1.67) |
| Switzerland | 416 | 413 | 0 | 3 | 0 | 0 | 0 | 3 | 0 |
| United Kingdom | 16,612 | 16,453 | 0 (0.00) | 21 (0.13) | 17 | 0 | 17 (0.10) | 4 | 89 (0.54) |
| Total | 331,650 | 320,656 | 90 (0.03) | 5,168 (1.56) | 614 | 121 | 735 (0.22) | 4,433 | 4,466 (1.35) |
Active surveillance of clinically healthy wild birds was a legislative requirement for MSs from 2006 to 2010. Since June 2010, there is no longer an EU legislative requirement for MSs to test clinically healthy wild birds. Early positive findings in the 2014/15 HPAI H5 (clade 2.3.4.4a) epizootic resulted from active surveillance, although the absolute number was very low in comparison to the number of tested samples (3/5194 birds were found positive for H5 HPAI through active surveillance under the EU surveillance programme between October and December 2014). LPAIV surveillance in the Netherlands demonstrated a disconnect between wild bird findings and findings in poultry (Verhagen et al., 2017). Overall, in active surveillance, 94 birds have been reported positive with HPAI between 2006 and April 2017 (Table 11), 50 of these were during the H5 H5Nx HPAI epizootic in 2016–2017. In relation to the number of submitted samples, this corresponds to an HPAI positive proportion of 0.03% of all submitted samples.
Table 11.
Results of passive wild bird surveillance January 2006–April 2017
| Country | Total sampled | Negative | HPAI (% of sampled) | ALL LPAI (% of sampled) | H5 LPAI | H7 LPAI | Notifiable LPAI (% of sampled) | Non‐Notifiable LPAI | Unknown pathotype (% of sampled) |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 7,709 | 7283 | 284 (3.68) | 9 (0.12) | 3 | 1 | 4 (0.05 | 5 | 131 (1.70 |
| Belgium | 1,863 | 1,769 | 6 (0.32) | 45 (2.42) | 1 | 0 | 1 (0.05) | 44 | 43 (2.31) |
| Bulgaria | 530 | 507 | 22 (4.15) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 1 (0.19) |
| Croatia | 197 | 178 | 18 (9.14) | 1 (0.51) | 0 | 0 | 0 (0.00) | 1 | 0 (0.00) |
| Cyprus | 1,499 | 1,481 | 0 (0.00) | 18 (1.20) | 0 | 0 | 0 (0.00) | 18 | 0 (0.00) |
| Czech Republic | 3,905 | 3,831 | 66 (1.69) | 4 (0.10) | 0 | 1 | 1 (0.03 | 3 | 0 (0.00) |
| Denmark | 2,090 | 1,952 | 44 (2.11) | 7 (0.33 | 0 | 1 | 1 (0.05) | 6 | 20 (0.96) |
| Estonia | 125 | 125 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Finland | 1,461 | 1,435 | 21 (1.44) | 3 (0.21) | 0 | 0 | 0 (0.00) | 3 | 2 (0.14) |
| France | 6,308 | 5,934 | 145 (2.30) | 88 (1.40) | 12 | 7 | 19 (0.30) | 69 | 77 (1.22) |
| Germany | 57,031 | 54,846 | 1,485 (2.60) | 33 (0.06) | 22 | 2 | 24 (0.04) | 9 | 563 (0.99) |
| Greece | 1,559 | 1,543 | 12 (0.77) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 4 (0.26) |
| Hungary | 9,320 | 9,223 | 37 (0.40) | 21 (0.23) | 3 | 0 | 3 (0.03) | 18 | 2 (0.02) |
| Ireland | 1,957 | 1,862 | 12 (0.61) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 5 (0.26) |
| Italy | 12,099 | 12,036 | 25 (0.21) | 16 (0.13) | 0 | 1 | 1 (0.01) | 15 | 12 (0.10 |
| Latvia | 198 | 194 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Lithuania | 976 | 961 | 13 (1.33) | 1 (0.10) | 0 | 0 | 0 (0.00) | 1 | 1 (0.10) |
| Luxembourg | 544 | 543 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 1 (0.18) |
| Malta | 19 | 19 | 0 (0.00) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 0 (0.00) |
| Netherlands | 11,862 | 11,821 | 13 (0.11) | 20 (0.17) | 1 | 0 | 1 (0.01) | 19 | 7 (0.06) |
| Poland | 1,779 | 1,631 | 143 (8.04) | 2 (0.11) | 0 | 2 | 2 (0.11) | 0 | 2 (0.11) |
| Portugal | 2,566 | 2,559 | 1 (0.04) | 5 (0.19) | 0 | 0 | 0 (0.00) | 5 | 0 (0.00) |
| Romania | 2,082 | 1,900 | 177 (8.50) | 2 (0.10) | 2 | 0 | 2 (0.10) | 0 | 3 (0.14) |
| Slovakia | 2,396 | 2,309 | 82 (3.42) | 4 (0.17) | 0 | 0 | 0 (0.00) | 4 | 1 (0.04) |
| Slovenia | 2,417 | 2,175 | 217 (8.98) | 2 (0.08) | 1 | 1 | 2 (0.08) | 0 | 22 (0.91) |
| Spain | 7,112 | 7,052 | 3 (0.04) | 20 (0.28) | 6 | 1 | 7 (0.10) | 13 | 16 (0.22) |
| Sweden | 3,133 | 2,985 | 113 (3.61) | 8 (0.26) | 4 | 0 | 4 (0.13) | 4 | 7 (0.22) |
| Switzerland | 466 | 347 | 10 (2.15) | 0 (0.00) | 0 | 0 | 0 (0.00) | 0 | 109 (23.39) |
| United Kingdom | 15,295 | 15,195 | 16 (0.10) | 3 (0.02) | 2 | 0 | 2 (0.01) | 1 | 69 (0.45) |
| Total | 158,498 | 153,696 | 2,965 (1.87) | 312 (0.20) | 57 | 17 | 74 (0.05) | 238 | 1,098 (0.69) |
To increase the efficiency of active surveillance, the sampling strategy should be guided by better knowledge of the epidemiology of HPAI in wild birds. This would require an in depth analysis of the dynamics in space and time of the HPAI wild bird data, including affected species, indicated above. In addition, it is recommended to implement targeted active surveillance at epidemiologically important sites where bird populations are studied intensively. There is a need to combine an HPAI test result with information of the bird sourcing the sample (e.g. place in the wild bird contact network), as this will allow virus dynamics in wild bird populations to be better understood, which is essential to designing more efficient active wild bird surveillance programmes. Serological surveillance could play a valuable role in informing which wild bird species are more likely to be infected and shed HPAI, and even particular clades of H5 HPAI virus. Instead of active wild bird surveillance across the EU (as done in the past), a few priority locations within the EU could be identified (e.g. based on Figure 13) to implement targeted wild bird surveillance combining active virology tests (swabbing) with enhanced passive surveillance. The latter could for instance be done by lowering thresholds that MSs (e.g. the UK) have in place to ensure that laboratories are not flooded with samples or by implementing additional patrols to increase the likelihood of finding dead wild birds (as done by France during winter 2016/2017). Targeting efforts at a few priority locations may detect the presence of circulating AIV, when infection prevalence and sample sizes are sufficient, when these do not cause massive mortality among these birds. It would be useful to implement such a targeted approach in the EU (near the borders) and at global level, making the results available to the international community. Such a strategy should be well coordinated since it should combine research objectives with fast reporting of positive findings, allowing risk managers to increase preparedness.
3.7.4. Analysis of the current H5 and H7 LPAI surveillance in poultry
Current H5 and H7 LPAI serosurveillance, when conducted as representative rather than risk‐based sampling, has been designed to detect serological evidence of LPAIV in a country or region when the percentage of seropositive farms is at 5% or higher (with 95% confidence in some poultry types and 99% confidence in others) (Commission Decision 2010/367/EU). Consequently, in combination with H5 and H7 LPAI outbreak reports, it provides a year to year snapshot of the regional/national LPAI situation, can demonstrate LPAIV farm seroprevalence to be below 5% and would detect major changes in LPAIV trends across years. Comparisons of the positive findings between MSs are limited because of differences in the national surveillance programmes (such as variation in: sampling frame strata, random or non‐random selection of holdings to sample within strata, temporal variation in sampling, and ambiguities in reporting of denominator population data) (Gonzales et al., 2010). Some countries test more than the EU requirements, resulting in a higher likelihood of finding seropositives (e.g., IT, NL). In addition, legislation allows a risk‐based implementation of surveillance, which was implemented by 10 MS49 in 2014 (APHA, 2015). The chosen risk‐based approaches differ between countries (e.g. some may emphasise free‐range chickens, others waterfowl) and the weighting of the risk factors lacks underlying quantitative evidence, resulting in limited value in making comparisons of surveillance results.
It can be argued that LPAI surveillance provided advance warning of H5 LPAIV circulation in French duck flocks over several years (Annex A). Although LPAI surveillance can show major changes in LPAIV prevalence over the year or indicate the LPAIV level at regional level, it is not effective for ‘early warning’ of LPAIV infections at the holding level or detection before mutation to HPAI takes place. The reasons are that according to the legislation (1) only a sample of farms is tested, (2) a relatively small number of birds within a flock is tested, (3) tested farms are tested only once in a year and (4) the assay tests for antibodies that are only detectable when most virus shedding of the bird has been taken place (Gonzales et al., 2014). Consequently, at the time of seropositive detection, most virus production in a flock has already taken place. The latter is supported by the observation that upon a seropositive finding, when samples are collected for virus detection, a minority of the farms turns out positive in PCR. Even in the Netherlands where all farms are tested and free‐range farms even four times a year, most attempts fail to detect virus in seropositive farms. Also in cases where egg yolk is used to detect antibodies in a continuous flow, infected farms will on average be detected after the peak of virus production has taken place (Gonzales et al., 2014). Only a detection strategy based on adequate real time monitoring of production parameters (Gonzales et al., 2012a,b) or notification of mild clinical disease would have the potential for early detection of individual outbreaks. Nevertheless, because a drop in production and mild disease may be caused by many diseases other than LPAI, the specificity of such a system would be very low, resulting in many false alarms. In addition, in domestic waterfowl a drop in production parameters may be absent upon LPAI infection.
It is highly likely that most LPAIV outbreaks in the EU are not detected because of the small proportion of farms that are tested using active surveillance and the low sensitivity of passive surveillance due to the mild clinical signs. From the reasoning above, it can be concluded that it is not possible to design a surveillance programme for early detection of individual LPAI outbreaks that is practically feasible. LPAIV outbreaks often do not result in secondary spread, as was for example demonstrated for laying hens in the Netherlands (Gonzales et al., 2014); infection fades out without any control measure being implemented. Nevertheless, LPAIV infections may result in secondary spread, as was shown in turkeys in Italy (Comin et al. 2012) and Germany (personal communication T. Harder, September 2017), ducks in France, and laying hens in poultry dense regions (Gonzales et al., 2014). In those situations, major LPAI epidemics can arise, associated with continued virus replication with a continued risk of virus mutation to HPAIV. Consequently, a surveillance programme focussing on detecting clusters of connected LPAI outbreaks would be essential to curtail further LPAIV spread. Although such a surveillance strategy does not guarantee detection before LPAIV has mutated to HPAIV (see Section 3.6), the French HPAI epidemic in 2015/16 (Annex A) and the Italian H7N1 1999/2000 epidemic (Capua et al., 2003) have shown that continued LPAIV spread can eventually result in a HPAI epidemic. Because the effectiveness of detecting clusters of LPAIV outbreaks depends on the spread of LPAIV between farms, no single design surveillance programme can be developed for the whole of the EU. A risk‐based approach is required that at least takes into account poultry species, production type and density of poultry flocks. Therefore, there is a need to collect robust data on poultry populations across the EU. Based on, for example, the available control measures, this would enable development of the design prevalence (maximum number of LPAI‐infected farms before detection in a region). Once this number is determined and the number of farms in the region is clear, the number of herds to be sampled can be determined. However, in case of positive findings, follow‐up is necessary to find out whether this is an individual seropositive farm or a cluster of positive farms. AI surveillance planning and follow up should be based on the local situation but reporting should allow analysis and comparison across MSs.
To optimise detection of circulating AI viruses in the EU, the following improvements of annual surveillance are suggested:
Focus testing on Anseriformes as they have a much higher risk of AI introduction and serosurveillance might in addition to LPAIV detect circulation of HPAI viruses that do not evoke clear clinical signs in Anseriformes. Testing should be done at least once a year during a production stage where subsequent virus testing of the same flock is still possible;
Holdings repeatedly positive (consecutive years) should apply a strict holding‐specific biosecurity plan to reduce the risk of virus introduction and transmission and also should apply bucket sampling during the production rounds until they are again negative;
During high‐risk periods (AI in the region), bucket sampling should be applied on holdings with Anseriformes;
Collect robust data on populations (numbers of holdings/birds per species/production type and their location);
Harmonise the interpretation of definitions (e.g. risk factors, data variables) to improve reporting and subsequent analysis;
Make annual reporting of results more user‐friendly;
Reporting of quantitative data regarding the used risk‐based approach would facilitate analysis of the results and to make progress on developing the methodology.
3.8. Early detection of HPAI (TOR 2)
Early detection of HPAI in gallinaceous species relies on notification of clinical suspicion. For Anseriformes species, the sensitivity of ‘notifying clinical suspicions’ as a mechanism for early detection might be low for some HPAIV lineages. Subclinically infected domestic Anseriformes have a higher likelihood of continued spreading of HPAIV when compared with clinically infected gallinaceous birds. As a consequence, expanding early detection of HPAI in Anseriformes using serological surveillance and bucket sampling may help to timely detect and curtail HPAI in Anseriformes. More information is provided in Section 3.7 since early detection is related to surveillance.
3.9. Biosecurity to prevent HPAI and LPAI entry and spread (TOR 2,4)
This chapter gives overall recommendations on biosecurity measures, however holding‐specific guidance is very important since every holding is unique and might require specific adaptation of otherwise generic rules. Identifying the weak biosecurity points of a holding is essential as these determine in a chain of measures the overall biosecurity level of a holding. Advising personnel how to strengthen biosecurity in practice and generating awareness on the importance of sustained implementation will result in augmented biosecurity.
3.9.1.
Commercial chicken production with only indoor housing
The most feasible, sustainable and efficient measure to reduce the risk of AI entry into indoor commercial chicken holdings is preventing wild bird access to poultry, in particular by making the housing wild bird proof (Figure J.2, Appendix J). Protection against faecal droppings from flying wild birds is also an additional measure, in particular faecal dropping that rinses from the roof or are washed down after rainfall and enters the house through the ventilation or drain inlets. Other measures with a high feasibility and sustainability are separation of waterfowl from other poultry species and providing potable drinking water instead of untreated surface water. Clearly, these measures can be achieved with a single one‐off investment, and further on require limited efforts to sustain. The implementation of a hygiene lock (a separate distinct secondary doorway with a hygiene barrier for entry of people and equipment) for each poultry house, restricting access to the birds and biosecurity training for staff have the second, third and fourth highest rank for effectiveness to prevent AIV entry, respectively, although their feasibility and sustainability are scored lower. This suggests that adapting (routine) human behaviour is important to reducing the risk of virus entry into a poultry holding, although it is difficult to sustain raised awareness and alertness over a long period. However, it should be noted that biosecurity has is also relevant to other hazards in addition to AIV and hence should be implemented in routine practices where possible. In particular biosecurity training was considered very useful as it will teach people to understand the main risks, which is a first step towards effective risk mitigation. It is recommended that professional staff on poultry holdings attend general biosecurity trainings but also that they receive holding‐specific biosecurity advice, ideally from an expert (e.g. veterinarian) familiar with the particular holding to explain the practical implementation in the daily working environment. The 2016–2017 epidemics increased the awareness of professional staff across the EU of the risk of AIV entry whereas knowledge on the risk of AIV spread via fomites and equipment seems to be limited still.
Figure J.2.
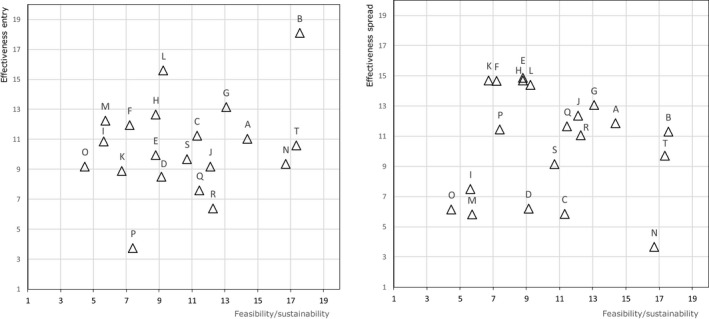
Average ranking of biosecurity measures applicable in a commercial chicken holding with only indoor housing (the letter IDs are explained in Table J.1)
The highest ranked measures to prevent spread of the virus are to contain poultry and fomites (i.e. those that were in contact with poultry) during transport, cleaning and disinfection of equipment, biosecurity training, cleaning and disinfection of transport vehicles, and use of a hygiene lock for each poultry house. When defining a biosecurity plan for a holding, biosecurity measures reducing the contact rate with AIV should be combined with measures reducing the virus quantities. These measures should also be implemented in disease‐free time to prevent unnoticed AIV spread, in particular in Anseriformes flocks.
Commercial chicken production with outdoor access
The outcome of the ranking exercise indicated that restricting access and providing biosecurity training are the most feasible, sustainable and efficient measures to reduce the risk of AI entry and spread in commercial holdings where chickens have access to outdoor areas (Figure J.3, Appendix J). A hygiene lock for each production unit has the highest rank for preventing AIV entry and the second highest rank for preventing spread, but its feasibility and sustainability are ranked considerably lower. All experts agreed that the effectiveness of biosecurity measures is in general much lower when poultry have outdoor access compared with indoor only housing. Therefore, in high‐risk periods, the first recommendation is to restrict outdoor access as much as possible. In case it is not possible to confine all domestic birds, it is recommended to reduce the size of the outdoor area. Increasing poultry density in the remaining area is one way to make the environment less attractive to wild birds. Preventing direct contact between wild birds and poultry is also considered highly effective to reduce the risk of AIV entry. The use of netting50 is considered useful by experts and feasible as it is already implemented for instance in fruit orchards. Installing horizontal protection (e.g. a roof or canvas) is also useful, certainly in part of the outdoor area (i.e. where feed and water are provided if this cannot be performed indoors). Making the area around the holding (where possible) unattractive to wild birds prevents against roosting and nesting nearby. Furthermore, grass on the holding must be kept cut (wild birds eat grass seeds, forage in long grass) and fallen fruit should be removed around the holding. Placing flashing or rotating lights or using acoustic devices at the entry points will deter wild birds from entering, but rotation is needed since wild birds will quickly get used to these. Anseriformes should be strictly separated from Galliformes.
Figure J.3.
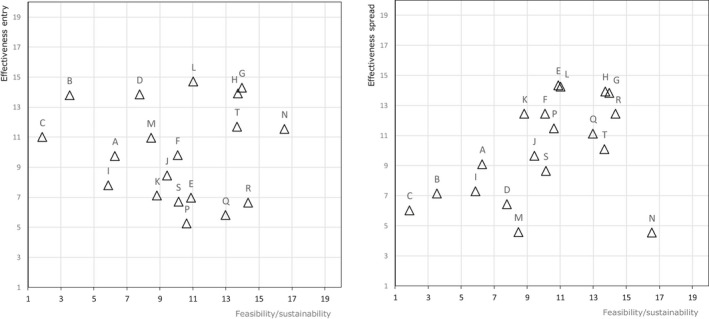
Average ranking of biosecurity measures applicable in a commercial chicken holding with outdoor access (the letter IDs are explained in Table J.1)
Commercial production of turkeys and Anseriformes
Ranking the biosecurity measures for commercial turkey or Anseriformes production would have a similar outcome as that described above for chickens. A few points related particularly to biosecurity in keeping turkeys and ducks are explained below.
As already described in the Statement on AI (EFSA, 2017a), turkey holdings may be at higher risk than chicken farms for AI introductions because of the constant need for introduction of clean bedding during the production cycle of turkeys and their high ventilation requirement (Mendes et al., 2013). The most critical point is the way bedding material is brought in. Especially in regions with high numbers of wild birds (e.g. geese and ducks) or due to an attractiveness of the holding for gulls and crows, the premises of the holdings and the area in front of the poultry house may be highly contaminated with potentially infectious faecal material of wild birds. Contact of poultry with this material, e.g. through vehicle wheels of lifters/tractors, must be prevented. Several solutions to improve biosecurity here are conceivable including to roll out a canvas cover outside before bringing the bedding in, and to store this cover afterwards. It is recommended to store bedding as much as possible in the same building where the turkeys are kept (or as close as possible to it) to reduce the risk of introducing wild bird faecal material from the outside into the poultry house. A practical solution used in several MS is building a wall of straw or otherwise packaged bedding material in the poultry house at the beginning of the production cycle and breaking this gradually down when new bedding is provided to the birds. There are also a few turkey holdings with a closed production system in the EU using a pipe‐filling system to distribute bedding.51 The disadvantage of such system is the high amount of dust produced while bringing bedding in, as this can increase the incidence of respiratory problems on birds. New bedding materials with a higher absorption capacity have also been explored (e.g. straw pellets, wood shavings, chopped straw). In addition, bedding storage, if kept separately from the turkey houses, must be proofed from wild birds, rodents and predators. It is virtually impossible to disinfect/decontaminate bedding material to which wild birds have had access.
Many turkey fattening facilities use natural ventilation with an open rooftop or tunnel; these systems require that all air inlets (e.g. chimneys) are wild bird proof. Changing to mechanical ventilation systems in controlled housing environments might help to reduce the risk for airborne AI introduction, although it should be combined with strict implementation of other biosecurity measures since there have also been AI outbreaks reported in holdings with controlled housing environments. There is ample practical information available in the public domain on ventilation systems that can be used in turkey production (ZDG, 2013; AviagenTurkeys, 2015). It should be mentioned that currently the importance of aerogenic transmission of AIV between holdings is not clear.
Due to the different fattening cycles of female (15–16 weeks) and male (21 weeks) turkeys, ‘brood and move’ routines are in place at holdings where both sexes are reared together. Loading of female turkeys during the final growing period of males inevitably leads to intense contact to loading crews and processing plant trucks and creates a high risk of AIV introduction. Reduction of such risks would require separate fattening of female and male turkeys; otherwise highest biosecurity rules for loading crews and trucks would have to be implemented. These would focus on difficult‐to‐handle very high hygiene standard including use of disinfected crates and containers, disinfecting trucks at the entrance, shower in and shower out for all visitors, farm clothing and farm boots, etc.
Artificial insemination is common practice on turkey breeding farms and performed on a weekly basis. Most of the breeding companies in the EU house male and female turkeys on the same farm. If artificial insemination is carried out by staff employed on the holding and movement from farm to farm is excluded, risks of introduction of AIV are considered to be low. However, odd cases of transmission of human influenza to turkeys through contaminated instruments have been reported (Pantin‐Jackwood et al., 2010). Replenishing breeding stock from outside, of course, demands highest biosecurity measures including quarantine management of newly introduced stock.
Confinement of breeding ducks and in particular geese is difficult due to their physiological need to access water pools and daylight,52 although many systems are entirely indoor, with water periodically provided in ‘troughs’. A key problem in current duck production is the high demand for clean bedding (as for turkeys) and the movement of the birds from rearing to production and finally to fattening/moulting houses.53 Using a plastic tunnel and having a concrete floor between houses at the same holding, when disinfected prior to movement, could reduce the risk of contact with wild bird faecal droppings. However, often, ducks are moved between holdings (even at large scale between different MS) during the production cycle (i.e. from large pre‐fattening flocks to many small fattening flocks and again to collection places before slaughter), which bears a high risk for AIV spread. Transport of day‐old ducks happens in closed vehicles, which are in most cases suited to be disinfected. However, large vehicles need large disinfectant tanks, which often can be fixed only on the outside of the vehicle what could cause challenges is wintertime. Collecting reusable material (e.g. plastic boxes/cages) on a round trip with more holdings on the way could be a high risk activity. These materials should to be collected on the way back, when the vehicle is empty. Vehicles transporting live birds other than day‐old birds are opened. If the birds’ craw is not empty at the time of loading, faeces may be scattered off. Vehicle cleaning and disinfection after live bird transport (liver ducks/geese for fattening, reared turkey for fattening, pre‐reared breeders) is a bigger task than an average holding supplied with disinfectant tools can handle. General lorry wash stations often do not serve live animal transport vehicles. These vehicles and their boxes are properly washed and disinfected at the slaughterhouses and at the home garage, however regular checks are important to maintain high quality. Establishment of supervised regional live animal truck wash stations would be useful.
In Hungary, depending on the duck species, natural or artificial insemination is used. Introducing new males to breeder flocks happens in pekin duck holdings. However, it is difficult to find available males. When there is no direct epizootic threat present, nor the Hungarian authority, nor the Food Business Operators require AI tests prior to the transport of the new males, although precautions are taken (e.g. quarantine requirements) to avoid Pasteurella multocida infection. The animal keepers are motivated to be careful in AI peacetimes as well. Artificial insemination is used in the Hungarian mulard duck industry, and now in increasing number also at goose breeder holdings. The sperm producing males are often kept in the same holding, sometimes in the same building, in cages, separated from the females. Operating all in‐all out system is not realistic, given that different age group males are needed to be reared to maintain the continuous sperm supply.
Non‐commercial (backyard) poultry production with outdoor access
For backyard holdings, biosecurity training has been assigned the highest overall rank when considering feasibility, sustainability and effectiveness to reduce AIV entry (Figure J.4, Appendix J). Preventing direct and indirect contacts between domestic and wild birds was identified as most efficient to prevent entry but has a lower rank for feasibility and sustainability. Protection against faecal droppings from flying wild birds would also be efficient to prevent virus entry, although it has very low feasibility and sustainability. This low feasibility is mainly due to the cultural practice of keeping poultry in completely free‐range conditions on the family property in several MSs. It is recommended to confine and at least limit the outdoor access area of domestic birds in high‐risk periods (see above). Restricting access is considered effective to reduce the risk of AIV entry and spread. Health monitoring and appropriate carcass disposal are specifically effective to reduce the risk of virus spread.
Figure J.4.
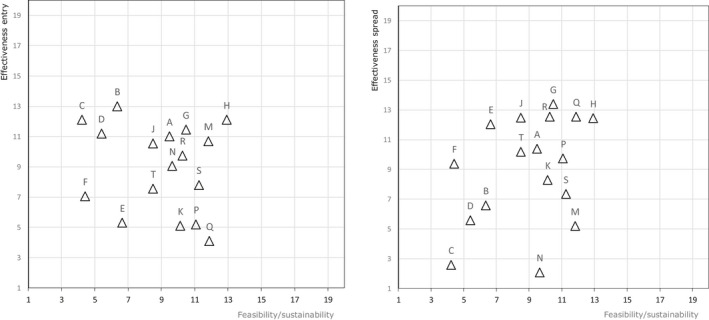
Average ranking of biosecurity measures applicable in a non‐commercial poultry holding (backyard) (the letter IDs are explained in Table J.1)
In some MSs, poultry dealers (people buying poultry from different breeding facilities, keeping the animals at their own facility and distributing them subsequently to people within the area) are the main distributors of domestic birds to backyards. Such distribution networks have been identified for severe risk of introduction and spread of AIV and hence strict biosecurity measures have to be implemented. It is recommended that poultry dealers buy birds only from sources that can provide a health certificate and ensure traceability of the birds (at least at batch level). Pre‐ordering of fixed numbers of poultry should be encouraged to ensure that no unsold poultry returns to the distributor's premises. The poultry dealers should receive a biosecurity training including how to clean and disinfect crates and vehicles and how to recognise clinical signs, which should facilitate early detection of AI cases. A veterinarian should assist the poultry dealer in the supervision of the flocks and provide biosecurity advice. Mixing of gallinaceous and Anseriformes birds should not occur.
Small‐scale commercial holdings
Small backyard holdings that directly sell poultry or poultry products in the local area, e.g. to their neighbours, may have increased contact rates at their premises if selling is performed at the farm. However, it is likely that it involves only people that do not rear poultry themselves. Hence, the risks of incursion and spread of AIV are considered low. Small‐scale commercial holdings which also market products through local retailing shops that collect poultry or poultry products from more than one holding in the area, may have higher risks for AIV entry and spread as a result of increasing numbers of contacts and linked production lines. The highest risk commodity is live poultry. Selling only slaughtered poultry should be encouraged, and public awareness should be increased concerning the prevention of access of backyard chickens to uncooked offal from purchased poultry carcasses (Harder et al., 2009).
Within small‐scale commercial holdings, the same biosecurity measures should be applied as in larger commercial holdings. There should be physical separation between the area where farming activities take place and the vending area. Cleaning and disinfection of the vending area should be strictly implemented.
Hunting and use of decoy birds
The effect of hunting on the risk of AIV spread depends on the type and location of shooting. In general, shooting may increase local movement rates of game birds and cause increased (indirect) contacts between wildlife and poultry on farming premises, trigger migration or increase the contact rate between potentially infected and non‐infected wildlife (Cox and Afton, 1997; Vaananen, 2001). Therefore, any type of shooting or any other activity disturbing wild birds (e.g. walking dogs, water sports, etc.) within zones where HPAIV has been detected in wild birds might increase the risk of virus spread. Galliformes and Anseriformes that are raised for shooting (game birds, usually mallards) should be batch‐tested before release as they could carry AIV without showing clinical signs (Vittecoq et al. 2012). Risk‐based testing of game birds, in particular Anseriformes, could be implemented before trading to reduce the probability of unnoticed AIV spread. EU legislation is already in place for intra‐EU trade in game birds (Commission Decision 2006/605/EC), stating that birds younger than one month should be tested by virology (20 cloacal and 20 tracheal swabs) during the week before dispatch to other MSs, plus health examination during the 24 h before dispatch. This is considered a safe practice and it is recommended to implement virological testing of swabs before game bird release in the wild within the country of rearing.
The use of live decoys for duck hunting will lead to AIV spread when the decoys are infected during the hunt and then bring the virus back to captive flocks at the hunter's place of residence (other decoys, backyard poultry flocks or professional poultry premises). It may therefore be appropriate to ban decoy transport in HPAI‐infected or high‐risk areas during AIV outbreaks. Under some circumstances, live decoys could still be used for hunting in AIV‐infected zones if they are kept on the hunting site at all times, since it is the transport that is the main problem.
At all times, carrying of hunted wildfowl, defeathering, gutting and butchering of carcasses are activities associated with a high risk of contact to, and dispersal of, AIV if the hunted bird is infected. Soiling of shoes and clothes, transport boxes and vehicles (car boot) is likely. Therefore, poultry farmers and workers on poultry farms attending wild bird hunting should seriously consider whether such activities might not pose difficulties in controlling risk for their holdings. In any case, hunters must strictly observe biosecurity measures to ensure that exposure of poultry to any of the items mentioned above is prevented. Hunting bans could be considered when separation of hunting and poultry rearing activities cannot be applied rigorously during high‐risk periods.
Biosecurity measures that could be applied in a zoo
Zoos with avian species on display pose a particular problem to prevention and control, including biosecurity measures:
often mixed species are shown outside in areas mimicking natural habitats;
feeding in the open may be provided in a way to display the birds to visitors;
described habitats and feeding measures will attract wild birds which may have unhindered contact with zoo birds;
zoo birds often have substantial financial value or are important to maintain genetic diversity in breeding and restocking programmes which may interfere with culling measures.
Practices of keeping birds in zoos, on the contrary, may also have advantages in terms of raising biosecurity:
exotic species may require year‐round indoor rearing;
smaller aviaries already provide access limitation for wild birds which may easily be further enforced (e.g. with impenetrable roofing);
often stables are available for wintering the birds from open habitats;
animal keepers usually are experienced and more compliant in observing biosecurity measures.
Very few preventive measures are at hand that can be applied in zoos without interfering with the display of the birds. Vaccination is only possible following permission by the European Commission and the usefulness of the licensed vaccines may be questioned. Also, AIV‐antibody‐positive birds (H5, H7) will no longer be eligible for exchange breeding programmes outside the EU.
Therefore, most of the reinforced biosecurity measures applied to avian collections at zoos have been applied after an outbreak of notifiable AI had been diagnosed (Globig et al., 2016). EU legislation grants exemption from culling of affected avian collections under defined conditions (laid down in Article 13 of Directive 2005/94/EC derogations from culling for certain holdings). These essentially include a risk assessment by the competent veterinary authority indicating that keeping the collection or only culling part of it will not further increase risks of incursion to neighbouring poultry holdings or spill‐back infection to wild birds. Specific measures taken by affected zoos comprised:
dividing the avian population into smaller epidemiological units;
confining all birds indoors with separate housing for small epidemiological units;
assigning separate staff to each of the epidemiological units;
prohibit sharing of staff, feed, bedding, machinery and equipment between the different epidemiological units;
-
discouraging/scaring off further visits of (aquatic) wild birds by
-
1
– draining open water bodies or
-
2
– horizontal screening of larger water bodies with warning tape
-
3
– confine all feeding in the zoo indoors including the animals in the children's’ area.
-
1
Closing the zoo for the public for a number of days has been instrumental to install all measures. Re‐opening is possible when all of the avian collection has been appropriately dealt with. Prolonged access restriction of the public to larger halls/spheres where zoo birds move unrestrained may be considered.
Improving knowledge on biosecurity
There are very limited data on the effect of biosecurity measures in preventing AIV entry and spread. Therefore, the text above describes the outcome of expert opinions. Performing case–control studies during outbreaks and collecting data via questionnaires are considered potentially very useful to improve our knowledge. Questionnaires carried out by independent organisations (e.g. universities,54 poultry associations55) often get answers that better reflect reality when compared with questionnaires carried out by the authorities. Providing feedback to farmers on the outcomes of the questionnaires will help them to improve their biosecurity implementation. It is recommended to look at biosecurity with a broader context, beyond AI, to assure motivation for poultry farmers also outside the AI high‐risk periods.
3.10. Establishment of a control & monitoring area and risk zones (TOR 2)
The protection measures in relation to HPAIV findings in wild birds were assessed based on the HPAI H5N8 2016/2017 outbreak (EFSA, 2016a). In this chapter, additional scientific guidance is provided on zoning around wild bird cases or poultry outbreaks and on exit strategy to lift protection measures.
3.10.1. Reconsider zoning
Restriction zoning around wild bird cases (Commission Decision 2006/563/EC) is no longer constructive when several cases are diagnosed within a short time frame in the same region (see EFSA, 2016a). Depending on the prevailing host species affected, a larger and possibly even transnational area of heightened alertness should be implemented instead. In this area, intensified passive monitoring would be required to gather data on the actual incidence of infection in wild bird populations. This may require specific input from ornithologists. Data would inform authorities on the grading and duration of reinforced biosecurity measures for poultry holdings.
Meticulous management of restriction zoning around infected poultry holdings (Commission Decision 2006/415/EC56) remains pivotal to prevent secondary spread which is the greatest threat to commercial poultry production systems. Descriptions of zones and measures should be easily graspable for people directly affected (local language) but need to be conceivable for MS as well, in particular when crossing MS boundaries. Further thoughts are required to control outbreaks with strains of stronger zoonotic propensities than the H5 HPAI 2.3.4.4a and b clades. The animal health aspects would not change but protection of humans would be critical and could be based on the ECDC toolkit.57 Such strains with increased zoonotic potential currently circulate in Egypt (clade 2.2.1.2), South‐East Asia (H5N6, clade 2.3.4.4c) and China (H7N9). In the light of previous experience, human infections must be expected should these strains reach the EU and spread through the same channels as in 2016/2017. In particular, poultry farmers and workers such as catching crews would be at highest risk before the incursion has been detected. In addition, after declaration of the outbreak, personnel on duty collecting and disposing dead wild birds as well as culling crews are exposed to the virus. Maintenance of reinforced biosecurity and wearing of enhanced personal protection equipment58 is problematic when culling large poultry holdings with heavy poultry individuals (turkey fatteners > 14 weeks, breeding geese). In cases where human‐to‐human transmission of highly zoonotic strains cannot fully be excluded, quarantine measures may be extended to farmers and workers. These additional measures would grossly delay actions and gradually deplete the pool of available staff that is off service during quarantine periods.
3.10.2. Exit strategy to lift protection measures
To date, there are two main risk factors identified for the incursion of notifiable AI into poultry holdings:
primary introduction by direct or indirect contact via infected wild birds;
secondary spread between poultry holdings.
The grading and duration of protection measures should be related to the infection pressure through both of these pathways. So, knowledge on the incidence (i.e. the number of new infections/time unit) of infections along these pathways is required.
Secondary spread between poultry holdings at national level often has a local or regional restriction element. Therefore, a regional scaling and adaptation of protection measures may be justified. The situation is more complicated when assessing risks of spread through wild bird populations. These are much more volatile than poultry populations and, if migrating species are involved, may affect a much wider geographic range and therefore will require supranational assessment. In addition, data on the incidence are much more difficult to collect.
EU and national legislation require risk assessments to justify actions of the competent veterinary authorities, especially if exemptions from or lifting of actions are demanded. Often regional and even local authorities are responsible for carrying out such assessment which is conceivable as it is the local authorities that should have the most thorough knowledge of the local conditions. However, such assessments can be heavily influenced by factors widely exceeding local geographic restrictions, be they transnational trading activities or wild bird migration. The principal basis for any risk assessment in terms of defining exit scenarios would ideally be the quantification of the viral load in the ‘environment’ (combined poultry holdings and wild bird populations as well virus contamination of the environment) as a proxy of the risk of incursion/spread. Such data, however, cannot be measured directly. They have to be extrapolated considering various criteria such as:
an epidemic curve (requires continuous, intensified passive surveillance of wild bird populations), i.e. knowledge on the dynamic of the epizootic;
knowledge on regional wild bird and poultry population structures and densities;
knowledge on regional intensity and structures of poultry trading activities.
Expertise and a harmonised and structured approach of collating and interpreting such data on an EU but at least on a national level59 would aid in terms of transparency of the decisions taken. Naturally, this process would require epidemiological, ornithological and virological data integration by expert groups. In addition, stakeholder expertise might be required as well. So, such processes can probably not be compressed into simple and harmonised algorithms and decision trees.
4. Conclusions
4.1.
4.1.1.
Mapping of HPAI and LPAI outbreaks during the last 5 years (TOR 5)
In the last decade, several clades of HPAIV H5 and members of the Eurasian lineage of HPAIV H7 have been detected in Europe. The majority of recent HPAI outbreaks on EU territory are of an epidemic, sporadic character. HPAIV H5 affected poultry and wild birds, whereas HPAIV H7 was only found in poultry.
Distinct HPAI clades of goose/Guangdong‐like H5 subtype viruses are endemic/present in poultry populations in several subcontinental regions outside the EU, e.g. South‐East and possibly Central Asia, China, Egypt, West Africa. Some of the H5 virus clades circulating in these areas bear zoonotic potential and may cause lethal disease in humans.
None of the HPAI viruses recently detected in the EU revealed significant zoonotic potential. However highly zoonotic HPAIV are circulating in Asia (H7N9, H5N6 and H5N1) and in Egypt (H5N1) that may be introduced to Europe.
H5 and H7 LPAI viruses are endemic in the European wild bird population.
Potentially zoonotic LPAI viruses of subtype H9N2 (G1 lineage) are enzootic in poultry in many parts of Asia, the Middle East and Northern Africa. Although not detected so far in Europe, introduction is possible.
HPAI introduction via migratory and residential wild birds (TOR 1)
Outbreaks of HPAI H5 in poultry were initiated by primary incursions of infected migratory wild birds into Europe, but intrinsic generation from a LPAI precursor virus and secondary spread between poultry holdings has been observed as well.
Four different geographical routes for the entry of wild birds into the EU have been defined. According to results obtained from the epizootic model generated in this opinion, the north‐eastern and eastern routes have been associated with a high risk of H5 HPAIV‐infected wild birds entering the EU. No H5 HPAIV incursion has been observed so far from the southern and north‐western routes.
Upon introduction of HPAIV into a wild bird population of sufficient size within the EU, amplification and further wild bird‐associated geographical spread of the virus may take place.
An association was identified between the HPAIV occurrence in wild birds and the likelihood of infection of poultry holdings, which is supported by the association between detections in wild birds and poultry in the field.
According to expert opinion, prevention of access of poultry to water bodies could result in an estimated threefold reduction in HPAI entry probability. Combining this biosecurity measure with confining poultry to indoor housing was estimated to further reduce the HPAI entry probability twofold, and adding routine or high biosecurity would result in a further estimated reduction of 4‐ and 44‐fold, respectively.
The estimated impact of biosecurity measures was considered independent of the HPAI virus characteristics.
LPAI introduction via migratory and/or residential wild birds (TOR 4 and TOR 5)
For LPAIV, endemically infected wild bird populations play an important role as a source of primary incursions, but secondary spread by undiagnosed infected poultry flocks must be considered as well.
According to results obtained from the epizootic model generated in this opinion, LPAIV can reach similar maximum prevalence levels in wild bird populations when compared with HPAIV.
At the same prevalence in the wild bird reservoir, the risk of LPAIV infection of a poultry holding was estimated to be lower than that of HPAIV.
The impact of implementing biosecurity on lowering the probability of LPAIV entry into a holding is considered similar to that of HPAIV.
HPAI and LPAI introduction via non‐wild bird pathways (TOR 1, TOR 3 and TOR 5)
The risk of HPAIV and LPAIV introduction into the EU through non‐wild bird pathways is estimated to be lower compared with the wild bird pathway.
The only non‐wild bird pathways that were considered to have a non‐negligible risk of HPAI introduction are intra‐EU movements and Third country trade of semen, intra‐EU movements of manure originating from holdings with Anseriformes species.
The only non‐wild bird pathways that were considered to have a non‐negligible risk of LPAI introduction are intra‐EU movements of live poultry and day‐old chicks, intra‐EU movements of manure originating from holdings with any species.
Introduction of AI into a poultry holding via feed and bedding is considered non‐negligible when accessible by wild birds during storage or any point during the transport route.
Pigeons are rarely found to be infected during either epizootics or in endemic areas where H5 and H7 viruses circulate, hence they do not play an important role in the spread of AIV.
Illegal introductions of HPAIV‐infected commodities (e.g. birds of prey, pet birds, unprocessed poultry meat) have been detected at the EU border.
HPAI and LPAI transmission and spread (TOR 6)
The transmission rate between animals within a flock is assessed to be higher for HPAI viruses than LPAI viruses.
Quantitative information on between‐flock transmission on farms is lacking.
Spread of HPAI viruses between farms is highly likely in the absence of control measures. In most cases, LPAIV remain restricted to a single farm, although horizontal spread has been observed in several occasions.
Mutation from LPAI to HPAI (TOR 7)
In very few cases, it could be proven that HPAI outbreaks were caused by intrinsic mutation of LPAIV to HPAIV and since 2005 the secondary spread of such HPAI viruses in the EU was limited except for one event which has led to recurrent HPAIV outbreaks in a single region of France.
No specific factors related to host species, environmental conditions or viral lineage were identified and likewise no molecular markers that would be useful predictors of increased risk of a specific LPAIV to mutate to an HPAI phenotype were recognised. However, emergence of HPAI viruses from LPAI precursors in Europe seems to have occurred more frequently for LPAI viruses of the H7 subtype.
Current knowledge does not allow a prediction as to if, and when, LPAI will mutate to HPAI.
HPAI and LPAI surveillance (TOR 2, TOR 4 and TOR 8)
Introduction of HPAIV in gallinaceous poultry populations inevitably results in severe clinical disease and high mortality, whereas the clinical manifestation and mortality in anseriform poultry depends on the phenotypic characteristics of a HPAI virus.
Passive surveillance through notification of suspicious clinical signs/mortality is the most effective method for early detection of HPAI outbreaks in gallinaceous poultry.
For effective surveillance in anseriform poultry, passive surveillance through notification of suspicious clinical signs/mortality needs to be accompanied by serological surveillance and/or a virological surveillance programme of birds found dead (bucket sampling).
Risk‐based surveillance is useful as it targets flocks where AI introduction is considered to be higher, although there is limited quantitative (EU‐wide) evidence to weight the risk factors.
Reporting of risk‐based surveillance approaches are not currently detailed enough to allow robust analysis and comparison of the results.
There is currently a lack of data on non‐affected holdings and houses within the affected regions, which is required in order to establish the magnitudes of risk of infection for the various potential risk factors, such as location, holding‐ and flock sizes, biosecurity measures, etc.
Current LPAI serological surveillance programme is useful to detect major changes in regional LPAI occurrence and does result in the detection of active H5 or H7 infection in a minority (around 20%) of follow‐ups conducted.
The serological surveillance is unfit for early warning of LPAI outbreaks at the individual holding level. Serosurveillance could be effective in detecting clusters of LPAIV‐infected holdings.
Early warning of LPAI at individual holding outbreak level is practically not possible given that the clinical signs are not specific and seroconversion comes too late.
Passive surveillance is an appropriate method for HPAI surveillance in wild birds if the HPAIV infections are associated with mortality.
Active wild bird surveillance has a very low efficiency in detecting HPAI.
When HPAIV has been detected in poultry within a given geographical area active wild bird surveillance could play a role in detecting HPAIV infections in wild birds that are not associated with mortality as a possible source of virus introduction.
A relative risk map of predicted H5 HPAIV occurrences in wild birds in Europe has been generated based on the wild bird events reported between 2005 and 2017, which could contribute to identification of priority locations in the EU where targeted active wild bird surveillance could be implemented during wild bird migration periods.
Early detection of HPAI (TOR 2)
Recognition and reporting of suspicion, sampling, testing and reporting of results should be done in a timely manner.
For gallinaceous species, early detection of HPAI relies on notification of clinical suspicion.
For Anseriformes species, the sensitivity of ‘notifying clinical suspicions’ as a mechanism for early detection can be low for some HPAIV lineages.
Subclinically infected domestic Anseriformes have a higher likelihood of continued spreading HPAIV when compared with clinically infected gallinaceous poultry.
Biosecurity to prevent HPAI and LPAI entry and spread (TOR 2 and TOR 4)
Biosecurity measures were found to play a key role in preventing AI spread from wild birds to poultry. Human diligence is pivotal to select, implement and maintain effective biosecurity measures.
While certain general biosecurity principles universally apply to poultry holdings, unique features for each holding need to be considered for optimised protection.
According to expert opinion, the most feasible, sustainable and efficient measure to reduce the risk of AI entry in indoor poultry holdings is to prevent direct and indirect wild bird contact. Other measures with a high feasibility and sustainability are separation of waterfowl from other poultry species, provision of potable drinking water instead of surface water, the implementation of a hygiene lock for each poultry house, and biosecurity training of staff.
According to expert opinion, the highest ranked measures to prevent secondary spread of the virus are to contain poultry and fomites (i.e. materials that were in contact with poultry) during transport, cleaning and disinfection of equipment, biosecurity training, cleaning and disinfection of transport vehicles, and the use of a hygiene lock for each poultry house.
Outdoor poultry holdings bear an increased risk of AI incursions and the applicable biosecurity measures are more limited.
According to expert opinion, restricting access to persons and providing biosecurity training are the most feasible, sustainable and efficient measures to reduce the risk of AI entry and spread in commercial holdings where poultry has access to outdoor areas.
According to expert opinion, for backyard holdings, biosecurity training has been assigned the highest overall rank to prevent AI entry and spread.
The risk of AIV introduction and spread will remain high in production processes when movement of animals, restricting access throughout the whole production cycle and/or contact with wild birds is not reduced.
Establishment of a control & monitoring area and risk zones (TOR 2)
There is no scientific evidence to guide the sizes of a control and monitoring zone upon finding HPAIV in wild birds because it depends on the dynamics of the epizootic and the affected bird species.
Establishing small‐sized (e.g. few km) restriction zones may be instrumental to be able to implement increased surveillance activities in poultry in this zone which has been shown to be effective to identify further cases.
In the progression of a wild bird epizootic, establishing wider zones, rather than a succession of small, restricted zones may be more appropriate.
Informing poultry keepers on the detection of HPAIV in wild birds in the region will increase their awareness of the risk of virus introduction into their holding.
Protection of persons becomes crucial in case of outbreaks with HPAIV having increased zoonotic potential.
5. Recommendations
5.1.
5.1.1.
Mapping of HPAI and LPAI outbreaks during the last 5 years (TOR 5)
A close observation of the global AI‐related epidemiology is recommended to inform competent authorities and guide strategic preventive measures. This may include collaborative wild bird surveillance programmes with third countries in Eastern Europe and Central Asia.
Establishing a harmonised data collection system, integrating outbreak notification data, wild bird findings and epidemiological parameters, will aid in providing timely epidemiological analysis.
Limiting data confidentiality claims will facilitate data exchange between MSs.
HPAI introduction via migratory and residential wild birds (TOR 1)
To reduce the probability of AI introduction in poultry having outdoor access, it is recommended to provide for holding capacity to separate wild birds and poultry during high‐risk periods.
To reduce the probability of AI introduction in poultry, it is recommended to discourage (new) poultry rearing in areas with a high density of wild Anseriforme populations.
To reduce the spread of AI between holdings, it is recommended to limit transport and mixing of birds from different origins during the production cycle.
HPAI and LPAI introduction via non‐wild bird pathways (TOR 1, TOR 3 and TOR 5)
It is recommended to perform studies on virus perseverance in semen and faeces/manure (unprocessed, storage, composting, effect of cleaning and disinfection procedures).
It is recommended that MSs trading poultry semen report their national rules (based on OIE recommendations) and an estimate of the volumes traded so that a risk‐based approach can be developed to assess the risk of AI introduction and spread via semen.
HPAI and LPAI transmission and spread (TOR 6)
It is recommended to perform epidemiological studies to assess the effect of risk factors influencing between‐flock and between‐farm spread. Collection of standardised epidemiological data at the EU level from the holdings (e.g. location) and their houses (e.g. affected or not, number of susceptible birds, population structure) would be required on an ongoing basis.
Mutation from LPAI to HPAI (TOR 7)
Besides epidemiological data, it is also recommended to standardise and improve collection of virological information across the EU from LP and HP outbreaks such as HA/NA subtype and viral genetic sequences to increase our ability to assess the role of specific viral, environmental and host factors on the pathogenicity evolution.
It is recommended to perform research applying a holistic approach and investigating the broader influenza genetic spectrum, as well as the contribution of environmental and host‐related aspects in the emergence of HPAI viruses.
HPAI and LPAI surveillance (TOR 2, TOR 4 and TOR 8)
Member States should focus their annual serological surveillance programme in Anseriformes and game bird populations where those populations exist.
Serosurveillance should aim at detecting clusters of LPAIV‐infected farms in order to identify those LPAI events with continuous between farm spread.
Epidemiological follow‐up of serological positives should be carried out at positive holdings to determine if there is clustering of AI‐infected holdings in space and/or time regardless of whether the seropositive birds are still at the holding or whether active virus infection has been detected. If the group (i.e. epidemiological unit such as shed or flock) of poultry that tested positive on serology are not available for PCR testing, then any other poultry (in particular seronegative birds) still remaining on that holding should be tested.
It is recommended to quantify the weighting of the risk factors used to design risk‐based surveillance across EU to facilitate analysis of the results and to compare results of MSs.
Targeted active wild bird surveillance through virology tests (swabbing) combined with enhanced passive surveillance at a few priority regions in the EU may detect, if infection prevalence and sample sizes are sufficient, the presence of circulating AIV when these do not cause massive mortality among these birds.
Early detection of HPAI (TOR 2)
Virological testing of dead anseriforme poultry collected in holdings on a daily/weekly base (i.e. bucket sampling) is recommended during outbreak and other high‐risk periods. Pooling of such samples for PCR‐directed diagnosis may be useful.
Biosecurity to prevent HPAI and LPAI entry and spread (TOR 2 and TOR 4)
Restrict wild bird access to poultry and open water bodies need to be avoided on the premises.
Use hygiene locks and restrict access of people to poultry houses. Limit contacts with other poultry holdings (fomite, material, workers).
It is recommended that professional staff of poultry holdings attend general biosecurity trainings but also receive holding‐specific biosecurity advice ideally from an expert (e.g. veterinarian) familiar with the particular holding.
Hobby keepers should receive information to achieve a minimal understanding of biosecurity to prevent entry and/or spread of AIV in their backyards and during markets and/or shows.
It is recommended to confine and at least limit the outdoor access area of domestic birds in high‐risk periods (see above).
If poultry cannot be confined during high‐risk periods, it is recommended to prevent direct contact between wild birds and poultry by reducing the size of the outdoor area and/or by using netting. Feed and water should be provided under a roof or a horizontal fabric.
Biosecurity training and improved control of catching crews and other ‘mobile’ staff (e.g. insemination teams) may be useful to limit indirect spread of HP and LPAIV during large‐scale operations in commercialised poultry holdings.
Game bird hunting activities must be fully separated from rearing poultry.
Game birds should be tested for AI before release.
Online biosecurity questionnaires could be used by farmers to check their current biosecurity level and subsequently to improve it based on the received feedback.
Establishment of a control & monitoring area and risk zones (TOR 2)
Upon detection of HPAIV in wild birds, it is recommended that control and in particular monitoring areas are established based on the ecological habitat and flight distance of the affected bird species.
During an epizootic of HPAIV in wild birds, it is recommended to test samples from new species and non‐previously reported areas. Testing in reported areas can be restricted to check viral presence in relation to an exit strategy.
Sharing data and expertise at national and EU level on exit strategies would aid in terms of harmonised and structured approaches as well as interpretation of available data. Collaboration between authorities and stakeholders is crucial.
Glossary and Abbreviations
- Categories of wild birds
Water or non‐water birds (see tables in Appendix B)
- Entering wild birds
Wild birds entering the local area considered in the model
- Foraging area
An area with a surface of 4 ha used by wild birds for foraging (it includes any land cover except water/wetland, artificial, and woodland)
- Formal EKE
EKE following the steps: initiation, pre‐elicitation (definition of the elicitation protocol), elicitation and detailed reporting (see details in Appendix B.8 of the Uncertainty Guidance; EFSA, 2016a)
- Hatching eggs
Eggs for incubation laid by poultry
- Infected bird
Bird in which AIV is detectable
- Migratory wild bird
Wild bird living at least part of the year outside the EU
- Poultry
Fowl, turkeys, guinea fowl, ducks, geese, quails, pigeons, pheasants, partridges and ratites (ostriches, emus, etc.) reared or kept in captivity for breeding, re‐stocking or the production of meat or eggs for consumption
- Poultry holding
In the model, a poultry holding is considered to consist of the poultry houses and the surroundings within a radius of 1 km (=3.14 km2 surface)
- Resident wild bird
Wild bird living within the EU during the whole year
- Semi‐formal EKE
EKE following minimal requirements of: predefined question and expert board, fully documented (see details in Appendix B.8 of the Uncertainty Guidance; EFSA, 2016a)
- Virtual contact area
A virtual contact area is defined as an area of about 4 ha in size in which wild birds forage (see more details in Section C.5.2, Appendix C)
- Wild bird reservoir
Migratory and residential wild birds available in an area
- Worst‐case poultry holding
A poultry holding with outdoor housing (the place where poultry is kept is surrounded by fences but there is no horizontal protection to prevent access of wild birds), the presence of domestic waterfowl and the presence of a water body accessible to domestic waterfowl and wild birds on the poultry holding
- ABP
animal by‐product
- ADNS
Animal Disease Notification System
- AI
avian influenza
- AIV
avian influenza virus
- BIP
border inspection post
- CN
Combined Nomenclature
- CS
cleavage site
- dpi
days post‐infection
- ECDC
European Centre for Disease Prevention and Control
- EID50
median egg infectious dose
- EKE
Expert knowledge elicitation
- EMPRES
Emergency Prevention System for Animal Health
- FAO
Food and Agriculture Organization of the United Nations
- gs/GD
original goose/Guangdong
- HA
haemagglutinin
- HPAI
highly pathogenic avian influenza
- IVPI
intravenous pathogenicity index
- LPAI
low pathogenic avian influenza
- MC
Monte Carlo
- MNWB
migratory non‐water birds
- MS
Member State
- MWB
migratory water birds
- NPLE
non‐pasteurised liquid egg
- OIE
World Organization for Animal Health
- PAP
processed animal protein
- PCR
polymerase chain reaction
- PFU
plaque‐forming units
- PVS
private veterinary services
- RBS
risk‐based surveillance
- RNW
residential non‐water birds
- RWB
residential water birds
- SIR
susceptible‐infective‐recovered
- SPG
specific pathogen‐free
- TCID50
median tissue culture infectious dose
- TOR
Terms of Reference
- TSE
transmissible spongiform encephalopathy
- TTE
testing to exclude
- WG
Working Group
Appendix A – Reported HPAI and LPAI outbreaks in the last 5 years
1.
The tables in this appendix are based on the EMPRES‐I database (FAO/EMPRES‐i, 2014c).
A.1. HPAI outbreaks in domestic and wild bird species
Table A.1.
Reported HPAI outbreaks in domestic birds in the different continents from January 2010 to June 2017 (FAO/EMPRES‐i, 2014b)
| Region | Subtype | Year | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||
| Africa | H5N1 | 443 | 376 | 100 | 93 | 364 | 706 | 455 | 199 | 2,736 |
| H5N2 | 46 | 5 | 2 | 2 | 55 | |||||
| H5N8 | 1 | 80 | 81 | |||||||
| H7N1 | 1 | 1 | ||||||||
| HPAIe | 1 | 1 | ||||||||
| Total | 444 | 422 | 106 | 95 | 364 | 706 | 458 | 279 | 2,874 | |
| Americas | H5 | 1 | 1 | |||||||
| H5N1 | 1 | 1 | ||||||||
| H5N2 | 12 | 227 | 239 | |||||||
| H5N8 | 1 | 3 | 4 | |||||||
| H7N3 | 44 | 64 | 1 | 2 | 30 | 1 | 142 | |||
| H7N9 | 2 | 2 | ||||||||
| Total | 44 | 64 | 15 | 233 | 30 | 3 | 389 | |||
| Asia | H5 | 2 | 1 | 3 | 1 | 7 | ||||
| H5N1a | 1,328 | 1516 | 444 | 392 | 406 | 259 | 312 | 162 | 4,819 | |
| H5N2b | 6 | 1 | 10 | 564 | 47 | 122 | 750 | |||
| H5N3 | 1 | 26 | 27 | |||||||
| H5N6c | 1 | 36 | 129 | 131 | 385 | 682 | ||||
| H5N8d | 44 | 510 | 43 | 126 | 723 | |||||
| H7N9 | 25 | 25 | ||||||||
| HPAIe | 1 | 2 | 2 | 5 | ||||||
| Total | 1,330 | 1,518 | 450 | 394 | 500 | 1,489 | 535 | 822 | 7,038 | |
| Europe | H5 | 8 | 4 | 12 | ||||||
| H5N1 | 2 | 2 | 14 | 8 | 2 | 28 | ||||
| H5N2 | 30 | 6 | 3 | 39 | ||||||
| H5N5 | 6 | 6 | ||||||||
| H5N6 | 1 | 1 | ||||||||
| H5N8 | 10 | 3 | 373 | 746 | 1,132 | |||||
| H5N9 | 21 | 4 | 25 | |||||||
| H7N7 | 6 | 2 | 3 | 11 | ||||||
| Total | 2 | 6 | 12 | 70 | 402 | 762 | 1,254 | |||
| Oceania | H5 | 1 | 1 | |||||||
| H7N2 | 2 | 2 | ||||||||
| H7N7 | 1 | 1 | ||||||||
| Total | 1 | 1 | 2 | 4 | ||||||
| Total | 1,777 | 1,940 | 601 | 561 | 891 | 2,498 | 1,425 | 1,866 | 11,559 | |
In 2015, the serotypes H5N1 and H5N2 were reported for one outbreak and subtypes H5N1 and H5N6 were reported for another outbreak; in 2016, one outbreak involved both domestic and in wild birds species.
In 2015, the subtypes H5N2 and H5N1 were reported for one outbreak the serotypes H5N2 and H5N6 were reported for another outbreak.
In 2015, the subtypes H5N6 and H5N1 were reported in four outbreaks; the subtypes H5N6, H5N1 and H5N2 were reported in two outbreaks; the subtypes H5N6 and H5N2 were reported in five outbreaks; the subtypes H5N6 and H5N8 were reported in one outbreak.
In 2015, the subtypes H5N8, H5N1 and H5N6 were reported for one outbreak; in 2017, one outbreak involved both domestic and wild birds species.
Unspecified.
Table A.2.
Reported HPAI outbreaks in wild birds in the different continents from January 2010 to June 2017 (FAO/EMPRES‐i, 2014b)
| Region | Subtype | Year | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||
| Africa | H5N1 | 4 | 4 | |||||||
| H5N8 | 2 | 5 | 7 | |||||||
| H7N1 | 1 | 1 | ||||||||
| Total | 7 | 5 | 12 | |||||||
| Americas | H5 | 7 | 7 | |||||||
| H5N1 | 2 | 2 | ||||||||
| H5N2 | 1 | 35 | 1 | 1 | 38 | |||||
| H5N8 | 20 | 20 | ||||||||
| H7N3 | 2 | 1 | 3 | |||||||
| Total | 2 | 1 | 65 | 1 | 1 | 70 | ||||
| Asia | H5 | 1 | 1 | |||||||
| H5N1a | 13 | 67 | 26 | 5 | 2 | 7 | 2 | 2 | 124 | |
| H5N2 | 2 | 1 | 3 | |||||||
| H5N6 | 3 | 9 | 170 | 182 | ||||||
| H5N8b | 30 | 4 | 15 | 24 | 73 | |||||
| HPAI | 2 | 1 | 1 | 4 | ||||||
| Total | 13 | 69 | 26 | 5 | 33 | 17 | 27 | 197 | 387 | |
| Europe | H5 | 1 | 1 | 7 | 9 | |||||
| H5N1 | 4 | 2 | 10 | 16 | ||||||
| H5N5 | 4 | 7 | 11 | |||||||
| H5N8 | 3 | 2 | 289 | 670 | 964 | |||||
| H5N9 | 3 | 3 | ||||||||
| H7 | 1 | 1 | ||||||||
| Total | 4 | 2 | 1 | 4 | 12 | 294 | 687 | 1,004 | ||
| Total | 17 | 69 | 30 | 6 | 38 | 94 | 329 | 890 | 1,473 | |
In 2016, one outbreak involved both domestic and in wild birds species.
In 2017, one outbreak involved both domestic and wild birds species.
A.2. LPAI outbreaks in domestic and wild bird species
Table A.3.
Reported LPAI outbreaks in domestic birds in the different continents from January 2010 to June 2017 (FAO/EMPRES‐i, 2014a)
| Region | Subtype | Year | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |||
| Africa | H5 | 1 | 1 | |||||||
| H5N2 | 3 | 17 | 12 | 31 | 16 | 3 | 82 | |||
| H7 | 1 | 2 | 3 | |||||||
| H7N1 | 2 | 12 | 1 | 15 | ||||||
| H7N2 | 2 | 2 | ||||||||
| H7N7 | 10 | 3 | 13 | |||||||
| H9 | 7 | 7 | ||||||||
| H9N2 | 15 | 50 | 15 | 3 | 4 | 11 | 1 | 99 | ||
| Total | 22 | 55 | 54 | 20 | 36 | 29 | 6 | 222 | ||
| Americas | H5 | 3 | 3 | |||||||
| H5N1 | 1 | 1 | ||||||||
| H5N2 | 1 | 4 | 1 | 1 | 7 | |||||
| H5N8 | 1 | 1 | ||||||||
| H7 | 1 | 1 | ||||||||
| H7N3 | 1 | 1 | 1 | 3 | ||||||
| H7N6 | 2 | 2 | ||||||||
| H7N7 | 1 | 1 | ||||||||
| H7N8 | 9 | 9 | ||||||||
| H7N9 | 9 | 9 | ||||||||
| LPAI | 1 | 2 | 3 | |||||||
| Total | 1 | 2 | 1 | 2 | 7 | 14 | 13 | 40 | ||
| Asia | H5 | 1 | 1 | 2 | ||||||
| H5N2 | 4 | 2 | 9 | 8 | 1 | 22 | 5 | 9 | 60 | |
| H5N3 | 1 | 1 | ||||||||
| H5N6 | 1 | 1 | 2 | |||||||
| H7 | 1 | 1 | 2 | |||||||
| H7N2 | 1 | 1 | 1 | 3 | ||||||
| H7N3 | 2 | 1 | 3 | |||||||
| H7N6 | 1 | 1 | ||||||||
| H7N7 | 6 | 6 | ||||||||
| H7N9 | 161 | 356 | 81 | 19 | 125 | 742 | ||||
| H9N2 | 255 | 636 | 124 | 151 | 19 | 1 | 1 | 1,187 | ||
| LPAI | 1 | 1 | ||||||||
| Total | 268 | 641 | 133 | 322 | 380 | 104 | 26 | 136 | 2,010 | |
| Europe | H5 | 7 | 1 | 1 | 1 | 10 | ||||
| H5N1 | 6 | 3 | 17 | 8 | 34 | |||||
| H5N2 | 1 | 4 | 4 | 9 | 11 | 4 | 33 | |||
| H5N3 | 2 | 3 | 7 | 10 | 22 | |||||
| H5N8 | 2 | 2 | ||||||||
| H5N9 | 5 | 1 | 6 | |||||||
| H7 | 2 | 2 | 1 | 1 | 1 | 7 | ||||
| H7N1 | 1 | 1 | 2 | 1 | 1 | 6 | ||||
| H7N3 | 1 | 1 | ||||||||
| H7N7 | 21 | 1 | 3 | 4 | 29 | |||||
| H7N9 | 1 | 1 | ||||||||
| LPAI | 5 | 1 | 6 | |||||||
| Total | 4 | 27 | 12 | 18 | 10 | 19 | 43 | 24 | 157 | |
| Oceania | H5 | 1 | 1 | |||||||
| H5N3 | 1 | 1 | ||||||||
| Total | 1 | 1 | 2 | |||||||
| Total | 273 | 692 | 201 | 396 | 412 | 166 | 112 | 179 | 2,431 | |
Appendix B – Composition of water and non‐water birds
1.
Wild bird species present in the EU have been grouped in two main groups of animals: water birds (ducks, geese, swans, grebes, etc.) and non‐water birds (raptors, song birds, waders, gulls, terns, storks, cranes, etc.) according to their general behaviour, habitat preference and known susceptibility to AIV.
B.1. Water birds
Table B.1.
List of species according to the subgroup, the order and family they belong to in the water birds group
| Sub‐category | Order | Family |
|---|---|---|
| Dabbling ducks | Anseriformes | Anatidae |
| Diving ducks | ||
| Shelducks | ||
| Seaducks | ||
| Geese | ||
| Swans | ||
| Mergansers | ||
| Grebes | Podicipediformes | Podicipedidae |
| Coot and other Rallidae | Gruiformes | Rallidae |
| Cormorants | Suliformes | Phalacrocoracidae |
| Pelicans, Herons, Spoonbills, Ibises | Pelecaniformes | Pelecanidae, Ardeidae, Threskiornithidae |
B.2. Non‐water birds
Although waders are water birds, they are in this opinion included in the non‐water birds group because their behaviour is different from that of Anatidae, in particular, towards the use of poultry farm premises (Caron et al., 2017).
B.2..1.
B.2..1.1.
Population of non‐water birds
A representative population of 10,000 non‐water birds within Europe would be composed of various groups of species. In an earlier stage of this work, it was estimated by working group experts with ornithological background that a typical breakdown of species would be as follows (Table B.3):
Table B.3.
Composition of a population of 10,000 non‐water birds
| Group of non‐water birds species | Number | Percentage% |
|---|---|---|
| Songbirds | 9,350 | 93.50 |
| Gulls‐Storks‐Cranes | 500 | 5.00 |
| Waders | 100 | 1.00 |
| Raptors | 50 | 0.50 |
| Total | 10,000 | 100.00 |
Appendix C – Model description
1.
For the sake of clarity, the description of the theoretical model (Sections 1, C.1., C.3.–C.4) is provided separately from model parameterisation (Section C.5). The model setting is based on evidence and expert knowledge and has a level of generality. Therefore, in principle, it might be applied any time assumptions are realistically met, whilst the way parameters are estimated is specific for the current assessment.
C.1. Model setting
The structure of the model reflects the circumstantial scientific evidence available and knowledge of the involved experts. Some assumptions though have been introduced in order to keep an acceptable balance between realism and level of complexity.
Most of the data available to estimate parameters of the model suffered from limitations in terms of representativeness of the target population and different types of bias. These limitations represent sources of uncertainty that have been expressed for each input parameter in the model in the form of uncertainty probability distributions. Available data have been used to support an expert judgement process (EFSA, 2016a). In addition to the possible flaws affecting inputs to the model, other potential uncertainties related to model structural aspects (e.g. assumptions on the mechanism underpinning the dynamic of AI infection in wild bird population) have been identified. All these sources of uncertainty, that are not inherent to the evidence, could not be addressed directly as input to the model. Their potential impact has been taken into consideration when analysing model outputs and assessed quantitatively whenever possible.
The main objective of the model is to describe the entrance of AI viruses into Europe through migratory wild birds, the possible dynamic of AI in both migratory and resident wild bird populations, and to assess the risk of transmission from wild birds to poultry during one migration season. In particular, HPAIV clade 2.3.4.4 entry via the NE entry route during the 2016–2017 migration60 was modelled (see Sections C.5 and C.6) as well as entry of LPAIV (see Sections C.5 and C.7).
The contributions of the various wild bird species to the probability of introduction and infection were considered to differ substantially, so ideally separate assessments were sought for. In general, there was a lack of comprehensive data on the course of AI infections at individual wild bird species level. Therefore, only two broad categories of wild birds, water birds and non‐water birds (see Tables B.1 and B.2 in Appendix B), were formed and considered as reflecting major differences in behaviour, habitat preference, susceptibility to infection and contact frequency with poultry.
During the migration season, migratory water birds and non‐water birds enter the EU via one of the entry routes (see Section 3.2.1) and mix with resident wild birds. Several studies on the movements, spatio‐temporal size and distribution of wild bird populations have been published mostly based on ringing data (e.g. Scott and Rose 1996; Wetlands International, 2017a), but these studies are limited to a few bird species or restricted to a few geographic areas. A European comprehensive overview is lacking. Therefore, a model of a generalised local wild bird population is considered representing those birds associated with the same feeding area or the preferred habitat (here called the ‘virtual contact area’).
The model allows to estimate the probability of AIV introduction via migratory birds and then to describe the AI dynamics in a local wild bird population. To this scope, for HPAI, different population scenarios are used (see Table 1, Section 3.2.1) based on alternative size (total number of birds) and composition (share of water versus non‐water and migratory versus non‐migratory birds) of the bird population associated with a virtual contact area (see definition in Section C.5.2). Bird population size is regulated through a carrying capacity, the maximum size of wild bird population that can be present in the virtual contact area. For each scenario size (i.e. carrying capacity value), population composition in the model was altered by assuming comparable shares of residential versus migratory birds and water versus non‐water birds. Only for LPAI, three different initial prevalence levels were considered (0.2%, 2% and 6%) one of which combined with two proportions of naïve population (0.5 and 1) (see Table 3, Section 3.3.2).
Based on the observation that detection of AI cases in wild non‐water birds is rare (e.g. EU annual report on AI surveillance (APHA, 2015)), it was decided to consider only water birds as a potential source of AI introduction in the EU via wild birds. The model describes the mechanism of AI transmission among wild birds via contact with any contaminated matrix (e.g. faeces on feeding grounds, cloacal and oropharyngeal excretions, contaminated water, scavenging on carcasses of birds that succumbed to AI, etc.). These infection sources (hereafter named infectious excretions) are assumed to be the route of transmission, embedding also the effect of direct bird‐to‐bird contacts. The model only considers defecation of wild birds when they reside on land or in water, since the proportion of defecation when flying is considered to be small. The possible impact of defecation during flight is included in the uncertainty analysis.
The susceptible‐infective‐recovered (SIR) theory is used to describe the transmission of the infection among the two wild bird groups, water and non‐water birds, interpreting the transition between S and I as the transmission rate β, whose expected value is expressed here as the probability that a bird gets infected in a day. The latter is estimated combining probability of a susceptible bird of getting in contact with infectious excretions with probability of acquiring the infection given the contact occurs. Considering the assumption that infectious excretions are the only route of infection, a new compartment (named G in the model) was added to the traditional SIR model in order to capture the persistence of the excretion infectivity beyond the infectivity of the bird. This enables the possibilities that virus can be transmitted to susceptible wild birds without the presence of infectious birds in the defined area. The transition between I and G is regulated by the shedding period, whilst that from G to S is ruled by the duration of the persistence of the excretions in the environment (see Section D.1.9).
The model describes the dynamics of the epidemic in the wild bird reservoir and allows calculating the prevalence of infected wild birds for each day during the migratory season for both the water and the non‐water wild bird populations. The infection might subsequently be transmitted to poultry via any possible route, for instance direct and indirect wild bird–poultry interaction, movement of people, vehicles, machinery, etc., acting as fomites. Transmission by any of these routes implies the presence of excretions of at least one infectious wild bird in the poultry holding. The number of wild birds, both water and non‐water birds, that can enter into the holding area daily (and subsequently produce excretions) was estimated by experts. The number of infected wild birds was then derived from the estimated number of wild birds circulating in the area and the prevalence calculated with the model.
Based on evidence, it was considered that the level of biosecurity in a poultry holding would affect the risk of an AI incursion given the number of infected birds entering the poultry holding (e.g. Parker et al., 2012). Therefore, that probability was estimated separately for a holding without biosecurity measures and for four scenarios reflecting stepwise increased implementation of biosecurity measures (see Section 3.9) aiming at preventing the introduction of the virus into the holding's poultry population.
As presented in Figure C.1, the model provides an estimate for the:
Entry assessment: probability of HPAI introduction across the EU boundaries via wild birds;
AI dynamics in the EU wild bird reservoir: HPAI or LPAI prevalence in wild bird populations during the migratory season;
Exposure assessment and poultry holding incursion: probability of HPAI or LPAI infection of poultry holdings due to the exposure to infected wild birds.
Figure C.1.
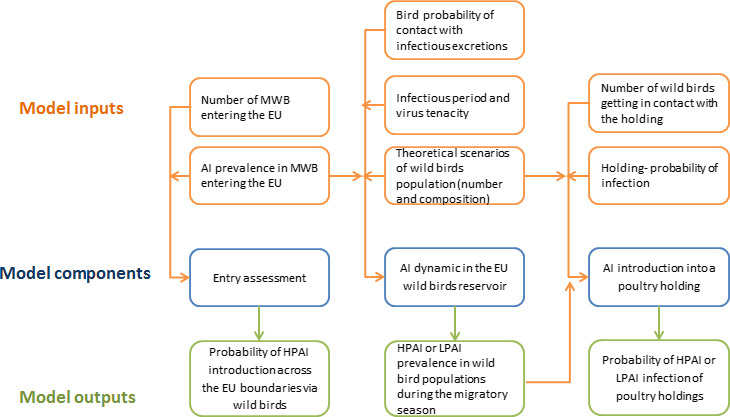
Schematic overview of the model inputs, components and outputs
For each of these outcomes, details are provided in the Sections C.1., C.3.–C.4.
Two main sources of uncertainty have been addressed in the model: those inherent to the evidence used as an input and the ones due to the setting of the model and the assumptions done. It must be acknowledged indeed that choices made for the structure of the model and related assumptions reflect different needs including balance between complexity and realism. Hence, they can introduce uncertainty in the results. Uncertainty related to the evidence has been addressed expressing model parameters as uncertainty probability distribution and combining these using simulations with MC algorithm. The approach taken to analyse the second type of uncertainties varies depending on the level of realism of the assumption, the expected impact on the outcome and the feasibility of quantifying it, as indicated in the Tables C.1, C.2–C.3 reported under the uncertainty analysis of each model outcome.
Table C.1.
Expected impact of assumptions on the ‘entry assessment’ compartment of the model on the model outcome
| Assumption | Expected impact | Explanation |
|---|---|---|
| Only migratory water birds are potential source of AI introduction in the EU via wild birds | Assessed quantitatively using EKE on model outcome | The uncertainty distribution is very much concentrated (uncertainty is limited). Considering also the migratory non‐water birds would squeeze, the distribution even more towards 1 further decreasing the uncertainty |
Table C.2.
Expected impact of assumptions on the ‘AI dynamics’ compartment of the model on the model outcome
| Assumption | Expected impact | Explanation |
|---|---|---|
| The entrance of migratory susceptible wild birds into the EU during migration season is modelled with a constant rate | Negligible impact |
1) The composition of the migratory wild birds does not affect the output 2) Different species arriving at different time covering all the migration period |
| Once birds land in the virtual area they stay there for the whole season (125 days duration) | Negligible impact | Birds will stay long enough in the area to allow transfer of the virus. Prolonging their stay has limited impact |
| Entry of infected migratory WB in the EU is clustered on one day | Assessed quantitatively | Worst‐case scenario |
| The distribution of birds by day of infectivity is uniform at the time they cross the EU border | Most realistic (negligible impact) | No evidence for a link between infection and host fitness |
| Once a wild bird got infected, it becomes able to transmit infection to a susceptible bird via its excretions from the next day | Most realistic (negligible impact) | Based on experimental data (Keawcharoen et al., 2008) |
| Random pattern assumed for the release of the infected excretions and for the contact birds‐excretions | Most realistic (negligible impact) | From data defecation rate, every 5 min, and they move randomly (e.g. about 7 min in Durant et al. 2006) |
| Contact with excretions is the only route of infection. | Most realistic (negligible impact) | Direct and indirect transmission of virus occurs via excretions |
| Groups of wild bird species are assumed to forage in a similar surface of the virtual contact area during a given day | Most realistic (negligible impact) | The probability of a wild bird to get in contact with contaminated excretions was estimated for groups of wild bird species with different behaviours |
| Probability of getting in contact with infected excretions excreted by birds from the same category is higher | Most realistic (negligible impact) | Birds of the same category share the same ecological niches which has only partial overlap with the other category |
| Quantity of excretion is not considered to differ between the different categories of birds | Negligible impact | Sharing the same ecological niches for birds of the same category has a much higher influence on the probability of contact compared to the quantity excreted |
| Infectivity of the excretions in the environment is assumed to be maintained for a number of days after release. During the persistence period level of infectivity of the excretions is assumed to be constant. | Worst‐case scenario | Evidence indicate a decrease of infectivity (Nazir et al., 2010) |
| Number of infected excretions is assumed to be directly proportional to the number of birds shedding the virus (infected) plus number of birds that recovered from infection but whose excretions are still infectious | Negligible impact | Sharing the same ecological niches for birds of the same category has a much higher influence on the probability of contact compared to the quantity excreted |
| AI infection confers immunity to birds once birds recover from the disease | Negligible impact | Quantity of virus excreted by a bird re‐infected with the same clade in a migration season is considered negligible to influence the outcome of the model; low prevalence low probability of reinfection |
| All migratory non‐water birds are susceptible when they enter the EU | Most realistic (negligible impact) | Categories were set up also on the probability they bring in the infection; evidence: very few non water birds found infected with HPAI 2.3.4.4 clade |
| All residential wild birds (both water birds and non‐water birds) are susceptible to the infection at the start of the migration season; the influence of heterologous immunity and previous exposure is not considered | Worst‐case scenario | Immunity would reduce viral amplification |
| Population dynamics of wild birds do not include baseline wild bird mortality | Worst‐case scenario | Including baseline mortality will decrease the size of the susceptible population. Analysis of the impact on the outcome of the model was negligible |
| Population dynamics of wild birds do not consider hatch | Most realistic (negligible impact) | Hatching does not occur during the migration or winter seasons |
Table C.3.
Expected impact of assumptions on the ‘poultry introduction’ compartment of the model on the model outcome
| Assumption | Expected impact | Explanation |
|---|---|---|
| Probability of infection of a poultry holding is assumed to be a function of the holding's biosecurity level | Most realistic (negligible impact) | Extrapolation from data for LPAI |
| Probability of infection of a poultry holding is assumed to be a function of the number of infected wild birds landing daily into the holding area | Most realistic (negligible impact) | Regional origin of the virus (e.g. no HPAI clade 2.3.4.4. of America origin was found in EU holdings) |
| No overlap among virtual contact areas and holding premises was considered | Assessed quantitatively | The number of wild water birds on the holding will increase, which could shift the distribution on the probability to introduce the virus to the right |
| Contact with excretions is assumed to be the only route of infection | Most realistic (negligible impact) | The definition of excretions is very broad, covering both direct and indirect virus transfer |
| Defecation by wild birds occurs on land or in water but not during flight | Most realistic (negligible impact) | Many wild birds species spent much more time on land/water during a day compared to their flight time. Defecation mainly occurs directly after feeding |
C.2. Entry assessment
C.2.1. Assumptions
For the sake of simplicity and considering, the limitations in the available data, the following assumptions have been made in the ‘entry assessment’ component of the model:
-
1
The entrance of migratory wild birds into the EU during a migratory season was modelled with a constant rate.
-
2
Only migratory water birds are a potential source of AIV introduction in the EU.
C.2.2. Description
It is assumed that a proportion of migratory water birds crossing the EU border daily, is infected with a prevalence of infection πMWB described by an uncertainty distribution. The latter distribution is assumed not to change over the migratory season.
The probability of HPAI introduction in the EU (ProbE) is expressed as the probability that at least one infected water bird enters the EU during the migratory season:
LPAI is considered endemic within the EU, hence this compartment of the model is not applicable.
C.2.3. Uncertainty analysis
As described previously, two types of uncertainties were addressed:
the one expressing limitations in the knowledge of the model inputs;
the one expressing the impact of assumptions of the assessment and the model on the outcome.
Uncertainty related to the evidence used to estimate the two parameters in the model – total number of migratory water birds and AI prevalence – has been expressed using probability distributions estimated via a process of Expert Knowledge Elicitation (EKE) (Table C.1). Then uncertainty distributions have been combined using Monte Carlo (MC) simulations.
The approach taken to analyse the second type of uncertainties varies depending on the level of realism of the assumption, the expected impact on the outcome and the feasibility of quantifying it as indicated in the table below.
The impact of the only uncertainty related to assumptions that was considered needed to assess quantitatively was estimated via a semi‐formal EKE and reported in Appendix E (model outputs).
C.3. AI dynamics in the wild bird reservoir
C.3.1. Assumptions
For the sake of simplicity and considering the limitations in the available data, the following assumptions have been made in the ‘AI dynamics’ compartment of the model:
-
3
For HPAI, the dynamic of the transmission of the virus among wild birds during the autumn migratory season is investigated.
-
4
For LPAI, the dynamic of the transmission of the virus among wild birds is investigated during period having the length of the autumn migratory season.
-
5
For HPAI, only migratory water birds are a potential source of AIV introduction in the EU.
-
6
For LPAI, only water birds are a potential source of AIV introduction into a free local area.
-
7
For HPAI, the entrance of susceptible migratory wild birds into the EU during the migration season was modelled with a constant rate.
-
8
For LPAI, the entrance of susceptible wild birds in the local area during the investigated period was modelled with a constant rate.
-
9
For HPAI, the entrance of migratory infected birds occurs in a single occasion during the migratory season.
-
10
For LPAI, the entrance of infected birds can occur in several occasions during the considered period.
-
11
The prevalence of HPAIV‐infected migratory wild birds at the time they cross the EU border is assumed to be constant during the migration season. The experts could not identify reliable information in order to model the variability of the prevalence across the migration season.
-
12
The prevalence of LPAIV‐infected water birds entering the local area is assumed to be constant during the considered period.
-
13
The distribution of birds by day of infectivity is uniform at the time they cross the EU border (for HPAI) or they enter the local area (for LPAI). The experts could not identify reliable information in order to model the distribution differently.
-
14
Once a wild bird got infected, it becomes able to transmit infection to a susceptible bird via its excretions from the following day onwards.
-
15
The quantity of virus excreted does not differ among ‘categories of wild birds’ (water birds (WB) versus non‐water birds (NWB)).
-
16
Contact with excretions is assumed to be the only route of infection, although it can happen in different ways. Bird‐to‐bird direct contact is considered to be implicitly covered by contact with excretions and is therefore not separately treated.
-
17
The probability of a wild bird to get infected is assumed not to depend on the time spent during a given day in a virtual contact area.
-
18
The probability of getting in contact with infectious excretions is assumed to be higher within the same category of birds (WB with WB and NWB with NWB) and lower between categories (WB with NWB and NWB with WB). The rationale for this is that birds of the same category share habitats and feeding area longer or to a greater extent than birds from different categories.
-
19
Infectivity of the excretions in the environment is assumed to be maintained for a number of days after release, also beyond recovery of the infected bird. During the persistence period, the level of infectivity of the excretions is assumed to be constant, which is considered to reflect a worst‐case scenario given the availability of evidence that infectivity normally decreases (although the kinetics vary between different matrices and environmental conditions).
-
20
The number of infectious excretions is assumed to be directly proportional to the number of birds shedding the virus (infected) plus the number of infected birds that are no longer infectious but whose excretions are still infectious;
-
21
AI infection confers immunity to birds once birds recover from the disease.
-
22
All NWBs are susceptible when they enter the EU (HPAI) or a local free area (LPAI). The influence of heterologous immunity and previous exposure is not considered unless explicitly stated.
-
23
All residential (HPAI) or locally present (LPAI) wild birds (both WBs and NWBs) are susceptible to the infection prior to arrival of infected water birds; the influence of heterologous immunity and previous exposure is not considered unless explicitly stated.
-
24
All wild birds visit virtual contact areas daily during the migration season.
-
25
A random pattern is assumed for the release of the infected excretions in the virtual contact area and for the contact of birds with them; the quantity of excretions has not been considered for the different categories of birds.
-
26
For both HPAI and LPAI, baseline wild bird mortality (including natural mortality plus hunting) occurring during the migration season is not considered in the model since its impact has been assessed considering a worst‐case scenario analysis and came out to be negligible on the amplification dynamic. Hence, the size of the wild bird population in each scenario fluctuates within the carrying capacity only on the basis of the daily arrival of wild birds and, only for HPAI, for the mortality due to AI infection. Hatch rate is ignored given the period of the year considered for the reference scenario (fall‐winter season).
C.3.2. Description
Contact with infected excretions is considered the only route of AI transmission. The AI dynamic in wild bird population is described within virtual contact areas visited daily by wild birds. These are places where the contact with infected excretions can take place. Different wild bird population scenarios in a virtual contact area have been simulated based on different carrying capacities (i.e. maximum number of birds that would aggregate in the area) and various compositions for the proportion of migratory birds (pM) and water birds (pWB) (see Table 1, Section 3.2.2). For the sake of simplicity, the index related to the type of scenario is omitted throughout the formulas reported below.
In the virtual contact area and for each simulated population, daily arrival of susceptible migratory wild birds over the migration season is described using a constant arrival rate. HPAI is considered to be an epizootic event in the virtual contact area and therefore the entrance of migratory infected birds is described to occur in a single occasion during the migratory season. The same epizootic model was used to simulate LPAIV entry in free local areas.
The number of infected migratory water birds carrying HPAIV and entering in Europa in one single occasion is given by:
where:
MWB is the number of migratory water birds arriving in a virtual contact area during the whole migratory season.
IMWB is the number of infected migratory water birds arriving in a virtual contact area during the whole migratory season.
Consequently, the number of susceptible migratory water birds (SMWBt) and non‐water birds (SMWBt) entering a virtual contact area at time t (in days) during the migration season via one of the entry routes is assumed to be constant in t and, respectively:
where:
N = maxbirds capacity of the theoretical area
pM = percentage of migratory birds
pWB = percentage of water birds
MNWB = number of migratory non water birds arriving in a virtual contact area during the whole migratory season.
The size of these population subgroups depends on the maximum population capacity N and the percentages pM and pWB considered in each scenario. In order to explore the potential influence of population size in the virtual contact area, two different times of entrance of infected migratory water birds were considered: at the beginning (day 1) and in the middle (day 60) of the migration season.
For the LPAI model, it is considered that introduction of LPAIV into a free local area within the EU can occur via infected water birds entering the area. These birds are further referred to as ‘entering birds’ since they can be migratory birds arriving from countries outside the EU or birds previously resident in neighbouring areas and locally moving to the virtual area under consideration.
Different wild bird population scenarios in a virtual local area have been simulated based on different carrying capacities (i.e. maximum number of birds that can land in the area), three initial AI prevalence levels (0.2%, 2% and 6%) and different proportions of naïve birds (reflected in two initial proportions of susceptible birds (0.5 and 1)) (see Table 5, Section 3.2.2). The wild bird population composition was kept fixed as for the proportion of entering/present wild birds (0.9/0.1) and water/non‐water birds (0.9/0.1) since this was considered the worst‐case scenario.
In the virtual local area and for each simulated population, daily arrival of entering wild birds over the considered period is described using a constant arrival rate and the entrance of infected birds is described to occur in several occasions during the considered period.
The total number of infected entering water birds carrying LPAIV and entering the virtual local area during the considered period is given by:
For each day of the considered period, the number of entering LPAI‐infected birds is simulated with a multinomial distribution, allocating IEWB infected water birds in 125 days. Consequently, the number of susceptible water birds (EWBt) and non‐water birds (ENWBt) entering a virtual local area at time t (in days) during the considered period is assumed to be constant in t and, respectively:
where:
proportion of naïve birds
N = maxbirds capacity of the theoretical area
PLength = length in days of the considered period
pE = percentage of birds entering local area from EU‐infected areas or from outside the EU.
Finally, the number of immune water birds (EWBt) and non‐water birds (ENWBt) entering a virtual local area at time t (in days) during the considered period is assumed to be constant in t and, respectively:
Given the assumptions above, it was considered that the model best describing AIV dynamic in the wild bird reservoir in the virtual contact area is a SIR model. However a new compartment (named G) was added to the traditional SIR model in order to capture the persistence of the excretion infectivity beyond the infectivity of the bird. The transition between S (Susceptible) and I (Infected) is assumed to occur at transition rate β, whose expected value is expressed as the product of the probability of contact of wild birds with infected excretions and probability of a wild bird to get infected given a contact with infected excretions.
The probability for a WB or a NWB of getting in contact with infected excretions shed by WB and NWB in a virtual contact area is expressed as a non‐linear function of the total infected excretions released in a virtual area and still infective at time t. It is assumed that infectivity of excretions can persist up to an approximated maximum of 13 days (see Section D.1.9). Therefore, at time t, birds can be infected not only by virus shed in the same day but also by virus persisting in the environment in the following 13 days. The release of excretions is assumed to occur randomly in space and with a frequency and amount that is the same for the two categories of birds. Therefore, each day, the number of infected birds is assumed to be a proxy of the virus newly excreted (I) while the number of birds recovered from infection, but whose excretions are still infective, is a proxy of the excretions in the persistence period beyond bird infectivity (G). The level of infectivity of the excretions is assumed to be constant over the period of persistence. Although not corresponding to reality, this last assumption is more likely to underestimate the probability of infection because infectivity is likely to decrease over time
where WB, WB; NWB, WB; NWB, NWB; and WB, NWB indicate the possible bird‐to‐excretion contact types via excretions (first acronym indicates the source of infection, the second one the infection recipient) and:
IWB = number of infected WB
INWB = number of infected NWB
GWB = number of WB already recovered whose excretions are still infectious
GNWB = number of NWB already recovered whose excretions are still infectious.
The probability of infection, respectively, for a WB and a NWB is expressed as the product of the conditional probability that a susceptible WB or NWB becomes infected given a contact with infectious excretions and the probability of a contact as defined above:
where
coefficient for the probability that a susceptible wild bird becomes infected given a contact with excretions containing LPAI infectious virus with respect to HPAI‐infected excretion.
The reduction coefficient is equal to 1 for HPAI model, while for LPAI model, it describes the factor of decrease of the probability that a susceptible wild bird becomes infected given a contact with excretions containing LPAI infectious virus with respect to the same probability calculated for a susceptible wild bird coming in contact with HPAI‐infected excretion.
The transition between I (Infected) and G (recovered with infected excretions) is regulated by the rate of recovery (inverse of the shedding period). Move from compartment G to R (Recovered) is ruled by the rate of persistence (inverse of persistence duration).
From day t = 1 onwards, the daily change in the size of the four populations of Susceptible (S), Infected (I), Recovered with infected excretions (G) and Recovered with non‐infected excretions (R) is described by the following differential equations:
a) for WBs:
For HPAI, the proportion of naïve birds is pnv = 100%. For LPAI, the same formulas apply replacing SMWB(t) with SEWB(t) and RMWB(t) with REWB(t).
b) for NWBs:
For HPAI, the proportion of naïve birds is pnv = 100%. For LPAI, the same formulas apply replacing SMNWB(t) with SENWB(t) and RMNWB(t) with RENWB(t). For each day of the migration season, the SIR model allows calculating the prevalence of infected wild birds (both for WBs and NWBs). The starting values are:
a) for WBs:
where RWB = N × (1 − pM) × pWB is the number of residential water birds present in a virtual contact area over the migratory season (for HPAI) or the considered period (for LPAI).
b) for NWBs:
where RNWB = N × (1 − pM) × (1 − pWB) is the number of residential non water birds present in a virtual contact area over the migratory season (for HPAI) or the considered period (for LPAI).
The prevalence of infected WBs at day t = k is given by:
where:
DWBd are WB dead due to AI disease given by:
The prevalence of infected NWBs at day d = k is given by:
where
DNWBd is the number of NWB dead by AI disease given by:
C.3.3. Uncertainty analysis
Two types of uncertainty have been addressed in the model:
Uncertainty on the evidence used to estimate the parameters in the model.
Uncertainty related to the assumptions made in the model and in the overall assessment.
Uncertainty related to model parameters has been expressed using probability distributions estimated via EKE (Table C.2). Then, these uncertainty distributions have been combined in the model using MC simulations.
The approach taken to analyse the uncertainties related to the assumptions made in the ‘AI dynamics’ compartment of the model varies depending on the level of realism of the assumption, the expected impact on the outcome and the feasibility of quantifying it as indicated in the table below.
It was not needed to estimate the combined impact of the uncertainties related to the assumptions since they were considered either reflecting the worst‐case scenario or expected to have a negligible impact on the model/assessment outcome.
C.4. AI introduction into a poultry holding
C.4.1. Assumptions
For the sake of simplicity and considering the limitations in the available data, the following assumptions have been made in the ‘poultry introduction’ compartment of the model:
-
27
The presence of excretions in the holding premises is assumed to initiate infection (covering both ‘direct’ and ‘indirect’ virus transfer).
-
28
The probability of infection of a poultry holding is assumed to be a function of the holding's biosecurity level and of the number of infected wild birds landing daily into the holding area. The transfer of infectious excretions from wild birds to poultry can happen via any mechanism.
-
29
No overlap was assumed among virtual contact areas and holding premises.
-
30
Defecation by wild birds occurs on land or in water but not during flight.
C.4.2. Description
The model describes the probability that a poultry holding with a specified level of biosecurity becomes AI infected via wild birds. Since the WBs and NWBs are assumed to have a different propensity of landing into a poultry holding (1 km radius area), the probability is computed separately for the two groups of birds.
The probability of infection of a poultry holding located in the proximity of a virtual contact area is assumed to be a function of the holding's biosecurity level and of the number of infected wild birds landing daily into the holding area and releasing infectious excretions. Four different biosecurity levels have been considered (see Figure 7, Section 3.2.2). Based on expert judgement (see Section D.4), the relationship between the probability of infection of a poultry holding and the number of infected wild birds is defined as a non‐linear function, with different regression coefficients at different levels of biosecurity.
Therefore, the probability that any biosecurity bn‐type poultry holding becomes infected via wild birds on day t is given by:
where:
coefficient for the probability for a worst‐case holding to get the infection given the presence of LPAI‐infected wild bird with respect to HPAI‐infected wild bird.
The reduction coefficient is equal to 1 for HPAI model, while for LPAI model it describes the factor of decrease of the probability that a worst‐case holding becomes infected given the presence of LPAI‐infected wild bird with respect to the same probability calculated for a worst‐case holding coming in contact with HPAI‐infected wild bird.
The probability that any biosecurity bn ‐type poultry holding becomes infected via wild birds during the whole migration season (named as seasonal probability in the main text) is given by:
C.4.3. Uncertainty analysis
Uncertainties related to the evidence used to estimate the parameters in the model, has been expressed using probability distributions estimated via EKE (Table C.3). Then, uncertainty distributions have been combined using MC simulations.
The approach taken to analyse the second type of uncertainties varies depending on the level of realism of the assumption, the expected impact on the outcome and the feasibility of quantifying it as indicated in Table C.3 below.
The impact of the only uncertainty related to assumptions that was considered needed to assess quantitatively was estimated via a semi‐formal EKE and reported in Appendix E (model outputs).
C.5. Model parameterisation
This section reports the methods used to estimate model parameters. It reflects the structure of the model that is arranged in three compartments: entry assessment, AI amplification in the wild bird reservoir, AI introduction into a poultry holding.
Existing data were available for few parameters of them, which were later kept constant in the model. Most of the parameters entering the model were estimated by experts either via semi‐formal or formal EKE. As output of the EKE process, an estimate of quartiles plus lower and upper bounds of the uncertainty distribution for the elicited parameter was provided by the experts, first individually and then collectively after a discussion finalised by reaching a consensus.
Those values were subsequently used to identify – among a set of reasonable parametric distributions – the one best fitting the elicited quantiles (based on the minimisation of the squared distance from theoretical quantiles) and to estimate its parameters. All the analyses were performed using statistical software R, package rriskDistributions and a modified version of package SHELF 2.01 (O'Hagan, 2016).
The list of parameters used in the simulation model, together with their estimated uncertainty distributions is provided in Sections C.6, C.7, D.2 and D.3.
C.5.1. Entry assessment
The prevalence of HPAI (clade 2.3.4.4, 2.3.2.1c or 2.2.1.x) infection in WB at the entry point into the EU and the number of MWB entering the EU in a migration season, were estimated by an EKE process and described by an uncertainty probability distribution.
C.5.2. AI dynamics in the wild bird reservoir
Virtual contact area
For the scope of this assessment, a virtual contact area is defined as an area of about 4 ha in size in which wild birds forage. It is assumed to include either the first of the following items or a combination of the first item with one of the following two:
a foraging area with a surface of 4 ha where wild birds are supposed to gather in order to feed on grass/crops/shrubland (foraging area). These areas are assumed to have any cover but water/wetland, artificial cover, bareland or woodland;
water/wetland area;
any NWB typical habitat other than foraging areas (i.e. woodland, shrubland).
Virus transfer between wild birds visiting the virtual contact area is possible even if they are not present at the same moment, for instance via exposure to contaminated faeces.
Probability of contact
The probability of contact with infected excretions is estimated separately for WB and NWB, according to the following bird‐to‐bird contact types: 1. WB, WB; 2. NWB, WB; 3. NWB, NWB; 4. WB, NWB (where the first acronym indicates the source of infection, the second one the infection recipient) defined as follows:
Contact WB, WB: Contact of WBs with virus shed by other WBs occurring in a theoretical area defined as a foraging area and/or wetland/water area;
Contact NWB, NWB: Contact of NWBs with virus shed by other NWBs occurring in a theoretical area defined as a foraging area and or any other NWB typical habitat (i.e. woodland, shrubland);
Contact WB, NWB: Contact between NWB and virus shed by WB occurring in a theoretical area defined as a foraging area;
Contact NWB, WB: Contact between WB and virus shed by NWB occurring in a theoretical area defined as a foraging area.
The probabilities of contact related to the various bird‐to‐bird contact types have been elicited by experts, in the form of uncertainty distributions (see Section D.1.4), taking into account the following considerations. As for transmission occurring via water (only for WBs), dilution by the volume of the water body, possible currents and sedimentation to the ground would grossly reduce the virus density but the virus may persists longer in the water61 compared to on the soil. Therefore, as a general rule agreed in the expert group, the immediate probability to get into contact with infected excretions in the water, given the number of infected birds, was considered lower than the analogous probability of contact occurring on the soil in the foraging areas, but it was considered to last longer in time in the water compartment. The two probabilities have not been estimated separately.
Habitats other than water and foraging areas were considered as places where contact with infected excretions could also take place, but only for NWBs. Within the group of experts estimating this parameter uncertainty distribution, it was agreed that the probability of contact would be lower in such places compared to the foraging areas. The two probabilities thought have not been estimated separately.
Experts were asked to estimate the uncertainty for different levels of exposure to infected excretions, namely virus shed by 1, 10 and 100 birds for each quantile of the uncertainty distribution.
A non‐linear regression model provided the best fit for each quantile separately for WB and NWB for each bird‐to‐excretion contact type:
For HPAI, the initial conditions used to run the SIR model, assuming that all infected migratory WBs enter EU the first day of the migratory season, for a theoretical area and a population scenario are given below:
-
Number of infected at time 1 for:
-
1
– WBs is I1,WB = MWB × πMWB
-
2
– NWBs is I1,NWB = 0
-
1
-
Number of susceptible at time 1 for:
-
1
– WBs is S1,WB = MWB1 + RWB
-
2
– NWBs is S1,NWB = MNWB1 + RNWB
-
1
Where RWB and RNWB indicate residential WB and NWB.
All susceptible birds can potentially get in contact with infected excretions
Number of recovered for both WB and NWB is:
Meaning that there are no recovered birds at the start of the migration season.
Similar initial conditions are used for LPAI, where MWB/MNWB are replaced by EWB/ENWB.
Model scenarios for HPAI
Different scenarios in terms of size (10–100,000 birds) and composition of wild bird populations are considered in each virtual contact area in order to assess AI infection amplification in the wild bird reservoir. Population composition in the scenario was altered by assuming different proportions of migratory birds (pM) and water birds (pWB). The scenarios represent extreme situations, identified by 10% and 90% of migratory/resident and water/non‐water birds in the wild bird reservoir (see Table 1, Section 3.2.2).
The total number of migratory WBs entering the virtual contact area during the migration season is given by the population size multiplied by the proportion of migratory birds and the proportion of WBs. Similar calculations are done for NWBs (see example Table C.4).
Table C.4.
Population size and different wild bird composition for the considered scenarios given a population size of 100,000 wild birds. The number of migratory water birds (MWB), migratory non‐water birds (MNWB), residential water birds (RWB) and residential non‐water birds (RNW) are calculated applying the proportions of migratory birds (pM) and water birds (pWB) to the population size
| Scenario | Population size | pM | pWB | RWB | RNWB | MWB | MNWB |
|---|---|---|---|---|---|---|---|
| 1 | 100,000 | 0.9 | 0.9 | 9,000 | 1,000 | 81,000 | 9,000 |
| 2 | 100,000 | 0.9 | 0.1 | 1,000 | 9,000 | 9,000 | 81,000 |
| 3 | 100,000 | 0.1 | 0.9 | 81,000 | 9,000 | 9,000 | 1,000 |
| 4 | 100,000 | 0.1 | 0.1 | 9,000 | 81,000 | 1,000 | 9,000 |
Model scenarios for LPAI
For the LPAI simulations, scenarios are selected to analyse the effect of virus prevalence in the wild bird population (since this parameter had a large impact on the outcome of the HPAI model) and the effect of protective immunity (since this an important biological question). A description is provided in Section 3.3.2.
Table C.5.
Population size, wild bird composition, prevalence of infected entering water bird and proportion of naïve wild population for the considered scenarios given a population size of 100,000 wild birds. The number of migratory water birds (MWB), migratory non‐water birds (MNWB), residential water birds (RWB) and residential non‐water birds (RNW) are calculated applying the proportions of migratory birds (pM)and water birds (pWB) to the population size
| Scenario | Population size | pM | pWB | RWB | RNWB | MWB | MNWB | πEWB | pnv |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 100,000 | 0.9 | 0.9 | 9,000 | 1,000 | 81,000 | 9,000 | 6% | 1 |
| 2 | 100,000 | 0.9 | 0.9 | 9,000 | 1,000 | 81,000 | 9,000 | 2% | 1 |
| 3 | 100,000 | 0.9 | 0.9 | 9,000 | 1,000 | 81,000 | 9,000 | 2% | 0.5 |
| 4 | 100,000 | 0.9 | 0.9 | 9,000 | 1,000 | 81,000 | 9,000 | 0.2% | 1 |
C.5.3. AI introduction into a poultry holding
Poultry holding area
For the purpose of this assessment, the poultry holding is determined by a 1 km radius circle from the centre of the holding and has a surface of 3.14 km2. Infected excretions released by wild birds within the poultry holding area may enter into a production unit via any means (e.g. boots, car wheels, etc.).
Probability of infection
Under the assumption that there is a non‐linear relationship between the probability that a poultry holding becomes infected and the number of infected birds landing in the holding premises, the approach previously adopted to estimate the probability of a contact with infected matrices was also used here. Parameters expressing the probability of infection were estimated given the presence of 1, 10 and 100 infected birds in the poultry holding area.
Parameter estimates for the probability of infection given exposure to infected excretions of WB and NWB are given Sections D.1.13 and D.1.14, for HPAI and LPAI, respectively.
C.6. Variables used to model HPAI
The model described above was also used to assess entry of HPAI clades 2.3.4.4, 2.3.2.1c and 2.2.1.2 into the EU via the NE route. Only the parameters ‘number of infected water birds entering the EU’ and ‘the infectious period for WBs and NWBs’ and ‘mortality rate due to the disease for WBs and NWBs’ were considered to be different between the clades. The estimated values for all parameters used in the model are provided in Tables C.7 (clade 2.3.4.4) and C.8 (clades 2.3.2.1c and 2.2.1.2).
C.7. Variables used to model LPAI
Table C.8.
List of variables used in the LPAI simulation model
| Parameter | Description | Source | Uncertainty distribution shape | U distr par1 | U distr par2 | U distr par3 | U distr par4 | More info | |
|---|---|---|---|---|---|---|---|---|---|
| MSLength | Length (in days) of the fall–winter migration season for wild birds entering the EU through the north‐east border | Semi‐formal EKE | Constant | 125 | NA | NA | NA | Table D.1 | |
| Prob(contact NWB,NWB ) | Probability that a water bird comes in contact with LPAI infectious excretions | Semi‐formal EKE | Beta | α = 0.72 | β = 69.84 | NA | NA | Section D.1.4 | |
| Prob(contact NWB,WB ) | Probability that a non‐water bird comes in contact with LPAI infectious excretions | Semi‐formal EKE | Beta | α = 0.55 | β = 74.55 | NA | NA | Section D.1.4 | |
| Prob(contact WB,NWB ) | Semi‐formal EKE | beta | α = 0.56 | β = 45.26 | NA | NA | Section D.1.4 | ||
| Prob(contact WB,WB ) | Semi‐formal EKE | beta | α = 0.56 | β = 35.64 | NA | NA | Section D.1.4 | ||
| 1/r NWB | Reciprocal of the duration in days of the LPAI shedding period in non‐water birds | Semi‐formal EKE | tnorm | β = 2.69 | Lower bound = 0.97 | Upper bound = 16.85 | Section D.1.6 | ||
| 1/r WB | Reciprocal of the duration in days of the LPAI shedding period in water birds | Semi‐formal EKE | tnorm | α = 5.06 | β = 3.61 | Lower bound = 1.87 | Upper bound = 25.13 | Section D.1.6 | |
| md NWB | Mortality rate in non‐water birds due to LPAI disease | Literature | Constant | 0.0025 | NA | NA | NA | Section D.1.8 | |
| md WB | Mortality rate in water birds due to LPAI disease | Literature | Constant | 0.001 | NA | NA | NA | Section D.1.8 | |
| Prob(Inf NWB | contact) | Probability that a susceptible non‐water bird becomes infected with LPAI given a contact with excretions containing infectious virus in a forage area | Semi‐formal EKE | beta | α = 0.78 | β = 1596.73 | NA | NA | Section D.1.10 | |
| Prob(Inf WB | contact) | Probability that a susceptible water bird becomes infected with LPAI given a contact with excretions containing infectious virus in a forage area | Semi‐formal EKE | beta | α = 0.74 | β = 235.89 | NA | NA | Section D.1.10 | |
|
|
Number of non‐water birds landing into a holding | Formal EKE | lnorm | Mean = 6.75 | SD = 1.05 | NA | NA | Table D.1 | |
|
|
Number of water birds landing into a holding | Formal EKE | Weibull | Scale = 0.46 | Shape = 111.36 | NA | NA | Table D.1 | |
| β b0 | Regression coefficient for the probability for a worst‐case holding to get the LPAI infection given the presence of LPAI‐infected wild bird | Formal EKE | beta | α = 0.39 | β = 615.11 | NA | NA | Table D.1 | |
| Preservation | Virus preservation | Literature | Constant | 13 | NA | NA | NA | Section D.1.3 | |
|
|
Reduction coefficient for the probability that a susceptible water bird becomes infected given a contact with excretions containing LPAI infectious virus with respect to HPAI‐infected excretion | Formal EKE | 0.01 | 0.03 | 0.05 | 0.1 | 0.5 | Section D.3 | |
|
|
Reduction coefficient for the probability for a worst‐case holding to get the infection given the presence of LPAI‐infected wild bird with respect to HPAI‐infected wild bird | Formal EKE | 0 | 0.08 | 0.1 | 0.3 | 1 | Section D.3 |
Appendix D – Model input values
D.1. Identified scientific evidence
D.1.1. Length of the fall–winter migration season
It was determined as 125 days (e.g. mid‐August to mid‐December), reflecting the average fall–winter migratory season in terms of duration and weather conditions based on the fall–winter migratory seasons which occurred since fall 2005 until the end of winter 2015 (Kear, 2005).
For the LPAI model, also a period of 125 days was used but without specifying when it occurs during the year. Keeping the length of the time period for the LPAI and HPAI the same facilitates comparison of the model outcomes. However, as LPAI viruses can circulate throughout the year in the wild bird population (data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished), the 125‐day period considered in the LPAI model can occur at any time point during a year. Using scenarios with different prevalences reflects periods in the year with high/medium/low prevalence (see Section 3.3.1).
D.1.2. Number of HPAIV clade 2.3.4.4 infected water birds
Data were used from 2014 to 2015 since the estimation of this parameter has been done in March 2016.
Data sources are (i) the European Commission database on wild birds and (ii) the NewFluBird database maintained at the Friedrich Loeffler Institute (DE) (Duncan et al., 2017a).
Virus clade 2.3.4.4 (H5N8) has been detected in the EU in wild birds in the year 2014 for the first time. In total, 37,080 wild birds of around 200 species were sampled in 2014 and 2015, and among those 10 animals were found as HPAIV H5N8 infected in 2014 and 2015.
The number and the proportion of sampled and positive wild birds belonging to the different group of species is reported in Table D.1.
Table D.1.
Total number and percentage of sampled and H5N8 infected wild birds in EU in 2014 and 2015 by group of species and according to the surveillance stream
| Group of species | Number of sampled wild birds (% of sampled wild birds) | Number of H5N8 infected wild birds | ||
|---|---|---|---|---|
| Passive surveillance | Active surveillance | Passive surveillance | Active surveillance | |
| Waterbirds | 1,548 (34.9) | 27,180 (83.2) | 1 | 8 |
| Songbirds | 1,416 (32.0) | 2,806 (8.6) | – | – |
| Other | 636 (14.4) | 388 (1.2) | – | – |
| Raptors | 552 (12.5) | 50 (0.2) | – | – |
| Gulls–Storks–Cranes | 263 (5.9) | 2,206 (6.8) | – | 1 |
| Waders | 16 (0.1) | 19 (0.1) | – | – |
| Total | 4,431 (100) | 32,649 (100) | 1 | 9 |
D.1.3. Prevalence of LPAIV in water birds
There is a high variation in (H5, H7 and H9) LPAIV prevalence between different seasons and in geographical location within the EU since the prevalence is influenced by many factors like wild bird population composition, weather and climate, vegetation structure, etc. (Perez‐Ramirez et al., 2012; Lambrecht et al., 2016).
LPAIV have been detected in more than 100 wild bird species but Anseriformes (particularly ducks, geese and swans) and Charadriiformes (particularly gulls, terns and waders) appear to constitute the most relevant species in LPAIV dynamics (Fouchier and Munster, 2009). These wild bird orders are, in this scientific opinion, included in the water and non‐water bird groups, respectively. Dabbling ducks of the Anas genus has been found to be the more frequently infected with LPAIV than other birds (Krauss et al., 2004; Munster et al., 2007; Parmley et al., 2008; Busquets et al., 2010b). This is likely linked with habitat preference and foraging behaviour (filtering surface water) that facilitate faecal–oral virus transmission. A seasonal pattern in AIV prevalence in mallards has been reported, with peaks in summer and autumn (Swieton et al., 2017).
A lot of data were collected on LPAIV prevalence in water birds to prepare for the formal EKE to elicit this parameter (e.g. data received as per procurement, coordinated by Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished) and experts identified additional studies (Barral et al., 2008; Lebarbenchon, 2008; Busquets et al., 2010b; Perez‐Ramirez et al., 2010, 2012; Jurado‐Tarifa et al., 2014; Swieton et al., 2017). Although the availability of a large data set, it was not possible to elicit an average prevalence across the EU given the variability of LPAIV prevalence in time and space.
Surveillance studies, mainly targeting mallards, indicated an annual peak in late summer and early autumn due to an increased susceptible population, followed by low infection during winter and a small increase during spring (Latorre‐Margalef et al., 2014). In mute swans, higher numbers of antibody‐positive birds are found in summer and winter (e.g. Lambrecht et al., 2016).
The H5+H7+H9 LPAIV prevalence as used for the model was based on mallards sampled at Ottenby in southern Sweden during fall migration, period August–December 2002–2015. The estimated weekly H5+H7+H9 LPAIV prevalence was estimated at 2.0%, and maximum weekly H5+H7+H9 LPAIV prevalence was estimated at 5.9%. The minimum H5+H7+H9 LPAIV prevalence value was estimated based on mallards sampled at the same site but in the spring. To estimate the H5+H7+H9 LPAIV prevalence, LPAIV general prevalence was extracted from weekly prevalence data as provided by Jonas Waldenström (data received as per procurement, Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, unpublished) and LPAIV subtype distribution from mallards sampled at the same sample site was extracted from Table 1 in Latorre‐Margalef et al. (2014).
D.1.4. Probability contact between wild birds and infectious excretions
The quantity of faeces excreted by water birds in one day largely varies between species and can be up to 75 g fresh weight per individual. A forage area of 4 ha is considered in the model and the surface of the forage area covered by excretions spread by 1, 10 or 100 infected water birds can vary from 50 to 500 m2. The experts took the following assumptions into account when estimating the variable: the faeces (virus) is dropped randomly in the foraging area, increasing the number of birds in a foraging area increases the surface covered by excretions and birds walk randomly in the foraging area.
D.1.5. Shedding period of HPAIV
Table D.2.
HPAI H5N8 virus detection in oropharyngeal (O) or cloacal (C) swabs of different water bird species after experimental infection via the intranasal route (positive/total number of birds)
| Species | Swab | 2 dpi | 3 dpi | 5 dpi | 7 dpi | 9 dpi | 11 dpi | Study |
|---|---|---|---|---|---|---|---|---|
| Muscovy duck | O | 4/4 | 4/4 | 4/4 | 3/4 | 2/4 | A | |
| C | 4/4 | 4/4 | 4/4 | 3/4 | 1/4 | A | ||
| Mallard | O | 4/4 | 4/4 | 0/4 | 0/2 | B | ||
| C | 1/4 | 3/4 | 0/4 | 0/2 | B | |||
| Baikal teal | O | 1/2 | 0/1 | 0/1 | 0/1 | 0/1 | C | |
| C | 1/2 | 0/1 | 0/1 | 0/1 | 0/1 | C | ||
| Domestic duck | O | See figure A of study C | C | |||||
| C | See figure B of study C | C | ||||||
D.1.6. Shedding period of LPAIV
Table D.3.
HPAI H5N8 virus detection in oropharyngeal (O) or cloacal (C) swabs of different non‐water bird species after experimental infection via the intranasal route (positive/total number of birds)
| Species | Swab | 2 dpi | 5 dpi | 7 dpi | 9 dpi | 11 dpi | Study |
|---|---|---|---|---|---|---|---|
| Layer chicken | O | 8/8 | 6/6 | NR | NR | NR | A |
| C | 7/8 | 6/6 | 1/1 | NR | NR | A | |
| Korean native chicken | O | 2/5 | 2/5 | 1/3 | 1/2 | 1/2 | A |
| C | 2/5 | 1/3 | 1/3 | 0/2 | 1/2 | A | |
| Quail | O | 4/4 | NR | NR | NR | NR | A |
| C | 4/4 | NR | NR | NR | NR | A |
NR: not reported.
Study A: strain A/Baikal teal/Korea/K14‐E016/2014 (Lee et al., 2016).
Analysis of HPAI and LPAI transmission based on reported outbreaks, indicate a longer infectious period for LPAI than for HPAI (Comin et al., 2011; Saenz et al., 2012). Henaux and Samuel (2011) reported longer median virus shedding times for LPAI‐infected ducks (10–11.5 days in oral and cloacal swabs) than HPAI‐infected ducks (5 days) and geese (7.5) days (Henaux and Samuel, 2011). The authors used LPAI data from studies published between 1978 and 1987. The tables below provides data on H5, H7 and H9 LPAIV shedding following inoculation as reported in more recent studies.
Table D.4.
H5, H7 or H9 LPAIV detection in oropharyngeal (O), cloacal (C) or faecal (F) swabs of different water bird species after experimental infection (positive/total number of birds)
| Species | LPAIV | Route | Swab | 2 dpi | 3 dpi | 5 dpi | 7 dpi | 9 dpi | 11 dpi | 14 dpi | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mallard | H5N2 A/mallard/MN/3555779/0 | IN | O | 5/5 | 5/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | França et al. (2012) |
| C | 4/5 | 4/5 | 5/5 | 4/5 | 2/5 | 0/5 | 0/5 | ||||
| IT | O | 5/5 | 5/5 | 1/5 | 0/5 | 0/5 | 0/5 | 0/5 | |||
| C | 5/5 | 4/5 | 5/5 | 5/5 | 0/5 | 0/5 | 0/5 | ||||
| IO | O | 5/5 | 5/5 | 4/5 | 2/5 | 0/5 | 0/5 | 0/5 | |||
| C | 5/5 | 5/5 | 5/5 | 2/5 | 1/5 | 0/5 | 0/5 | ||||
| IC | O | 5/5 | 4/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | |||
| C | 5/5 | 5/5 | 5/5 | 3/5 | 0/5 | 0/5 | 0/5 | ||||
| II | O | 5/5 | 3/5 | 4/5 | 0/5 | 0/5 | 0/5 | 0/5 | |||
| C | 5/5 | 5/5 | 5/5 | 5/5 | 2/5 | 0/5 | 0/5 | ||||
| Mallard |
H7N7 A/mallard/Sweden/7206/2004 |
IE | C | 6/6 | 6/6 | 6/6 | 6/6 | 6/6 | 5/6 | 2/6 | Jourdain et al. (2010) |
| F | 6/6 | 6/6 | 6/6 | 4/6 | 4/6 | 4/6 | 3/6 | ||||
| Pekin ducks |
H7N2 A/chicken/NJ/15086‐3/1994 |
ICh | O | + | + | + | + | + | + | + | Spackman et al. (2010) |
| C | + | + | + | + | + | – | – | ||||
|
H7N2 A/turkey/NY/4450‐4/1994 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | + | + | + | + | + | + | ||||
|
H7N2 A/chicken/NY/3112‐1/1995 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | + | + | + | + | + | + | ||||
|
H7N3 A/chicken/NY/12273‐11/1999 |
ICh | O | + | + | + | + | + | + | + | ||
| C | + | + | + | + | + | + | + | ||||
|
H7N2 A/chicken/NY/30749‐3/2000 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | + | + | + | + | – | – | ||||
|
H7N2 A/guinea hen/MA/148081‐11/2002 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | + | + | + | – | – | + | ||||
|
H7N2 A/chicken/PA/9801289/1998 |
ICh | O | + | + | + | + | – | – | – | ||
| C | + | + | – | – | – | – | – | ||||
|
H7N2 A/turkey/VA/SEP‐67/2002 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | – | – | – | – | – | – | ||||
|
H7N2 A/chicken/MD/MinhMa/2004 |
ICh | O | + | + | + | + | + | – | – | ||
| C | + | – | – | + | – | – | – | ||||
|
H7N8 A/mallard/OH/421/1987 |
ICh | O | + | + | – | – | – | – | – | ||
| C | – | – | + | + | + | + | + | ||||
|
H7N3 A/pintail/MN/423/1999 |
ICh | O | + | + | + | + | + | + | + | ||
| C | + | + | + | + | + | + | + | ||||
|
H7N9 A/ruddy turnstone/DE/1538/2000 |
ICh | O | + | + | + | + | + | + | + | ||
| C | + | – | + | + | + | + | + | ||||
| Mallard |
H5N2 A/mallard/MN/355779/00 |
ICh | O | 2/5 | 5/5 | 4/5 | ND | 0/5 | ND | 0/5 | Costa et al. (2011) |
| C | 2/5 | 5/5 | 5/5 | ND | 3/5 | ND | 1/5 | ||||
|
H7N3 A/mallard/MN/182761/98 |
ICh | O | 1/5 | 1/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||
| C | 0/5 | 0/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||||
| Redhead duck |
H5N2 A/mallard/MN/355779/00 |
ICh | O | 5/5 | 5/5 | 2/5 | ND | 3/5 | ND | 0/5 | |
| C | 2/5 | 2/5 | 2/5 | ND | 2/5 | ND | 1/5 | ||||
|
H7N3 A/mallard/MN/182761/98 |
ICh | O | 4/5 | 3/5 | 0/5 | ND | 1/5 | ND | 0/5 | ||
| C | 0/5 | 0/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||||
| Wood duck |
H5N2 A/mallard/MN/355779/00 |
ICh | O | 3/5 | 3/5 | 2/5 | ND | 0/5 | ND | 0/5 | |
| C | 0/5 | 0/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||||
|
H7N3 A/mallard/MN/182761/98 |
ICh | O | 2/5 | 2/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||
| C | 1/5 | 0/5 | 0/5 | ND | 0/5 | ND | 0/5 | ||||
| Mallard |
H5N2 A/mallard/MN/346250/00 |
OINO | O | 3/4 | 0/4 | 3/4 | 0/4 | ND | ND | ND | Achenbach and Bowen (2011) |
| C | 3/4 | 3/4 | 3/4 | 0/4 | ND | ND | ND | ||||
| Contact | O | 1/4 | 3/4 | 2/4 | 0/4 | ND | ND | ND | |||
| C | 0/4 | 4/4 | 4/4 | 0/4 | ND | ND | ND | ||||
|
H7N3 A/Ruddy turnstone/ReedsBeachNJ/00 |
OINO | O | 3/4 | 4/4 | 4/4 | 0/4 | ND | ND | ND | ||
| C | 4/4 | 4/4 | 3/4 | 0/4 | ND | ND | ND | ||||
| Contact | O | 4/4 | 4/4 | 4/4 | 0/4 | ND | ND | ND | |||
| C | 4/4 | 4/4 | 3/4 | 1/4 | ND | ND | ND |
IN: intranasal; IT: intratracheal; IO: intraocular; IC: intracloacal; II: intraingluvial; IE: intra‐oesophagus; ICh: intrachoanal; ND: non‐determined; OINO: orally, intranasally and ocularly; +: mean log10 virus shed titres of 13–15 birds > 0.25; –: mean log10 virus shed titres of 13–15 birds < 0.25.
Table D.5.
H5, H7 or H9 LPAIV detection in oropharyngeal (O) or cloacal (C) swabs of different non‐water bird species after experimental infection (positive/total number of birds)
| Species | LPAIV | Route | Swab | 2 dpi | 3 dpi | 5 dpi | 7 dpi | 9 dpi | 11 dpi | 14 dpi | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laughing gull |
H5N2 A/mallard/MN/355779/00 |
ICh | O | 5 | 5 | 4 | ND | 2 | ND | 0 | Costa et al. (2011) |
| C | 3 | 4 | 5 | ND | 1 | ND | 0 | ||||
|
H7N3 A/mallard/MN/182761/98 |
ICh | O | 2 | 2 | 0 | ND | 0 | ND | 0 | ||
| C | 0 | 0 | 0 | ND | 0 | ND | 0 | ||||
| Gyr‐saker hybrid falcon |
H7N2 A/Anas plathyrhynchos/Spain/1877/2009 |
Feed | O | + | + | + | + | + | – | ND | Bertran et al. (2012) |
| NC | O | + | + | + | + | + | + | ND | |||
| Finches |
H9N2 A/environment/Bangladesh/9306/2010 |
ON | O | 4/5 | ND | 3/5 | ND | 0/5 | ND | ND | Lenny et al. (2015) |
| Parakeets |
H9N2 A/environment/Bangladesh/9306/2010 |
ON | O | 5/5 | ND | 4/5 | ND | 0/5 | ND | ND |
Feed: feeding; NC: nasochoanal; ICh: intrachoanal; ND: non‐determined; ON: oculonasally; +: inverted Ct value < 35; –: inverted Ct value > 35.
D.1.7. Mortality rate HPAIV in wild birds
The AI consortium extracted data from 30 studies (Duncan et al., 2017a) and overviews are provided in Tables D.6 and D.7. Only studies where birds have been experimentally infected were considered (n = 22). The mortality rate has been computed as the average of the ratio by study between the number of birds died because of the infection, and the number of birds experimentally inoculated. The minimum and the maximum are provided for completeness. Please bear in mind that values provided in the two tables below are from studies that differ in terms of inoculation dose, species inoculated, number of animals inoculated, general setting, etc.
Table D.6.
Mortality rate in water birds and non‐water birds by clade based on data extracted from the scientific literature by the AI consortium (Duncan et al., 2017a)
| Viral clade and group of wild bird species | Average mortality rate | Min. mortality rate | Max. mortality rate | No. of birds inoculated in the studies | No. of experimental studies |
|---|---|---|---|---|---|
| 2.2.1 | 0.79 | 0.125 | 1 | 91 | 10 |
| Non‐water bids | 0.79 | 0.71 | 0.88 | 23 | 2 |
| Water birds | 0.79 | 0.12 | 1 | 68 | 8 |
| 2.3.2.1 | 0.66 | 0 | 1 | 189 | 26 |
| Non‐water bids | 1 | 1 | 1 | 8 | 2 |
| Water birds | 0.63 | 0 | 1 | 181 | 24 |
| 2.3.4.4 | 0.16 | 0 | 1 | 62 | 15 |
| Non‐water bids | 0.75 | 0.5 | 1 | 8 | 2 |
| Water birds | 0.07 | 0 | 0.5 | 54 | 13 |
| Grand total | 0.54 | 0 | 1 | 342 | 51 |
Table D.7.
Morbidity and mortality in water birds following experimental infection with H5, H7 or H9 LPAIV
| Species | HN‐type | Dose (EID50 and TCID50) | Route | Morbidity/mortality | Reference |
|---|---|---|---|---|---|
| Pekin duck | H5N3 | 3.3–1.6 × 105/L air | AER | No | Kuiken (2013) |
| H5N2 | 1 × 105 | NAS or ORA | No | ||
| H2N2 | 1 × 107 | ORA or REC | No | ||
| Mallard | H5N2 | 1 × 107 EID50 | ORA and TRA | No | |
| H5N2 | 1 × 108.7 EID50 | OES | No | ||
| H5N1 | 1.25 × 105 EID50 | IV | No | ||
| H5N1 | 1.1 × 106 EID50 | IV | No | ||
| H4N6 and H5N2 | 1 × 106 EID50 | OCU, NAS and PHA | No | ||
| H7N7 | 1 × 108.7 EID50 | OES | No | ||
| H5N9 | 1.5 × 106 PFU | OES and PHA | No | ||
| H3N8 and H5N2 | 1 × 105–1 × 106 EID50 | NAS | No | ||
| Comm. Layer type duck | H5N2 | 1 × 107.2 EID50 | NAS | No | |
| Cherry Valley dom. Duck | H5N1 | 1 × 108 EID50 | NAS | No | |
| Pekin duck | H5N1 | 106 EID50 (23 days old pekin duck) | ED and NAS | No | Ferreira et al. (2010) |
| Mule duck | H7N1 | 106 EID50 (7‐day‐old mule duck) |
AER: aerosol; IV: intravenous; NAS: intranasal; OCU: supraocular; OES: intra‐oesophageal; ORA: intraoral; PHA: intrapharyngeal; REC: intrarectal; TRA: intratracheal; ED: eye drop; EID50: median egg infectious dose; TCID50: median tissue culture infectious dose; PFU: plaque‐forming units; n.a., not appropriate.
For clade 2.2.1, one of the three studied included for computing the estimates was performed on pigeons. Due to the well‐documented low mortality caused by the infection with HPAI virus in pigeons, this study was excluded from the estimates.
D.1.8. Mortality rate LPAIV in wild birds
Infection with LPAIV generally causes no major clinical signs in wild birds. The Tables D.7 and D.8 below give an overview of the reported morbidity and mortality reported in water and non‐water birds, respectively, following inoculation with H5, H7 or H9 LPAIV.
Table D.8.
Morbidity and mortality in non‐water birds following experimental infection with H7 LPAIV
| Species | HN‐type | Dose (EID50/mL) | Route | Morbidity/mortality | Reference |
|---|---|---|---|---|---|
| Captive‐reared gyr‐saker hybrid falcon | H7N2 | 106 | Nasochoanal and by natural feeding | No | Bertran et al. (2012) |
D.1.9. AIV preservation
Although there is evidence that the AI virus can persist in the environment for a period of up to several weeks and even months depending on the matrix and the environmental conditions (Stallknecht and Brown, 2009; Nazir et al., 2010, 2011; Wood et al., 2010; EFSA, 2016a), a persistence of 13 days was used as an approximation in the model. The approximation includes that the infectivity is kept constant which does not reflect the observed gradual decay but compensates for the shortened period.
D.1.10. Probability wild bird infection given contact with HPAIV‐infectious excretions
Table D.9 provides information on the virus concentration in oral and cloacal swabs of falcons infected with HPAI H5N1 under experimental conditions.
Table D.9.
HPAIV shedding of non‐water birds following experimental inoculation
| Species | HPAIV | Route | Swab | Virus concentration | Test method | Reference |
|---|---|---|---|---|---|---|
| Gyr‐saker hybrid falcon |
H5N1 A/Great crested grebe/Basque Country/06.03249/2006) |
Feed | O | ± 26 (inverted Ct value) | RT‐PCR | Bertran et al. (2012) |
| NC | O | ± 28 (inverted Ct value) | RT‐PCR |
Feed: feeding; NC: nasochoanal.
D.1.11. Probability wild bird infection given contact with LPAIV‐infectious excretions
In wild birds, LPAIV is thought to preferentially infect cells lining the intestinal tract, leading to virus shedding mainly via faeces (Fouchier and Munster, 2009). Transmission via the faecal–oral route is considered the primary mode of LPAIV transmission in many bird species, although transmission via respiratory secretions may also be relevant for particular land‐based bird species (Ellstrom et al., 2008).
Achenbach and Bowen (2011) describe transmission of LPAIV H7N3 from inoculated mallards to chickens, red‐winged blackbirds and other mallards that were co‐housed (Achenbach and Bowen, 2011). In a similar experiment, LPAIV H5N2 was only transmitted from mallards to chickens and mallards via direct or indirect transmission, but not to red‐winged blackbirds.
Lenny et al. (2015) reported that finches and parakeets inoculated with H9N2 subsequently excreted the virus but were not able to infect naïve birds of the same species that were kept in the same cage (Lenny et al., 2015).
Tables D.10 and D.11 give an overview of the reported virus concentration shed by water and non‐water birds following inoculation with H5, H7 or H9 LPAIV.
Table D.10.
LPAIV shedding of water birds following experimental inoculation
| Species | LPAIV | Route | Swab | Virus concentrationA | Reference |
|---|---|---|---|---|---|
| Mallard |
H7N7 A/mallard/Sweden/7206/2004 |
IE | C | ± 21 (inverted Ct value) | Jourdain et al. (2010) |
| F | ± 15 (inverted Ct value) | ||||
| Mallard |
H5N2 A/mallard/MN/3555779/0 |
IN | O | ± 4.5 EID50/mL | França et al. (2012) |
| C | ± 4.9 EID50/mL | ||||
| IT | O | ± 3.5 EID50/mL | |||
| C | ± 4.9 EID50/mL | ||||
| IO | O | ± 4.4 EID50/mL | |||
| C | ± 6.5 EID50/mL | ||||
| IC | O | ± 3.8 EID50/mL | |||
| C | ± 6.2 EID50/mL | ||||
| II | O | ± 4.2 EID50/mL | |||
| C | ± 6.5 EID50/mL | ||||
| Pekin ducks |
H7N2 A/chicken/NJ/15086‐3/1994 |
ICh | O | ± 2.5 EID50/mL | Spackman et al., 2010 |
| C | ± 3.0 EID50/mL | ||||
|
H7N2 A/turkey/NY/4450‐4/1994 |
ICh | O | ± 3.8 EID50/mL | ||
| C | ± 3.8 EID50/mL | ||||
|
H7N2 A/chicken/NY/3112‐1/1995 |
ICh | O | ± 2.5 EID50/mL | ||
| C | ± 2.5 EID50/mL | ||||
|
H7N3 A/chicken/NY/12273‐11/1999 |
ICh | O | ± 2.1 EID50/mL | ||
| C | ± 3.0 EID50/mL | ||||
|
H7N2 A/chicken/NY/30749‐3/2000 |
ICh | O | ± 3.5 EID50/mL | ||
| C | ± 1.8 EID50/mL mL | ||||
|
H7N2 A/guinea hen/MA/148081‐11/2002 |
ICh | O | ± 4.0 EID50/mL | ||
| C | ± 1.5 EID50/mL | ||||
|
H7N2 A/chicken/PA/9801289/1998 |
ICh | O | ± 2.0 EID50/mL | ||
| C | ± 1.5 EID50/mL | ||||
|
H7N2 A/turkey/VA/SEP‐67/2002 |
ICh | O | ± 3.8 EID50/mL | ||
| C | ± 0.3 EID50/mL | ||||
|
H7N2 A/chicken/MD/MinhMa/2004 |
ICh | O | ± 2.8 EID50/mL | ||
| C | ± 1.6 EID50/mL | ||||
|
H7N8 A/mallard/OH/421/1987 |
ICh | O | ± 1.9 EID50/mL | ||
| C | ± 0.9 EID50/mL | ||||
|
H7N3 A/pintail/MN/423/1999 |
ICh | O | ± 4.1 EID50/mL | ||
| C | ± 4.2 EID50/mL | ||||
|
H7N9 A/ruddy turnstone/DE/1538/2000 |
ICh | O | ± 3.5 EID50/mL | ||
| C | ± 5.0 EID50/mL | ||||
| Mallard |
H5N2 A/mallard/MN/346250/00 |
OINO | O | 2.3 EID50/mL | Achenbach and Bowen (2011) |
| C | 5.9 EID50/mL | ||||
| Contact | O | 1.9 EID50/mL | |||
| C | 4.6 EID50/mL | ||||
|
H7N3 A/Ruddy turnstone/ReedsBeachNJ/00 |
OINO | O | 3.3 EID50/mL | ||
| C | 4.4 EID50/mL | ||||
| Contact | O | 2.9 EID50/mL | |||
| C | 6.5 EID50/mL |
IN: intranasal; IT: intratracheal; IO: intraocular; IC: intracloacal; II: intraingluvial; IE: intra‐oesophagus; O: oral; C: cloacal; F: fecal.
A: mean value at day with highest virus excretion detected via RT‐PCR.
Table D.11.
LPAIV shedding of non‐water birds following experimental inoculation
| Species | LPAIV | Route | Swab | Virus concentration | Test method | Reference |
|---|---|---|---|---|---|---|
| Gyr‐saker hybrid falcon |
H7N2 A/Anas plathyrhynchos/Spain/1877/2009 |
Feed | O | ± 24 (inverted Ct value) | RT‐PCR | Bertran et al. (2012) |
| NC | O | ± 22 (inverted Ct value) | RT‐PCR |
Feed: feeding; NC: nasochoanal.
D.1.12. Number of wild birds landing into a holding
The probability for wild birds to land in a poultry holding is affected by several factors, such as the land use and ecological environment around the holding, the species‐specific behaviour, attractiveness of the area (e.g. food availability), altitude of the holding, etc. (Busani et al., 2009; Mughini‐Gras et al., 2014). Tables D.12 and D.13 give an overview of water and non‐water birds observed in immediate barn area of poultry holdings in Canada (Burns et al., 2012).
Table D.12.
Water birds observed in immediate barn area of poultry holdings in Canada (Burns et al., 2012)
| Sub‐category | Order | Species (Number of birds in immediate barn area) |
|---|---|---|
| Dabbling ducks | Anseriformes | Mallard (6) |
| Geese | Canada goose (11) | |
| Swans | Trumpeter swan (4) | |
| Pelicans, Herons, Spoonbills, Ibises | Pelecaniformes | Great blue heron (6) |
Table D.13.
Non‐water birds observed in immediate barn area of poultry holdings in Canada (Burns et al., 2012)
| Sub‐category | Order | Species (Number of birds in immediate barn area) |
|---|---|---|
| Raptors | Falconiformes | Peregrine falcon (3),American kestrel (5) |
| Accipitriformes | Red‐tailed hawk (10), Bald eagle (9) | |
| Song birds | Passeriformes | European starling (144), North‐western crow (45), American robin (33), House finch (26), House sparrow (58), White crowned sparrow (25), Dark‐eyed junco (27), Song sparrow (18), Savannah sparrow (28), Golden‐crowned sparrow (8), Spotted towhee (8), American goldfinch (17), Black‐capped chickadee (14), Brewers blackbird (4), Yellow‐rumped warbler (5), American pipit (5), Red‐winged blackbird (28), Barn swallow (10), Chestnut‐backed chickadee (4), Bewick's wren (3), Brown‐headed cowbird (10), Pine siskin (3), Townsend's solitaire (3), Stellar's jay (1), Horned lark (48), Common grackle (13), American crow (14), Blue jay (7), Chipping sparrow (8), Northern cardinal (7), Gray catbird (2), Yellow warbler (2) |
| Columbiformes | Rock dove (35), Mourning dove (10) | |
| Waders | Charadriiformes | Glaucous‐winged gull (14), Ring‐billed gull (2), Killdeer (18) |
| Gulls‐Terns‐Storks‐Cranes | Charadriiformes | Mew gull (3) |
D.1.13. Regression coefficient probability HPAIV infection worst‐case holding
The concentration of HPAI H5N8 virus excreted by a water bird in one standard day‐ excretion is for 5 days, high loads are excreted for only 2 days. Peak titres are 1,000,000 TCID50/gr faeces (0.5 day); average titres over 5 days per gr faeces are 1,000. The infective dose by groups of birds was 250–1,000 TCID50 (for domestic waterfowl) and 5,000–20,000 TCID50 (for gallinaceous poultry) (Kim et al., 2014; Li et al., 2014; Kanehira et al., 2015; Kang et al., 2015; Lee et al., 2016; Sun et al., 2016).
Further scientific evidence is provided in the systematic literature review performed by the AI consortium, identifying and synthesising the evidence on risk factors of HPAI and LPAI introductions into European poultry holdings (data received as per procurement, Linnaeus University (SE), Erasmus Medical Centre (NL), OC/EFSA/ALPHA2015/01 supplemented with NP/EFSA/ALPHA/2015/04, Gonzales et al., 2017).
D.1.14. Regression coefficient probability LPAIV infection worst‐case holding
The concentration of LPAIVs shed by mallards and falcons experimentally infected with LPAI H5N2 and H7N2, respectively, is given in Table D.14. Further scientific evidence is provided in the systematic literature review performed by the AI consortium, identifying and synthesising the evidence on risk factors of HPAI and LPAI introductions into European poultry holdings (Duncan et al., 2017b).
Table D.14.
LPAI virus excretion of wild birds following experimental infection
| Species | Dose | Route | HN‐type | Virus excretion (Peak titres expressed in log10 EID50/mL or TCID50/g of tissue) | Reference |
|---|---|---|---|---|---|
| Mallards |
IN, IT and IO: 106 EID50/0.1 mL; IC and II: 106 EID50/1 mL |
IN, IT, IO, IC and II | H5N2 |
OP: 2.5–4.8 EID50/mL; CL: 3.0–7.0 EID50/mL |
França et al. (2012) |
| Captive‐reared gyr‐saker hybrid falcon | 106 EID50/mL | Nasochoanal and natural feeding routes | H7N2 | 103.2–107.2 TCID50/g of tissue | Bertran et al. (2012) |
IN: intranasal; IT: intratracheal, IO: intraocular, IC: intracloacal; II: intraingluvial; EID50: median egg infectious dose; TCID50: median tissue culture infectious dose.
D.1.15. Migratory wild birds entering the EU during a migratory season
There is a lack of information on the number of migratory wild water birds entering the EU via the NE route during a migratory season, covering the bird species listed in Section B.1 (Appendix B). There is also large variation expected between years. Therefore, it was decided to select theoretical scenarios reflecting different real world conditions (see Section C.5.2, Appendix C).
D.2. Estimates for the HPAI model
Tables D.15, D.16 and D.17 show the collection of estimates for the HPAI model parameters.
Table D.15.
Estimates for HPAI provided in the EKEs by working group members or event participants
| Variable | Description | Question description | Clade | LB | Q1 | Median | Q3 | UB | Estimate done by |
|---|---|---|---|---|---|---|---|---|---|
| MNWB | Total number of migratory non‐water birds entering the EU during fall–winter migratory season through the north‐east border | Total number of migratory non‐water birds entering the EU during fall–winter migratory season through the north‐east border. Number of birds in 1,000 units | 2.3.4.4 | 175,000 | 390,000 | 515,000 | 590,000 | 700,000 | Semi‐formal EKE |
| Mslength | Length (in days) of the fall–winter migratory season for wild birds entering the EU through the north‐east border | Average over last 100 year of length in days of the fall–winter migratory season for wild bird of the water bird group entering in EU through north‐east border | 2.3.4.4 | 125 | Data | ||||
| MWB | Total number of migratory water birds entering the EU during fall–winter migratory season through the north‐east border | Total number of migratory water birds entering the EU during fall–winter migratory season through the north‐east border. Number of birds in 1,000 units | 2.3.4.4 | 17,500 | 26,500 | 37,250 | 46,500 | 55,000 | Semi‐formal EKE |
| NWBin | Number of non‐water birds landing into a holding | Estimate the average number, over 2016–2017 fall–winter migratory season (5 months duration), of non‐water birds (migratory and/or residential) that will land on any holding surface of a ‘worst‐case’ poultry holding per day | 2.3.4.4 | 200 | 400 | 750 | 2,000 | 10,000 | Workshop |
| Prob(contactNWB,NWB)1 | Probability that a non‐water bird comes in contact with the excretions of 1 infected non‐water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 1 infected non‐water bird in a foraging area (see definition above) and in other potential area in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 2 | 14.5 | 175 | 350 | 1,750 | Semi‐formal EKE |
| Prob(contactNWB,NWB)10 | Probability that a non‐water bird comes in contact with the excretions of 10 infected non‐water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 10 infected non‐water bird in a foraging area (see definition above) and in other potential area in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 17.5 | 130 | 1,500 | 2,700 | 7,000 | Semi‐formal EKE |
| Prob(contactNWB,NWB)100 | Probability that a non‐water bird comes in contact with the excretions of 100 infected non‐water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 100 infected non‐water bird in a foraging area (see definition above) and in other potential area in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 150 | 1,450 | 5,498.5 | 6,249 | 9,500 | Semi‐formal EKE |
| Prob(contactNWB,WB)1 | Probability that a water bird comes in contact with the excretions of 1 infected non‐water bird in a foraging area | Think of a standard population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 1 infected non‐water bird in a foraging area (see definition above) in a standard day (number out of 10,000) | 2.3.4.4 | 1 | 10 | 51 | 210 | 600 | Semi‐formal EKE |
| Prob(contactNWB,WB)10 | Probability that a water bird comes in contact with the excretions of 10 infected non‐water bird in a foraging area | Think of a standard population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 10 infected non‐water bird in a foraging area (see definition above) in a standard day (number out of 10,000) | 2.3.4.4 | 7.5 | 65 | 485 | 1,475 | 4,000 | Semi‐formal EKE |
| Prob(contactNWB,WB)100 | Probability that a water bird comes in contact with the excretions of 100 infected non‐water bird in a foraging area | Think of a standard population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 100 infected non‐water bird in a foraging area (see definition above) in a standard day (number out of 10,000) | 2.3.4.4 | 75 | 525 | 3,475 | 5,749 | 8,500 | Semi‐formal EKE |
| Prob(contactWB,NWB)1 | Probability that a non‐water bird comes in contact with the excretions of 1 infected water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 1 infected water bird in a foraging area (see definition above) in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 3 | 16 | 112.5 | 275 | 900 | Semi‐formal EKE |
| Prob(contactWB,NWB)10 | Probability that a non‐water bird comes in contact with the excretions of 10 infected water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 10 infected water bird in a foraging area (see definition above) in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 30 | 160 | 1,125 | 2,750 | 8,999.5 | Semi‐formal EKE |
| Prob(contactWB,NWB)100 | Probability that a water bird comes in contact with the excretions of 100 infected water bird in a foraging area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might come in contact with the excretions shedded by 100 infected water bird in a foraging area (see definition above) in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 100 | 1,000 | 5,998.5 | 6,499 | 7,499.5 | Semi‐formal EKE |
| п_MWB | Prevalence of infected HPAIV water birds at the moment they cross the EU border | Think of a population of 1,000,000 migratory water birds crossing the EU north‐east border during the 2016/2017 fall–winter migration season. Estimate the number of these birds that will be HPAI H5N8 infected at the time they cross the north‐east EU border | 2.3.2.1c | 0 | 15 | 100 | 500 | 1,000 | Semi‐formal EKE |
| п_MWB | Prevalence of infected HPAIV water birds at the moment they cross the EU border | Think of a population of 1,000,000 migratory water birds crossing the EU north‐east border during the 2016/2017 fall–winter migration season. Estimate the number of these birds that will be HPAI H5N8 infected at the time they cross the north‐east EU border | 2.2.1.2 | 0 | 2 | 8 | 25 | 200 | Semi‐formal EKE |
| Prob(contactWB,WB)1 | Probability that a water bird comes in contact with the excretions of 1 infected water bird in a foraging area | Think of a population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 1 infected water bird in a foraging area (see definition above) or in a water body in a standard day (number out of 10,000) | 2.3.4.4 | 1.5 | 19.5 | 150 | 400 | 1,500 | Semi‐formal EKE |
| ShedWB | Duration in days of the shedding period in water birds | Total number of days a HPAI‐infected water bird will shed infectious virus | 2.3.2.1c | 0 | 2 | 4 | 6 | 14 | Semi‐formal EKE |
| ShedWB | Duration in days of the shedding period in water birds | Total number of days a HPAI‐infected water bird will shed infectious virus | 2.2.1.2 | 0 | 2 | 4 | 6 | 14 | Semi‐formal EKE |
| Prob(contactWB,WB)10 | Probability that a water bird comes in contact with the excretions of 10 infected water bird in a foraging area | Think of a population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 10 infected water bird in a foraging area (see definition above) or in a water body in a standard day (number out of 10,000) | 2.3.4.4 | 12 | 170 | 1,450 | 3,500 | 6,499.5 | Semi‐formal EKE |
| ShedNWB | Duration in days of the shedding period in non‐water birds | Total number of days a HPAI‐infected non‐water bird will shed the infectious virus | 2.3.2.1c | 0 | 1 | 3 | 5 | 10 | Semi‐formal EKE |
| ShedNWB | Duration in days of the shedding period in non‐water birds | Total number of days a HPAI‐infected non‐water bird will shed the infectious virus | 2.2.1.2 | 0 | 1 | 3 | 5 | 10 | Semi‐formal EKE |
| Prob(contactWB,WB)100 | Probability that a water bird comes in contact with the excretions of 100 infected water bird in a foraging area | Think of a population of 10,000 water birds. Indicate the number of these birds that might come in contact with the excretions shedded by 100 infected water bird in a foraging area (see definition above) or in a water body in a standard day (number out of 10,000) | 2.3.4.4 | 112.5 | 1,275 | 6,498.5 | 7,249 | 7,499.5 | Semi‐formal EKE |
| π_MWB | Prevalence of infected HPAIV water birds at the moment they cross the EU border | Think of a population of 1,000,000 migratory water birds crossing the EU north‐east border during the 2016/2017 fall–winter migration season. Estimate the number of these birds that will be HPAI H5N8 infected at the time they cross the north‐east EU border | 2.3.4.4 | 0 | 16.94 | 114.42 | 463.67 | 5,000 | Formal EKE |
| Prob(PHb0 inf)1 | Probability for a worst‐case holding to get the infection given the presence of 1 HPAI‐infected wild bird | Think of 10,000 worst‐case poultry holdings (see definition above). Given exposure of the holding to 1 H5N8 infected wild bird, estimate the number of worst‐case poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 0.58 | 4.03 | 18.84 | 5,000 | Formal EKE |
| Prob(PHb0 inf)10 | Probability for a worst‐case holding to get the infection given the presence of 10 HPAI‐infected wild bird | Think of 10,000 worst‐case poultry holdings (see definition above). Given exposure of the holding to 10 H5N8 infected wild bird, estimate the number of worst‐case poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 5.19 | 23.78 | 112.99 | 5,000 | Formal EKE |
| Prob(PHb0 inf)100 | Probability for a worst‐case holding to get the infection given the presence of 100 HPAI‐infected wild bird | Think of 10,000 worst‐case poultry holdings (see definition above). Given exposure of the holding to 100 H5N8 infected wild bird, estimate the number of worst‐case poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 43.17 | 183.44 | 854.36 | 5,000 | Formal EKE |
| Prob(PHb1 inf) | Probability for a holding to get the infection given the presence of 100 HPAI‐infected wild bird if access by wild birds to the water bodies was prevented | Consider the scenario described in Q21c and take the median number of worst‐case poultry holdings you estimated as a starting point for this question. If access by poultry (including waterfowl) to the water bodies was prevented in these poultry holdings, what would be the number of such poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 16.94 | 61.31 | 190.15 | 5,000 | Formal EKE |
| Prob(PHb2 inf) | Probability for a holding to get the infection given the presence of 100 HPAI‐infected wild bird if in addition access of poultry to any outdoor area were prevented | Consider the scenario described in Q22 and take the median number of poultry holdings you estimated as a starting point for this question. If access by poultry (including waterfowl) to the water bodies AND access of poultry to any outdoor area were prevented in these poultry holdings, what would be the number of such poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 7.21 | 29.38 | 110.54 | 5,000 | Formal EKE |
| Prob(PHb3 inf) | Probability for a holding to get the infection given the presence of 100 HPAI‐infected wild bird if in addition routine (daily average practiced) biosecurity measures were applied (disinfection of boots, changing clothes, washing hands) | Consider the scenario described in Q23 and take the median number of poultry holdings you estimated as a starting point for this question. If access by poultry (including waterfowl) to the water bodies AND access of poultry to any outdoor area were prevented AND routine (daily average practiced, e.g. on a layer/fattening holding) biosecurity measures were applied (e.g. disinfection of boots, changing clothes, washing hands, standard filtering provisions) in these poultry holdings, what would be the number of such poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 2.39 | 7.75 | 28.21 | 5,000 | Formal EKE |
| Prob(PHb4 inf) | Probability for a holding to get the infection given the presence of 100 HPAI‐infected wild bird if in addition high biosecurity measures were applied (as practised in nucleus or breeding herds) (rigorous implementation of showering in, complete exclusion of wild birds to feed, bedding and animal by‐products, separation of houses) | Consider the scenario described in Q23 and take the median number of poultry holdings you estimated as a starting point for this question. If access by poultry (including waterfowl) to the water bodies AND access of poultry to any outdoor area were prevented AND high biosecurity measures were applied (as practised in nucleus or breeding holding) (e.g. rigorous implementation of showering in; complete exclusion of wild birds to feed, bedding and animal by‐products; separation of houses) in these poultry holdings, what would be the number of such poultry holdings that will get infected in one day | 2.3.4.4 | 0 | 0.0026 | 0.6694 | 6.6169 | 5,000 | Formal EKE |
| ProbInf_NWB|contact | Probability that a susceptible non‐water bird becomes infected given a contact with excretions containing infectious virus in a forage area | Think of a standard population of 10,000 non‐water birds (see definition above). Indicate the number of these birds that might become infected, provided they get in contact with excretions containing infectious virus in a foraging area (see definition above) in a standard day (number out of 10,000). Think of the various groups of birds separately (i.e. raptors, songbirds, waders, gulls–storks–cranes) and then sum them up | 2.3.4.4 | 0 | 0.0001 | 0.000325 | 0.00065 | 0.0125 | Semi‐formal EKE |
| ProbInf_WB|contact | Probability that a susceptible water bird becomes infected given a contact with excretions containing infectious virus in a forage area | Think of a population of 10,000 water birds. Indicate the number of these birds that might become infected, provided they get in contact with excretions containing infectious virus in a foraging area (see definition above) in a standard day (number out of 10,000) | 2.3.4.4 | 0.000025 | 0.00055 | 0.002125 | 0.004 | 0.025 | Semi‐formal EKE |
| ShedNWB | Duration in days of the shedding period in non‐water birds | Total number of days a HPAI‐infected non‐water bird will shed the infectious virus | 2.3.4.4 | 0 | 2.7 | 5 | 7.6 | 14 | Semi‐formal EKE |
| ShedWB | Duration in days of the shedding period in water birds | Total number of days a HPAI‐infected water bird will shed infectious virus | 2.3.4.4 | 0 | 3 | 5 | 7 | 14 | Semi‐formal EKE |
| WBin | Number of water birds landing into a holding | Estimate the average number, over 2016–2017 fall–winter migratory season (5 months duration), of water birds (migratory and/or residential) that will land on any holding surface of a worst‐case poultry holding | 2.3.4.4 | 0.1 | 5.5 | 50 | 300 | 1,000 | Formal EKE |
Table D.16.
Regression coefficient estimates for probability of contact with infected excretion for each bird‐to excretion contact type
| Route of infection | Uncertainty distribution centile probability | Correlation between empirical and predicted | Beta estimate |
|---|---|---|---|
| From NWB to WB | 0.01 | 0.9999944 | 7.53E‐05 |
| 0.25 | 0.9998164 | 5.41E‐04 | |
| 0.5 | 0.999827 | 4.28E‐03 | |
| 0.75 | 0.9939367 | 8.89E‐03 | |
| 0.99 | 0.9963089 | 4.84E‐02 | |
| From WB to WB | 0.01 | 0.999985 | 1.13E‐04 |
| 0.25 | 0.9996709 | 1.37E‐03 | |
| 0.5 | 0.9976682 | 1.08E‐02 | |
| 0.75 | 0.9546119 | 1.69E‐02 | |
| 0.99 | 0.9802862 | 1.09E‐01 | |
| From NWB to NWB | 0.01 | 0.9998891 | 1.51E‐04 |
| 0.25 | 0.999852 | 1.56E‐03 | |
| 0.5 | 0.9913423 | 8.32E‐03 | |
| 0.75 | 0.9606092 | 1.11E‐02 | |
| 0.99 | 0.9981449 | 1.26E‐01 | |
| From WB to NWB | 0.01 | 0.979903 | 1.03E‐04 |
| 0.25 | 0.9985441 | 1.06E‐03 | |
| 0.5 | 0.9991636 | 9.30E‐03 | |
| 0.75 | 0.9637193 | 1.20E‐02 | |
| 0.99 | 0.9939418 | 1.92E‐01 |
Table D.17.
Regression coefficient estimates for probability of wild bird to poultry holding contact
| Bird‐to‐bird contact type | Uncertainty distribution centile probability | Correlation between empirical and predicted | Beta estimate |
|---|---|---|---|
| From wild bird to poultry holding | 0.25 | 0.9998668 | 4.335122e‐05 |
| 0.5 | 0.9998462 | 1.857073e‐04 | |
| 0.75 | 0.9998513 | 8.960164e‐04 |
D.3. Estimates for the LPAI model
Estimates from the HPAI model were used also in the LPAI model for the following variables: Prob(contactNWB,NWB), Prob(contactNWB,WB), Prob(contactWB,NWB), Prob(contactWB,WB), , (Tabe D.18).
Table D.18.
Estimates for LPAI provided in the EKEs by working group members or event participants
| Variable | Description | LB | Q1 | Median | Q3 | UB | Estimate done by | |
|---|---|---|---|---|---|---|---|---|
| MSLength | Length (in days) of AIV transmission period considered in the LPAI model | Fixed to 125 days (same as for HPAI) | Arbitrary | |||||
| 1/rNWB | Reciprocal of the duration in days of the LPAI shedding period in non‐water birds | 1 | 3 | 4 | 6 | 14 | Semi‐formal EKE | |
| 1/rWB | Reciprocal of the duration in days of the LPAI shedding period in water birds | 2 | 4 | 6 | 8 | 21 | Semi‐formal EKE | |
| mdNWB | Mortality rate in non‐water birds due to LPAI disease | 0.0025 | 0.0025 | 0.0025 | 0.0025 | 0.0025 | Literature | |
| mdWB | Mortality rate in water birds due to LPAI disease | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | Literature | |
| Prob(InfNWB|contact) | Probability that a susceptible non‐water bird becomes infected with LPAI given a contact with excretions containing infectious virus in a forage area | 0 | 0.0001 | 0.000325 | 0.00065 | 0.0125 | Semi‐formal EKE | |
| Prob(InfWB|contact) | Probability that a susceptible water bird becomes infected with LPAI given a contact with excretions containing infectious virus in a forage area | 0.000025 | 0.00055 | 0.002125 | 0.004 | 0.025 | Semi‐formal EKE | |
|
|
Number of non‐water birds landing into a holding | Estimate from HPAI was used | ||||||
|
|
Number of water birds landing into a holding | Estimate from HPAI was used | ||||||
|
|
Reduction coefficient for the probability that a susceptible water bird becomes infected given a contact with excretions containing LPAI infectious virus with respect to HPAI‐infected excretion | 0.01 | 0.03 | 0.05 | 0.1 | 0.5 | Formal EKE | |
|
|
Reduction coefficient for the probability for a worst‐case holding to get the infection given the presence of LPAI‐infected wild bird with respect to HPAI‐infected wild bird | 0 | 0.08 | 0.1 | 0.3 | 1 | Formal EKE | |
D.4. Estimates for biosecurity impact in the HPAI and LPAI model
The main output of the EKE considered is the probability of a poultry holding to get infected due to exposure of the holding to 100 H5N8 infected wild birds when implementing stepwise biosecurity measures.
Distributions are obtained from the formal EKE procedure, in particular, the experts had been asked to estimate the number of poultry holdings that will get infected in 1 day, given exposure of the holding to 100 H5N8 infected wild birds and given a specific level of biosecurity. The range estimated by the expert was taken fixed for all the four biosecurity steps considered.
For each scenario considered, the linear pool distribution was obtained averaging with equal weight all the best fitting expert's distributions. Then, the median and the quantiles of the fitted distribution were calculated and used, together with range, in order to fit a theoretical distribution. The median and quantiles calculation implies an approximation of the linear pool distribution: the goodness of the approximation can be measured with the value of the area under the curve, which should be theoretically equals to 1. The goodness of the approximation decreases when the range is much bigger than the interquartile range, indeed the area under the curve moves from 0.9636 (related to worst case of biosecurity) to 0.7544 (related to the highest level of biosecurity). In Table D.19, the fitted distribution together with the estimated parameters are reported.
Table D.19.
Characteristics for the elicited distributions
| Biosecurity level | Description of biosecurity level | Distribution | λ | κ |
|---|---|---|---|---|
| 1 | Effect preventing access of domestic poultry to water bodies | Weibull | 0.64 | 112.33 |
| 2 | Effect indoor housing | Weibull | 0.57 | 60.01 |
| 3 | Effect implementation routine biosecurity | Weibull | 0.62 | 15.74 |
| 4 | Effect implementation high biosecurity | Weibull | 0.2 | 2.12 |
Appendix E – Model outputs
E.1. HPAI clade 2.3.4.4
E.1.1. Entry assessment
Figure E.1.
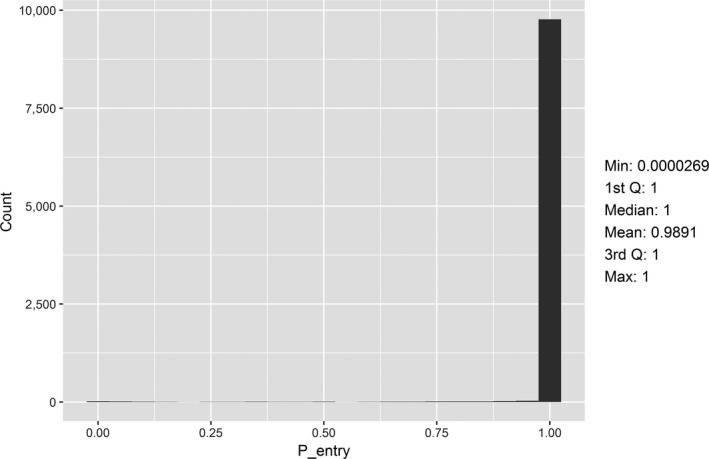
Probability of clade 2.3.4.4 HPAIV introduction into the EU via migratory wild birds
E.1.2. Amplification in the wild bird reservoir
E.1.3. Introduction into poultry holding
E.2. HPAI clades 2.2.1.2 and 2.3.2.1c
E.2.1. Introduction into poultry holding
Figure E.6.
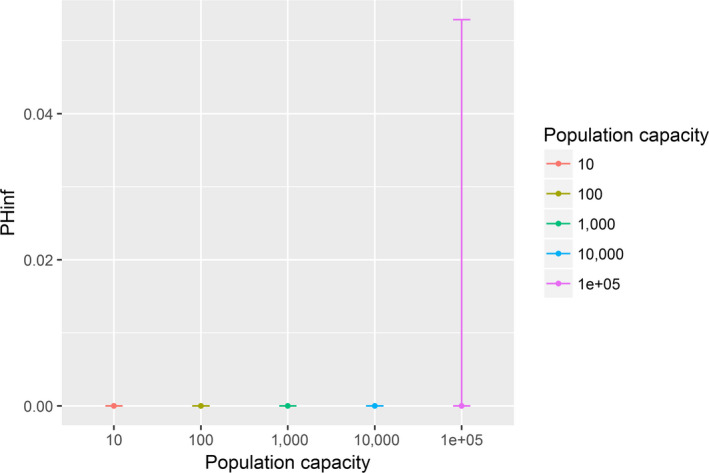
Probability of a poultry holding without implementing biosecurity becoming infected with HPAIV clade 2.2.1.2 via wild birds over the entire migratory season, when this holding is located in an area where 10–105 wild birds are present (consisting of 90% migratory birds, 90% water birds; scenario 1)
Figure E.7.
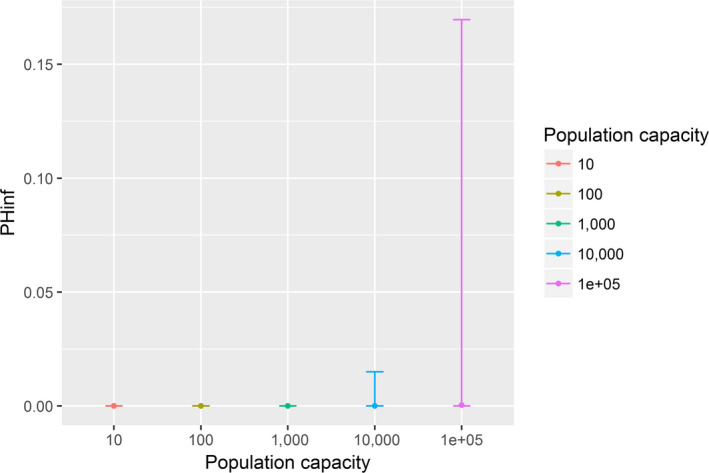
Probability of a poultry holding without implementing biosecurity becoming infected with HPAIV clade 2.3.2.1c via wild birds over the entire migratory season, when this holding is located in an area where 10–105 wild birds are present (consisting of 90% migratory birds, 90% water birds; scenario 1)
E.3. LPAI
E.3.1. Amplification in the wild bird reservoir
Figure E.8.
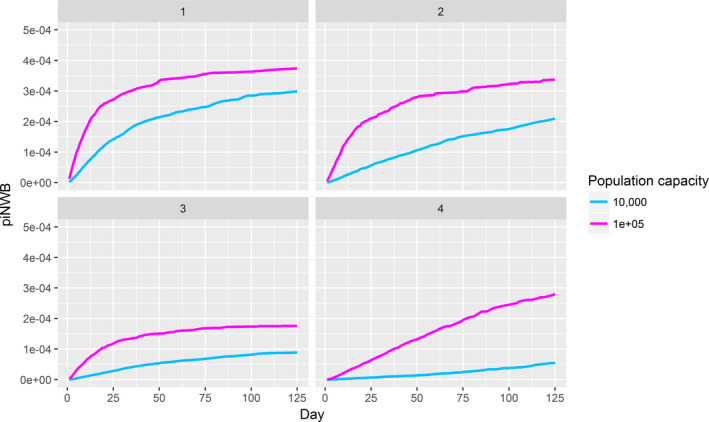
Modelled LPAI 95th percentile prevalence in wild non‐water birds. Different population capacities (10,000 and 100,000) and scenarios (1–4, see definition in Table 7 in Section 3.3.2) are presented
E.3.2. Introduction into poultry holding
Figure E.10.
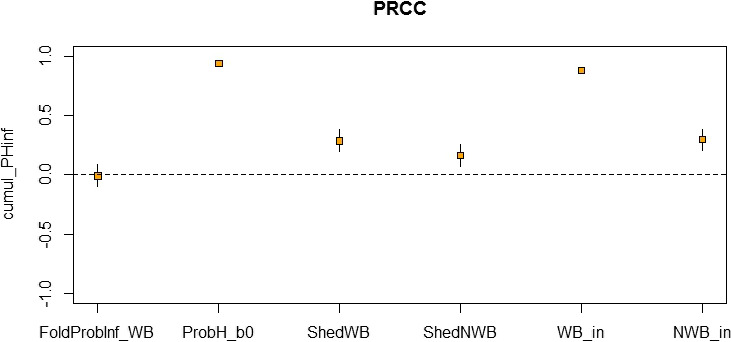
Sensitivity analysis on the seasonal probability that a poultry holding without biosecurity becomes LPAI infected after entry of infected wild birds. A wild bird population capacity of 100,000 and a prevalence of infected entering water birds of 0.6% birds are considered
Appendix F – AI introduction non‐wild bird pathways
F.1. Definitions
The parameters that have been identified as relevant to qualitatively assess the risk of introduction of AI via non‐wild bird pathways are here listed together with the relevant definitions.
Import volume: amount of the commodity that is traded. Analyses are carried out separately for imports from third countries and intra‐EU movements.
For the current assessment, the trade volumes have been extracted from open access databases, for 2014, 2015 and 2016 to look for the pattern in annual levels. These are presented in the sections below. It should be noted that the main database for this, UN Comtrade (the United Nations International Trade Statistics Database), will not be exact volumes as these reports are based on Combined Nomenclature (CN) codes (Council Regulation (EEC) No 2658/8762). Nevertheless, it gives a good approximation to the countries involved in trade and relative volumes. Section F.21 of this Appendix provides further information on the different trade pathways and the third countries which are approved for trade to the EU.
Probability of testing (according to EU legislation): likelihood that the commodity will be tested for AIV presence during transport considering the procedures described in the current EU legislation.
Tests that can be used (according to EU legislation): list of methods that can be used according to the Avian Influenza Diagnostic Manual (2006/437/EC) to test AIV presence in the given commodity.
Probability of virus detection (given AIV presence in commodity): likelihood that AIV will be detected in the commodity when AIV is present, using the listed tests.
Probability of virus preservation during transport: likelihood that AIV infectivity is maintained after transport/quarantine measures of a commodity that is considered contaminated or infected.
Probability of poultry exposure to the commodity: likelihood that poultry is in contact with the commodity that is considered contaminated or infected (generally, this refers to commercial poultry being fed on commercial feed).
Probability of AIV introduction into a commercial poultry holding via the commodity (small scale–large scale): likelihood that a holding of kept birds (poultry or zoo) after contact with a commodity considered infected becomes AIV infected. This parameter represents the overall result from the assessment of the previous parameters.
Pathways and commodities:
The 10 pathways and the corresponding commodities other than wild birds that have been taken into consideration in Section 3.4 as possible means of AIV introduction into a commercial poultry holding are:
Live birds: captive birds such as Passeriformes, birds of prey, Psittacines, peacocks, swans, wild ducks, wild geese, snipe, woodcocks, grouse, birds as pets, racing pigeons (not for consumption) and zoo birds (i.e. originating in an approved body, institute or centre)
Live poultry: breeding, production birds, hatching eggs and day‐old chicks of all poultry types (gallinaceous poultry, Anseriformes, ratites, gamebirds)
Meat and eggs for human consumption: meat, table eggs
Semen
Feathers, skin and down: raw feathers (with some treatment), treated skin feathers and down
Feed
Bedding
Manure
Pharmaceuticals (vaccines)
Other animal by‐products (bones, feet, casings, pet food (not livestock feed)).
Definition of ‘commodity’: the means by which there is potential to introduce AIV into a commercial poultry holding
For each commodity, the different parameters identified as relevant to qualitatively assess the risk of introduction of AI via non‐wild bird pathways have been evaluated and assessed for both HPAI and LPAI in two scenarios: (i) Third country trade and (ii) Intra‐EU movements, with a description of the entry and exposure to poultry pathways, given the EU rules and requirements for trade.
Illegal introduction is briefly described in the text but is not included in the qualitative assessment due to a lack of data.
When there are no differences in the parameter assessments between HP and LP in the same scenario, the results of the assessment of the commodity refer to AI in general (HPAI+LPAI). If there are differences in the parameters (for example where testing for H5N1 HPAI only is required), then the two pathogenic strains are assessed separately.
The volume of trade is important to consider for the aggregated risk which can increase the risk level if there is a large volume. Certain commodities, such as meat, table eggs and hatching eggs are less important for the spread of LPAI because infection in the parent flock will not lead to infection in the commodity.
For many commodities, such as live poultry, captive birds, hatching eggs and day‐old chicks entering the EU from third countries, there are only a limited number of countries which are approved for trade. These countries are listed in Part1, Annex I of Commission Regulation (EC) No 798/2008, where certificate requirements are listed.
F.2. Psittaciformes (CN code 010632)
This commodity covers all psittacine species. It includes, e.g. parrots, lovebirds, macaws and parakeets. Birds must be bred in captivity and cannot be taken from the wild (Article 5 of Commission Regulation (EU) No 139/2013 has replaced (EC) No 318/200763). It does not include pet birds.
F.2.1. Background info
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and Psittaciformes (topic) for the period 2005–2015 (restricting to documents in English only). The abstracts of three publications returned from this search string were screened and the extracted information from the relevant papers is reported below. Also, the EMPRES‐i database was consulted for the period 1‐1‐2005 to 15‐6‐2015. Experts provided additional scientific evidence that was not retrieved by the performed searches.
Reported AI cases in Psittaciformes since 2005
The scarcity of publications on avian influenza in Psittaciformes in the last 10 years indicates that these birds are rarely reported to be infected. Consultation of the EMPRES‐i database and screening of the scientific literature identified only few reports. Hawkins et al. (2006) reported the isolation of a LPAI H5N2 virus from a 3‐month‐old red‐lored Amazon parrot (Amazona autumnalis autumnalis) suffering from severe lethargy and probably illegally imported into the US (Hawkins et al., 2006).
Kaleta et al. (2007) has published a review on AIVs in birds of the order Psittaciformes, providing an overview table on natural infections of AIVs in psittacine birds that were found dead upon arrival in importing countries, in quarantine stations or in pet shops (period 1973–2006) (Kaleta et al., 2007).
In the UK in 2006, an African Grey parrot was tested positive for H5N1 HPAI while in quarantine. In 2013, a mixed consignment of psittacines and Passeriformes, were illegally introduced into Austria and stopped at Vienna Airport. Of the ~ 100 birds, 60 were alive, and of the dead birds, 4 tested positive for H5N1 HPAI (Bundesministerium für Gesundheit, 2013).
Clinical signs of AI infections in Psittaciformes
Intranasal infection of chickens, ducks and turkeys with 0.2 mL of 106 EID50 of the LPAI H5N2 virus isolated from the red‐lored Amazon parrot (see above) did not cause any clinical signs, although it could replicate to high titres in these birds and efficiently transmit to contact control cage mates (Pillai et al., 2008). Parakeets were also shown to be susceptible to intranasal inoculation of LPAI H7N9 virus (Jones et al., 2014). Virus was shed via the oropharyngeal route for 6 days but the birds remained free of disease signs. Parakeets intranasally infected with 106 EID50 of a H9N2 strain showed sporadic clinical signs (lethargy, hunched posture, laboured breathing) and shed virus oropharyngeally for 6 days, although in quantities 2–3 logs lower than chickens (Lenny et al., 2015).
Experimental infection of budgerigars (Melopsittacus undulatus) via the intranasal route with H5N1 HPAIV showed similar pathogenicity compared to chickens and quails (Isoda et al., 2006). Two out of three strains induced severe nervous disorders in some animals and mortality in all birds by 5 days post‐inoculation. The third H5N1 strain used in this study was probably not able to infect any budgerigar given the absence of seroconversion 14 days post‐inoculation.
International trade and intra‐EU transport
The OIE Terrestrial Code chapter 10.4 (OIE, 2010) describes the recommendations for importation of live birds other than poultry. In essence, regardless of the avian influenza status of the country of origin, an international veterinary certificate is required, attesting: (i) that the birds showed no clinical sign of avian influenza infection at the day of shipment, (ii) that the birds were kept in isolation since they were hatched or for at least 21 days prior to shipment and showed no clinical signs of avian influenza, (iii) that a statistically valid sample of the bird consignment was tested to demonstrate freedom from avian influenza infection and (iv) that the birds are transported in new or appropriately sanitised containers.
EU rules (Commission Decision 2007/25/EC) are in place for the movement of pet birds (i.e accompanied by their owner). Birds which are destined for special breeding programmes or for Approved Bodies, Institutes or Centres or from registered bodies (Council Directive 92/65/EEC) into and between Member States. There is a gap in the requirements for pet birds, in that the veterinary certificate which covers the country of origin, the quarantine or testing requirements and an owner attestation is required is only required for third country trade, and the country of origin can be one of any on the OIE list. For intra‐community trade, there is no such requirement for a veterinary certificate. Therefore, there is a risk that such birds, if they have had contact with infected wild birds or poultry while in the country of origin, could potentially be incubating avian influenza viruses. However, these species are not generally considered to play a major role in transmission of such viruses to other birds and particularly not poultry.
For other captive birds, Regulation (EC) No 139/2013 applies for non‐poultry birds. There is some debate over the definition of certain ‘poultry’ species which are not specifically used for production of birds destined for the food chain. Therefore, it is possible some poultry type birds are being moved under incorrect certification. Nevertheless, such birds must originate in an approved premises and be destined for an approved quarantine unit, accompanied by a health certificate and having already undergone testing for avian influenza virus with negative results. Birds are placed in quarantine for 30 days on arrival.
Reported AI introductions linked to import of Psittaciformes
There have been no reported or known introductions of avian influenza to EU poultry flocks through the import of such birds. It is unlikely they would be in contact with poultry, and EU quarantine rules assure the birds, in quarantine, would either not survive an HPAIV infection if they are susceptible, or that testing would identify positive birds. In conclusion, it is considered extremely unlikely that psittacine birds actively shedding infectious AIV would leave an EU quarantine unit. Thus, the risk that AI outbreaks in EU poultry flocks ensue via this pathway is considered negligible.
F.2.2. Third country trade – AI
Import volume: 600–1,000 Psittaciformes; based on average annual trade over 3 years. Birds should originate in registered or approved breeding establishments (see Directive 2009/158/EC). CITES regulations may apply as well. Countries approved for the imports of such birds are those listed in Part 1 of Annex I of Regulation (EC) No 798/2008. Australia, parts of Brazil, Canada, Chile, Israel, New Zealand, Tunisia, USA as well as, Argentina and part of the Philippines (listed in Annex I to Regulation (EC) No 139/2013) are authorised for imports, provided the authorities have approved captive bird breeding establishments. Probability of testing (according to EU legislation): All birds are subject to approved virus detection tests with negative results 7–14 days prior to the shipment. On arrival, birds are transported directly to an approved quarantine facility where they remain for 30 days (Commission Implementing Regulation (EC) No 139/2013). During quarantine, and without use of sentinel birds, imported birds must be examined virologically (serological testing not being appropriate). Tracheal/oropharyngeal and/or cloacal swabs (or faeces) must be taken from at least 60 birds or from all birds if the consignment is less than 60 birds, during the first 7 to 15 days of the quarantine. Therefore, the probability of testing is non‐negligible.
Tests that can be used (according to EU legislation): Any AI test in accordance to diagnostic manual can be used; all virological testing of samples taken during quarantine must be carried out in official laboratories designated by the competent authority using diagnostic procedures in accordance with the diagnostic manual for avian influenza. For virological examination, pooling of samples up to a maximum of five samples of individual birds in one pool is allowed. Faecal material must be pooled separately from other organ and tissue samples.
Probability of virus detection (given AIV presence in commodity): The random sampling size is chosen to guarantee detection of an infection at a prevalence of 5% with 95% confidence. Any clinical signs would be reported to the Competent Authorities and prompt further individual sampling. It is considered non‐negligible that virus will be detected, given the testing requirements.
Probability of virus preservation during transport: Thirty days in quarantine means even if the birds had been infected in the period between testing in place of origin and time of shipment, enough time should have elapsed to cover the incubation period (21 days according to the OIE and Commission Regulation (EC) No 94/200564) and birds would either be detected as ‘infected’ or would have recovered, eliminated the virus and be seropositive. Therefore this is considered extreme unlikely.
Probability of poultry exposure to the commodity: Direct contact with commercial poultry is considered extreme unlikely. Indirect contact with poultry type birds (e.g. in zoos or backyard holdings) considered unlikely.
Probability of AIV introduction into a poultry holding via the commodity: Extreme unlikely.
F.2.3. Intra‐EU movements‐ AI
Trade volume: Between 150,000 and 200,000 individual animals per year. Birds must be certified as originating in a holding where no signs or reports of AIV in the previous 30 days have occurred. Holdings must be registered or approved. This does not include any birds travelling as pets with their owners.
Probability of testing (according to EU legislation): No testing required, therefore this parameter is very unlikely.
Tests that can be used (according to EU legislation): Not applicable (NA).
Probability of virus detection (given AIV presence in commodity): NA.
Probability of virus preservation during transport: if birds were infected and if they were clinically well at the time of the transport, the birds could still be infective at the time of movement into a MS given the short transport times. Therefore, under certain circumstances this parameter can be scored as non‐negligible.
Probability of poultry exposure to the commodity: Direct contact to commercial poultry is considered extreme unlikely. Indirect contact to poultry, for example on backyard premises is considered unlikely. Contact with other kept birds: non‐negligible.
Probability AIV introduction into a poultry holding via the commodity: there are no reports of EU origin psittacines from registered or approved breeding facilities becoming infected with AIV, but given the uncertainty and the lack of testing requirements, this is considered very unlikely.
F.3. Birds of prey (CN code 0101631)
This commodity covers all birds of prey species. CITES regulations may apply as well. Birds must be bred in captivity and cannot be taken from the wild.
F.3.1. Background info
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and prey or falcon or owl or buzzard or hawk or harrier (topic) for the period 2005–2015 (restricting to documents in English only). The abstracts of 21 publications were screened and experts provided additional scientific evidence that was not retrieved by the performed searches. The extracted information from the relevant papers is reported below and in Table F.1.
Ducatez et al. (2007) identified 48 dead or sick vultures in Burkina Faso of which at least 17 were confirmed to be HPAI positive. HPAI H5N1 viruses were isolated from vultures and affected poultry farms in the same region and were phylogenetically similar. The authors mention that virus transmission from poultry to vultures could have happened. The feeding behaviour of vultures could also be involved in transmission within domestic poultry and/or transmission between poultry holdings, although clear supporting scientific evidence is lacking.
Khan et al. (2009) reported an outbreak of HPAI H5N1 in Houbara bustards, which were introduced into the Kingdom of Saudi Arabia for hunting purposes by falcons. Thirty‐eight out of 41 bustards died as well as 10 out of 16 falcons that came in contact with these birds. However, molecular analysis indicated that there were two different HPAI H5N1 viruses cocirculating in the falconry and poultry sectors in Saudi Arabia (Monne et al., 2008); therefore, there was no clear link between the birds of prey and poultry outbreaks.
Shivakoti et al. (2010) found an emaciated mountain hawk‐eagle in Japan from which they isolated HPAI H5N1. Nine days later, on HPAI outbreak in poultry farms started. However, there are no clear links between the infected hawk‐eagle and the poultry farms.
Reid et al. (2011) reported HPAI H5N1 isolated from a common buzzard in Bulgaria (29 March 2010), with 99.9% similarity with the HA gene sequence of HPAI H5N1 (clade 2.3.2) isolates from an outbreak in backyard chicken flocks in Romania (13–29 March 2010).
Kohls et al. (2011) found AIV‐RNA of subtypes H6, H9 or H13 in swabs of gulls (4.1%) and ducks (3.8%) hunted by falconry birds such as Gyrfalcon and peregrine falcon during two successive hunting seasons (2006–2008), whereas all 54 falconry birds were negative.
Bertran et al. (2012) successfully infected gyr‐saker hybrid falcons with HPAI H5N1 or LPAI H7N2, via the nasal choanal route or via ingestion of previously infected chicks.
van den Brand et al. (2015) detected H5N1‐infected common buzzards (12/385) and peregrine falcons (2/6) during an H5N1 outbreak in wild water birds in Germany (2006), whereas seven other species were negative: Eurasian sparrow hawk (111), common kestrel (38), undetermined species of buzzard (36), white‐tailed sea eagle (19), undetermined species of raptor (12), northern goshawk (10), red kite (3), rough‐legged buzzard (3) and western marsh‐harrier (1). The results suggested that H5N1 outbreaks in wild water birds are more likely to lead to exposure to and mortality from H5N1 in raptors such as common buzzards and peregrine falcons that hunt or scavenge medium‐sized birds, than in raptors that hunt small birds and do not scavenge such as Eurasian sparrow hawks and common kestrels. The authors hypothesised that the raptors became infected by feeding on H5N1‐contaminated carcasses, as reported for gulls (Brown et al., 2006).
Naguib et al. (2015) reported an outbreak of HPAI H5N1 clade 2.3.2.1c in hunting falcons in Dubai.
Ip et al. (2015) reported presence of HPAIV H5N8 and reassortants thereof in wild bird species, including birds of prey as well as in hunting falcons, in the U.S. in 2014.
During the H5N8 outbreak in 2015/6 in the EU also several cases of a large avian predator species, White‐tailed eagle, have been reported.
Clinical signs of AI infections in birds of prey
International trade and intra‐EU transport regarding birds of prey
EU rules are in place for the movement of pet birds (i.e. accompanied by their owner) (Commission Decision 2007/25/EC) or birds which are destined for special breeding programmes or for Approved Bodies (Council Directive 92/65/EEC) into the Union. There is a gap in the requirements for pet birds, in that the veterinary certificate which covers the country of origin, the quarantine or testing requirements and an owner attestation is only required for third country trade, and the country of origin can be one of any on the OIE list. For intra‐community trade, there is no such requirement for a veterinary certificate. Therefore, there is a risk that such birds, if they have had contact with infected wild birds or poultry while in the country of origin or if fed on contaminated poultry chicks, could potentially be incubating avian influenza viruses; however, these species are not generally considered to play a major role in transmission of such viruses to poultry, because the birds of prey would be hunting and not having prolonged contact with live birds. This differs, of course, when such birds are destined to join a zoological collection or falconry.
For other captive birds, Regulation (EU) No 139/2013 applies for imports from third countries of non‐poultry birds. Such birds must originate in an approved premises and be destined for an approved quarantine unit, accompanied by a health certificate and having already undergone testing for avian influenza virus with negative results. Birds are placed in quarantine for 30 days on arrival.
Reported AI introductions into poultry linked to import of birds of prey
There are no known reports of direct transmission of avian influenza viruses to poultry by imports of birds of prey. They are generally considered to be spill‐over hosts as a result of consuming infected or contaminated birds themselves. Birds of prey, that were tested positive for HPAI, have been implicated in a case of illegal introduction into the EU at Brussels airport in 2005 (Van Borm et al., 2005).
F.3.2. Third country trade – AI
Import volume: Fewer than 500 birds per annum.
Probability of testing (according to EU legislation): All birds are subject to approved virus detection tests with negative results 7–14 days prior to the shipment. On arrival, birds are transported directly to an approved quarantine facility where they remain for 30 days (Regulation (EU) No 139/2013). During quarantine, and without use of sentinel birds, imported birds must be examined virologically (serological testing not being appropriate). Tracheal/oropharyngeal and/or cloacal swabs (or faeces) must be taken from at least 60 birds or from all birds if the consignment is less than 60 birds, during the first 7–15 days of the quarantine. Therefore, the probability of testing is non‐negligible.
Tests that can be used (according to EU legislation): Any AI test in accordance to diagnostic manual can be used. All virological testing of samples taken during quarantine must be carried out in official laboratories designated by the competent authority using diagnostic procedures in accordance with the diagnostic manual for avian influenza. For virological examination, pooling of samples up to a maximum of five samples of individual birds in one pool is allowed. Faecal material must be pooled separately from other organ and tissue samples.
Probability of virus detection (given AIV presence in commodity): The random sampling size is chosen to guarantee detection of an infection at a prevalence of 5% with 95% confidence. Any clinical signs would be reported to the Competent Authorities and prompt further individual sampling. Virus detection is considered non‐negligible and allows for the small uncertainty that other birds are present in the quarantine facility and in contact with infected birds and are not tested or show no clinical signs.
Probability of virus preservation during transport: Given the requirements for quarantine, this level is considered to be extreme unlikely.
Probability of poultry exposure to the commodity: Direct contact to commercial poultry extreme unlikely. Indirect contact to poultry unlikely. Contact with other kept birds: non‐negligible. Captive birds should not be kept with commercial poultry in the EU. Poultry workers should not keep captive birds at home and therefore poultry exposure is considered extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: Given the import requirements for testing and quarantine and exposure to poultry overall this is considered extreme unlikely.
F.3.3. Intra‐EU movements – AI
Import volume: Around 500–1,500 birds per annum. This does not include any birds travelling as pets with their owners.
Probability of testing (according to EU legislation): Very unlikely as there are no requirements
Tests that can be used (according to EU legislation): NA
Probability of virus detection (given AIV presence in commodity): NA
Probability of virus preservation during transport: Non‐negligible
Probability of poultry exposure to the commodity: Extreme unlikely
Probability of AIV introduction into a poultry holding via the commodity: Very unlikely to account for the uncertainty around the lack of testing requirements.
F.4. Other birds (Traces code 010639)
This commodity covers bird consignments other than poultry, Psittaciformes, ratites or birds of prey ((but including for instance pigeons 01063910) and other birds, such as Passeriformes, peacocks, swans, wild ducks, wild geese, snipe, woodcocks, grouse, quails, pheasants and partridges). The commodity is transported without the presence of the owner.
F.4.1. Background information
A literature search has been carried out in Web of Science using the string ‘Avian’ (title) and (pigeon*) or (columb*), and ‘spread’ (topic) and ‘influenza’ (topic) from 2006 to onwards (without restrictions the documents for language). Forty‐one records resulted and after screening for relevance of the abstracts and full‐texts, nine articles were considered relevant as they contained test results of pigeons or Columbiformes. Table F.2 summarises the evidence reported on the role of pigeons in AIV, amplification and/or shedding in eight papers. The ninth paper, Hernandez‐Jover et al. (2015), is considered separately because it is a quantitative risk assessment, which estimates the probability of introduction of LPAIVs from wild birds into poultry exhibition flocks in Australia, and the subsequent spread to other poultry flocks using scenario trees and Monte Carlo stochastic simulation modelling (Hernandez‐Jover et al., 2015). The authors reported that ‘the median probability of LPAI spread through movement of birds in flocks keeping waterfowl and turkeys was estimated by the authors to be 0.28 (0.123–0.541) and 0.230 (0.104–0.421), respectively. A lower probability was estimated for chicken (0.087; 0.027–0.202) and pigeon (0.0003; 3.0 × 10−5 to 0.0008) flocks. The sensitivity analysis indicates that the prevalence of LPAI in wild waterfowl and the probability of contact of domestic birds with wild waterfowl are the most influential parameters on the probability of exposure, while the probability of spread is mostly influenced by the probability of movement of birds and the probability of the exhibitor detecting and reporting LPAI.’
Natural infections of Passeriformes are interpreted as a consequence of spill‐over infection from infected poultry holdings (Han et al., 2012; Slusher et al., 2014); there are no reports of self‐sustained transmission chains in Passeriformes temporally and geographically independent of the presence of HPAIV in poultry holdings. Special cases have involved small passerine species such as the muniahs in Buddhist animal release rites in Hong Kong and elsewhere in South‐East Asia (Gutiérrez et al., 2011). The role of some passerine species such as starlings, sparrows, and swallows acting as bridging hosts between water bird habitats and poultry holdings has been debated controversially (Forrest et al., 2010; Caron et al., 2015). Although some of these species were found to be susceptible in experimental infection (e.g. Fujimoto et al., 2015), there are no reports that actually link naturally infected passerines with incursions of HPAIV into poultry holdings. In this respect, members of the Corvidae family comprising crows, jays and magpies should be mentioned here as they are also passerine birds and have more frequently been found infected with HPAIV probably as a consequence of scavenging on carcasses of other infected birds (e.g. Khan et al., 2014). It is an acknowledged data gap for the role of bridging species in causing incursion of AI into poultry holdings.
F.4.2. Third country trade – AI
Import volume: ~ 600–1,000 per annum birds other than poultry, Psittaciformes or birds of prey.
Probability of testing (according to EU legislation): non‐negligible, as this is required for third country imports.
Tests that can be used (according to EU legislation): any AI test in accordance to OIE diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.4.3. Intra‐EU movements – AI
Import volume: 20,000–25,000 per annum of birds other than poultry, Psittaciformes or birds of prey. The different species cannot be differentiated using the Comtrade database.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): NA.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely which accounts for the lack of testing requirements.
F.5. Pet birds (consignment with presence of owner)
This commodity covers bird consignments other than poultry, Psittaciformes or birds of prey (but including for instance pigeons and Passeriformes), that are transported with the presence of the owner (no more than five birds per consignment). It is regulated by Commission Decision 2007/25/EC.
For intra‐EU movements of pet birds, there are no rules, except for the number of birds which can travel with each person. For third country imports into the EU, a veterinary certificate and owner declaration are required, showing the country of origin and the period of quarantine or results of testing for H5N1 by PCR or serology.. The two pathogenic strains are separated for the purpose of this category because of different requirements for third country origin pets to have a (negative) test certificate for H5N1.
F.5.1. Background information
The animal species included in this commodity are the same already described in the background information of previous sections but the use of these animals is different. As pet birds, they would be going directly to the owner's household and would be extremely unlikely to be exposed to commercial poultry.
F.5.2. Third country trade – HPAI
Import volume: no requirement to record as trade statistics – information may be available from national statistics but given the parameter values it was not considered necessary to collect these data.
Probability of testing (according to EU legislation): non‐negligible for H5N1.
Tests that can be used (according to EU legislation): either PCR or serology, in accordance to diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible only for H5N1.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.5.3. Intra‐EU movements – HPAI
Import volume: no requirement to record as Trade statistics – information may be available from national statistics but given the parameter values it was not considered necessary to collect these data.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very likely (other virus else than H5N1).
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.5.4. Third country trade – LPAI
Import volume: no requirement to record Trade statistics.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely which accounts for the lack of testing required.
F.5.5. Intra‐EU trade – LPAI
Import volume: no requirement to record Trade statistics.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): n/a.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely, as only self‐certification as disease free is required for intra‐EU movements MSs.
F.6. Hatching eggs – Gallus gallus (Traces code 040711)
This commodity covers eggs for incubation, laid by Gallus gallus.
F.6.1. Background information (for Gallus gallus and other poultry species)
The information here reported considers also hatching eggs laid by other poultry species (described in the following Section F.7).
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and hatching (topic) for the period 2005–2015 (restricting to documents in English only). The abstract of 35 publications were screened and it was noticed that most were describing in‐ovo vaccination studies. The full text of a few relevant publications was screened and the extracted information is reported below.
Vertical AI transmission and AI susceptibility of hatching poultry eggs
There is only limited proof of true vertical AI transmission. No AIVs were detected in the faecal samples of newly hatched ducklings in two duck holdings where avian influenza virus was circulating (Marinova‐Petkova et al., 2016). Most LPAIV and virtually all HPAIV infections are lethal to the embryo although the mean death times may differ (Cobb, 2011) and hatching of HPAI infected eggs has not been convincingly documented. This does not exclude the presence of LPAIV and HPAIV in the egg white and yolk or on the shell of embryonating eggs originating from infected layers. Scientific evidence comes from unpublished studies cited by Swayne and Beck (2004), suggesting the presence of HPAI H5N2 virus in 85% to 100% of eggs laid 3–4 days after experimental inoculation of poultry with HPAI virus. In addition, H5N2 HPAI virus has been recovered from the yolk and albumen of eggs from naturally infected chicken flocks (Cappucci et al., 1985) and from experimentally infected hens (Beard et al., 1984). Promkuntod et al. (2006) reported the detection of H5N1 in Japanese quail eggs. It is not clear whether a viraemic stage of infection is required to deposit AIV inside an egg or whether local infection of the oviduct would be sufficient as well. Most LPAI and HPAI viruses cause shell malformation, reduction or cessation, respectively, of egg production (e.g. Gonzales et al., 2012a,b), and/or hatching (e.g. Cai et al., 2011), further limiting the potential for vertical transmission of AI virus.
Newly hatched birds are more susceptible to infectious diseases than older birds because of an immature immune system. On the other hand, embryos and hatchling may be specifically protected by maternally derived AI‐specific antibodies (for instance, if they were derived from layers repeatedly vaccinated, (Abdelwhab et al., 2012)), although it seems that high maternal antibody‐titres are required to provide full clinical protection (De Vriese et al., 2010; Maas et al., 2011).
Cobb (2011) provides an overview on the spread of pathogens through trade in poultry hatching eggs. Reference is made to chapter 6.4 of the OIE Terrestrial Animal Health Code (OIE, 2009) describing hygiene and disease security procedures for poultry breeding flocks and hatcheries. Similar procedures are laid down in Council Directive 2009/158/EC Annex II.
International trade and intra‐EU transport
The OIE Terrestrial Code chapter 10.4 (OIE, 2010) describes the recommendations for importation of hatching eggs of poultry. In essence, the birds should have been derived from a flock and kept in an AI‐free area (at least 21 days prior to the collection of the eggs) and should be transported in new or appropriately sanitised containers.
Once an AI outbreak is suspected or confirmed within the EU, no poultry may enter or leave the affected holding or be transported within the protection zone without an authorisation from the competent authority, observing appropriate biosecurity measures such as to minimise any risk of the spread of avian influenza (Council Directive 2005/94/EC).
Legislation on imports of live poultry, day‐old chicks and hatching eggs, table eggs, egg products, and the meat of game birds and poultry is laid down in Commission Regulation (EC) No 798/2008. This includes a list of approved countries for which there are certificates to allow the import of such consignments. Some countries are regionalised, some have special additional measures. Hatching eggs must similarly have originated from breeding flocks free of notifiable AI and have been present in a hatchery for 6 weeks prior to import. In the case of hatching eggs, the live poultry hatched from such eggs must similarly be isolated for a period of 3 weeks. Testing is not required; as it is presumed that infected eggs will not hatch or that the infected chicks will not survive.
For movements within the EU for live poultry, day‐old chicks and hatching eggs, Directive 2009/158/EC applies.
Reported AI introductions linked to import of hatching poultry eggs
No reports were found. The import rules and expert scientific opinion on the hatchability of infected eggs suggest this route is unlikely, although birds are not tested once hatched.
Nevertheless, the possible fomite transfer during transport should not be discounted. Particularly if birds are less likely to show clinical signs and the birds are housed in a hatchery or rearing unit together with other birds.
F.6.2. Third country trade – AI
Import volume: 25–30 million eggs per year.
Probability of testing (according to EU legislation): extreme unlikely, but quarantine required for 30 days during which time the hatchery must record hatchability and report any suspicion of notifiable disease.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: extreme unlikely, given the quarantine requirements.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely, which accounts for the aggregated risk because of the volume of eggs which are transported and the potential for fomite contamination.
F.6.3. Intra‐EU movements – HPAI
Import volume: 1.1 billion eggs per year.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: very unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding/hatchery via the commodity: unlikely, which accounts for the volume and therefore the aggregated risk score. However, with the HPAI strains the required veterinary inspection should detect any clinical signs.
F.6.4. Intra‐EU movements – LPAI
Import volume: 1.1 billion eggs per year.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): unlikely.
Probability of virus preservation during transport: non‐negligible for fomite transfer.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding/hatchery via the commodity: even taking into account the fomite risk and the large volume of eggs, it was scored as unlikely because they will not be infected, although fomite contamination (egg shells, crates, etc.) cannot be discounted.
F.7. Hatching eggs – other (Traces code 040719)
This commodity covers eggs for incubation, laid by poultry species other than Gallus gallus.
F.7.1. Background information
See Section F.6.1.
F.7.2. Third country trade – AI
Import volume: 5 million of non‐gallinaceous poultry; 200,000–300,000 for both game birds and non‐poultry.
Probability of testing (according to EU legislation): extreme unlikely, but quarantine required for 30 days.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: extreme unlikely, due to quarantine requirements.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely which accounts for the aggregated score of a large volume of trade.
F.7.3. Intra‐EU movements – AI
Import volume: 120–170 million per annum of which 15 million non‐poultry hatching eggs and 50 million for game birds hatching eggs.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: very unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: unlikely, which accounts for the aggregated score of a large volume of trade.
F.8. Day‐old‐chickens (Traces code 010511 or 010512)
This commodity covers all Gallus gallus individuals less than 72 h old, not yet fed. It covers four commodity codes, depending on grandparent, parent, layers or other breeding stock.
F.8.1. Background information (for chickens and other poultry species)
The information reported in this section considers also individuals less than 72 h old of other poultry species.
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and ‘Day‐old poultry’ (topic) for the period 2005–2015 (restricting to documents in English only). The abstract of 61 publications were screened and it was noticed that most were describing studies vaccinating 1‐day‐old poultry. The full text of a few relevant publications was screened and the extracted information is reported below.
AI susceptibility day‐old poultry
In general, day‐old chickens are more susceptible to infectious diseases than older birds because of an immature immune system. For instance, in newly hatched birds, the activation, phagocytosis and bactericidal activities of heterophils and macrophages were shown to be age‐dependent in that they increase with age (Kodama et al., 1976; Wells et al., 1998; Kogut et al., 2002).
Yamamoto et al. (2007a,b) inoculated day‐old call ducks with HPAI H5N1 and reported neurological signs (drowsiness, ataxia and intermittent generalised seizure) and corneal opacity followed by mortality between 3 and 7 days post‐infection.
In the past, intracerebral injection of AI viruses in day‐old chicks was performed to determine the pathogenicity of the virus (e.g. Afzal et al., 2012). However, the intracerebral pathogenicity index is not recommended any longer by the OIE (2015).
International trade and intra‐EU transport
International trade and intra‐EU transport rules reported for Sections F.6 and F.7 apply also to this commodity.
Only a limited list of countries or regions is approved for the import into the EU of poultry (breeding, production, day‐old chicks (DOCs) and hatching eggs). DOCs must originate from a parent flock free of HPAI or vaccinated under a vaccination plan agreed by the European Commission; no such plans are currently agreed. The parent flock should have been present at premises free of HPAI for 6 weeks and there should have been no other HPAI outbreaks in the surrounding 10 km for 30 days. Once the consignments are imported into the EU, the Regulation requires that the birds be kept at the destination for a minimum of three weeks (not more than 2 months) with sampling and (negative) testing for AI, during which time there is also monitoring for clinical signs. For intra‐EU movements, day‐old chicks must originate from a hatchery where eggs come from flocks in an approved breeding establishment, have been held for 6 weeks and which are subject to a monthly inspection and 72–h inspection or 72 premovement veterinary examination.
Small consignments of fewer than 20 units are not subject to the same requirements, but must originate from flocks which have been present in the EU since hatching or for at least 3 months, and must show no clinical signs of disease.
Reported AI introductions linked to import of day‐old‐poultry
The import conditions, requiring isolation and testing on arrival means this is an unlikely route for introduction of AI into the EU poultry sector.
Nevertheless, the possible fomite transfer during transport should not be discounted, particularly if birds are less likely to show clinical signs and the birds are kept in a hatchery or rearing unit together with other birds. However, the quarantine requirements mean infected birds would show clinical signs while in quarantine and therefore will not be placed on a poultry farm.
F.8.2. Third country trade – AI
Import volume: 200,000–400,000 of birds per year.
Probability of testing (according to EU legislation): non‐negligible; quarantine required for 21 days and negative testing.
Tests that can be used (according to EU legislation): according to the diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.8.3. Intra‐EU movements – HPAI
Import volume: 500–700 million birds per year.
Probability of testing (according to EU legislation): extreme unlikely – not required by the legislation.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: very unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: unlikely to account for the aggregated risk score and high volume of trade and because with the HPAI strains the required veterinary inspection should detect any clinical signs.
F.8.4. Intra‐EU movement – LPAI
Import volume: 500–700 million birds per year.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: non‐negligible.
F.9. Day‐old‐poultry (ducks or geese)
This commodity covers all ducks or geese less than 72 h old, not yet fed. The import rules are the same as for day‐old chicks from gallinaceous poultry.
F.9.1. Background information
See Section F.8.1.
F.9.2. Third country trade – AI
Import volume: 50,000–200,000 birds per year.
Probability of testing (according to EU legislation): non‐negligible.
Tests that can be used (according to EU legislation): according to the diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.9.3. Intra‐EU movement – HPAI
Import volume: 50–60 million birds per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): very unlikely.
Probability of virus preservation during transport: very unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: unlikely.
F.9.4. Intra‐EU movement – LPAI
Import volume: 50–60 million birds per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: non‐negligible.
F.10. Poultry weighing more than 185 g/more than 72 h old – Gallus gallus (Traces code 010594)
This commodity covers all Gallus gallus individuals reared or kept in captivity for breeding or for production, which are more than 185 g in weight/more than 72 h old (therefore not considered day old chicks).
F.10.1. Background information (for Gallus gallus and other poultry species)
The information reported in this section considers also individuals reared or kept in captivity for breeding or production purposes.
The background information of this commodity is covered by the legislation (Council Directive 2009/158/EC) which requires live poultry to enter quarantine on arrival in the EU and be tested (negative) prior to leaving.
AI susceptibility of older poultry
Naïve birds from all poultry species are considered susceptible to AIV infection. At least for HPAIV H5N1‐infected ducks, several studies report an age‐dependent evolution in AI susceptibility with the presence of mortality and/or clinical signs in young animals and no/milder mortality and/or clinical signs in older birds (Pantin‐Jackwood and Swayne, 2007; Pantin‐Jackwood et al., 2007; Löndt et al., 2010). This can be related to a variety of factors including the virus strain and host‐specific factors such as immune competence (Kothlow and Kaspers, 2008). Reemers et al. (2010) inoculated 1‐ and 4‐week old chickens with H9N2 via the nasal route. The results suggest that the strength of virus‐induced host responses is affected by maturation of the (respiratory) immune system and may be a key factor in age‐dependent host responses to AI infection.
International trade and intra‐EU transport
International trade and intra‐EU transport rules reported for Sections F.6, F.7 and F.8 apply also to this commodity.
For live poultry (breeding or production), the birds must originate in a territory free of HPAI and not be vaccinated against HPAI, they must have been present at the premises for at least 3 months or since hatching, the premises must be free of HPAI and not within 10 km of an outbreak of HPAI in the past 30 days. Once the consignments of live poultry are imported into the EU, the Regulation requires that the birds be kept at the destination for a minimum of three weeks with sampling and (negative) testing for AI, during which time there is also monitoring for clinical signs.
Reported AI introductions linked to import of older poultry
There are no reported AIV introductions to EU poultry holdings through the imports of breeding poultry. The list of approved countries is limited and the birds must enter quarantine for 21 days prior to entering a poultry holding.
Nevertheless, the possible fomite transfer during transport should not be discounted, particularly if birds are less likely to show clinical signs and the birds are in hatchery or rearing unit with other birds.
F.10.2. Third country trade – AI
Import volume: 800,000–1 million birds per annum.
Probability of testing (according to EU legislation): non‐negligible.
Tests that can be used (according to EU legislation): any AI test in accordance to diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.10.3. Intra‐EU movements – HPAI
Import volume: 400–500 million birds per annum.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): birds should exhibit clinical signs.
Probability of virus preservation during transport: unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: unlikely because with the HPAI strains the required veterinary inspection should detect any clinical signs.
F.10.4. Intra‐EU movements – LPAI
Import volume: 400–500 million birds per annum.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): clinical signs are unlikely to be observed.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: non‐negligible.
F.11. Poultry weighing more than 185 g – not Gallus gallus (Traces code 010599 and 010633)
This commodity covers non‐Gallus gallus species reared or kept in captivity for breeding ((e.g. turkeys 01059930), guinea fowl (01059950), ducks (01059910), geese (01059920). Also, included are the ratites, under CN code 010633).
F.11.1. Background information
Same as for Section F.10.1.
The background information of this commodity is covered by the legislation (2009/158/EC), which requires live poultry to enter quarantine and be tested (negative) prior to leaving.
F.11.2. Third country trade – AI
Import volume: < 200 birds per annum.
Probability of testing (according to EU legislation): non‐negligible.
Tests that can be used (according to EU legislation): any AI test in accordance to diagnostic manual.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.11.3. Intra‐EU movements – HPAI
Import volume: 15–18 million birds per annum.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): birds should exhibit clinical signs
Probability of virus preservation during transport: unlikely.
Probability of poultry exposure to the commodity: non‐negligible
Probability of AIV introduction into a poultry holding via the commodity: unlikely because with the HPAI strains the required veterinary inspection should detect any clinical signs.
F.11.4. Intra‐EU movements – LPAI
Import volume: 15–18 million birds per annum.
Probability of testing (according to EU legislation): very unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): clinical signs are unlikely to be observed.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: non‐negligible.
F.12. Meat (Traces code 0207)
This commodity covers the raw meat of all poultry, fowls, turkeys, guinea fowls, ducks, geese, quails, pigeons, pheasants and partridges reared or kept in captivity for the production of meat. Only fresh and frozen meat is accounted for (cooked meat is not considered a relevant commodity for AIV transmission; therefore, it has not been assessed).
F.12.1. Background information
This section provides scientific evidence relevant to assess the possible spread of HPAI via meat of poultry. An analysis of the risk for a food‐borne infection to humans is beyond this opinion. Pensaert and Van Reeth (2010) did an analysis for the H1N1 virus and concluded that turkey meat infected with this virus is not a food‐borne threat. Harder et al. (2016) provided a review on the same topic related to HPAIV H5N1.
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and meat (topic) for the period 2005–2015. The abstract of 32 publications were screened and the full text of relevant publications was screened. The extracted information is reported below.
AI detection in meat of poultry
HPAI infections induce systemic infections in poultry and virus can be identified in many tissues. Many HPAI viruses are mainly dispersed in the neuronal and respiratory system, heart and pancreas although virus can sometimes also be detected in muscle tissue (Swayne and Beck, 2005; Antarasena et al., 2006; Toffan et al., 2008; Löndt et al., 2010; Pasick et al., 2010; Kapczynski et al., 2013). Even if muscle cells are not the primary permissive cell type, blood vessel endothelial cells in muscle tissue might harbour infectious virus.
A few papers were found describing the presence of infectious virus in meat after an experimental HPAI infection (see Table F.3). On the other hand, LPAI induces mainly localised respiratory and gastrointestinal infections (Mo et al., 1997; Alexander, 2000; Swayne and Beck, 2005) and virus is not to be expected to be disseminated systemically. Therefore, it is not surprising that no papers were identified on the presence of LPAI in meat. Nevertheless, remnants of lung and kidney tissues in frozen carcasses produced from LPAIV‐infected poultry may still harbour infectious virus. However, Zepeda and Salman (2007) performed a quantitative release assessment and reported that the probability of LPAI introduction through chicken meat imports is ‘insignificant’.
Table F.3.
Overview of identified publications reporting AI virus titres in meat of poultry
| Virus type | Species | Infection | Age | Virus titre in meat (log10 EID50/g) | Reference |
|---|---|---|---|---|---|
| HPAI H5N1 | Chicken | Experimental | 3–4 weeks | 7.3 | Swayne and Beck (2005) |
| HPAI H5N1 | Chicken | Experimental | 4 weeks | 7.5–8.0 | Thomas and Swayne (2007) |
| HPAI H5N1 | Chicken | Experimental | NS | 8.9 | Brown et al. (2008) |
| HPAI H5N1 | Chicken | Experimental | 3–4 weeks | 1.9–7.5 | Das et al. (2008) |
| HPAI H5N1 | Chicken | Experimental | NS | 9.0–9.2 and 10.4 (both per 30 g) | Bertran and Swayne (2014) |
| HPAI H5N2 | Chicken | Experimental | 3‐to‐4 weeks | 2.7–3.2 | Swayne and Beck (2005) |
| HPAI H7N3 | Chicken | Experimental | NS | 7.3–7.6 (per 30 g) | Swayne and Beck (2005) |
| HPAI H7N7 | Chicken | Experimental | NS | 7.5–7.8 (per 30 g) | Swayne and Beck (2005) |
NS: not specified.
AI tenacity in meat of poultry
Ejaz et al. (2007) infected 10‐weeks‐old broiler chickens intranasally with LPAI H9N2. After 10 days post‐inoculation, the animals were euthanised and their carcasses were cut into small pieces and frozen at −20°C. At a weekly basis, samples were thawed and presence of the virus was analysed through embryonated egg inoculation. Infectious virus was detected in bone marrow and legs (until 6 weeks post‐storage), neck and wings (until 4 weeks post‐storage), and breast (until 2 weeks post‐storage).
Nazir et al. (2011) spiked duck breast meat with H4N6, H5N1 and H6N8 LPAIVs and analysed the residual infectivity on cell culture at regular intervals for a maximum of 24 weeks. A linear regression model was used to analyse the data and to calculate the time required for 90% loss of virus infectivity (T90 values). Incubation at 20°C resulted in T90 values of 3, 2 and 3 days for H4N6, H5N1 and H6N8 viruses, respectively, whereas incubation at 0°C resulted in T90 values of 40, 54 and 81 days for H4N6, H5N1 and H6N8 viruses, respectively.
Beato et al. (2012) experimentally infected chickens, turkeys and ducks with HPAI H7N1 via the oronasal route and collected pectoral muscles. The samples were stored at 4°C and infectious virus was re‐isolated in chicken, turkey and duck meat (kept at 4°C) for 135, 90 and 75 days, respectively.
AI inactivation from meat of poultry
Thomas and Swayne (2007) infected chickens via the intranasal route with HPAI H5N1 and detected the virus in thigh and breast muscle samples. Thermal inactivation of the virus was independent of the meat type. Cooking heavily contaminated meat (as tested in this study) requires 13.3 min and 21.9 s at 60°C and 70°C, respectively, to achieve an 11‐log EID50 reduction of the H5N1 virus titre. These time periods are around two‐ and fourfold lower, respectively, than the minimum time described in the USDA time‐temperature guidelines to cook chicken meat for a 7‐log reduction of Salmonella.
Isbarn et al. (2007) performed a study suggesting that inactivation of HPAI H7N7 in a chicken meat suspensions is more efficient using a combination of temperature and high hydrostatic pressure compared to using temperature only. The generated mathematical inactivation model predicts a virus inactivation by 7 logs after 1 min exposure to 460 MPa and 15°C.
Thomas et al. (2008) analysed thermal inactivation of HPAI and LPAI H5N2 in meat spiked with the virus and in meat from intranasally infected White Leghorn chickens. For HPAI, inactivation took longer in meat from infected chickens than in spiked meat. Linear regression models predicted that the current US guidelines for cooking chicken meat to achieve a 7‐log reduction of Salmonella also would effectively inactivate the AI strains tested. Both H5N2 viruses tested and HPAI H5N1 were effectively inactivated in chicken meat held at 70 or 73.9°C for less than 1 s.
There are also alternative methods to heat inactivation. For instance, Brahmakshatriya et al. (2009) performed a preliminary study using electron beam irradiation.
In case of AI outbreaks, on‐farm composting seems to become an alternative method to destroy infected carcasses without and reducing the risk of virus spread by limiting transport of infected material (Bendfeldt et al., 2006; Flory and Peer, 2010; Ahmed et al., 2012). Khan et al. (2013) reported that dialysis cassettes could be used as tools to analyse AI virus inactivation by exposing it to temperature, gas and pH conditions. The availability of this kind of tools will facilitate the optimisation of alternative culling (and manure treatment) methods and the evaluation of their biosafety aspects.
Transmission of AI via feeding of infected poultry meat
Swayne and Beck (2005) inoculated 3‐ to ‐4‐week‐old chickens by the intranasal route with LPAI H7N2, HPAI H5N2 or HPAI H5N1 viruses. Viral titres of 2.7 and 3.2 log10 EID50/g were detected in breast and thigh muscle tissue of the HPAI H5N2‐infected chickens whereas no virus was detected in muscle tissue of the LPAI H7N2 inoculated animals. Feeding meat of the HPAI H5N2‐infected chickens to chickens deprived from food for 12‐h chickens did not result in the transmission of the virus to the naïve animals: none of the chickens developed clinical signs or produced antibodies to the AIV. On the other hand, on average 7.3 log10 EID50/g was detected in breast muscle of HPAI H5N1 intranasally inoculated chickens. Feeding HPAI H5N1 contaminated breast meat to 10 naïve chickens resulted in death of nine animals and presence of HPAI H5 virus was confirmed in oropharyngeal and cloacal swabs.
Swayne (2006) analysed HPAI H5N1 thermal inactivation in spiked breast or thigh chicken meat using the heating block of a thermocycler. Virus concentrations ranging from 2.8 to 6.8 log10 EID50/g were tested. The reduction of virus infectivity titres was dependent on virus concentration and no HPAI virus was isolated after heating at 70°C for 1 s.
Brown et al. (2008) fed two herring gulls (Larus argentatus) 5 g meat (8.9 log10 EID50/g) taken from a chicken that died after an intranasal HPAI H5N1 inoculation. Virus transmission took place since HPAI H5N1 was isolated from oropharyngeal and cloacal swabs (both with a mean duration of 3 days).
Kwon et al. (2009) fed piglets with breast and thigh muscle meat from chickens that died from infection with H5N1 virus. The piglets did not show clinical signs but virus was detected in nasal swabs in two out of four animals on day 3 post‐infection only. In addition, H5N1‐neutralising antibodies were detected in serum samples, suggesting that the animals became infected asymptomatically following consumption of raw H5N1‐infected poultry meat.
Bertran and Swayne (2014) performed an experiment to determine the mean infectious and lethal doses for ferrets following consumption of breast meat from chickens that were infected with HPAI H5N1 24 h earlier via the intranasal route. One out of three ferrets became ill when exposed to meat containing 6.8 or 10.92 log10 EID50/g HPAI H5N1 Mong/05 virus and none of the animals died. Only exposure to meat containing 10.96 log10 EID50/g HPAI H5N1 VN/04 resulted in clinical signs (3/3 animals) and mortality (2/3 animals). In a second experiment, fed ferrets with meat of chickens that were infected with HPAI H5N1, H7N3 or H7N7. Seroconversion was detected 14 days post‐meat feeding in the ferrets exposed to meat containing HPAI H5N1, H7N3 (and virus isolation in nasal or rectal samples of some animals) but not in the H7N7 exposed animals.
International trade and intra‐EU transport
The OIE Terrestrial Code chapter 10.4 (OIE, 2010) describes the recommendations for importation of fresh meat of poultry. In essence, poultry should have been kept in a HPAI‐free area and have been slaughtered in an approved abattoir in a HPAI‐free area. However, trade in poultry meat from a country, zone or compartment affected by LPAI is allowed, provided the animals did not exhibit any sign suggestive of AI infection during ante and post‐mortem inspection.
Imports into the EU of poultry meat should be done according to Commission Regulation (EC) No 798/2008. A limited number of countries are approved and health certificates contain guarantees that the meat does not originate in a flock with avian influenza. Nevertheless, no testing is required and particularly in the case of duck or goose meat, there would be a residual risk that birds were consigned to slaughter in good faith while incubating disease.
For EU trade in poultry meat and eggs and egg products, there is ‘placing on the market’ and no veterinary certification, e.g. for meat all slaughterhouse that trade within the EU must be approved and are under competent authority supervision, no certificate is issued, but a commercial document with stamp must accompany the products.
Poultry meat (fresh or frozen) and table eggs are frequently intercepted in passenger luggage (information from UK Border Force seizures). However, even those may be expected to be infected with a higher likelihood than their parallel legal commodities, the volumes involved are far lower.
The article 10.4.25 (OIE, 2016a,b) of the OIE Terrestrial Code describes that, for processed poultry meat, a range of industry standard temperatures between 60.0°C and 73.9°C can inactivate the avian influenza viruses, e.g. achieve a 7‐log reduction, between 507 and 0.51 s, respectively.
Reported AI introductions linked to meat of ducks
Mase et al. (2005) reported the isolation of HPAI H5N1 (approximately 0.5–4.5 log10 EID50/g) from duck meat processed for human consumption, imported to Japan from China.
Beato et al. (2006) described the isolation of LPAI H10N7 virus from a duck carcass smuggled from China into Italy.
Harder et al. (2009) reported an HPAI H5N1 (clade 2.2) outbreak during July–August 2007 involving several poultry holdings of which two were large duck‐fattening farms. The infected ducks did not show clinical signs, neither was mortality increased in the flock. Some of the birds had already been sent to slaughter prior to infection being detected and were not recalled as they were thought to have been slaughtered before the incursion time point of the virus. Culling of more than 750,000 animals was done to terminate the outbreak. In December 2007, the same virus (with very high sequence homology) was identified in three backyard chicken holdings in a different region of Germany. Epidemiological analysis established the chickens had access to uncooked offal from commercial deep‐frozen duck carcasses. Phylogenetic analysis established a direct link between the outbreaks in ducks in summer and in chickens in winter 2007, suggesting that infected duck meat originating from the duck‐fattening farms affected by the outbreaks in August 2007 might have caused the outbreaks in December 2007.
F.12.2. Third country trade – HPAI
Import volume: 0.15 million tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): NA.
Probability of virus preservation during transport: non‐negligible for frozen meat.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely.
F.12.3. Intra‐EU movement – HPAI
Import volume: 3 million tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): NA.
Probability of virus preservation during transport: non‐negligible for frozen meat.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely.
The probability of introduction of LPAI through the meat has not been assessed because considered not relevant in both Third countries trade and intra‐EU movements.
F.13. Table eggs (Traces code 040721, 29 or 90)
This commodity covers eggs and egg products for human consumption, which are produced by fowl (Gallus gallus), turkeys, guinea fowl, ducks, geese, quails, pigeons, pheasants and partridges. It regards fresh table eggs only and excludes anything which is processed.
F.13.1. Background information
This section provides scientific evidence relevant to assess the possible spread of HPAI via eggs or egg products. An analysis of the risk for a food‐borne infection to humans is beyond the scope of this opinion.
A search was performed in the Web of Science using the string ‘avian influenza’ (title), egg and contamination (topic) for the period 2005–2015. The abstract of 44 publications were screened and the full text of relevant publications was screened. The extracted information is reported below.
AI detection in poultry eggs and egg products
HPAI virus has been detected and recovered in the internal contents of chicken, quail, duck and geese eggs laid by infected birds or might remain infectious up to three days on contaminated eggshells (Bean et al., 1985; Cappucci et al., 1985; Li et al., 2006; Promkuntod et al., 2006; Tiwari et al., 2006; Kilany et al., 2010). No virus was recovered in eggs collected during an HPAI H5N2 outbreak in turkeys (Cappucci et al., 1985). Exposure of birds to (broken) contaminated eggs may lead to a new infection and further spread of the virus.
AI inactivation from eggs or egg products
HPAIV perseverance is reduced with increasing temperature, reduced humidity and acidic pH (McDevitt et al., 2010; Beato et al., 2012)). Products with high percentage of egg white limit the temperature of pasteurisation in order to maintain a desirable consistency and viscosity. A guidance on ‘good manufacturing practice for liquid, concentrated, frozen and dried egg products used as food ingredients’ has been published by the European Egg Processors Association (EEPA, 2011).
Chmielewski et al. (2011) reported predicted reductions of 5.7 and 7.8 log10 for H5N2 HPAIV and H7N2 LPAIV, respectively, in egg white achieved by applying the USDA pasteurisation standard of 57°C for 6.3 min.
Chmielewski et al. (2013) described that the pasteurisation processes for fortified, sugared, plain, salted egg yolk and homogenised whole egg products spiked with HPAI H5N2 resulted in more than 5‐log reductions in virus at the lower temperature‐longer times of USDA‐approved Salmonella pasteurisation processes. A more than 5‐log reduction of HPAIV was detected for the five products at the higher temperatures–shorter times of USDA‐approved pasteurisation processes.
International trade and intra‐EU transport
The OIE Terrestrial Code chapter 10.4 (OIE, 2010) describes the recommendations for importation of eggs for human consumption. In essence, eggs should be produced and packed in an AI‐free area and are transported in new or appropriately sanitised packaging materials. If the eggs are produced in HPAI‐free areas, their surfaces should be sanitised (in accordance with OIE Code Chapter 6.4). For the importation of egg products of poultry, regardless of the AI status of the country of origin, the commodity fulfils the requirements for importation of eggs for human consumption or the commodity has been processed to ensure the destruction of AI (heat inactivation achieving a 7‐log reduction of AI, see OIE Code Article 10.4.25 for technical information) and contact with any source of AIV is prevented (OIE, 2010).
Reformulated egg products such as omelette mixes, noodles, cake mixes are usually made from pasteurised egg products. Implementing of a second pasteurisation process after the reformulation is considered a good practice.
In the US, analyses have been performed to simulate the effect of incorporating a holding time before egg movement in conjunction with targeted active surveillance as a novel approach to move eggs from flocks within a control area with a low likelihood of them being contaminated with HPAI. Malladi et al. (2012) used a stochastic disease transmission model to estimate the HPAI disease prevalence, mortality and fraction of internally contaminated eggs at various days after the infection of a layer flock. The outcome suggested a significant reduction in the number of internally contaminated eggs moved from an HPAI‐infected but undetected flock with each additional day of holding time since the likelihood to detect the outbreak in the flock increases. Furthermore, Malladi et al. (2015) used quantitative simulation models to evaluate the movement of potentially contaminated hatching eggs from a breeder located in an HPAI‐affected area, given that active surveillance, elevated biosecurity and a 2‐day on‐farm holding were employed. The mean model predicted the number of internally contaminated hatching eggs released per movement from an HPAI‐infected turkey breeder ranged from 0 to 0.008 under the tested scenarios.
Non‐pasteurised liquid egg (NPLE) is a commodity for which movements from a HPAI‐affected area to a pasteurisation facility in a disease‐free area may be permitted considering the inactivation of HPAI virus via pasteurisation. Weaver et al. (2015) estimated HPAIV concentrations in NPLE for different scenarios of disease detection in an affected holding. The HPAIV concentration seemed to be highest when the disease was identified in a flock based on high mortality recognised via passive surveillance. The virus concentration was considered to be diluted in a tanker‐truck containing eggs from diseased and non‐diseased flocks when transported to the pasteurisation plant. In all simulation scenarios, the HPAIV concentration was below 5 log10 EID50/mL NPLE.
Legislation: See hatching eggs.
Reported AI introductions linked to import of table eggs
Thomas et al. (2005) reported the outcome of an epidemiological investigation into the spread of HPAI H7N7 in the Netherlands in 2003, and identified an increased risk of HPAIV introduction in layer finisher poultry holdings. The authors mention as possible explanation the high number of contacts between these farms, in particular via cardboard egg trays used for removal of eggs during the epidemic.
F.13.2. Third country trade – AI
Import volume: Very variable – 700,000–2.5 million per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: very unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely.
F.13.3. Intra‐EU movements – AI
Import volume: Very variable – 15–50 million per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: very unlikely.
Probability of AIV introduction into a poultry holding via the commodity: as birds successfully producing high quality table eggs are rarely viraemic, the eggs would not contain high levels of virus and could be contaminated but not infected; therefore it is the fomite risk which leads to a probability of very unlikely. Moreover, the probability of AIV incursion is considered very unlikely, although the volumes are high.
F.14. Semen
This commodity includes mainly the semen of turkeys which is the most widely used one in the poultry industry and can lead to AI infection through artificial insemination.
F.14.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ and semen (topic) for the period 2005–2015. The abstract of 15 publications were screened but none of them was considered relevant.
AI detection in poultry semen
No studies were identified in the period 2005–2015; however, there is scientific literature on the infection of turkeys after being artificially inseminated and this was considered an important route for transmission of pandemic influenza A (H1N1) into turkeys (Pantin‐Jackwood et al., 2010).
International trade and intra‐EU transport
For the semen used in the artificial insemination practices, there are no harmonised rules among the MSs; specific national rules apply and they should take into account the OIE code: AIV for 21 day freedom and no clinical signs at time of collection.
The OIE Terrestrial Code chapter 10.4 (OIE, 2010) describes the recommendations for importation of poultry semen for human consumption. In essence, semen should be derived from donor poultry without clinical signs of avian influenza and kept in an AI‐free.
Reported AI introductions linked to import of poultry semen
Not identified for the period 2005–2015.
F.14.2. Third country trade – HPAI
Import volume: no data available as there are no harmonised rules or certificates.
Probability of testing (according to EU legislation): Extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: considering the lack of data on the volumes used and the lack of trade rules the risk of this pathway for HPAI cannot be rejected, hence the risk might be considered non‐negligible, given the level of uncertainty. It is important to emphasise that the probability of ‘non‐negligible’ is between 10% and 100%, and the high level of uncertainty is therefore reflected in this scoring.
F.14.3. Intra‐EU movement – HPAI
Import volume: no data available as there are no harmonised rules or certificates.
Probability of testing (according to EU legislation): Extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: considering the lack of data on the volumes used and the lack of trade rules the risk of this pathway for HPAI cannot be rejected, hence the risk might be considered non‐negligible, given the level of uncertainty. It is important to emphasise that the probability of ‘non‐negligible’ is between 10% and 100%, hence the uncertainty.
The probability of introduction of LPAI through the semen has not been assessed because considered not relevant in both Third countries trade and intra‐EU movements, as there is no viraemia associated with infection.
F.15. Manure
This commodity concerns the unprocessed manure of poultry, which is a category 2 animal by‐product.
F.15.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and ‘manure’ (topic) for the period 2005–2015. The abstract of 17 publications were screened and the full text of relevant publications was screened. The extracted information is reported below.
AI tenacity and inactivation from manure
Chumpolbanchorn et al. (2006) spiked chicken manure with HPAI H5N1 and found that the virus lost its infectivity within 24 h or 15 min when samples were kept at 25°C or 40°C, respectively. Exposure to ultraviolet light at 4–5 ∞w/cm2 for 4 h did not destroy viral infectivity in manure.
Guan et al. (2009) reported that AIV infectivity was inactivated in compost within 7 days when temperatures reached at least 50°C. Direct contact of the infectious specimens with compost (using nylon mesh bags) speeded up the inactivation process.
Elving et al. (2012) spiked chicken manure with HPAI H7N1 and reported a 12‐log10 reduction of the virus after 6.4 h, 1.7 h or 29 min when the composting material consisted of manure and straw and was kept at 35°C, 45°C or 55°C. A similar reduction was observed after 7.6 h, 9.8 h or 30 min when embryonated eggs were added to the composting material. Similar inactivation rates were observed for bacteriophage Ø6, suggesting that it might be used as an indicator of AI inactivation during thermal treatments.
International trade and intra‐EU transport
According to Commission Regulation (EU) No 142/2011, the importation into and the transit through the Union of unprocessed poultry manure is prohibited, therefore this pathway is not relevant in the case of Third‐countries trade.
For intra‐EU movements of unprocessed processed manure, the operator must apply by sending a form set out in Regulation (EU) No 142/2011 (Annex XVI, Chapter III, Section 10) for the consent of the competent authorities of the MS of origin and the MS of destination. If the movement is approved, the MS of origin should inform the MS of destination under Article 48(1) and (3) of (EC) No 1069/2009 (Annex XI, Chapter 1, Section 1 point 1) through the use of Traces by entering the commercial document. Attached to the document is a model health attestation according to Regulation (EU) No 142/2011 (Annex XI, Chapter 1, Section 1 point 1). As a derogation to these requirements, two MSs which share a common border may authorise the dispatch of manure between farms located in border regions of those two MSs (subject to appropriate conditions for the control of any possible risks to public or animal health, such as obligations for the operators concerned to keep appropriate records), without the requirement for the health attestation and recording in Traces, but the volumes and frequency of this event are not known, and it is this uncertainty which drives the risk level.
The product itself consists of a mixture of bird manure, poultry feed, sawdust and bedding material that accumulates at the bottom of or on manure conveyor belts in poultry sheds and therefore can potentially include dead poultry. If not treated or pelleted, it can contain high levels of virus if taken from an infected poultry farm, and should always be stacked to reduce viral load (Avian Influenza Directive 2005/94/EC), in accordance with disease control measures.
Therefore, for manure originating in holdings with HPAI‐infected galliforme species, it would be expected that the disease would have been confirmed and all manure treated. For manure originating from holdings with HPAI in anseriform species or LPAI in any poultry species, it is possible that disease would not be detected.
Reported AI introductions linked to import of poultry manure
None
F.15.2. Third country trade – AI
Not relevant.
F.15.3. Intra‐EU movement – AI
Import volume: no data available as there are no harmonised rules or certificates.
Probability of testing (according to EU legislation): Extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability AIV introduction into a poultry holding via the commodity: for manure originating from holdings with HPAI in anseriform species or LPAI in any poultry species, it is considered a non‐negligible pathway taking also into account reduced virus perseverance in manure. In terms of exposure directly to commercial poultry, it cannot be discounted as a potential pathway if the manure is spread near a poultry farm or if there is access to wild waterfowl.
F.16. Skin, feathers and down (Traces code 0505)
This commodity regards two subcategories:
the raw feathers, with some treatment, and
the skin and feathers and down, which are unworked, but disinfected or treated.
The risk of AIV may be different between them two.
F.16.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and ‘feather’ (topic) for the period 2005–2015. The abstracts of 51 publications were screened and the full text of relevant publications was extracted. The information is reported below. It was noticed that this search strategy is missing studies that report for instance presence of viral antigen and RNA in feather follicles as a diagnostic matrix when analysing the tissue distribution of viral antigens (e.g. Kalthoff et al., 2008). Although such missed studies might confirm the data presented below, it is considered that that the most important studies are covered with the used search strategy.
AI detection in feathers
A few papers describing the presence of viral antigen and/or infectious virus in feathers after a natural or experimental HPAI infection (see Table F.4). On the other hand, LPAI induces localised respiratory and gastrointestinal infections. Therefore, it is not surprising that no papers were identified on the presence of LPAI in feathers.
Table F.4.
Identified publications on avian influenza and feather for the period 2005–2015
| Virus type | Clade | Species | Infection | Age birds | Viral antigen | Virus isolation | Reference |
|---|---|---|---|---|---|---|---|
| HPAI H5N1 | 2.2 | Pekin ducks | Experimental | 4 and 24 weeks | Yes | NS | Aiello et al. (2013) |
| 2.3.2 and 2.3.4 | Chickens, Pekin and Muscovy ducks | Natural | NS | Yes | Yesa | Slomka et al. (2012a), Slomka et al. (2012b) | |
| NS | Sturm‐Ramirez et al. (2005) | ||||||
| NS | Call ducks | Experimental | 1‐day‐old, 2 and 4 weeks | Yes | NS | Yamamoto et al. (2007a), Yamamoto et al. (2007b) | |
| NS | Japanese domestic ducks | Experimental | 4 weeks | Yes | Yes | Yamamoto et al. (2008) | |
| 2.3.2 | Whooper swans | Natural | Juvenile and adult | Yes | NS | Yamamoto et al. (2009) | |
| 2.2 and 2.3.2 | Domestic ducks | Experimental | 4 weeks | Yes | Yes | Yamamoto et al. (2010) | |
| HPAI H7N1 | NA | Chicken | Experimental | 8 and 12 weeks | Yes | Yesb | Busquets et al. (2010a) |
| H4N6 | NA | Aythya ferina, Anas clypeata, Anas crecca | Natural | Hatching year age | Not reported | Yes | De Marco et al. (2014) |
| H3N8 | NA | Anas crecca | Natural | Not reported | Not reported | Yes | De Marco et al. (2014) |
| H?N2 | NA | Anas crecca | Natural | Not reported | Not reported | Yes | De Marco et al. (2014) |
| H2N? | NA | Anas platyrhynchos | Natural | Not reported | Not reported | Yes | De Marco et al. (2014) |
Feather samples were collected at four HPAI H5N1‐infected poultry holdings in Vietnam, virus was isolated from 19/23 and 19/20 chicken and 10/13 and 10/10 duck feather samples, respectively, in the 2011 and the 2012 study.
Lower detection of viral RNA in 12 weeks old compared to 8 weeks old group; detection in the older group was related to the level of calcification of the feather shaft.
NS, not specified; NA, not applicable
AI tenacity in feathers
Busquets et al. (2010a,b) reported that the viral RNA load in feather pulp was statistically significantly higher than in oropharyngeal and cloacal swabs from 1 dpi to 6 days post‐mortem. Infectious HPAI H5N1 virus was detected in feather pulp from 2 days post‐infection onwards and retained its infectivity for as long as 5–6 days post‐mortem at an environmental temperature of 22–23°C.
Yamamoto et al. (2010) sampled feather samples from HPAI H5N1‐infected ducks at 3 days post‐infection. Infectious viruses persisted for the longest period in feathers, compared with drinking water and faeces, at both 4°C and 20°C. Viral infectivity persisted in the feathers for 160 days s at 4°C and for 10–15 days at 20°C, for both clades. Viral RNA in feathers was more stable than the infectivity.
Transmission of AI via feathers
Oral inoculation of nine 2‐week‐old call ducks with feathers collected from a call duck that died 4 days after an intravenous infection resulted in the infection of seven out of nine birds, confirmed via histological lesions with viral antigens, virus isolation from cloacal swabs and HI antibody production (Yamamoto et al., 2007a,b). Similar clinical signs were observed as in call ducks infected via the intranasal route with the same virus.
International trade and intra‐EU transport
The OIE Terrestrial Code chapter 10.4 (2016) describes the recommendations for importation of feathers and down (OIE, 2016b). In essence, the commodity should originate from poultry kept and slaughtered in a HPAI‐free area, have been processed to ensure destruction of AI virus (washed and steam‐dried at 100°C for 30 min, fumigation with formalin (10% formaldehyde) for 8 h, irradiation with a dose of 20 kilogray, any equivalent treatment which has been demonstrated to inactivate AIV) and precautions were taken to avoid contact of the commodity with any source of AI.
According to Commission Regulation (EU) No 142/2011, the importation into and the transit through the Union of untreated feathers and parts of feathers and down is prohibited, while treated feathers and down (hot steam at 100°C for 30 min) may be placed on the market with no restrictions.
Treatments comprise physical and/or chemical processes ensuring complete viral inactivation. Therefore provided the treatment is carried out correctly, these products have a negligible risk. The majority of such products are destined not only for technical use, in the furnishings and furniture businesses but also for decorating clothing or fly fish lures.
For imports of treated feathers, parts of feathers and down, no health certificate is required. For LPAI, these commodities may be contaminated rather than infected.
There is an anomaly with the trade code. According to the Vet Checks Regulation (Commission Decision 2007/275/EC) EX 0505), EX 0505 is described as ‘Skins and other parts of birds, with their feathers or down, feathers and parts of feathers (whether or not with trimmed edges) and down, not further worked than cleaned, disinfected or treated for preservation; powder and waste of feathers or parts of feathers’. Therefore, it is considered that feathers and parts of feathers and down have been treated by ‘another method’ but not undergone a full steam treatment. This is why, once imported, these products have to go to an approved establishment in the EU for further treatment. Therefore they may appear in the trade database as ‘raw’ as they haven't been fully processed or if ‘other’ is a viable option under the Combined Nomenclature (CN) codes (Council Regulation (EEC) No 2658/87).
Reported AI introductions linked to import of feathers
No report found.
F.16.2. Third country trade – HPAI
A) Raw feathers – (with some treatment)
Import volume: 0.7–1 tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability virus preservation during transport: extreme unlikely.
Probability poultry exposure to the commodity: extreme unlikely.
Probability AIV introduction into a poultry holding via the commodity: extreme unlikely.
B) Skin and feathers or down unworked, but disinfected or treated
Import volume: 10–11 tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability virus preservation during transport: extreme unlikely.
Probability poultry exposure to the commodity: extreme unlikely.
Probability AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.16.3. Intra‐EU trade – HPAI
A) Raw feathers – (with some treatment)
Import volume: 7–8 tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: very unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely, taking into account the high volume.
B) Skin and feathers or down unworked, but disinfected or treated
Import volume: 95,000–115,000 tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): n/a.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: very unlikely, taking into account the high volume.
The probability of introduction of LPAI through raw feathers with some treatment and treated skin, feathers and down has not been assessed because considered not relevant in both third countries trade and intra‐EU movements, as these commodities may be contaminated rather than infected.
F.17. Feed
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002, defines ‘feed’ (or ‘feedingstuff’) as any substance or product, including additives, whether processed, partially processed or unprocessed, intended to be used for oral feeding to animals. For the scope of this assessment, where the commodity refers to commercially available pelleted feed, this is considered unlikely to be a risk for AIV transmission provided that no wild bird contact will occur during storage.
This commodity also includes poultry products used for feeding other animal species (except for poultry tallow), and feed specifically for poultry (pelleted or not).
F.17.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and ‘feed’ (topic) for the period 2005–2015 (restricting to documents in English only). The abstracts of two relevant publications returned from this search string were screened and the extracted information is reported below.
Conraths et al. (2016) conducted epidemiological outbreak investigations in highly pathogenic avian influenza virus of the subtype H5N8 (HPAIV H5N8)‐affected commercial holdings and a zoo in Germany, for the period of 2014–2015, in order to identify potential routes of entry of the pathogen. The epidemiological team assessed retrospectively the risk factors for introduction, in a qualitative manner for risk analysis, with the World Organization for Animal Health (OIE) using risk scores (0, negligible; 1, low; 2 medium; 3, high). The mean risk scores were agreed by the team for each risk factor, and the risk of introduction of AIV into the affected premises by contaminated feed was negligible (0) with low uncertainty. This is because feedstuff was heat‐treated during the process of pelleting.
On the other hand, there is limited information available on the likelihood of AIV‐contaminated ingredients to become inactivated in non‐pelleted chicken feed. It is known however that in general the likelihood of the infectious virus to be introduced into flocks via the feed seems to be very low to low (Toro et al., 2016). Toro et al. (2016) describe that despite in the environment factors such as heat, extreme pH values, dryness, and a diverse variety of disinfectants inactivate quickly the virus (Shahid et al., 2009; Zou et al. 2013), the presence of organic material increases the virus resistance to physical and chemical inactivation.
This study (Toro et al., 2016), by conducting four different trials, showed that disinfection with commercially available feed disinfectants – Termin‐8 (a blend of formaldehyde, propionic acid, terpenes, and surfactant) and Finio (a blend of approved phytochemicals and carboxylic acids) – effectively inactivates the virus if present in non‐pelleted chicken feed. Furthermore, since the stability of the AI virus in chicken feed is known to be limited, it was evaluated the effect of protein to the virus suspension, by adding skim milk powder. Results showed that although protein prolonged the stability of the infectious virus in untreated feed to 24 h at 24°C, the feed disinfectants were nevertheless capable of inactivating the virus.
Third country trade and intra‐EU movements
The OIE Terrestrial Code chapter 10.4 (2016) describes the recommendations of products of poultry origin, other than feather meal and poultry meal, intended for use in animal feeding, or for agricultural or industrial use (OIE, 2016b). In essence, regardless of the avian influenza status of the country of origin, an international veterinary certificate is required, attesting that: (i) these commodities (e.g. live animals, products of animal origin, animal genetic material, biological products an pathological material) were processed in an avian influenza free country, zone or compartment from poultry which were kept in an avian influenza free country, zone or compartment from the time they were hatched until the time of slaughter or for at least the 21 days preceding slaughter, (ii) these commodities have been processed to ensure the destruction of avian influenza virus using: (a) moist heat treatment for 30 min at 56°C, or (b) any equivalent treatment which has been demonstrated to inactivate avian influenza virus. Also that (iii) the necessary precautions were taken to avoid contact of the commodity with any source of avian influenza virus.
In general, this is considered extremely unlikely to be a suitable pathway for incursion of AI into commercial poultry. However, the product itself could be exposed to virus‐infected wild birds or virus‐contaminated environment while being transported or on arrival when stored; therefore, in these circumstances, this pathway is considered likely. However, it is considered as part of the general wild bird indirect exposure pathways, which highlights the importance of biosecurity around storing feed away from possible contamination.
Reported AI introductions linked to feed trade
None have been directly ascribed to commercially available pelleted poultry feed. However the contamination of feed at the poultry holding should always be considered a potential pathway in epidemiological investigations (Avian Influenza Directive). It therefore stands to reason that if contamination could occur at any point along the transport chain, this should also be considered.
F.17.2. Third country trade – AI
Import volume: Around 700,000–900,000 tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: it is considered extreme unlikely but feed might become contaminated during storage; therefore, under certain circumstances it could be non‐negligible.
F.17.3. Intra‐EU movements – AI
Import volume: Around 2.4–2.6 million tonnes per annum.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: it is considered extreme unlikely but feed might become contaminated during storage; therefore, under certain circumstances it could be non‐negligible.
F.18. Bedding
This commodity includes hay (CN code EX 1214 90) and straw (CN code EX 1213 00 00), which means that of the entire commodity range, only hay or straw requires a vet check on arrival, as this constitutes animal derived material.
F.18.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and bedding (topic) for the period 2005–2016 (restricting to documents in English only). Only one abstract of three publications found was considered relevant from this search string and the extracted information from this paper is reported below.
Conraths et al. (2016) conducted epidemiological outbreak investigations in highly pathogenic avian influenza virus of the subtype H5N8 (HPAIV H5N8)‐affected commercial holdings and a zoo in Germany, for the period of 2014–2015, in order to identify potential routes of entry of the pathogen. The epidemiological team assessed retrospectively the risk factors for introduction of the virus, in a qualitative manner for risk analysis, with the World Organization for Animal Health (OIE) using risk scores (0, negligible; 1, low; 2 medium; 3, high). The mean risk scores were agreed by the team for each risk factor, and the risk of introduction of the AIV via indirect contact with materials, such as bedding material, contaminated with wild bird‐infected faeces used from affected premises into the commercial poultry holding was estimated to be highest (1.33) with the highest uncertainty (1.67), than those from water and feed (negligible risk and low uncertainty). It should also be noted that before the outbreak occurred in late October 2014, large aggregations of wild birds had been observed on the pastures around the affected farms and the ground on the premise surroundings was considerably contaminated.
Third country and intra‐EU trade
The OIE Terrestrial Code chapter 10.4 (2016) for Avian Influenza does not describe any AI‐specific recommendation or measure, however EU rules requires all hay and straw imported from third countries to be subject to veterinary checks on arrival at the BIP (2007/275/EC). The list of countries which may export this commodity is limited to: Australia, Belarus, Canada, Switzerland, Chile, Greenland, Iceland, New Zealand, Serbia, Ukraine, USA and parts of South Africa. For Serbia, Ukraine and Belarus, only pelleted product intended for combustion (494/2014/EU amending 136/2004/EC). Hay and straw must only enter the EU from third countries with a commercial document; however there are no consignments notified in the UN Comtrade database from third countries.
For intra‐EU movements, large volumes of such a commodity are moved in the EU.
In general, the risk of introduction of AIV via this pathways is considered extreme unlikely, however, similarly to the feed pathway, if contamination occurs at time of harvest, during transport or storage, through contact with infected wild birds or contaminated vermin and because of the intended exposure to poultry, this pathway is scored as non‐negligible, with some uncertainty based on the time since harvest and the transport conditions which may affect viral perseverance.
Reported AI introductions linked to trade in bedding
None have been directly ascribed to commercially available bedding. However, the contamination of bedding at the poultry holding should always be considered a potential pathway in epidemiological investigations (Avian Influenza Directive). It therefore stands to reason that if contamination could occur at any point along the transport chain, this should also be considered.
F.18.2. Third country trade – AI
Commission Regulation (EC) No 136/200465 regulates imports of hay and straw: they must be certified, not necessarily treated. They may be subject to testing but not specified which pathogens.
Import volume: 15–50 thousand tonnes.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: it is considered extreme unlikely but contamination of imported bedding with wild bird faeces cannot currently be eliminated as a potential pathway; therefore, under certain circumstances it could be non‐negligible.
F.18.3. Intra‐EU movements AI
Bedding undergoes to MSs’ national rules; commercial documents are required for intra‐EU movements.
Import volume: 850–900 thousand tonnes.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: non‐negligible.
Probability of poultry exposure to the commodity: non‐negligible.
Probability of AIV introduction into a poultry holding via the commodity: it is considered extreme unlikely but contamination of imported bedding with wild bird faeces cannot currently be eliminated as a potential pathway; therefore, under certain circumstances it could be non‐negligible.
F.19. Pharmaceuticals
This commodity refers to vaccines, which are approved by EMEA. All vaccinated birds and all registered vaccines must be notified to the European Commission and each MS must submit plans for approval indicating vaccine to be used and keep records of birds vaccinated (captive birds in zoos and collections or in special circumstances, some poultry).
Only one vaccine for avian influenza is approved for use in the EU: the Nobilis Influenza H5N2 for use in chickens. It is an inactivated vaccine manufactured in the EU. Nevertheless, other live vaccines for use in poultry (e.g. against Newcastle Disease) are often produced in eggs (SPF eggs) and contamination could occur, but is thought improbable given the controls in place with Good Manufacturing Practices with pharmaceutical companies and veterinary medicine authorities.
All authorised vaccines are assessed for quality, safety and efficacy. As part of the quality assessment, the applicant has to provide information on any potential extraneous agents and there is a monograph which outlines which agents to test for and ensure that there is no risk. Also, if material(s) of animal origin is used during production of the vaccine then information has to be provided and a TSE risk assessment performed. If they are live vaccines that have been attenuated in some way, the potential to revert to virulence is assessed based on the safety data provided. Any concerns on safety such that a product could revert to a virulent state would be noted and taken into account when determining the benefit/risk of the product, and whether an authorisation is given. The final product tests in place should give assurance that there is no contamination, but in case it does, there are steps in place to manage the risk from such events. The tests that are performed on authorised live/inactivated vaccines reassures that there is no risk of extraneous agents, and these tests include one for influenza A.
Finished medicinal products, such as vaccines, are not covered by veterinary legislation for import and therefore there are no import data available. Intermediate products derived from Category 3 material and intended for technical uses in medical devices, in vitro diagnostics, laboratory reagents and cosmetics are included (See Commission Decision 2007/275/EC) but these products are not considered a risk of introducing avian influenza to poultry as they are destined for laboratories.
F.19.1. Background information
A search was performed in the Web of Science using the string ‘avian influenza’ (title) and ‘pharmaceutical’ (topic) for the period 2005–2015 (restricting to documents in English only). The publications found from this search string were not considered relevant for this subject, mainly because the risk of introduction of AIV to poultry via pharmaceuticals is unlikely to occur, therefore not reported in this context.
Third country and intra‐EU trade
The OIE Terrestrial Code chapter 10.4 (2016) (Infection with Avian Influenza viruses) does not describe any recommendations or measures related to pharmaceuticals. However there are recommendations in the OIE Terrestrial Manual chapter 1.1.8 (2015) on the principle of veterinary vaccine production and in chapter 3.7.2 on the minimum requirements for the production and quality control of vaccines. In addition, EU rules are in place for the trade and imports of pharmaceuticals (Directive 2001/82/EC) which requires national authorities to authorise veterinary medicines, to carry out any tests to ensure compliance and to grant market authorisation or EU marketing authorisation Regulation (EC) No 726/2004.
Reported AI introductions linked to trade in pharmaceuticals
None identified from the available literature or a search of epidemiological reports into AI outbreaks.
F.19.2. Third country trade – AI
Import volume: no data available.
Probability of testing (according to EU legislation): non‐negligible if approved.
Tests that can be used (according to EU legislation): PCR.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.19.3. Intra‐EU movements – AI
Import volume: no data available.
Probability of testing (according to EU legislation): non‐negligible if approved.
Tests that can be used (according to EU legislation): PCR.
Probability of virus detection (given AIV presence in commodity): non‐negligible.
Probability of virus preservation during transport: unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
F.20. Other animal by‐products obtained by poultry
This commodity refers to the animal by‐products as defined by Regulation 2009/1069/EU of the European Parliament and of the Council of 21 October 2009: animal by‐products (ABPs) means entire bodies or parts of animals, products of animal origin or other products obtained from animals, which are not intended for human consumption, including oocytes, embryos and semen.
This section refers to ‘other ABPs’, such us: processed meat, processed parts of birds (including feathers, skin, feet, casings, blood), but they were discounted as not being a significant pathway (and scored as extremely unlikely for HPAI and not relevant for LPAI) as they were either for treatment, processing (e.g. for pet food) or for human consumption.
F.20.1. Background information
ABPs’ rules (Regulations (EC) No 1069/2009 and (EU) No 142/2011) about the disposal of category 1 and 2 waste means high‐risk material will be rendered. Category 3 ABPs are also considered a very low risk category for AIV transmission. Unprocessed pet food such as dog chews made of poultry meat or frozen day‐old chicks are frequently traded and imported, with health certification. Both represent an extremely unlikely pathway for the introduction of HPAIV (and not relevant for LPAIV) into commercial poultry.
Third country and intra‐EU trade
Not available.
Reported AI introductions linked to trade in ABMs
None attributed, according to a search of the literature and available epidemiological reports of outbreaks.
F.20.2. Third country trade and intra‐EU movement – HPAI
Import volume: no data available.
Probability of testing (according to EU legislation): extreme unlikely.
Tests that can be used (according to EU legislation): NA.
Probability of virus detection (given AIV presence in commodity): extreme unlikely.
Probability of virus preservation during transport: extreme unlikely.
Probability of poultry exposure to the commodity: extreme unlikely.
Probability of AIV introduction into a poultry holding via the commodity: extreme unlikely.
The probability of introduction of LPAI through the ABMs has not been assessed because considered not relevant in both Third countries trade and intra‐EU movements.
F.21. Overview imports of some commodities from third countries
Table F.5.
Lists of countries which are approved for the import of certain live poultry, hatching eggs, poultry meat and table eggs into the EU, according to Regulation (EC) No 798/2008: X = Whole country; R = Regions only; Y = additional measures required
| Country | Breeding poultry | Breeding ratites | Day‐old‐chicks | Day‐old‐ratites | Hatching eggs poultry | Hatching eggs ratites | SPF eggs | Slaughter poultry or game stocking | Slaughter ratites | Less than 20 poultry | Poultry meat | Poultry meat (MSM) | Ratite meat | Ratite meat (MSM) | Wild game meat | Wild game meat (MSM) | Eggs for the table | Egg products |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albania | X | X | ||||||||||||||||
| Argentina | X | X | X | X | X | X | ||||||||||||
| Australia | X | Y | X | Y | X | Y | X | X | X | Y | Y | X | X | |||||
| Brazil | R | R | R | R | R | R | X | R | R | R | R | R | R | R | R | |||
| Botswana | Y | Y | Y | X | Y | X | X | |||||||||||
| Belarus | X | X | ||||||||||||||||
| Canada | R | R | R | R | R | R | X | R | R | R | R | R | R | X | X | |||
| Switzerland | ||||||||||||||||||
| Chile | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| China | R | R | X | |||||||||||||||
| Greenland | X | X | X | |||||||||||||||
| Hong Kong | X | |||||||||||||||||
| Israel | Y | Y | Y | Y | Y | Y | X | Y | Y | Y | Y | Y | Y | X | ||||
| India | X | |||||||||||||||||
| Iceland | X | X | X | |||||||||||||||
| Rep of Korea | X | X | ||||||||||||||||
| Moldova | X | |||||||||||||||||
| Montenegro | X | |||||||||||||||||
| Madagascar | X | X | X | X | ||||||||||||||
| Malaysia | R | R | ||||||||||||||||
| Macedonia | X | X | ||||||||||||||||
| Mexico | X | X | ||||||||||||||||
| Namibia | X | X | X | X | X | X | X | |||||||||||
| New Caledonia | X | |||||||||||||||||
| New Zealand | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Saint Pierre Michelon | X | |||||||||||||||||
| Serbia | X | |||||||||||||||||
| Russia | X | X | X | |||||||||||||||
| Singapore | X | |||||||||||||||||
| Thailand | X | X | X | Y | X | X | ||||||||||||
| Tunisia | X | X | X | X | X | X | X | X | X | X | X | |||||||
| Turkey | X | |||||||||||||||||
| Ukraine | X | X | X | X | X | |||||||||||||
| United States | R | R | R | R | R | R | X | R | R | R | R | R | R | X | X | |||
| Uruguay | X | X | X | X | ||||||||||||||
| South Africa | Y | Y | Y | X | Y | X | X | |||||||||||
| Zimbabwe | Y | Y | Y |
F.22. Overview of the qualitative assessment of the risk of introduction of AI via non‐wild bird pathways
Table F.6.
Overview of the qualitative assessment of the risk of introduction of AI via non‐wild bird pathways
| Movement | Virus type | Import volume | Probability of testing | Tests that can be used | Probability of virus detection | Probability of virus preservation during transport | Probability of poultry exposure to the commodity | Aggregated score: probability of AIV introduction into a commercial poultry holding via the commodity (small scale – large scale) |
|---|---|---|---|---|---|---|---|---|
| PATHWAY: Live (non‐poultry) birds | ||||||||
| COMMODITY: Psittaciformes | ||||||||
| Third country trade | AI | 600–1,000 per annum | Non‐Negligible | Any ai test in accordance to diagnostic manual | Non‐negligible | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| Intra‐EU movement | AI | 150,000–200,000 birds per year | Very Unlikely | na | na | Non‐negligible | Extreme unlikely | Very unlikely |
| COMMODITY: Birds of prey | ||||||||
| Third country trade | AI | Fewer than 500 per annum | Non‐Negligible | Any AI test in accordance to diagnostic manual | Non‐negligible | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| Intra‐EU movement | AI | Around 500–1,500 per annum | Very Unlikely | na | na | Non‐negligible | Extreme unlikely | Very unlikely |
| COMMODITY: Other captive birds, destined for approved bodies | ||||||||
| Third country trade | AI | ~ 600–1,000 per annum | Non‐negligible | Any AI test in accordance to OIE diagnostic manual | Non‐negligible | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| Intra‐EU movement | AI | 20,000–25,000 per annum of birds other than poultry, Psittaciformes or birds of prey (each) | Very unlikely | na | na | Non‐negligible | Extreme unlikely | Very unlikely |
| COMMODITY: Birds as pets | ||||||||
| Third country trade | HPAI | No requirement to record Trade statistics | Testing required for H5N1 only | Either PCR or serology, in accordance to diagnostic manual | Non‐negligible only for H5N1 | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| LPAI | No requirement to record Trade statistics | Testing required for H5N1 only | Either PCR or serology, in accordance to diagnostic manual for H5N1 | Very unlikely | Non‐negligible | Extreme unlikely | Extreme unlikely | |
| Intra‐EU movement | HPAI | No requirement to record Trade statistics | Extreme unlikely | na | Very unlikely | Non‐negligible | Extreme unlikely | Very unlikely |
| LPAI | No requirement to record trade statistics | Extreme unlikely | na | Very unlikely | Non‐negligible | Extreme unlikely | Very unlikely | |
| PATHWAY: Live poultry | ||||||||
| COMMODITY: Hatching eggs – Gallus gallus | ||||||||
| Third country trade | AI | 25–30 million eggs per year | Extreme unlikely, but quarantine required | na | Very unlikely | Extreme unlikely | Non‐negligible | Very unlikely |
| Intra‐EU movement | HPAI | 1.1 billion per annum | Extreme unlikely | na | Very unlikely | Very unlikely | Non‐negligible | Unlikely |
| LPAI | 1.1 billion per annum | extreme unlikely | na | Unlikely | Non‐negligible for fomite transfer | Non‐negligible | Unlikely | |
| COMMODITY: Hatching eggs – other | ||||||||
| Third country trade | AI | 5 million of non‐Gallus poultry; 200–300,000 for both game birds and non‐poultry | Extreme unlikely, but quarantine required | na | Very unlikely | Extreme unlikely | Non‐negligible | Very unlikely |
| Intra‐EU movement | AI | 120–170 million per annum of which 15 million non‐poultry and 50 million for game birds | Extreme unlikely | na | Very unlikely | Very unlikely | Non‐negligible | Unlikely |
| COMMODITY: Day‐old chickens | ||||||||
| Third country trade | AI | 200,000–400,000 of birds per year | Non‐negligible, but quarantine required | According to the diagnostic manual | Non‐negligible | Extreme unlikely | Non‐negligible | Extreme unlikely |
| Intra‐EU movement | HPAI | 500–700 million birds per year | Extreme unlikely | na | Very unlikely | Very unlikely | Non‐negligible | Unlikely |
| LPAI | 500–700 million birds per year | Extreme unlikely | na | Very unlikely | Non‐negligible | Non‐negligible | Non‐negligible | |
| COMMODITY: Day‐old poultry (ducks or geese) | ||||||||
| Third country trade | AI | 50–200,000 birds per annum | Non‐negligible | According to the diagnostic manual | Non‐negligible | Extreme unlikely | Non‐negligible | Extreme unlikely |
| Intra‐EU movement | HPAI | 50–60 million birds per annum | Extreme unlikely | na | Very unlikely | Very unlikely | Non‐negligible | Unlikely |
| LPAI | 50–60 million birds per annum | Extreme unlikely | na | Unlikely | Non‐negligible | Non‐negligible | Non‐negligible | |
| COMMODITY: Breeding or production poultry – Gallus gallus | ||||||||
| Third country trade | AI | 800,000–1 million birds per annum | Non‐negligible | Any AI test in accordance to diagnostic manual | Non‐negligible | Extreme unlikely | Non‐negligible | Extreme unlikely |
| Intra‐EU movement | HPAI | 400–500 million birds per annum | Very unlikely | na | Birds should exhibit clinical signs | Unlikely | Non‐negligible | Unlikely |
| LPAI | 400–500 million birds per annum | Very unlikely | na | Clinical signs are unlikely to be observed | Non‐negligible | Non‐negligible | Non‐negligible | |
| COMMODITY: Breeding or production poultry – not Gallus gallus | ||||||||
| Third country trade | AI | < 200 birds per annum | Non‐negligible | Any AI test in accordance to diagnostic manual | Non‐negligible | Extreme unlikely | Non‐negligible | Extreme unlikely |
| Intra‐EU movement | HPAI | 15–18 million birds per annum | Very unlikely | na | Birds should exhibit clinical signs | Unlikely | Non‐negligible | Unlikely |
| LPAI | 15–18 million birds per annum | Very unlikely | na | Clinical signs are unlikely to be observed | Non‐negligible | Non‐negligible | Non‐negligible | |
| PATHWAY: Meat and eggs for human consumption | ||||||||
| COMMODITY: Meat | ||||||||
| Third country trade | HPAI | 0.15 million tonnes per annum | Extreme unlikely | na | na | Likely for frozen meat | Extreme unlikely | Very unlikely |
| LPAI | Not relevant | Not relevant | ||||||
| Intra‐EU movement | HPAI | 3 million tonnes per annum | Extreme unlikely | na | na | Likely for frozen meat | Extreme unlikely | Very unlikely |
| LPAI | Not relevant | Not relevant | ||||||
| COMMODITY: Table eggs | ||||||||
| Third country trade | AI | Very variable – 700,000–2.5 million per annum | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Very unlikely | Very unlikely |
| Intra‐EU movement | AI | Very variable – 15–50 million per annum | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Very unlikely | Very unlikely |
| PATHWAY: Semen | ||||||||
| COMMODITY: Semen | ||||||||
| Third country trade | HPAI | No data available as there are no harmonised rules or certificates | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Non‐negligible |
| LPAI | Not relevant | Not relevant | ||||||
| Intra‐EU movement | HPAI | No data available as there are no harmonised rules or certificates | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Non‐negligible |
| LPAI | Not relevant | Not relevant | ||||||
| PATHWAY: Manure | ||||||||
| COMMODITY: Manure | ||||||||
| Third country trade | AI | Not relevant – only processed manure allowed | ||||||
| Intra‐EU movement | AI | No data availablea | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Non‐negligible |
| PATHWAY: Feathers, skin and down | ||||||||
| COMMODITY: Raw feathers (with some treatment) | ||||||||
| Third country trade | HPAI | 0.7–1 tonnes per annum | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| LPAI | Not relevant | Not relevant | ||||||
| Intra‐EU movement | HPAI | 7–8 tonnes per annum | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Very unlikely | Very unlikely |
| LPAI | Not relevant | Not relevant | ||||||
| COMMODITY: Treated skin feathers and down | ||||||||
| Third country trade | HPAI | 10–11 tonnes per annum | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| LPAI | Not relevant | Not relevant | ||||||
| Intra‐EU movement | 95,000–115,000 tonnes per annum | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Extreme unlikely | Very unlikely (same score as raw feathers to account for the greater volume) | |
| LPAI | Not relevant | Not relevant | ||||||
| PATHWAY: Feed | ||||||||
| COMMODITY: Feed | ||||||||
| Third country trade | AI | Around 700,000–900,000 tonnes per annum | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Extreme unlikely (Non‐negligible(a)) |
| Intra‐EU movement | AI | Around 2.4–2.6 million tonnes per annum | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Extreme unlikely (Non‐negligible(a)) |
| PATHWAY: Bedding | ||||||||
| COMMODITY: Bedding | ||||||||
| Third country trade | AI | 15–50 thousand tonnes | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Extreme unlikely (Non‐negligible(a)) |
| Intra‐EU movement | AI | 850–900 thousand tonnes | Extreme unlikely | na | Extreme unlikely | Non‐negligible | Non‐negligible | Extreme unlikely (Non‐negligible(a)) |
| PATHWAY: Pharmaceuticals | ||||||||
| COMMODITY: Vaccines | ||||||||
| Third country trade | AI | No data available | Non‐negligible if approved | PCR | Non‐negligible | Unlikely | Extreme unlikely | Extreme unlikely |
| Intra‐EU movement | AI | No data available | Non‐negligible if approved | PCR | Non‐negligible | Unlikely | Extreme unlikely | Extreme unlikely |
| PATHWAY: Other animal by‐products | ||||||||
| COMMODITY: Bones, feet, casings, pet food (not livestock feed) | ||||||||
| Third country trade | HPAI | No data available | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| LPAI | No data available | Not relevant | ||||||
| Intra‐EU movement | HPAI | No data available | Extreme unlikely | na | Extreme unlikely | Extreme unlikely | Extreme unlikely | Extreme unlikely |
| LPAI | No data available | Not relevant | ||||||
Na: not applicable.
The health attestation for unprocessed manure traded between MSs is attached to the commercial document in TRACES, but the code cannot distinguish between other Cat 2 ABPs; therefore, data are not available.
Appendix G – AI transmission and spread parameters
1.
Appendix H – LPAI to HPAI mutation
1.
GISAID is acknowledged for making the genetic sequence information available that has been used in this analysis (GISAID, 2017) (Figures H.1–H.4).
Appendix I – HPAI and LPAI surveillance
I.1. Surveillance components
I.1.1. Poultry passive surveillance
Passive surveillance for notifiable avian disease in domestic poultry relies on notifications of clinically affected poultry to the competent authority by private veterinary surgeons or animal owners. Overall sensitivity of this surveillance component is dictated by private veterinary services (PVS)’ understanding their responsibilities in the detection or exclusion of notifiable disease and the engagement of different poultry sectors with national veterinary authorities.
Notifiable passive surveillance in poultry has very high sensitivity of detection of HPAI in gallinaceous poultry as these species are particularly susceptible to HPAI and consistently show severe clinical signs within 1–2 days of infection.66 The extent to which notifiable passive surveillance in poultry is sensitive in other poultry species, in particular Anseriformes, is dependent on the virulence of the circulating virus as well as host‐specific and environmental factors.67 The H5N8 HPAI virus circulating in Europe in 2014 (clade 2.3.4.4a) was not associated with enhanced mortality in wild birds and as a corollary it is likely that notifiable passive surveillance was less sensitive in Anseriformes during that event. However, in the H5N8 HPAI epizootic in 2016/2017 (clade 2.3.4.4b), anseriforme poultry were more frequently identified by this surveillance component, and many migratory and residential waterfowl were found dead due to HPAI (EFSA, ECDC, EURL, 2017a, 2017b).
The sensitivity of this surveillance component in the detection of LPAI follows a similar trend. Passive notifiable surveillance in poultry is unlikely to detect circulating LPAI in poultry populations unless the LPAI virus is unusually virulent causing non‐negligible levels of mortality or associated with considerable changes in production factors such as egg production, and/or feed and water intake. Where industry is particularly engaged and levels of coordination between industry and the competent authority are high, this sensitivity of detection of LPAI can be enhanced (see production monitoring section below). In comparison, the likelihood of passive notifiable surveillance in poultry detecting LPAI in farmed anseriforme populations is very low, unless there is significant concurrent disease at the holding which may serve to complicate the clinical picture.
Passive notifiable poultry surveillance is the front line methodology for early detection of HPAI in gallinaceous and to a lesser extent anseriforme poultry. While it is difficult to quantify its efficacy at EU level, Member State (MS) level or even between different poultry sectors within a MS, it should be noted that this component has been responsible for the vast majority of the identification of premises infected with HPAI since 2005.
I.1.2. Testing To Exclude (TTE) notifiable avian disease
TTE is a surveillance component designed to complement existing passive notifiable surveillance. It allows a PVS to send samples to either private or public laboratories to exclude the presence of notifiable avian disease without notifying at the competent authority. This can only be done where the PVS does not formally suspect notifiable disease otherwise the conventional passive notifiable cascade must be carried out. It should also be used with particular caution when the risk of avian influenza incursion into poultry premises is high, for example during an HPAI epizootic in that country or in contiguous countries.
One of the major benefits of TTE to producers is that while the classic notifiable disease surveillance requires premises to be put under movement restrictions, testing to exclude the presence of notifiable disease does not have this requirement. Should the holding subsequently test positive for notifiable avian disease then this would trigger the usual notifiable disease response and would trigger restrictions and confirmatory testing if required.
TTE is a useful tool to increase LPAI surveillance sensitivity and helps to build trust among government, producers and industry. The cost of TTE testing is usually covered by the private sector and has a lower priority for testing than conventional notifiable disease testing.
TTE is not covered by existing EU legislation but is used by a number of (private and/or public) organisations in some MSs (e.g. the UK, DE, the NL, IT) to empower decision making at PVS level and improve the overall sensitivity of detection where atypical or mild clinical presentation can complicate a successful differential diagnosis. This approach should only be used in case of non‐specific clinical signs (not to be used for instance with increased mortality or when AIV has been detected in the area).
I.1.3. Production parameter monitoring
MSs are advised to use production parameter monitoring as a form of syndromic surveillance in order to initiate the standard notifiable passive surveillance cascade described above. The legislation (Commission Implementing Decision (EU) 2017/263)68 describes the monitoring of mortality data, egg production records and feed/water intake as a method for the early detection of LPAI in gallinaceous poultry and HPAI in anseriforme poultry. This is currently undertaken consistently by at least a few MSs and would almost certainly result in higher sensitivity of detection of both notifiable and non‐notifiable disease. A research project in the NL is analysing this approach (no results available at this stage). Moreover, increased usage of this form of surveillance would also be likely to affect timeliness of detection, since production parameter monitoring, could also play a role in the detection of strains of AI that may subsequently mutate from an LPAI variant to a HPAI variant and will help in tracing back the incursion time point.
Adopting production parameter monitoring does come with an array of upfront costs as well as requiring an engaged industry together with coordination with the competent authority that may limit its use to heavily commercialised and intensively managed sectors. The requirements for managing commercially sensitive production data at a suitably confidential level alongside the cost in analysing this data with an inconsistent threat of incursion from HPAI and LPAI is likely to have hindered its earlier adoption. Furthermore, a non‐negligible false alarm rate where production parameter monitoring flags up holdings where AI is not present could seriously damage confidence in this component and would require careful examination of baseline data and any threshold drops in production that would flag up further examination.
Production parameter monitoring and TTE act synergistically and could, if used together, provide a useful approach to increasing LPAI surveillance sensitivity by using individual farm's baseline data and looking at production drops and empower PVS/Owner to trigger exclusion testing if necessary. The reluctance of industry to share confidential production data makes it unlikely that the public sector would be involved in this monitoring and would therefore dictate industry responsibility in the monitoring of production factors and take action when required.
Production parameter monitoring is a useful complement to the current system of notifiable disease surveillance in poultry but would require significant resource to set up for MSs that do not currently capture the data required to implement it effectively. In future years, if epizootics of the size and impact of the 2016/2017 event continue to occur, it may become a useful tool for particular sectors in heavily impacted MSs for the early detection of disease.
I.1.4. Annual active serological surveillance
In addition to mandatory notifiable avian disease surveillance (see Appendix I, Section I.1.1), MSs are also mandated to carry out annual serological testing on a number of poultry premises every year (Commission Decision 2010/367/EU). This testing can either be carried out on a representative cross‐section of their poultry population or alternatively MSs can use a risk‐based methodology designed to target ‘sentinel’ or higher risk populations of poultry that may be more vulnerable depending on risk strata such as geographical, demographic or ornithological factors. The objective of this surveillance component is to inform the competent authority of circulating avian influenza virus with a view to controlling the disease and to complement the existing notifiable disease surveillance by identifying evidence of historical infection in populations that escaped previous detection. This is particularly valuable for identification of HPAI in anseriforme populations where subclinical infection may preclude identification by passive notifiable avian disease surveillance. This also applies to the detection of H5 and H7 LPAI in gallinaceous poultry which may also present subclinically. This active surveillance programme in poultry is looking to identify AIV‐specific antibodies against subtypes H5 and/or H7 in the tested poultry and therefore is not designed as a method of early detection of AI infection and is unlikely to detect LPAI early enough to prevent mutation to HPAI, particularly where this may occur on a single farm. However, unlike passive surveillance where the number of investigations and the populations sampled will vary substantially year to year within a given region or country, active serological surveillance provides a consistent sampling frame (i.e. denominator data) which can provide evidence towards disease freedom which can be useful for trade purposes.
To increase this component's effectiveness, positive serological findings have to be followed up with further epidemiological investigations and (where possible) further sampling and virological testing to detect or rule out the presence of active virus infections. This programme of active serological surveillance has been in place since 2003 (Commission Decision 2002/649/EC) and since that time a minority of seropositive holdings have subsequently been found to have active virus on the holding. In 2013–2015 29.7% (33/111) of seropositive holdings were found to be infected with H5 or H7 virus on follow‐up testing (24.7% 23/93) for H5 seropositive holdings and 55.6% (10/18) for H7 seropositive holdings). In the remainder of cases, either the poultry have been removed from the holding (e.g. to slaughter) and could not be tested or no active virus infection was found on the premises. Serological testing is unable to differentiate between antibodies to HPAI and LPAI of the same H‐type, but given that gallinaceous poultry are likely to present severe clinical disease if infected with HPAI, it emphasises the need for testing anseriforme populations with this surveillance for detection of exposure to both LPAI and HPAI.
In the 2015/2016 H5 HPAI incident in France, the significant event of widespread AIV H5 infection in the south‐west of the country was preceded by several years of serological H5‐positives in this area of the country. Unfortunately, as these animals were tested prior to slaughter, further samples for virological testing were only taken in around half of the cases although epidemiological investigations were always conducted (see Annex A). It is still unclear as to when, where, or on how many occasions this circulating LPAIV mutated into HPAIV; however, it is informative to note that there was some evidence of H5 AI in this area before this extensive incident occurred (see Annex A). A key lesson from the French surveillance programme and outbreaks is that stricter follow up of seropositive holdings and implementation of measures is required to prevent AIV circulation on holdings over more than 1 year to prevent mutation into HPAI.
As currently implemented, there is a wide range of interpretation of the sampling frame for this surveillance component. This allows individual MSs to tailor their surveillance programme specific to their requirements depending on their demographic and poultry sector composition or alternatively to the sector which they decide is most vulnerable to introduction of AIV. While this has some benefits to MSs, it hinders effective surveillance evaluation of this component at EU level. For example, while testing laying hens or other gallinaceous poultry may be of use in identifying past exposure to LPAI, when there has been repeated exposure of HPAI in anseriforme populations which could show subclinical disease, it may be more useful to monitor anseriforme populations using serological surveillance given a limited amount of resource.
The criteria for risk‐based sampling considered by MSs vary considerably but can be categorised as follows:
Geographical – including proximity to waterbodies, contiguous countries and proximity to high density of migratory wild birds
Demographic – densely populated poultry areas
Production type/biosecurity level – free Range, Anseriforme and Game bird holdings, mixed poultry species holdings
Trade – targeting holdings with international trade (intra‐EU and Third countries)
Timing of sampling – timing sampling to coincide with seasonal production
Reactive sampling – increasing burden of sampling if contiguous areas report AI
The scientific evidence regarding the relative importance of these risk strata is unclear and may vary across the EU. The implementation of a risk‐based approach is often difficult as there are not enough and/or not reliable data and knowledge available to determine the relevant weight of each risk factor. The data provided by France (see Annex A) show that only seropositive anseriforme flocks were detected in 2012–2015, but the sampling scheme was not changed accordingly. Having a good knowledge of the poultry populations (number and location) is required to define an appropriate sampling plan.
The annual serologically surveillance programme is a useful tool in monitoring the ongoing health of poultry populations and is particularly useful following an outbreak to reassure the competent authority and trading partners that disease is not circulating in poultry populations. However, when there is evidence of HPAI circulating in Europe, MSs should focus their sampling on anseriforme populations or game bird holdings which may harbour subclinical infection.
I.1.5. Passive wild bird surveillance
Passive wild bird surveillance describes the convenience sampling of dead or moribund wild birds and was last described by Commission Decision 2010/367/EU. It often provides the initial evidence of incursion of HPAIV into Europe (EFSA, ECDC, EURL, 2017a, 2017b). MS use a risk‐based approach to identify dead or moribund wild birds and identify a target number of birds they intend to submit over the year. During epizootics where mortality in wild birds has been recorded, this component has a proven track record of being very useful. Wild bird mortality was identified in this way during the 2005–2007 H5N1 HPAI (2.2 clade) and 2016/2017 H5 HPAI (2.3.4.4b clade). While this surveillance was less effective during the 2014 H5N8 incursion (2.3.4.4a clade), there were detections of dead pelicans infected with H5N1 HPAIV (2.3.2.1c clade) in Bulgaria and Romania.
In the recent 2016/2017 epizootic, poultry outbreaks and positive wild bird submissions showed a strong spatial correlation (EFSA, ECDC, EURL, 2017a, 2017b); however, positive wild bird submissions were not always indicative of subsequent poultry incursions. Passive wild surveillance is less useful in epizootics not associated with high levels of wild bird mortality.
There are also some known unavoidable biases with this convenience sampling technique in that larger wild birds (swans, geese, raptors, gulls, pelicans) are more likely to be detected and submitted. Regional mass mortality events in smaller water birds may alleviate this bias, as these events are less likely to remain unreported (nine reported incidents of > 50 dead wild birds in 2016/2017 H5 HPAI epizootic). In addition to these biases, MSs take alternative approaches to sampling which will also affect which wild birds are found and where they are found. The probability of finding wild bird carcasses is also dependent on habitat types, which differ substantially across the EU. In addition, MSs should (according to Commission Decision 2010/367/EU) target specific species with known associations with HPAI. A list of ‘Target Species’ is provided in the legislation. Sampling and testing of wild bird faeces from the environment has been carried out as a means of active surveillance and may be considered as an approach that provokes little disturbance to natural habitats and birds. However, detection rates are expected to be low and, in positive cases, would require determination of the bird species by genetic means (mitochondrial DNA).
The risk‐based approach used by MSs to inform their collection of wild birds for this component helps to maximise sensitivity of detection by using geographical, ornithological and other ecological risk factors. While empirical data on risk factors to drive risk‐based sampling are lacking, there is some scientific consensus on the important factors to be considered and these are explicitly stated in the legislation. The inherent non‐representative sampling also makes it not possible to extrapolate the findings to the live wild bird population over any geographical region; this is also complicated by the sheer volume of species of wild birds and their associated clinical response to AIV infection. It is important to note that even in extensive epizootics such as the 2016/2017 H5 HPAI (2.3.4.4b clade) virus, there will be significant variation in the prevalence of AIV within wild bird populations and, as such, negative results from dead wild birds can play an important role in establishing and withdrawing interventions during an AI outbreak.
I.1.6. Active Wild Bird Surveillance
Active surveillance of clinically healthy wild birds was a legislative requirement for MSs between 2006 and 2010; however, since June 2010, there is no longer any formal requirement for this surveillance component to be carried out. Nevertheless, many MSs do still carry out this activity as a complement to their passive wild bird surveillance programme. The primary goal of this component is to detect changes in the epidemiology of AIV subtypes. MSs could (according to Commission Decision 2010/367/EU) target specific species with known associations with HPAI. These ‘target’ species include the species in which HPAI had been detected at the highest rate in the EU at the time of publication (i.e. June 2010), plus raptor species with large breeding populations in Europe. Targeting these species may well be more sensitive than testing wild birds at random. An update of the list of target species is suggested (EFSA, ECDC, EURL, 2017a, 2017b).
Targeting healthy wild birds is also useful in epizootics where wild bird mortality is not a defining feature of the incursion and could be the only way of finding evidence that disease is present in wild bird populations, as in 2014 with positive wild birds being found in Germany and the Netherlands through active wild bird surveillance of clinically healthy birds. Unlike passive surveillance of dead birds, active surveillance could potentially provide an evidence of infection prevalence in a defined area if sufficient sampling is carried out and a sampling strategy with little bias would be available. However, so far, at EU level, active surveillance of wild birds has a very low sensitivity of detection of HPAIV with PCR testing of clinically healthy birds unlikely to find active HPAIV. During the period where active surveillance was mandatory (2006–2010), MSs found 39 HPAIV positives from 246,952 submissions. Serological surveillance could play a valuable role in informing which wild bird species are more likely to be infected and shed HPAI, and even particular clades of H5 HPAI virus, when applied at a few high‐risk locations across the EU (Gilbert et al., 2014; Poen et al., 2016). While getting estimates of disease prevalence in a defined area can be useful, especially after an outbreak when case detections have slowed, to see whether virus is possibly circulating in absence of clinical disease, sufficient testing of wild birds, so as to detect a minimum level of disease, could be prohibitively expensive.
I.2. Poultry population densities
All MSs provided data to EFSA on the number of holdings for several poultry species. This is described in the scientific report (EFSA, ECDC, EURL, 2017a, 2017b), from which the figures below are copied.
I.3. Serological surveillance data
Figure I.3.
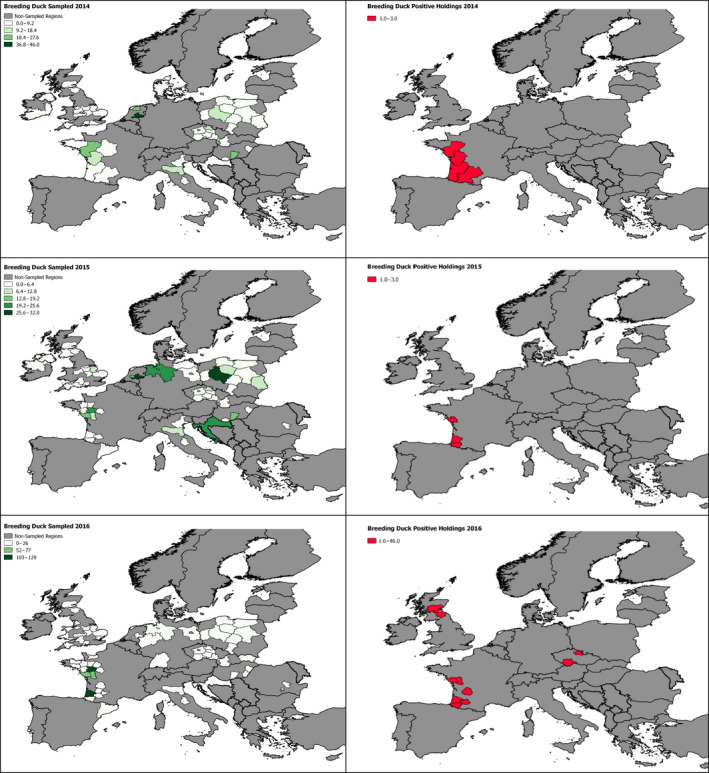
Breeding duck serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.4.
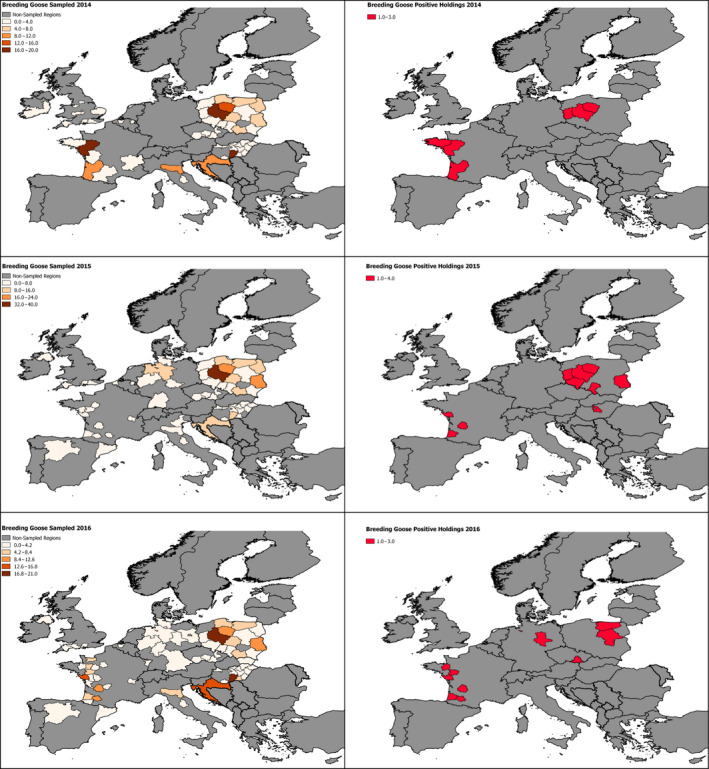
Breeding goose serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.5.
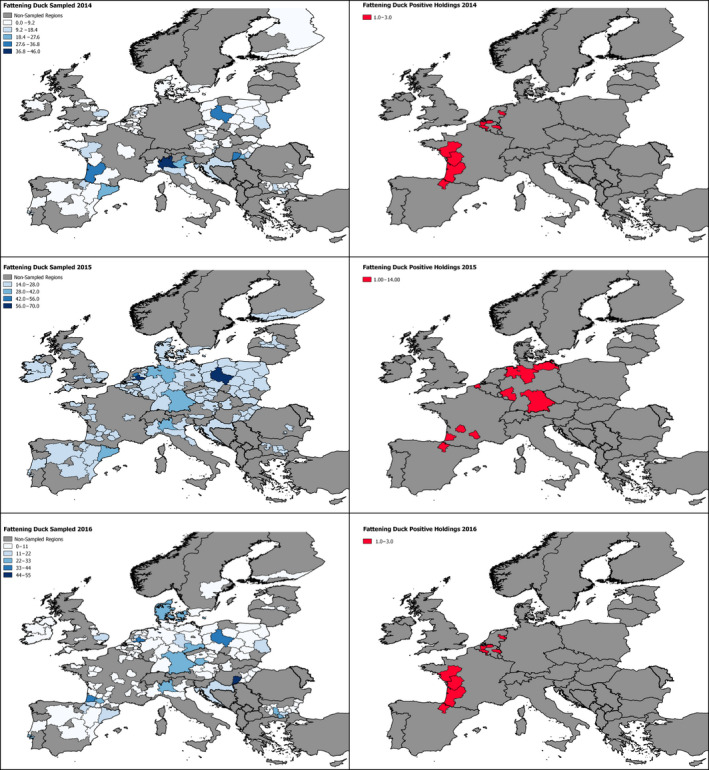
Fattening duck serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.6.
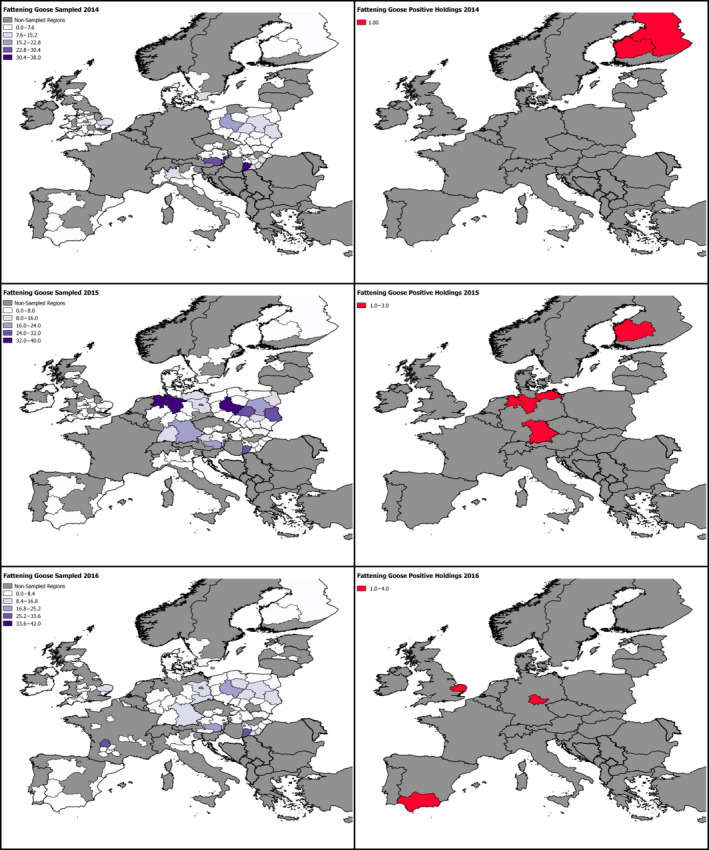
Fattening goose serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.7.
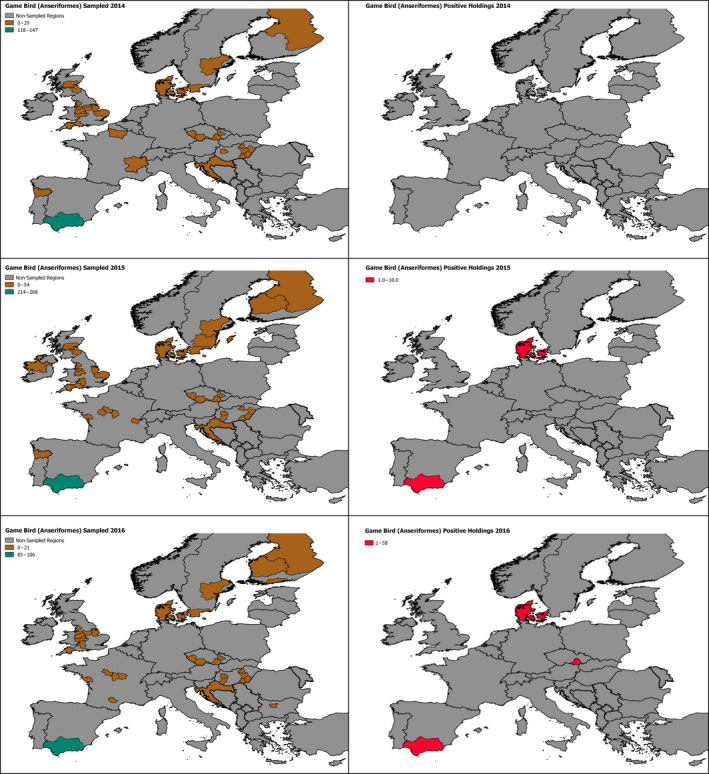
Game bird (Anseriformes) serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.8.
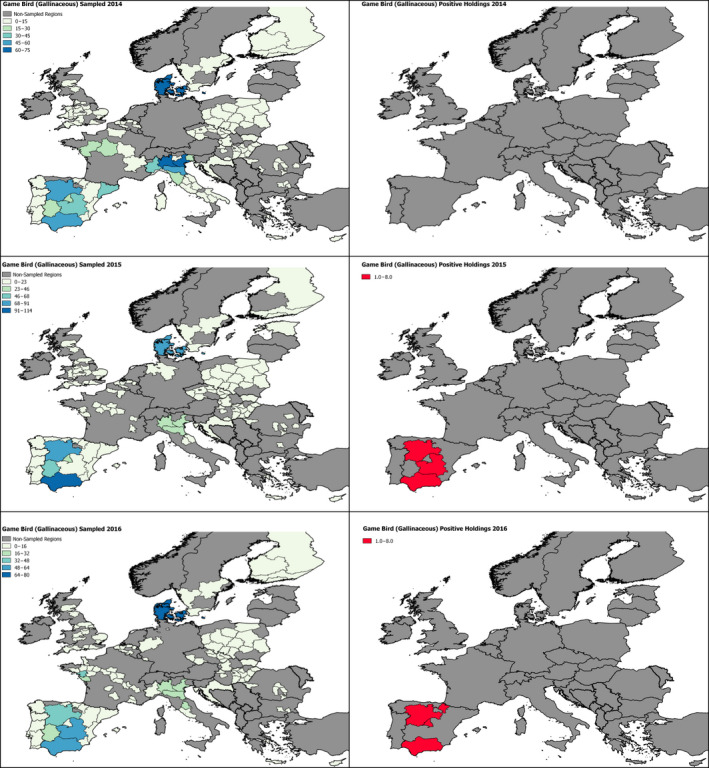
Game bird (Gallinaceous) serological surveillance sampling and regions with positive holdings detected, 2014–2016
Figure I.9.
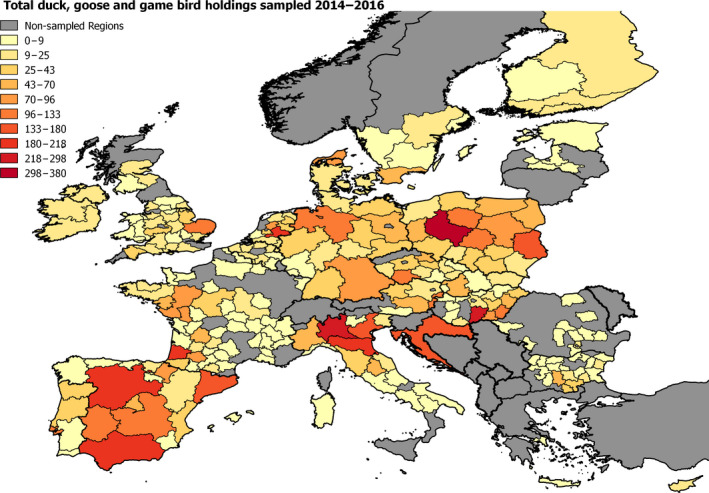
Map showing intensity of serological surveillance activity in farmed anseriforme poultry and all game birds between 2014 and 2016
Figure I.10.
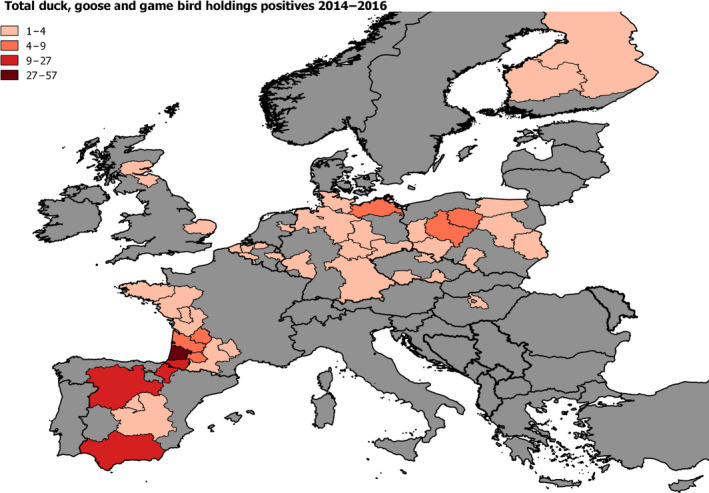
Map showing regions with holdings showing prior exposure to AI in farmed anseriforme poultry and all game birds, 2014–2016
Appendix J – Biosecurity
1.
Table J.1.
Description of the identified biosecurity measures commercial and non‐commercial holdings with indoor housing only or with outdoor access to poultry The table is based on the measures identified in the AI Statement (EFSA, 2017a), which was then edited and further elaborated
| ID | Biosecurity measure | Indoor – commercial holdings | Outdoor – commercial holdings | Non‐commercial holdings (backyards) |
|---|---|---|---|---|
| A | Prevent access to pests and mammals | Integrated pest control should actively be implemented in the all the zones of the holding (guaranteeing no poultry access) and access of other domestic animals (such as dogs or cats) and wild animals (such as fox, marten, badger) should be prevented by closing doors of the buildings and use of covering material. Baits and/or chemical pest control should always be used in accordance with the manufacturer's instructions | Buildings should have a closing doors system to prevent the access of other animals and rodent control should be implemented. Fences around the outdoor area should be used | |
| B | Prevent direct wild bird contact | It is assured by construction of bird proof roof, walls, windows and/or fences when poultry are housed indoors | In the outdoor area, fences and/or nets can be used with a maximum mesh diameter of 25 mm. Access of poultry to water bodies that could be visited by wild (water) birds should always be prevented. Feed (provided in containers) and water for poultry must be indoor to prevent the attraction of wild birds | |
| C | Prevention of direct contact with faecal droppings from flying wild birds | It is provided by construction of a roof and walls or fences when poultry are housed indoors. This includes no contact with faecal droppings entered with rain, etc. | In the outdoor area, horizontal fabric (e.g. canvas roof) should be used | |
| D | Make environment unattractive to wild birds | Prevent roosting and nesting of wild birds by making the holding environment unattractive to them. For example, clean spilled feed, keep grass on the holding cut (wild birds eat grass seeds, forage in long grass), select trees and shrubs to minimise wild bird attraction, remove fallen fruit around the holding, use persuasive elements. Placing flashing or rotating lights at the entry points will deter wild birds from entering. Water drainage is implemented to prevent uncovered water accumulation. Ensure there are no hobby poultry flocks on the holding | ||
| E | Contain poultry or fomites that were in contact with poultry during transport |
Vehicles transporting poultry, (hatching) eggs, carcasses or waste products (e.g. manure, litter) should be closed, preventing any loss of material (including via wind) during transport Ideally, access of these vehicles should be limited to small zones of the holding, preventing contact with animals and materials that will be introduced in the poultry houses. Rendering trucks should not enter a holding but instead remain on the public road The frequency of these transports should be reduced as much as possible (e.g. one visit of large volume instead of two visits) and vehicles driving from one poultry holding to another should be prevented where possible |
All materials with contact to poultry and poultry products should be transported in boxes. All transport material should be single use or cleaned and disinfected after use | |
| F | Clean and disinfect transport vehicles |
Vehicles transporting poultry, (hatching) eggs, carcasses or waste products (e.g. manure, litter) should be empty (except for poultry and hatching egg delivery) clean and disinfected when entering the zone of the holding where the poultry is present Vehicles transporting feed or bedding have ideally only access to the zones of the holding without presence of poultry and stop as close as possible to the place of destination (e.g. storage building) on a paved ground surface. They should not have access to the zones of the holding where the poultry is present Cleaning and disinfection of the wheels, wheel arches and footsteps/rests is implemented when entering/leaving the holding. Paths should be cleaned and disinfected after passage of the vehicle. The frequency of these transports should be reduced as much as possible (e.g. one visit of large volume instead of two visits) and vehicles driving from one poultry holding to another should be prevented where possible. In cases where feed is supplied in a recipient (e.g. big bag), it is recommended to use new recipients |
Transport is mainly done by the owner. Vehicles should be clean and must not go in the outdoor area | |
| G | Restricted access | Only staff and essential professional visitors (e.g. veterinarians, drivers, technicians, inspectors or catchers) can access the holding. Ideally, the borders of the zones of the holding without the presence of poultry are clearly marked with signs and the borders of the zones where the poultry is present are physically separated (e.g. fence, barrier in hygiene lock). Signs and physical separation have the scope to remind and enforce staff and professional visitors to implement the necessary biosecurity measures | Only owners (resident family) and essential professional visitors (e.g. veterinarians or inspectors) can access the area where poultry is present | |
| H | Biosecurity training | Persons working at the holding or giving regular service to the holding, should have participated in a general biosecurity training (e.g. via a course) adapted to poultry production and understanding the implications to animal health and welfare, human health and food safety. A holding‐specific biosecurity plan should be available. The roles and responsibilities of the staff should be clearly defined/explained accordingly and professional visitors should be informed on the holding's layout and biosecurity plan before entering the professional and production zones of the holding. Occasional visitors should be instructed and escorted by trained persons | Owners should know recommended biosecurity measures (e.g. via leaflet information material) | |
| I | Previous poultry contact |
Persons can only enter the holding if they had no contact with poultry, poultry waste and/or a poultry processing material at another holding (including backyard) within the previous 24 h. This time could be enlarged in a high‐risk situation, e.g. depending on the production system, virus characteristics, contact with wild birds (e.g. bird ringing or hunting), etc. The farm should provide a visitor book to register. Professional visitors should record their visit and keep their own record of poultry holding visits |
Not applicable | |
| J | Clean clothing and footwear | Clothing and footwear should always be clean (=free of organic material) before entry of the holding. Appropriate footwear hygiene measures can be applied (e.g. disposable overshoes) | The owner must change clothing and footwear after contacts or use protective cloths (e.g. overalls, disposable overshoes) during contact with poultry, poultry waste and/or a poultry processing material at another holding (including backyard) or wild birds. Hands should be washed with soap | |
| K | Cleaning and disinfection equipment | Any movable equipment should be cleaned and disinfected when entering and leaving a poultry production unit (e.g. use colour codes per poultry house) and per holding (no equipment should be used on multiple farms). All other stationary equipment should be cleaned and disinfected after each production cycle | All stationary equipment should be cleaned and disinfected at regular intervals (e.g. feed container) | |
| L | Hygiene lock to production unit |
Clothing, footwear (capable of being washed or disinfected) should be changed, hands should be washed and disinfected and hair should be covered before and after entry of the production unit. Clothing and footwear should be production unit‐specific. Persons can enter the production unit only when wearing personal protection equipment such as (disposable) coveralls or overalls, head covering and boots belonging to the holding. These should be cleaned and disinfected before/after use. Providing tools for scraping the soles of footwear will facilitate their cleaning. Place a hygiene line (a clear physical barrier, e.g. bench or low wall) is suggested, as a line is very easily crossed and therefore ignored) at the entrance of each poultry house: footwear and clothing have to remain at each side of this line in the hygiene lock These measures should be valid for all ‘entry’ points |
Not applicable | |
| M | Closed bedding storage | Should be achieved in closed facilities with concrete floor to prevent access of animals (including wild birds and rodents). In addition, rodent control should be applied | ||
| N | Potable drinking water | Water supplied to poultry should be potable or comply with the national water quality standard. Poultry should not have access to surface and rain water. Surface water should not be used for cleaning | Feed and water supply should be provided indoors. Water supplied to poultry should be potable or comply with the national water quality standard. Poultry should not have access to surface and rain water. Surface water should not be used for cleaning | Supplied to poultry should be potable or comply with the national water quality standard. Poultry should not have access to surface water |
| O | Filtration of incoming air | Filtration of incoming air can be achieved per production unit in some specific housing designs, via (e.g. HEPA) filters | Non‐applicable | Non‐applicable |
| P | Protected waste storage | Storage of manure and used bedding should not be stored on the premises and immediately removed after a production cycle. Otherwise, it should be done in a way to prevent access of animals. The collection of manure should also be done in a way to prevent access of animals | Manure and used bedding/litter should be stored covered | |
| Q | Carcass disposal | Should be done in a vermin‐proof structure, remote from the production units and close to the public road (accessible from outside the farm). It should be on a solid surface to allow proper cleaning and disinfection. Cooling is recommended as it facilitates longer storage and subsequently less frequent carcass transport because frequent carcass transport has a higher risk than keeping carcasses longer at a holding. The carcass disposal area should be distanced/separated from poultry area of the holding | A veterinarian needs to be contacted in case of increased mortality in the flock or a disease is suspected. Suspected carcasses (except samples) should not be moved from the backyard. Carcasses should be disposed in a safe way, e.g. buried, after sampling | |
| R | Health monitoring | Starts by checking the health certificates for new poultry and/or hatching eggs when arriving at a holding. The place of origin should be documented and it is recommended to keep the number of suppliers as limited as possible. Quarantine should be implemented for new poultry and/or hatching eggs when arriving at a holding, in particular when new animals will be introduced in a flock. Monitoring of the poultry health status should be continued throughout the production cycle under the supervision of a veterinarian | The owner of the backyard should buy poultry from professional suppliers with regular health monitoring to assure the birds are healthy, the invoice should be kept. A veterinarian should be consulted in case of clinical signs | |
| S | Flock management | Should be based on the ‘all‐in, all‐out’ principle, preferably at holding level. This means that all birds have a similar age and the houses will be emptied, cleaned (including removal of manure) and disinfected after each production cycle. Proper drying period after disinfection is required. Movement of feed between flocks should be avoided. Removal of carcasses, broken eggs and rejected eggs should be done at least daily | Should be based on the ‘all‐in, all‐out’ principle, preferably at holding level. This means that all birds have a similar age and the houses will be emptied, cleaned (including removal of manure) and disinfected after each production cycle. A sanitary vacuum of 14 days is recommended. Movement of feed between flocks should be avoided. Removal of carcasses, broken eggs and rejected eggs should be done at least daily | Removal of carcasses, broken eggs and rejected eggs should be done at least daily. New birds should be isolated in quarantine before being added to a flock |
| T | Separation of poultry species | Mixing of poultry species should be prevented by keeping each species and production type in a separate house. In particular mixing of ducks or geese with other poultry species is forbidden. Separation of production systems on a holding is recommended | Mixing of poultry species should be prevented by keeping each species in a separate house/outdoor area. In particular mixing of ducks or geese with other poultry species should be prevented | |
Some specific recommendations:
Feed is in most cases stored in a closed environment. It is recommended that each holding has his own bag to collect dust when feed is blown into silos.
If closed bedding storage is not feasible, preventing of wild bird access is the minimum to achieve (e.g. roof and netting).
Catching crews are often visiting more than one holding per day. They should at least wash their hands, change clothes and footwear and wear (single‐use) hair rotection.
Annex A – Avian Influenza surveillance in France from 2012 to 2015
1.
Annex A can be found in the online version of this output, under the section ‘Supporting information’, at: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4991/full
Supporting information
Avian Influenza surveillance in France from 2012 to 2015
Suggested citation: EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke H‐H, Velarde A, Willeberg P, Winckler C, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Baldinelli F, Barrucci F, Fabris C, Martino L, Mosbach‐Schulz O, Verdonck F, Morgado J and Stegeman JA, 2017. Scientific opinion on avian influenza. EFSA Journal 2017;15(10):4991, 233 pp. 10.2903/j.efsa.2017.4991
Requestor: European Commission
Question numbers: EFSA‐Q‐2015‐00214 and EFSA‐Q‐2016‐00348
Panel members: Dominique Bicout, Anette Bøtner, Andrew Butterworth, Paolo Calistri, Klaus Depner, Sandra Edwards, Bruno Garin‐Bastuji, Margaret Good, Christian Gortázar Schmidt, Virginie Michel, Miguel Angel Miranda, Simon More, Søren Saxmose Nielsen, Mohan Raj, Liisa Sihvonen, Hans Spoolder, Jan Arend Stegeman, Hans‐Hermann Thulke, Antonio Velarde, Preben Willeberg and Christoph Winckler.
Acknowledgements: The Panel wishes to thank the members of the Working Group on avian influenza: Dominique Bicout, Andrew Breed, Adam Brouwer, Anette Bøtner, Jeroen Dewulf, Matthieu Guillemain, Timm Harder, Andrew David Hart, Isabella Monne, Helen Roberts, Jan Arend Stegeman, Hans‐Hermann Thulke, Martin Wikelski for the preparatory work on this scientific output and the hearing experts: Patrick Daniel, Teun Fabri, Nicolas Gaidet, Barbara Grabkowsky, Vittorio Guberti, Marie Guyot, Ursula Hoefle, Adeline Huneau Salaun, Thijs Kuiken, Hartmut Meyer, Virginie Michel, Ioana Neghirla, Éric Niqueux, Yali Si, Brigita Slavec, Krzysztof Smietanka, Christoph Staubach, Anna Luca Vescei, Josanne Verhagen, Sophie Von Dobschuetz and ECDC staff member Cornelia Adlhoch and the AI Consortium (Erasmus University, NL; Wageningen University, NL; Animal & Plant Health Agency, UK; Friederich‐Loeffler‐Institut, DE; Linnaeus University, SE; Istituto Zooprofilattico Sperimentale delle Venezie, IT) for the support provided to this scientific output and Adeline Huneau‐Salaün, Anne Bronner for writing the Annex on the AI surveillance in France from 2012 to 2015.
Amendment: This output has been updated to include data received from Finland after the date of publication. These do not impact on the overall outcomes. The original is available on request.
Adopted: 14 September 2017
Amended: 14 December 2017
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder:
Figure 1: © FAO
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.5018/full
This publication is linked to the following EFSA Supporting Publications article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1282/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1283/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1284/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1285/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1286/full, http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2017.EN-1287/full
Notes
Council Directive 2005/94/EC of 20 December 2005 on Community measures for the control of avian influenza and repealing Directive 92/40/EEC. OJ L 10, 14.1.2006, p. 16–65.
Commission Decision 2005/734/EC of 19 October 2005 laying down biosecurity measures to reduce the risk of transmission of highly pathogenic avian influenza caused by Influenza virus A subtype H5N1 from birds living in the wild to poultry and other captive birds and providing for an early detection system in areas at particular risk. OJ L 274, 20.10.2005, p. 105–107.
Commission Decision 2006/563/EC of 11 August 2006 concerning certain protection measures in relation to highly pathogenic avian influenza of subtype H5N1 in wild birds in the Community and repealing Decision 2006/115/EC. OJ L 222, 15.8.2006, p. 11.
Commission Decision 2020/367/EC of 25 June 2010 on the implementation by Member States of surveillance programmes for avian influenza in poultry and wild birds. OJ L 166, 1.7.2010, p. 22–32.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1–24.
Regulation EU 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). OJ L 84, 31.3.2016, p. 1–208.
http://ec.europa.eu/food/animals/animal-diseases/control-measures/avian-influenza/index_en.htm, last accessed on 13 June 2016 (European Commission, 2016).
Commission Regulation (EU) No 2011/142 of 25 February 2011 implementing Regulation 2009/1069/EC of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 54, 26.2.2011, p. 1–254.
Commission Decision 2006/437/EC of 4 August 2006 approving a Diagnostic Manual for avian influenza as provided for in Council Directive 2005/94/EC. OJ L 237, 31.8.2006, p. 1–27.
What is in the EU Regulation to date: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32010D0367&rid=1
Commission Decision 2007/268/EC of 13 April 2007 on the implementation of surveillance programmes for avian influenza in poultry and wild birds to be carried out in the Member States and amending Decision 2004/450/EC. OJ L 115, 3.5.2007, p. 3–17.
MSs using an enzyme‐linked immunosorbent assay (ELISA) to detect AI in serum will likely detect H9‐specific antibodies but do not have the obligation to notify H9 findings. If H9N2 would be identified, sequencing would be required to differentiate genogroup G1 viruses from other genogroups. Analysis of H9 viruses would be more relevant for human health aspects, but this is beyond the current objectives of AI surveillance in poultry.
There is no sound algorithm available to combine qualitative risk scores.
http://platform.gisaid.org (GISAID, 2017)
The word feral is often used for birds of likely captive origin having established viable (but often non‐migratory) populations in the wild, such as, for example some Greylag geese in the Netherlands, or Wood duck in the UK.
The lack of consideration of the migratory non‐water birds as a vehicle of HPAI introduction (Figure 4) was assessed as a source of uncertainty potentially affecting the outcome of the model.
Viral persistence for 13 days in the environment is an approximation from published data on viral perseverance (see Section D.1.9, Appendix D). Analysis in an earlier version of the model indicated that extending virus persistence to 60 days increased the number of infected birds whereas the kinetics of the epidemic remained identical.
Under the model conditions and assumptions, the critical number is around 1,700 for 95% quantile. While for median the critical number is around 11,000. Extrapolation of this value to the real world is difficult given the high uncertainty.
For each simulated prevalence distribution, the 95% simulation quantile from the right tail of the distribution is considered as a worst case: any particular sample from this distribution have 5% chance of being greater than this value.
Total population size at the end of the migratory season is 10,000 wild birds.
Due to the assumptions taken into account (see Appendix C). For instance, introduction of juveniles was not considered.
Reaching the critical number of susceptible residential birds after entry of susceptible migratory birds for around 10 days (population capacity 100,000 birds; median 0.000, 95th percentile 0.023 at day 125; see Figure 5, scenario 2).
The presence of 9,000 susceptible resident water birds (population capacity 100,000 birds; median 0.000, 95th percentile 0.005 at day 125; see Figure 5, scenario 4).
Looking at the 95th percentile, less than 1/1,000 holdings without biosecurity would become infected by HPAI H5N8 clade 2.3.4.4 when the wild bird reservoir contains 1,000 wild birds (in scenario 1) (see Table E.1, Appendix E).
For instance, disinfection of boots, changing clothes, washing hands, etc.
For instance, rigorous implementation of showering in, complete exclusion of wild birds to feed, bedding and animal by‐products, separation of houses, etc.
Median numbers of 4080, 3525 and 300 for the HPAIV clades 2.3.4.4, 2.3.2.1c and 2.2.1.2, respectively.
Median 0, 95th percentile 15 and 170 infected per 1,000 holdings considering wild bird populations of 10,000 and 100,000 birds, respectively.
Median 0, 95th percentile 0 and 53 infected per 1,000 holdings considering wild bird populations of 10,000 and 100,000 birds, respectively.
95th percentile 0 considering wild bird populations of 100,000 birds for scenarios 2, 3 and 4.
Water bird population estimates retrieved from www.wpe.wetlands.org on 10 January 2017.
Retrieved from www.wpe.wetlands.org on 18 April 2017.
Uncertainty distribution extreme percentiles.
Commission Regulation (EC) No 798/2008 of 8 August 2008 laying down a list of third countries, territories, zones or compartments from which poultry and poultry products may be imported into and transit through the Community and the veterinary certification requirements. OJ L 226, 23.8.2008, p. 1–94.
Commission Implementing Regulation (EU) No 139/2013 of 7 January 2013 laying down animal health conditions for imports of certain birds into the Union and the quarantine conditions thereof. OJ L 47, 20.2.2013, p. 1–17.
Commission Decision 2007/25/EC of 22 December 2006 as regards certain protection measures in relation to highly pathogenic avian influenza and movements of pet birds accompanying their owners into the Community. OJ L 8, 13.1.2007, p. 29–34.
Council Directive 92/65/EEC of 13 July 1992 laying down animal health requirements governing trade in and imports into the Community of animals, semen, ova and embryos not subject to animal health requirements laid down in specific Community rules referred to in Annex A (I) to Directive 90/425/EEC. OJ L 268, 14.9.1992, p. 54–72.
Council Directive 2009/158/EC of 30 November 2009 on animal health conditions governing intra‐Community trade in, and imports from third countries of, poultry and hatching eggs. OJ L 343, 22.12.2009, p. 74–113.
Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff. OJ L 256, 7.9.1987, p. 1–675.
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002. OJ L 300, 14.11.2009, p. 1–33.
Including both experimental and field data.
TTE is a surveillance component designed to complement existing passive notifiable surveillance. It allows a PVS to send samples to either private or public laboratories to exclude the presence of notifiable avian disease without notifying at the competent authority. This can only be done where the PVS does not formally suspect notifiable disease otherwise the conventional passive notifiable cascade must be carried out. It should also be used with caution when the risk of avian influenza incursion into poultry premises is high, for example during an HPAI epizootic in that country or in contiguous countries. More information is provided in Section I.1.2.
For instance, during the 2016/2017 epidemic, some MSs stopped reporting the number of wild birds found dead within an area where an infected wild bird was detected and hence considered affected.
BE, BG, DE, FI, FR, IT, LI, the NL, RO, the UK.
The diameter (e.g. 25 mm) should be selected to prevent access of local and migrating wild water birds. Netting could be opened and closed depending on the risk, as performed in fruit production sector. Song birds might sit on the nets but experts indicate that this is not likely for most water birds (which have the highest risk to excrete AIV).
Dussau‐distribution composed of a straw bale shredder system standing outside and a pipe‐filling system inside the turkey house.
This need is included in welfare regulations of several countries.
For instance, in Barbari duck production in Hungary, there are three laying cycles in a lifetime, interrupted twice by 14 weeks of moulting (in the first round 25–30 weeks, in the second 22 weeks and in the third 19 weeks of production). The total life cycle lasts around 3 years, the rearing period included. (info provided by Anna Luca Vecsei, 18 August 2017).
For example ‘Biocheck poultry’ http://www.biocheck.ugent.be/v4/about/poultry/ which is available in several languages (Biocheck (Ghent University), 2017).
Hygiënescan AVINED.
Commission Decision 2006/415/EC of 14 June 2006 concerning certain protection measures in relation to highly pathogenic avian influenza of the subtype H5N1 in poultry in the Community and repealing Decision 2006/135/EC. OJ L 164, 16.6.2006, p. 51–60.
https://ecdc.europa.eu/en/publications-data/toolkit-investigation-cases-avian-influenza-humans, last accessed on 27 August 2017.
For more information, see Adlhoch et al. (2016).
It is noted that Directive 2005/94/EC requires that Member States have established expert groups in their contingency plans.
The modelling was done before the onset of the 2016–2017 HPAI outbreaks.
Waterbodies have in general relative constant, low temperatures, which tend to prolong the survival of the virus, and desiccation of the virus cannot occur in water, as it can happen on the ground.
Council Regulation (EEC) No 2658/87 of 23 July 1987 on the tariff and statistical nomenclature and on the Common Customs Tariff. OJ L 256, 7.9.1987, p. 1–675.
Commission Regulation (EC) No 318/2007 of 23 March 2007 laying down animal health conditions for imports of certain birds into the Community and the quarantine conditions thereof. OJ L 84, 24.3.2007, p. 7–29.
Commission Regulation (EC) No 92/2005 of 19 January 2005 implementing Regulation (EC) No 1774/2002 of the European Parliament and of the Council as regards means of disposal or uses of animal by‐products and amending its Annex VI as regards biogas transformation and processing of rendered fats. OJ L 19, 21.1.2005, p. 27–33.
Commission Regulation (EC) No 136/2004 of 22 January 2004 laying down procedures for veterinary checks at Community border inspection posts on products imported from third countries. OJ L 21, 28.1.2004, p. 11–23.
Initial clinical presentation of HPAI infection in chickens and turkeys may be sudden death with little or no other preceding clinical signs or gross pathology. Alternatively, there may be acute disease with signs of systemic disease including respiratory symptoms (ocular/nasal discharges, coughing, dyspnoea and swelling of infraorbital sinuses and/or head), apathy, depression, lethargy, cyanosis and reductions in feed and water intake. Nervous signs such as ataxia, paralysis of the wings and pedalling movements of the legs may be seen whilst in laying birds there may be a significant drop in both the quantity and quality of egg production. In these birds, ‘yolk peritonitis’ may be seen in gross pathology.
In other poultry species, clinical presentation can be more variable with ducks and geese generally unlikely to show the levels of acute disease seen in gallinaceous poultry. However, it should be noted that infection of ducks with H5N1 (2.3.2.1c clade) and H5N8 (2.3.4.4b clade) viruses can result in systemic infection and death. More often than in galliforme birds neurological disorders will develop (ataxia, torticollis, shivering, etc.). Reductions of feed and water intake are not considered in ‘passive surveillance’ but in ‘production parameter monitoring’.
Commission Implementing Decision (EU) 2017/263 of 14 February 2017 on risk mitigating and reinforced biosecurity measures and early detection systems in relation to the risks posed by wild birds for the transmission of highly pathogenic avian influenza viruses to poultry. OJ L 39, 16.2.2017, p. 6–11.
References
- Abdelwhab EM, Grund C, Aly MM, Beer M, Harder TC and Hafez HM, 2012. Influence of maternal immunity on vaccine efficacy and susceptibility of one day old chicks against Egyptian highly pathogenic avian influenza H5N1. Veterinary Microbiology, 155, 13–20. [DOI] [PubMed] [Google Scholar]
- Abdelwhab EM, Veits J and Mettenleiter TC, 2013. Avian influenza virus NS1 A small protein with diverse and versatile functions. Virulence, 4, 583–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwhab EM, Veits J and Mettenleiter TC, 2014. Prevalence and control of H7 avian influenza viruses in birds and humans. Epidemiology and Infection, 142, 896–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwhab EM and Abdel‐Moneim AS, 2015. Epidemiology, ecology and gene pool of influenza A virus in Egypt: will Egypt be the epicentre of the next influenza pandemic? Virulence, 6, 6–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelwhab EM, Hassan MK, Abdel‐Moneim AS, Naguib MM, Mostafa A, Hussein ITM, Arafa A, Erfan AM, Kilany WH, Agour MG, El‐Kanawati Z, Husseini HA, Selim AA, Kholousy S, El‐Naggar H, El‐Zoghby EF, Samy A, Iqbal M, Eid A, Ibraheem EM, Pleschka S, Veits J, Nasef SA, Beer M, Mettenleiter TC, Grund C, Ali MM, Harder TC and Hafez HM, 2016. Introduction and enzootic of A/H5N1 in Egypt: virus evolution, pathogenicity and vaccine efficacy ten years on. Infection Genetics and Evolution, 40, 80–90. [DOI] [PubMed] [Google Scholar]
- Abolnik C, Londt BZ, Manvell RJ, Shell W, Banks J, Gerdes GH, Akol G and Brown IH, 2009. Characterisation of a highly pathogenic influenza A virus of subtype H5N2 isolated from ostriches in South Africa in 2004. Influenza and Other Respiratory Viruses, 3, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abolnik C, 2014. A current review of avian influenza in pigeons and doves (Columbidae). Veterinary Microbiology, 170, 181–196. [DOI] [PubMed] [Google Scholar]
- Achenbach JE and Bowen RA, 2011. Transmission of avian influenza A viruses among species in an artificial barnyard. PLoS ONE, 6, e17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adlhoch C, Gossner C, Koch G, Brown I, Bouwstra R, Verdonck F, Penttinen P and Harder T, 2014. Comparing introduction to Europe of highly pathogenic avian influenza viruses A(H5N8) in 2014 and A(H5N1) in 2005. Eurosurveillance, 19, 2–6. [DOI] [PubMed] [Google Scholar]
- Adlhoch C, Brown IH, Angelova SG, Balint A, Bouwstra R, Buda S, Castrucci MR, Dabrera G, Dan A, Grund C, Harder T, van der Hoek W, Krisztalovics K, Parry‐Ford F, Popescu R, Wallensten A, Zdravkova A, Zohari S, Tsolova S and Penttinen P, 2016. Highly pathogenic avian influenza A(H5N8) outbreaks: protection and management of exposed people in Europe, 2014/15 and 2016. Eurosurveillance, 21, 2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal F, Saeed A, Sharif MA, Ayub N and Hassan S, 2012. Pathogenicity of avian influenza virus H5N1 2007 isolates from Pakistan. Asian Pacific Journal of Tropical Biomedicine, 2, S380–S382. [Google Scholar]
- Ahmed ZAM, Hussin HA, Rohaim MA and Nasr SAES, 2012. Efficacy of composting dead poultry and farms wastes infected with avian influenza virus H5N1. American‐Eurasian Journal of Agricultural and Environmental Sciences, 12, 588–596. [Google Scholar]
- Aiello R, Beato MS, Mancin M, Rigoni M, Romero Tejeda A, Maniero S, Capua I and Terregino C, 2013. Differences in the detection of highly pathogenic avian influenza H5N1 virus in feather samples from 4‐week‐old and 24‐week‐old infected Pekin ducks (Anas platyrhynchos var. domestica). Veterinary Microbiology, 165, 443–447. [DOI] [PubMed] [Google Scholar]
- Al‐Attar MY, Danial FA and Al‐Baroodi SY, 2008. Detection of antibodies against avian influenza virus in wild pigeons and starlings. Journal of Animal and Veterinary Advances, 7, 448–449. [Google Scholar]
- Alexander DJ and Spackman D, 1981. Characterization of influenza‐a viruses isolated from turkeys in England during march‐may 1979. Avian Pathology, 10, 281–293. [DOI] [PubMed] [Google Scholar]
- Alexander DJ, 2000. A review of avian influenza in different bird species. Veterinary Microbiology, 74, 3–13. [DOI] [PubMed] [Google Scholar]
- Alkhamis M, Willeberg P, Carlsson U, Carpenter T and Perez A, 2012. Alternative scan‐based approaches to identify space‐time clusters of highly pathogenic avian influenza virus H5N1 in wild birds in Denmark and Sweden in 2006. Avian Diseases, 56, 1040–1048. [DOI] [PubMed] [Google Scholar]
- Alkhamis M, Perez A, Batey N, Howard W, Baillie G, Watson S, Franz S, Focosi‐Snyman R, Onita I, Cioranu R, Turcitu M, Kellam P, Brown IH and Breed AC, 2013. Modeling the association of space, time, and host species with variation of the HA, NA, and NS genes of H5N1 highly pathogenic avian influenza viruses isolated from birds in Romania in 2005–2007. Avian Diseases, 57, 612–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM and May RM, 1991. Infectious diseases of humans: dynamics and control. Oxford University Press, Oxford, UK. 757 pp. [Google Scholar]
- Anonymous , 2016. Avian influenza outbreaks continue in France. Veterinary Record, 178, 200. [DOI] [PubMed] [Google Scholar]
- Antarasena C, Sirimujalin R, Prommuang P, Blacksell SD, Promkuntod N and Prommuang P, 2006. Tissue tropism of a Thailand strain of high‐pathogenicity avian influenza virus (H5N1) in tissues of naturally infected native chickens (Gallus gallus), Japanese quail (Coturnix coturnix japonica) and ducks (Anas spp.). Avian Pathology, 35, 250–253. [DOI] [PubMed] [Google Scholar]
- APHA (Animal and Plant Health Agency), 2014. H5N8 highly pathogenic avian influenza outbreak (AIV2014/01) in breeding ducks. England, UK. 25 pp. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/412086/ai-epi-report-nov2014.pdf
- APHA (Animal and Plant Health Agency), 2015. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2014. Weybridge, United Kingdom. 131 pp. Available online: http://www.academia.edu/24284623/Annual_Report_on_surveillance_for_avian_influenza_in_poultry_and_in_wild_birds_in_Member_States_of_the_European_Union_in_2014
- Arafa AS, Naguib MM, Luttermann C, Selim AA, Kilany WH, Hagag N, Samy A, Abdelhalim A, Hassan MK, Abdelwhab EM, Makonnen Y, Dauphin G, Lubroth J, Mettenleiter TC, Beer M, Grund C and Harder TC, 2015. Emergence of a novel cluster of influenza A(H5N1) virus clade 2.2.1.2 with putative human health impact in Egypt, 2014/15. Eurosurveillance, 20, 2–8. [DOI] [PubMed] [Google Scholar]
- Artois M, Bicout D, Doctrinal D, Fouchier R, Gavier‐Widen D, Globig A, Hagemeijer W, Mundkur T, Munster V and Olsen B, 2009. Outbreaks of highly pathogenic avian influenza in Europe: the risks associated with wild birds. Revue Scientifique Et Technique‐Office International Des Epizooties, 28, 69–92. [DOI] [PubMed] [Google Scholar]
- AviagenTurkeys , 2015. How to set up farm biosecurity? Available online: http://www.aviagenturkeys.com/uploads/2015/11/05/CL21%20Biosecurity%20Poster%20EN%20V1.pdf [Accessed: 18 May 2017].
- Barr DA, Kelly AP, Badman RT, Campey AR, Orourke MD, Grix DC and Reece RL, 1986. Avian influenza on a multi‐age chicken farm. Australian Veterinary Journal, 63, 195–196. [DOI] [PubMed] [Google Scholar]
- Barral M, Alvarez V, Juste RA, Agirre I and Inchausti I, 2008. First case of highly pathogenic H5N1 avian influenza virus in Spain. Bmc Veterinary Research, 4, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean WJ, Kawaoka Y, Wood JM, Pearson JE and Webster RG, 1985. Characterization of virulent and avirulent a‐chicken‐pennsylvania‐83 influenza a viruses potential role of defective interfering rna species in nature. Journal of Virology, 53, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard CW, Brugh M and Johnson DC, 1984. Laboratory studies with the Pennsylvania avian influenza viruses (H5N2). Proceedings of the United States Animal Health Association, 88, 462–473. [Google Scholar]
- Beato MS, Terregino C, Cattoli G and Capua I, 2006. Isolation and characterization of an H10N7 avian influenza virus from poultry carcasses smuggled from China into Italy. Avian Pathology, 35, 400–403. [DOI] [PubMed] [Google Scholar]
- Beato MS, Mancin M, Bertoli E, Buratin A, Terregino C and Capua I, 2012. Infectivity of H7 LP and HP influenza viruses at different temperatures and pH and persistence of H7 HP virus in poultry meat at refrigeration temperature. Virology, 433, 522–527. [DOI] [PubMed] [Google Scholar]
- Becker WB, 1966. The isolation and classification of Tern virus: influenza virus A/Tern/South Africa/1961. Journal of Hygiene, 64, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendfeldt ES, Peer RW and Flory GA, 2006. In‐house composting as a rapid response to avian influenza. BioCycle, 47, 38–42. [Google Scholar]
- Bertran K and Swayne DE, 2014. High doses of highly pathogenic avian influenza virus in chicken meat are required to infect ferrets. Veterinary Research, 45, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran K, Busquets N, Abad FX, de la Fuente JG, Solanes D, Cordon I, Costa T, Dolz R and Majo N, 2012. Highly (H5N1) and low (H7N2) pathogenic avian influenza virus infection in falcons via nasochoanal route and ingestion of experimentally infected prey. PLoS ONE, 7, e32107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins SN, Dusek RJ, White CL, Gidlewski T, Bodenstein B, Mansfield KG, DeBruyn P, Kraege D, Rowan E, Gillin C, Thomas B, Chandler S, Baroch J, Schmit B, Grady MJ, Miller RS, Drew ML, Stopak S, Zscheile B, Bennett J, Sengl J, Brady C, Ip HS, Spackman E, Killian ML, Torchetti MK, Sleeman JM and Deliberto TJ, 2016. Widespread detection of highly pathogenic H5 influenza viruses in wild birds from the Pacific Flyway of the United States. Scientific Reports, 6, 28980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi YH, Zhang ZJ, Liu WJ, Yin YB, Hong JM, Li XD, Wang HM, Wong G, Chen JJ, Li YF, Ru WD, Gao RY, Liu D, Liu YX, Zhou BP, Gao GF, Shi WF and Lei FM, 2015. Highly pathogenic avian influenza A(H5N1) virus struck migratory birds in China in 2015. Scientific Reports, 5, 12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi YH, Chen JJ, Zhang ZJ, Li MX, Cai TL, Sharshov K, Susloparov I, Shestopalov A, Wong G, He YB, Xing Z, Sun JQ, Liu D, Liu YX, Liu L, Liu WJ, Lei FM, Shi WF and Gao GF, 2016. Highly pathogenic avian influenza H5N1 Clade 2.3.2.1c virus in migratory birds, 2014–2015. Virologica Sinica, 31, 300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biocheck (Ghent University) , 2017. Biocheck.ugent Poultry. Available online: http://www.biocheck.ugent.be/v4/about/poultry/ [Accessed: 18 May 2017].
- Bonfante F, Fusaro A, Zanardello C, Patrono LV, De Nardi R, Maniero S and Terreqino C, 2014. Lethal nephrotropism of an H10N1 avian influenza virus stands out as an atypical pathotype. Veterinary Microbiology, 173, 189–200. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Monne I, Tamba M, Santucci U, Massi P, Patregnani T, Piccolomini LL, Natalini S, Ferri G, Cattoli G and Marangon S, 2014. Highly pathogenic H7N7 avian influenza in Italy. Veterinary Record, 174, 382–388. [DOI] [PubMed] [Google Scholar]
- Bos ME, Nielen M, Koch G, Stegeman A and De Jong MC, 2008. Effect of H7N1 vaccination on highly pathogenic avian influenza H7N7 virus transmission in turkeys. Vaccine, 26, 6322–6328. [DOI] [PubMed] [Google Scholar]
- Bos ME, Nielen M, Koch G, Bouma A, De Jong MC and Stegeman A, 2009. Back‐calculation method shows that within‐flock transmission of highly pathogenic avian influenza (H7N7) virus in the Netherlands is not influenced by housing risk factors. Preventive Veterinary Medicine, 88, 278–285. [DOI] [PubMed] [Google Scholar]
- Bos ME, Te Beest DE, van Boven M, van Beest Holle MR, Meijer A, Bosman A, Mulder YM, Koopmans MP and Stegeman A, 2010. High probability of avian influenza virus (H7N7) transmission from poultry to humans active in disease control on infected farms. Journal of Infectious Diseases, 201, 1390–1396. [DOI] [PubMed] [Google Scholar]
- Bottcher‐Friebertshauser E, Garten W, Matrosovich M and Klenk HD, 2014. The hemagglutinin: a determinant of pathogenicity. In: Compans RW and Oldstone MBA (eds.). Influenza Pathogenesis and Control ‐. Vol I. Springer International Publishing, Switzerland. pp. 3–34. [DOI] [PubMed] [Google Scholar]
- Bouma A, Claassen I, Natih K, Klinkenberg D, Donnelly CA, Koch G and van Boven M, 2009. Estimation of transmission parameters of H5N1 avian influenza virus in chickens. Plos Pathogens, 5, e1000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwstra RJ, Koch G, Heutink R, Harders F, van der Spek A, Elbers AR and Bossers A, 2015. Phylogenetic analysis of highly pathogenic avian influenza A(H5N8) virus outbreak strains provides evidence for four separate introductions and one between‐poultry farm transmission in the Netherlands, November 2014. Eurosurveillance, 20, 31–42. [DOI] [PubMed] [Google Scholar]
- Bouwstra R, Gonzales JL, de Wit S, Stahl J, Fouchier RAM and Elbers ARW, 2017. Spatial‐environmental risk analysis of introduction of low pathogenic avian influenza virus infections on poultry farms in the Netherlands, 2007–2013. Emerging Infectious Diseases, 23, 1510–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragstad K, Jørgensen PH, Handberg K, Hammer AS, Kabell S and Fomsgaards A, 2007. First introduction of highly pathogenic H5N1 avian influenza A viruses in wild and domestic birds in Denmark, Northern Europe. [DOI] [PMC free article] [PubMed]
- Brahmakshatriya V, Lupiani B, Brinlee JL, Cepeda M, Pillai SD and Reddy SM, 2009. Preliminary study for evaluation of avian influenza virus inactivation in contaminated poultry products using electron beam irradiation. Avian Pathology, 38, 245–250. [DOI] [PubMed] [Google Scholar]
- Briand F, Schmitz A, Ogor K, Le Prioux A, Guillou‐Cloarec C, Guillemoto C, Allee C, Le Bras M, Hirchaud E, Quenault H, Touzain F, Cherbonnel‐Pansart M, Lemaitre E, Courtillon C, Gares H, Daniel P, Fediaevsky A, Massin P, Blanchard Y, Eterradossi N, van der Werf S, Jestin V and Niqueux E, 2017. Emerging highly pathogenic H5 avian influenza viruses in France during winter 2015/16: phylogenetic analyses and markers for zoonotic potential. Eurosurveillance, 22, 2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brochet AL, Guillemain M, Lebarbenchon C, Simon G, Fritz H, Green AJ, Renaud F, Thomas F and Gauthier‐Clerc M, 2009. The potential distance of highly pathogenic avian influenza virus dispersal by mallard, common teal and Eurasian pochard. EcoHealth, 6, 449–457. [DOI] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Beck JR, Suarez DL and Swayne DE, 2006. Susceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza viruses. Emerging Infectious Diseases, 12, 1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE and Swayne DE, 2008. Experimental infections of herring gulls (Larus argentatus) with H5N1 highly pathogenic avian influenza viruses by intranasal inoculation of virus and ingestion of virus‐infected chicken meat. Avian Pathology, 37, 393–397. [DOI] [PubMed] [Google Scholar]
- Brown JD, Stallknecht DE, Berghaus RD and Swayne DE, 2009. Infectious and lethal doses of H5N1 highly pathogenic Avian influenza virus for house sparrows (Passer domesticus) and rock pigeons (Columbia livia). Journal of Veterinary Diagnostic Investigation, 21, 437–445. [DOI] [PubMed] [Google Scholar]
- Bundesministerium für Gesundheit , 2013. Highly pathogenic Avian Influenza (H5N1) in birds smuggled from Indonesia. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20130614_pres_hpai_austria.pdf
- Burns TE, Ribble C, Stephen C, Kelton D, Toews L, Osterhold J and Wheeler H, 2012. Use of observed wild bird activity on poultry farms and a literature review to target species as high priority for avian influenza testing in 2 regions of Canada. The Canadian Veterinary Journal, 53, 158–166. [PMC free article] [PubMed] [Google Scholar]
- Busani L, Dalla Pozza M, Bonfanti L, Toson M, Ferrè N and Marangon S, 2007. Intervention strategies for low‐pathogenic avian influenza control in Italy. Avian Diseases, 51, 470–473. [DOI] [PubMed] [Google Scholar]
- Busani L, Valsecchi MG, Rossi E, Toson M, Ferre N, Pozza MD and Marangon S, 2009. Risk factors for highly pathogenic H7N1 avian influenza virus infection in poultry during the 1999–2000 epidemic in Italy. Veterinary Journal, 181, 171–177. [DOI] [PubMed] [Google Scholar]
- Busquets N, Abad FX, Alba A, Dolz R, Allepuz A, Rivas R, Ramis A, Darji A and Majo N, 2010a. Persistence of highly pathogenic avian influenza virus (H7N1) in infected chickens: feather as a suitable sample for diagnosis. Journal of General Virology, 91, 2307–2313. [DOI] [PubMed] [Google Scholar]
- Busquets N, Alba A, Napp S, Sánchez A, Serrano E, Rivas R, Núñez JI and Majó N, 2010b. Influenza A virus subtypes in wild birds in North‐Eastern Spain (Catalonia). Virus Research, 149, 10–18. [DOI] [PubMed] [Google Scholar]
- Butt AM, Siddique S, Idrees M and Tong YG, 2010. Avian influenza A (H9N2): computational molecular analysis and phylogenetic characterization of viral surface proteins isolated between 1997 and 2009 from the human population. Virology Journal, 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YB, Song HC, Ye JQ, Shao HX, Padmanabhan R, Sutton TC and Perez DR, 2011. Improved hatchability and efficient protection after in ovo vaccination with live‐attenuated H7N2 and H9N2 avian influenza viruses. Virology Journal, 8, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelle J, Zhao DL, Gilbert M, Nelson MI, Newman SH, Takekawa JY, Gaidet N, Prosser DJ, Liu Y, Li P, Shu YL and Xiao XM, 2014. Risks of avian influenza transmission in areas of intensive free‐ranging duck production with wild waterfowl. EcoHealth, 11, 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappucci DT Jr, Johnson DC, Brugh M, Smith TM, Jackson CF, Pearson JE and Senne DA, 1985. Isolation of avian influenza virus (subtype H5N2) from chicken eggs during a natural outbreak. Avian Diseases, 29, 1195–1200. [PubMed] [Google Scholar]
- Capua I and Marangon S, 2000. The avian influenza epidemic in Italy, 1999–2000: a review. Avian Pathology, 29, 289–294. [DOI] [PubMed] [Google Scholar]
- Capua I, Marangon S, della Pozza M, Terregino C and Cattoli G, 2003. Avian influenza in Italy 1997–2001. Avian Diseases, 47, 839–843. [DOI] [PubMed] [Google Scholar]
- Caron A, Cappelle J, Cumming GS, de Garine‐Wichatitsky M and Gaidet N, 2015. Bridge hosts, a missing link for disease ecology in multi‐host systems. Veterinary Research, 46, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron A, Cappelle J and Gaidet N, 2017. Challenging the conceptual framework of maintenance hosts for influenza A viruses in wild birds. Journal of Applied Ecology, 54, 681–690. [Google Scholar]
- Chang H, Dai FY, Liu ZL, Yuan FZ, Zhao SY, Xiang X, Zou FC, Zeng BQ, Fan YT and Duan G, 2014. Seroprevalence survey of avian influenza A (H5) in wild migratory birds in Yunnan province, Southwestern China. Virology Journal, 11, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LJ, Lin XD, Guo WP, Tian JH, Wang W, Ying XH, Wang MR, Yu B, Yang ZQ, Shi M, Holmes EC and Zhang YZ, 2016. Diversity and evolution of avian influenza viruses in live poultry markets, free‐range poultry and wild wetland birds in China. Journal of General Virology, 97, 844–854. [DOI] [PubMed] [Google Scholar]
- Chmielewski RA, Beck JR and Swayne DE, 2011. Thermal inactivation of avian influenza virus and Newcastle disease virus in a fat‐free egg product. Journal of Food Protection, 74, 1161–1168. [DOI] [PubMed] [Google Scholar]
- Chmielewski RA, Beck JR and Swayne DE, 2013. Evaluation of the US Department of Agriculture's egg pasteurization processes on the inactivation of high‐pathogenicity avian influenza virus and Velogenic Newcastle disease virus in processed egg products. Journal of Food Protection, 76, 640–645. [DOI] [PubMed] [Google Scholar]
- Chumpolbanchorn K, Suemanotham N, Siripara N, Puyati B and Chaichoune K, 2006. The effect of temperature and UV light on infectivity of avian influenza virus (H5N1, Thai field strain) in chicken fecal manure. Southeast Asian Journal of Tropical Medicine and Public Health, 37, 102–105. [PubMed] [Google Scholar]
- Claes G, Welby S, Van Den Berg T, Van Der Stede Y, Dewulf J, Lambrecht B and Marché S, 2013. The impact of viral tropism and housing conditions on the transmission of three H5/H7 low pathogenic avian influenza viruses in chickens. Epidemiology Infection, 141, 2428–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes G, Lambrecht B, Dewulf J, van den Berg T and Marché S, 2015. Extended transmission of two H5/H7 low pathogenic avian influenza viruses in chickens. Epidemiology and Infection, 143, 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes F, Morzaria SP and Donis RO, 2016. Emergence and dissemination of clade 2.3.4.4 H5Nx influenza viruses ‐ how is the Asian HPAI H5 lineage maintained. Current Opinion in Virology, 16, 158–163. [DOI] [PubMed] [Google Scholar]
- Cobb SP, 2011. The spread of pathogens through trade in poultry hatching eggs: overview and recent developments. Revue scientifique et technique (International Office of Epizootics), 30, 165–175. [DOI] [PubMed] [Google Scholar]
- Comin A, Klinkenberg D, Maragon S, Toffan A and Stegeman A, 2011. Transmission dynamics of low pathogenicity avian influenza infections in Turkey flocks. PLoS ONE, 6, e26935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comin A, Stegeman A, Marangon S and Klinkenberg D, 2012. Evaluating surveillance strategies for the early detection of low pathogenicity avian influenza infections. PLoS ONE, 7, e35956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conraths FJ, Sauter‐Louis C, Globig A, Dietze K, Pannwitz G, Albrecht K, Horeth‐Bontgen D, Beer M, Staubach C and Homeier‐Bachmann T, 2016. Highly pathogenic avian influenza H5N8 in Germany: outbreak investigations. Transboundary and Emerging Diseases, 63, 10–13. [DOI] [PubMed] [Google Scholar]
- Costa TP, Brown JD, Howerth EW and Stallknecht DE, 2011. Variation in viral shedding patterns between different wild bird species infected experimentally with low‐pathogenicity avian influenza viruses that originated from wild birds. Avian Pathology, 40, 119–124. [DOI] [PubMed] [Google Scholar]
- Cox RR and Afton AD, 1997. Use of habitats by female northern pintails wintering in southwestern Louisiana. Journal of Wildlife Management, 61, 435–443. [Google Scholar]
- Dalby AR and Iqbal M, 2015. The European and Japanese outbreaks of H5N8 derive from a single source population providing evidence for the dispersal along the long distance bird migratory flyways. PeerJ, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A, Spackman E, Thomas C, Swayne DE and Suarez DL, 2008. Detection of H5N1 high‐pathogenicity avian influenza virus in meat and tracheal samples from experimentally infected chickens. Avian Diseases, 52, 40–48. [DOI] [PubMed] [Google Scholar]
- de Jong MCM, Stegeman A, van der Goot J and Koch G, 2009. Intra‐ and interspecies transmission of H7N7 highly pathogenic avian influenza virus during the avian influenza epidemic in the Netherlands in 2003. Revue Scientifique Et Technique‐Office International Des Epizooties, 28, 333–340. [DOI] [PubMed] [Google Scholar]
- De Koeijer A, Arnold M, Gonzales J and Boender GJ, 2017. Data analysis and predictive modelling of HPAI H5 and H7 outbreaks in the EU 2005‐2015. EFSA supporting publication, EN‐1285. [Google Scholar]
- De Marco MA, Delogu M, Sivay M, Sharshov K, Yurlov A, Cotti C and Shestopalov A, 2014. Virological evaluation of avian influenza virus persistence in natural and anthropic ecosystems of Western Siberia (Novosibirsk Region, Summer 2012). PLoS ONE, 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nardi M, Hill A, von Dobschuetz S, Munoz O, Kosmider R, Dewe T, Harris K, Freidl G, Stevens K, van der Meulen K, Stäerk KDC, Breed A, Meijer A, Koopmans M, Havelaar A, van der Werf S, Banks J, Wieland B, van Reeth K, Dauphin G and Capua I and the Fc , 2014. Development of a risk assessment methodological framework for potentially pandemic influenza strains (FLURISK). EFSA Supporting Publications 2014:11, 571E–n/a. [Google Scholar]
- De Vriese J, Steensels M, Palya V, Gardin Y, Dorsey KM, Lambrecht B, Van Borm S and van den Berg T, 2010. Passive protection afforded by maternally‐derived antibodies in chickens and the antibodies’ interference with the protection elicited by avian influenza inactivated vaccines in progeny. Avian Diseases, 54, 246–252. [DOI] [PubMed] [Google Scholar]
- Domanska‐Blicharz K, Minta Z, Smietanka K, Marche S and van den Berg T, 2010. H5N1 high pathogenicity avian influenza virus survival in different types of water. Avian Diseases, 54, 734–737. [DOI] [PubMed] [Google Scholar]
- Ducatez MF, Tarnagda Z, Tahita MC, Sow A, de Landtsheer S, Londt BZ, Brown IH, Osterhaus A, Fouchier RAM, Ouedraogo JBB and Muller CP, 2007. Genetic characterization of HPAI (H5N1) viruses from poultry and wild vultures, Burkina Faso. Emerging Infectious Diseases, 13, 611–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan VG, Chen R, Spiro DJ, Sengamalay N, Zaborsky J, Ghedin E, Nolting J, Swayne DE, Runstadler JA, Happ GM, Senne DA, Wang RX, Slemons RD, Holmes EC and Taubenberger JK, 2008. The evolutionary genetics and emergence of avian influenza viruses in wild birds. Plos Pathogens, 4, e1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan D, Harris K, Poen M, Kowalezyk S, Fouchier R, Staubach C and Kuiken T, 2017a. Report about HPAI introduction into Europe, HPAI detection in wild birds and HPAI spread between European holdings in the period 2005–2015. EFSA external scientific report 2017a:EN‐1284. [Google Scholar]
- Duncan D, Harris K, Poen M, Kowalezyk S, Fouchier R, Staubach C and Kuiken T, 2017b. LPAI detection in wild birds and LPAI spread between European holdings in the period 2005–2015. EFSA external scientific report:EN‐1286. [Google Scholar]
- Durant D, Kersten M, Fritz H, Juin H and Lila M, 2006. Constraints of feeding on Salicornia ramosissima by wigeon Anas penelope: an experimental approach. Journal of Ornithology, 147, 1–12. [Google Scholar]
- EEPA (European Egg Processors Association), 2011. Guide to good manufacturing practice for “liquid, concentrated, frozen and dried egg products” used as food ingredients (non‐ready to eat egg products). Available online: https://ec.europa.eu/food/sites/food/files/safety/docs/biosafety_fh_guidance_guide_good-practice-haccp-eepa_en.pdf [Accessed: 15 July 2016].
- EFSA (European Food Safety Authority), 2005. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related to animal health and welfare aspects of Avian Influenza. EFSA Journal 2005;3(9):266. 10.2903/j.efsa.2005.266 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2006a. Opinion of the Scientific Panel Animal Health and Welfare (AHAW) related with the migratory birds and their possible role in the spread of highly pathogenic avian influenza. EFSA Journal 2006;4(5):357, 46 pp. 10.2903/j.efsa.2006.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2006b. Opinion of the Scientific Panel on Animal Health and Welfare (AHAW) on a request from the Commission related with animal health and welfare risks associated with the import of wild birds other than poultry into the European Union. EFSA Journal 2006;4(11):410, 55 pp. 10.2903/j.efsa.2006.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), 2008. Animal health and welfare aspects of avian influenza and the risk of its introduction into the EU poultry holdings ‐ Scientific opinion of the Panel on Animal Health and Welfare. EFSA Journal 2008;6(6):715, 162 pp. 10.2903/j.efsa.2008.715 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), 2016a. Workshop on the introduction of HPAI via wild birds. EFSA supporting publication 2016:EN‐1052, 46 pp. 10.2903/j.efsa.2016.EN-1299 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority), online, 2016b. Uncertainty Guidance. Available online: https://www.efsa.europa.eu/en/topics/topic/uncertainty-assessment
- EFSA (European Food Safety Authority), More S, Bicout D, Bøtner A, Butterworth A, Calistri P, Depner K, Edwards S, Garin‐Bastuji B, Good M, Gortázar Schmidt C, Michel V, Miranda MA, Nielsen SS, Raj M, Sihvonen L, Spoolder H, Thulke H H, Velarde A, Willeberg P, Winckler C, Adlhoch C, Baldinelli F, Breed A, Brouwer A, Guillemain M, Harder T, Monne I, Roberts H, Cortinas Abrahantes J, Mosbach‐Schulz O, Verdonck F, Morgado J and Stegeman A, 2017a. Urgent request on avian influenza. EFSA Journal 2017;15(1):4687, 31 pp. 10.2903/j.efsa.2017.4687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority), ECDC (European Centre for Disease Prevention and Control), EURL (European Reference Laboratory on Avian Influenza) , Brown I, Mulatti P, Smietanka K, Staubach C, Willeberg P, Adlhoch C, Candiani D, Fabris C, Zancanaro G, Morgado J and Verdonck F. 2017b. Scientific report: Avian influenza overview Oct 2016 ‐ Aug 2017, 2017. EFSA Journal 2017;15(10):5018, 101 pp. 10.2903/j.efsa.2017.5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejaz R, Ahmed Z, Siddique N and Naeem K, 2007. Chicken meat as a source of avian influenza virus persistence and dissemination. International Journal of Poultry Science, 6, 871–874. [Google Scholar]
- Ellstrom P, Latorre‐Margalef N, Griekspoor P, Waldenstrom J, Olofsson J, Wahlgren J and Olsen B, 2008. Sampling for low‐pathogenic avian influenza A virus in wild Mallard ducks: oropharyngeal versus cloacal swabbing. Vaccine, 26, 4414–4416. [DOI] [PubMed] [Google Scholar]
- Elving J, Emmoth E, Albihn A, Vinneras B and Ottoson J, 2012. Composting for avian influenza virus elimination. Applied and Environmental Microbiology, 78, 3280–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Zoghby EF, Aly MM, Nasef SA, Hassan MK, Arafa AS, Selim AA, Kholousy SG, Kilany WH, Safwat M, Abdelwhab EM and Hafez HM, 2013. Surveillance on A/H5N1 virus in domestic poultry and wild birds in Egypt. Virology Journal, 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission , 2016. Avian Influenza. Available online: https://ec.europa.eu/food/animals/animal-diseases/control-measures/avian-influenza_en [Accessed: 13 June 2016]
- FAO EMPRES watch (Food and Agriculture Organization of the United Nations ‐ Emergency Prevention System for Animal Health), 2016. Highly pathogenic avian influenza (H5N1 HPAI) spread in the middle east: risk assessment. Available online: http://www.fao.org/ag/againfo/programmes/en/empres/home.asp
- FAO/EMPRES‐i (Food and Agriculture Organization of the United Nations ‐ Emergency Prevention System for Animal Health), 2014a. Avian Influenza database. Available online: http://empres-i.fao.org/eipws3g/
- FAO/EMPRES‐i (Food and Agriculture Organization of the United Nations ‐ Emergency Prevention System for Animal Health), 2014b. Reported HPAI outbreaks in domestic birds. Available online: http://empres-i.fao.org/eipws3g/
- FAO/EMPRES‐i (Food and Agriculture Organization of the United Nations ‐ Emergency Prevention System for Animal Health), 2014c. Reported LPAI outbreaks in domestic birds. Available online: http://empres-i.fao.org/eipws3g/
- Ferreira HL, Pirlot JF, Kaspers B, Kothlow S, van den Berg T and Larnbrecht B, 2010. Development of specific enzyme‐linked immunosorbent assays to evaluate the duck immune response after experimental infection with H5N1 and H7N1 low pathogenic avian influenza viruses. Avian Diseases, 54, 660–667. [DOI] [PubMed] [Google Scholar]
- Flory GA and Peer RW, 2010. Verification of poultry carcass composting research through application during actual avian influenza outbreaks. Ilar Journal, 51, 149–157. [DOI] [PubMed] [Google Scholar]
- Forrest HL, Kim JK and Webster RG, 2010. Virus shedding and potential for interspecies waterborne transmission of highly pathogenic H5N1 influenza virus in sparrows and chickens. Journal of Virology, 84, 3718–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchier RAM and Munster VJ, 2009. Epidemiology of low pathogenic avian influenza viruses in wild birds. Revue Scientifique Et Technique‐Office International Des Epizooties, 28, 49–58. [DOI] [PubMed] [Google Scholar]
- Fouchier RAM, Schneeberger PM, Rozendaal FW, Broekman JM, Kemink SAG, Munstert V, Kuiken T, Rimmelzwaan GF, Schutten M, van Doornum GJJ, Koch G, Bosman A, Koopmans M and Osterhaus A, 2004. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proceedings of the National Academy of Sciences of the United States of America, 101, 1356–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- França M, Poulson R, Brown J, Howerth EW, Berghaus RD, Carter D and Stallknecht DE, 2012. Effect of different routes of inoculation on infectivity and viral shedding of lpai viruses in mallards. Avian Diseases, 56, 981–985. [DOI] [PubMed] [Google Scholar]
- Franҫa MS and Brown JD, 2014. Influenza pathobiology and pathogenesis in avian species. In: Compans RW and Oldstone MBA (eds.). Influenza Pathogenesis and Control ‐. Vol I. Springer International Publishing, Cham, Germany. pp. 221–242. [Google Scholar]
- Fries AC, Nolting JM, Danner A, Webster RG, Bowman AS, Krauss S and Slemons RD, 2013. Evidence for the circulation and inter‐hemispheric movement of the H14 subtype influenza A virus. PLoS ONE, 8, e59216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto Y, Usui T, Ito H, Ono E and Ito T, 2015. Susceptibility of wild passerines to subtype H5N1 highly pathogenic avian influenza viruses. Avian Pathology, 44, 243–247. [DOI] [PubMed] [Google Scholar]
- Gaidet N, Cattoli G, Hammoumi S, Newman SH, Hagemeijer W, Takekawa JY, Cappelle J, Dodman T, Joannis T, Gil P, Monne I, Fusaro A, Capua I, Manu S, Micheloni P, Ottosson U, Mshelbwala JH, Lubroth J, Domenech J and Monicat F, 2008. Evidence of infection by H5N2 highly pathogenic avian influenza viruses in healthy wild waterfowl. Plos Pathogens, 4, e1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidet N, Caron A, Cappelle J, Cumming GS, Balanca G, Hammoumi S, Cattoli G, Abolnik C, de Almeida RS, Gil P, Fereidouni SR, Grosbois V, Tran A, Mundava J, Fofana B, El Mamy ABO, Ndlovu M, Mondain‐Monval JY, Triplet P, Hagemeijer W, Karesh WB, Newman SH and Dodman T, 2012. Understanding the ecological drivers of avian influenza virus infection in wildfowl: a continental‐scale study across Africa. Proceedings of the Royal Society B‐Biological Sciences, 279, 1131–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garske T, Clarke P and Ghani AC, 2007. The transmissibility of highly pathogenic avian influenza in commercial poultry in industrialised countries. PLoS ONE, 2, e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafouri SA, Langeroudi AG, Maghsoudloo H, Tehrani F, Khaltabadifarahani R, Abdollahi H and Fallah MH, 2017. Phylogenetic study‐based hemagglutinin (HA) gene of highly pathogenic avian influenza virus (H5N1) detected from backyard chickens in Iran, 2015. Virus Genes, 53, 117–120. [DOI] [PubMed] [Google Scholar]
- Gilbert M, Koel BF, Bestebroer TM, Lewis NS, Smith DJ and Fouchier RAM, 2014. Serological evidence for non‐lethal exposures of mongolian wild birds to highly pathogenic avian influenza H5N1 virus. PLoS ONE, 9, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GISAID (Global Initiative on Sharing All Influenza Data), 2017. GISAID platform. Available online: http://platform.gisaid.org [DOI] [PMC free article] [PubMed]
- Globig A, Staubach C, Beer M, Koppen U, Fiedler W, Nieburg M, Wilking H, Starick E, Teifke JP, Werner O, Unger F, Grund C, Wolf C, Roost H, Feldhusen F, Conraths FJ, Mettenleiter TC and Harder TC, 2009. Epidemiological and ornithological aspects of outbreaks of highly pathogenic avian influenza virus H5N1 of Asian lineage in wild birds in Germany, 2006 and 2007. Transboundary and Emerging Diseases, 56, 57–72. [DOI] [PubMed] [Google Scholar]
- Globig A, Fereidouni SR, Harder TC, Grund C, Beer M, Mettenleiter TC and Starick E, 2013. Consecutive natural influenza A virus infections in sentinel mallards in the evident absence of subtype‐specific hemagglutination inhibiting antibodies. Transboundary and Emerging Diseases, 60, 395–402. [DOI] [PubMed] [Google Scholar]
- Globig A, Starick E, Homeier T, Pohlmann A, Grund C, Wolf P, Zimmermann A, Wolf C, Heim D, Schlößer H, Zander S, Beer M, Conraths FJ and Harder TC, 2016. Epidemiological and molecular analysis of an outbreak of highly pathogenic avian influenza H5N8 clade 2.3.4.4 in a german zoo: effective disease control with minimal culling. Transboundary and Emerging Diseases, 1–12. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Elbers AR, Bouma A, Koch G, de Wit JJ and Stegeman JA, 2010. Low‐pathogenic notifiable avian influenza serosurveillance and the risk of infection in poultry ‐ a critical review of the European Union active surveillance programme (2005–2007). Influenza and Other Respiratory Viruses, 4, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales JL, Elbers AR, Bouma A, Koch G, de Wit JJ and Stegeman JA, 2012a. Transmission characteristics of low pathogenic avian influenza virus of H7N7 and H5N7 subtypes in layer chickens. Veterinary Microbiology, 155, 207–213. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Elbers ARW, van der Goot JA, Bontje D, Koch G, de Wit JJ and Stegeman JA, 2012b. Using egg production data to quantify within‐flock transmission of low pathogenic avian influenza virus in commercial layer chickens. Preventive Veterinary Medicine, 107, 253–259. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Boender GJ, Elbers AR, Stegeman JA and de Koeijer AA, 2014. Risk‐based surveillance for early detection of low pathogenic avian influenza outbreaks in layer chickens. Preventive Veterinary Medicine, 117, 251–259. [DOI] [PubMed] [Google Scholar]
- Gonzales JL, Elbers ARW, and Beerens N, 2017. Risk factors of primary introduction of highly pathogenic and low pathogenic avian influenza virus into European poultry holdings, considering at least material contaminated by wild birds and contact with wild birds. EFSA Supporting publication EN‐1282, 24 pp. [Google Scholar]
- Gonzales JL, van der Groot JA, Stegeman JA, Elbers AR and Koch G, 2011. Transmission between chickens of an H7N1 low pathogenic avian influenza virus isolated during the epidemic of 1999 in Italy. Veterinary Microbiology, 152, 187–190. [DOI] [PubMed] [Google Scholar]
- Gu M, Chen HZ, Li QH, Huang JQ, Zhao MJ, Gu XB, Jiang KJ, Wang XQ, Peng DX and Liu XF, 2014. Enzootic genotype S of H9N2 avian influenza viruses donates internal genes to emerging zoonotic influenza viruses in China. Veterinary Microbiology, 174, 309–315. [DOI] [PubMed] [Google Scholar]
- Guan J, Chan M, Grenier C, Wilkie DC, Brooks BW and Spencer JL, 2009. Survival of avian influenza and Newcastle disease viruses in compost and at ambient temperatures based on virus isolation and real‐time reverse transcriptase PCR. Avian Diseases, 53, 26–33. [DOI] [PubMed] [Google Scholar]
- Guillemain M, Bertout JM, Christensen TK, Poysa H, Vaananen VM, Triplet P, Schricke V and Fox AD, 2010. How many juvenile Teal Anas crecca reach the wintering grounds? Flyway‐scale survival rate inferred from wing age‐ratios. Journal of Ornithology, 151, 51–60. [Google Scholar]
- Guillemain M and Elmberg J, 2014. The teal. T & AD Poyser, London, UK. 304 pp. [Google Scholar]
- Guillemain M, Arzel C, Mondain‐Monval JY, Schricke V, Johnson AR and Simon G, 2006. Spring migration dates of teal Anas crecca ringed in the Camargue, southern France. Wildlife Biology, 12, 163–169. [Google Scholar]
- Guillemain M, Fox AD, Poysa H, Vaananen VM, Christensen TK, Triplet P, Schricke V and Korner‐Nievergelt F, 2013. Autumn survival inferred from wing age ratios: Wigeon juvenile survival half that of adults at best? Journal of Ornithology, 154, 351–358. [Google Scholar]
- Gutiérrez RA, Sorn S, Nicholls JM and Buchy P, 2011. Eurasian Tree sparrows, risk for H5N1 virus spread and human contamination through Buddhist ritual: an experimental approach. PLoS ONE, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JS, Ip HS, Franson JC, Meteyer C, Nashold S, TeSlaa JL, French J, Redig P and Brand C, 2009. Experimental infection of a North American raptor, American kestrel (Falco sparverius), with highly pathogenic avian influenza virus (H5N1). PLoS ONE, 4, e7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y, Hou G, Jiang W, Han C, Liu S, Chen J, Li J, Zhang P, Huang B, Liu Y and Chen J, 2012. A survey of avian influenza in tree sparrows in China in 2011. PLoS ONE, 7, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder TC, Teuffert J, Starick E, Gethmann J, Grund C, Fereidouni S, Durban M, Bogner KH, Neubauer‐Juric A, Repper R, Hlinak A, Engelhardt A, Nockler A, Smietanka K, Minta Z, Kramer M, Globig A, Mettenleiter TC, Conraths FJ and Beer M, 2009. Highly pathogenic avian influenza virus (H5N1) in frozen duck carcasses, Germany, 2007. Emerging Infectious Diseases, 15, 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder T, Maurer‐Stroh S, Pohlmann A, Starick E, Höreth‐Böntgen D, Albrecht K, Pannwitz G, Teifke J, Gunalan V, Lee RTC, Sauter‐Louis C, Homeier T, Staubach C, Wolf C, Strebelow G, Höper D, Grund C, Conraths FJ, Mettenleiter TC and Beer M, 2015. Influenza A(H5N8) virus similar to strain in korea causing highly pathogenic avian influenza in Germany. Emerging Infectious Diseases, 21, 860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder TC, Buda S, Hengel H, Beer M and Mettenleiter TC, 2016. Poultry food products—a source of avian influenza virus transmission to humans? Clinical Microbiology and Infection, 22, 141–146. [DOI] [PubMed] [Google Scholar]
- Hawkins MG, Crossley BM, Osofsky A, Webby RJ, Lee CW, Suarez DL and Hietala SK, 2006. Avian influenza A virus subtype H5N2 in a red‐lored Amazon parrot. Journal of the American Veterinary Medical Association, 228, 236–241. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hiromoto Y, Chaichoune K, Patchimasiri T, Chakritbudsabong W, Prayoonwong N, Chaisilp N, Wiriyarat W, Parchariyanon S, Ratanakorn P, Uchida Y and Saito T, 2011. Host cytokine responses of pigeons infected with highly pathogenic Thai avian influenza viruses of subtype H5N1 isolated from wild birds. PLoS ONE, 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine HG, Foord AJ, Wang JN, Valdeter S, Walker S, Morrissy C, Wong FYK and Meehan B, 2015. Detection of highly pathogenic zoonotic influenza virus H5N6 by reverse‐transcriptase quantitative polymerase chain reaction. Virology Journal, 12, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henaux V and Samuel MD, 2011. Avian influenza shedding patterns in waterfowl: implications for surveillance, environmental transmission, and disease spread. Journal of Wildlife Diseases, 47, 566–578. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Jover M, Schemann K, East IJ and Toribio J, 2015. Evaluating the risk of avian influenza introduction and spread among poultry exhibition flocks in Australia. Preventive Veterinary Medicine, 118, 128–141. [DOI] [PubMed] [Google Scholar]
- Imai M and Kawaoka Y, 2012. The role of receptor binding specificity in interspecies transmission of influenza viruses. Current Opinion in Virology, 2, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip HS, Torchetti MK, Crespo R, Kohrs P, DeBruyn P, Mansfield KG, Baszler T, Badcoe L, Bodenstein B, Shearn‐Bochsler V, Killian ML, Pedersen JC, Hines N, Gidlewski T, DeLiberto T and Sleeman JM, 2015. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerging Infectious Diseases, 21, 886–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isbarn S, Buckow R, Himmelreich A, Lehmacher A and Heinz V, 2007. Inactivation of avian influenza virus by heat and high hydrostatic pressure. Journal of Food Protection, 70, 667–673. [DOI] [PubMed] [Google Scholar]
- Isoda N, Sakoda Y, Kishida N, Bai GR, Matsuda K, Umemura T and Kida H, 2006. Pathogenicity of a highly pathogenic avian influenza virus, A/chicken/Yamaguchi/7/04 (H5N1) in different species of birds and mammals. Archives of Virology, 151, 1267–1279. [DOI] [PubMed] [Google Scholar]
- iTOL (Interactive Tree of Life), 2016. Sharing data: shared projects for EFSA_SO_Avian‐Influenza. Available online: http://itol.embl.de/shared/EFSA_SO_Avian_Influenza
- JNCC (Joint Nature Conservation Committee), 2001. A 6.15 Bewick's Swan Cygnus columbianus bewickii. JNCC, Peterborough, UK. 4 pp. Available online: http://jncc.defra.gov.uk/pdf/UKSPA/UKSPA-A6-15.pdf
- Jones JC, Sonnberg S, Kocer ZA, Shanmuganatham K, Seiler P, Shu YL, Zhu HC, Guan Y, Peiris M, Webby RJ and Webster RG, 2014. Possible role of songbirds and parakeets in transmission of influenza A(H7N9) virus to humans. Emerging Infectious Diseases, 20, 380–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain E, Gunnarsson G, Wahlgren J, Latorre‐Margalef N, Brojer C, Sahlin S, Svensson L, Waldenstrom J, Lundkvist A and Olsen B, 2010. Influenza virus in a natural host, the Mallard: experimental infection data. PLoS ONE, 5, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurado‐Tarifa E, Napp S, Gomez‐Pacheco JM, Fernandez‐Morente M, Jaen‐Tellez JA, Arenas A and Garcia‐Bocanegra I, 2014. Surveillance of influenza viruses in waterfowl used as Decoys in Andalusia, Spain. PLoS ONE, 9, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaleta EF, Pena KMB, Yilmaz A, Redmann T and Hofheinz S, 2007. Avian influenza A viruses in birds of the order Psittaciformes: reports on virus isolations, transmission experiments and vaccinations and initial studies on innocuity and efficacy of oseltamivir in ovo. Deutsche Tierarztliche Wochenschrift, 114, 260–267. [PubMed] [Google Scholar]
- Kalthoff D, Breithaupt A, Teifke JP, Globig A, Harder T, Mettenleiter TC and Beer M, 2008. Pathogenicity of highly pathogenic avian influenza virus (H5N1) in adult mute swans. Emerging Infectious Diseases, 14, 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D, Bogs J, Harder T, Grund C, Pohlmann A, Beer M and Hoffmann B, 2014a. Nucleic acid‐based detection of influenza A virus subtypes H7 and N9 with a special emphasis on the avian H7N9 virus. Eurosurveillance, 19, 30–42. [DOI] [PubMed] [Google Scholar]
- Kalthoff D, Bogs J, Grund C, Tauscher K, Teifke JP, Starick E, Harder T and Beer M, 2014b. Avian influenza H7N9/13 and H7N7/13: a comparative virulence study in chickens, pigeons, and ferrets. Journal of Virology, 88, 9153–9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammon A, Heidari A, Dayhum A, Eldaghayes I, Sharif M, Monne I, Cattoli G, Asheg A, Farhat M and Kraim E, 2015. Characterization of avian influenza and Newcastle disease viruses from poultry in libya. Avian Diseases, 59, 422–430. [DOI] [PubMed] [Google Scholar]
- Kanehira K, Uchida Y, Takemae N, Hikono H, Tsunekuni R and Saito T, 2015. Characterization of an H5N8 influenza A virus isolated from chickens during an outbreak of severe avian influenza in Japan in April 2014. Archives of Virology, 160, 1629–1643. [DOI] [PubMed] [Google Scholar]
- Kang HM, Park HY, Lee KJ, Choi JG, Lee EK, Song BM, Lee HS and Lee YJ, 2014. Characterization of H7 Influenza A Virus in wild and domestic birds in Korea. PLoS ONE, 9, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HM, Lee EK, Song BM, Jeong J, Choi JG, Jeong J, Moon OK, Yoon H, Cho Y, Kang YM, Lee HS and Lee YJ, 2015. Novel reassortant influenza A(H5N8) viruses among inoculated domestic and wild ducks, South Korea, 2014. Emerging Infectious Diseases, 21, 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Lau EHY, Guan W, Yang Y, Song T, Cowling BJ, Wu J, Peiris M, He J and Mok CKP, 2017. Epidemiology of human infections with highly pathogenic avian influenza A(H7N9) virus in Guangdong, 2016 to 2017. Eurosurveillance, 22, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapczynski DR, Pantin‐Jackwood M, Guzman SG, Ricardez Y, Spackman E, Bertran K, Suarez DL and Swayne DE, 2013. Characterization of the 2012 highly pathogenic avian influenza H7N3 virus isolated from poultry in an outbreak in Mexico: pathobiology and vaccine protection. Journal of Virology, 87, 9086–9096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BS, Russier M, Jeevan T, Marathe B, Govorkova EA, Russell CJ, Kim‐Torchetti M, Choi YK, Brown I, Saito T, Stallknecht DE, Krauss S and Webby RJ, 2016. Novel highly pathogenic avian A(H5N2) and A(H5N8) influenza viruses of clade 2.3.4.4 from North America have limited capacity for replication and transmission in mammals. Msphere, 1, pii: e00003‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kear J, 2005. Bird Families of the World: Ducks, Geese, and Swans. Oxford University Press, New York, USA. 930 pp. [Google Scholar]
- Keawcharoen J, van Riel D, van Amerongen G, Bestebroer T, Beyer WE, van Lavieren R, Osterhaus A, Fouchier RAM and Kuiken T, 2008. Wild ducks as long‐distance vectors of highly pathogenic avian influenza virus (H5NI). Emerging Infectious Diseases, 14, 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan OA, Shuaib MA, Rhman SSA, Ismail MM, Hammad YA, Baky MHA, Fusaro A, Salviato A and Cattoli G, 2009. Isolation and identification of highly pathogenic avian influenza H5N1 virus from Houbara bustards (Chlamydotis undulata macqueenii) and contact falcons. Avian Pathology, 38, 35–39. [DOI] [PubMed] [Google Scholar]
- Khan MA, Jeong KH, Ahn H, Lee YJ, Kim EJ, Glanville TD, Kim JH and Choi DY, 2013. Development of an avian influenza virus inactivation evaluation method using dialysis cassette in animal manure and mortality disposal systems. Biosystems Engineering, 114, 60–64. [Google Scholar]
- Khan SU, Berman L, Haider N, Gerloff N, Rahman MZ, Shu B, Rahman M, Dey TK, Davis TC, Das BC, Balish A, Islam A, Teifke JP, Zeidner N, Lindstrom S, Klimov A, Donis RO, Luby SP, Shivaprasad HL and Mikolon AB, 2014. Investigating a crow die‐off in January‐February 2011 during the introduction of a new clade of highly pathogenic avian influenza virus H5N1 into Bangladesh. Archives of Virology, 159, 509–518. [DOI] [PubMed] [Google Scholar]
- Khan SU, Anderson BD, Heil GL, Liang S and Gray GC, 2015. A systematic review and meta‐analysis of the seroprevalence of influenza A(H9N2) infection among humans. Journal of Infectious Diseases, 212, 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kido H, Okumura Y, Takahashi E, Pan HY, Wang SY, Yao DB, Yao M, Chida J and Yano M, 2012. Role of host cellular proteases in the pathogenesis of influenza and influenza‐induced multiple organ failure. Biochimica Et Biophysica Acta‐Proteins and Proteomics, 1824, 186–194. [DOI] [PubMed] [Google Scholar]
- Kilany WH, Arafa A, Erfan AM, Ahmed MS, Nawar AA, Selim AA, Khoulosy SG, Hassan MK, Aly MM, Hafez HM and Abdelwhab EM, 2010. Isolation of highly pathogenic avian influenza H5N1 from table eggs after vaccinal break in commercial layer flock. Avian Diseases, 54, 1115–1119. [DOI] [PubMed] [Google Scholar]
- Kim HR, Kim BS, Bae YC, Moon OK, Oem JK, Kang HM, Choi JG, Lee OS and Lee YJ, 2011. H5N1 subtype highly pathogenic avian influenza virus isolated from healthy mallard captured in South Korea. Veterinary Microbiology, 151, 386–389. [DOI] [PubMed] [Google Scholar]
- Kim Y‐I, Pascua PNQ, Kwon H‐I, Lim G‐J, Kim E‐H, Yoon S‐W, Park S‐J, Kim SM, Choi E‐J, Si Y‐J, Lee O‐J, Shim W‐S, Kim S‐W, Mo I‐P, Bae Y, Lim YT, Sung MH, Kim C‐J, Webby RJ, Webster RG and Choi YK, 2014. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerging Microbes and Infections, 3, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida N, Sakoda Y, Shiromoto M, Bai GR, Isoda N, Takada A, Laver G and Kida H, 2008. H2N5 influenza virus isolates from terns in Australia: genetic reassortants between those of the Eurasian and American lineages. Virus Genes, 37, 16–21. [DOI] [PubMed] [Google Scholar]
- Klopfleisch R, Werner O, Mundt E, Harder T and Teifke JP, 2006. Neurotropism of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) in experimentally infected pigeons (Columbia livia f. domestica). Veterinary Pathology, 43, 463–470. [DOI] [PubMed] [Google Scholar]
- Kodama H, Sato G and Mikami T, 1976. Age‐dependent resistance of chickens to Salmonella invitro ‐ phagocytic and bactericidal activities of splenic phagocytes. American Journal of Veterinary Research, 37, 1091–1094. [PubMed] [Google Scholar]
- Kogut M, Rothwell L and Kaiser P, 2002. Differential effects of age on chicken heterophil functional activation by recombinant chicken interleukin‐2. Developmental and Comparative Immunology, 26, 817–830. [DOI] [PubMed] [Google Scholar]
- Kohls A, Hafez HM, Harder T, Jansen A, Lierz P, Lueschow D, Schweiger B and Lierz M, 2011. Avian influenza virus risk assessment in falconry. Virology Journal, 8, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothlow S and Kaspers B, 2008. Chapter 14. The avian respiratory immune system. In: Davison F, Kaspers B, Schat KA (eds.). Avian Immunology. Elsevier Ltd., London, UK. pp. 273–288. [Google Scholar]
- Krauss S, Walker D, Pryor SP, Niles L, Chenghong L, Hinshaw VS and Webster RG, 2004. Influenza A viruses of migrating wild aquatic birds in North America. Vector‐Borne and Zoonotic Diseases, 4, 177–189. [DOI] [PubMed] [Google Scholar]
- Krauss S, Obert CA, Franks J, Walker D, Jones K, Seiler P, Niles L, Pryor SP, Obenauer JC, Naeve CW, Widjaja L, Webby RJ and Webster RG, 2007. Influenza in migratory birds and evidence of limited intercontinental virus exchange. Plos Pathogens, 3, 1684–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Stallknecht DE, Slemons RD, Bowman AS, Poulson RL, Nolting JM, Knowles JP and Webster RG, 2016. The enigma of the apparent disappearance of Eurasian highly pathogenic H5 clade 2.3.4.4 influenza A viruses in North American waterfowl. Proceedings of the National Academy of Sciences of the United States of America, 113, 9033–9038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, 2013. Is low pathogenic avian influenza virus virulent for wild waterbirds? Proceedings of the Royal Society B‐Biological Sciences, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YK, Kang MS, Kang MI, Lipatov AS and Swayne DE, 2009. Mammalian models for studies of transmission of highly pathogenic avian influenza A (H5N1) viruses with meat from infected poultry. Proceedings of the 4th Asian Society of Veterinary Pathologists Conference Bangkok, Thailand, pp. 462–463.
- Lam TTY, Zhou BP, Wang J, Chai YJ, Shen YY, Chen XC, Ma C, Hong WS, Chen Y, Zhang YJ, Duan L, Chen PW, Jiang JF, Zhang Y, Li LF, Poon LLM, Webby RJ, Smith DK, Leung GM, Peiris JSM, Holmes EC, Guan Y and Zhu HC, 2015. Dissemination, divergence and establishment of H7N9 influenza viruses in China. Nature, 522, 102‐U265. [DOI] [PubMed] [Google Scholar]
- Lambrecht B, Marche S, Houdart P, van den Berg T and Vangeluwe D, 2016. Impact of age, season, and flowing vs. stagnant water habitat on avian influenza prevalence in mute swan (Cygnus olor) in Belgium. Avian Diseases, 60, 322–328. [DOI] [PubMed] [Google Scholar]
- Lang AS, Lebarbenchon C, Ramey AM, Robertson GJ, Waldenstrom J and Wille M, 2016. Assessing the role of seabirds in the ecology of influenza A viruses. Avian Diseases, 60, 378–386. [DOI] [PubMed] [Google Scholar]
- Latorre‐Margalef N, Tolf C, Grosbois V, Avril A, Bengtsson D, Wille M, Osterhaus A, Fouchier RAM, Olsen B and Waldenstrom J, 2014. Long‐term variation in influenza A virus prevalence and subtype diversity in migratory mallards in northern Europe. Proceedings of the Royal Society B‐Biological Sciences, 281, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebarbenchon C, 2008. Emerging diseases and ecosystems: ecology of avian influenza viruses in the Camargue (South of France). PhD thesis, Montpellier 2 University, France, 227 pp.
- Lee DH, Park JK, Youn HN, Lee YN, Lim TH, Kim MS, Lee JB, Park SY, Choi IS and Song CS, 2011. Surveillance and isolation of HPAI H5N1 from wild mandarin ducks (Aix galericulata). Journal of Wildlife Diseases, 47, 994–998. [DOI] [PubMed] [Google Scholar]
- Lee DH, Kwon J‐H, Park J‐K, Lee Y‐N, Yuk S‐S, Lee J‐B, Park S‐Y, Choi I‐S and Song D‐S, 2012. Characterization of low‐pathogenicity H5 and H7 Korean avian influenza viruses in chickens. Poultry Science, 91, 3086–3090. [DOI] [PubMed] [Google Scholar]
- Lee DH, Torchetti MK, Winker K, Ip HS, Song CS and Swayne DE, 2015. Intercontinental spread of asian‐origin H5N8 to North America through Beringia by migratory birds. Journal of Virology, 89, 6521–6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Bahl J, Torchetti MK, Killian ML, Ip HS, DeLiberto TJ and Swayne DE, 2016. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014–2015. Emerging Infectious Diseases, 22, 1283–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenny BJ, Shanmuganatham K, Sonnberg S, Feeroz MM, Alam SM, Hasan MK, Jones‐Engel L, McKenzie P, Krauss S, Webster RG and Jones JC, 2015. Replication capacity of avian influenza A(H9N2) virus in pet birds and mammals, Bangladesh. Emerging Infectious Diseases, 21, 2174–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I and Bork P, online, 2016. iTOL v3 (Interactive Tree of Life). Available online: http://itol.embl.de/ [DOI] [PMC free article] [PubMed]
- Li Y, Lin Z, Shi J, Qi Q, Deng G, Li Z, Wang X, Tian G and Chen H, 2006. Detection of Hong Kong 97‐like H5N1 influenza viruses from eggs of vietnamese waterfowl. Archives of Virology, 151, 1615–1624. [DOI] [PubMed] [Google Scholar]
- Li J, zu Dohna H, Anchell NL, Adams SC, Dao NT, Xing Z and Cardona CJ, 2010. Adaptation and transmission of a duck‐origin avian influenza virus in poultry species. Virus Research, 147, 40–46. [DOI] [PubMed] [Google Scholar]
- Li XY, Shi JZ, Guo J, Deng GH, Zhang QY, Wang JL, He XJ, Wang KC, Chen JM, Li YY, Fan J, Kong HU, Gu CY, Guan YT, Suzuki Y, Kawaoka Y, Liu LL, Jiang YP, Tian GB, Li YB, Bu ZG and Chen HL, 2014. Genetics, receptor binding property, and transmissibility in mammals of naturally isolated H9N2 avian influenza viruses. Plos Pathogens, 10, e1004508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lierz M, Hafez HM, Klopfleisch R, Lüschow D, Prusas C, Teifke JP, Rudolf M, Grund C, Kalthoff D, Mettenleiter T, Beer M and Harder T, 2007. Protection and virus shedding of falcons vaccinated against highly pathogenic avian influenza A virus (H5N1). Emerging Infectious Diseases, 13, 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh E, Ek‐Kommonen C, Vaananen VM, Vaheri A, Vapalahti O and Huovilainen A, 2014. Molecular epidemiology of H9N2 influenza viruses in Northern Europe. Veterinary Microbiology, 172, 548–554. [DOI] [PubMed] [Google Scholar]
- Londt BZ, Banks J and Alexander DJ, 2007. Highly pathogenic avian influenza viruses with low virulence for chickens in in vivo tests. Avian Pathology, 36, 347‐U348. [DOI] [PubMed] [Google Scholar]
- Löndt BZ, Núñez A, Banks J, Alexander DJ, Russell C, Richard‐ Löndt AC and Brown IH, 2010. The effect of age on the pathogenesis of a highly pathogenic avian influenza (HPAI) H5N1 virus in Pekin ducks (Anas platyrhynchos) infected experimentally. Influenza and Other Respiratory Viruses, 4, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lycett SJ, Bodewes R, Pohlmann A, Banks J, Banyai K, Boni MF, Bouwstra R, Breed AC, Brown IH, Chen HL, Dan A, DeLiberto TJ, Diep N, Gilbert M, Hill S, Ip HS, Ke CW, Kida H, Killian ML, Koopmans MP, Kwon JH, Lee DH, Lee YJ, Lu L, Monne I, Pasick J, Pybus OG, Rambaut A, Robinson TP, Sakoda Y, Zohari S, Song CS, Swayne DE, Torchetti MK, Tsai HJ, Fouchier RAM, Beer M, Woolhouse M and Kuiken T and Global Consortium H5N8 Related , 2016. Role for migratory wild birds in the global spread of avian influenza H5N8. Science, 354, 213–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas R, Rosema S, van Zoelen D and Venema S, 2011. Maternal immunity against avian influenza H5N1 in chickens: limited protection and interference with vaccine efficacy. Avian Pathology, 40, 87–92. [DOI] [PubMed] [Google Scholar]
- MAFFT version 7 (Multiple alignment program for amino acid or nucleotide sequences), online, 2017. Multiple sequence alignment and NJ/UPGMA phylogeny. Available online: http://mafft.cbrc.jp/alignment/server/
- Malladi S, Weaver JT, Goldsmith T, Hueston W, Voss S, Funk J, Der C, Bjork KE, Clouse TL, Hennessey M, Sampedro F, Lee B and Halvorson DA, 2012. The impact of holding time on the likelihood of moving internally contaminated eggs from a highly pathogenic avian influenza infected but undetected commercial table‐egg layer flock. Avian Diseases, 56, 897–904. [DOI] [PubMed] [Google Scholar]
- Malladi S, Weaver JT, Alexander CY, Middleton JL, Goldsmith TJ, Snider T, Tilley BJ, Gonder E, Hermes DR and Halvorson DA, 2015. Quantitative estimation of the number of contaminated hatching eggs released from an infected, undetected turkey breeder hen flock during a highly pathogenic avian influenza outbreak. Avian Diseases, 59, 355–367. [DOI] [PubMed] [Google Scholar]
- Mannelli A, Busani L, Toson M, Bertolini S and Marangon S, 2007. Transmission parameters of highly pathogenic avian influenza (H7N1) among industrial poultry farms in northern Italy in 1999–2000. Preventive Veterinary Medicine, 81, 318–322. [DOI] [PubMed] [Google Scholar]
- Mansour SMG, ElBakrey RM, Ali H, Knudsen DEB and Eid AAM, 2014. Natural infection with highly pathogenic avian influenza virus H5N1 in domestic pigeons (Columba livia) in Egypt. Avian Pathology, 43, 319–324. [DOI] [PubMed] [Google Scholar]
- Marchenko VY, Susloparov IM, Kolosova NP, Goncharova NI, Shipovalov AV, Durymanov AG, Ilyicheva TN, Budatsirenova LV, Ivanova VK, Ignatyev GA, Ershova SN, Tulyahova VS, Mikheev VN and Ryzhikov AB, 2015. Influenza A(H5N8) virus isolation in Russia, 2014. Archives of Virology, 160, 2857–2860. [DOI] [PubMed] [Google Scholar]
- Marchenko VY, Susloparov IM, Kolosova NP, Goncharova NI, Shipovalov AV, Ilyicheva TN, Durymanov AG, Chernyshova OA, Kozlovskiy LI, Chernyshova TV, Pryadkina EN, Karimova TV, Mikheev VN and Ryzhikov AB, 2016. Highly pathogenic influenza H5N1 virus of clade 2.3.2.1c in Western Siberia. Archives of Virology, 161, 1645–1649. [DOI] [PubMed] [Google Scholar]
- Marinova‐Petkova A, Georgiev G, Seiler P, Darnell D, Franks J, Krauss S, Webby RJ and Webster RG, 2012. Spread of influenza virus A (H5N1) clade 2.3.2.1 to Bulgaria in common buzzards. Emerging Infectious Diseases, 18, 1596–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova‐Petkova A, Georgiev G, Petkov T, Darnell D, Franks J, Kayali G, Walker D, Seiler P, Danner A, Graham A, McKenzie P, Krauss S, Webby RJ and Webster RG, 2016. Influenza surveillance on ‘foie gras’ duck farms in Bulgaria, 2008–2012. Influenza and Other Respiratory Viruses, 10, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase M, Tsukamoto K, Imada T, Imai K, Tanimura N, Nakamura K, Yamamoto Y, Hitomi T, Kira T, Nakai T, Kiso M, Horimoto T, Kawaoka Y and Yamaguchi S, 2005. Characterization of H5N1 influenza A viruses isolated during the 2003–2004 influenza outbreaks in Japan. Virology, 332, 167–176. [DOI] [PubMed] [Google Scholar]
- Maurer‐Stroh S, Lee RT, Gunalan V and Eisenhaber F, 2013. The highly pathogenic H7N3 avian influenza strain from July 2012 in Mexico acquired an extended cleavage site through recombination with host 28S rRNA. Virology Journal, 10, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt J, Rudnick S, First M and Spengler J, 2010. Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Applied and Environmental Microbiology, 76, 3943–3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes AS, Moura DJ, Naas IA, Morello GM, Carvalho TMR, Refatti R and Paixao SJ, 2013. Minimum ventilation systems and their effects on the initial stage of turkey production. Brazilian Journal of Poultry Science, 15, 7–13. [Google Scholar]
- Millman C, Christley R, Rigby D, Dennis D, O'Brien SJ and Williams N, 2017. “Catch 22”: biosecurity awareness, interpretation and practice amongst poultry catchers. Preventive Veterinary Medicine, 141, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo IP, Brugh M, Fletcher OJ, Rowland GN and Swayne DE, 1997. Comparative pathology of chickens experimentally inoculated with avian influenza viruses of low and high pathogenicity. Avian Diseases, 41, 125–136. [PubMed] [Google Scholar]
- Mondal S, Xing Z and Cardona C, 2013. A comparison of virulance of influenza A virus isolates from mallards in experimentally inoculated turkeys. Avian Diseases, 57, 790–796. [DOI] [PubMed] [Google Scholar]
- Monne I, Fusaro A, Al‐Blowi MH, Ismai MM, Khan OA, Dauphin G, Tripodi A, Salviato A, Marangon S, Capua I and Cattoli G, 2008. Co‐circulation of two sublineages of HPAI H5N1 virus in the Kingdom of Saudi Arabia with unique molecular signatures suggesting separate introductions into the commercial poultry and falconry sectors. Journal of General Virology, 89, 2691–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monne I, Fusaro A, Nelson MI, Bonfanti L, Mulatti P, Hughes J, Murcia PR, Schivo A, Valastro V, Moreno A, Holmes EC and Cattolia G, 2014. Emergence of a highly pathogenic avian influenza virus from a low‐pathogenic progenitor. Journal of Virology, 88, 4375–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monne I, Meseko C, Joannis T, Shittu I, Ahmed M, Tassoni L, Fusaro A and Cattoli G, 2015. Highly pathogenic avian influenza A(H5N1) virus in poultry, Nigeria, 2015. Emerging Infectious Diseases, 21, 1275–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughini‐Gras L, Bonfanti L, Mulatti P, Monne I, Guberti V, Cordioli P and Marangon S, 2014. Environmental correlates of H5N2 low pathogenicity avian influenza outbreak heterogeneity in domestic poultry in Italy. PLoS ONE, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulatti P, Bos ME, Busani L, Nielen M and Marangon S, 2010. Evaluation of interventions and vaccination strategies for low pathogenicity avian influenza: spatial and space‐time analyses and quantification of the spread of infection. Epidemiology and Infection, 138, 813–824. [DOI] [PubMed] [Google Scholar]
- Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, Osterhaus A and Fouchier RAM, 2005. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerging Infectious Diseases, 11, 1545–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Baas C, Lexmond P, Waldenstrom J, Wallensten A, Fransson T, Rimmelzwaan GF, Beyer WEP, Schutten M, Olsen B, Osterhaus A and Fouchier RAM, 2007. Spatial, temporal, and species variation in prevalence of influenza A viruses in wild migratory birds. Plos Pathogens, 3, 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyka D, Pantin‐Jackwood M, Spackman E, Smith D, Rula O, Muzyka N and Stegniy B, 2016. Isolation and genetic characterization of avian influenza viruses isolated from wild birds in the azov‐black sea region of Ukraine (2001–2012). Avian Diseases, 60, 365–377. [DOI] [PubMed] [Google Scholar]
- Naguib MM, Kinne J, Chen HL, Chan KH, Joseph S, Wong PC, Woo PCY, Wernery R, Beer M, Wernery U and Harder TC, 2015. Outbreaks of highly pathogenic avian influenza H5N1 clade 2.3.2.1c in hunting falcons and kept wild birds in Dubai implicate intercontinental virus spread. Journal of General Virology, 96, 3212–3222. [DOI] [PubMed] [Google Scholar]
- Nao N, Yamagishi J, Miyamoto H, Igarashi M, Manzoor R, Ohnuma A, Tsuda Y, Furuyama W, Shigeno A, Kajihara M, Kishida N, Yoshida R and Takada A, 2017. Genetic predisposition to acquire a polybasic cleavage site for highly pathogenic avian influenza virus hemagglutinin. MBio, 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazir J, Haumacher R, Ike A, Stumpf P, Bohm R and Marschang RE, 2010. Long‐term study on tenacity of avian influenza viruses in water (distilled water, normal saline, and surface water) at different temperatures. Avian Diseases, 54, 720–724. [DOI] [PubMed] [Google Scholar]
- Nazir J, Haumacher R, Ike AC and Marschang RE, 2011. Persistence of avian influenza viruses in lake sediment, duck feces, and duck meat. Applied and Environmental Microbiology, 77, 4981–4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell DG, Elvers KT, Dopfer D, Hansson I, Jones P, James S, Gittins J, Stern NJ, Davies R, Connerton I, Pearson D, Salvat G and Allen VM, 2011. Biosecurity‐based interventions and strategies to reduce Campylobacter spp. on poultry farms. Applied Environmental Microbiology, 77, 8605–8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman SH, Iverson SA, Takekawa JY, Gilbert M, Prosser DJ, Batbayar N, Natsagdorj T and Douglas DC, 2009. Migration of whooper swans and outbreaks of highly pathogenic avian influenza H5N1 virus in Eastern Asia. PLoS ONE, 4, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niqueux E, Picault JP, Amelot M, Allée C, Lamandé J, Guillemoto C, Pierre I, Massin P, Blot G, Briand FX, Rose N and Jestin V, 2014. Quantitative transmission characteristics of different H5 low pathogenic avian influenza viruses in Muscovy ducks. Veterinary Microbiology, 168, 78–87. [DOI] [PubMed] [Google Scholar]
- Nunez A, Brookes SM, Reid SM, Garcia‐Rueda C, Hicks DJ, Seekings JM, Spencer YI and Brown IH, 2016. Highly pathogenic avian influenza H5N8 clade 2.3.4.4 virus: equivocal pathogenicity and implications for surveillance following natural infection in breeder ducks in the United Kingdom. Transboundary and Emerging Diseases, 63, 5–9. [DOI] [PubMed] [Google Scholar]
- O'Hagan T, online, 2016. Tony O'Hagan ‐ SHELF: the sheffield elicitation framework. Available online: http://www.tonyohagan.co.uk/shelf/
- OIE (World Organization for Animal Health), 2009. Chapter 6. 4. Hygiene and disease security procedures in poultry breeding flocks and hatcheries. In: OIE (ed.). Terrestrial Animal Code, Paris, France. pp. 249–256. [Google Scholar]
- OIE (World Organization for Animal Health), 2010. Chapter 10.4. Avian Influenza. In: OIE (ed.). Terrestrial Animal Health Code, Paris, France. pp. 1–20. [Google Scholar]
- OIE (World Organization for Animal Health), 2015. Chapter 2.3.4. Avian influenza (infection with avian influenza viruses). In: OIE (ed.). Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Paris, France. pp. 5–7. [Google Scholar]
- OIE (World Organization for Animal Health), online‐a, 2016a. Chapter 10.4 Infection with avian influenza viruses. In: OIE (ed.). Terrestrial Animal Health Code, Paris, France. pp. 1–18. [Google Scholar]
- OIE (World Organization for Animal Health), online‐b, 2016b. Highly pathogenic avian influenza, United States of America. Available online: http://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=20837
- OIE WAHID (World Animal Health Information Database Interface), online, 2013. Data after 2004 (WAHIS Interface). Available online:http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home
- Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus A and Fouchier RAM, 2006. Global patterns of influenza A virus in wild birds. Science, 312, 384–388. [DOI] [PubMed] [Google Scholar]
- Ottaviani D, de la Rocque S, Khomenko S, Gilbert M, Newman SH, Roche B, Schwabenbauer K, Pinto J, Robinson TP and Slingenbergh J, 2010. The Cold European Winter of 2005–2006 assisted the spread and persistence of H5N1 influenza virus in wild birds. EcoHealth, 7, 226–236. [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ and Swayne DE, 2007. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Diseases, 51, 250–259. [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Suarez DL, Spackman E and Swayne DE, 2007. Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5NI viruses in ducks. Virus Research, 130, 151–161. [DOI] [PubMed] [Google Scholar]
- Pantin‐Jackwood M, Wasilenko JL, Spackman E, Suarez DL and Swayne DE, 2010. Susceptibility of turkeys to pandemic‐H1N1 virus by reproductive tract insemination. Virology Journal, 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantin‐Jackwood MJ, Miller PJ, Spackman E, Swayne DE, Susta L, Costa‐Hurtado M and Suarez DL, 2014. Role of poultry in the spread of novel H7N9 influenza virus in China. Journal of Virology, 88, 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker CD, Reid SM, Ball A, Cox WJ, Essen SC, Hanna A, Mahmood S, Slomka MJ, Irvine RM and Brown IH, 2012. First reported detection of a low pathogenicity avian influenza virus subtype H9 infection in domestic fowl in England. Veterinary Record, 171, 372. [DOI] [PubMed] [Google Scholar]
- Parmley EJ, Bastien N, Booth TF, Bowes V, Buck PA, Breault A, Caswell D, Daoust PY, Davies JC, Elahi SM, Fortin M, Kibenge F, King R, Li Y, North N, Ojkic D, Pasick J, Pryor SP, RobInson J, Rodrigue J, Whitney H, Zimmer P and Leighton FA, 2008. Wild bird influenza survey, Canada, 2005. Emerging Infectious Diseases, 14, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasick J, Handel K, Robinson J, Copps J, Ridd D, Hills K, Kehler H, Cottam‐Birt C, Neufeld J, Berhane Y and Czub S, 2005. Intersegmental recombination between the haemagglutinin and matrix genes was responsible for the emergence of a highly pathogenic H7N3 avian influenza virus in British Columbia. Journal of General Virology, 86, 727–731. [DOI] [PubMed] [Google Scholar]
- Pasick J, Robinson J, Hooper‐McGrevy K, Wright P, Kitching P, Handel K, Copps J, Ridd D, Kehler H, Hills K and Cottam‐Birt C, 2007. The roles of national and provincial diagnostic laboratories in the eradication of highly pathogenic H7N3 avian influenza virus from the Fraser Valley of British Columbia, Canada. Avian Diseases, 51, 309–312. [DOI] [PubMed] [Google Scholar]
- Pasick J, Berhane Y, Hisanaga T, Kehler H, Hooper‐McGrevy K, Handel K, Neufeld J, Argue C and Leighton F, 2010. Diagnostic test results and pathology associated with the 2007 Canadian H7N3 highly pathogenic avian influenza outbreak. Avian Diseases, 54, 213–219. [DOI] [PubMed] [Google Scholar]
- Pensaert M and Van Reeth K, 2010. A scientific evaluation of pork, pork products and turkey meat as a possible source of foodborne infection with novel H1N1 (nH1N1) influenza virus in humans. Scientific report submitted to EFSA. 10.2903/sp.efsa.2010.EN-55/epdf [DOI]
- Perez A, Alkhamis M, Carlsson U, Brito B, Carrasco‐Medanic R, Whedbee Z and Willeberg P, 2011. Global animal disease surveillance. Spat Spatiotemporal Epidemiol, 2, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Ramirez E, Gerrikagoitia X, Barral M and Hofle U, 2010. Detection of low pathogenic avian influenza viruses in wild birds in Castilla‐La Mancha (south central Spain). Veterinary Microbiology, 146, 200–208. [DOI] [PubMed] [Google Scholar]
- Perez‐Ramirez E, Acevedo P, Allepuz A, Gerrikagoitia X, Alba A, Busquets N, Diaz‐Sanchez S, Alvarez V, Abad FX, Barral M, Majo N and Hofle U, 2012. Ecological factors driving avian influenza virus dynamics in Spanish Wetland ecosystems. PLoS ONE, 7, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JL, McCracken KG, Pruett CL, Rohwer S, Drovetski SV, Zhuravlev YN, Kulikova I, Gibson DD and Winker K, 2012. A parapatric propensity for breeding precludes the completion of speciation in common teal ( Anas crecca, sensu lato). Molecular Ecology, 21, 4563–4577. [DOI] [PubMed] [Google Scholar]
- Pillai SPS, Suarez DL, Pantin‐Jackwood M and Lee CW, 2008. Pathogenicity and transmission studies of H5N2 parrot avian influenza virus of Mexican lineage in different poultry species. Veterinary Microbiology, 129, 48–57. [DOI] [PubMed] [Google Scholar]
- Pillai SPS, Pantin‐Jackwood M, Suarez DL, Saif YM and Lee CW, 2010a. Pathobiological characterization of low‐pathogenicity H5 avian influenza viruses of diverse origins in chickens, ducks and turkeys. Archives of Virology, 155, 1439–1451. [DOI] [PubMed] [Google Scholar]
- Pillai SPS, Pantin‐Jackwood M, Yassine HM, Saif YM and Lee CW, 2010b. The high susceptibility of turkeys to influenza viruses of different origins implies their importance as potential intermediate hosts. Avian Diseases, 54, 522–526. [DOI] [PubMed] [Google Scholar]
- Poen MJ, Verhagen JH, Manvell RJ, Brown I, Bestebroer TM, van der Vliet S, Vuong O, Scheuer RD, van der Jeugd HP, Nolet BA, Kleyheeg E, Muskens GJDM, Majoor FA, Grund C and Fouchier RAM, 2016. Lack of virological and serological evidence for continued circulation of highly pathogenic avian influenza H5N8 virus in wild birds in the Netherlands, 14 November 2014 to 31 January 2016. Euro Surveillance: Bulletin Europeen sur les maladies Transmissibles = European Communicable Disease Bulletin, 21, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post J, Peeters B, Cornelissen J, Vervelde L and Rebel JMJ, 2013. Contribution of the NS1 gene of H7 avian influenza virus strains to pathogenicity in chickens. Viral Immunology, 26, 396–403. [DOI] [PubMed] [Google Scholar]
- Promkuntod N, Antarasena C, Prommuang P and Prommuang P, 2006. Isolation of avian influenza virus a subtype H5N1 from internal contents (Albumen and allantoic fluid) of Japanese quail (Coturnix coturnix japonica) eggs and oviduct during a natural outbreak. In: Blouin EF and Maillard JC (eds.). Impact of Emerging Zoonotic Diseases on Animal Health. Blackwell Publishing, Oxford. pp. 171–173. [DOI] [PubMed] [Google Scholar]
- Pu J, Wang SG, Yin YB, Zhang GZ, Carter RA, Wang JL, Xu GL, Sun HL, Wang M, Wen C, Wei YD, Wang DD, Zhu BL, Lemmon G, Jiao YN, Duan SS, Wang Q, Du Q, Sun M, Bao JN, Sun YP, Zhao JX, Zhang H, Wu G, Liu JH and Webster RG, 2015. Evolution of the H9N2 influenza genotype that facilitated the genesis of the novel H7N9 virus. Proceedings of the National Academy of Sciences of the United States of America, 112, 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulit‐Penaloza JA, Sun XJ, Creager HM, Zeng H, Belser JA, Maines TR and Tumpey TM, 2015. Pathogenesis and transmission of novel highly pathogenic avian influenza H5N2 and H5N8 viruses in ferrets and mice. Journal of Virology, 89, 10286–10293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaut A, online, 2007. FigTree v. 1.4.2. Available online: http://tree.bio.ed.ac.uk/software/figtree/
- Reemers SS, van Leenen D, Koerkamp MJG, van Haarlem D, van de Haar P, van Eden W and Vervelde L, 2010. Early host responses to avian influenza A virus are prolonged and enhanced at transcriptional level depending on maturation of the immune system. Molecular Immunology, 47, 1675–1685. [DOI] [PubMed] [Google Scholar]
- Reid SM, Shell WM, Barboi G, Onita I, Turcitu M, Cioranu R, Marinova‐Petkova A, Goujgoulova G, Webby RJ, Webster RG, Russell C, Slomka MJ, Hanna A, Banks J, Alton B, Barrass L, Irvine RM and Brown IH, 2011. First reported incursion of highly pathogenic notifiable avian influenza A H5N1 viruses from clade 2.3.2 into European poultry. Transboundary and Emerging Diseases, 58, 76–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant LA, Fuckar NS, Osterhaus A, Dobson AP and Kuiken T, 2010. Spatial and temporal association of outbreaks of H5N1 influenza virus infection in wild birds with the 0 DEGREES C isotherm. Plos Pathogens, 6, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Herfst S, van den Brand JMA, Lexmond P, Bestebroer TM, Rimmelzwaan GF, Koopmans M, Kuiken T and Fouchier RAM, 2015. Low virulence and lack of airborne transmission of the dutch highly pathogenic avian influenza virus H5N8 in ferrets. PLoS ONE, 10, e0129827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard M, Fouchier R, Monne I and Kuiken T, 2017. Mechanisms and risk factors for mutation from low to highly pathogenic avian influenza virus. EFSA external scientific report, EN‐1287.
- Ridgill SC and Fox AD, 1990. Cold weather movements of waterfowl in Western Europe. IWRB Special Publication, 1–89.
- Ronquist F and Huelsenbeck JP, 2003. MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. [DOI] [PubMed] [Google Scholar]
- Saenz RA, Essen SC, Brookes SM, Iqbal M, Wood JL, Grenfell BT, McCauley JW, Brown IH and Gog JR, 2012. Quantifying transmission of highly pathogenic and low pathogenicity H7N1 avian influenza in turkeys. PLoS ONE, 7, e45059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Tanikawa T, Uchida Y, Takemae N, Kanehira K and Tsunekuni R, 2015. Intracontinental and intercontinental dissemination of Asian H5 highly pathogenic avian influenza virus (clade 2.3.4.4) in the winter of 2014–2015. Reviews in Medical Virology, 25, 388–405. [DOI] [PubMed] [Google Scholar]
- SCoFCAH (Standing Committee on the Food Chain and Animal Health), online, 2012. Low Pathogenic Avian Influenza (LPAI) H5 epidemic in Italy. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/reg-com_ahw_20121204_pres_avian_influenza_italy.pdf [Accessed: 14 April 2017]
- Scott DA and Rose PM, 1996. Atlas of Anatidae populations in Africa and Western Eurasia. Wetlands International Publication, Wageningen, The Netherlands. 336 pp. [Google Scholar]
- Shahid MA, Abubakar M, Hameed S and Hassan S, 2009. Avian influenza virus (H5N1); effects of physico‐chemical factors on its survival. Virology Journal, 6, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheta BM, Fuller TL, Larison B, Njabo KY, Ahmed AS, Harrigan R, Chasar A, Aziz SA, Khidr AAA, Elbokl MM, Habbak LZ and Smith TB, 2014. Putative human and avian risk factors for avian influenza virus infections in backyard poultry in Egypt. Veterinary Microbiology, 168, 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shichinohe S, Itoh Y, Nakayama M, Ozaki H, Soda K, Ishigaki H, Okamatsu M, Sakoda Y, Kida H and Ogasawara K, 2016. Comparison of pathogenicities of H7 avian influenza viruses via intranasal and conjunctival inoculation in cynomolgus macaques. Virology, 493, 31–38. [DOI] [PubMed] [Google Scholar]
- Shivakoti S, Ito H, Otsuki K and Ito T, 2010. Characterization of H5N1 highly pathogenic avian influenza virus isolated from a Mountain Hawk Eagle in Japan. Journal of Veterinary Medical Science, 72, 459–463. [DOI] [PubMed] [Google Scholar]
- Shriner SA, Root JJ, Lutman MW, Kloft JM, VanDalen KK, Sullivan HJ, White TS, Milleson MP, Hairston JL, Chandler SC, Wolf PC, Turnage CT, McCluskey BJ, Vincent AL, Torchetti MK, Gidlewski T and DeLiberto TJ, 2016. Surveillance for highly pathogenic H5 avian influenza virus in synanthropic wildlife associated with poultry farms during an acute outbreak. Scientific Reports, 6, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si YL, Wang TJ, Skidmore AK, de Boer WF, Li L and Prins HHT, 2010. Environmental factors influencing the spread of the highly pathogenic avian influenza H5N1 virus in wild birds in Europe. Ecology and Society, 15, 16. [Google Scholar]
- Slomka MJ, To TL, Tong HH, Coward VJ, Hanna A, Shell W, Pavlidis T, Densham ALE, Kargiolakis G, Arnold ME, Banks J and Brown IH, 2012a. Challenges for accurate and prompt molecular diagnosis of clades of highly pathogenic avian influenza H5N1 viruses emerging in Vietnam. Avian Pathology, 41, 177–193. [DOI] [PubMed] [Google Scholar]
- Slomka MJ, To TL, Tong HH, Coward VJ, Mawhinney IC, Banks J and Brown IH, 2012b. Evaluation of lateral flow devices for identification of infected poultry by testing swab and feather specimens during H5N1 highly pathogenic avian influenza outbreaks in Vietnam. Influenza and Other Respiratory Viruses, 6, 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomka MJ, Hanna A, Mahmood S, Govil J, Krill D, Manvell RJ, Shell W, Arnold ME, Banks J and Brown IH, 2013. Phylogenetic and molecular characteristics of Eurasian H9 avian influenza viruses and their detection by two different H9‐specific Real Time reverse transcriptase polymerase chain reaction tests. Veterinary Microbiology, 162, 530–542. [DOI] [PubMed] [Google Scholar]
- Slusher MJ, Wilcox BR, Lutrell MP, Poulson RL, Brown JD, Yabsley MJ and Stallknecht DE, 2014. Are passerine birds reservoirs for influenza A viruses? Journal of Wildlife Diseases, 50, 792–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smietanka K, Minta Z, Swieton E, Olszewska M, Jozwiak M, Domanska‐Blicharz K, Wyrostek K, Tomczyk G and Pikula A, 2014. Avian influenza H9N2 subtype in Poland ‐ characterization of the isolates and evidence of concomitant infections. Avian Pathology, 43, 427–436. [DOI] [PubMed] [Google Scholar]
- Soliman A, Saad M, Elassa E, Amir E, Plathonoff C, Bahgat V, El‐Badry M, Ahmed LS, Fouda M, Gamaleldin M, Mohamed NA, Salyer S, Cornelius C and Barthel R, 2012. Surveillance of avian influenza viruses in migratory birds in Egypt, 2003–09. Journal of Wildlife Diseases, 48, 669–675. [DOI] [PubMed] [Google Scholar]
- Spackman E, Gelb J Jr, Preskenis LA, Ladman BS, Pope CR, Pantin‐Jackwood MJ and McKinley ET, 2010. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virology Journal, 7, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spekreijse D, Bouma A, Stegeman JA, Koch G and de Jong MC, 2011a. The effect of inoculation dose of highly pathogenic avian influenza virus strain H5N1 on the infectiousness of chickens. Veterinary Microbiology, 147, 59–66. [DOI] [PubMed] [Google Scholar]
- Spekreijse D, Bouma A, Koch G and Stegeman JA, 2011b. Airborne transmission of a highly pathogenic avian influenza virus strain H5N1 between groups of chickens quantified in an experimental setting. Veterinary Microbiology, 152, 88–95. [DOI] [PubMed] [Google Scholar]
- Spekreijse D, Bouma A, Koch G and Stegeman A, 2013. Quantification of dust‐borne transmission of highly pathogenic avian influenza virus between chickens. Influenza Other Respiratory Viruses, 7, 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K, Raman R, Jayaraman A, Viswanathan K and Sasisekharan R, 2013. Quantitative description of glycan‐receptor binding of influenza A virus H7 hemagglutinin. PLoS ONE, 8, e49597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ssematimba A, Hagenaars TJ, de Wit JJ, Ruiterkamp F, Fabri TH, Stegeman JA and de Jong MCM, 2013. Avian influenza transmission risks: analysis of biosecurity measures and contact structure in Dutch poultry farming. Preventive Veterinary Medicine, 109, 106–115. [DOI] [PubMed] [Google Scholar]
- Stallknecht DE and Brown JD, 2009. Tenacity of avian influenza viruses. Revue Scientifique Et Technique‐Office International Des Epizooties, 28, 59–67. [DOI] [PubMed] [Google Scholar]
- Starick E, Beer M, Hoffmann B, Staubach C, Werner O, Globig A, Strebelow G, Grund C, Durban M, Conraths FJ, Mettenleiter T and Harder T, 2008. Phylogenetic analyses of highly pathogenic avian influenza virus isolates from Germany in 2006 and 2007 suggest at least three separate introductions of H5N1 virus. Veterinary Microbiology, 128, 243–252. [DOI] [PubMed] [Google Scholar]
- Stech O, Veits J, Weber S, Deckers D, Schroer D, Vahlenkamp TW, Breithaupt A, Teifke J, Mettenleiter TC and Stech J, 2009. Acquisition of a polybasic hemagglutinin cleavage site by a low‐pathogenic avian influenza virus is not sufficient for immediate transformation into a highly pathogenic strain. Journal of Virology, 83, 5864–5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stech O, Veits J, Abdelwhab ESM, Wessels U, Mettenleiter TC and Stech J, 2015. The neuraminidase stalk deletion serves as major virulence determinant of H5N1 highly pathogenic avian influenza viruses in chicken. Scientific Reports, 5, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensels M, Van Borm S and Van den Berg TP, 2006. Avian influenza: mini‐review, European control measures and current situation in Asia. Verhandelingen ‐ Koninklijke Academie voor Geneeskunde van Belgie, 68, 103–120. [PubMed] [Google Scholar]
- Stegeman A, Bouma A, Elbers ARW, de Jong MCM, Nodelijk G, de Klerk F, Koch G and van Boven M, 2004. Avian influenza A virus (H7N7) epidemic in the Netherlands in 2003: course of the epidemic and effectiveness of control measures. Journal of Infectious Diseases, 190, 2088–2095. [DOI] [PubMed] [Google Scholar]
- Stegeman A, Velkers F, Bouwstra R and Elbers A, 2015. Transmission characteristics of Highly Pathogenic Avian Influenza (H5N8) virus during outbreaks in the Netherlands in 2014. Proceedings of the 14th International Symposium on Veterinary Epidemiology and Economics (ISVEE 14), Merida, Yucatan, Mexico, 3–7 November 2015, 1.
- Steinhauer DA, 1999. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology, 258, 1–20. [DOI] [PubMed] [Google Scholar]
- Sturm‐Ramirez KM, Hulse‐Post DJ, Govorkova EA, Humberd J, Seiler P, Puthavathana P, Buranathai C, Nguyen TD, Chaisingh A, Long HT, Naipospos TSP, Chen H, Ellis TM, Guan Y, Peiris JSM and Webster RG, 2005. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? Journal of Virology, 79, 11269–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Gu M, Liu D, Cui J, Gao GF, Zhou J and Liu X, 2017. Epidemiology, evolution, and pathogenesis of H7N9 influenza viruses in five epidemic waves since 2013 in China. Trends in Microbiology, 25, 1–16. [DOI] [PubMed] [Google Scholar]
- Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee CW, Manvell RJ, Mathieu‐Benson C, Moreno V, Pedersen JC, Panigrahy B, Rojas H, Spackman E and Alexander DJ, 2004. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerging Infectious Diseases, 10, 693–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Pu J, Hu J, Liu L, Xu G, Gao GF, Liu X and Liu J, 2016. Characterization of clade 2.3.4.4 highly pathogenic H5 avian influenza viruses in ducks and chickens. Veterinary Microbiology, 182, 116–122. [DOI] [PubMed] [Google Scholar]
- Swayne DE and Beck JR, 2004. Heat inactivation of avian influenza and Newcastle disease viruses in egg products. Avian Pathology, 33, 512–518. [DOI] [PubMed] [Google Scholar]
- Swayne DE and Beck JR, 2005. Experimental study to determine if low‐pathogenicity and high‐pathogenicity avian influenza viruses can be present in chicken breast and thigh meat following intranasal virus inoculation. Avian Diseases, 49, 81–85. [DOI] [PubMed] [Google Scholar]
- Swayne DE, 2006. Microassy for measuring thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. International Journal of Food Microbiology, 108, 268–271. [DOI] [PubMed] [Google Scholar]
- Swieton E, Wyrostek K, Jozwiak M, Olszewska‐Tomczyk M, Domnska‐Blicharz K, Meissner W, Wlodarczyk R, Minias P, Janiszewski T, Minta Z and Smietanka K, 2017. Surveillance for avian influenza virus in wild birds in poland, 2008–15. Journal of Wildlife Diseases, 53, 330–338. [DOI] [PubMed] [Google Scholar]
- Tassoni L, Fusaro A, Milani A, Lemey P, Awuni JA, Sedor VB, Dogbey O, Commey ANO, Meseko C, Joannis T, Minoungou GL, Ouattara L, Haido AM, Cisse‐Aman D, Couacy‐Hymann E, Dauphin G, Cattoli G and Monne I, 2016. Genetically different highly pathogenic avian influenza A(H5N1) viruses in West Africa, 2015. Emerging Infectious Diseases, 22, 2132–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ME, Bouma A, Ekker HM, Fonken AJM, Stegeman JA and Nielen M, 2005. Risk factors for the introduction of high pathogenicity Avian Influenza virus into poultry farms during the epidemic in the Netherlands in 2003. Preventive Veterinary Medicine, 69, 1–11. [DOI] [PubMed] [Google Scholar]
- Thomas C and Swayne DE, 2007. Thermal inactivation of H5N1 high pathogenicity avian influenza virus in naturally infected chicken meat. Journal of Food Protection, 70, 674–680. [DOI] [PubMed] [Google Scholar]
- Thomas C, King DJ and Swayne DE, 2008. Thermal inactivation of avian influenza and Newcastle disease viruses in chicken meat. Journal of Food Protection, 71, 1214–1222. [DOI] [PubMed] [Google Scholar]
- Thontiravong A, Kitikoon P, Wannaratana S, Tantilertcharoen R, Tuanudom R, Pakpinyo S, Sasipreeyajan J, Oraveerakul K and Amonsin A, 2012. Quail as a potential mixing vessel for the generation of new reassortant influenza A viruses. Veterinary Microbiology, 160, 305–313. [DOI] [PubMed] [Google Scholar]
- Tiensin T, Nielen M, Vernooij H, Songserm T, Kalpravidh W and Chotiprasantintara S, 2007. Transmission of highly pathogenic avian influenza virus H5N1 within flocks during the 2004 epidemic in Thailand. Journal of Infectious Diseases, 196, 1679–1784. [DOI] [PubMed] [Google Scholar]
- Tiwari A, Patnayak DP, Chander Y, Parsad M and Goyal SM, 2006. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Diseases, 50, 284–287. [DOI] [PubMed] [Google Scholar]
- Toffan A, Beato MS, De Nardi R, Bertoli E, Salviato A, Cattoli G, Terregino C and Capua I, 2008. Conventional inactivated bivalent H5/H7 vaccine prevents viral localization in muscles of turkeys infected experimentally with low pathogenic avian influenza and highly pathogenic avian influenza H7N1 isolates. Avian Pathology, 37, 407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H, van Santen VL and Breedlove C, 2016. Inactivation of avian influenza virus in nonpelleted chicken feed. Avian Diseases, 60, 846–849. [DOI] [PubMed] [Google Scholar]
- Tosh C, Nagarajan S, Kumar M, Murugkar HV, Venkatesh G, Shukla S, Mishra A, Mishra P, Agarwal S, Singh B, Dubey P, Tripathi S and Kulkarni DD, 2016. Multiple introductions of a reassortant H5N1 avian influenza virus of clade 2.3.2.1c with PB2 gene of H9N2 subtype into Indian poultry. Infection Genetics and Evolution, 43, 173–178. [DOI] [PubMed] [Google Scholar]
- USDA APHIS VS (Services USDoAAaPHISV), online, 2015. Epidemiologic and other analyses of hpai‐affected poultry flocks: July 15, 2015 Report. Available online: https://www.aphis.usda.gov/animal_health/animal_dis_spec/poultry/downloads/Epidemiologic-Analysis-July-15-2015.pdf
- Vaananen VM, 2001. Hunting disturbance and the timing of autumn migration in Anas species. Wildlife Biology, 7, 3–9. [Google Scholar]
- van der Groot JA, van Boven M, Stegeman A, van de Water SGP, de Jong MCM and Koch G, 2008. Transmission of highly pathogenic avian influenza H5N1 virus in Pekin ducks is significantly reduced by a genetically distant H5N2 vaccine. Virology, 382, 91–97. [DOI] [PubMed] [Google Scholar]
- Van Borm S, Thomas I, Hanquet G, Lambrecht N, Boschmans M, Dupont G, Decaestecker M, Snacken R and van den Berg T, 2005. Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerging Infectious Diseases, 11, 702–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Brand JMA, Krone O, Wolf PU, van de Bildt MWG, van Amerongen G, Osterhaus A and Kuiken T, 2015. Host‐specific exposure and fatal neurologic disease in wild raptors from highly pathogenic avian influenza virus H5N1 during the 2006 outbreak in Germany. Veterinary Research, 46, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera S, Bulla I, Ziller M, Pohlmann A, Harder T and Stanke M, 2014. ClassyFlu: classification of influenza A viruses with discriminatively trained profile‐HMMS. PLoS ONE, 9, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot JA, De Jong MCM, Koch G and Van Boven M, 2003. Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2). Epidemiology and Infection, 131, 1003–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Groot JA, Koch G, de Jong MCM and van Boven M, 2005. Quantification of the effect of vaccination on transmission of avian influenza (H7N7) in chickens. Proceedings of the National Academy of Sciences of the United tates of America, 102, 18141–18146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Groot JA, van Boven M, Koch G and de Jong MCM, 2007. Variable effect of vaccination against highly pathogenic avian influenza (H7N7) virus on disease and transmission in pheasants and teals. Vaccine, 25, 8318–8325. [DOI] [PubMed] [Google Scholar]
- Veits J, Weber S, Stech O, Breithaupt A, Graber M, Gohrbandt S, Bogs J, Hundt J, Teifke JP, Mettenleiter TC and Stech J, 2012. Avian influenza virus hemagglutinins H2, H4, H8, and H14 support a highly pathogenic phenotype. Proceedings of the National Academy of Sciences of the United States of America, 109, 2579–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JH, van der Jeugd HP, Nolet BA, Slaterus R, Kharitonov SP, de Vries PP, Vuong O, Majoor F, Kuiken T and Fouchier RA, 2015. Wild bird surveillance around outbreaks of highly pathogenic avian influenza A(H5N8) virus in the Netherlands, 2014, within the context of global flyways. Eurosurveillance, 20, pii: 21069. [DOI] [PubMed] [Google Scholar]
- Verhagen JH, Lexmond P, Vuong O, Schutten M, Guldemeester J, Osterhaus AD, Elbers AR, Slaterus R, Hornman M, Koch G and Fouchier RA, 2017. Discordant detection of avian influenza virus subtypes in time and space between poultry and wild birds; towards improvement of surveillance programs. PLoS ONE, 12, e0173470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittecoq M, Grandhomme V, Champagnon J, Guillemain M, Crescenzo‐Chaigne B, Renaud F, Thomas F, Gauthier‐Clerc M and van der Werf S, 2012. High Influenza A Virus Infection Rates in Mallards Bred for Hunting in the Camargue. South of France, Plos One. 7 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenström J, Kuiken T and Wille M, 2017. Narrative overview on wild bird migration in the context of highly pathogenic avian influenza incursion into the European Union. EFSA external publication, EN‐1283.
- Wan HQ and Perez DR, 2006. Quail carry sialic acid receptors compatible with binding of avian and human influenza viruses. Virology, 346, 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan HQ, Sorrell EM, Song HC, Hossain MJ, Ramirez‐Nieto G, Monne I, Stevens J, Cattoli G, Capua I, Chen LM, Donis RO, Busch J, Paulson JC, Brockwell C, Webby R, Blanco J, Al‐Natour MQ and Perez DR, 2008. Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS ONE, 3, e2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YB, Dai ZY, Cheng H, Liu ZX, Pan ZC, Deng WK, Gao TS, Li XT, Yao YG, Ren J and Xue Y, 2013. Towards a better understanding of the novel avian‐origin H7N9 influenza A virus in China. Scientific Reports, 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Zhang T, Li X, Jiang Z, Jiang Q, Chen Q, Tu X, Chen Z, Chang J, Li L and Xu B, 2014. Serological evidence of H7, H5 and H9 avian influenza virus co‐infection among herons in a city park in Jiangxi, China. Scientific Reports, 4, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver JT, Malladi S, Spackman E and Swayne DE, 2015. Risk reduction modeling of high pathogenicity avian influenza virus titers in nonpasteurized liquid egg obtained from infected but undetected chicken flocks. Risk Analysis, 35, 2057–2068. [DOI] [PubMed] [Google Scholar]
- Wells LL, Lowry VK, Deloach JR and Kogut MH, 1998. Age‐dependent phagocytosis and bactericidal activities of the chicken heterophil. Developmental and Comparative Immunology, 22, 103–109. [DOI] [PubMed] [Google Scholar]
- Werner O, Starick E, Teifke J, Klopfleisch R, Prajitno TY, Beer M, Hoffmann B and Harder TC, 2007. Minute excretion of highly pathogenic avian influenza virus A/chicken/Indonesia/2003 (H5N1) from experimentally infected domestic pigeons (Columbia livia) and lack of transmission to sentinel chickens. Journal of General Virology, 88, 3089–3093. [DOI] [PubMed] [Google Scholar]
- Wetlands International , online‐a, 2017a. Water bird population estimates online database. Available online: http://wpe.wetlands.org/ [Accessed: 18 April 2017].
- Wetlands International , online‐b, 2017b. Waterbird population estimates online database. Available online: http://wpe.wetlands.org/ [Accessed: 10 January 2017].
- Wibawa H, Bignham J, Huradji H, Lowther S, Payne J, Harper J, Junaidi A, Middleton D and Meers J, 2014. Experimentally infected ducks show efficient transmission of Indonesian H5N1 highly pathogenic avian influenza virus, but lack persistent viral shedding. PLoS ONE, 9, e83417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille M, Tolf C, Avril A, Latorre‐Margalef N, Wallerstrom S, Olsen B and Waldenstrom J, 2013. Frequency and patterns of reassortment in natural influenza A virus infection in a reservoir host. Virology, 443, 150–160. [DOI] [PubMed] [Google Scholar]
- Wille M, van Run P, Waldenstrom J and Kuiken T, 2014. Infected or not: are PCR‐positive oropharyngeal swabs indicative of low pathogenic influenza A virus infection in the respiratory tract of Mallard Anas platyrhynchos? Veterinary Research, 45, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willeberg P, Perez A, Thurmond M, Ascher M, Carpenter T and AlKhamis M, 2010. Visualization and analysis of the Danish 2006 highly pathogenic avian influenza virus H5N1 wild bird surveillance data by a prototype avian influenza bioportal. Avian Diseases, 54, 433–439. [DOI] [PubMed] [Google Scholar]
- Wood JP, Choi YW, Chappie DJ, Rogers JV and Kaye JZ, 2010. Environmental persistence of a highly pathogenic avian influenza (H5N1) virus. Environmental Science and Technology, 44, 7515–7520. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Kitagawa K, Ikenaga N, Yamada M, Mase M and Narita M, 2007a. Severe nonpurulent encephalitis with mortality and feather lesions in call ducks (Anas platyrhyncha var. domestica) inoculated intravenously with H5N1 highly pathogenic avian influenza virus. Avian Diseases, 51, 52–57. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Kitagawa K, Ikenaga N, Yamada M, Mase M and Narita M, 2007b. Pathogenesis in call ducks inoculated intranasally with H5N1 highly pathogenic avian influenza virus and transmission by oral inoculation of infective feathers from an infected call duck. Avian Diseases, 51, 744–749. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Okamatsu M, Miyazaki A, Yamada M and Mase M, 2008. Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerging Infectious Diseases, 14, 1671–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Yamada M and Ito T, 2009. Zoonotic risk for influenza A (H5N1) infection in wild swan feathers. Journal of Veterinary Medical Science, 71, 1549–1551. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Yamada M and Mase M, 2010. Comparative pathology of chickens and domestic ducks experimentally infected with highly pathogenic avian influenza viruses (H5N1) isolated in Japan in 2007 and 2008. Jarq‐Japan Agricultural Research Quarterly, 44, 73–80. [Google Scholar]
- Yang L, Zhu WF, Li XD, Bo H, Zhang Y, Zou SM, Gao RB, Dong J, Zhao X, Chen WB, Dong LB, Zou XH, Xing YC, Wang DY and Shu YL, 2017. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. Journal of Virology, 91, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee KS, Cardona CJ and Carpenter TE, 2009. Transmission of low‐pathogenicity avian influenza virus of subtype H6N2 from chickens to Pekin ducks and Japanese quail (Coturnix coturnix japonica). Avian Pathology, 38, 59–64. [DOI] [PubMed] [Google Scholar]
- Yoon SW, Webby RJ and Webster RG, 2014. Evolution and ecology of influenza A viruses. In: Compans RW and Oldstone MBA (eds.). Influenza Pathogenesis and Control ‐. Vol I. Springer International Publishing, Switzerland. pp. 359–375. [Google Scholar]
- Youn HN, Lee YN, Lee DH, Park JK, Yuk SS, Lee HJ, Yeo JM, Yang SY, Lee JB, Park SY, CHoi IS and Song CS, 2012. Effect of intranasal administration of Lactobacillus fermentum CJL‐112 on horizontal transmission of influenza virus in chickens. Poultry Science, 91, 2517–2522. [DOI] [PubMed] [Google Scholar]
- Ypma RJF, Jonges M, Bataille A, Stegeman A, Koch G, van Boven M, Koopmans M, van Ballegooijen WM and Wallinga J, 2013. Genetic data provide evidence for wind‐mediated transmission of highly pathogenic avian influenza. Journal of Infectious Diseases, 207, 730–735. [DOI] [PubMed] [Google Scholar]
- Yu H, Zhou YJ, Li GX, Ma JH, Yan LP, Wang B, Yang FR, Huang M and Tong GZ, 2011. Genetic diversity of H9N2 influenza viruses from pigs in China: a potential threat to human health? Veterinary Microbiology, 149, 254–261. [DOI] [PubMed] [Google Scholar]
- ZDG (Zentralverband der Deutschen Geflugelwirtschaft e.V.), 2013. Bundeseinheitliche Eckwerte fur eine freiwillige Vereinbarung zur Haltung von Mastputen. ZDG, Berlin, Germany, 40 pp. Available online: http://www.zdg-online.de/
- Zepeda C and Salman MD, 2007. Assessing the probability of the presence of low pathogenicity avian influenza virus in exported chicken meat. Avian Diseases, 51, 344–351. [DOI] [PubMed] [Google Scholar]
- Zhao G, Pan J, Gu X, Zhao K, Chen H, Chen S, Wang X, Peng D and Liu X, 2013. Prevalence of low pathogenic avian influenza virus in one live bird market in Eastern China from 2002–2011. Avian Diseases, 57, 155–158. [DOI] [PubMed] [Google Scholar]
- Zhao BH, Zhang X, Zhu WF, Teng Z, Yu XL, Gao Y, Wu D, Pei EL, Yuan ZA, Yang L, Wang DY, Shu YL and Wu F, 2014. Novel avian influenza A(H7N9) virus in tree sparrow, shanghai, China, 2013. Emerging Infectious Diseases, 20, 850–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu HC, Lam TTY, Smith DK and Guan Y, 2016. Emergence and development of H7N9 influenza viruses in China. Current Opinion in Virology, 16, 106–113. [DOI] [PubMed] [Google Scholar]
- Zhu W, Zhou J, Li Z, Yang L, Li X, Huang W, Zou S, Chen W, Wei H, Tang J, Liu L, Dong J, Wang D and Shu Y, 2017. Biological characterisation of the emerged highly pathogenic avian influenza (HPAI) A(H7N9) viruses in humans, in mainland China, 2016 to 2017. Eurosurveillance, 22, 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou SM, Guo JF, Gao RB, Dong LB, Zhou JF, Zhang Y, Dong J, Bo H, Qin K and Shu YL, 2013. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virology Journal, 10, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Avian Influenza surveillance in France from 2012 to 2015


