Table 1.
Specification summary of the substances in the flavouring group evaluation 7, Revision 5
| FL‐no | EU register name | Structural formula | FEMA no CoE no CAS no | Phys. form Mol. formula Mol. weight | Solubilitya Solubility in ethanolb | Boiling point, °Cc Melting point, °C ID test Assay minimum | Refrac. Indexd Spec. gravitye | Specification comments |
|---|---|---|---|---|---|---|---|---|
| 02.077 | Pentan‐3‐ol |

|
2349 584‐02‐1 |
Liquid C5H12O 88.15 |
Slightly soluble Freely soluble |
115 MS 98% |
1.407–1.413 0.815–0.822 |
|
| 02.124 | 6‐Methylhept‐5‐en‐2‐ol |

|
10264 1569‐60‐4 |
Liquid C8H16O 128.21 |
Slightly soluble Freely soluble |
77 (20 hPa) MS 95% |
1.447–1.453 0.848–0.854 |
Racemate (EFFA, 2002a) |
| 02.131 | But‐3‐en‐2‐ol |

|
598‐32‐3 |
Liquid C4H8O 72.11 |
Slightly soluble Freely soluble |
90 MS 95% |
1.409–1.415 0.831–0.837 |
Racemate (EFFA, 2016) |
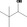
| 02.142 | 3,3‐Dimethylbutan‐2‐ol |
|
464‐07‐3 |
Liquid C6H14O 102.18 |
Slightly soluble Freely soluble |
120 MS 95% |
1.410–1.416 0.814–0.820 |
Racemate (EFFA, 2002a) |
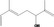
| 02.145 | 2,6‐Dimethylocta‐1,5,7‐trien‐3‐ol |
|
29414‐56‐0 |
Liquid C10H16O 152.24 |
Slightly soluble Freely soluble |
240 MS 95% |
1.484–1.490 0.895–0.901 |
Racemate Mixture of E/Z stereoisomers: 50–80% (E) (EFFA, 2012) |
| 02.148 | Dodecan‐2‐ol |

|
11760 10203‐28‐8 |
Liquid C12H26O 186.34 |
Insoluble Freely soluble |
129 (15 hPa) 19 MS 95% |
1.438–1.444 0.829–0.835 |
Racemate (EFFA, 2002a) |
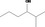
| 02.177 | 2‐Methylhexan‐3‐ol |
|
10266 617‐29‐8 |
Liquid C7H16O 116.20 |
Slightly soluble Freely soluble |
144 MS 95% |
1.418–1.424 0.820–0.826 |
Racemate (EFFA, 2002a) |
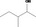
| 02.182 | 3‐Methylpentan‐2‐ol |
|
10276 565‐60‐6 |
Liquid C6H14O 102.18 |
Insoluble Freely soluble |
134 MS 95% |
1.415–1.421 0.827–0.833 |
Racemate (EFFA, 2010) |
| 02.183 | 4‐Methylpentan‐2‐ol |
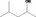
|
10279 108‐11‐2 |
Liquid C6H14O 102.18 |
Slightly soluble Freely soluble |
132 MS 99% |
1.407–1.414 0.802–0.808 |
Racemate (EFFA, 2002a) |
| 02.187 | Non‐1‐en‐3‐ol |

|
10291 21964‐44‐3 |
Liquid C9H18O 142.24 |
Practically insoluble or insoluble Freely soluble |
195 MS 98% |
1.438–1.444 0.835–0.845 |
Racemate (EFFA, 2016) |
| 02.190 | Nonan‐3‐ol |

|
10290 624‐51‐1 |
Liquid C9H20O 144.26 |
Practically insoluble or insoluble Freely soluble |
195 MS 95% |
1.425–1.431 0.818–0.824 |
Racemate (EFFA, 2010) |
| 02.194 | Octa‐1,5‐dien‐3‐ol |

|
83861‐74‐9 |
Liquid C8H14O 126.20 |
Practically insoluble or insoluble Freely soluble |
187 MS 95% |
1.441–1.447 0.832–0.838 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: 60–90% (E) (EFFA, 2012) |
| 02.211 | Undeca‐1,5‐dien‐3‐ol |

|
56722‐23‐7 |
Liquid C11H20O 168.28 |
Practically insoluble or insoluble Freely soluble |
244 NMR 95% |
1.456–1.462 0.872–0.878 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: 60–90% (E) (EFFA, 2012) CASrn refers to the (Z)‐isomer only. CASrn in the Union List to be changed to 319497‐21‐7 |
| 02.255 | (Z)‐4‐Hepten‐2‐ol |

|
66642‐85‐1 |
Liquid C7H14O 114.19 |
Insoluble Freely soluble |
154 MS 92% |
1.433–1.453 0.832–0.852 |
Racemate (EFFA, 2017) Mixture of E/Z stereoisomers: (Z)‐isomer (approx. 92%), (E)‐isomer (approx. 4%). Minor constituents 2‐heptanol (< 1), trans‐3‐hepten‐2‐ol (< 1%), cis‐3‐hepten‐2‐ol (< 1%) (EFFA, 2010) CAS nr does not specify the geometrical isomer Name in the Union List to be changed to 4‐Hepten‐2‐ol |
| 07.072 | 6‐Methylheptan‐3‐one |
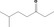
|
2143 624‐42‐0 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
162 MS 95% |
1.412–1.418 0.813–0.819 |
|
| 07.084 | Pentan‐3‐one |

|
2350 96‐22‐0 |
Liquid C5H10O 86.13 |
Partly soluble Freely soluble |
102 MS 99% |
1.389–1.395 0.812–0.818 |
|
|
07.150 2074 |
Decan‐2‐one |

|
4271 11055 693‐54‐9 |
Liquid C10H20O 156.27 |
Insoluble Freely soluble |
210 MS 98% |
1.423–1.429 0.821–0.827 |
|
| 07.156 | 2,6‐Dimethyloct‐6‐en‐3‐one (mixture of E and Z) |
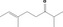
|
90975‐15‐8 |
Liquid C10H18O 154.25 |
Insoluble Freely soluble |
80 (13 hPa) NMR 95% |
1.442–1.448 0.823–0.829 |
Mixture of E/Z isomers: 50–80% (E) (EFFA, 2017) |
| 07.157 | 6,10‐Dimethylundecan‐2‐one |

|
11068 1604‐34‐8 |
Liquid C13H26O 198.35 |
Insoluble Freely soluble |
121 (16 hPa) MS 95% |
1.433–1.439 0.828–0.834 |
Racemate (EFFA, 2002a) |
| 07.158 | Dodecan‐2‐one |

|
11069 6175‐49‐1 |
Liquid C12H24O 184.32 |
Insoluble Freely soluble |
119 (13 hPa) 20 MS 99% |
1.431–1.437 0.825–0.835 |
|
| 07.160 | Heptadecan‐2‐one |

|
11089 2922‐51‐2 |
Solid C17H34O 254.46 |
Insoluble Freely soluble |
144 (1 hPa) 48 MS 95% |
n.a. n.a. |
|
| 07.161 | Hex‐1‐en‐3‐one |

|
1629‐60‐3 |
Liquid C6H10O 98.14 |
Practically insoluble or insoluble Freely soluble |
128 MS 95% |
1.420–1.426 0.849–0.855 |
|
| 07.162 | Hex‐5‐en‐2‐one |

|
109‐49‐9 |
Liquid C6H10O 98.14 |
Slightly soluble Freely soluble |
128 MS 95% |
1.418–1.424 0.839–0.845 |
|
| 07.178 | 3‐Methylbutan‐2‐one |

|
11131 563‐80‐4 |
Liquid C5H10O 86.13 |
Slightly soluble Freely soluble |
94 MS 95% |
1.387–1.393 0.801–0.807 |
|
| 07.181 | 6‐Methylheptan‐2‐one |

|
11146 928‐68‐7 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
167 MS 95% |
1.412–1.418 0.813–0.819 |
|
| 07.182 | 5‐Methylheptan‐3‐one |

|
541‐85‐5 |
Liquid C8H16O 128.21 |
Insoluble Freely soluble |
158 MS 95% |
1.418–1.424 0.816–0.824 |
Racemate (EFFA, 2002a) |
| 07.185 | 3‐Methylpentan‐2‐one |
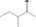
|
11157 565‐61‐7 |
Liquid C6H12O 100.16 |
Insoluble Freely soluble |
117 MS 95% |
1.398–1.404 0.810–0.816 |
Racemate (EFFA, 2002a) |
| 07.189 | Nonan‐4‐one |
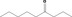
|
11161 4485‐09‐0 |
Liquid C9H18O 142.24 |
Insoluble Freely soluble |
188 MS 95% |
1.416–1.422 0.821–0.827 |
|
| 07.198 | Pseudo‐ionone |

|
4299 11191 141‐10‐6 |
Liquid C13H20O 192.30 |
Insoluble Freely soluble |
144 (16 hPa) MS 95% |
1.529–1.535 0.894–0.903 |
Mixture of E/Z stereoisomers: > 50% EE (EFFA, 2012) |
| 07.199 | Tetradecan‐2‐one |

|
11192 2345‐27‐9 |
Solid C14H28O 212.37 |
Insoluble Freely soluble |
146 (16 hPa) 33 MS 95% |
n.a. n.a. |
|
| 07.201 | Tridec‐12‐en‐2‐one |

|
60437‐21‐0 |
Liquid C13H24O 196.33 |
Insoluble Freely soluble |
129 (13 hPa) NMR 95% |
1.441–1.447 0.815–0.821 |
|
| 07.204 | 3,3,6‐Trimethylhepta‐1,5‐dien‐4‐one |
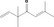
|
546‐49‐6 |
Liquid C10H16O 152.24 |
Practically insoluble or insoluble Freely soluble |
181 MS 95% |
1.462–1.468 0.867–0.873 |
|
| 07.205 | 6,10,14‐Trimethylpentadecan‐2‐one |

|
11205 502‐69‐2 |
Liquid C18H36O 268.48 |
Insoluble Freely soluble |
174 (13 hPa) MS 95% |
1.445–1.451 0.834–0.840 |
Racemate (EFFA, 2002a) |
| 07.210 | 1‐Nonene‐3‐one |

|
24415‐26‐7 |
Liquid C9H16O 140.22 |
Insoluble Freely soluble |
80 (16 hPa) MS 95% |
1.436–1.442 0.826–0.830 |
|
| 07.236 | (Z)‐5‐Octen‐2‐one |

|
11171 22610‐86‐2 |
Liquid C8H14O 126.20 |
Practically insoluble or insoluble Freely soluble |
115 NMR 95% |
1.431–1.437 0.842–0.848 |
|
|
07.239 1840 |
[R‐e]‐5‐Isopropyl‐8‐methylnona‐6,8‐dien‐2‐one |
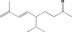
|
4331 2278‐53‐7 |
Liquid C13H22O 194.31 |
Practically insoluble or insoluble Freely soluble |
238 MS 95% |
1.471–1.477 0.846–0.852 |
|
| 07.262 | 9‐Decen‐2‐one |
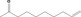
|
4706 35194‐30‐0 |
Liquid C10H18O 154 |
Slightly soluble Soluble |
206.3 IR NMR MS 99% |
1.426–1.446 0.834–0.854 |
|
| 09.304 | sec‐Heptyl isovalerate |
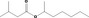
|
10806 238757‐71‐6 |
Liquid C12H24O2 200.32 |
Insoluble Freely soluble |
235 NMR 95% |
1.423–1.429 0.867–0.873 |
Racemate (EFFA, 2002a) |
| 09.323 | sec‐Butyl acetate |
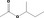
|
10527 105‐46‐4 |
Liquid C6H12O2 116.16 |
Slightly soluble Freely soluble |
111 MS 95% |
1.385–1.391 0.867–0.873 |
Racemate (EFFA, 2002a) |
| 09.325 | sec‐Butyl butyrate |

|
10528 819‐97‐6 |
Liquid C8H16O2 144.21 |
Slightly soluble Freely soluble |
152 MS 95% |
1.399–1.405 0.858–0.864 |
Racemate (EFFA, 2002a) |
| 09.328 | sec‐Butyl formate |
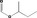
|
10532 589‐40‐2 |
Liquid C5H10O2 102.13 |
Slightly soluble Freely soluble |
94 MS 95% |
1.386–1.392 0.877–0.883 |
Racemate (EFFA, 2002a) |
| 09.332 | sec‐Butyl hexanoate |

|
10533 820‐00‐8 |
Liquid C10H20O2 172.27 |
Insoluble Freely soluble |
82 (21 hPa) NMR 95% |
1.408–1.414 0.861–0.867 |
Racemate (EFFA, 2002a) |
| 09.386 | sec‐Hept‐4(cis)‐enyl acetate |

|
94088‐33‐2 |
Liquid C9H16O2 156.22 |
Insoluble Freely soluble |
185 MS 95% |
1.412–1.418 0.854–0.860 |
Racemate (EFFA, 2002a) |
| 09.388 | sec‐Heptyl acetate |

|
10802 5921‐82‐4 |
Liquid C9H18O2 158.24 |
Insoluble Freely soluble |
172 MS 95% |
1.406–1.412 0.862–0.868 |
Racemate (EFFA, 2002a) |
| 09.391 | sec‐Heptyl hexanoate |

|
10805 6624‐58‐4 |
Liquid C13H26O2 214.35 |
Insoluble Freely soluble |
126 (20 hPa) MS 95% |
1.421–1.427 0.851–0.857 |
Racemate (EFFA, 2002a) |
| 09.604 | Isopropyl decanoate |

|
10730 2311‐59‐3 |
Liquid C13H26O2 214.35 |
Insoluble Freely soluble |
88 (3 hPa) MS 95% |
1.421–1.427 0.851–0.857 |
|
| 09.605 | Isopropyl dodecanoate |

|
10233‐13‐3 |
Liquid C15H30O2 242.40 |
Insoluble Freely soluble |
105 (1 hPa) MS 95% |
1.427–1.433 0.851–0.857 |
|
| 09.606 | Isopropyl hexadecanoate |

|
10732 142‐91‐6 |
Liquid C19H38O2 298.51 |
Insoluble Freely soluble |
342 13 MS 95% |
1.433–1.439 0.852–0.858 |
|
| 09.608 | Isopropyl octanoate |

|
10731 5458‐59‐3 |
Liquid C11H22O2 186.29 |
Insoluble Freely soluble |
124 (53 hPa) MS 95% |
1.414–1.420 0.853–0.859 |
|
| 09.609 | Isopropyl valerate |

|
18362‐97‐5 |
Liquid C8H16O2 144.21 |
Insoluble Freely soluble |
165 MS 95% |
1.398–1.404 0.855–0.861 |
|
| 09.676 | sec‐Octyl acetate |

|
10799 2051‐50‐5 |
Liquid C10H20O2 172.27 |
Practically insoluble or insoluble Freely soluble |
193 MS 95% |
1.409–1.415 0.857–0.863 |
Racemate (EFFA, 2010) |
| 09.880 | (Z)‐Hept‐4‐enyl‐2 butyrate |
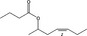
|
94088‐12‐7 |
Liquid C11H20O2 184.28 |
Practically insoluble or insoluble Freely soluble |
224 MS 95% |
1.414–1.420 0.852–0.858 |
Racemate (EFFA, 2010) |
|
09.926 2070 |
Octan‐3‐yl formate |

|
4009 84434‐65‐1 |
Liquid C9H18O2 158.24 |
Practically insoluble or insoluble Freely soluble |
71 (9 hPa) IR NMR MS 98% |
1.413–1.417 0.865–0.875 |
Racemate (EFFA, 2010) |
FL‐no: FLAVIS number; FEMA: Flavor and Extract Manufacturers Association; CoE: Council of Europe; CAS: Chemical Abstract Service; ID: identity; MS: mass spectrometry; NMR: nuclear magnetic resonance; IR: infrared spectroscopy.
Solubility in water, if not otherwise stated.
Solubility in 95% ethanol, if not otherwise stated.
At 1 atm (1,013.25 hPa), if not otherwise stated.
At 20°C, if not otherwise stated.
At 25°C, if not otherwise stated.
