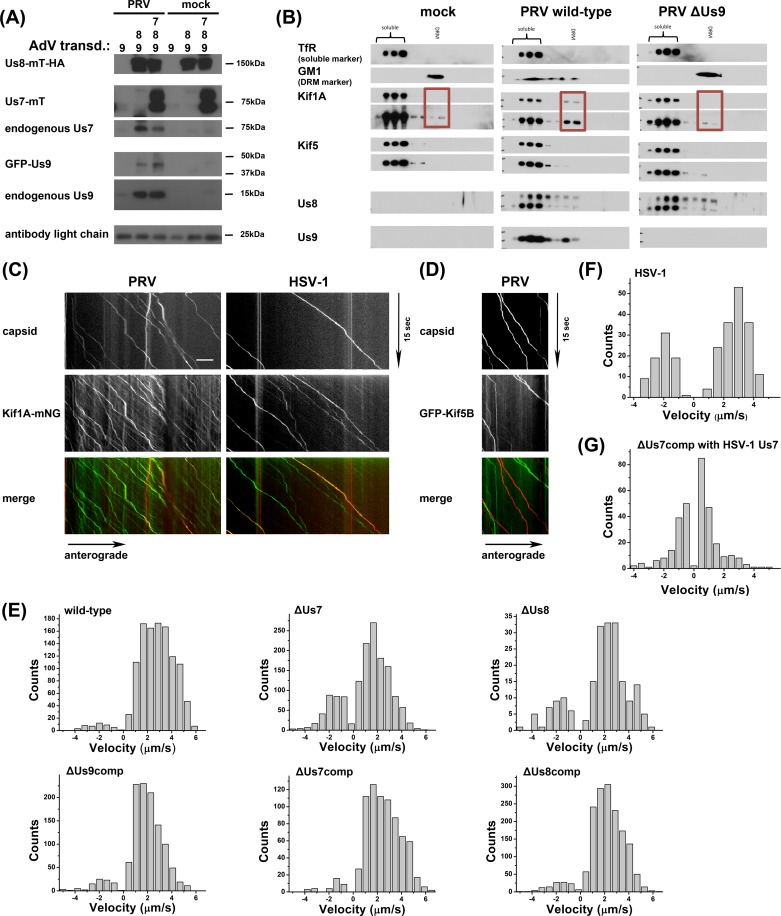
Fig 3.
(A) Immunoprecipitates of anti-HA bead fractions of PK15 lysates transduced with mock (lanes 1 & 4), Us8-mT-HA and GFP-Us9 (lanes 2 & 5), or Us8-mT-HA, GFP-Us9, and Us7-mT (lanes 3 & 6) and either PRV infected (lanes 1–3) or uninfected (lanes 4–6). Imunoprecipitates were processed for Western blotting and the membrane was incubated with antibodies against HA-tag, GFP-tag, Us7, and Us9. (B) DRM fraction isolation from SCG neuronal cultures as described in [Lyman et al., PLoS Path, 2008] using mock infected cells or cells infected with wild-type or ΔUs9 PRV. Fractions were processed for Western blotting and membranes were incubated with antibodies against Kif1a, Kif5, Us8, Us9 and TfR (soluble marker) or Cholera Toxin B (CTX-B, DRM marker). (C) TIRF microscopy of live SCG axons grown in compartmentalized cultures. Cells were transduced with full-length Kif1a-mNG, infected with red capsid tagged PRV or HSV-1 and imaged at ~19frames/s at 12-14hpi. Axonal co-transport of Kif1a was observed with both viral capsids. (D) Same as Fig 3C except with neurons transduced with full-length GFP-Kif5B instead of Kif1a-mNG and infected with red capsid tagged PRV. No axonal co-transport was observed. (E) Motility analysis of segment velocities during capsid egress as described in Scherer et al., JVI, 2016 for PRV wild-type, mutant, and compensation (comp) conditions. (F) Same as in (E) but for HSV-OK14 egress. (G) Same as in (E), but for ΔUs7 PRV compensation with HSV-1 Us7.