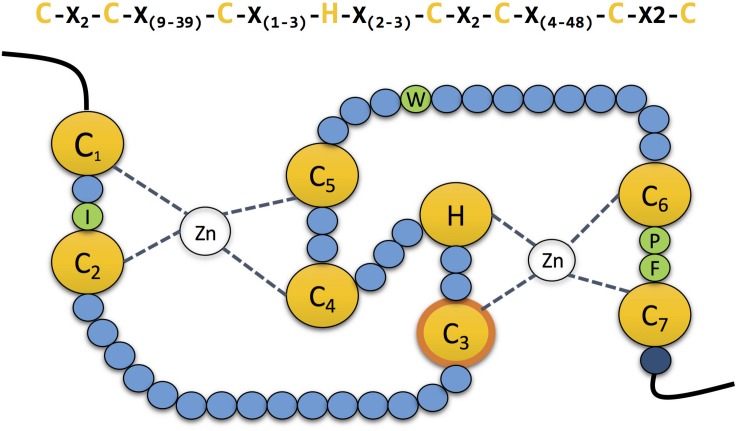
FIGURE 2.
The zinc coordinating residues in RING domains. Schematic representation of the cross-brace” topology of RING domains. The RING domain contains seven conserved cysteines and one histidine (yellow) which are involved in the coordination of two atoms of zinc. The third cysteine mediates the ubiquitin transfer in the second RING domain in RBR E3 ubiquitin ligases (contour labelled in orange). Four conserved residues (green) guide the interaction with the E2 conjugating enzyme. Mutation of the last residue of the domain (dark blue), which is normally a positively charged arginine or lysine, compromises the stability of the adjacent cysteine, affecting the coordination of the zinc atom.