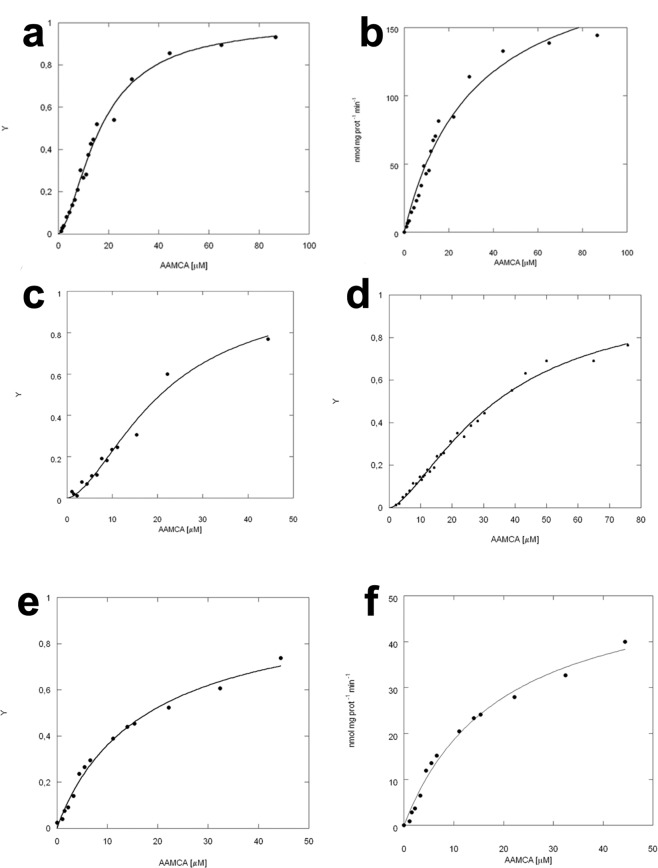
Figure 2.
Dependence of rFAAH activity on substrate concentration. (a) Dependence of rFAAH activity on substrate concentration, interpolated through the Hill equation; (b) rFAAH shows a canonical sigmoidal curve in the presence of increasing concentration of the AAMCA substrate, that is not fitted equally well by non-linear regression through the Michaelis-Menten equation; (c) rFAAH in the presence of URB597 at a homodimer:inhibitor 1:0.5 molar ratio shows a sigmoidal behaviour; (d) Kinetic analysis of rFAAH F432A mutant indicates that P432 residue is involved in the catalytic activity of the enzyme, but not in the modulation of cooperativity; (e) Kinetic analysis of rFAAH W445Y mutant interpolated through the Hill formalism; (f) Kinetic analysis of rFAAH W445Y mutant shows loss of the sigmoidal behaviour, leading to a canonical hyperbolic Michaelis-Menten enzyme without any cooperativity.