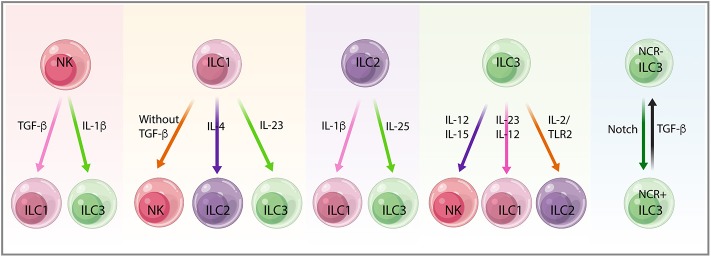
Figure 5.
The plasticity between innate lymphoid cell (ILC) subsets. Mature ILCs display substantial plasticity depending on the distinct cytokines, the microenvironment milieus, and the diverse context of cancer. Natural killer (NK) cells convert into non-cytotoxic ILC1s upon transforming growth factor-beta (TGF-β) stimulation in the tumor microenvironment (TME) in mouse fibrosarcoma and melanoma. NK cells also convert into ILC3s in response to IL-1β, preventing stage 3 NK cells from stage 4 mature NK cells and retaining ILC3-like phenotype. ILC1s show plasticity toward all other ILCs subtypes, including NK cells, ILC2s, and ILC3s with the absence of TGF-β, the accumulation of IL-4, and under the stimulation of IL-23, respectively. Conversion of ILC2s to ILC1s needs IL-1β, whereas ILC2s in response to IL-25 differentiate into IL-17-producing ILC3s. ILC3 treatment with IL-15 and IL-12 leads to NK cell conversion, whereas plasticity from ILC3s toward ILC1s and ILC2s depends on IL-23, IL-12, and IL-2/TLR2 signals. NCR−ILC3 and NCR+ILC3 conversion needs Notch and TGF-β signals, indicating the bidirectional plasticity of ILC3s in the face of different challenges.