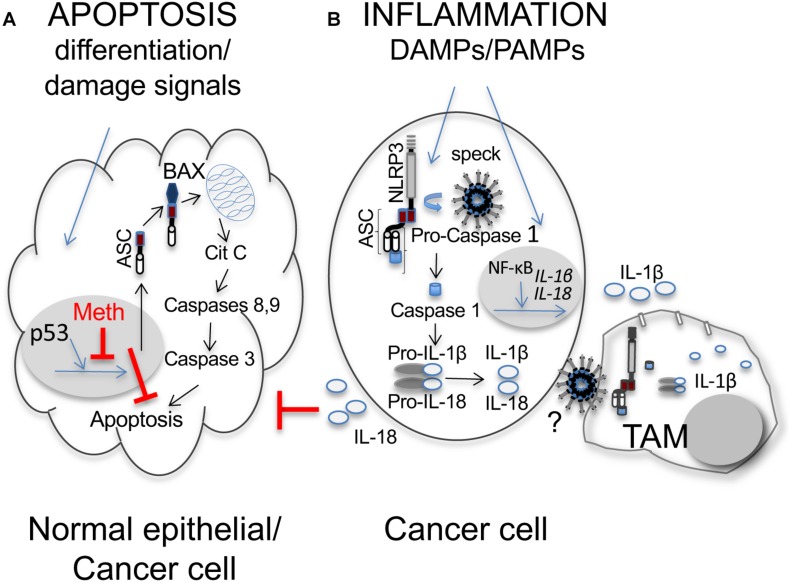
FIGURE 1.
Dual role of ASC/TMS1 in cancer. (A) Pro-apoptotic function. In normal epithelial cells differentiation and stress/damage cell signals induce the onco-suppressor p53 to activate transcription of several genes, among these ASC. Activated ASC binds to Bax and the ASC-Bax complex translocates to mitochondria mediating Citochrome C (Cit C) release. Cit C activates the initiator caspases-8 or -9, which in turn activate caspase-3 and the apoptotic cascade. At difference with normal epithelial cells, in cancer cell methylation (Meth) of the promoter region of ASC induces gene silencing and inhibits apoptosis, thus contributing to cell survival and tumor development (Hanahan and Weinberg, 2011). (B) Pro-inflammatory function in cancer cells. In cancer cells and myeloid cells (such as TAMs), recognition of pathogen- or damage-associated molecular patterns (PAMPs, DAMPs) induces the assembly/polymerization of the inflammasome molecular complex, which in cancer cells is otherwise often constitutively activated as a result of genetic lesions (Kolb et al., 2014; Kantono and Guo, 2017; Karan, 2018; Karki and Kanneganti, 2019). The inflammasome is composed by a multimerized module formed by a sensor Nod Leucine-Rich Repeat-containing receptor, such as NLRP3, bound to the pyrin domain of the adaptor ASC, which in turn is bound, through its CARD domain, to pro-caspase-1. The multimerized complex forms a speck that induces caspase-1 activation. Caspase-1 can then catalyze the proteolytic cleavage and activation of IL-1β and IL-18. Sensing of PAMPS or DAMPS also induces activation of NF-κB, which translocates to the nucleus and activates transcription of pro-IL-1β and pro-IL-18. ASC specks and inflammatory cytokines (IL-1β and IL-18) are released from cancer cells, through mechanisms not completely elucidated (?), and captured by TAMs contributing to massive IL-1β release with auto-activation of cancer cells, as well as triggering of other immune or stromal cell components in the tumor microenvironment. IL-18 can contribute to cancer cell proliferation by inhibiting caspase-8 mediated apoptosis.