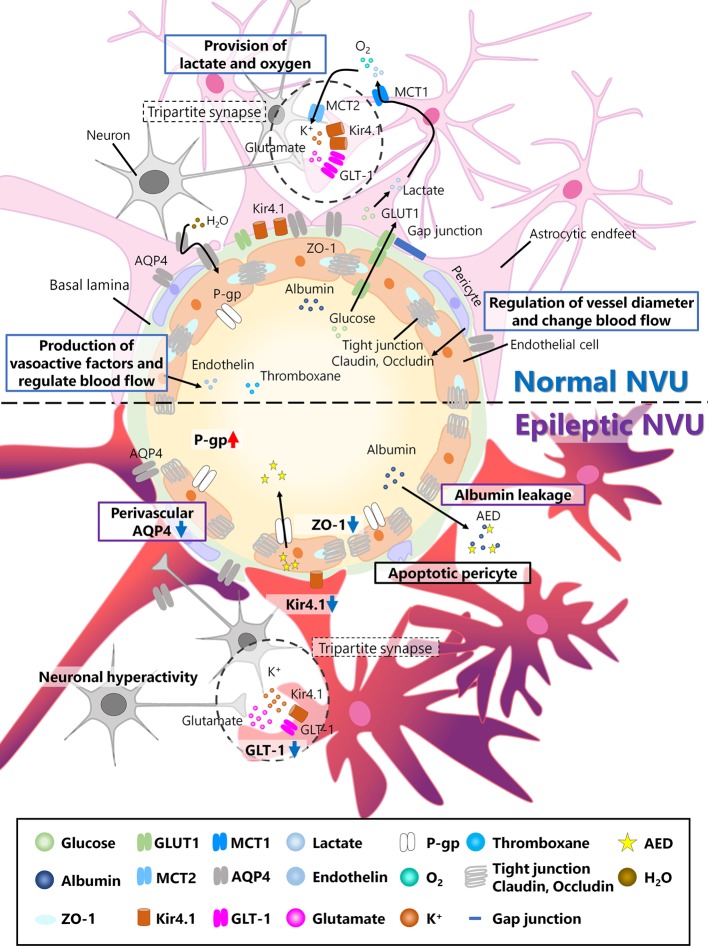
Figure 1.
Neurovascular unit (NVU) in the normal and epileptic brain. The NVU is comprised of neurons, astrocytes, pericytes, and endothelial cells. The basal lamina also exists between endothelial cells and astrocytes and is covered with astrocytic endfeet in the normal NVU. In the normal NVU, pericytes regulate the vessel diameter and change in blood flow. Endothelial cells are connected by tight junction proteins such as claudin and occludin, and ZO-1 is a type of occludin. Glucose is transported from the blood flow to astrocytes via glucose transporter type 1 (Glut1), metabolized into lactate, and discharged into extracellular space via monocarboxylate transporter 1 (MCT1). This lactate is transported to neurons via MCT2. Additionally, oxygen is also provided to neurons by astrocytes. Astrocytes and pre/post synapses comprise tripartite synapses. At tripartite synapses, potassium ions and glutamate released from neurons flow into astrocytes via inwardly rectifying potassium (Kir) 4.1 channels or glutamate transporter 1 (GLT-1), both of which are expressed around astrocytic endfeet. Additionally, aquaporin (AQP) 4, which is also expressed in astrocytes, regulates water transport. Endothelial cells produce vasoactive factors, such as thromboxane and endothelin, and regulate blood flow. In epileptic NVUs, ZO-1 expression is downregulated, leading to the apoptosis of pericytes, which induces blood–brain barrier (BBB) leakage. Albumin released from the blood flow into the brain parenchyma in the epileptic brain can be a cause of drug resistance. Kir 4.1 and GLT-1 are downregulated in astrocytes, which fail to regulate ion and neurotransmitter homeostasis. Perivascular AQP4 downregulation induces an imbalance in water influx. P-gp, which is expressed in endothelial cells and is associated with antiepileptic drug (AED) excretion, is upregulated in the epileptic NVU.