Figure 3.
Enzyme Activity of Recombinant Human TKFC Protein Expressed and Purified from Escherichia coli, and Western Blot of TKFC in Subject Fibroblasts
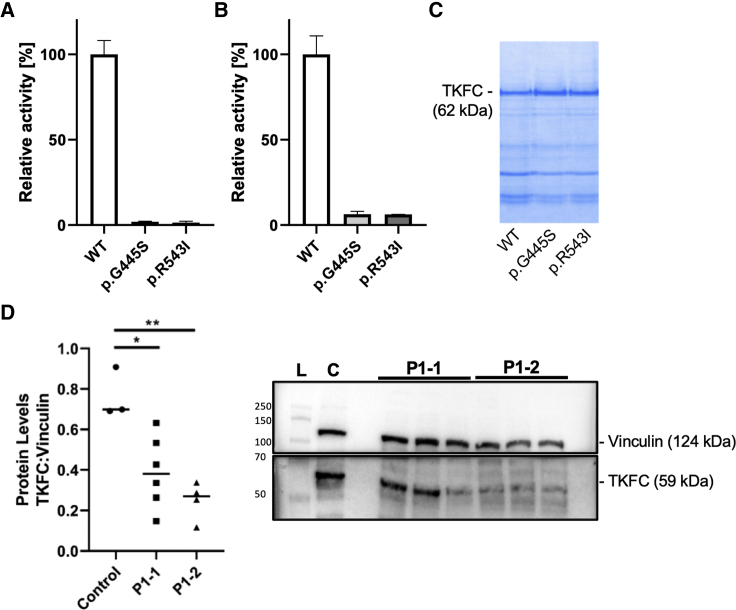
(A–C) Enzyme activity was measured using either 10 mmol/L D-glyceraldehyde (A) or 10 mmol/L dihydroxyacetone (B) as substrate. Equal amounts of recombinant protein was used for activity assays as adjusted by polyacrylamide electrophoresis (representative gel in C). Bars show average activity, error bars standard deviation. Recombinant protein was isolated in three replicates in case of wild-type (WT) and the p.Gly445Ser variant and in two replicates for the p.Arg543Ile variant.
(D) TKFC protein studies in family 1 show significant reduction of TKFC protein levels in P1-1 and P1-2 compared to metabolic disease control subjects. Protein levels were calculated as a ratio of TKFC levels to vinculin levels. Data are expressed as median with individual data points. One-way ANOVA with Tukey’s multiple comparisons test post hoc was performed to determine significance as indicated by ∗p < 0.05 and ∗∗p < 0.01. Representative western blot shows lane 1, protein ladder (L); lane 2, metabolic control (C); lanes 4–6, affected individual P1-1; lanes 7–9: affected individual P1-2.