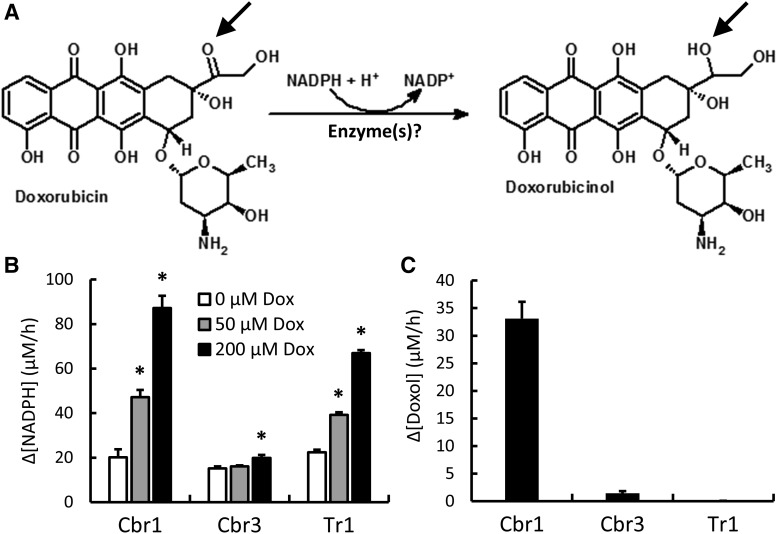
Fig. 1.
Reduction of Dox to its cardiotoxic metabolite Doxol. (A) Diagram showing the formation of Doxol via the two-electron reduction of the C-13 carbonyl group of Dox to an alcohol (arrows). (B) Dox-dependent NADPH oxidation by purified Cbr1, Cbr3, and Tr1. Reaction conditions were 200 µM NADPH, indicated concentrations of Dox, and 1 µM Cbr1, 1.9 µM Cbr3, or 1.9 µM Tr1 (Cbr3 and Tr1 histograms were normalized to 1 µM enzyme). Change in A340 was monitored for 1 hour at 37°C, and the rate of NADPH oxidation was calculated from the linear phase of the time course. (C) NADPH-dependent Doxol formation by purified Cbr1, Cbr3, and Tr1. Reaction conditions were 200 µM NADPH, 200 µM Dox, and 1 µM enzyme; incubations were for 1 hour at 37°C. Doxol levels were measured by LC-MS/MS. Error bars represent 1 S.D.; n = 3 for all reactions; *P < 0.05 by Student’s t test.