Table 2.
Adverse events from the REFLECT trial in hepatocellular carcinoma
| Adverse event | Lenvatinib arm | Sorafenib arm | ||
|---|---|---|---|---|
| All grades, % | Grade ≥3, % | All grades, % | Grade ≥3, % | |
| PPES | 27 | 3 | 52 | 11 |
| Diarrhea | 39 | 4 | 46 | 4 |
| Hypertension | 42 | 23 | 30 | 14 |
| Decreased appetite | 34 | 5 | 27 | 1 |
| Decreased weight | 31 | 8 | 22 | 3 |
| Fatigue | 30 | 4 | 25 | 4 |
| Alopecia | 3 | 0 | 25 | 0 |
| Proteinuria | 25 | 6 | 11 | 2 |
| Dysphonia | 24 | 1 | 12 | 0 |
| Nausea | 20 | 1 | 14 | 1 |
| Abdominal pain | 17 | 2 | 18 | 3 |
| Decreased platelet | 18 | 5 | 12 | 3 |
| Increased AST | 14 | 5 | 17 | 8 |
| Hypothyroidism | 16 | 0 | 2 | 0 |
| Vomiting | 16 | 1 | 8 | 1 |
| Constipation | 16 | 1 | 11 | 0 |
| Rash | 10 | 0 | 16 | 1 |
| Increased bilirubin | 15 | 7 | 13 | 5 |
| TEAE (TR, %) | 57 | 49 | ||
| Serious TEAE (TR, %) | 18 | 10 | ||
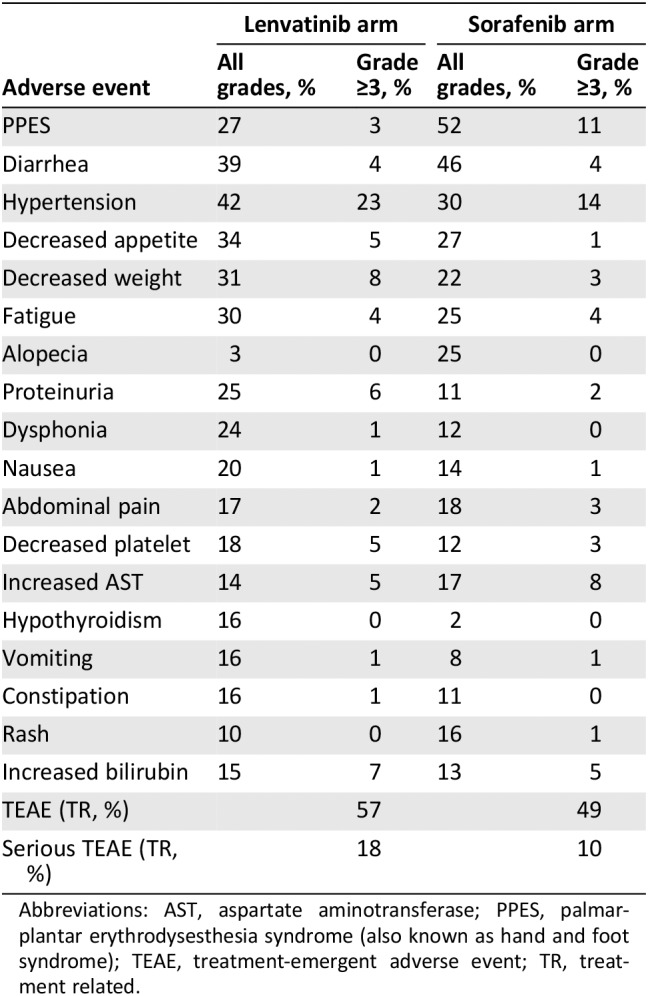
Abbreviations: AST, aspartate aminotransferase; PPES, palmar‐plantar erythrodysesthesia syndrome (also known as hand and foot syndrome); TEAE, treatment‐emergent adverse event; TR, treatment related.
