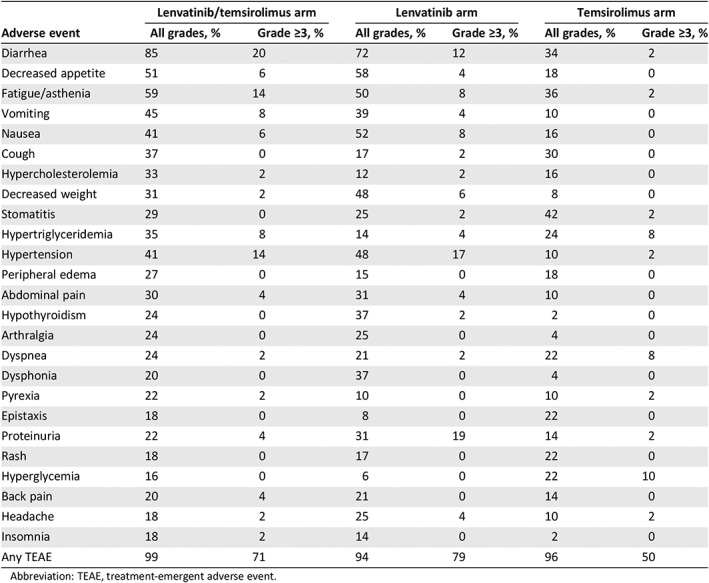
Table 3.
Adverse events from the phase II study of lenvatinib plus everolimus for renal cell carcinoma
| Adverse event | Lenvatinib/temsirolimus arm | Lenvatinib arm | Temsirolimus arm | |||
|---|---|---|---|---|---|---|
| All grades, % | Grade ≥3, % | All grades, % | Grade ≥3, % | All grades, % | Grade ≥3, % | |
| Diarrhea | 85 | 20 | 72 | 12 | 34 | 2 |
| Decreased appetite | 51 | 6 | 58 | 4 | 18 | 0 |
| Fatigue/asthenia | 59 | 14 | 50 | 8 | 36 | 2 |
| Vomiting | 45 | 8 | 39 | 4 | 10 | 0 |
| Nausea | 41 | 6 | 52 | 8 | 16 | 0 |
| Cough | 37 | 0 | 17 | 2 | 30 | 0 |
| Hypercholesterolemia | 33 | 2 | 12 | 2 | 16 | 0 |
| Decreased weight | 31 | 2 | 48 | 6 | 8 | 0 |
| Stomatitis | 29 | 0 | 25 | 2 | 42 | 2 |
| Hypertriglyceridemia | 35 | 8 | 14 | 4 | 24 | 8 |
| Hypertension | 41 | 14 | 48 | 17 | 10 | 2 |
| Peripheral edema | 27 | 0 | 15 | 0 | 18 | 0 |
| Abdominal pain | 30 | 4 | 31 | 4 | 10 | 0 |
| Hypothyroidism | 24 | 0 | 37 | 2 | 2 | 0 |
| Arthralgia | 24 | 0 | 25 | 0 | 4 | 0 |
| Dyspnea | 24 | 2 | 21 | 2 | 22 | 8 |
| Dysphonia | 20 | 0 | 37 | 0 | 4 | 0 |
| Pyrexia | 22 | 2 | 10 | 0 | 10 | 2 |
| Epistaxis | 18 | 0 | 8 | 0 | 22 | 0 |
| Proteinuria | 22 | 4 | 31 | 19 | 14 | 2 |
| Rash | 18 | 0 | 17 | 0 | 22 | 0 |
| Hyperglycemia | 16 | 0 | 6 | 0 | 22 | 10 |
| Back pain | 20 | 4 | 21 | 0 | 14 | 0 |
| Headache | 18 | 2 | 25 | 4 | 10 | 2 |
| Insomnia | 18 | 2 | 14 | 0 | 2 | 0 |
| Any TEAE | 99 | 71 | 94 | 79 | 96 | 50 |
Abbreviation: TEAE, treatment‐emergent adverse event.
