Table 4.
Concept under active exploration
| Drug(s) | n | Cancer type | Phase | Primary objective | NCT no. |
|---|---|---|---|---|---|
| Lenvatinib/pembrolizumab | 29 | Advanced gastric | II | ORR | 03609359 |
| Lenvatinib/TACE | 336 | HCC | III | OS | 03905967 |
| Lenvatinib/pembrolizumab | 24 | Gastroesophageal | IIa | OS | 03321630 |
| Lenvatinib/cetuximab | 16 | Squamous cell carcinoma (head/neck, skin) | I/Ib | MTD | 03524326 |
| Lenvatinib/pembrolizumab | 329 | Selected solid tumor | I/II | MTD/DLT/ORR | 02501096 |
| Lenvatinib/pembrolizumab | 60 | DTC | II | CR, ORR | 02973997 |
| Lenvatinib/everolimus vs. lenvatinib/pembrolizumab vs. sunitinib | 1,050 | RCC | III | PFS | 02811861 |
| Lenvatinib/pembrolizumab vs. chemotherapy | 780 | Endometrial | III | PFS, OS | 03517449 |
| Lenvatinib/eribulin | 30 | STS | Ib/II | ORR | 03526679 |
| Lenvatinib/pembrolizumab | 694 | Urothelial | III | PFS, OS | 03898180 |
| Lenvatinib/capecitabine/radiation | 28 | Rectal | I | MTD, DLT | 02935309 |
| Lenvatinib/pembrolizumab | 660 | Melanoma, first line | III | PFS, OS | 03820986 |
| Lenvatinib/pembrolizumab | 100 | Melanoma, second line | II | ORR | 03776136 |
| Lenvatinib/pembrolizumab or placebo | 750 | HCC | III | PFS, OS | 03713593 |
| Lenvatinib/pembrolizumab or chemotherapy | 720 | Advanced, recurrent endometrial | III | PFS, OS | 03884101 |
| Lenvatinib/everolimus | 40 | DTC | II | PFS | 03139747 |
| Lenvatinib/nivolumab | 50 | HCC | II | ORR, PR, CR | 03841201 |
| Lenvatinib ± carboplatin, pemetrexed/pembrolizumab | 726 | Nonsquamous, non‐small cell lung cancer | III | PFS, OS | 03829319 |
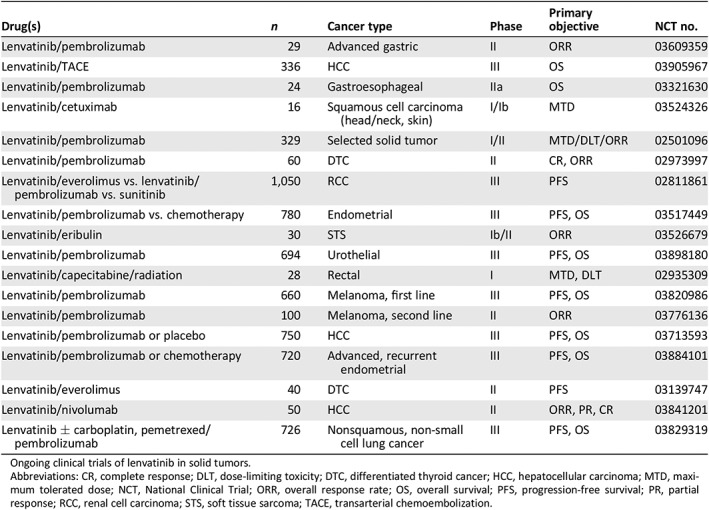
Ongoing clinical trials of lenvatinib in solid tumors.
Abbreviations: CR, complete response; DLT, dose‐limiting toxicity; DTC, differentiated thyroid cancer; HCC, hepatocellular carcinoma; MTD, maximum tolerated dose; NCT, National Clinical Trial; ORR, overall response rate; OS, overall survival; PFS, progression‐free survival; PR, partial response; RCC, renal cell carcinoma; STS, soft tissue sarcoma; TACE, transarterial chemoembolization.
