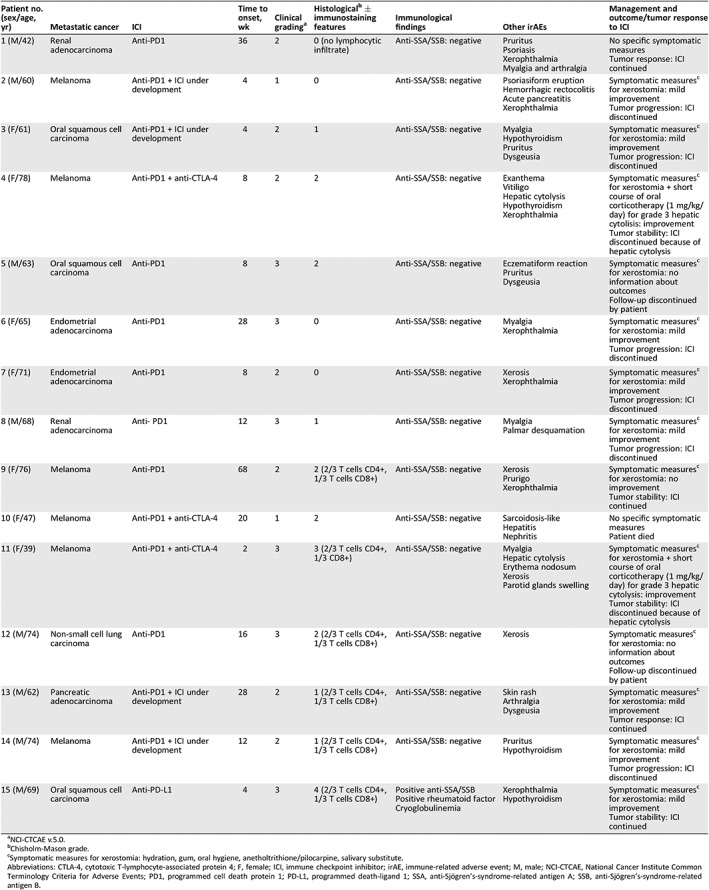
Table 1.
Clinical, histological, and immunostaining features of sicca syndrome in patients treated with ICIs
| Patient no. (sex/age, yr) | Metastatic cancer | ICI | Time to onset, wk | Clinical gradinga | Histologicalb ± immunostaining features | Immunological findings | Other irAEs | Management and outcome/tumor response to ICI |
|---|---|---|---|---|---|---|---|---|
| 1 (M/42) | Renal adenocarcinoma | Anti‐PD1 | 36 | 2 | 0 (no lymphocytic infiltrate) | Anti‐SSA/SSB: negative |
|
|
| 2 (M/60) | Melanoma | Anti‐PD1 + ICI under development | 4 | 1 | 0 | Anti‐SSA/SSB: negative |
|
|
| 3 (F/61) | Oral squamous cell carcinoma | Anti‐PD1 + ICI under development | 4 | 2 | 1 | Anti‐SSA/SSB: negative |
|
|
| 4 (F/78) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 8 | 2 | 2 | Anti‐SSA/SSB: negative |
|
|
| 5 (M/63) | Oral squamous cell carcinoma | Anti‐PD1 | 8 | 3 | 2 | Anti‐SSA/SSB: negative |
|
|
| 6 (F/65) | Endometrial adenocarcinoma | Anti‐PD1 | 28 | 3 | 0 | Anti‐SSA/SSB: negative |
|
|
| 7 (F/71) | Endometrial adenocarcinoma | Anti‐PD1 | 8 | 2 | 0 | Anti‐SSA/SSB: negative |
|
|
| 8 (M/68) | Renal adenocarcinoma | Anti‐ PD1 | 12 | 3 | 1 | Anti‐SSA/SSB: negative |
|
|
| 9 (F/76) | Melanoma | Anti‐PD1 | 68 | 2 | 2 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 10 (F/47) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 20 | 1 | 2 | Anti‐SSA/SSB: negative |
|
|
| 11 (F/39) | Melanoma | Anti‐PD1 + anti‐CTLA‐4 | 2 | 3 | 3 (2/3 T cells CD4+, 1/3 CD8+) | Anti‐SSA/SSB: negative |
|
|
| 12 (M/74) | Non‐small cell lung carcinoma | Anti‐PD1 | 16 | 3 | 2 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 13 (M/62) | Pancreatic adenocarcinoma | Anti‐PD1 + ICI under development | 28 | 2 | 1 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 14 (M/74) | Melanoma | Anti‐PD1 + ICI under development | 12 | 2 | 1 (2/3 T cells CD4+, 1/3 T cells CD8+) | Anti‐SSA/SSB: negative |
|
|
| 15 (M/69) | Oral squamous cell carcinoma | Anti‐PD‐L1 | 4 | 3 | 4 (2/3 T cells CD4+, 1/3 T cells CD8+) |
Positive anti‐SSA/SSB Positive rheumatoid factor Cryoglobulinemia |
|
|
NCI‐CTCAE v.5.0.
Chisholm‐Mason grade.
Symptomatic measures for xerostomia: hydration, gum, oral hygiene, anetholtrithione/pilocarpine, salivary substitute.
Abbreviations: CTLA‐4, cytotoxic T‐lymphocyte‐associated protein 4; F, female; ICI, immune checkpoint inhibitor; irAE, immune‐related adverse event; M, male; NCI‐CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; PD1, programmed cell death protein 1; PD‐L1, programmed death‐ligand 1; SSA, anti‐Sjögren's‐syndrome‐related antigen A; SSB, anti‐Sjögren's‐syndrome‐related antigen B.
