ABSTRACT
The plant hormone gibberellin (GA) stimulates developmental transitions including seed germination, flowering, and the transition from juvenile to adult growth stage. This study provided evidence that GA and the GA receptor GID1 (GA-INSENSITIVE DWARF1) are also needed for the embryo-to-seedling transition in Arabidopsis. The ga1-3 GA biosynthesis mutant fails to germinate unless GA is applied, whereas the gid1abc triple mutant fails to germinate because it cannot perceive endogenous or applied GA. Overexpression of the GID1a, GID1b, and GID1c GA receptors rescued the germination of a small percentage of ga1-3 seeds without GA application, and this rescue was improved by dormancy-breaking treatments, after-ripening and cold stratification. While GID1 overexpression stimulated ga1-3 seed germination, this germination was aberrant suggesting incomplete rescue of the germination process. Cotyledons emerged before the radicle, and the resulting “ghost” seedlings failed to develop a primary root, lost green coloration, and eventually died. The development of ga1-3 seedlings overexpressing GID1 was rescued by pre-germinative but not post-germinative GA application. Since the gid1abc mutant also exhibited a ghost phenotype after germination was rescued by cutting the seed coat, we concluded that both GA and GID1 are needed for the embryo-to-seedling transition prior to emergence from the seed coat.
KEYWORDS: Gibberellin, GID1, dormancy, seed germination, embryo-to-seedling transition
Introduction
Plant embryos survive for long periods of time in a quiescent and developmentally arrested state in dry seeds (reviewed by Bewley et al.1). This allows their prolonged storage for use in propagating crop plants. This state of suspended animation must be reversed during germination. The term germination in the strict sense refers to the event when the embryonic root or radicle emerges from the seed coat. However, the germination process begins with water uptake, and involves many steps needed to reactivate metabolism and commence seedling development. These processes include cellular and DNA repair, the initiation of respiration, the initiation of RNA transcription and protein translation, the induction of hydrolytic enzymes for the mobilization of stored reserves, loosening of barrier tissues, and cell expansion culminating in radicle emergence. Radical emergence results from enzymatic weakening of the seed coat coupled with the internal force generated by the elongation of the embryonic axis. Given the complexity of the process, many different defects could potentially prevent germination, especially in aged seeds. Once germinated, a seedling must proceed through all critical developmental stages associated with growth, flowering, and fertility to complete its life cycle and ensure the next generation of viable seeds.
Seed dormancy can prevent the germination of viable seeds under favorable environmental conditions (reviewed in Finkelstein et al.2). Dormancy prevents germination out of season and ensures species survival of natural catastrophes as seeds in the soil. The seeds of many temperate species are dormant at maturity, then acquire the ability to germinate through dormancy-breaking processes including after-ripening (a period of dry storage) and cold imbibition of water (also called cold stratification or moist chilling). The length of time required for dormancy loss through dry after-ripening is genetically determined. This together with transcriptomic studies suggests that measurable differences in regulatory factors control the change in germination potential with after-ripening of dry seeds.3,4 The plant hormone abscisic acid (ABA) induces dormancy during seed maturation and maintains dormancy in mature seeds (reviewed in Finkelstein et al.2), whereas the plant hormone gibberellin A (GA) stimulates germination and has been implicated in dormancy loss through after-ripening and cold stratification.5–7 Seeds of GA biosynthetic mutants fail to germinate unless GA is applied in Arabidopsis and tomato.8,9
The Arabidopsis GA1 gene encodes copalyl synthase, an early enzyme needed for GA biosynthesis.9,10 Mutations in this and other biosynthesis genes cause lack of de novo GA biosynthesis and GA-rescued phenotypes, including failure to germinate, dwarfism, delay in or failure to flower, and infertility.9,11 The ga1-3 allele is a 5-kb deletion, resulting in a null allele with trace levels of GA hormone.12,13 These phenotypes reveal that GA stimulates germination, cell division and elongation, and flower development. GA also stimulates many developmental events in the plant life cycle including embryo development, the transition from embryo to seedling development, the development of leaf primordia in the shoot apical meristem, and the juvenile to adult phase transition.14–18
GA stimulates plant growth and development by targeting negative regulators of GA responses, called DELLA (Asp-Glu-Leu-Leu-Ala) proteins, for destruction by the ubiquitin-proteasome pathway.19–21 GA signaling in Arabidopsis requires GA biosynthesis, the three GA receptors (GID1a, GID1b, and GID1c), and the F-box protein SLY1 (SLEEPY1) for proteolytic DELLA destruction (reviewed in Hauvermale et al.22). GA binding to the GID1 receptor causes a conformational change in GID1 allowing the receptor to bind DELLA protein. The SLY1 F-box protein recognizes and binds DELLA when in complex with GID1. Thus, GA and GID1 stimulate DELLA polyubiquitination by the SCFSLY1 E3 ubiquitin ligase, leading to DELLA destruction by the 26S proteasome pathway.19,23,24 This lifts DELLA repression of GA responses including seed germination. Loss of GA perception in the gid1abc triple mutant and loss of GA signaling in the sly1 mutant lead to similar phenotypes as those seen in the ga1-3 mutant, except that these phenotypes cannot be rescued by GA application.25,26,27 The failure of germination in gid1abc and sly1 mutants can only be rescued by artificially removing the seed coat.
Previous work showed that overexpression of the three GA receptors in the GA biosynthesis mutant ga1-3 increased sensitivity to GA stimulation of seed germination.5 This effect was stronger when seeds first experienced the dormancy-breaking treatments of cold stratification and dry after-ripening. Subsequently, we made the anecdotal observation that ga1-3 seeds in which HA-tagged GID1a, GID1b, or GID1c were overexpressed on the 35 promoter (HA:GID1-OE) were able to germinate at a very low efficiency. This phenomenon of germination in a GA-deficient mutant was not noticed initially because the resulting seedlings were small and failed to grow. This study characterized GID1 rescue of ga1-3 germination, and the post-germination developmental arrest we have termed the ga1-3 “ghost” phenotype.
Materials and methods
Germplasm
Genotypes included in this study were wild-type (Landsberg erecta or Ler), ga1-3, and ga1-3 HA:GID1a-OE, ga1-3 HA:GID1b-OE, and ga1-3 HA:GID1c-OE, as previously described. 9,28,29,5 The GID1-OE overexpression lines carry the HA:GID1(a-c) translational fusion constructs overexpressed on the Cauliflower Mosaic Virus 35S promoter. The gid1abc triple mutant in the Columbia (Col) ecotype is fully infertile. It is maintained as a gid1a heterozygote with the genotype gid1b-1/gid1b-1 gid1c-2/gid1c-2 gid1a-1/+.25
Growth conditions
Seeds were plated on MS-agar (0.5x Murishige and Skoog salts, 0.8% agar) plates buffered with MES pH 5.5 (2-(N-morpholino)ethanesulfonic acid, Sigma-Aldrich), subjected to 3 days of cold stratification at 4°C to obtain uniform germination. After cold stratification, plates were imbibed in the light at 22°C until they reached the 4 leaf stage, at which time they were transferred to soil for propagation. To promote germination, ga1-3 seeds were plated in the presence of 10 µM GA3. Plants were grown in potting soil in ConvironTM growth chambers as in19. Both ga1-3 and ga1-3 HA:GID1 overexpression lines were sprayed once a week with 10 µM GA3 to rescue fertility until they reached the green silique stage. Mature seeds were stored in open tubes for 2 weeks at room temperature, then stored in closed tubes either at room temperature for after-ripening or at −20°C to preserve dormancy.
Germination assays
For germination experiments evaluating the effects of GA and dormancy breaking treatments on seed germination in ga1-3 and ga1-3 HA:GID1-OE lines, three biological replicates of at least 100 seeds each were surface sterilized with 10% bleach/0.01% SDS for 10 minutes, washed with sterile water, and then plated on MS-agar plates supplemented with the indicated concentration of GA4. Stocks of 10 mM GA4 (Sigma) were solubilized in 70% ethanol and stored at −20°C until use. Plates were either cold stratified (imbibed at 4°C for 3 days) and then imbibed in the light at 22°C or placed directly into the light without cold stratification. Seed germination was scored daily over 9 days. The mean percent germination was calculated using three biological replicates.
For characterization of the ghost-like phenotype in gid1abc triple mutant seedlings, three biological replicates of at least 100 seeds each were surface sterilized with 10% bleach/0.01% SDS for 10 minutes and plated on MS-agar plates. Plates were cold stratified at 4°C for 3 days and then imbibed in the light at 22°C. Seed germination was monitored over the course of 9 days and then seedling development was tracked for an additional 12 days.
GA treatment of ga1-3 HA:GID1-OE seedlings
ga1-3 HA:GID1-OE seeds that germinated in the absence of GA were transferred to MS-agar plates containing 1 µM GA4 where indicated. Alternatively, 1 µM GA4 was added to MS-agar plates containing ungerminated ga1-3 and ga1-3 HA:GID1-OE seeds. All plates were placed in the light at 22°C for an additional 12 days. Seed germination and seedling growth were monitored daily.
GA dose-response curves of ga1-3 HA:GID1-OE lines
To evaluate the minimum amount of GA4 needed to rescue the ghost phenotype, ga1-3 HA:GID1- OE seeds were after-ripened for 2 months, then plated in the presence of 0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, or 1 µM GA4. The 0 µM GA4 MS-agar plates contained 70% ethanol at the maximum concentration used in the dose-response curve to serve as a “mock” control for the effect of the solvent. Up to 5000 seeds from each ga1-3 HA:GID1-OE were screened to ensure a total of 100 seedlings for each condition. Seeds were surface sterilized with 10% bleach/0.01% SDS for 10 minutes and plated on MS agar plates with or without GA4. Plates were cold stratified for 3 days at 4°C and then placed in the light at 22°C. Seed germination and seedling growth were monitored daily for a total of 12 days.
Micrographs
All seedling photographs were taken with a Leica MZ6 light microscope with 8X magnification using the Infinity1 camera and Infinity Capture software (Luminera).
Statistical analysis
For all germination experiments, data was transformed using a square root transformation. For both germination and GA dose-response experiments, statistical significance was assessed using an analysis of variance (ANOVA) with a Tukey’s all pairwise comparison (SAS v9.4).
Results
GID1 overexpression, after-ripening, and cold stratification stimulate ga1-3 seed germination without GA
To characterize the HA:GID1-OE rescue of ga1-3 germination, the effect of overexpression without and with the dormancy-breaking treatments of dry after-ripening and cold-stratification was examined. An after-ripening time course was conducted for seeds of wild-type (WT) Ler, ga1-3, ga1-3 HA:GID1a-OE, ga1-3 HA:GID1b-OE, and ga1-3 HA:GID1b-OE followed by germination at 22°C for 9 days without GA hormone. Germination was examined with and without cold stratification for 3 days at 4°C before incubation at 22°C. Germination of ga1-3 HA:GID1-OE seeds occurred within 5–9 days of imbibition in the light at 22ºC while Ler WT germinated within 24 hrs (Figure S1). The combination of long after-ripening and cold stratification resulted in no more than 2% germination in untransformated ga1-3, whereas GID1 gene overexpression resulted in 1.3 to 9.4% germination (n = 100; Table 1).
Table 1.
The effects of HA:GID1-OE on ga1-3 germination with cold and after-ripening.
| |
2Percent Germination with after-ripening |
||||
|---|---|---|---|---|---|
| Genotype | 1Treatment | 2 wk | 8 wk | 24 wk | 52 wk |
| Ler | NC | 100 | 100 | 100 | 100 |
| C | 100 | 100 | 100 | 100 | |
| ga1-3 | NC | 0.0 | 0.3 | 0.0 | 0.0 |
| C | 0.0 | 0.5 | 1.3 | 2.0 | |
| ga1-3 + 1 µM GA4 | NC | 98.0 | 98.0 | 99.0 | 100 |
| C | 99.0 | 98.5 | 99.0 | 100 | |
| HA:GID1a-OE | NC | 0.0 | 1.5 | 2.9 | 4.0 |
| C | 1.3 | 3.8 | 3.8 | 4.0 | |
| HA:GID1b-OE | NC | 0.0 | 0.7 | 6.7 | 6.0 |
| C | 3.8 | 9.4 | 7.0 | 6.0 | |
| HA:GID1c-OE | NC | 0.0 | 1.3 | 5.1 | 6.0 |
| C | 0.6 | 2.8 | 5.2 | 6.0 | |
Based on an ANOVA with Tukey’s pairwise comparison, each GID1 overexpression construct significantly stimulated ga1-3 germination, and this effect was enhanced by both after-ripening and cold stratification (Table 1; Table 2). At 2 and 8 weeks (wk) of after-ripening, GID1 gene overexpression only stimulated ga1-3 germination when the seeds were cold stratified. GID1 overexpression significantly stimulated ga1-3 germination both with and without cold stratification when seeds had been after-ripened for 24 and 52 wk (p < .0001; Table 1; Figure S2). The strongest stimulation of germination was seen with GID1b overexpression when seed dormancy was broken through 8 wk of after-ripening in concert with cold stratification. Interestingly, the ga1-3 HA:GID1b-OE germination increased with 8 wk after-ripening, then decreased with longer 24 and 52 wk of after-ripening (Figure S2).
Table 2.
An ANOVA summary from a Tukey’s pairwise comparison determining significant interactions among and between genotypes, treatments (cold or no cold), and after-ripening.
| Source | DF | Type I SS | Mean Square | F Value | Pr > F |
|---|---|---|---|---|---|
| Genotype (Geno) | 3 | 31.18774071 | 10.39591357 | 110.61 | <0.0001 |
| Treatment (Treat) | 1 | 10.24538460 | 10.24538460 | 109.01 | <0.0001 |
| After-ripening (AR) | 3 | 31.72070122 | 10.57356707 | 112.50 | <0.0001 |
| Rep | 2 | 0.29601524 | 0.14800762 | 1.57 | 0.2152 |
| Geno*Treat | 3 | 2.28134864 | 0.76044955 | 2.88 | 0.0406 |
| Geno*AR | 9 | 4.79955619 | 0.53328402 | 2.12 | 0.0376 |
| Geno*Treat*AR | 24 | 18.34487438 | 0.76436977 | 8.13 | <0.0001 |
Germination of ga1-3 without GA results in aberrant seedling development that cannot be rescued by post-germination GA application
While GID1 overexpression did allow ga1-3 seeds to germinate at a low frequency in the absence of GA, the resulting seedlings failed to develop normally. During wild-type Ler germination, seedlings emerged radicle first, then the cotyledons expanded, the radicle elongated into a root, and the first leaves developed (Figure 1). The ga1-3 and ga1-3 HA:GID1-OE seedlings emerged cotyledons-first rather than radicle first. Thus, it appeared that cotyledon expansion rather than radicle elongation generated the force needed to emerge from the seed coat. After germination, the cotyledons did not expand normally, and the radicle and hypocotyl did not elongate (Figure 2). Many of these abnormal seedlings had white-tipped cotyledons that never became green. The radicles remained small, resembling those of an embryo (Figure 3). Eventually, these seedlings lost their green coloration, became translucent, and died. Thus, we refer to this as a “ghost” seedling phenotype. To summarize, GID1 overexpression rescued ga1-3 germination in the strict sense of emerging from the seed coat but was unable to fully rescue the germination process since seedlings were unable to complete the embryo-to-seedling transition.
Figure 1.
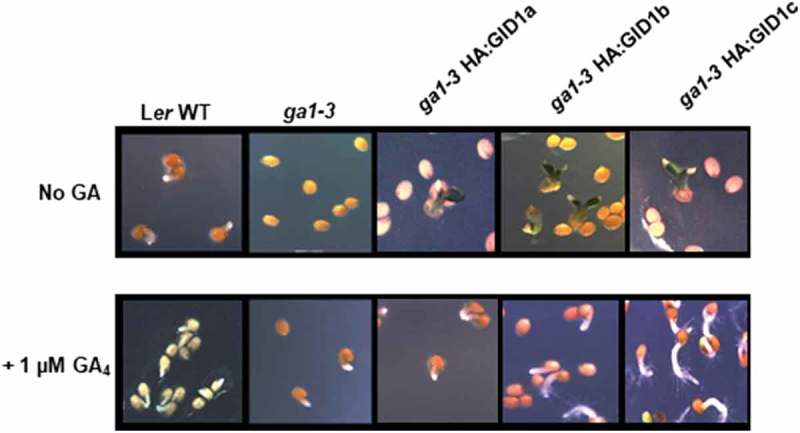
ga1-3 HA:GID1-OE germination resulted in abnormal seedlings. Normal germination of Ler and of ga1-3 in the presence of 1 µM GA4 (radicle emergence first), is contrasted with abnormal germination of ga1-3 GID1-OE seeds in the absence of GA. ga1-3 GID1-OE seeds germinated cotyledons first, and cotyledons had white tips. This “ghost” phenotype was rescued in ga1-3 GID1-OE lines when seeds were imbibed in the presence of 1 µM GA4.
Figure 2.
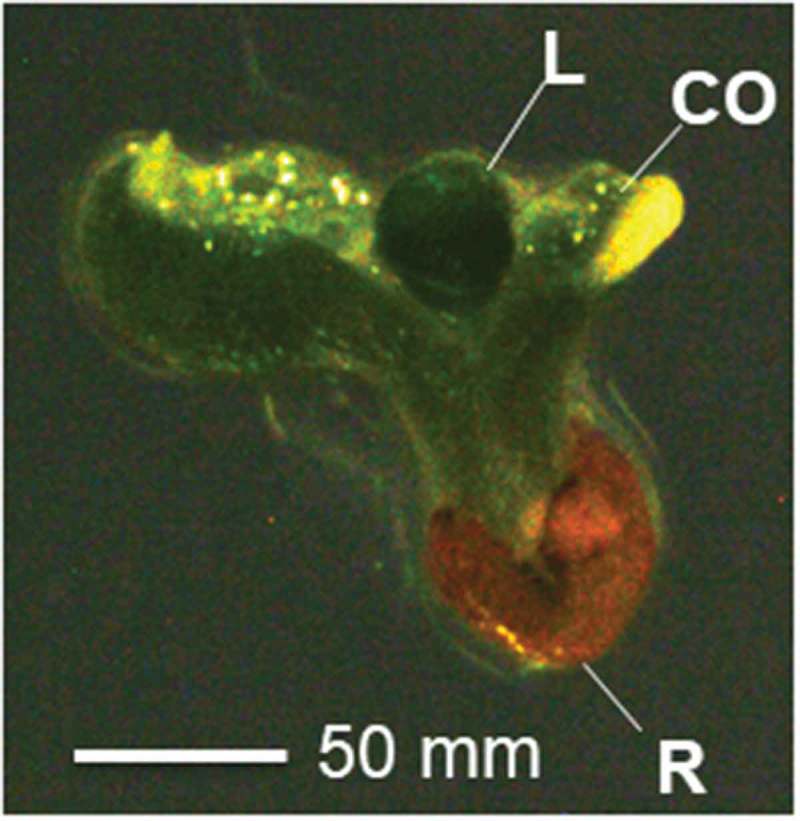
An abnormal “ghost” ga1-3 HA:GID1b-OE seedling. In the absence of GA, ga1-3 HA:GID1-OE seeds germinated with cotyledons (CO) emerging before the radicle/root (r). The resulting seedlings showed failure in hypocotyl elongation and cotyledon expansion, although first leaves (l) did emerge. These seedlings eventually died. Scale bar = 50 mm.
Figure 3.
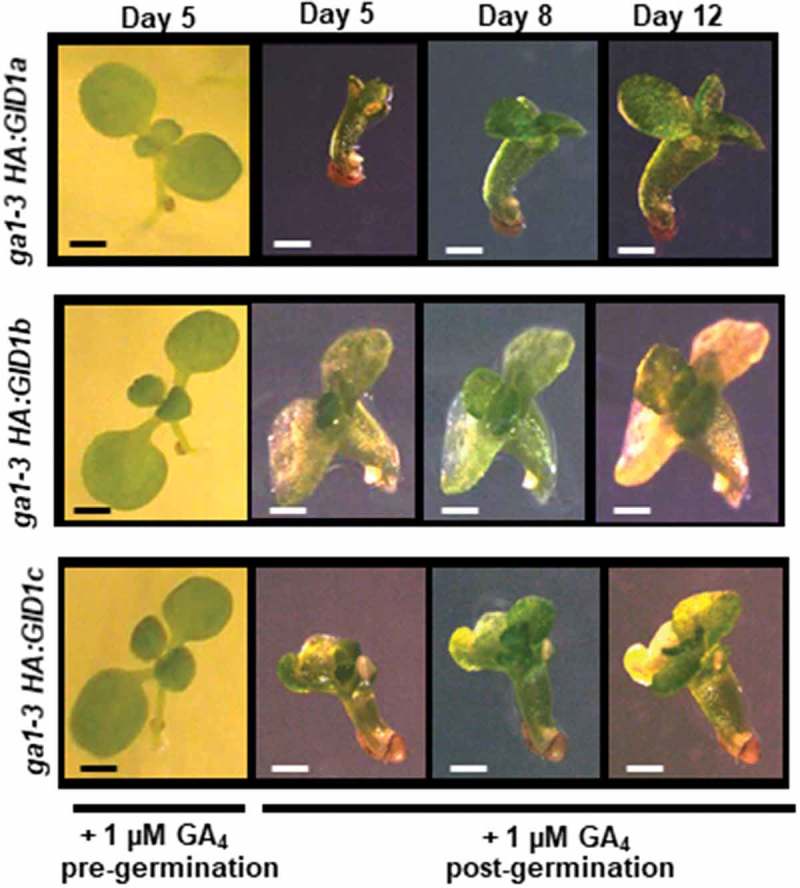
Post-germinative GA application failed to rescue ga1-3 HA:GID1-OE seedling development. When germinated in the presence of 1 µM GA4 ga1-3 HA:GID1-OE lines developed a normal seedling phenotype. When GA4 was added after germination seedling growth and development was abnormal, and seedlings gradually lost green coloration and died. Black or white scale bars = 50 mm.
To determine whether GA application could rescue the germination and abnormal growth of the ga1-3 HA:GID1-OE overexpression lines, seeds and seedlings were transferred to plates containing 1 µM GA4. GA treatment fully rescued germination and normal post-germinative growth of the seeds that failed to germinate after imbibing for 10 days at 22°C in the absence of GA (Figure 1; Figure 3). In contrast, ghost ga1-3 HA:GID1-OE seedlings that germinated in the absence of GA, failed to recover after 5–12 days of incubation in the light in the presence of GA (Figure 3). Although the true leaves of ga1-3 HA:GID1-OE ghost seedlings began to grow, there was no additional development of the primary root, and seedling death associated with cotyledon bleaching occurred within 10 to 14 days after germination. These results suggest that GA hormone is needed prior to germination to stimulate the transition from embryo to seedling development.
Next a GA dose-response experiment was used to determine the GA4 concentration required during germination to rescue the post-germinative seedling development of ga1-3 HA:GID1a-OE, ga1-3 HA:GID1b-OE, and ga1-3 HA:GID1c-OE. These seeds were after-ripened for 2 months, then cold stratified before germinating on GA.5 Seeds were imbibed for 12 days on MS-agar containing increasing concentrations of GA4 and 100 of the resulting seedlings were scored for normal versus ghost seedling phenotype. Three replicates of 100 germinated seeds each were examined for each GA concentration (Figure 4). Note that since ga1-3 HA:GID1c-OE had 2% germination without GA, it was necessary to imbibe 5000 seeds to obtain 100 germinated seedlings. It appeared that the concentration of GA needed to exceed 50% germination under these conditions was also sufficient to rescue the ghost phenotype, 0.01 µM for ga1-3 HA:GID1b-OE and 0.1 µM for ga1-3 HA:GID1a-OE and ga1-3 HA:GID1c-OE. The ga1-3 HA:GID1b-OE line was most responsive and the ga1-3 HA:GID1a-OE line least responsive to GA rescue of the ghost seedling phenotype. The minimum GA concentration at which ghosts were still observed was 0.005 µM for ga1-3 HA:GID1b-OE, 0.05 µM for ga1-3 HA:GID1c-OE, and 0.1 µM for ga1-3 HA:GID1a-OE. When the ghost phenotype was observed over 12 days of imbibition at 0.005 µM GA4, it was observed the ga1-3 HA:GID1b-OE ghost seedlings were able to develop a short root before dying, suggesting that a partial rescue was possible (Figure S3).
Figure 4.
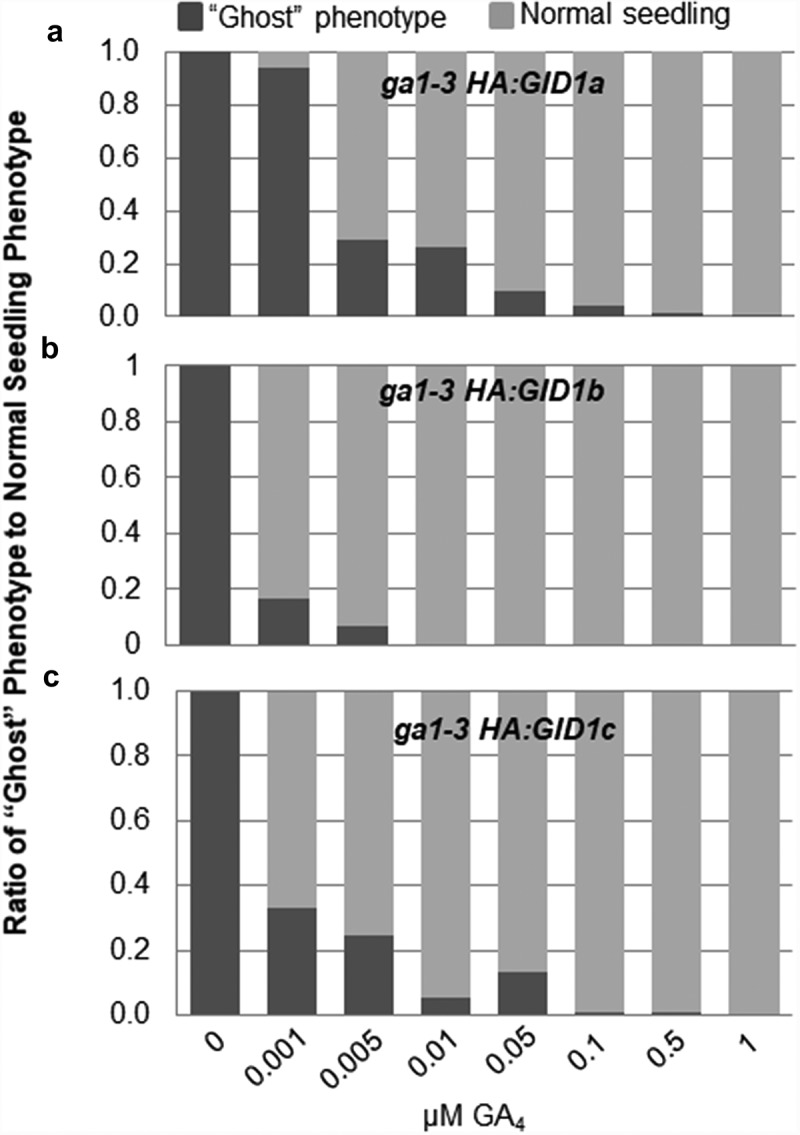
The ghost seedling phenotype is rescued by GA in a dose-dependent manner. Two month after-ripened (a) ga1-3 HA:GID1a-OE, (b) ga1-3 HA:GID1b-OE, and (c) ga1-3 HA:GID1c-OE seeds were plated without GA4 or with the indicated GA4 concentration. Plates were cold stratified for 3 days in the dark, and then moved to 22°C in the light. The ratio of “ghosts” (dark gray) to normal seedlings (light gray) is shown.
Germination of the gid1abc triple mutant frequently results in a ghost-like seedling
If GA signaling is required for seedling growth and development, then we would expect a strong GA-insensitive mutant to exhibit a ghost phenotype like that observed in ga1-3. The gid1abc triple mutant is infertile and cannot germinate unless the seed coat is cut. Thus, the gid1abc triple is maintained as a gid1a heterozygote with the genotype, gid1b/gid1b gid1c/gid1c gid1a/+ (Figure 5(a)).25 The progeny of the heterozygote segregated 17% ungerminated seeds. It may be that this is slightly less than the expected 25% because gid1a and gid1b are about 22 Mb apart on Arabidopsis chromosome 3. In order to examine the post-germinative growth of the gid1abc homozygous triple mutant, seed coats of 63 ungerminated seeds were nicked with a sharp pair of dissection tweezers. All of the nicked seeds produced seedlings, of which only 4 produced “normal” seedlings exhibiting the GA dwarf phenotype, dark green cotyledons and true leaves, and elongated roots (Figure 5(c)). The remaining seedlings arrested in seedling development and resembled the ga1-3 ghost seedlings (Figure 5(b)). These seedlings failed to produce an elongated root, became glassy or translucent, lost their green coloration, and died without establishing seedling growth on MS-agar. Thus, 93.7% of gid1abc triple mutants exhibited a ghost seedling phenotype when seed germination was forced by cutting the seed coat. This indicates that GA perception by GID1 is needed for efficient transition from embryo to seedling development during the germination process.
Figure 5.
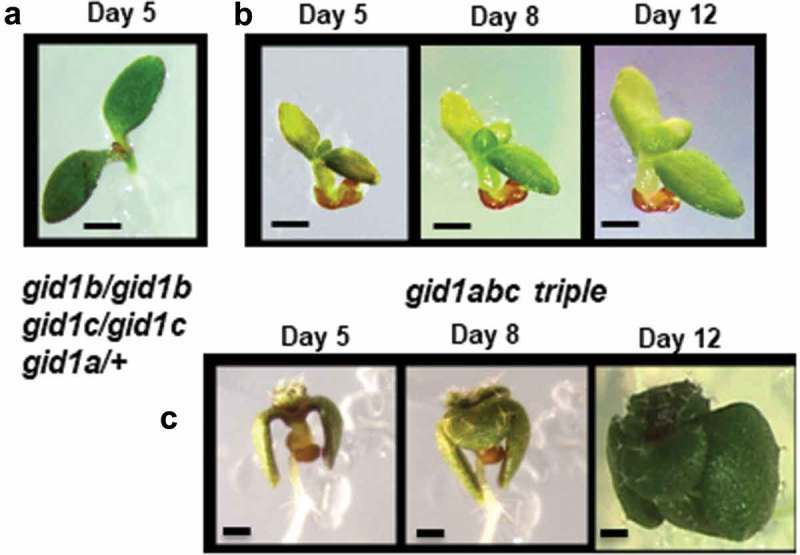
Ghost phenotype in a gid1abc triple mutant seedling. (a) A gid1b/b c/c a/+ heterozygous seedlings displayed a wild-type phenotype, whereas (b) the homozygous gid1abc triple mutant displayed a ghost-seedling phenotype similar to ga1-3 HA:GID1-OE lines, including cotyledon-first germination, the failure of root elongation, and loss of green coloration in the cotyledons and first leaves. (c) A small percentage of gid1abc triple mutant completed the embryo-to-seedling transition, developing roots and shoots. Bar = 50 mm.
Discussion
This study provided evidence that GA hormone functions prior to seedling emergence to stimulate the embryo-to-seedling developmental transition. Previous work suggested that GA is mainly needed to overcome the seed coat barrier during germination since ga1-3, sly1-2, and gid1abc seed germination can be rescued by testa weakening or removal of the seed coat.23,25,30–32 However, absence of GID1a, GID1b, and GID1c in gid1abc led to an inability to efficiently transition to seedling development after the seed coat was cut (Figure 5). Thus, GA signaling is required for this transition. Moreover, exogenous GA application could only rescue ga1-3 HA:GID1-OE seedling growth and development if applied before seedling emergence from the seed coat. Thus, GA signaling stimulates the embryo-to-seedling transition prior to germination in the strict sense. This suggests that GA may play a critical role in reactivating metabolism and/or development during the germination process before germination in the strict sense. Future work will need to investigate which essential processes needed during germination fail during ghost seedling germination.
Overexpression of GID1a, GID1b, and GID1c was not expected to rescue ga1-3 seed germination because GA receptors were not expected to function in the absence of GA biosynthesis. Surprisingly, we found that GID1 gene overexpression led to a low level of seed germination in ga1-3, in the absence of GA biosynthesis and application. This suggests either that GID1 proteins have some limited ability to function in germination without GA, or that the residual GA levels in ga1-3 seeds are sufficient to trigger some GA signaling and germination. It is known that GID1 gene overexpression has no negative effect on the embryo-to-seedling transition since wild-type Ler transformed with HA:GID1-OE constructs complete normal plant development without arresting in the embryo-to-seedling transition.29
While Arabidopsis GID1a and GID1b depend on GA-binding to interact with and target DELLA proteins for destruction, GID1b protein can bind DELLA protein with low affinity without GA.33 GA enhances GID1b binding to DELLA. This GA-independent function may be why GID1b overexpression results in both the strongest increase in GA sensitivity during germination and in the strongest rescue of ga1-3 and sly1-2 seed germination (Figure 3).5 However, we cannot rule out the possibility that GID1 proteins have functions that occur in the absence of GA. That GID1b overexpression stimulates germination in a GA-deficient mutant is interesting given that gid1 double mutant analysis revealed that GID1b can act as a negative regulator of seed germination, especially in the dark.34 This suggests that the role of GID1b in regulating germination can depend on environmental context.
While the ga1-3 HA:GID1-OE ghost seedlings were able to emerge from the seed coat, this germination was aberrant in that cotyledon expansion, not radicle elongation, provided the force needed to fracture the seed coat. Similar cases of cotyledon-first germination have been observed. The GA-insensitive sly1 F-box mutants fail to germinate unless the seed coat is cut.23 After nicking the sly1 seed coat, it was observed that the seedlings germinated cotyledons first and that primary root elongation was either absent or delayed. The resulting sly1 seedlings often have problems becoming established on soil, suggesting these seedlings may have difficulties with the embryo-to-seedling transition similar to, but milder than those of the gid1abc triple mutant. Cotyledon-first germination was observed when ga1-3 germination was partly rescued by mutations in DELLA genes, negative regulators of GA signaling. Penfield et al.35 showed that higher dormancy was associated with smaller cotyledons in multiple dormant Arabidopsis backgrounds, including ga1−3, rescued to varying degrees by mutations in the five DELLA genes. This suggested that dormancy loss stimulates germination through increased cotyledon expansion. If DELLA-imposed dormancy blocks cotyledon expansion, then this suggests that GID1 gene overexpression may partly lift DELLA-imposed dormancy allowing cotyledon expansion to break open the seed coat. Moreover, this might explain why dormancy-breaking treatments of cold stratification and after-ripening enhanced the cotyledon-first germination of ga1−3 HA:GID1-OE lines. Previous work showed that increasing GA sensitivity with after-ripening is associated with increased accumulation of GID1 proteins, suggesting that GID1 plays a role in increasing germination potential.5
The ghost phenotype could be caused by many possible flaws in development, such as failure to initiate photosynthesis, cell division, or failure in shoot or root development. GA stimulates many aspects of plant development that might be needed for the embryo-to-seedling transition, including the transition to leaf development in the shoot apical meristem,36 the mobilization of stored reserves during the embryo-to-seedling transition,37 and stimulation of root meristem enlargement and root growth.38 Aberrations in any of these functions may have caused the ghost phenotype. Future work will need to investigate the precise mechanisms causing the ga1-3 ghost phenotype.
Several lines of evidence suggest that GA signaling may be needed to trigger auxin-stimulated root growth and development during germination. Weaker alleles of the Arabidopsis auxin-insensitive mutant monopteros showed a failure in seedling root development similar to ga1-3 ghosts and reminiscent of the phenotypes observed when auxin transport is inhibited.39 Interestingly, GA appears to stimulate root responsiveness to indole-3-acetic acid (IAA).40,41 Moreover, there appears to be a connection between GA and control of auxin transport.42,43 Previous work suggested that GA stimulates root gravitropism based on the fact that asymmetric GA localization in roots stimulated asymmetric localization of PIN2 auxin transporters by controlling PIN2 protein stability. Future work will need to examine if the ghost phenotype is associated with either defects in auxin transport or auxin-regulated vascular development.
Funding Statement
This work was supported by the NSF, Directorate for Biological Sciences [085098].
Acknowledgments
The authors thank Tracy Harris for expert technical assistance. Thanks are due to Wenjing Ge, Carl Walker, Kimberly Garland-Campbell, and members of the Steber lab for helpful comments on the research and manuscript. This work was supported by NSF Award #085098 (to CMS).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Bewley DJ, Black M.. Seeds: physiology of development and germination. New York, NY: Springer; 2013. [Google Scholar]
- 2.Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:1–9. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- 3.Fennimore SA. Genetic basis for seed dormancy. Seed Sci Res. 1998;8:173-182. [Google Scholar]
- 4.Nelson SK, Ariizumi T, Steber CM. Biology in the dry seed: transcriptome changes associated with dry seed dormancy and dormancy loss in the arabidopsis GA-insensitive sleepy1-2 mutant. Front Plant Sci. 2017;8:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauvermale AL, Tuttle KM, Takebayashi Y, Seo M, Steber CM. Loss of Arabidopsis thaliana seed dormancy is associated with increased accumulation of the GID1 GA hormone receptors. Plant Cell Physiol. 2015;56:1773–1785. doi: 10.1093/pcp/pcv084. [DOI] [PubMed] [Google Scholar]
- 6.Urbanova T, Leubner-Metzger G. Gibberellins and seed germination. In Annual Plant Reviews Book Series, Volume 49: The Gibberellins. West Sussex, UK: John Wiley & Sons, Ltd; 2018. [Google Scholar]
- 7.Yamauchi Y, Ogawa M, Kuwahara A, Hanada A, Kamiya Y, Yamaguchi S. Activation of gibberellin biosynthesis and response pathways by low temperature during imbibition of Arabidopsis thaliana seeds. Plant Cell. 2004;16:367–378. doi: 10.1105/tpc.018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groot SPC, Karssen CM. Gibberellins regulate seed germination in tomato by endosperm weakening: a study with gibberellin-deficient mutants. Planta. 1987;171:525–531. doi: 10.1007/BF00392302. [DOI] [PubMed] [Google Scholar]
- 9.Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana (L.) Heynh. Theor Appl Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- 10.Karssen CM, Zagorski S, Kepczynski J, Groot SPC. Key role for endogenous gibberellins in the control of seed germination. Ann Bot. 1989;63:71–80. doi: 10.1093/oxfordjournals.aob.a087730. [DOI] [Google Scholar]
- 11.Sakamoto T, Miura K, Itoh H, Tatsumi T, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Agrawal GK, Takeda S, Abe K, et al. An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004;134:1642–1653. doi: 10.1104/pp.103.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeevaart JAD, Talon M. Gibberellin mutants in Arabidopsis thaliana. In: Karssen CM, van Loon LC, Vreugdenhil D, editors. Progress in Plant Growth Regulation. Current Plant Science and Biotechnology in Agriculture, vol 13. Dordrecht, The Netherlands: Springer; 1992. [Google Scholar]
- 13.Sun T, Kamiya Y.. The arabidopsis ga1 locus encodes the cyclase ent-kaurene synthetase a of gibberellin biosynthesis. Plant Cell. 1994;6:1509-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galinha C, Bilsborough G, Tsiantis M. Hormonal input in plant meristems: A balancing act. Semin Cell Dev Biol. 2009;20:1149–1156. doi: 10.1016/j.semcdb.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Ogas J, Kaufmann S, Henderson J, Somerville C. PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci. 1999;96:13839–13844. doi: 10.1073/pnas.96.24.13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakamoto T, Kobayashi M, Itoh H, Tagiri A, Kayano T, Tanaka H, Iwahori S, Matsuoka M. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 2001;125:1508–1516. doi: 10.1104/pp.125.3.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telfer A, Bollman KM, Poethig RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development. 1997;124:645–654. [DOI] [PubMed] [Google Scholar]
- 19.McGinnis KM, Thomas SG, Soule JD, Strader LC, Zale JM, Sun T-P, Steber CM. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell. 2003;15:1120–1130. doi: 10.1105/tpc.010827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow T-Y, Hsing Y-IC, Kitano H, Yamaguchi I, et al. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- 21.Ueguchi-Tanaka M, Matsuoka M. The perception of gibberellins: clues from receptor structure. Curr Opin Plant Biol. 2010;13:503–508. doi: 10.1016/j.pbi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Hauvermale AL, Ariizumi T, Steber CM. Gibberellin signaling: a theme and variations on DELLA repression. Plant Physiol. 2012;160:83–92. doi: 10.1104/pp.112.200956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steber CM, Cooney SE, McCourt P. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics. 1998;149:509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun T-P. DELLA proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004;135:1008–1019. doi: 10.1104/pp.104.039578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willige BC, Ghosh S, Nill C, Zourelidou M, Dohmann EMN, Maier A, Schwechheimer C. The DELLA domain of GA INSENSITIVE mediates the interaction with the GA INSENSITIVE DWARF1A gibberellin receptor of Arabidopsis. Plant Cell. 2007;19:1209–1220. doi: 10.1105/tpc.107.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariizumi T, Steber CM.. Seed germination of GA-insensitive sleepy1 mutants does not require RGL2 protein disappearance in arabidopsis. Plant Cell. 2007;19:791-804. doi: 10.1105/tpc.106.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iuchi S, Suzuki H, Kim Y, Iuchi A, Kuromori T, Ueguchi-Tanaka M, Asami T, Yamaguchi I, Matsuoka M, Kobayashi M, et al. Multiple loss-of-function of Arabidopsis gibberellin receptor AtGID1s completely shuts down a gibberellin signal. Plant Pathol J. 2007;50:958-966. [DOI] [PubMed] [Google Scholar]
- 28.Cao D, Hussain A, Cheng H, Peng J. Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta. 2005;223:105–113. doi: 10.1007/s00425-005-0057-3. [DOI] [PubMed] [Google Scholar]
- 29.Ariizumi T, Murase K, Sun T-P, Steber CM. Proteolysis-independent downregulation of DELLA repression in Arabidopsis by the gibberellin receptor GIBBERELLIN INSENSITIVE DWARF1. Plant Cell. 2008;20:2447–2459. doi: 10.1105/tpc.108.058487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bethke PC, Libourel IGL, Aoyama N, Chung -Y-Y, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1188. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debeaujon I, Koornneef M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000;122:415–424. doi: 10.1104/pp.122.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Sci Res. 2005;15:281–307. doi: 10.1079/SSR2005218. [DOI] [Google Scholar]
- 33.Yamamoto Y, Hirai T, Yamamoto E, Kawamura M, Sato T, Kitano H, Matsuoka M, Ueguchi-Tanaka M. A rice gid1 suppressor mutant reveals that gibberellin is not always required for interaction between its receptor, GID1, and DELLA proteins. Plant Cell. 2010;22:3589–3602. doi: 10.1105/tpc.110.074542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ge W, Steber CM. Positive and negative regulation of seed germination by the Arabidopsis GA hormone receptors, GID1a, b, and c. Plant Direct. 2018; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penfield S, Li Y, Gilday AD, Graham S, Graham IA. Arabidopsis ABA INSENSITIVE4 regulates lipid mobilization in the embryo and reveals repression of seed germination by the endosperm. Plant Cell. 2006;18:1887–1899. doi: 10.1105/tpc.106.041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hay A, Tsiantis M. KNOX genes: versatile regulators of plant development and diversity. Development. 2010;137:3153–3165. doi: 10.1242/dev.030049. [DOI] [PubMed] [Google Scholar]
- 37.Park J, Oh D, Dassanayake M, Nguyen KT, Ogas J, Choi G, Sun T. Gibberellin signaling requires chromatin remodeler PICKLE to promote vegetative growth and phase transitions. Plant Physiol. 2017;173:1463–1474. doi: 10.1104/pp.16.01471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moubayidin L, Perilli S, Dello Ioio R, Di Mambro R, Costantino P, Sabatini S. The rate of cell differentiation controls the Arabidopsis root meristem growth phase. Curr Biol. 2010;20:1138–1143. doi: 10.1016/j.cub.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Hardtke C, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu X, Harberd NP. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature. 2003;421:740–743. doi: 10.1038/nature01387. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Zhu C, Gan L, Ng D, Xia K. GA3 enhances root responsiveness to exogenous IAA by modulating auxin transport and signaling in Arabidopsis. Plant Cell Rep. 2015;34:483–494. doi: 10.1007/s00299-014-1728-y. [DOI] [PubMed] [Google Scholar]
- 42.Löfkea C, Zwiewkab M, Heilmannd I, Van Montagub MCE, Teichmanna T, Friml J. Asymmetric gibberellin signaling regulates vacuolar trafficking of PIN auxin transporters during root gravitropism. Proc Natl Acad Sci. 2013;110:3627–3632. doi: 10.1073/pnas.1300107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willige BC, Isono E, Richter R, Zourelidou M, Schwechheimer C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell. 2011;23:2184–2195. doi: 10.1105/tpc.111.086355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


