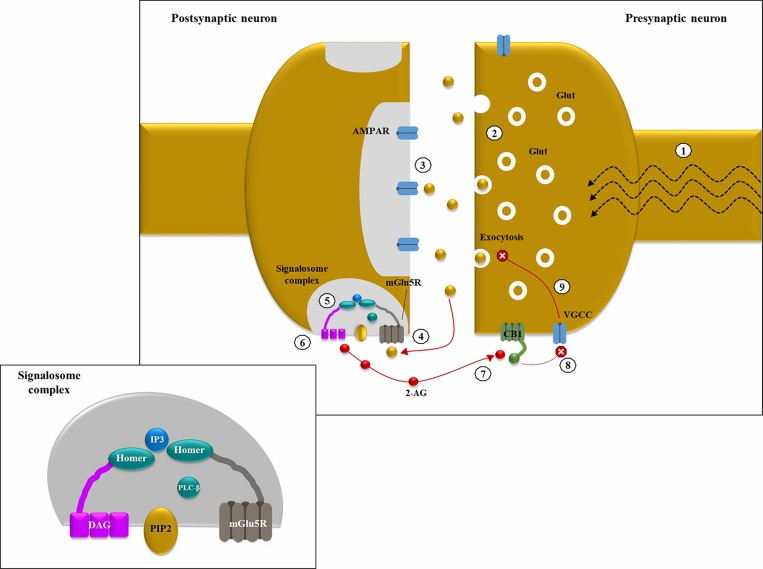
Figure 1.
Endocannabinoid-mediated negative feedback in epilepsy: (1) in excitatory synapses, depolarization induces Glutamate (Glut) release into the synaptic cleft, thanks to the increase in intracellular Ca2+ levels mediated by the opening of voltage-gated calcium channels (VGCC); (2) in hyperexcitable states, like epileptic seizures, a large amount of neurotransmitter is released from the presynaptic neuron; (3) under basal conditions, Glut binds primarily to intra-synaptic ionotropic receptors (AMPARs); (4) in case of hyperexcitability with Glut “spill over”, group 1 metabotropic Glut receptors (ie, mGlu5Rs) located at pery-synpatic level are activated by ligand binding; (5) mGLU5Rs are anchored together with phospholipase C β (PLCβ) and diacylglycerol lipase α (DGLα) thanks to the scaffolding protein HOMER, forming a sopramolecular complex known as “2-AG signalosome” (illustrated in the smaller panel); (6) mGlu5R activation increases diacyl glycerol (DAG) synthesis by PLCβ and its following conversion into 2-arachydonoyl glycerol (2-AG), catalyzed by DGLα; (7) 2-AG acts as a retrograde messenger and binds pre-synaptic CB1 (coupled with Gi/o); (8) CB1 activation inhibits VGCC thus reducing intracellular Ca2+ levels; (9) ↓ Ca2+ levels determine a decrease in Glut exocytosis. This negative feedback mechanism could be protective against Glut-mediated excitotoxicity in hyperexcitable states.