Abstract
An advanced glycation end products (AGE)/a receptor for AGE (RAGE) axis plays a central role in the pathogenesis of diabetic vascular remodeling. This study was conducted to clarify the role of RAGE in nondiabetic atherosclerosis. We used the aortic and coronary atherosclerotic lesions of Watanabe heritable hyperlipidemic (WHHL) rabbits prone to myocardial infarction (WHHLMI) at 1 to 14 months. Immunohistochemistry demonstrated the significant expression of RAGE as early as at 1 month with the stronger expression at 3 and 7 months, which was remarkably diminished at 14 months. RAGE expression was concordant with AGE accumulation. The major original sources of RAGE expression were macrophages and smooth muscle cells in addition to endothelial cells, and RAGE expression was distributed in the areas of phospholipid products, a component of oxidized LDL and nitrotyrosine. The concentrations of serum AGE did not alter significantly with aging. These findings suggested the expression of RAGE was induced by hyperlipidemia and oxidative stress independent of diabetes in WHHLMI rabbits. Additionally, our in vitro study showed that silencing of RAGE tended to attenuate oxidized-LDL-triggered PAI-1 expression in human cultured macrophages, as well as oxidized-LDL-induced tissue factor expression in peritoneal macrophages, suggesting a possible role of RAGE in prothrombogenic molecular regulation. In conclusion, the present study provides in vivo evidence that RAGE plays an integral role in the initiation and progression of nondiabetic atherosclerosis, suggesting that RAGE may be a novel target for treating not only diabetic but also nondiabetic vascular complications.
Keywords: Advanced glycation end products (AGE), RAGE, Atherosclerosis, WHHLMI rabbits, Oxidized LDL
Introduction
Major risk factors such as diabetes, hyperlipidemia and hypertension are recognized to increase atherothrombotic disorders. Especially, diabetes increases the morbidity and mortality of cardiovascular dis-eases1,2). There is increasing evidence that inflammation, thrombogenicity and proteolysis are associated with the progression and instability of atherosclerotic plaques in diabetes3-6). Several mediators such as monocyte chemoattractant protein-1 (MCP-1), plasminogen activator inhibitor 1 (PAI-1), tissue factor (TF) and matrix metalloproteinase-9 (MMP-9) have been shown to contribute to the process of diabetic vascular complica-tions3-6). Hyperlipidemia is also a well-known risk factor for cardiovas-cular diseases, and lipid-lowering therapy has been shown to prevent the risk of cardiovascular diseas-es6,7). A hyperlipidemia-dependent atherosclerotic animal model, Watanabe heritable hyperlipidemic (WHHL) rabbits prone to myocardial infarction (WHHLMI), has been em-ployed for the research of hyperlipidemic atherosclerosis8).
Advanced glycation end products (AGE) are nonenzymatically glycated and oxidized modifiers of proteins and lipids9). Activation of a major receptor of AGE (RAGE) enhances inflammatory cell infiltration including macrophages and T cells and oxidant stress, and an AGE/RAGE axis plays a central role in the pathogenesis of diabetic vascular complications10,11). However, it remains to be elucidated whether RAGE mediates vascular responses in nondiabetic atherosclerosis associated with hyperlipidemia
In this study, we attempted to clarify the role of RAGE in hyperlipidemia-dependent atherosclerosis using WHHLMI rabbits. Furthermore, the effect of the knockdown of RAGE on atherosclerosis-related molecular expression was investigated in human monocyte-derived macrophages and RAGE−/−mouse peritoneal macrophages.
Materials and Methods
Animals
Male and female WHHLMI rabbits with no sex-related differences in atherosclerosis at 1 to 14 months were bred at the Institute for Experimental Animals, Kobe University Graduate School of Medicine8). New Zealand White (NZW) rabbits were obtained from CLEA Japan Inc. (Tokyo, Japan). The animals were kept in rooms equipped with laminar-flow filters at a temperature of ≈22°C and were fed a standard rabbit chow in the Experimental Animal Laboratory of Fukushima Medical University8,12). The RAGE targeting construct and the generation of RAGE−/− mice were described previous-ly13,14). C57BL/6J wild-type and RAGE−/− mice were produced by mating C57BL/6J RAGE+/− mice, weaned at age 4-5 weeks and maintained in a temperature-controlled (22°C) fa-cility in the Osaka City University Graduate School of Medicine. Procedures in this study were approved by the animal care and use committee at the Osaka City University Graduate School of Medicine. The experiments of RAGE−/− mice were also approved by the Animal Research Committee at Fukushima Medical University.
Study Protocol of WHHLMI Rabbits
One to 14 month-old rabbits were selected on the basis of their serum cholesterol levels, which ranged from 13.0 to 18.1 mmol/l, to make the degrees of atherosclero-sis8,15). Rabbits were anesthetized by an intravenous injection of 50 mg/kg sodium pentobarbital. The thoracic aortas and coronary arteries of the rabbits were removed and used for immunohistochemical analysis of RAGE and its cellular origins in atheromatous plaques. These experiments were carried out under the control of the Animal Research Committee in accordance with the Guidelines on Animal Experiments of Kobe University Graduate School of Medicine and Fukushima Medical University, the Japanese Government Animal Protection and Management Law (No. 105), as well as the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996).
Immunohistochemistry
Immunohistochemical staining was performed as previously described8,12). Briefly, rabbits were anesthetized and perfused with lactated Ringer’s solution and then Bouin’s fixative by use of a perfusion apparatus at a constant pressure of 100 mmHg. After perfusion-fixation, the atherosclerotic lesions of the aortae and coronary arteries were excised and then immersed in Bouin’s fixative for at least 24 hours. After immersion-fixation, the atherosclerotic lesions of descending thoracic aortae and coronary arteries were embedded in paraffin and cut into 4-µm-thick section. The sections were used for immunohistochemical analysis. Sections were reacted at 4°C overnight with monoclonal antibodies against rabbit macrophages (RAM11, Dako Cytomation, Carpinteria, CA) diluted 1:100, smooth muscle α-actin (1A4, 1:100 dilution, Dako Cytomation) and endothelial cells (CD31, 1:100 dilution, Dako Cytomation), phospholipid products, a component of oxidized LDL (ox-LDL) (DLH3)16) diluted 1:100, AGE (6D12, 1:100 dilution, Trans Genic Inc., Kumamoto, Japan) and nitrotyrosine (Kamiya Biomedical Co., Seattle, WA) diluted 1:100, and with polyclonal antibody against RAGE (Millipore, Billerica, MA) diluted 1:400. A streptavidin-biotinylated horseradish peroxidase system (Nichirei, Tokyo, Japan) was used and antibody binding was visualized with 3, 3´-diaminobenzidine and hydrogen peroxide (DAB SUBSTRATE KIT FOR PEROXIDASE, Vector Laboratories, Burlingame, CA).
RAGE Expression and Cellular Components of Atheromatous Plaques
We defined the atheromatous lesion between endothelial cells and internal elastic laminae of arteries as plaque area under a light microscope at magnification ×100 as described previously8,12,15). The percent area of RAGE expression was defined as the RAGE positive area to the total plaque area. In the same way, the percent areas of macrophages and smooth muscle cells were defined as the RAM11-positive and 1A4-positive area to the total plaque area, respectively. Image analysis was performed to quantify the immunoreactive area using Image J 1.34 (National Institutes of Health, Bethesda, MD).
Mesurement of Serum AGE Levels in WHHLMI Rabbits
The level of serum AGE was measured using ELISA as described previously17). Briefly, a 96-well microtiter plate was coated by overnight incubation at 4°C with monoclonal antibody, which detects non-carboxymethyllysine (CML) AGE. Then 100 µl of rabbit serum was added to each well and incubated for 2 hours at room temperature with gentle shaking on a horizontal rotary shaker. Immunoreactivity of each fraction was read from the calibration curve and was expressed as AGE unit (U) per ml, with one unit corresponding to the amount of antibody reactive material found in AGE-bovine serum albumin at a protein concentration of 1 µg/ml.
Isolation of Peripheral Blood Monocytes and Culture of Monocyte-Derived Macrophages
Human peripheral blood monocytes were isolated by density centrifugation and adherent method from normal healthy volunteers and the purity of isolated monocytes was greater than 95% as determined by flow cytometry and cytohistochemistry as described previously18,19). Human monocytes were plated in collagen type I-coated 6-well plate (BD Biosciences, Bedford, MA) and incubated in a humidified incubator at 37°C in RPMI 1640 medium containing 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA) for up to 30-72 hours to differentiate into macrophages. Cells were more than 90 % viable as assessed with trypan blue exclusion. Ethical approval was obtained from Fukushima Medical University for the study.
Preparation of Oxidized LDL and Lipoprotein Deficient Serum (LPDS)
Oxidized LDL and LPDS were prepared as described previously20). Human LDL (density = 1.019-1.063 g/ml) and LPDS (density > 1.21 g/ml) were isolated from serum of fasting normolipidemic volunteers by sequential ultracentrifugation. Oxidized LDL was prepared by incubating native LDL for 24 hours at 4°C in phosphate-buffered saline (PBS) containing 5 µmol/l CuSO4, then extensively dialyzed against PBS and sterilized by filtration. LPDS was dialyzed against saline containing 20 mmol/l CaCl2 at room temperature and against saline to remove CaCl2. Then, the sample was incubated with silicic acid at 37°C for 4 hours, adjusted to 50 mg/ml with saline and then sterilized by filtration for use.
Fluorescent Immunohistochemistry
Monocytes were cultured in type I collagen-coated chamber slides (BD BioCoatTM, BD Biosciences) with RPMI 1640 medium containing 10% FBS for 72 hours. Cytospin sample slides of monocytes were also prepared. Fluorescent immunohistochemistry of RAGE in monocytes and monocyte-derived macrophages was performed as described previously21). The slides were fixed in 4% formaldehyde for 60 minutes and were not permeabilized, and incubated with anti-RAGE antibody (Millipore) for 20 minutes. After three washes in PBS, Alexa-Fluor 594-conjugated secondary antibody (Molecular Probes, Eugene, OR) was reacted. Stained slides were stored in the dark until they were analyzed by a confocal microscope (Olympus, Tokyo, Japan) with argon (488 nm) lasers.
Knockdown of RAGE
RAGE expression was silenced by small interfering RNA (siRNA) 5´-CACUGCAGUCGGAGCUAAUGG-3´ (sense strand)22). Monocyte-derived macrophages were transfected with double-strand siRNA in serum-free medium mixed with lipofectamine (Invitrogen) according to the manufacturer’s instructions. Four hours after transfection, monocyte-derived macrophages were incubated in a medium containing 2 mg/ml LPDS for 24 hours and subsequently stimulated by 50 µg/ml oxidized LDL. Alternatively, cells were treated with an irrelevant siRNA 5´-GUACCGCACGUCAUUCGCAUC-3´ (sense strand) as a negative control.
Western Blotting
The expression of RAGE, PAI-1, TF and β-actin was determined by Western blotting as described previously23,24). Oxidized LDL (50 µg/ml) was added to nearly confluent human monocytes-derived macrophages in collagen type I-coated 6-well plates and incu-bated for 18 hours with or without silencing of RAGE by siRNA. The whole cells were collected and solubilized with a hypotonic lysis buffer and the protein concentrations were measured by the Bradford method. Aliquots containing 20 µg of proteins were subjected to SDS/polyacrylamide gel electrophoresis. For Western blotting, we used specific antibodies to RAGE (Santa Cruz Biotechnology Inc., CA) diluted 1:200, to PAI-1 (Molecular Innovations, Southfield, MI) diluted 1:1,000, to TF (Santa Cruz Biotechnology) diluted 1:200, and to β-actin (Santa Cruz Biotechnology) diluted 1:2,000. The signals from immunoreactive bands were visualized by an Amersham ECL system (Amersham Pharmacia Biotech UK Ltd., Buckinghamshire, England).
Isolation of Mouse Peritoneal Macrophages
We used RAGE−/− mice and wild-type littermate mice at the age of 7 weeks to isolate peritoneal macrophages. RAGE−/− and wild-type mice were injected with 2 ml of thioglycollate medium (Becton, Dickinson and Company, Sparks, MD) into the peritoneal cavities. After 3 days, the mice were sacrificed and injected with 5 ml PBS into peritoneal cavities to collect peritoneal macrophages. Cells were cultured with or without 5 µg/ml oxidized LDL for 18 hours in RPMI 1640 medium containing 2 mg/ml lipoprotein deficient bovine calf serum (Biomedical Technologies Inc., Stoughton, MA).
Densitometric Analysis
After scanning blots into a computer (CANON Canoscan 8400F, Canon Inc., Tokyo, Japan), the optical densities of individual immunoblots were analyzed using Image J 1.34 (National Institutes of Health, Bethesda, MD) as described previously23,24).
Statistical Analysis
Statistical analyses were performed using ANOVA with Scheffé’s post hoc test and un-paired Student’s t-test. A level of P<0.05 was considered significant. Data are represented as means±S.D.
Results
Atheromatous Plaques with Aging
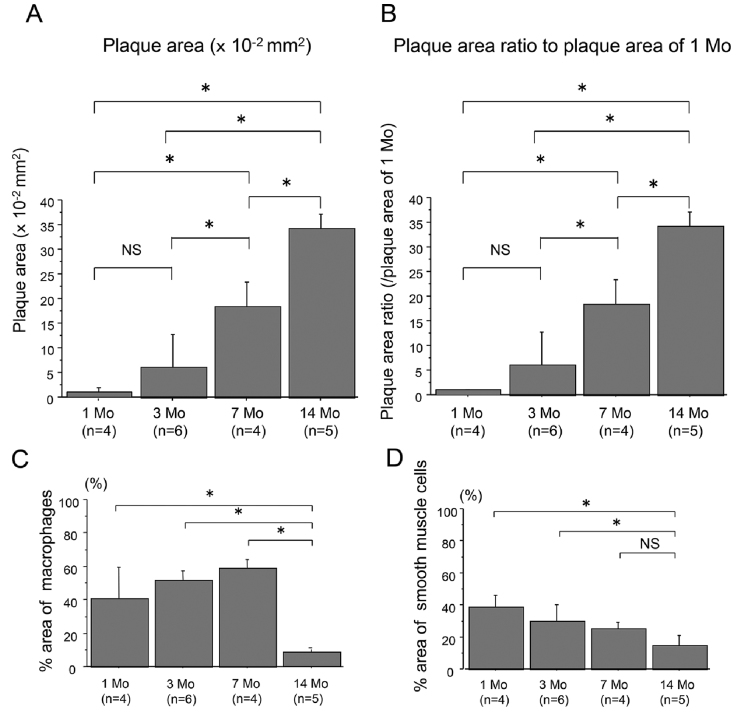
Atheromatous plaques of the aortae of WHHLMI rabbits were significantly found as early as at 1 month and accelerated at 3 through 14 months (Figs. 1A and 1B). The data also show the infiltration of cellular components including macrophages and smooth muscle cells (Figs. 1C and 1D). However, at 14 months, the atheromatous plaques consisted of decreased cellular components and increased extracellular fibrous and lipid components compared to 7 months (Figs. 1C and 1D), which was consistent with the previous report15).
Fig. 1.
Quantitative analysis of the absolute plaque areas (A) and proportions (B) of aortic plaques of WHHLMI rabbits with aging (1, 3, 7 and 14 months, n=4 to 6), as well as % area of macrophages (C) and smooth muscle cells (D). The proportions of plaques were determined by the control values at 1 month (B). Measured values are expressed as mean±S.D. *P<0.05. Mo, months and NS, not significant.
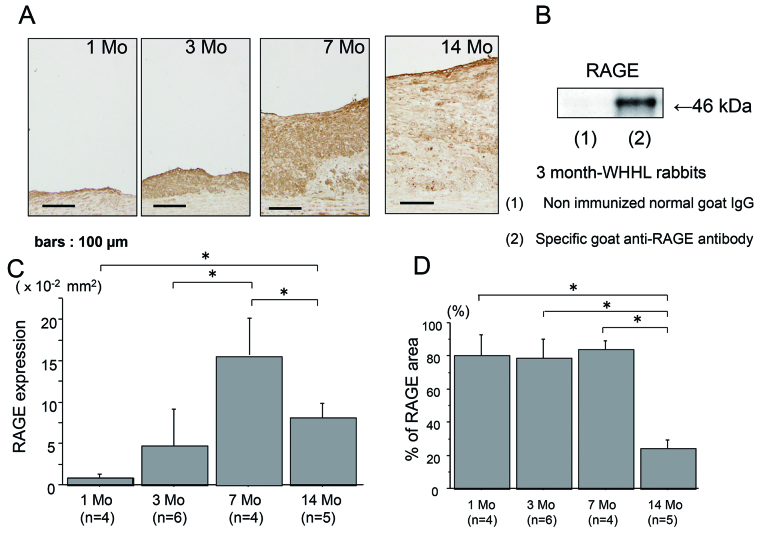
RAGE expression
The expression of RAGE was noted as early as at 1 month in hyperlipidemia-dependent atherosclerotic lesions and progressively increased at 3 and 7 months (Fig. 2A), and the intensive expression of RAGE in the aortae of 3 months of WHHLMI rabbits as determined by Western blotting (Fig. 2B). However, the atherosclerotic plaques at 14 months had the decreased expression of RAGE compared with 7 months (Figs. 2C and 2D).
Fig. 2.
The expression of RAGE in the aortic atherosclerotic plaques of WHHLMI rabbits with aging by immunohistochemistry (A) and Western blotting (B). A: RAGE expression at 1, 3, 7 and 14 months. B: Western blotting of RAGE in the aortae of 3-month-WHHLMI rabbits. Immunoblot was probed with a specific goat anti-RAGE antibody (lane 2), whereas nonimmunized normal goat IgG was used as a negative control antibody (lane 1). RAGE was identified at the band of 46 kD in the atherosclerotic lesions of 3-month-WHHLMI rabbit. C and D: Quantitative analysis of RAGE expression of area (C) and proportion (D) in the atherosclerotic plaques of WHHLMI rabbits at 1, 3, 7 and 14 months (n=4 to 6, each month). Measured values are represented as mean±S.D. *P<0.05.
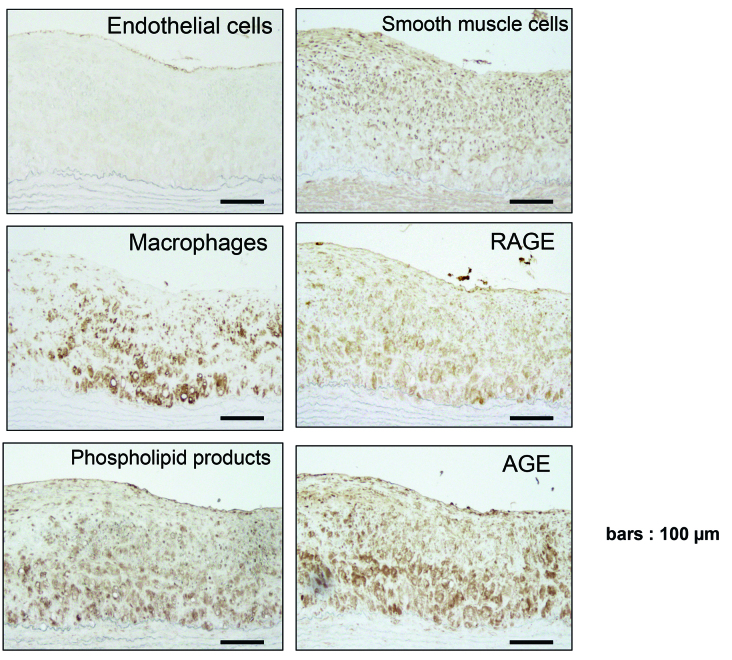
RAGE, AGE and Cellular Component Expression
Fig. 3 shows the cellular components and the expression of RAGE, AGE and phospholipid products at 7 months in the atheromatous plaques by immunohistochemistry. The expression of RAGE appeared to be distributed in the areas of cellular components including endothelial cells, smooth muscle cells and macrophages. In addition, RAGE and macrophage were distributed in phospholipid products expressing area. Interestingly, AGE and RAGE were accumulated in nondiabetic hyperlipidemia-dependent atheromatous plaques.
Fig. 3.
Immunohistochemistry of the aortic atherosclerotic plaques of 7-month-WHHLMI rabbits. Results were from one representative out of four 7-month-WHHLMI rabbits. Immunostainings for endothelial cells (CD31), smooth muscle α-actin (1A4), AGE, RAGE, macrophages (RAM-11), phospholipid products (DLH3) and RAGE and AGE are shown.
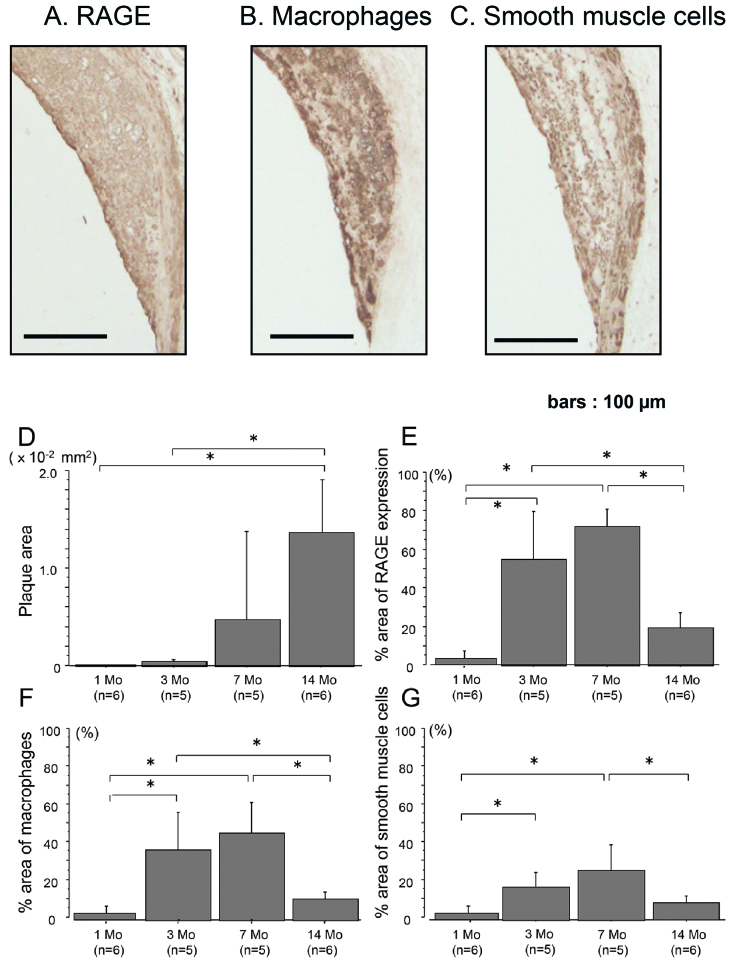
RAGE Expression in Coronary Atheromatous Plaques
The components of coronary atheromatous plaques and the original sources of RAGE were similar to those of aortic plaques at 7 months. The abundant infiltration of macrophages and smooth muscle cells were found in the coronary atherosclerotic lesions. RAGE expression was identified as the cellular sources of macrophages and smooth muscle cells in addition to endothelial cells (Figs. 4A, 4B and 4C). The data also show the quantitative analysis of the area of coronary atheromatous plaques (Fig. 4D), and RAGE expression (Fig. 4E) and its cellular sources with aging (Figs. 4F and 4G). RAGE expression was progressively increased with aging up to 7 months and markedly decreased at the age of 14 months, which was consistent with the extent of cellular infiltration.
Fig. 4.
A, B, C: RAGE expression (A) and cellular components including macrophages (B) and smooth muscle cells (C) in coronary atherosclerotic plaques of 7-month-WHHLMI rabbits as determined by immunohistochemistry. Results were the representative sections of the coronary atheromatous lesions from 5 rabbits of 7-month-WHHLMI. D, E, F, G: Quantitative analyses of the amounts of plaques (D), and the proportion of RAGE expression (E) and cellular components including macrophages (F) and smooth muscle cells (G) in coronary plaques of WHHLMI rabbits with aging (1, 3, 7 and 14 months, n=5 to 6). Values are represented as mean±S.D. *P<0.05.
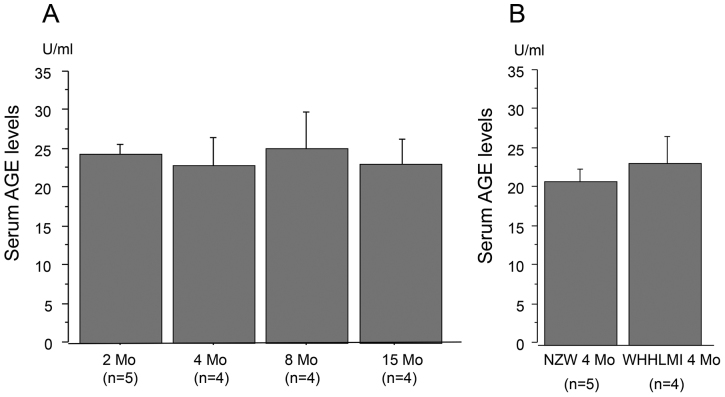
Serum AGE Levels
Fig. 5A demonstrated the serum AGE levels at 2, 4, 8 and 15 months of WHHLMI rabbits, indicating no significant difference in the serum AGE levels among the four groups. Fig. 5B shows the serum AGE levels of WHHLMI rabbits and control NZW rabbits at 4 months. There was no significant difference between two groups. The results suggested that the expression of RAGE in hyperlipidemia-dependent atherosclerotic lesions was not related to the serum AGE levels.
Fig. 5.
A: The serum AGE levels of WHHLMI rabbits with aging (2, 4, 8 and 15 months, n=4 to 5). B: The serum AGE levels of WHHLMI rabbits (n=4) and NZW rabbits (n=5) at 4 months. Data are expressed as mean±S.D.
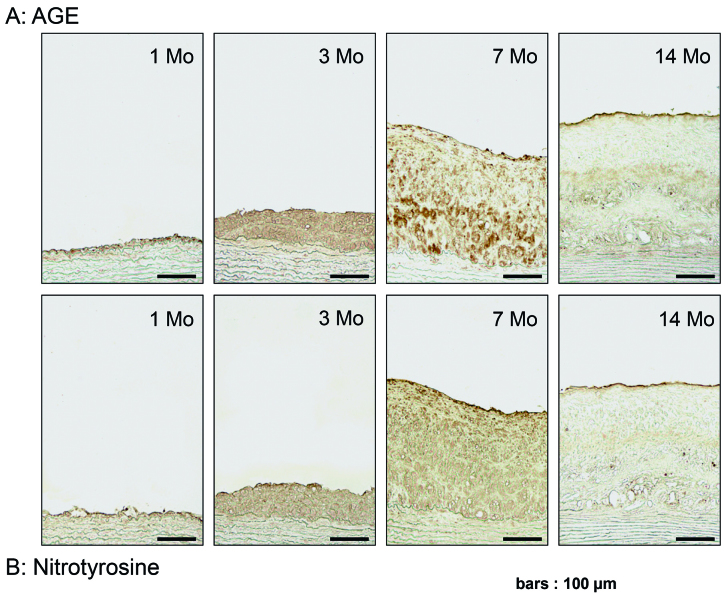
AGE Accumulation and Nitrotyrosine Expression with Aging
Fig. 6A shows that the deposition of AGE was increased with aging and that AGE was predominantly recognized at the cellular components infiltrating into atheromatous plaques in WHHLMI rabbit aortae. The deposition of AGE was noted at endothelial cells as early as at 1 month. The whole expression of AGE was increased with aging at 3 and 7 months, and diminished at the age of 14 months. Moreover, immuno-histochemistry demonstrated the expression of indirect oxidant marker nitrotyrosine with ag-ing25), which was similar to AGE deposition (Fig. 6B). The data suggested that AGE were produced by oxidative stress and that the profiles of AGE accumulation and nitrotyrosine expression were similar to RAGE expression.
Fig. 6.
AGE accumulation (A) and nitrotyrosine expression (B) in aortic atherosclerotic plaques with aging as determined by immunohistochemistry. Results were the representative sections of the aortic atheromatous lesions from 1, 3, 7 and 14 months of WHHLMI rabbits.
Role of RAGE in Atherosclerosis-Related Molecular Expression
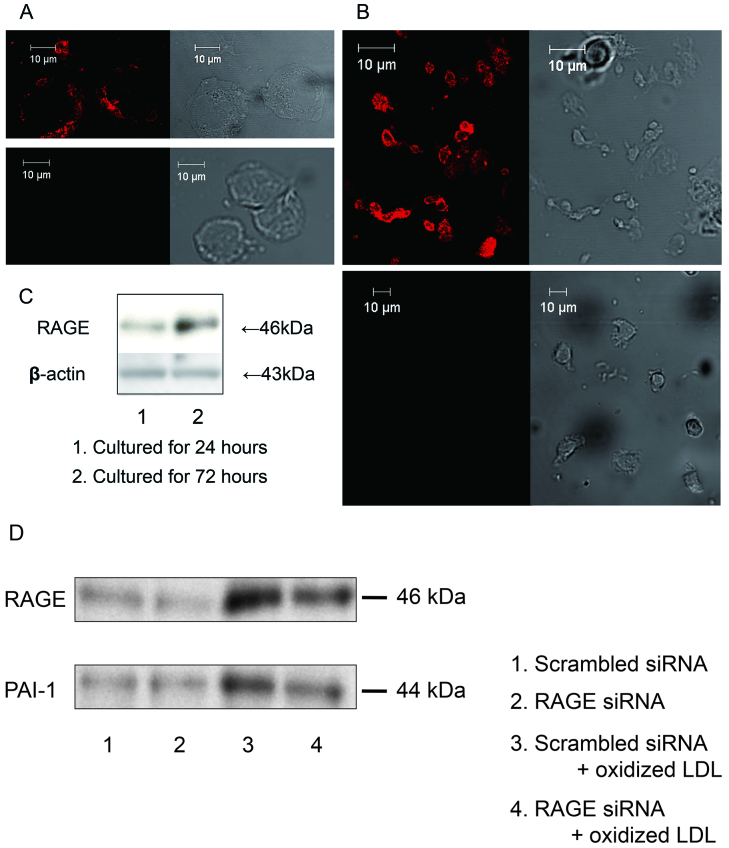
Fluorescent immunohistochemistry revealed the expression of RAGE on plasma membrane both of human monocytes and monocyte-derived macrophages (Figs. 7A and 7B). RAGE expression was strongly identified in macrophages cultured for 72 hours rather than for 24 hours by Western blotting (Fig. 7C), suggesting that RAGE expression was increased by the differentiation into macrophages. Fig. 7D shows that oxidized LDL increased the expression of RAGE and PAI-1 in human monocyte-derived macrophages. The silencing of RAGE in the macrophages tented to block PAI-1 expression.
Fig. 7.
A: Immunofluorescent detection of RAGE in human circulating monocytes (upper left panel, specific anti-RAGE antibody; upper right panel, phase contrast; lower left panel, non immunized normal goat IgG; lower right panel, phase contrast). B: Immunofluorescent detection of RAGE in monocyte-derived macrophages cultured for 72 hours (upper left panel, specific anti-RAGE antibody; upper right panel, phase contrast; lower left panel, non immunized normal goat IgG; lower right panel, phase contrast). C: The expression of RAGE was determined by Western blotting after 24 and 72 hours of culture. D: Effects of knockdown of RAGE on oxidized LDL-triggered PAI-1 expression in cultured macrophages. Isolated monocytes were incubated for 18 hours in the presence or absence of 50 µg/ml oxidized LDL after transfection of RAGE siRNA or scrambled siRNA. PAI-1 expression was determined by Western blotting. Immunoblots are from one experiment representative from three separate experiments.
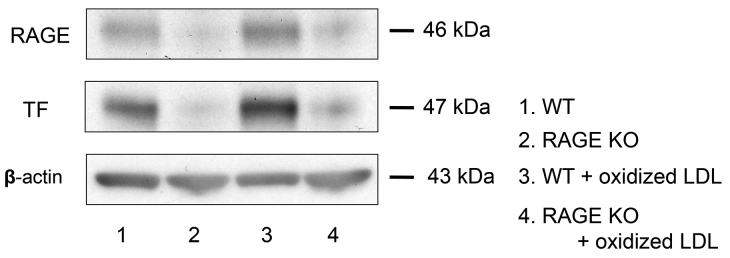
In addition, oxidized LDL promoted RAGE and TF expression significantly in peritoneal macrophages from wild mice. The knockout of RAGE tented to decrease oxidized-induced TF expression in peritoneal macrophages from RAGE−/− mice (Fig. 8).
Fig. 8.
The role of RAGE in TF expression in the absence or presence of oxidized LDL in peritoneal macrophages of RAGE−/− and wild-type mice. The macrophages were incubated for 18 hours with or without 5 µg/ml oxidized LDL. TF and RAGE expression was determined by Western blotting. In peritoneal macrophages from RAGE−/− mice, TF expression was very low at basal condition and the response to oxidized LDL was less compared to wild-type mice. Representative immunoblots from three separate experiments are shown.
Discussion
We show here that RAGE expression was associated with the initiation and progression of atherosclerosis in WHHLMI rabbits, as well as the RAGE-mediated atherosclerosis-related molecular expression.
The present study clearly shows that RAGE expression is concordant with cellular infiltration of macrophages and smooth muscle cells in the atherosclerotic lesions of nondiabetic WHHLMI rabbits. This is consistent with the previous reports on nondiabetic human and animal atherosclerotic lesions11,26). AGE are shown to be generated by oxidative stress, and Calkin et al. reported that administration of antioxidant attenuated the AGE accumulation in mice9,27-29). Our data demonstrated that AGE was distributed in the areas of phospholipid products and nitrotyrosine in the atherosclerotic lesions. The present study suggested that hyperlipidemia triggered-oxidative stress may accelerate AGE generation and RAGE expression in cellular components29-31), especially macrophages in the nondiabetic atherosclerosis.
It is well acknowledged that atheromatous RAGE expression is accelerated with high plasma glucose levels and stronger in diabetics than nondiabetics5,26). Soro-Paavonen et al. have shown that knockout of RAGE attenuates the development of atherosclerosis in diabetic mice10). These suggested a central role of RAGE in the atherosclerosis complicated with diabetes. In addition, several studies have suggested that RAGE expression is involved in inflammatory cell infiltration, thrombogenicity and plaque destability through various signaling pathways in diabetic and nondiabetic atherosclerotic lesions32-36). Harja et al. have demonstrated that endothelial RAGE modulates vascular and inflammatory responses independent of diabetes in apoE−/− mice, suggesting the important role of RAGE in the pathogenesis of nondiabetic atherosclerosis11).
We demonstrated that the significant expression of RAGE was recognized in endothelial cells as early as at 1 month in the aortic atherosclerotic lesions of WHHLMI rabbits, as well as at 3 and 7 months. This finding concurred with the results reported by Roy et al. that RAGE is expressed in the endothelial cells of the aortic atherosclerotic lesions of diabetic 2-month-WHHL rabbits37).
The glucose metabolism in WHHLMI rabbits is a critical issue in this study, which was extensively investigated in adult (10- to 15-month-old) and middle-aged (17- to 21-month-old) WHHLMI rab-bits38). Briefly, changes in blood sugar levels were similar among the three groups, adult normal and WHHIMI rabbits and middle-aged WHHIMI rabbits. In terms of glucose tolerance, there were two groups in both adult and middle-aged WHHLMI rabbits. The rabbits with high fasting immunoreactive insulin (IRI) levels showed insulin resistance as determined by HOMA-R and Matsuda-insulin sensitivity index (ISI). However, overall, there was no significant difference in glucose tolerance among the three groups (normal adult rabbits, and adult and middle-aged WHHIMI rabbits). Also, we have the data regarding the glucose metabolism including fasting blood sugar levels, IRI concentrations and HOMA-R in 6-month, 12-month and 18-month-old WHHIMI rabbits, demonstrating that the changes in glucose metabolism are similar among the three groups (unpublished data).
Endothelial dysfunction accelerates the development of atherosclerosis including monocyte chemotaxis, monocytes adhesion to endothelial cells and proteoly-sis19,23,39). Moreover, MCP-1, vascular cell adhesion molecule-1 (VCAM-1) and MMP-2 are modulated by RAGE in endothelial cells under nondiabetic conditions11). Taken together, our results regarding strong expression of RAGE in endothelial cells suggested that endothelial RAGE may initiate and accelerate nondiabetic atherosclerosis independent of diabetes.
In the present study, we showed that RAGE expression was accelerated with cellular infiltration and that the atherosclerotic lesions with reduced cellular components at 14 months had marked decrease in RAGE expression. However, we found no significant difference in the serum AGE levels with aging, as well as between WHHLMI rabbits and normal NZW rabbits. These results suggested that serum AGE levels were not a determinant of RAGE expression in atherosclerotic lesions.
We attempted to clarify the issue what regulates RAGE expression in nondiabetic atheromatous plaques. Since Oka et al. reported that the plasma cholesterol and oxidized LDL levels are markedly high as early as 1 month with the persistent high levels in WHHL rabbits40), one possibility may be hyperlipidemia which impairs endothelial function and accumulates macrophages in plaques39). Although Roy et al. found RAGE expression in atherosclerotic lesions of diabetic WHHL rabbits37), we demonstrated the expression of RAGE in endothelial cells and macrophages of pure hyperlipidemia-dependent atherosclerotic animal model independent of diabetes. Our in vitro study demonstrated that oxidized LDL increased RAGE expression in macrophages. These findings suggest the regulation of RAGE expression by hyperlipidemia and oxidized LDL.
Another possibility of the mechanism of RAGE expression in nondiabetic athrosclerotic lesions may be oxidative stress28), which nonenzymatically generates AGE. It has been shown that statin suppresses RAGE expression by decreasing oxidant stress-dependent AGE generation in human diabetic atherosclerotic plaques36). Statin has been also reported to attenuate RAGE expression probably via decreased oxidant stress in the absence of lipid-lowering effect29,41). In the present study, RAGE expression and AGE accumulation were associated with nitrotyrosine expression with cellular infiltration in the atherosclerotic lesions. This finding suggested that the accumulation of peroxynitrate-mediated protein oxidation reflected hyperlipidemia-dependent oxidative stress, which may induce AGE accumulation and RAGE expression in plaques.
Burke et al. have suggested that RAGE expression may be involved in the plaque instability in diabetes since stronger RAGE expression in plaques was found in sudden death diabetic subjects than nondiabetics26). Our study using WHHLMI rabbits suggested the possibility that the intensive expression of RAGE in endothelial cells and macrophages of coronary atherosclerotic lesions may contribute to inflammation, thrombogenicity and extracellular matrix degradation in plaques, in turn, plaque destabilization. Further study is required for a better understanding of the mechanism by which RAGE signaling leads to plaque instability.
In conclusion, we show that RAGE may play an integral role in the pathogenesis of atherosclerosis in WHHLMI rabbits independent of diabetes, suggesting that RAGE may be a good target to treat diabetic and nondiabetic vascular complications.
Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (21590935 and 20790538), Fukushima Medical University Research Project, Takeda Science Foundation and The Uehara Memorial Foundation.
Conflict of Interest
The authors declare that they have no conflict of interest relevant to this article.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Eng J Med, 339: 229-234, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA, 287: 2570-2581, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature, 414: 813-820, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Sobel BE, Woodcock-Mitchell J, Schneider DJ, Holt RE, Marutsuka K, Gold H. Increased plasminogen activator inhibitor type 1 in coronary artery atherectomy speciments from type 2 diabetic compared with nondiabetic patients: a potential factor predisposing to thrombosis and its persistence. Circulation, 97: 2213-2221, 1998. [DOI] [PubMed] [Google Scholar]
- 5.Cipollone F, Iezzi A, Fazia M, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation, 108: 1070-1077, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. The SANDS randomized trial. JAMA, 299: 1678-1689, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90056 participants in 14 randomized trials of statins. Lancet, 366: 1267-1278, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbit). Arterioscler Thromb Vasc Biol, 23: 1239-1244, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation, 114: 597-605, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Soro-Paavonen A, Watson AMD, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes, 57: 2461-2469, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest, 118: 183-194, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shindo J, Ishibashi T, Yokoyama K, et al. Granulocyte-macrophage colony-stimulating factor prevents the pro-gression of atherosclerosis via changes in the cellular and extracellular composition of atherosclerotic lesions in Watanabe heritable hyperlipidemic rabbits. Circulation, 99: 2150-2156, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Shoji T, Koyama H, Morioka T, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes, 55: 2245-2255, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto Y, Doi T, Kato I, et al. Receptor for advanced glycation end products is a promising target of diabetic nephropathy. Ann N Y Acad Sci, 1043: 562-566, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Shiomi M, Ito T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of develop-ment: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis, 207: 1-7, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Itabe H, Takeshima E, Iwasaki H, et al. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. J Biol Chem, 269: 15274-15279, 1994. [PubMed] [Google Scholar]
- 17.Takeuchi M, Makita Z, Bucala R, et al. Immunological evidence that non-carboxymethyllysine advanced glycation end-products are produced from short chain sugars and dicarbonyl compounds in vivo. Mol Med, 6: 114-125, 2000. [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata K, Ishibashi T, Sakamoto T, et al. Effects of blockade of the renin-angiotensin system on tissue factor and plasminogen activator inhibitor-1 synthesis in human cultured monocytes. J Hypertens, 19: 775-783, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Ishibashi T, Sakamoto T, Ohkawara H, et al. Integral role of RhoA activation in monocyte adhesion-triggered tissue factor expression in endothelial cells. Arterioscler Thromb Vasc Biol, 23: 681-687, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama K, Ishibashi T, Yi-qiang L, Nagayoshi A, Teramoto T, Maruyama Y. Interleukin-1 beta and interleukin-6 increase levels of apolipoprotein B mRNA and decrease accumulation of its protein in culture medium of HepG2 cells. J Lipid Res, 39: 103-113, 1998. [PubMed] [Google Scholar]
- 21.Okumura AJ, Hatsuzawa K, Tamura T, et al. Involvement of a novel Q-SNARE, D12, in quality control of the endomembrane system. J Biol Chem, 281: 4495-4506, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Naito Y, Yamada T, Ui-Tei K, Morishita S, Saigo K. siDirect: highly effective, target-specific siRNA design software for mammalian RNA interference. Nucleic Acids Res, 32(Web Server issue): W124-W129, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakamoto T, Ishibashi T, Sakamoto N, et al. Endogenous NO blockade enhances tissue factor expression via increased Ca2+ influx through MCP-1 in endothelial cells by monocyte adhesion. Arterioscler Thromb Vasc Biol, 25: 2005-2011, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto T, Ishibashi T, Sugimoto K, et al. RhoA-dependent PAI-1 gene expression induced in endothelial cells by monocyte adhesion mediates geranylgeranyl transferase I and Ca2+ signaling. Atherosclerosis, 193: 44-54, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res, 75: 291-302, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics. A postmortem study. Arterioscler Thromb Vasc Biol, 24: 1266-1271, 2004. [DOI] [PubMed] [Google Scholar]
- 27.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes, 40: 405-412, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MM, Heinecke JW. Production of Nε-(carboxymethyl) lysine is impaired in mice deficient in NADPH oxidase. A role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes, 52: 2137-2143, 2003. [DOI] [PubMed] [Google Scholar]
- 29.Calkin AC, Giunti S, Sheehy KJ, et al. The HMG-CoA reductase inhibitor rosuvastatin and the angiotensin receptor antagonist candesartan attenuate atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes via effects on advanced glycation, oxidative stress and inflammation. Diabetologia, 51: 1731-1740, 2008. [DOI] [PubMed] [Google Scholar]
- 30.Faria A, Persaud SJ. Cardiac oxidative stress in diabetes: Mechanisms and therapeutic potential. Pharmacol Ther, 172: 50-62, 2017. [DOI] [PubMed] [Google Scholar]
- 31.Matsui T, Higashimoto Y, Nishino Y, et al. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes, 66: 1683-1695, 2017. [DOI] [PubMed] [Google Scholar]
- 32.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE. A Scaffold for the macrovascular complications of diabetes and beyond. Circ Res, 93: 1159-1169, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AM, Yan SD, Wautier JL, Stern D. Activation of receptor for advanced glycation end products: a mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ Res, 84: 489-497, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Naka Y, Bucciarelli LG, Wendt T, et al. RAGE axis: animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol, 24: 1342-1349, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Aikawa M, Rabkin E, Sugiyama S, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation, 103: 276-283, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Cuccurullo C, Iezzi A, Fazia ML, et al. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol, 26: 2716-2723, 2006. [DOI] [PubMed] [Google Scholar]
- 37.Roy H, Bhardwaj S, Babu M, et al. VEGF-A, VEGF-D, VEGF receptor-1, VEGF receptor-2, NF-κB, and RAGE in atherosclerotic lesions of diabetic Watanabe heritable hyperlipidemic rabbits. FASEB J, 20: 2159-2161, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Shiomi M, Kobayashi T, Kuniyoshi N, et al. Myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits with mesenteric fat accumulation are a novel animal model for metabolic syndrome. Pathobiol, 79: 329-38, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arterioscler Thromb Vasc Biol, 27: 266-274, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Oka K, Yasuhara M, Suzumura K, Tanaka K, Sawamura T. Antioxidants suppress plasma levels of lectinlike oxidized low-density lipoprotein receptor-ligands and reduce atherosclerosis in Watanabe heritable hyperlipidemic rab-bits. J Cardiovasc Pharmacol, 48: 177-183, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Lim S, Barter P. Antioxidant effects of statins in the management of cardiometabolic disorders. J Atheroscler Thromb, 21: 997-1010, 2014. [DOI] [PubMed] [Google Scholar]