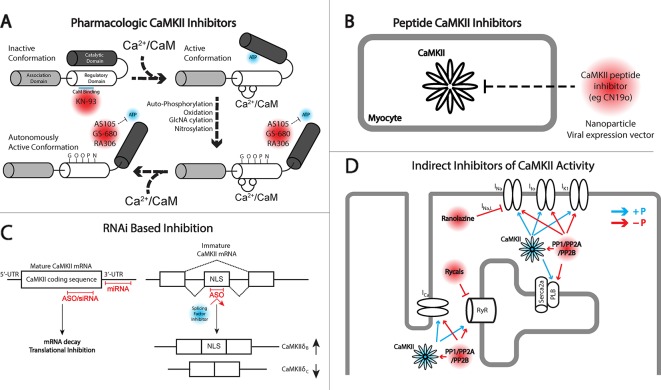
Figure 1.
Different approaches for targeting Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling. (A) Depiction of inactive and active monomers of CaMKII showing the association, regulatory, and catalytic domains. The association domain is responsible for interaction with other CaMKII monomers and is necessary for forming the holoenzyme structure, consisting of 12 monomers. In its inactive state, the catalytic domain is obscured by interaction with the regulatory domain. This interaction is disrupted upon the binding of Ca2+/CaM leading to autophosphorylation by neighboring CaMKII monomers, in addition to other posttranslational modifications including oxidation, GlcNAcylation, and nitrosylation, maintaining CaMKII activation even upon release of Ca2+/CaM. KN-93 is a known allosteric inhibitor of CaM binding and therefore preferentially targets CaMKII in the inactive state. CaMKII inhibitors AS105, GS-680, and RA306 are novel pyrimidine–based, ATP-competitive inhibitors that inhibit the activated catalytic domain of CaMKII and represent potential therapeutic agents for translational CaMKII inhibition. (B) Peptide inhibitors of CaMKII (e.g., CN19o, refined from CaMKIItide) show favorable selectivity and potency for CaMKII inhibition but face challenges in delivery and bioavailability. Both viral gene delivery and novel advances in nanoparticles offer opportunities for delivery of these agents to the heart. (C) RNA interference (RNAi) is a novel approach for inhibiting CaMKII activity at the transcript level. Antisense oligonucleotides (ASOs), small interfering RNA (siRNA), and miRNAs provide opportunity for degrading CaMKII transcripts and/or inhibiting protein translation. ASOs can also be used to interfere with recruitment of splicing factors to enhance concentrations of CaMKIIδB which has been shown to have cardioprotective effects. (D) Indirect inhibition of CaMKII can be achieved by regulating downstream targets of CaMKII kinase activity. An example comes from the late Na+ current (INa,L) inhibitor ranolazine, or the RyR stabilizing agents, rycals. Alternatively, protein phosphatases may be targeted to antagonize kinase activity in cardiomyocytes. The development of phosphatase activators for cancer therapeutics may offer opportunity for drug applications in cardiovascular disease.