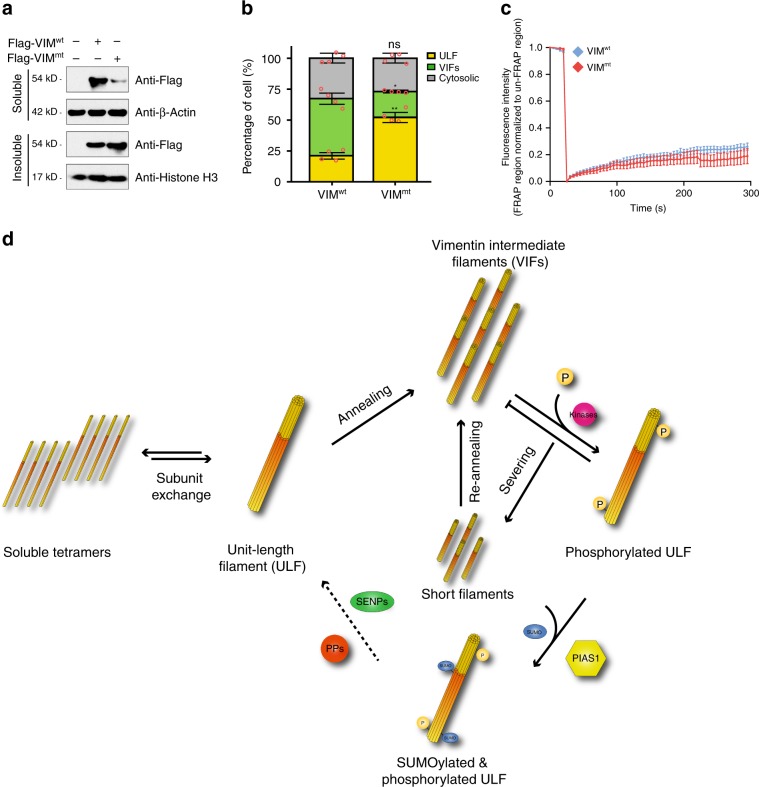
Fig. 7. SUMOylation of vimentin regulates its dynamic assembly.
a HeLa cells were transfected with an empty Flag vector as a negative control, Flag-VIMwt, and Flag-VIMmt, and were separated into RIPA-soluble and -insoluble fractions. VIM protein levels were examined by western blotting. b MCF-7 cells were transfected with Emerald wild-type vimentin (VIMwt) and Emerald vimentin K439/445R, double mutant (VIMmt), and the proportion of unit-length filament (ULF), vimentin intermediate filament (VIF), and cytosolic vimentin between VIMwt and VIMmt were calculated under a microscope. Data represent the mean ± SD, error bars represent SD, ns nonsignificant, *p < 0.05, **p < 0.01, Student’s t-test, n = 4 biologically independent samples. c Fluorescence recovery after photobleaching (FRAP) assay of Emerald wild-type vimentin (VIMwt) and Emerald vimentin K439/445R, double mutant (VIMmt) in MCF-7 cells. Line plot shows average fluorescence at each time point ± SD. Differences between values for VIMwt and VIMmt were not statistically significant at all time points by Student’s t-test. n = 7 biologically independent cells. d Model of the VIM dynamic assembly and disassembly. Vimentin is maintained in equilibrium between unit-length filament (ULF) and soluble tetramers (subunit exchange step). Vimentin filaments elongate by end-to-end annealing of ULF to form mature vimentin intermediate filament (VIF; annealing step). The phosphorylation-dependent shortening of VIF (severing step) involves phosphorylation on Ser-39 and Ser-56 of vimentin by several kinases, including Akt1. The short filaments can re-anneal with another ULF to form new VIF (re-annealing step). However, the phosphorylated ULF is not amenable to the re-annealing process. These phosphorylated ULF products are subject to PIAS1-mediated SUMOylation, stimulating the dephosphorylation of the phosphorylated ULF, and subsequently re-enter to either subunit exchange process or VIF maturation process. Source data are provided as a Source Data file.