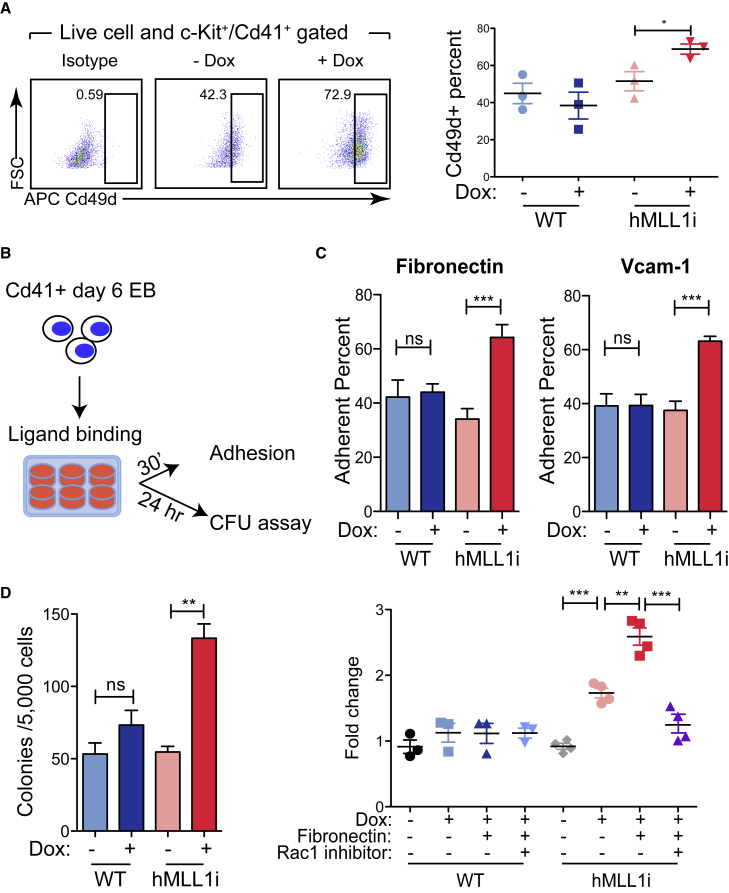
Figure 6.
hMLL1 Induction Specifically Promotes Integrin-Mediated Cell Adhesion and Further Expands CFU
(A) Flow cytometry to detect Cd49d expression in c-Kit+/Cd41+ gated EB cells. Quantification of one representative experiment from three independent differentiation experiments is shown on the right. Data show the average ± SEM, n = triplicate cultures.
(B) Experimental procedure to test integrin function. Day 6 EB Cd41+ enriched WT or hMLL1-inducible cells were cultured on integrin ligand (fibronectin or Vcam1)-coated plates for the indicated time and tested for adhesion or CFU content.
(C) Cell adhesion of Cd41+ enriched progenitors to fibronectin and Vcam1. Data are representative of four independent experiments and presented as the average of triplicate cultures ± SEM.
(D) CFU assay using sorted Cd41+ EB progenitors following 24 h adhesion. Adherent cells were harvested with dissociation buffer and pooled with remaining suspension cells, then counted for CFU assay. Left: both hMLL1-inducible and WT cells were adhered to fibronectin fragment and then followed by CFU assay. One representative experiment of three is shown as the average ± SEM, n = 3 triplicate cultures. Right: quantification of CFU fold changes with Dox, fibronectin fragment, or Rac1 inhibitor (NSC23766, 10 μM). The graph show data pooled from 3 to 4 independent experiments (n = 3 for WT; n = 4 for hMLL1i, with each data point representing the average of triplicate cultures) representing the overall average ± SEM.